Abstract
Coronavirus disease 2019 (COVID-19), which is a respiratory illness associated with high mortality, has been classified as a pandemic. The major obstacles for the clinicians to contain the disease are limited information availability, difficulty in disease diagnosis, predicting disease prognosis, and lack of disease monitoring tools. Additionally, the lack of valid therapies has further contributed to the difficulties in containing the pandemic. Recent studies have reported that the dysregulation of the immune system leads to an ineffective antiviral response and promotes pathological immune response, which manifests as ARDS, myocarditis, and hepatitis. In this study, a novel platform has been described for disseminating information to physicians for the diagnosis and monitoring of patients with COVID-19. An adjuvant approach using compounds that can potentiate antiviral immune response and mitigate COVID-19-induced immune-mediated target organ damage has been presented. A prolonged beneficial effect is achieved by implementing algorithm-based individualized variability measures in the treatment regimen.
Abbreviations: COVID-19, coronavirus disease 2019; CoV, coronavirus; ARDS, acute respiratory distress syndrome; SARS, severe acute respiratory syndrome; MERS, Middle East respiratory syndrome; RT-PCR, reverse transcriptase polymerase chain reaction; IFNβ, interferon-beta; RNA, ribonucleic acid; dsRNA, double-stranded RNA; TLR, toll-like receptors; RIG-I, retinoic acid-inducible gene I; NF-κB, nuclear factor kappa-light-chain; IRF, interferon regulatory factor; IFN, interferon; JAK-STAT, Janus kinase signal transducer and activation of transcription; IFNR, type I IFN receptor; PRM, patterns recognition molecules; IL, interleukin; NK cells, natural killer cells; Th cells, T helper cells; APC, antigen-presenting cells; CTL, cytotoxic T cells; ICU, intensive care unit; IP-10, IFN gamma-induced protein 10; MCP, monocyte chemoattractant protein; TNF, tumor necrosis factor; NLR, neutrophil-lymphocyte ratio; Tregs, regulatory T cells; CD, cluster of differentiation; NKT, NK T cells; HLH, hemophagocytic lymphohistiocytosis; S-1P, sphingosine-1-phosphate; GC, glucosylceramide; HCC, hepatocellular carcinoma; SphK, sphingosine kinase; MT, microtubules; HBC, hyperimmune bovine colostrum; IG, immunoglobulin; HRV, heart rate variability; SK1, sphingokinase 1
Keywords: COVID-19, Coronavirus, Digital health, Antiviral therapy, Antiviral response, ARDS
1. Introduction
Coronavirus (CoV) disease (COVID-19) is a respiratory illness associated with a high mortality rate in some populations. The major obstacles for the clinicians to contain this pandemic are the lack of reliable information, limitations of diagnosis, and accurate prediction of disease prognosis. Additionally, the lack of therapies for this disease and high infectivity have contributed to severe outbreaks worldwide, especially in regions with an elderly population or those where the implementation of infection-limiting strategies has been delayed. In this study, a novel platform has been described for disseminating information on the disease to aid the diagnosis and monitoring of patients with COVID-19. An adjuvant approach using compounds that can potentiate the antiviral immune response and mitigate the COVID-19-associated immune-mediated target organ damage has also been described.
1.1. COVID-19: major problems in developing therapies
Globally, RNA viruses, which are a major threat to human health, infect hundreds of millions of individuals and cause millions of deaths annually. An outbreak of CoV infection that causes respiratory illness was reported in Wuhan, Hubei, China in 2019, a disease that is termed COVID-19 [1], [2], [3]. COVID-19 was declared a pandemic in January 2020 [4], [5]. The estimated case fatality ratio among medically attended patients is approximately 2% [6]. The mean reproduction number (R0) of the virus is estimated to range from 2.24 (1.96–2.55) to 3.58 (2.89–4.39) and the reporting rate has increased 2–8-fold [7]. Current estimates indicate that severe acute respiratory syndrome-CoV-2 (SARS-CoV-2) has a median incubation period of 3 days (range: 0–24 days) with potential asymptomatic transmission [8].
The clinical manifestation of COVID-19 varies from simple respiratory tract infection to septic shock with acute respiratory distress syndrome (ARDS)-like characteristics. The most common symptoms of COVID-19 include fever, cough, and shortness of breath [9]. Real-time polymerase chain reaction (RT-PCR) is used to detect the etiological agent of acute respiratory infections. The diagnosis of patients with COVID-19 is challenging for the healthcare system. The accurate diagnosis of COVID-19 aids in containing and preventing the spread of this epidemic. A study reported that the SARS-CoV-2 testing capacity of most European Union (EU) laboratories was 8275 RT-PCR tests per week [10].
Various studies have reported that both humoral and cell-mediated immune responses protect against COVID-19. Most data on the development of treatment and vaccination strategies for COVID-19 were derived from studies on two related coronaviruses (SARS-CoV and Middle East respiratory syndrome-coronavirus (MERS-CoV)). COVID-19 is phylogenetically related to SARS-CoV. Compared with SARS-CoV, COVID-19-CoV binds to the same host receptor with a lower affinity [11]. Previous studies have reported that the antibody response elicited against the S protein of SARS-CoV protected from the mouse models against the infection [12], [13]. However, the antibody response was short-lived in patients with SARS-CoV [14]. In contrast, T cell response is reported to offer long-term protection. Hence, there is increased interest in developing T cell-modulating prospective drugs and vaccines against SARS [15]. Currently, there are no safe and reliable vaccines or a combination of vaccines to protect against SARS-CoV and MERS-CoV [16].
To contain the COVID-19 pandemic, there is a need to develop a rapid and easily available diagnostic and screening tool [17]. Currently, the virus is detected using RT-PCR, which has varying diagnostic accuracies depending on the kits [18]. To increase the accuracy of diagnostic tests, the samples must be collected from both the upper and lower respiratory tracts [19]. In contrast to other strains of CoVs, SARS-CoV-2 cannot be detected in the blood, urine, or fecal specimens [20].
The initial indications for COVID-19 testing were based on clinical and epidemiological criteria. However, these parameters were not efficient as asymptomatic patients could spread the virus to their close contacts and infected cases without known contacts have been reported [21]. Hence, drive-through centers for mass screening of the population was implemented [22].
Most patients with COVID-19 develop a mild illness. Hence, there is a need to identify patients who will develop severe disease. There are no known biomarkers to predict the disease progression [23], [24], [25]. Patients susceptible to severe forms of COVID-19 are associated with comorbidities, including diabetes, hypertension, and cardiovascular disease [26].
There are no specific treatment strategies for COVID-19, especially for symptomatic cases [27]. Currently, various in vitro, in vivo, and clinical studies are examining potential therapeutic agents against SARS-CoV and MERS-CoV. Interferon-beta (IFNβ) was reported to be the most potent for reducing MERS-CoV replication in vitro [9]. Additionally, in vitro studies have demonstrated that viral RNA replication and transmission are disrupted upon treatment with the adenosine analog Remdesivir [28], [29]. Recent studies examining the pathogenesis of the disease have challenged the efficacy of currently employed therapeutic options [30], [31].
1.2. Digital platform for disseminating information to health care providers for determining disease risk and severity and monitoring disease progression
To contain the COVID-19 pandemic, there is a need for digital technologies to disseminate information and access remote care [32], [33]. An adaptive learning technology (Area9 Lyceum Aps part of Area9 Group), which included an educational module on the COVID-19 pandemic, was developed using the AACC Learning Lab for Laboratory Medicine on NEJM Knowledge [34]. The module on the underlying Rhapsode™ platform is an open-source tool and presents learners with the latest factual information on SARS-CoV-2 and COVID-19. Additionally, the platform contains authoritative references to the World Health Organization and other data sources. The topics presented include the origins, transmission mode, symptoms, complications, diagnosis, and prevention of COVID-19. Based on the adaptive learning model, the completion of the module ensures competency in the material and the time for completion of the module varies depending on each learner adapting to their existing strengths and weaknesses.
Altus Care (a product of Area9 Innovation Aps part of Area9 Group) allows easy digitization of treatment plans or research protocols and remote implementation of these plans. The physicians or healthcare team do not need to physically attend patients and can monitor any disease remotely with this platform. This platform can be used to simultaneously treat multiple patients. The Altus Care platform has been combined with several treatment algorithms.
An algorithm approach is being developed to randomly select individuals for testing and subsequently to identify patients at risk of developing severe infection. Additionally, the platform can be potentially used for risk stratification of patients and identification of patients who are at risk to develop the severe form of the disease. A closed-loop system is being established that does not require on-line supervision but enables intervention when needed. This system is expected to enable the selection of high-risk populations for testing and careful monitoring, which contributes to the efficient utilization of the healthcare system resources.
1.3. Role of the cellular and humoral immune systems in COVID-19
CoVs, which contain a positive-sense RNA, have the largest RNA genome among all viruses [35]. The replication of CoVs is mediated by the attachment of the viral spike protein to the host cell receptors, which subsequently results in the release of the viral genome into the cell [36].
COVID-19 shares characteristics with other CoVs, which assist in immune evasion and survival. Additionally, viral infection leads to immune dysregulation with the immune-mediated injury manifesting as ARDS, myocarditis, and hepatitis. Other manifestations have also been attributed to immune activity.
Innate immune cells must recognize viral invasion through pathogen-associated molecular patterns (PAMPs) to mount an effective antiviral response. PAMPs, such as viral genomic RNA or the intermediates of viral replication, including double-stranded RNA (dsRNA), are recognized by endosomal RNA receptors (toll-like receptor (TLR)3 or TLR7) or cytosolic RNA sensor (RIG-I/MDA5) during CoV infection. This leads to the activation of the downstream signaling cascades, especially NF-κB and IRF3, and the nuclear translocation of downstream signaling molecules. The translocated signaling molecules promote the expression of type I IFNs and other proinflammatory cytokines, which comprise the antiviral defense at the entry site [37], [38]. Type I IFN receptor (IFNR) activates the signal transduction through the activation of the JAK-STAT pathway via signal transducer and activator of transcription (STAT)1 and STAT2. This complex along with interferon regulatory factor 9 (IRF9) is translocated to the nucleus to initiate the transcription of IFN-stimulated genes, which suppress viral replication and dissemination [38]. Innate immunity may also share antibody-like characteristics with mannose-binding lectins, which function as pattern recognition molecules (PRMs) against SARS [39]. T cell-secreted cytokines, especially interleukin (IL)-12, promotes IFN secretion. Thus, IFN is the link between the innate and adaptive immune responses 40, [41].
The infection rate and disease severity among pediatric patients are lower than those among adults [42]. There are no reported severe cases of COVID-19 among young children, who have an effective innate immune response. This indicates that the innate immune response is a critical factor that determines the disease outcome [37], 40 . Consistently, children have a low threshold for the induction of IFN-γ secretion by the NK cells [43]. The robust secretion of IFN-γ mediates an effective innate antiviral response. The downstream cascade of IFN-γ is associated with the regulation of viral replication and the induction of an effective adaptive immune response [44] . Thus, the immune response against the virus is rapid during the incubation period in children, which inhibits viral replication and decreases the viral titers [45].
In adaptive immunity, the Th1 type immune response is critical for clearing the viral infection. The cytokine microenvironment generated by the antigen-presenting cells (APCs) determines the T cell responses. Helper T (Th) cells mediate the overall adaptive response, while cytotoxic T cells (CTLs) eliminate the virus-infected cells. The immune response, especially the production of neutralizing antibodies, has a protective role by limiting the infection at the later phase and preventing re-infection [37]. Recent studies have demonstrated that the Th1 type response is critical to control SARS-CoV and MERS-CoV infections and that it may have a similar role in controlling SARS-CoV-2.
The serum levels of IL-6 and C-reactive protein were upregulated in 52% and 84% of patients with COVID-19, respectively [46]. Increased total neutrophils and decreased total lymphocytes are correlated with disease severity and death [1]. Patients needing intensive care unit (ICU) exhibited higher plasma levels of innate cytokines, such as IP-10, MCP-1, MIP-1A, and TNFα.2, which further supported the role of proinflammatory cytokines in disease progression and severity [1], [37] .
One study reported that of the 452 patients with COVID-19, 286 who were diagnosed with severe infection exhibited low lymphocyte counts and percentages of monocytes, eosinophils, and basophils, high leukocyte counts and neutrophil-lymphocyte ratio (NLR), and upregulated levels of infection-related biomarkers and inflammatory cytokines [47]. In addition to lymphopenia, some studies have reported that patients with COVID-19 exhibit leukopenia [48]. The decreased numbers of T cells, Th cells, and suppressor T cells were correlated with disease severity. In severe cases, the percentage of naïve Th cells increased, while that of memory Th cells decreased. Additionally, the decreased numbers of regulatory T cells (Tregs) are correlated with disease severity [47].
In severe COVID-19 cases, the serum inflammatory biomarkers were downregulated with the absence of CD4+, CD8+, and Treg cells in the peripheral blood, especially Treg cells [37], [49]. The T cell phenotypes in convalescent patients indicated a CD4+ and CD8+ response with highly active cells that can robustly secrete inflammatory cytokines and exert cytotoxic effects [37], 50. The number and functions of T cells were downregulated in patients with COVID-19, especially among elderly patients and patients requiring ICU [51].
These findings on the changes in the immune response to COVID-19 support the current observations and indicate that CoVs are adapted to evade immune detection and suppress the antiviral immune response [52]. This explains the long incubation period of CoVs when compared with that of influenza (2–11 days on average) [53]. CoVs interfere with multiple steps during the initial innate immune response, including RNA sensing, type I IFN production [54] and STAT1/2 activation downstream of IFNR [55] as evidenced by suppressive markers. This delayed type I IFN response inhibits adaptive immune activation [56]. Prolonged viral persistence exacerbates inflammatory responses, which may lead to immune exhaustion and immune suppression as feedback regulatory mechanisms. A biased Th2 type response also favors a poor outcome of the disease.
In patients infected with SARS-CoV and MERS-CoV, type I IFN-mediated immune responses are suppressed, which results in increased mortality [57]. The increased influx of inflammatory cells is associated with fatal pneumonia 40, [56]. Genomic analysis has revealed that CoV infection can trigger the IFN signaling pathway [58]. CoVs evade the immune response by inhibiting the IFN signaling pathway [59] and enclosing their dsRNA in vesicles, which leads to decreased IFN induction [59]. Active viral replication results in the activation of type I IFN and the influx of neutrophils and macrophages, which promote the secretion of proinflammatory cytokines [37], [60].
The upregulated cytokine production at the advanced disease stages and neutrophil influx result in ARDS, which is characterized by pulmonary infiltrates and moderate to severe hypoxemia [61]. Studies examining the ARDS phases have revealed an initial inflammatory infiltrate and alveolar exudate, which results in the gradual replacement of the pulmonary tissue with scar tissue and hyaline membranes [62], [63].
Immune dysregulation during the progression of ARDS may be similar to that during the pathogenesis of sepsis and septic shock [64], [65]. The inefficient immunomodulation in patients with ARDS can be attributed to the network of cells and cytokines involved in ARDS pathogenesis and various insults involved in the etiology of ARDS [66].
NK and NKT cells (NK T cells) cells are involved in the early pathogenesis of ARDS and tissue injury associated with viral infections [67], [68], [69], [70]. NK cells account for approximately 10% of the total resident lymphocytes in the lungs [71]. Viral infection promotes the robust activation of NK cells [72]. The activation of NK cells has been well-studied in patients with pulmonary influenza infection. In the influenza virus-infected lungs, NK cells augment CTL activation and secrete inflammatory cytokines [73]. The mild pulmonary infection in most patients with influenza can be attributed to NK cell-mediated viral clearance. NK cells may also contribute to the dysregulated immune response in patients progressing to severe pulmonary infection and ARDS [73], [74]. ARDS is also characterized by enhanced levels of inflammatory cytokines, which is partially attributed to the cytokine secretion by the NK cells. The levels of cytokine determine the progression to pneumonia or ARDS [75]. The cytotoxic activity of NK cells in influenza-infected explants has been demonstrated against lung macrophages. Lung macrophages mediate NK-driven inflammatory response, a process terms as "pulling the stops" during lung inflammation in ARDS [73], [76]. Further evidence of severe immune dysregulation in COVID-19 disease is observed in the secondary hemophagocytic lymphohistiocytosis (HLH)-like “cytokine storm” manifested in the most critically ill patients [77]. The activated NKT cells can reduce the activity of Tregs in ARDS models [78]. [79], [80]. NKT cells are critical for influenza infection clearance [81]. These major differences between the innate immune outcomes can be attributed to the improved evasion mechanisms in CoVs. Additionally, these innate immune cells can secrete excessive dysregulated cytokines. Tregs can counteract the pathological changes initiated by NK and NKT cells and activate the inflammatory cascade downstream, including other innate and adaptive immune cells [82]. ARDS may result from deficiencies in Treg activation [83]. In particular, Tregs secrete anti-inflammatory cytokines, which results in regulated and functional inflammation [84]. Treg dysfunctions may result from a lack of Treg localization. Some studies have demonstrated that Tregs do not localize to the lung in patients with ARDS and remain in the peripheral blood [85]. The decreased number of Tregs in the blood relative to the proinflammatory Th17 cells was indicated as a marker of ARDS severity [86]. In influenza models, Tregs are activated and expanded in non-lethal models [87], which results in the positive modulation of the immune response by suppressing inflammation [88]. Tregs can prevent excessive tissue damage during influenza infection in mice but do not affect the antiviral immune response [89].
These immunobiological findings have led to the examination of immunosuppression and immunomodulation in ARDS. The therapeutic effects of glucocorticoids have been examined against ARDS. Some studies have demonstrated the beneficial effects of glucocorticoids. However, these beneficial benefits have not been conclusively demonstrated. Currently, glucocorticoids are not clinically used to treat ARDS [90], [91].
Several findings have indicated that the progression of COVID-19 is associated with immune dysregulation. Histological analysis of patients with severe COVID-19 revealed typical ARDS pathology with signs of both mononuclear cell infiltration and direct virus-induced damage. The infiltration of mononuclear cells has also been reported in other tissues, including the liver and heart [50]. During the early phase of COVID-19 infection, alveolar edema, vascular congestion, and infiltration of mononuclear inflammatory cells and multinucleated giant cells are observed [92].
Liver inflammation is observed in approximately 60% of patients requiring ICU due to COVID-19-induced pneumonia. In contrast, liver inflammation is not observed in a high proportion of clinically benign patients [93]. This phenotype can be explained by drug-induced hepatitis or virus-induced liver injury. However, elevated cytokine secretion and immune dysregulation could also account for this phenotype, which may lead to severe pulmonary disease (presumably with severe cytokine and immune cell activation ("HLH type")) with other organs [77]. The cases of COVID-19-associated myocarditis and encephalitis have also been reported, which further suggested pathological immune activation or viral pleiotropy.
Thus, the dysregulation of innate and Tregs cell functions is involved in ARDS, which has been validated in the models of influenza pneumonia [94]. COVID-19 may manifest as ARDS, which may be partially mediated through immune dysfunction. These results suggest that immune modulation may augment antiviral immune response and allow rapid viral clearance. Additionally, immune modulation may attenuate the pathological uncontrolled immune-mediated target organ dysfunction.
1.4. Augmenting the antiviral cellular immune response and mitigating the immune-mediated target organ damage using an adjuvant approach in the gut
The gut provides a platform for generating immune signals that impact the systemic immune system [95], [96], [97]. Adjuvants, including non-absorbable compounds, have been used to modulate the intestinal immune signals in several preclinical and clinical trials using non-absorbable compounds [95], [96], [97], [98], [99], [100], [101], [102], [103], [104], [105]. Three types of adjuvants, namely glycosphingolipids, low-dose colchicine, and hyperimmune colostrum that target the gut immune system have been proposed to augment the antiviral immune response and mitigate immune-mediated target organ damage in patients with COVID-19. These three adjuvants are expected to have a high safety profile and induce a potent antiviral response, as well as alleviate immune-mediated organ damage in patients with COVID-19.
1.4.1. Immunomodulation through the sphingolipid pathway in COVID-19-associated pneumonia and ARDS
In addition to the structural functions, the “Sphinx-like” sphingolipids exhibit bioactive properties, including immunomodulatory activity [106]. "Sphingolipid rheostat" has a critical role in immunomodulation [107], [108]. Sphingolipids are critical for the functions of immune cells involved in the initial antiviral response and the initiation and progression of ARDS. The sphingolipid rheostat has a critical function in the immune cells. According to this paradigm, ceramide (a major functional sphingolipid) accumulates in the cell as part of a "zero-sum game" with sphingosine, a sphingolipid that is generated from the ceramidase-mediated cleavage of fatty acids from ceramide [109]. Other catabolic and anabolic mechanisms can also generate these sphingolipids. However, the counter-regulatory functions of these mechanisms tend to shift the balance to ceramide or sphingosine synthesis [110]. The phosphorylation of sphingosine to sphingosine-1-phosphate (S-1P) promotes cell survival. In the immune cells, S-1P is a major inflammatory mediator [111], [112]. Ceramide promotes cellular apoptosis and conditionally exerts anti-inflammatory effects [113].
Glycosphingolipids, which are sphingolipids with a sugar moiety, have also been proposed to maintain the immune balance by regulating different arms of the immune system in opposing environments [110]. The effects of glycosphingolipids may be mediated by regulating the lipid rafts and the downstream signaling pathways [114], [115]. This system was described as a "two-faced" system for maintaining the immune balance in opposing settings. In this system, the same ligand or different ligands can target different or the same subset of cells [95], [116].
NKT cell subsets play a role in both inflammation and immune tolerance. The activation of the NKT cells results in the secretion of proinflammatory or anti-inflammatory cytokines, which determine their cellular and humoral milieu for immunity or tolerance. β-Glucosylceramide (GC), a glycosphingolipid, exerts a beneficial tolerogenic effect on the immune system in both animals and humans [117], [118]. Additionally, GC functions as an adjuvant for Treg induction and consequently alleviates adipose tissue-associated inflammation [119]. Furthermore, GC alleviates immunological disorders and may be associated with the "fine-tuning" of immune responses by altering the plasticity of NKT lymphocytes [120], [121]. GC-mediated dual effect on immunity was demonstrated using the models of colitis and hepatocellular carcinoma (HCC) [120]. Administration of GC alleviated colitis (tolerance) and suppressed HCC (immunity). A similar beneficial effect of GC treatment on two immune-opposing models of graft versus host disease has been reported [122]. GC can potentiate the antiviral vaccine response by exerting a potent adjuvant effect [123].
NK cells are dependent on the proinflammatory cytokine interleukin-2 (IL-2) for survival. The lack of IL-2 promotes apoptosis and increases cellular ceramide levels. Artificially elevation of ceramide levels promotes apoptosis independent of IL-2 levels [124]. In NK cell leukemia, NK cells are insensitive to apoptosis signals. However, treatment with ceramide induces apoptosis in the NK cells [125]. The opposite spectrum of the sphingolipid rheostat involves promoting NK cell survival and activity. S-1P and its receptor are required for lymph node NK cell localization and proinflammatory cytokine secretion [126], [127]. In some conditions, S-1P, which is crucial for NK cell migration and cytokine secretion, inhibits direct cytotoxicity [128].
There are limited studies on other sphingolipids in the NKT cells. The S1P receptor activity is crucial for NKT localization in the tissues [129]. Conversely, S1P synthesis in the target cells, which is observed in lymphoma, is associated with a reduction in cytokine secretion and cytotoxic effects, which may result in the decreased presentation of CD1d antigen [130].
Tregs are dependent on ceramide for their function and induction of immune tolerance. Additionally, Tregs have unique metabolic pathways to maintain elevated ceramide levels [131], [132], [133], [134]. In contrast, S-1P is a double-edged sword. The binding of S1-P to its receptor can suppress and enhance Treg activity. Previous studies have demonstrated that Treg suppression is associated with decreased S-1P levels [135]. The functions of S-1P have not been completely elucidated. Inhibition of sphingosine kinase (SphK), an enzyme that generates S-1P, was also associated with decreased Treg differentiation. This may be attributed to different conditions (e.g. acute vs. chronic inflammation), direct S-1P activity, or other effects resulting from SphK inhibition or knockdown.
These findings indicate the involvement of sphingolipids in inflammatory signaling, especially in cells involved in proinflammatory or anti-inflammatory responses against viral pneumonia and ARDS.
The therapeutic effects of sphingolipid modulation in viral pneumonia or ARDS have not been examined. However, the modulation of sphingolipid rheostat in the inflammatory cells can be a potential therapeutic strategy for COVID-19. In particular, interventions driving the rheostat toward ceramide and away from S-1P, such as inhibitors of SphK, have been examined in clinical trials [136].
The administration of ceramide to cells involved in the COVID-19 immune response may clear NK and NKT cells and induce apoptosis of active cells. Ceramide is crucial for the survival and the functions of Tregs, which appear later during the inflammatory process. Ceramide may prolong Treg action and alleviate immune dysregulation.
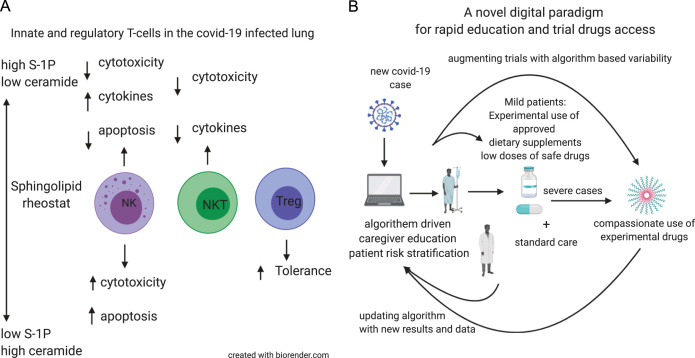
The levels of S-1P, which is involved in the early stages of inflammation, may decrease upon treatment with sphingolipid modulators [107]. During the early stages of infection, S-1P suppresses cytotoxicity and promotes cytokine secretion. In the NK cells, S-1P may fine-tune the immune response and promote their cytotoxicity against infected cells and inhibit excessive cytokine secretion, which promotes the development of ARDS. In the NKT cells, S-1P may enhance antigen presentation and NKT cell activity. During the later stages of inflammation, low S-1P levels may activate the Treg signaling although some contradictory results have been reported. Fig. 1A shows a schematic representation of the potential role of S-1P as a therapeutic target for patients with COVID-19.
Fig. 1.
(A) Schematic representation of the role of sphingoise-1 phosphate (S-1P in patients infected with COVID-19. (B) Schematic presentation of an algorithm-based approach for the diagnosis and treatment of patients with COVID-19 infection.
1.4.2. Low-dose colchicine
The cytoskeleton comprises microtubules (MTs), which are dynamic cytoplasmic tubular polymers, microfilaments, and intermediate filaments [137]. MTs are critical for the innate and adaptive immune responses and the dynamics of inflammatory cells [138], [139], [140], [141]. Colchicine is an orally administered potent anti-inflammatory drug. The mechanism of action of colchicine is the inhibition of tubulin polymerization and MT generation. Additionally, colchicine acts on the inflammatory chemokines and the inflammasome [142], [143]. The anti-tubulin effect of colchicine inhibits neutrophil function and hence is used to treat gout and familial Mediterranean fever (FMF) [144]. Although it is the main therapeutic agent for FMF, approximately 10–30% of patients with FMF do not respond to colchicine even when used at the highest tolerable dose [145], [146], [147], [148], [149]. Moreover, regular dosages of colchicine are associated with a high rate of toxicity [150], [151].
Low and non-absorbable dosages of colchicine exerted a systemic anti-inflammatory effect, which suggested that the localized effect on the gut MTs is a key regulator of the systemic immune system. Targeting MTs with a low-dose of colchicine alleviated the inflammatory response in immune-mediated hepatitis [152]. Colchicine can serve as a potent adjuvant to therapeutic drugs to alleviate non-responsiveness or partial responsiveness to therapies. Preliminary data suggest that the combination of colchicine and other drugs may potentiate long-term beneficial effects [152]. Recent studies have demonstrated the role of neutrophils and IL6 in COVID-19 pathogenesis and disease progression. Hence, a low-dose of colchicine can modulate the immune system in patients with COVID-19. Colchicine downregulated the expression of cytokines, such as IL1, IL6, and TNFα in in vitro models [153] and that of IL1b, IL6, and IL18 in vivo [154].
1.4.3. Hyperimmune colostrum: promoting an effective antiviral immune response against viral strains
Hyperimmune bovine colostrum (HBC), which contains a high level of antigen-specific IgG, is produced by immunizing cows during pregnancy. The immunomodulatory effect of HBC is reported to be similar to that of glycosphingolipids. Treatment with HBC was effective against enteric pathogens but not the non-pathogenic gut microbiota [155], [156]. HBC induces Tregs and alleviates systemic inflammation in the models of colitis, immune-mediated hepatitis, and type 2 diabetes [157], [158]. Clinical trials have demonstrated that HBC has a high safety profile and that it can alleviate systemic inflammation and the associated immune-mediated organ damage by promoting the activation of Tregs [159], 160.
IFN secretion may be critical for the immune response against CoV 40, [161]. In preclinical studies, naïve mice were fed with HBC to examine the induction of T cell clones that secrete IFN in response to two influenza strains. HBC augmented the secretion of IFN from the virus-specific positive T cell clones in the naïve mice (unpublished).
These data indicated that HBC may promote a potent anti-COVID-19 immune response and simultaneously induce Tregs and ameliorate immune-mediated organ damage. The high safety profile of HBC suggests that it can be used as a potent adjuvant in patients with mild to moderate COVID-19 and prevent disease spread.
1.5. Enhancing the therapy response based on individual variability patterns to ensure sustainable augmented antiviral responses and mitigation of immune-mediated organ damage
The interaction between multiple networks within healthy biological systems is dynamic [162], [163], [164], [165], [166]. Physiological functional systems exhibit highly irregular and complex dynamics [167]. According to nonlinear dynamics, the pathology of disease is correlated with the loss of complexity and the inability to retain the physiological state of the system [139], 168, [169], [170], [171], [172], [173]. Variations in the biological system, which are critical for optimal response to multiple internal and external triggers, contribute to regulated plasticity [110], [141], [170], [171].
Gene expression, which is regulated by stochastic factors, is affected by random fluctuations or “noise” [174], [175], [176], [177], [178], [179]. Similar to other biological systems, the immune system is dynamic with inherent variability, which is critical for its physiological function [170], [171], [172], [180], [181]. Intra- and inter-patient variability in the immune system has been reported at the cellular organelle and whole-organ levels [169], [170], [182], [183], [184], [185], [186]. Variability in the expression of lymphocyte biomarkers was reported in subjects with immune-associated disorders [187]. Heterogeneity in the founder cells contributes to the variability in immune cell proliferation and apoptosis. Variation in the activation of Tregs is a major obstacle for developing cell-based therapy against immune-mediated disorders [188]. High intra-group and inter-subject variabilities were demonstrated using the ex vivo cytokine release tests. The median coefficient of variation in the levels of IL-1β and IL-8 was 29% and 52%, respectively [189]. Dynamic instability is a feature of biological variability, which is also a characteristic of MTs [190]. Mitchison and Kirschner observed that MTs exhibit stochastic switching between growing and shrinking states, a behavior known as dynamic instability [191], [192], [193]. The growth and shortening rates of MTs are highly variable. The mean growth rate of individual MTs increased with increasing tubulin concentration. This large variability can be attributed to known random measurement errors. Hence, variability may be an inherent characteristic of the MTs [194], [195].
Previous studies have reported that individual cells exhibit variable drug response [196]. A study on a preclinical model revealed both intra-group and inter-experimental variabilities in the individualized response to concanavalin A, an immune inducer, and orally administered immunomodulatory agents. The individualized responses were observed using the serum levels of IFN-γ, TNF-α, and IL-10 and the expression of CD4+CD25+, CD8+CD25+, and CD3+NK1.1+ in the lymphocytes. Individual variability in response to immune triggers and immunomodulatory therapies has a critical function in this isolated system. These data further support the implementation of personalized variability-based platforms to improve the response to chronic therapies [197].
In humans, serum drug levels vary among patients [198], [199]. The inter-individual and intra-individual variabilities in drug response have been partially attributed to pharmacogenomics and pharmacodynamics of drugs, as well as to drug responsiveness [199], [200], [201], [202]. However, non-specific interactions between drugs and macromolecules in cells cannot be explained only through pharmacodynamics.
Intermittent dosing with drug “holidays” has clinical benefits, including decreased adverse drug effects [203]. The re-induction of anti-TNF response after a drug holiday has been suggested as a mechanism to overcome the loss of drug response [204], [205]. In a prospective trial involving 80 patients with inflammatory bowel disease receiving anti-TNF therapy, loss of clinical response was observed in 36% of controls receiving regular dosing and 13% of patients dosed using a model aimed at maintaining the drug level using a (de-)escalation dashboard. Clinical benefits were achieved irrespective of the lack of change in drug levels [206]. These indicate that dose alterations can lead to clinical improvement when compared with regular fixed dosing.
The unpredictability of the response to chronic medications can be attributed to the variability of the biological systems and the dynamic changes associated with immune processes [110], [139], [170], [173], [207], [208]. Immune modulatory dosing using regular fixed regimens may not be compatible with the physiological variability in the immune system and may further contribute to the loss of beneficial effects with time [209], [210]. Fixed dosing regimens were proposed to be incompatible with a high degree of variability, which is a characteristic of the immune system. To improve the long-term sustainable effects of chronic medications, patient-specific variability must be considered [110], [170], [180], [181].
A single platform integrating the use of these proposed immunomodulatory biological mechanisms and the overlying concept of "augmentation by randomness” is being developed, which may aid the therapeutic needs of COVID-19.
In the first step, healthcare providers are presented with a digital platform containing the latest data on the COVID-19 pandemic. Additionally, the platform can be used to identify patients at risk of infection based on clinical characteristics and subsequently select individuals for testing. Patients diagnosed with COVID-19 are stratified by the algorithm to three levels of severity based on their symptoms, physical examination results, viral load, immune parameters, and laboratory test results. Patients classified in the mild or moderate categories will be offered supportive care and a controlled, semi-random, and therapeutic regimen involving HBC dose determined by the algorithm. As HBC is classified as a food supplement, this step does not require regulatory approval and can be implemented immediately. The colostrum-based approach can be combined with other experimental drugs.
In the second step, the effect of low-dose colchicine on alleviating immune imbalance will be examined in patients with COVID-19. This step is based on the re-purposing of an approved drug with known efficacy but associated with a high rate of resistance or loss of clinical response. This eases the regulatory approval.
In the third step, glycosphingolipids and SK1 inhibitors will be developed as potential therapeutic drugs for potentiating the antiviral immune response.
As a part of the second and third steps, the algorithm will be improved by introducing personalized measures based on variability in the subject-related and disease-related parameters. Variabilities directly or indirectly associated with the function of MTs, including the quantification of MT irregularity, heart rate variability, chronobiology-linked parameters, and viral-related signatures will be quantified and implemented into the algorithm along with various biochemical markers of inflammation and other inputs [170], [171], [180], [181], [211], [212]. The irregularity in dosing and timing of drug administration based on personalized signatures is proposed to result in a beneficial sustainable effect and prevent resistance [211].
Fig. 1B shows a schematic of the use of an algorithm-based approach for the treatment of patients with COVID-19.
2. Summary
We established a novel digital health platform that can assist in the clinical management of patients with COVID-19, including diagnosis, stratification based on disease severity, and monitoring. Additionally, this novel platform enables the administration of three families of adjuvants that target the gut immune system to enhance antiviral immunity independent of viral genome mutations and alleviate immune-mediated lung, heart, and liver damages. This platform can be implemented within a short period as it is designed to ensure easy regulatory approval. Positive results from this system enable continuous algorithm improvement and refinement of the clinical response to these compounds in combination with additional antiviral agents under development.
Disclosure
YI is the founder of Oberon Sciences and a consultant for Teva, ENZO, Protalix, Betalin Therapeutics, Immuron, SciM, Natural Shield, Tiziana Pharma, Plantylight, and Exalenz Bioscience. MB and KJ are the founders of the Altus Care software platform developed by the Area9 Innovation Aps (part of Area9 Group).
References
- 1.Wu F., Zhao S., Yu B., Chen Y.M., Wang W., Song Z.G., Hu Y., Tao Z.W., Tian J.H., Pei Y.Y., Yuan M.L., Zhang Y.L., Dai F.H., Liu Y., Wang Q.M., Zheng J.J., Xu L., Holmes E.C., Zhang Y.Z. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579(7798):265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cao J., Hu X., Cheng W., Yu L., Tu W.J., Liu Q. Clinical features and short-term outcomes of 18 patients with corona virus disease 2019 in intensive care unit. Intensive Care Med. 2020;46:851–853. doi: 10.1007/s00134-020-05987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jones D.S. History in a crisis -lessons for Covid-19. N. Engl. J. Med. 2020 doi: 10.1056/NEJMp2004361. [DOI] [PubMed] [Google Scholar]
- 4.Giovanetti M., Benvenuto D., Angeletti S., Ciccozzi M. The first two cases of 2019-nCoV in Italy: where they come from? J. Med. Virol. 2020;92:518–521. doi: 10.1002/jmv.25699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothe C., Schunk M., Sothmann P., Bretzel G., Froeschl G., Wallrauch C., Zimmer T., Thiel V., Janke C., Guggemos W., Seilmaier M., Drosten C., Vollmar P., Zwirglmaier K., Zange S., Wölfel R., Hoelscher M. Transmission of 2019-nCoV infection from an asymptomatic contact in Germany. N. Engl. J. Med. 2020;382(10):970–971. doi: 10.1056/NEJMc2001468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lipsitch M., Swerdlow D.L., Finelli L. Defining the epidemiology of Covid-19—studies needed. N. Engl. J. Med. 2020;382:1194–1196. doi: 10.1056/NEJMp2002125. [DOI] [PubMed] [Google Scholar]
- 7.Zhao S., Lin Q., Ran J., Musa S.S., Yang G., Wang W., Lou Y., Gao D., Yang L., He D., Wang M.H. Preliminary estimation of the basic reproduction number of novel coronavirus (2019-nCoV) in China, from 2019 to 2020: a data-driven analysis in the early phase of the outbreak. Int. J. Infect. Dis. 2020;92:214–217. doi: 10.1016/j.ijid.2020.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guan W.-j, Ni Z.-y, Hu Y. Clinical characteristics of 2019 novel coronavirus infection in China. medRxiv. 2020 2020.2002.2006.20020974. [Google Scholar]
- 9.Hui D.S., I Azhar E., Madani T.A., Ntoumi F., Kock R., Dar O., Ippolito G., Mchugh T.D., Memish Z.A., Drosten C., Zumla A., Petersen E. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020;91:264–266. doi: 10.1016/j.ijid.2020.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reusken C., Broberg E.K., Haagmans B., Meijer A., Corman V.M., Papa A., Charrel R., Drosten C., Koopmans M., Leitmeyer K., On Behalf Of Evd-LabNet And E. Laboratory readiness and response for novel coronavirus (2019-nCoV) in expert laboratories in 30 EU/EEA countries, January 2020. Eurosurveillance. 2020;25(6) doi: 10.2807/1560-7917.ES.2020.25.6.2000082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhu N., Zhang D., Wang W., Li X., Yang B., Song J., Zhao X., Huang B., Shi W., Lu R., Niu P., Zhan F., Ma X., Wang D., Xu W., Wu G., Gao G.F., Tan W. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang Z.-y, Kong W.-p, Huang Y., Roberts A., Murphy B.R., Subbarao K., Nabel G.J. A DNA vaccine induces SARS coronavirus neutralization and protective immunity in mice. Nature. 2004;428(6982):561–564. doi: 10.1038/nature02463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Graham R.L., Becker M.M., Eckerle L.D., Bolles M., Denison M.R., Baric R.S. A live, impaired-fidelity coronavirus vaccine protects in an aged, immunocompromised mouse model of lethal disease. Nat. Med. 2012;18(12):1820–1826. doi: 10.1038/nm.2972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tang F., Quan Y., Xin Z.-T., Wrammert J., Ma M.J., Lv H., Wang T.B., Yang H., Richardus J.H., Liu W., Cao W.C. Lack of peripheral memory B cell responses in recovered patients with severe acute respiratory syndrome: a six-year follow-up study. J. Immunol. 2011;186(12):7264–7268. doi: 10.4049/jimmunol.0903490. [DOI] [PubMed] [Google Scholar]
- 15.Liu W.J., Zhao M., Liu K., Xu K., Wong G., Tan W., Gao G.F. T-cell immunity of SARS-CoV: implications for vaccine development against MERS-CoV. Antivir. Res. 2017;137:82–92. doi: 10.1016/j.antiviral.2016.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McAleer M. Multidisciplinary Digital Publishing Institute; 2020. Prevention is better than the cure: risk management of COVID-19. [Google Scholar]
- 17.Loeffeholz M.J.T.Y. Laboratory diagnosis of emerging human coronavirus infections - the state of the art. Emerg. Microbes Infect. 2020:1–26. doi: 10.1080/22221751.2020.1745095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gao P., Guan X.L., Huang R., Shang-Guan X.F., Luan J.W., Liu M.C., Xu H., Wang X.W. Risk factors and clinical characteristics of tacrolimus-induced acute nephrotoxicity in children with nephrotic syndrome: a retrospective case-control study. Eur. J. Clin. Pharmacol. 2020;76(2):277–284. doi: 10.1007/s00228-019-02781-3. [DOI] [PubMed] [Google Scholar]
- 19.Poissy J., Goffard A., Parmentier-Decrucq E., Favory R., Kauv M., Kipnis E., Mathieu D., van der Werf S., Guery B., MERS-CoV Biology G. Kinetics and pattern of viral excretion in biological specimens of two MERS-CoV cases. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2014;61(2):275–278. doi: 10.1016/j.jcv.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruning A.H.L., Aatola H., Toivola H., Ikonen N., Savolainen-Kopra C., Blomqvist S., Pajkrt D., Wolthers K.C., Koskinen J.O. Rapid detection and monitoring of human coronavirus infections. New Microbes New Infect. 2018;24:52–55. doi: 10.1016/j.nmni.2018.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salathé M., Althaus C.L., Neher R., Stringhini S., Hodcroft E., Fellay J., Zwahlen M., Senti G., Battegay M., Wilder-Smith A., Eckerle I., Egger M., Low N. COVID-19 epidemic in Switzerland: on the importance of testing, contact tracing and isolation. Swiss Med. Wkly. 2020;150 doi: 10.4414/smw.2020.20225. [DOI] [PubMed] [Google Scholar]
- 22.Kwon K.T., Ko J.H., Shin H., Sung M., Kim J.Y. Drive-through screening center for COVID-19: a safe and efficient screening system against massive community outbreak. J. Korean Med. Sci. 2020;35(11) doi: 10.3346/jkms.2020.35.e123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou F., Yu T., Du R., Fan G., Liu Y., Liu Z., Xiang J., Wang Y., Song B., Gu X., Guan L., Wei Y., Li H., Wu X., Xu J., Tu S., Zhang Y., Chen H., Cao B. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J., Qi T., Liu L., Ling Y., Qian Z., Li T., Li F., Xu Q., Zhang Y., Xu S., Song Z., Zeng Y., Shen Y., Shi Y., Zhu T., Lu H. Clinical progression of patients with COVID-19 in Shanghai, China. J. Infect. 2020;80:1. doi: 10.1016/j.jinf.2020.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Tao Z.W., Lei W., Liu Z.Y. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novelcoronavirus disease. Chin. Med. J. 2020 doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang C., Wang Y., Li X., Ren L., Zhao J., Hu Y., Zhang L., Fan G., Xu J., Gu X., Cheng Z., Yu T., Xia J., Wei Y., Wu W., Xie X., Yin W., Li H., Liu M., Xiao Y., Gao H., Guo L., Xie J., Wang G., Jiang R., Gao Z., Jin Q., Wang J., Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan P.C., Liu C., Raynaud P., Lo M.K., Spiropoulou C.F., Symons J.A., Beigelman L., Deval J. Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathog. 2018;14(2) doi: 10.1371/journal.ppat.1006889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Warren T.K., Jordan R., Lo M.K., Ray A.S., Mackman R.L., Soloveva V., Siegel D., Perron M., Bannister R., Hui H.C., Larson N., Strickley R., Wells J., Stuthman K.S., Van Tongeren S.A., Garza N.L., Donnelly G., Shurtleff A.C., Retterer C.J., Gharaibeh D., Zamani R., Kenny T., Eaton B.P., Grimes E., Welch L.S., Gomba L., Wilhelmsen C.L., Nichols D.K., Nuss J.E., Nagle E.R., Kugelman J.R., Palacios G., Doerffler E., Neville S., Carra E., Clarke M.O., Zhang L., Lew W., Ross B., Wang Q., Chun K., Wolfe L., Babusis D., Park Y., Stray K.M., Trancheva I., Feng J.Y., Barauskas O., Xu Y., Wong P., Braun M.R., Flint M., McMullan L.K., Chen S.S., Fearns R., Swaminathan S., Mayers D.L., Spiropoulou C.F., Lee W.A., Nichol S.T., Cihlar T., Bavari S. Therapeutic efficacy of the small molecule GS-5734 against Ebola virus in rhesus monkeys. Nature. 2016;531(7594):381–385. doi: 10.1038/nature17180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Singh A.K., Singh A., Shaikh A., Singh R., Misra A. Chloroquine and hydroxychloroquine in the treatment of COVID-19 with or without diabetes: a systematic search and a narrative review with a special reference to India and other developing countries. Diabetes Metab. Syndr. 2020;14(3):241–246. doi: 10.1016/j.dsx.2020.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu; W.L., H COVID-19: attacks the 1-beta chain of hemoglobin and captures the porphyrin to inhibit human heme metabolism. ChemRxiv. 2020 doi: 10.26434/chemrxiv.11938173.v8. [DOI] [Google Scholar]
- 32.Inkster B., O’Brien R., Selby E., Joshi S., Subramanian V., Kadaba M., Schroeder K., Godson S., Comley K., Vollmer S.J., Mateen B.A. Digital health management during and beyond the COVID-19 pandemic: opportunities, barriers, and recommendations. JMIR Ment. Health. 2020;7:19246. doi: 10.2196/19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Keesara S., Jonas A., Schulman K. Covid-19 and health care’s digital revolution. N. Engl. J. Med. 2020;382(23):82. doi: 10.1056/NEJMp2005835. [DOI] [PubMed] [Google Scholar]
- 34.An adpative learning course: COVID-19 N. Engl. J. Med. Area 2020 9. 〈https://area9lyceumcom/covid19_course/〉.
- 35.Anand K., Ziebuhr J., Wadhwani P., Mesters J.R., Hilgenfeld R. Coronavirus main proteinase (3CLpro) structure: basis for design of anti-SARS drugs. Science. 2003;300(5626):1763–1767. doi: 10.1126/science.1085658. [DOI] [PubMed] [Google Scholar]
- 36.Weiss S.R., Navas-Martin S. Coronavirus pathogenesis and the emerging pathogen severe acute respiratory syndrome coronavirus. Microbiol Mol. Biol. Rev. 2005;69(4):635–664. doi: 10.1128/MMBR.69.4.635-664.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prompetchara E., Ketloy C., Palaga T. Immune responses in COVID-19 and potential vaccines: lessons learned from SARS and MERS epidemic. Asian Pac. J. Allergy Immunol. 2020;38:1–9. doi: 10.12932/AP-200220-0772. [DOI] [PubMed] [Google Scholar]
- 38.de Wit E., van Doremalen N., Falzarano D., Munster V.J. SARS and MERS: recent insights into emerging coronaviruses. Nat. Rev. Microbiol. 2016;14(8):523–534. doi: 10.1038/nrmicro.2016.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ip W.K., Chan K.H., Law H.K., Tso G.H., Kong E.K., Wong W.H., To Y.F., Yung R.W., Chow E.Y., Au K.L., Chan E.Y., Lim W., Jensenius J.C., Turner M.W., Peiris J.S., Lau Y.L. Mannose-binding lectin in severe acute respiratory syndrome coronavirus infection. J. Infect. Dis. 2005;191(10):1697–1704. doi: 10.1086/429631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nezhad FM, P. Negahdaripour, M. Dehghani, Z. Farahmandnejad, M. Moghadami, M. Nezafat, N. Masoompour, SM, Therapeutic Approaches for COVID-19 Based on the Dynamics of Interferon-mediated Immune Responses, preprintsorg > medicine & pharmacology > other > 〈https://doi.org/1020944/preprints2020030206v1〉. 2020.
- 41.Tang F., Liu W., Zhang F., Xin Z.T., Wei M.T., Zhang P.H., Yang H., Ly H., Cao W.C. IL-12 RB1 genetic variants contribute to human susceptibility to severe acute respiratory syndrome infection among Chinese. PLoS One. 2008;3(5) doi: 10.1371/journal.pone.0002183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dong Y., Mo X., Hu Y., Qi X., Jiang F., Jiang Z., Tong S. Epidemiology of COVID-19 among children in China. Pediatrics. 2020;145 doi: 10.1542/peds.2020-0702. [DOI] [PubMed] [Google Scholar]
- 43.Dobbs K., Tabellini G., Calzoni E. Natural killer cells from patients with recombinase-activating gene and non-homologous end joining gene defects comprise a higher frequency of CD56(bright) NKG2A(+++) cells, and yet display increased degranulation and higher perforin content. Front. Immunol. 2017;8:798. doi: 10.3389/fimmu.2017.00798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Murira A., Lamarre A. Type-I interferon responses: from friend to foe in the battle against chronic viral infection. Front. Immunol. 2016;7:609. doi: 10.3389/fimmu.2016.00609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivarsson M.A., Loh L., Marquardt N. Differentiation and functional regulation of human fetal NK cells. J. Clin. Investig. 2013;123(9):3889–3901. doi: 10.1172/JCI68989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhou P., Yang X.L., Wang X.G. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579(7798):270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Guan W.J., Ni Z.Y., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 2020 doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qin C., Zhou L., Hu Z. Dysregulation of immune response in patients with COVID-19 in Wuhan, China. Clin. Infect. Dis. 2020 doi: 10.1093/cid/ciaa248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Z., Shi L., Wang Y., et al. Pathological findings of COVID-19 associated with acute respiratory distress syndrome, The Lancet Respiratory Medicine. [DOI] [PMC free article] [PubMed]
- 51.Diao B.W.C., Tan Y., Chen X., Liu Y., Ning L. Reduction and functional exhaustion of T cells in patients with coronavirus disease 2019 (COVID-19) medRxiv. 2020 doi: 10.3389/fimmu.2020.00827. (https://doi.org/2020.02.18.20024364) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alcami A., Koszinowski U.H. Viral mechanisms of immune evasion. Immunol. Today. 2000;21(9):447–455. doi: 10.1016/S0167-5699(00)01699-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lessler J., Reich N.G., Brookmeyer R., Perl T.M., Nelson K.E., Cummings D.A. Incubation periods of acute respiratory viral infections: a systematic review. Lancet Infect. Dis. 2009;9(5):291–300. doi: 10.1016/S1473-3099(09)70069-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cyranoski D. This scientist hopes to test coronavirus drugs on animals in locked-down Wuhan. Nature. 2020;577(7792):607. doi: 10.1038/d41586-020-00190-6. [DOI] [PubMed] [Google Scholar]
- 55.Chan J.F., Yuan S., Kok K.H. A familial cluster of pneumonia associated with the 2019 novel coronavirus indicating person-to-person transmission: a study of a family cluster. Lancet. 2020;395(10223):514–523. doi: 10.1016/S0140-6736(20)30154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39(5):529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Perlman S., Dandekar A.A. Immunopathogenesis of coronavirus infections: implications for SARS. Nat. Rev. Immunol. 2005;5(12):917–927. doi: 10.1038/nri1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kindler E., Thiel V. SARS-CoV and IFN: too little, too Late. Cell Host Microbe. 2016;19(2):139–141. doi: 10.1016/j.chom.2016.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Strayer D.R., Dickey R., Carter W.A. Sensitivity of SARS/MERS CoV to interferons and other drugs based on achievable serum concentrations in humans. Infect. Disord. Drug Targets. 2014;14(1):37–43. doi: 10.2174/1871526514666140713152858. [DOI] [PubMed] [Google Scholar]
- 60.Duan S., Thomas P.G. Balancing immune protection and immune pathology by CD8(+) T-cell responses to influenza infection. Front. Immunol. 2016;7:25. doi: 10.3389/fimmu.2016.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Matthay M.A., Zemans R.L., Zimmerman G.A. Acute respiratory distress syndrome. Nat. Rev. Dis. Primers. 2019;5(1):18. doi: 10.1038/s41572-019-0069-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Taubenberger J.K., Morens D.M. The pathology of influenza virus infections. Annu Rev. Pathol. 2008;3:499–522. doi: 10.1146/annurev.pathmechdis.3.121806.154316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gadre S.K., Duggal A., Mireles-Cabodevila E. Acute respiratory failure requiring mechanical ventilation in severe chronic obstructive pulmonary disease (COPD) Medicine. 2018;97(17) doi: 10.1097/MD.0000000000010487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lewis A.J., Billiar T.R., Rosengart M.R. Biology and metabolism of sepsis: innate immunity, bioenergetics, and autophagy. Surg. Infect. 2016;17(3):286–293. doi: 10.1089/sur.2015.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ruthman C.A., Festic E. Emerging therapies for the prevention of acute respiratory distress syndrome. Ther. Adv. Respir. Dis. 2015;9(4):173–187. doi: 10.1177/1753465815585716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Han S., Mallampalli R.K. The acute respiratory distress syndrome: from mechanism to translation. J. Immunol. 2015;194(3):855–860. doi: 10.4049/jimmunol.1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.van Erp E.A., van Kampen M.R., van Kasteren P.B., de Wit J. Viral infection of human natural killer cells. Viruses. 2019;11:3. doi: 10.3390/v11030243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zhang Y., Wallace D.L., de Lara C.M. In vivo kinetics of human natural killer cells: the effects of ageing and acute and chronic viral infection. Immunology. 2007;121(2):258–265. doi: 10.1111/j.1365-2567.2007.02573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fontana M.F., Pepper M. NKTeeing up B cell responses to viral infection. Immunity. 2018;48(2):198–200. doi: 10.1016/j.immuni.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tyznik A.J., Verma S., Wang Q., Kronenberg M., Benedict C.A. Distinct requirements for activation of NKT and NK cells during viral infection. J. Immunol. 2014;192(8):3676–3685. doi: 10.4049/jimmunol.1300837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hervier B., Russick J., Cremer I., Vieillard V.N.K. Cells in the human lungs. Front. Immunol. 2019;10:1263. doi: 10.3389/fimmu.2019.01263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.O’Sullivan T.E., Sun J.C. Generation of natural killer cell memory during viral infection. J. Innate Immun. 2015;7(6):557–562. doi: 10.1159/000375494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cong J., Wei H. Natural killer cells in the lungs. Front. Immunol. 2019;10:1416. doi: 10.3389/fimmu.2019.01416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Englert J.A., Bobba C., Baron R.M. Integrating molecular pathogenesis and clinical translation in sepsis-induced acute respiratory distress syndrome. JCI Insight. 2019;4:2. doi: 10.1172/jci.insight.124061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cohen L., Fiore-Gartland A., Randolph A.G. A modular cytokine analysis method reveals novel associations with clinical phenotypes and identifies sets of co-signaling cytokines across influenza natural infection cohorts and healthy controls. Front. Immunol. 2019;10:1338. doi: 10.3389/fimmu.2019.01338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Cooper G.E., Ostridge K., Khakoo S.I., Wilkinson T.M.A., Staples K.J. Human CD49a(+) lung natural killer cell cytotoxicity in response to influenza A virus. Front. Immunol. 2018;9:1671. doi: 10.3389/fimmu.2018.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mehta P., McAuley D.F., Brown M. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020 doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Toyama M., Kudo D., Aoyagi T. Attenuated accumulation of regulatory T cells and reduced production of interleukin 10 lead to the exacerbation of tissue injury in a mouse model of acute respiratory distress syndrome. Microbiol. Immunol. 2018;62(2):111–123. doi: 10.1111/1348-0421.12564. [DOI] [PubMed] [Google Scholar]
- 79.Aoyagi T., Yamamoto N., Hatta M. Activation of pulmonary invariant NKT cells leads to exacerbation of acute lung injury caused by LPS through local production of IFN-gamma and TNF-alpha by Gr-1+ monocytes. Int. Immunol. 2011;23(2):97–108. doi: 10.1093/intimm/dxq460. [DOI] [PubMed] [Google Scholar]
- 80.Paget C., Trottein F. Role of type 1 natural killer T cells in pulmonary immunity. Mucosal Immunol. 2013;6(6):1054–1067. doi: 10.1038/mi.2013.59. [DOI] [PubMed] [Google Scholar]
- 81.Artiaga B.L., Yang G., Hutchinson T.E. Rapid control of pandemic H1N1 influenza by targeting NKT-cells. Sci. Rep. 2016;6(1):37999. doi: 10.1038/srep37999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Nair S., Dhodapkar M.V. Natural killer T cells in cancer immunotherapy. Front. Immunol. 2017;8:1178. doi: 10.3389/fimmu.2017.01178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lin S., Wu H., Wang C., Xiao Z., Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front. Immunol. 2018;9:1545. doi: 10.3389/fimmu.2018.01545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lin S., Wu H., Wang C., Xiao Z., Xu F. Regulatory T cells and acute lung injury: cytokines, uncontrolled inflammation, and therapeutic implications. Front. Immunol. 2018;9 doi: 10.3389/fimmu.2018.01545. 1545-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Halter S., Aimade L., Barbié M. T regulatory cells activation and distribution are modified in critically ill patients with acute respiratory distress syndrome: a prospective single-centre observational study. Anaesth. Crit. Care Pain Med. 2020;39(1):35–44. doi: 10.1016/j.accpm.2019.07.014. [DOI] [PubMed] [Google Scholar]
- 86.Yu Z.-X., Ji M.-S., Yan J. The ratio of Th17/Treg cells as a risk indicator in early acute respiratory distress syndrome. Crit. Care. 2015;19(1):82. doi: 10.1186/s13054-015-0811-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Betts R.J., Prabhu N., Ho A.W.S. Influenza A virus infection results in a robust, antigen-responsive, and widely disseminated Foxp+ regulatory T cell response. J. Virol. 2012;86(5):2817. doi: 10.1128/JVI.05685-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.León B., Bradley J.E., Lund F.E., Randall T.D., Ballesteros-Tato A. FoxP3+ regulatory T cells promote influenza-specific Tfh responses by controlling IL-2 availability. Nat. Commun. 2014;5(1):3495. doi: 10.1038/ncomms4495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Arpaia N., Green J.A., Moltedo B. A distinct function of regulatory T cells in tissue protection. Cell. 2015;162(5):1078–1089. doi: 10.1016/j.cell.2015.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mokra D., Mikolka P., Kosutova P., Mokry J. Corticosteroids in acute lung injury: the dilemma continues. Int. J. Mol. Sci. 2019;20:19. doi: 10.3390/ijms20194765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Meduri G.U., Bridges L., Shih M.C., Marik P.E., Siemieniuk R.A.C., Kocak M. Prolonged glucocorticoid treatment is associated with improved ARDS outcomes: analysis of individual patients’ data from four randomized trials and trial-level meta-analysis of the updated literature. Intensive Care Med. 2016;42(5):829–840. doi: 10.1007/s00134-015-4095-4. [DOI] [PubMed] [Google Scholar]
- 92.Tian S., Hu W., Niu L., Liu H., Xu H., Xiao S.-Y., Pulmonary pathology of early-phase 2019 novel coronavirus (COVID-19) pneumonia in two patients with lung cancer, Journal of Thoracic Oncology. [DOI] [PMC free article] [PubMed]
- 93.Yang H.Y., Xu J., Li Y. [The preliminary analysis on the characteristics of the cluster for the corona virus disease]. Zhonghua Liu Xing Bing. Xue Za Zhi. 2020;41(0):623–628. doi: 10.3760/cma.j.cn112338-20200223-00153. [DOI] [PubMed] [Google Scholar]
- 94.Wu C., Chen X., Cai Y. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan. China JAMA Intern. Med. 2020 doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ilan Y. Immune rebalancing by oral immunotherapy: a novel method for getting the immune system back on track. J. Leukoc. Biol. 2019;105(3):463–472. doi: 10.1002/JLB.5RU0718-276RR. [DOI] [PubMed] [Google Scholar]
- 96.Ilan Y. Oral immune therapy: targeting the systemic immune system via the gut immune system for the treatment of inflammatory bowel disease. Clin. Transl. Immunol. 2016;5(1) doi: 10.1038/cti.2015.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ilan Y. Review article: novel methods for the treatment of non-alcoholic steatohepatitis - targeting the gut immune system to decrease the systemic inflammatory response without immune suppression. Aliment Pharmacol. Ther. 2016;44(11–12):1168–1182. doi: 10.1111/apt.13833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ilan Y., Shailubhai K., Sanyal A. Immunotherapy with oral administration of humanized anti-CD3 monoclonal antibody: a novel gut-immune system-based therapy for metaflammation and NASH. Clin. Exp. Immunol. 2018;193(3):275–283. doi: 10.1111/cei.13159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Almon E., Khoury T., Drori A. An oral administration of a recombinant anti-TNF fusion protein is biologically active in the gut promoting regulatory T cells: results of a phase I clinical trial using a novel oral anti-TNF alpha-based therapy. J. Immunol. Methods. 2017;446:21–29. doi: 10.1016/j.jim.2017.03.023. [DOI] [PubMed] [Google Scholar]
- 100.Lalazar G., Zigmond E., Weksler-Zangen S. Oral administration of beta-glucosylceramide for the treatment of insulin resistance and nonalcoholic steatohepatitis: results of a double-blind, placebo-controlled trial. J. Med. Food. 2017;20(5):458–464. doi: 10.1089/jmf.2016.3753. [DOI] [PubMed] [Google Scholar]
- 101.Ilan Y., Gingis-Velitski S., Ben Ya’aco A. A plant cell-expressed recombinant anti-TNF fusion protein is biologically active in the gut and alleviates immune-mediated hepatitis and colitis. Immunobiology. 2017;222(3):544–551. doi: 10.1016/j.imbio.2016.11.001. [DOI] [PubMed] [Google Scholar]
- 102.Ilan Y., Ben Ya’acov A., Shabbat Y., Gingis-Velitski S., Almon E., Shaaltiel Y. Oral administration of a non-absorbable plant cell-expressed recombinant anti-TNF fusion protein induces immunomodulatory effects and alleviates nonalcoholic steatohepatitis. World J. Gastroenterol. 2016;22(39):8760–8769. doi: 10.3748/wjg.v22.i39.8760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Israeli E., Zigmond E., Lalazar G. Oral mixture of autologous colon-extracted proteins for the Crohn’s disease: a double-blind trial. World J. Gastroenterol. 2015;21(18):5685–5694. doi: 10.3748/wjg.v21.i18.5685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lalazar G., Mizrahi M., Turgeman I. Oral administration of OKT3 MAb to patients with NASH, promotes regulatory T-cell induction, and alleviates insulin resistance: results of a Phase IIa blinded placebo-controlled trial. J. Clin. Immunol. 2015;35(4):399–407. doi: 10.1007/s10875-015-0160-6. [DOI] [PubMed] [Google Scholar]
- 105.Israeli E., Goldin E., Fishman S. Oral administration of non-absorbable delayed release 6-mercaptopurine is locally active in the gut, exerts a systemic immune effect and alleviates Crohn’s disease with low rate of side effects: results of double blind Phase II clinical trial. Clin. Exp. Immunol. 2015;181(2):362–372. doi: 10.1111/cei.12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ilan Y. Compounds of the sphingomyelin-ceramide-glycosphingolipid pathways as secondary messenger molecules: new targets for novel therapies for fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2016;310(11):G1102–G1117. doi: 10.1152/ajpgi.00095.2016. [DOI] [PubMed] [Google Scholar]
- 107.Ishay Y., Nachman D., Khoury T., Ilan Y. The role of sphingolipid pathway in liver fibrosis: an emerging new potential target for novel therapies. Am. J. Physiol. Cell Physiol. 2020 doi: 10.1152/ajpcell.00003.2020. [DOI] [PubMed] [Google Scholar]
- 108.Newton J., Lima S., Maceyka M., Spiegel S. Revisiting the sphingolipid rheostat: evolving concepts in cancer therapy. Exp. Cell Res. 2015;333(2):195–200. doi: 10.1016/j.yexcr.2015.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Schuchman E.H. Acid ceramidase and the treatment of ceramide diseases: the expanding role of enzyme replacement therapy. Biochim. Biophys. Acta. 2016;1862(9):1459–1471. doi: 10.1016/j.bbadis.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 110.Ilan Y. Beta-glycosphingolipids as mediators of both inflammation and immune tolerance: a manifestation of randomness in biological systems. Front. Immunol. 2019;10:1143. doi: 10.3389/fimmu.2019.01143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Weigert A., Olesch C., Brune B. Sphingosine-1-phosphate and macrophage biology-how the Sphinx Tames the big eater. Front. Immunol. 2019;10:1706. doi: 10.3389/fimmu.2019.01706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Aoki M., Aoki H., Ramanathan R., Hait N.C., Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat. Inflamm. 2016;2016 doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Gomez-Munoz A., Gangoiti P., Granado M.H., Arana L., Ouro A. Ceramide-1-phosphate in cell survival and inflammatory signaling. Adv. Exp. Med. Biol. 2010;688:118–130. doi: 10.1007/978-1-4419-6741-1_8. [DOI] [PubMed] [Google Scholar]
- 114.Lalazar G., Ben Ya’acov A., Livovsky D.M. Beta-glycoglycosphingolipid-induced alterations of the STAT signaling pathways are dependent on CD1d and the lipid raft protein flotillin-2. Am. J. Pathol. 2009;174(4):1390–1399. doi: 10.2353/ajpath.2009.080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Shuvy M., Ben Ya’acov A., Zolotarov L., Lotan C., Ilan Y. Beta glycosphingolipids suppress rank expression and inhibit natural killer T cell and CD8+ accumulation in alleviating aortic valve calcification. Int. J. Immunopathol. Pharmacol. 2009;22(4):911–918. doi: 10.1177/039463200902200406. [DOI] [PubMed] [Google Scholar]
- 116.Bandyopadhyay K., Marrero I., Kumar V. NKT cell subsets as key participants in liver physiology and pathology. Cell. Mol. Immunol. 2016;13(3):337–346. doi: 10.1038/cmi.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ilan Y., Maron R., Tukpah A.M. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl. Acad. Sci. USA. 2010;107(21):9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ilan Y., Zigmond E., Lalazar G. Oral administration of OKT3 monoclonal antibody to human subjects induces a dose-dependent immunologic effect in T cells and dendritic cells. J. Clin. Immunol. 2010;30(1):167–177. doi: 10.1007/s10875-009-9323-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ilan Y., Maron R., Tukpah A.-M. Induction of regulatory T cells decreases adipose inflammation and alleviates insulin resistance in ob/ob mice. Proc. Natl. Acad. Sci. USA. 2010;107(21):9765–9770. doi: 10.1073/pnas.0908771107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zigmond E., Preston S., Pappo O. Beta-glucosylceramide: a novel method for enhancement of natural killer T lymphoycte plasticity in murine models of immune-mediated disorders. Gut. 2007;56(1):82–89. doi: 10.1136/gut.2006.095497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zigmond E., Shalev Z., Pappo O., Alper R., Zolotarov L., Ilan Y. NKT lymphocyte polarization determined by microenvironment signaling: a role for CD8+ lymphocytes and beta-glycosphingolipids. J. Autoimmun. 2008;31(2):188–195. doi: 10.1016/j.jaut.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 122.Ilan Y., Ohana M., Pappo O. Alleviation of acute and chronic graft-versus-host disease in a murine model is associated with glucocerebroside-enhanced natural killer T lymphocyte plasticity. Transplantation. 2007;83(4):458–467. doi: 10.1097/01.tp.0000252783.66886.f3. [DOI] [PubMed] [Google Scholar]
- 123.Mizrahi M., Lalazar G., Ben Ya’acov A. Beta-glycoglycosphingolipid-induced augmentation of the anti-HBV immune response is associated with altered CD8 and NKT lymphocyte distribution: a novel adjuvant for HBV vaccination. Vaccine. 2008;26(21):2589–2595. doi: 10.1016/j.vaccine.2008.03.026. [DOI] [PubMed] [Google Scholar]
- 124.Taguchi Y., Kondo T., Watanabe M. Interleukin-2-induced survival of natural killer (NK) cells involving phosphatidylinositol-3 kinase-dependent reduction of ceramide through acid sphingomyelinase, sphingomyelin synthase, and glucosylceramide synthase. Blood. 2004;104(10):3285–3293. doi: 10.1182/blood-2004-03-0900. [DOI] [PubMed] [Google Scholar]
- 125.Watters R.J., Fox T.E., Tan S.F. Targeting glucosylceramide synthase synergizes with C6-ceramide nanoliposomes to induce apoptosis in natural killer cell leukemia. Leuk. Lymphoma. 2013;54(6):1288–1296. doi: 10.3109/10428194.2012.752485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fang V., Chaluvadi V.S., Ramos-Perez W.D. Gradients of the signaling lipid S1P in lymph nodes position natural killer cells and regulate their interferon-γ response. Nat. Immunol. 2017;18(1):15–25. doi: 10.1038/ni.3619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Drouillard A., Mathieu A.-L., Marçais A. S1PR5 is essential for human natural killer cell migration toward sphingosine-1 phosphate. J. Allergy Clin. Immunol. 2018;141(6):2265–2268. doi: 10.1016/j.jaci.2017.11.022. e2261. [DOI] [PubMed] [Google Scholar]
- 128.Lagadari M., Lehmann K., Ziemer M. Sphingosine-1-phosphate inhibits the cytotoxic activity of NK cells via Gs protein-mediated signalling. Int. J. Oncol. 2009;34(1):287–294. [PubMed] [Google Scholar]
- 129.Allende M., Zhou D., Kalkofen D. S1P1 receptor expression regulates emergence of NKT cells in peripheral tissues. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2008;22:307–315. doi: 10.1096/fj.07-9087com. [DOI] [PubMed] [Google Scholar]
- 130.Webb T., Kimball A., Sun W. Mantle cell lymphoma associated sphingosine-1 phosphate inhibits natural killer T cell mediated antitumor responses (TUM2P.1042) J. Immunol. 2015;194(1 Supplement):69.39. [Google Scholar]
- 131.Kue C.S., Lim H.X., Jung M.Y., Hong H.-J., Cho D., Kim T.S. C6-ceramide in combination with transforming growth factor-β enhances Treg cell differentiation and stable FoxP3 expression in vitro and in vivo. Immunobiology. 2013;218(7):952–959. doi: 10.1016/j.imbio.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 132.Apostolidis S.A., Rodriguez-Rodriguez N., Suarez-Fueyo A. Phosphatase PP2A is requisite for the function of regulatory T cells. Nat. Immunol. 2016;17(5):556–564. doi: 10.1038/ni.3390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Krummey S.M., Ford M.L. Tregs hold on to ceramide. Am. J. Transplant. 2016;16(7) 1945-1945. [Google Scholar]
- 134.Zhou Y., Salker M.S., Walker B. Acid Sphingomyelinase (ASM) is a negative regulator of regulatory T cell (Treg) development. Cell. Physiol. Biochem. 2016;39(3):985–995. doi: 10.1159/000447806. [DOI] [PubMed] [Google Scholar]
- 135.Liu G., Burns S., Huang G. The receptor S1P1 overrides regulatory T cell-mediated immune suppression through Akt-mTOR. Nat. Immunol. 2009;10(7):769–777. doi: 10.1038/ni.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haass N.K., Nassif N., McGowan E.M. Switching the sphingolipid rheostat in the treatment of diabetes and cancer comorbidity from a problem to an advantage. Biomed. Res. Int. 2015;2015 doi: 10.1155/2015/165105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pilhofer M., Ladinsky M.S., McDowall A.W., Petroni G., Jensen G.J. Microtubules in bacteria: ancient tubulins build a five-protofilament homolog of the eukaryotic cytoskeleton. PLoS Biol. 2011;9(12) doi: 10.1371/journal.pbio.1001213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Renkawitz J., Kopf A., Stopp J. Nuclear positioning facilitates amoeboid migration along the path of least resistance. Nature. 2019;568(7753):546–550. doi: 10.1038/s41586-019-1087-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019;43(7):739–748. doi: 10.1002/cbin.11157. [DOI] [PubMed] [Google Scholar]
- 140.Ilan-Ber T., Ilan Y. The role of microtubules in the immune system and as potential targets for gut-based immunotherapy. Mol. Immunol. 2019;111:73–82. doi: 10.1016/j.molimm.2019.04.014. [DOI] [PubMed] [Google Scholar]
- 141.Ilan Y. Microtubules: from understanding their dynamics to using them as potential therapeutic targets. J. Cell. Physiol. 2019;234(6):7923–7937. doi: 10.1002/jcp.27978. [DOI] [PubMed] [Google Scholar]
- 142.Ravelli R.B., Gigant B., Curmi P.A. Insight into tubulin regulation from a complex with colchicine and a stathmin-like domain. Nature. 2004;428(6979):198–202. doi: 10.1038/nature02393. [DOI] [PubMed] [Google Scholar]
- 143.Perico N., Ostermann D., Bontempeill M. Colchicine interferes with L-selectin and leukocyte function-associated antigen-1 expression on human T lymphocytes and inhibits T cell activation. J. Am. Soc. Nephrol. 1996;7(4):594–601. doi: 10.1681/ASN.V74594. [DOI] [PubMed] [Google Scholar]
- 144.Ben-Chetrit E., Levy M., Colchicine: 1998 update, in: Proceedings of the Paper presented at: Seminars in arthritis and rheumatism, 1998. [DOI] [PubMed]
- 145.Hentgen V., Grateau G., Kone-Paut I. Evidence-based recommendations for the practical management of Familial Mediterranean fever. Semin Arthritis Rheum. 2013;43(3):387–391. doi: 10.1016/j.semarthrit.2013.04.011. [DOI] [PubMed] [Google Scholar]
- 146.Tufan A., Babaoglu M.O., Akdogan A. Association of drug transporter gene ABCB1 (MDR1) 3435C to T polymorphism with colchicine response in familial Mediterranean fever. J. Rheumatol. 2007;34(7):1540–1544. [PubMed] [Google Scholar]
- 147.Erden A., Batu E.D., Sari A. Which definition should be used to determine colchicine resistance among patients with familial Mediterranean fever? Clin. Exp. Rheumatol. 2018;36(6 Suppl 115):97–102. [PubMed] [Google Scholar]
- 148.Ozen S., Kone-Paut I., Gul A. Colchicine resistance and intolerance in familial mediterranean fever: definition, causes, and alternative treatments. Semin Arthritis Rheum. 2017;47(1):115–120. doi: 10.1016/j.semarthrit.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 149.Ozer I., Mete T., Turkeli Sezer O. Association between colchicine resistance and vitamin D in familial Mediterranean fever. Ren. Fail. 2015;37(7):1122–1125. doi: 10.3109/0886022X.2015.1056064. [DOI] [PubMed] [Google Scholar]
- 150.Angelidis C., Kotsialou Z., Kossyvakis C. Colchicine pharmacokinetics and mechanism of action. Curr. Pharm. Des. 2018;24(6):659–663. doi: 10.2174/1381612824666180123110042. [DOI] [PubMed] [Google Scholar]
- 151.Alkadi H., Khubeiz M.J., Jbeily R. Colchicine: a review on chemical structure and clinical usage. Infect. Disord. Drug Targets. 2018;18(2):105–121. doi: 10.2174/1871526517666171017114901. [DOI] [PubMed] [Google Scholar]
- 152.Ilan YBI, T, A subject sepcific system and method for prevention of body adapatation for chronic treatment of disease, WO 2019/008571 Al, 2019. 〈https://patents.google.com/patent/WO2019008571A1/en〉.
- 153.Singh M., Pal S., Dhakad U., Srivastava R., Chattopadhyay N., Das S. Effect of colchicine on inflammation-mediating cytokines in human osteoarthritic chondrocytes (in vitro model) Osteoarthr. Cartil. 2018;26:S305–S306. [Google Scholar]
- 154.Martínez G.J., Robertson S., Barraclough J. Colchicine acutely suppresses local cardiac production of inflammatory cytokines in patients with an acute coronary syndrome. J. Am. Heart Assoc. 2015;4(8) doi: 10.1161/JAHA.115.002128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Otto W., Najnigier B., Stelmasiak T., Robins-Browne R.M. Randomized control trials using a tablet formulation of hyperimmune bovine colostrum to prevent diarrhea caused by enterotoxigenic Escherichia coli in volunteers. Scand. J. Gastroenterol. 2011;46(7–8):862–868. doi: 10.3109/00365521.2011.574726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sponseller J.K., Steele J.A., Schmidt D.J. Hyperimmune bovine colostrum as a novel therapy to combat Clostridium difficile infection. J. Infect. Dis. 2015;211(8):1334–1341. doi: 10.1093/infdis/jiu605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ben Ya’acov A., Lichtenstein Y., Zolotarov L., Ilan Y. The gut microbiome as a target for regulatory T cell-based immunotherapy: induction of regulatory lymphocytes by oral administration of anti-LPS enriched colostrum alleviates immune mediated colitis. BMC Gastroenterol. 2015;15:154. doi: 10.1186/s12876-015-0388-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Adar T., Ben Ya’acov A., Lalazar G. Oral administration of immunoglobulin G-enhanced colostrum alleviates insulin resistance and liver injury and is associated with alterations in natural killer T cells. Clin. Exp. Immunol. 2012;167(2):252–260. doi: 10.1111/j.1365-2249.2011.04511.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Mizrahi M., Shabat Y., Ben Ya’acov A. Alleviation of insulin resistance and liver damage by oral administration of Imm124-E is mediated by increased Tregs and associated with increased serum GLP-1 and adiponectin: results of a phase I/II clinical trial in NASH. J. Inflamm. Res. 2012;5:141–150. doi: 10.2147/JIR.S35227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Abdelmalek MF, BL Harrison, SA Powell, EE Rinella, ME Tobis, N. Peres, D. Kanellos, J. Lalazar, G. Sanya, AJ, Imm-124E improves metabolic endotoxemia and markers of liver injury in nonalcoholic steatohepatitis, Hepatology. 68 (1 Supp),108.
- 161.Yoshikawa T., Hill T.E., Yoshikawa N. Dynamic innate immune responses of human bronchial epithelial cells to severe acute respiratory syndrome-associated coronavirus infection. PLoS One. 2010;5(1) doi: 10.1371/journal.pone.0008729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Goldberger A.L., Amaral L.A., Hausdorff J.M., Ivanov P., Peng C.K., Stanley H.E. Fractal dynamics in physiology: alterations with disease and aging. Proc. Natl. Acad. Sci. USA. 2002;99(Suppl 1):2466–2472. doi: 10.1073/pnas.012579499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Martinez-Lavanchy P.M., Muller C., Nijenhuis I. High stability and fast recovery of expression of the TOL plasmid-carried toluene catabolism genes of Pseudomonas putida mt-2 under conditions of oxygen limitation and oscillation. Appl. Environ. Microbiol. 2010;76(20):6715–6723. doi: 10.1128/AEM.01039-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Kyriazis M. Biological ageing and clinical consequences of modern technology. Biogerontology. 2017;18(4):711–715. doi: 10.1007/s10522-017-9680-1. [DOI] [PubMed] [Google Scholar]
- 165.Kyriazis M. Practical applications of chaos theory to the modulation of human ageing: nature prefers chaos to regularity. Biogerontology. 2003;4(2):75–90. doi: 10.1023/a:1023306419861. [DOI] [PubMed] [Google Scholar]
- 166.Kyriazis M. Chaos theory. BMJ. 1992;304(6820):186. doi: 10.1136/bmj.304.6820.186-c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lipsitz L.A., Goldberger A.L. Loss of ‘complexity’ and aging. Potential applications of fractals and chaos theory to senescence. JAMA. 1992;267(13):1806–1809. [PubMed] [Google Scholar]
- 168.“Ten Principles of Complexity & Enabling Infrastructures” (PDF), by Professor Eve Mitleton-Kelly DCRP, London School of Economics, Archived from the original (PDF) on 29 December2009.
- 169.Ilan Y. Randomness in microtubule dynamics: an error that requires correction or an inherent plasticity required for normal cellular function? Cell Biol. Int. 2019 doi: 10.1002/cbin.11157. [DOI] [PubMed] [Google Scholar]
- 170.Ilan Y. Generating randomness: making the most out of disordering a false order into a real one. J. Transl. Med. 2019;17(1):49. doi: 10.1186/s12967-019-1798-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ilan Y. Advanced tailored randomness: a novel approach for improving the efficacy of biological systems. J. Comput. Biol. 2020;27(1):20–29. doi: 10.1089/cmb.2019.0231. [DOI] [PubMed] [Google Scholar]
- 172.Ilan Y. Overcoming randomness does not rule out the importance of inherent randomness for functionality. J. Biosci. 2019;44:6. [PubMed] [Google Scholar]
- 173.Ilan Y. Why targeting the microbiome is not so successful: can randomness overcome the adaptation that occurs following gut manipulation? Clin. Exp. Gastroenterol. 2019;12:209–217. doi: 10.2147/CEG.S203823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Vinuelas J., Kaneko G., Coulon A., Beslon G., Gandrillon O. Towards experimental manipulation of stochasticity in gene expression. Prog. Biophys. Mol. Biol. 2012;110(1):44–53. doi: 10.1016/j.pbiomolbio.2012.04.010. [DOI] [PubMed] [Google Scholar]
- 175.Boettiger A.N. Analytic approaches to stochastic gene expression in multicellular systems. Biophys. J. 2013;105(12):2629–2640. doi: 10.1016/j.bpj.2013.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Eldar A., Elowitz M.B. Functional roles for noise in genetic circuits. Nature. 2010;467(7312):167–173. doi: 10.1038/nature09326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Phillips N.E., Manning C., Papalopulu N., Rattray M. Identifying stochastic oscillations in single-cell live imaging time series using Gaussian processes. PLoS Comput. Biol. 2017;13(5) doi: 10.1371/journal.pcbi.1005479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wang G., Sun S., Zhang Z. Randomness in sequence evolution increases over time. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Streit A., Sommer R.J. Genetics: random expression goes binary. Nature. 2010;463(7283):891–892. doi: 10.1038/463891a. [DOI] [PubMed] [Google Scholar]
- 180.Kenig A., Ilan Y. A personalized signature and chronotherapy-based platform for improving the efficacy of sepsis treatment. Front. Physiol. 2019;10:1542. doi: 10.3389/fphys.2019.01542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Khoury T., Ilan Y. Introducing patterns of variability for overcoming compensatory adaptation of the immune system to immunomodulatory agents: a novel method for improving clinical response to anti-TNF therapies. Front. Immunol. 2019;10:2726. doi: 10.3389/fimmu.2019.02726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Singh N., Moneghetti K.J., Christle J.W., Hadley D., Froelicher V., Plews D. Heart rate variability: an old metric with new meaning in the era of using mhealth technologies for health and exercise training guidance. part two: prognosis and training. Arrhythm. Electro Rev. 2018;7(4):247–255. doi: 10.15420/aer.2018.30.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lees T., Shad-Kaneez F., Simpson A.M., Nassif N.T., Lin Y., Lal S. Heart rate variability as a biomarker for predicting stroke, post-stroke complications and functionality. Biomark. Insights. 2018;13 doi: 10.1177/1177271918786931. 1177271918786931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Herssens N., Verbecque E., Hallemans A., Vereeck L., Van Rompaey V., Saeys W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture. 2018;64:181–190. doi: 10.1016/j.gaitpost.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 185.Henriques T., Munshi M.N., Segal A.R., Costa M.D., Goldberger A.L. “Glucose-at-a-Glance”: new method to visualize the dynamics of continuous glucose monitoring data. J. Diabetes Sci. Technol. 2014;8(2):299–306. doi: 10.1177/1932296814524095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tosato F., Bernardi D., Sanzari M.C., Pantano G., Plebani M. Biological variability of lymphocyte subsets of human adults’ blood. Clin. Chim. Acta. 2013;424:159–163. doi: 10.1016/j.cca.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 187.Mitchell S., Roy K., Zangle T.A., Hoffmann A. Nongenetic origins of cell-to-cell variability in B lymphocyte proliferation. Proc. Natl. Acad. Sci. USA. 2018;115(12):E2888–E2897. doi: 10.1073/pnas.1715639115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fuchs A., Gliwinski M., Grageda N. Minimum information about T regulatory cells: a step toward reproducibility and standardization. Front. Immunol. 2017;8:1844. doi: 10.3389/fimmu.2017.01844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Liebers V., Kendzia B., Stubel H. Cell activation and cytokine release ex vivo: estimation of reproducibility of the whole-blood assay with fresh human blood. Adv. Exp. Med. Biol. 2018;1108:25–36. doi: 10.1007/5584_2018_225. [DOI] [PubMed] [Google Scholar]
- 190.Cirillo L., Gotta M., Meraldi P. The elephant in the room: the role of microtubules in cancer. Adv. Exp. Med. Biol. 2017;1002:93–124. doi: 10.1007/978-3-319-57127-0_5. [DOI] [PubMed] [Google Scholar]
- 191.Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- 192.Burbank K.S., Mitchison T.J. Microtubule dynamic instability. Curr. Biol. 2006;16(14):R516–R517. doi: 10.1016/j.cub.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 193.Mitchison T.J., Kirschner M.W. Some thoughts on the partitioning of tubulin between monomer and polymer under conditions of dynamic instability. Cell Biophys. 1987;11:35–55. doi: 10.1007/BF02797111. [DOI] [PubMed] [Google Scholar]
- 194.Gildersleeve R.F., Cross A.R., Cullen K.E., Fagen A.P., Williams R.C., Jr. Microtubules grow and shorten at intrinsically variable rates. J. Biol. Chem. 1992;267(12):7995–8006. [PubMed] [Google Scholar]
- 195.Pedigo S., Williams R.C., Jr. Concentration dependence of variability in growth rates of microtubules. Biophys. J. 2002;83(4):1809–1819. doi: 10.1016/S0006-3495(02)73946-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Elgart V., Lin J.R., Loscalzo J. Determinants of drug-target interactions at the single cell level. PLoS Comput. Biol. 2018;14(12) doi: 10.1371/journal.pcbi.1006601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.El-Haj M., Kanovitch D., Ilan Y. Personalized inherent randomness of the immune system is manifested by an individualized response to immune triggers and immunomodulatory therapies: a novel platform for designing personalized immunotherapies. Immunol. Res. 2019;67(4–5):337–347. doi: 10.1007/s12026-019-09101-y. [DOI] [PubMed] [Google Scholar]
- 198.Contin M., Alberghini L., Candela C., Benini G., Riva R. Intrapatient variation in antiepileptic drug plasma concentration after generic substitution vs stable brand-name drug regimens. Epilepsy Res. 2016;122:79–83. doi: 10.1016/j.eplepsyres.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 199.Del Bello A., Congy-Jolivet N., Danjoux M. High tacrolimus intra-patient variability is associated with graft rejection, and de novo donor-specific antibodies occurrence after liver transplantation. World J. Gastroenterol. 2018;24(16):1795–1802. doi: 10.3748/wjg.v24.i16.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Leino A.D., King E.C., Jiang W. Assessment of tacrolimus intrapatient variability in stable adherent transplant recipients: establishing baseline values. Am. J. Transplant. 2018 doi: 10.1111/ajt.15199. [DOI] [PubMed] [Google Scholar]
- 201.Gueta I., Markovits N., Yarden-Bilavsky H. High tacrolimus trough level variability is associated with rejections after heart transplant. Am. J. Transplant. 2018;18(10):2571–2578. doi: 10.1111/ajt.15016. [DOI] [PubMed] [Google Scholar]
- 202.Gueta I., Markovits N., Yarden-Bilavsky H. Intrapatient variability in tacrolimus trough levels after solid organ transplantation varies at different postoperative time periods. Am. J. Transplant. 2018 doi: 10.1111/ajt.15134. [DOI] [PubMed] [Google Scholar]
- 203.Rensing N., Han L., Wong M. Intermittent dosing of rapamycin maintains antiepileptogenic effects in a mouse model of tuberous sclerosis complex. Epilepsia. 2015;56(7):1088–1097. doi: 10.1111/epi.13031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Ben-Horin S., Kopylov U., Chowers Y. Optimizing anti-TNF treatments in inflammatory bowel disease. Autoimmun. Rev. 2014;13(1):24–30. doi: 10.1016/j.autrev.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 205.Ferriols-Lisart R., Ferriols-Lisart F. Dose modifications of anti-TNF drugs in rheumatoid arthritis patients under real-world settings: a systematic review. Rheumatol. Int. 2015;35(7):1193–1210. doi: 10.1007/s00296-015-3222-4. [DOI] [PubMed] [Google Scholar]
- 206.Strik A., Berends S., Mould D., Mathôt R., Ponsioen C., van den Brande J., Jansen J., Hoekman D., Brandse J., Löwenberg M., D’Haens G. DOP56 Dashboard driven vs. conventional dosing of infliximab in inflammatory bowel disease patients: the PRECISION trial. J. Crohn’s Colitis. 2019;13(Supplement):S063. [Google Scholar]
- 207.Stergiou N., Decker L.M. Human movement variability, nonlinear dynamics, and pathology: is there a connection? Hum. Mov. Sci. 2011;30(5):869–888. doi: 10.1016/j.humov.2011.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Ilan Y. Advanced tailored randomness: a novel approach for improving the efficacy of biological systems. J. Comput. Biol. 2019 doi: 10.1089/cmb.2019.0231. [DOI] [PubMed] [Google Scholar]
- 209.Weiner W.J., Koller W.C., Perlik S., Nausieda P.A., Klawans H.L. Drug holiday and management of Parkinson disease. Neurology. 1980;30(12):1257–1261. doi: 10.1212/wnl.30.12.1257. [DOI] [PubMed] [Google Scholar]
- 210.Toni T., Tidor B. Combined model of intrinsic and extrinsic variability for computational network design with application to synthetic biology. PLoS Comput. Biol. 2013;9(3) doi: 10.1371/journal.pcbi.1002960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gelman R., Bayatra A., Kessler A., Schwartz A., Ilan Y. Targeting SARS-CoV-2 Receptors as a means for reducing infectivity and improving antiviral and immune response: an algorithm-based method for overcoming resistance to antiviral agents. Emerg. Microbes Infect. 2020:1–21. doi: 10.1080/22221751.2020.1776161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ilan Y. Order through disorder: the characteristic variability of systems. Front. Cell Dev. Biol. 2020;8:186. doi: 10.3389/fcell.2020.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]