Significance Statement
AKI is common among hospitalized neonates and children and associated with adverse short- and long-term kidney outcomes. However, data for long-term outcomes of children with episodes of dialysis-treated AKI are limited. Using Ontario provincial health administrative databases, the authors identified 1688 pediatric survivors hospitalized and treated with dialysis for AKI episodes in 1996–2017, and followed them for a median of 9.6 years. Compared with matched hospitalized comparators, AKI survivors were at significantly increased risk of long-term kidney failure or death. The authors also observed significantly higher long-term rates of CKD and hypertension among the dialysis-treated AKI survivors. These findings support enhanced surveillance of kidney function and blood pressure after episodes of severe childhood AKI, with the aim of improving long-term kidney and patient survival.
Keywords: blood pressure, children, chronic kidney disease, clinical epidemiology, end stage kidney disease, hypertension, mortality, pediatric nephrology, acute renal failure, dialysis
Visual Abstract
Abstract
Background
AKI is common during pediatric hospitalizations and associated with adverse short-term outcomes. However, long-term outcomes among survivors of pediatric AKI who received dialysis remain uncertain.
Methods
To determine the long-term risk of kidney failure (defined as receipt of chronic dialysis or kidney transplant) or death over a 22-year period for pediatric survivors of dialysis-treated AKI, we used province-wide health administrative databases to perform a retrospective cohort study of all neonates and children (aged 0–18 years) hospitalized in Ontario, Canada, from April 1, 1996, to March 31, 2017, who survived a dialysis-treated AKI episode. Each AKI survivor was matched to four hospitalized pediatric comparators without dialysis-treated AKI, on the basis of age, sex, and admission year. We reported the incidence of each outcome and performed Cox proportional hazards regression analyses, adjusting for relevant covariates.
Results
We identified 1688 pediatric dialysis–treated AKI survivors (median age 5 years) and 6752 matched comparators. Among AKI survivors, 53.7% underwent mechanical ventilation and 33.6% had cardiac surgery. During a median 9.6-year follow-up, AKI survivors were at significantly increased risk of a composite outcome of kidney failure or death versus comparators. Death occurred in 113 (6.7%) AKI survivors, 44 (2.6%) developed kidney failure, 174 (12.1%) developed hypertension, 213 (13.1%) developed CKD, and 237 (14.0%) had subsequent AKI. AKI survivors had significantly higher risks of developing CKD and hypertension versus comparators. Risks were greatest in the first year after discharge and gradually decreased over time.
Conclusions
Survivors of pediatric dialysis–treated AKI are at higher long-term risks of kidney failure, death, CKD, and hypertension, compared with a matched hospitalized cohort.
AKI is common among critically ill children and neonates, occurring in approximately one-third of intensive care unit (ICU) admissions.1–7 Dialysis is administered in 6%–9% of pediatric AKI episodes.5,6 The incidence of dialysis-treated AKI has significantly increased among hospitalized children in Ontario, Canada, over the last two decades.8 Pediatric AKI, especially severe, dialysis-treated AKI, is associated with poor short-term outcomes. These include hospital mortality, duration of mechanical ventilation, length of hospital stay, and health care costs.5–7,9–13 In adults, AKI is strongly associated with long-term mortality, kidney failure, CKD, and hypertension.7,14–21 This has led to the development of post-AKI follow-up recommendations, including clinics and interventional trials, focused on preventing kidney and cardiovascular sequelae in AKI survivors.22,23
The risks of long-term kidney and cardiovascular sequelae after pediatric AKI remain uncertain. This is at least partially due to the fact that studies have lacked comparator cohorts, have had high losses to follow-up and/or have had short follow-up periods. Recent prospective cohort studies have conflicting results. The Translational Research Investigating Biomarker Endpoints in Acute Kidney Injury (TRIBE-AKI), Follow-Up Renal Assessment of Injury Long-Term After AKI (FRAIL-AKI), and Assessment, Serial Evaluation, and Subsequent Sequelae of AKI (ASSESS-AKI) studies all found that cardiac surgery–associated AKI survivors have similar 4–7-year kidney outcomes as children without AKI,24–26 with high CKD and hypertension prevalence in both groups. In a study of 277 Canadian children admitted to the pediatric ICU, those with AKI episodes had higher long-term risks of CKD and hypertension.27 Among >300 pediatric AKI survivors included in these studies, only five were reported to have dialysis-treated AKI. In fact, most pediatric AKI studies to date have focused on specific high-risk patient populations (e.g., AKI after cardiac surgery or nephrotoxin administration).24–26,28–30 There is a lack of long-term outcome data in children with AKI from any cause and children with dialysis-treated AKI. Although there is a clear signal of increased CKD risk among pediatric AKI survivors,13,31–34 the long-term outcomes after episodes of dialysis-treated AKI remain uncertain. Currently, few children receive follow-up care after an AKI episode and no specific pediatric follow-up guidelines exist.24,32
Pediatric patients may be an ideal population to study the long-term outcomes of AKI, given the low rates of preexisting CKD, diabetes, and cardiovascular risk factors. Children also have a long anticipated life expectancy after AKI episodes to manifest potential complications. Adult studies have also consistently shown that the risk of long-term kidney sequelae increases with greater AKI severity. This makes dialysis-treated AKI an appropriate exposure to determine whether a similar association exists in the pediatric population. We conducted a population-based matched-cohort study of hospitalized pediatric dialysis–treated AKI survivors to determine the risks of kidney failure, death, CKD, hypertension, subsequent AKI, and major adverse kidney events (MAKE) over two decades. We hypothesized that pediatric AKI survivors would have significantly higher long-term risks of kidney failure and death, compared with hospitalized patients without dialysis-treated AKI.
Methods
Setting and Design
We performed a population-based retrospective matched-cohort study of all hospitalized pediatric patients that experienced a dialysis-treated AKI between April 1, 1996, and March 31, 2017, in Ontario. Ontario is the largest province in Canada, with a pediatric population of 3 million and a universal health care system.35 This project was authorized under section 45 of Ontario’s Personal Health Information Protection Act. Our study is reported in accordance with the Reporting of studies Conducted using Observational Routinely collected health Data36 guidelines.
Study Population
Dialysis-treated AKI cohort
We identified all neonatal (0–28 days) and childhood (29 days to 18 years) hospitalizations in Ontario for any reason between April 1, 1996, and March 31, 2017. Birth hospitalizations were included if their length of stay was ≥5 days. Our study exposure was dialysis-treated AKI, identified through validated health administrative codes using a similar reported strategy.8 Briefly, dialysis-treated AKI was defined as the presence of at least one acute dialysis code (intermittent hemodialysis [HD], continuous renal replacement therapy [CRRT], or peritoneal dialysis [PD]) or a dialysis access code (for HD or PD catheter) that occurred between the index episode of care admission and discharge dates. For neonates, dialysis-treated AKI was defined only by the presence of at least one acute dialysis code. We did not use dialysis access codes to define AKI in neonates, based upon our previous observation that neonates may have access codes without subsequent acute dialysis codes billed during their index hospitalization (e.g., PD catheter insertion during cardiac surgery without subsequent dialysis).8 Hospitalized neonates that started dialysis after 28 days of life were classified as children.
Exclusion Criteria
From all eligible episodes of care (i.e., with or without dialysis-treated AKI), we excluded non-Ontario residents (due to lack of and/or incomplete records) and individuals with a diagnostic code for an inborn error of metabolism or poisoning, in whom dialysis may have been performed without the presence of AKI. We also excluded those who received any form of dialysis or a kidney transplant before admission, looking back to their date of birth or April 1988 (start of data availability). We excluded individuals who died or received a kidney transplant between the admission date and 90 days + 14 days (i.e., 104 days) postdischarge. The 14-day buffer was included to account for potential delays in hospital chart abstraction and coding. Among dialysis-treated AKI episodes only, we excluded individuals who continued to receive dialysis at 76 days from index dialysis start date to 104 days postdischarge (to exclude those who remained dialysis-dependent). Overall, these exclusions resulted in a cohort of pediatric dialysis–treated AKI survivors that remained alive and without evidence of kidney failure by 3 months postdischarge.
Comparator Cohort
After these exclusions, all remaining episodes of care without dialysis-treated AKI were considered for our comparator group. Starting from April 1996, each dialysis-treated AKI episode of care was matched chronologically with four comparator episodes on the basis of age at hospital discharge (neonates and children were matched separately; ±7 days for neonates and ±365 days for children), sex, and index hospitalization fiscal year. Once a dialysis-treated AKI episode was matched with four comparator episodes in unique individuals, all five individuals in the matched set were included in the study and not considered for further matching (i.e., sampling without replacement).
Data Sources
This study utilized data from multiple linked provincial health administrative databases housed at ICES . ICES is an independent, nonprofit research institute whose legal status under Ontario’s health information privacy law allows it to collect and analyze health care and demographic data, without consent, for health system evaluation and improvement. These databases contain the records of all Ontario residents with a valid health card (>99% of the province). Emigration from the province is very low (approximately 0.1% per year) and was the only reason for loss to follow-up. Demographic data were obtained from the Registered Persons Database. Hospitalization characteristics and comorbidity data were obtained from the Discharge Abstract Database (using International Classification of Diseases, Ninth and Tenth Revision codes) and Ontario Health Insurance Plan diagnostic and fee codes. The datasets were linked using unique encoded identifiers and analyzed at ICES. Complete and uncleaned data were available to investigators for the purpose of analysis. Descriptions of the databases and administrative codes used to define each variable are included in Supplemental Appendix 1.
Outcomes
Our primary outcome was a composite of all-cause mortality and kidney failure (defined as receipt of either chronic dialysis [at least two dialysis codes separated by 90–150 days] or kidney transplant). Given the low incidence of kidney failure after pediatric AKI in other studies,13,24–26 we anticipated that there would be insufficient sample size to perform multivariable analysis using kidney failure alone as our primary outcome. We therefore selected the composite primary outcome of kidney failure and death to achieve an adequate number of events. Secondary outcomes included de novo CKD (excluding children with preexisting CKD), de novo hypertension (excluding children with preexisting hypertension), MAKE (composite of all-cause mortality, kidney failure, or de novo CKD), and subsequent AKI. All primary and secondary outcomes were defined by the presence of validated administrative codes (see Supplemental Appendix 1).37–39
Demographics and Comorbidities
We evaluated the demographic and hospitalization characteristics of dialysis-treated AKI and comparator groups. Demographic variables included age, sex, gestational age, birthweight, neighborhood income quintile (by postal code),40 and rural status (community <10,000 persons).41 Index hospitalization variables included receipt of mechanical ventilation, extracorporeal membrane oxygenation (ECMO), cardiac surgery, Risk Adjustment for Congenital Heart Surgery (RACHS)-1 score ,42 stem cell or solid organ transplantation, sepsis, shock, hemolytic uremic syndrome, and malignancy. We evaluated ICU admission (presence and length of stay), length of hospital stay, dialysis duration, hospital site, and admission era (1996–2001, 2002–2006, 2007–2011, and 2012–2017). We looked back 5 years before index hospitalization (or to date of birth if age <5 years) for the following preexisting comorbidities: hypertension, CKD, malignancy, chronic liver disease, diabetes mellitus, nonrenal solid organ transplant, and cardiac surgery. We looked back 3 years to determine each individual’s Pediatric Medical Complexity Algorithm (PMCA) classification (complex chronic, noncomplex chronic, or nonchronic disease), using the “least conservative” strategy.43
Sample Size
A formal sample size calculation was not performed before the study. In order to maximize event numbers, we included all eligible individuals with dialysis-treated AKI within the province during our study period.
Statistical Analyses
We reported all continuous variables as mean (SD) and median (interquartile range [IQR]). Categoric variables were reported as frequencies (percentages). Standardized differences were used to identify any baseline differences between hospitalized dialysis-treated AKI and comparator groups. A standardized difference >10% was considered significant. After the index hospitalization, we identified primary and secondary outcomes at 104 days after hospital discharge date (i.e., day 0=104 days postdischarge) in both cohorts. This strategy was followed to remove individuals with kidney failure from our dialysis-treated AKI cohort and to avoid creating an immortal time bias compared with nonexposed individuals. The observation window continued until time of death, 2 years after last health care contact (a proxy indicator of emigration from the province), or March 31, 2018 (i.e., the end of data availability).
Multivariable Cox proportional hazards models were used to assess the association between dialysis-treated AKI and our primary outcome of kidney failure or death. We adjusted for the following variables in our Cox proportional hazards models: preexisting CKD, hypertension, diabetes, and malignancy; PMCA classification; and receipt of mechanical ventilation, cardiac surgery, ECMO, sepsis, and stem cell transplantation during the index hospitalization. All tests for significance were considered two sided, with the criterion for statistical significance set a priori at α=0.05. We did not adjust the α for multiple comparisons for secondary outcomes because these analyses were considered exploratory. When the assumption of proportionality was violated, the Cox proportional hazards model was time-stratified using data-driven time periods, such that the proportionality assumption was met within each time period. We also stratified using investigator-defined time periods (0–0.5 years, >0.5–1 year, >1–10 years, >10 years) for descriptive purposes.
We performed subgroup descriptive analysis to determine incidence rates for our primary outcome (kidney failure or death), stratified by the presence of cardiac surgery during index hospitalization, prior cardiac surgery, and prior malignancy. In addition, we created separate multivariable Cox proportional hazards models for our primary outcome among neonates and children. Our neonatal model was adjusted only for mechanical ventilation, cardiac surgery, ECMO, and sepsis. Kaplan–Meier curves in both the dialysis-treated AKI and comparator cohorts were plotted to estimate the survival probabilities for each outcome over time. Stratified log-rank tests were used to compare the equality of the survival curves in matched samples.44 All analyses were performed using SAS version 9.4 (SAS Institute, Cary, NC).
Results
Baseline Characteristics
We identified 1,257,295 unique individuals (0–18 years) that were hospitalized in Ontario between 1996 and 2017. After application of our exclusion criteria, 1,249,320 unique individuals remained eligible for inclusion (participant flow diagram; Supplemental Figure 1). We excluded 182 individuals who had evidence of chronic dialysis between 76 days post dialysis start date and 104 days postdischarge. Among these individuals, 123 had evidence of an AKI diagnostic code during their index hospitalization (suggesting that they experienced dialysis-treated AKI that progressed directly to kidney failure) and 59 individuals did not have any AKI diagnostic code (suggesting chronic dialysis initiation for kidney failure without an AKI episode). We ultimately identified 1688 patients (116 neonates and 1572 children) who survived a dialysis-treated AKI episode between 1996 and 2017 and matched them with 6752 hospitalized pediatric patients without dialysis-treated AKI. Among neonates with dialysis-treated AKI, the initial dialysis modality was PD in 59 cases (50.9%), CRRT in 46 (39.7%), and HD in 11 (9.5%). In children, the initial dialysis modality was PD in 688 cases (43.8%), HD in 542 (34.5%), and CRRT in 342 (21.8%). Within our matched comparator cohort, only 28 individuals (0.4%) were found to have administrative coding for AKI (without dialysis receipt) during their index episode of care. Because this affected only a small number of individuals, and the focus of our study was dialysis-treated AKI, these individuals were not excluded.
Complete baseline characteristics by AKI status are described in Table 1. Dialysis-treated AKI survivors had higher rates of preexisting hypertension, CKD, cardiac surgery, and complex chronic disease by PMCA than hospitalized comparators. During hospitalization, AKI survivors were more likely to be mechanically ventilated and receive ECMO. Among AKI survivors, 568 (33.6%) underwent cardiac surgery (median RACHS-1 score 3) and 857 (50.8%) were admitted to the neonatal or pediatric ICU. Median hospital length of stay was significantly longer in dialysis-treated AKI survivors versus comparators (16 versus 2 days). Median duration of dialysis was 13 (IQR, 6–26) days in dialysis-treated AKI survivors. Gestational age and birthweight data are described for the overall cohort in Table 1. For neonates specifically, the median gestational age for those with dialysis-treated AKI was 39 weeks (IQR, 37–40) versus 33 weeks (IQR, 29–37) for hospitalized comparators. Only 14 neonates (15%) in the dialysis-treated AKI cohort were born preterm (<37 weeks) and none were born extremely preterm (<28 weeks).
Table 1.
Patient and index hospitalization characteristics, by dialysis-treated AKI status
| Variable | Dialysis-treated AKI, N (%) | Comparators, N (%) | Standardized Difference (%)a |
|---|---|---|---|
| Total patients | 1688 | 6752 | — |
| Patient characteristics | |||
| Median age at index date, yr (IQR) | 5 (0–15) | 5 (1–15) | 3 |
| Sex (male) | 913 (54.1%) | 3652 (54.1%) | 0 |
| Income quintileb | |||
| 1 (lowest) | 395 (23.4%) | 1567 (23.2%) | 0 |
| 2 | 307 (18.2%) | 1339 (19.8%) | 4 |
| 3 | 342 (20.3%) | 1376 (20.4%) | 0 |
| 4 | 349 (20.7%) | 1294 (19.2%) | 4 |
| 5 (highest) | 295 (17.5%) | 1176 (17.4%) | 0 |
| Rural statusc | 264 (15.6%) | 877 (13.0%) | 8 |
| Gestational age, weeks | |||
| <28 | 7 (0.4%) | 92 (1.4%) | 10 |
| 28 to <32 | 13 (0.8%) | 128 (1.9%) | 15 |
| 32 to <37 | 104 (6.2%) | 380 (5.6%) | 5 |
| ≥37 | 571 (33.8%) | 2240 (33.2%) | 8 |
| Missing data | 993 (58.8%) | 3912 (57.9%) | 2 |
| Birth weight, g | |||
| <1000 | 8 (0.5%) | 106 (1.6%) | 11 |
| 1000 to <1500 | 24 (1.4%) | 178 (2.6%) | 10 |
| 1500 to <2500 | 130 (7.7%) | 443 (6.6%) | 6 |
| ≥2500 | 960 (56.9%) | 3861 (57.2%) | 4 |
| Missing data | 566 (33.5%) | 2164 (32.0%) | 3 |
| Preexisting comorbiditiesd | |||
| Hypertension | 96 (6.1%) | 178 (2.8%) | 16 |
| CKD | 41 (2.6%) | 25 (0.4%) | 18 |
| Malignancy | 137 (8.7%) | 414 (6.6%) | 8 |
| Chronic liver disease | 69 (4.4%) | 126 (2.0%) | 14 |
| Diabetes mellitus | 35 (2.2%) | 156 (2.5%) | 2 |
| Nonrenal solid organ transplantation | 9 (0.6%) | 12 (0.2%) | 6 |
| Cardiac surgery | 111 (7.1%) | 76 (1.2%) | 30 |
| PMCA classificatione | |||
| Nonchronic disease | 984 (62.6%) | 4807 (76.4%) | 30 |
| Noncomplex chronic disease | 124 (7.9%) | 687 (10.9%) | 10 |
| Complex chronic disease | 464 (29.5%) | 794 (12.6%) | 42 |
| Index hospitalization characteristics | |||
| Mechanical ventilationf | 906 (53.7%) | 364 (5.4%) | 125 |
| ECMO | 561 (33.2%) | 44 (0.7%) | 96 |
| Sepsis | 168 (10.0%) | 67 (1.0%) | 40 |
| Shock | 77 (4.6%) | 25 (0.4%) | 27 |
| Malignancy | 192 (11.4%) | 277 (4.1%) | 27 |
| Stem cell transplantation | 45 (2.7%) | <6 (< 0.1%) | — |
| Nonrenal solid organ transplantation | 48 (2.8%) | <6 (< 0.1%) | — |
| Hemolytic uremic syndrome | 183 (10.8%) | <6 (< 0.1%) | — |
| Cardiac surgery | 568 (33.6%) | 60 (0.9%) | 96 |
| RACHS-1 scoreg, median (IQR) | 3 (2–3) | 3 (2–3) | 31 |
| First dialysis modality | |||
| PD | 747 (44.3%) | — | — |
| HD | 553 (32.8%) | — | — |
| CRRT | 388 (23.0%) | — | — |
| Duration of dialysis, days | |||
| Mean±SD | 22.69±33.60 | — | — |
| Median (IQR) | 13 (6–26) | — | — |
| Hospital length of stay, days | |||
| Mean±SD | 30.53±45.85 | 6.02±14.09 | 72 |
| Median (IQR) | 16 (7–36) | 2 (1–4) | 165 |
| ICU admissionf(neonatal and pediatric) | 1111 (65.8%) | 596 (8.8%) | 146 |
| ICU length of stay | |||
| Mean±SD | 16.99±27.65 | 19.72±26.88 | 10 |
| Median (IQR) | 8 (3–19) | 5 (1–31) | 16 |
| Index hospitalization era | |||
| 1996–2001 | 524 (31.0%) | 2120 (31.4%) | 1 |
| 2002–2006 | 314 (18.6%) | 1259 (18.6%) | 0 |
| 2007–2011 | 348 (20.6%) | 1401 (20.7%) | 0 |
| 2012–2017 | 502 (29.7%) | 1972 (29.2%) | 1 |
| Index hospital siteh | |||
| The Hospital for Sick Children (Toronto) | 716 (42.4%) | 1002 (14.8%) | 64 |
| Children’s Hospital of Eastern Ontario (Ottawa) | 337 (20.0%) | 400 (5.9%) | 43 |
| London Health Sciences Centre (London) | 134 (7.9%) | 331 (4.9%) | 12 |
| McMaster Children’s Hospital (Hamilton) | 199 (11.8%) | 443 (6.6%) | 18 |
| Other | 779 (46.1%) | 4793 (71.0%) | 52 |
—, not applicable.
Standardized difference was used to compare pediatric dialysis–treated AKI survivors with hospitalized pediatric survivors without dialysis-treated AKI. Standardized differences are less sensitive to sample size than traditional hypothesis tests. They provide a measure of difference between groups with respect to a pooled standard deviation. A standardized difference >10% is considered a meaningful difference between groups. In this study, standardized differences were calculated using hospitalized comparators as the referent.
Income quintile was defined as neighborhood income quintile by postal code.
Rural status was defined as residence within a community <10,000 persons.41
Comorbidities in the 5 yr before the index date were considered.
PMCA classification is a validated algorithm, used to classify children with chronic disease according to medical complexity using administrative data.43
The number of individuals in the dialysis-treated AKI cohort that were admitted to the ICU or underwent mechanical ventilation were lower than expected. The administrative codes used to define these variables have not been validated in children and we suspect there is incomplete coding.
RACHS-1 score is used to stratify congenital heart surgery patients by risk of mortality; a higher score (from 1 to 4) indicates higher mortality risk.42
Index hospital site includes all hospital admissions during the index episode of care. Individuals transferred between hospitals would have both hospitals captured during their index episode.
Incidence of Long-Term Kidney Outcomes and Death
Median follow-up was 9.6 years (IQR, 4.0–16.3 years) for the entire cohort (9.1 years for dialysis-treated AKI survivors and 9.8 years for comparators). Kidney failure occurred in 44 dialysis-treated AKI survivors (2.6%), with an incidence of 2.72 events per 1000 person-years (py) (95% confidence interval [95% CI], 2.03 to 3.66), compared with ten comparators (0.1%; 0.14 events per 1000 py; 95% CI, 0.08 to 0.27) (Table 2). Death occurred in 113 dialysis-treated AKI survivors (6.7%) during follow-up (6.83 deaths per 1000 py; 95% CI, 5.68 to 8.22), compared with 161 comparators (2.4%; 2.32 deaths per 1000 py; 95% CI, 1.99 to 2.71). MAKE occurred in 297 dialysis-treated AKI survivors (18.3%, 20.95 cases per 1000 py); de novo CKD occurred in 213 dialysis-treated AKI survivors (13.1%, 15.02 cases per 1000 py); de novo hypertension occurred in 174 dialysis-treated AKI survivors (12.1%, 13.07 cases per 1000 py); and subsequent AKI (with or without dialysis) occurred in 237 AKI survivors (14.0%, 16.11 cases per 1000 py). Kaplan–Meier curves showed that dialysis-treated AKI survivors developed kidney failure or death (Figure 1, Supplemental Figures 2 and 3), MAKE (Figure 2), de novo CKD (Figure 3), and de novo hypertension (Figure 4) sooner, compared with comparators.
Table 2.
Kidney outcomes and all-cause mortality among hospital survivors, by dialysis-treated AKI status
| Outcome | Dialysis-treated AKI (n=1688) | Comparators (n=6752) | ||
|---|---|---|---|---|
| n (%)a | Incidence per 1000 py (95% CI) | n (%)b | Incidence per 1000 py (95% CI) | |
| Primary outcomes | ||||
| Kidney failure and death composite | 156 (9.2%) | 9.66 (8.26 to 11.30) | 170 (2.5%) | 2.45 (2.11 to 2.85) |
| Kidney failure | 44 (2.6%) | 2.72 (2.03 to 3.66) | 10 (0.1%) | 0.14 (0.08 to 0.27) |
| All-cause mortality | 113 (6.7%) | 6.83 (5.68 to 8.22) | 161 (2.4%) | 2.32 (1.99 to 2.71) |
| Secondary outcomes | ||||
| MAKEc | 297 of 1622 (18.3%) | 20.95 (18.69 to 23.47) | 253 of 6488 (3.9%) | 3.83 (3.39 to 4.34) |
| De novo CKDd | 213 of 1622 (13.1%) | 15.02 (13.13 to 17.18) | 113 of 6488 (1.7%) | 1.71 (1.42 to 2.06) |
| De novo hypertensiond | 174 of 1436 (12.1%) | 13.07 (11.27 to 15.17) | 169 of 5744 (2.9%) | 2.82 (2.42 to 3.28) |
| AKI during subsequent hospital encountere | 237 (14.0%) | 16.11 (14.18 to 18.29) | 138 (2.0%) | 2.01 (1.70 to 2.38) |
| Dialysis-treated AKI during subsequent hospital encounter | 97 (5.7%) | 6.06 (4.97 to 7.40) | 36 (0.5%) | 0.52 (0.38 to 0.72) |
Median follow-up time was 9.1 yr for the dialysis-treated AKI cohort.
Median follow-up time was 9.8 yr for the comparator cohort.
MAKE was defined as a composite of all-cause mortality, kidney failure, or de novo CKD.
De novo CKD and de novo hypertension were determined only for individuals without preexisting CKD and hypertension, respectively.
Hospital encounters included inpatient admissions and emergency department visits.
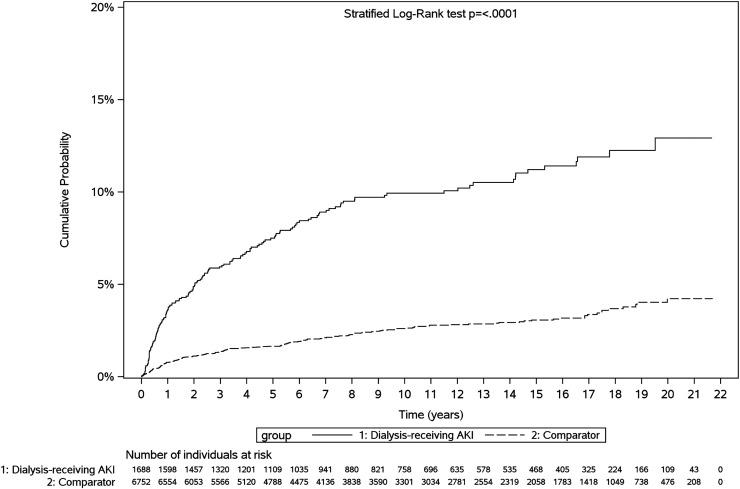
Figure 1.
Cumulative probability of kidney failure or death among dialysis-treated AKI survivors versus comparators. Dialysis-treated AKI survivors experienced kidney failure or death sooner post-discharge.
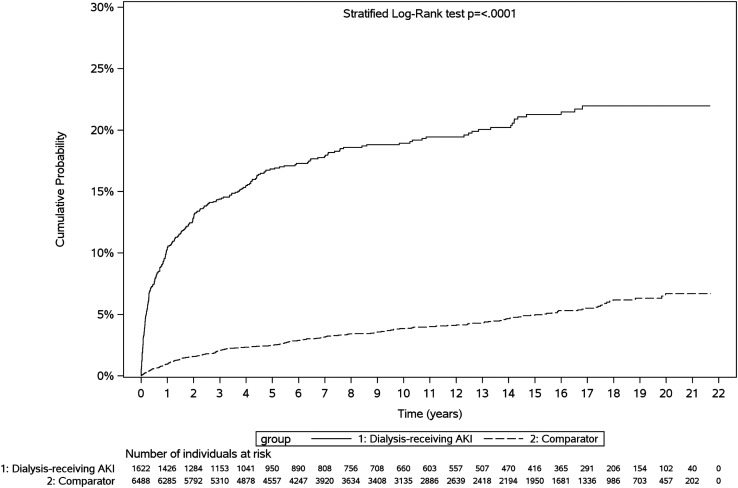
Figure 2.
Cumulative probability of MAKE among dialysis-treated AKI survivors versus comparators. MAKE was defined as a composite of all-cause mortality, kidney failure, or de novo CKD. Dialysis-treated AKI survivors experienced MAKE sooner post-discharge.
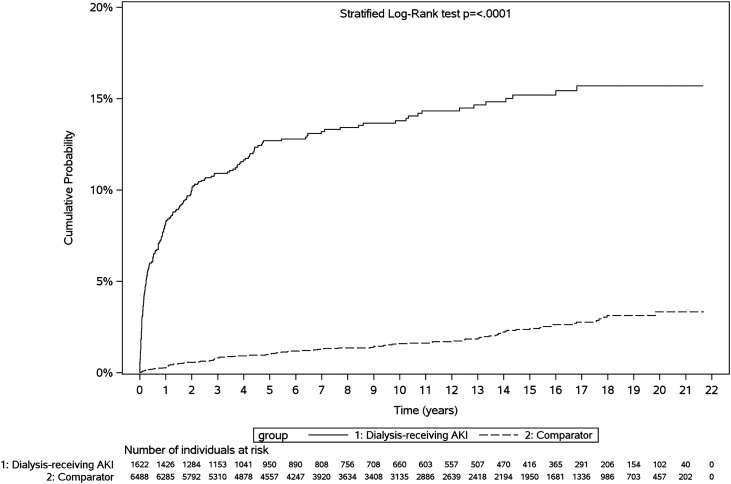
Figure 3.
Cumulative probability of de novo CKD among dialysis-treated AKI survivors versus comparators. “De novo” CKD was defined as a new diagnosis of CKD postdischarge in an individual without a diagnostic code for CKD during a 5-year lookback period before index hospitalization. Dialysis-treated AKI survivors experienced de novo CKD sooner post-discharge.
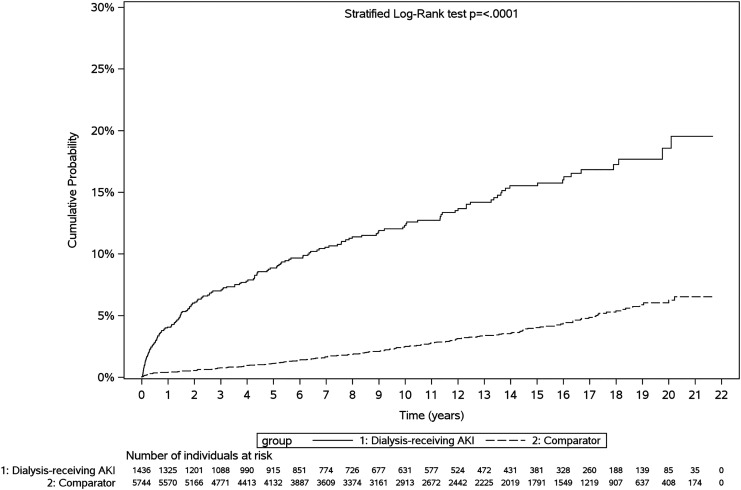
Figure 4.
Cumulative probability of de novo hypertension among dialysis-treated AKI survivors versus comparators. “De novo” hypertension was defined as a new diagnosis of hypertension postdischarge in an individual without a diagnostic code for hypertension during a 5-year lookback period before index hospitalization. Dialysis-treated AKI survivors experienced de novo hypertension sooner post-discharge.
Risks of Long-Term Kidney Outcomes and Death
Dialysis-treated AKI survivors were at an increased risk of kidney failure or death throughout follow-up, compared with hospitalized comparators, after adjustment for potential confounders (adjusted hazard ratio [aHR] 2.96; 95% CI, 2.20 to 3.97; P<0.0001). Dialysis-treated AKI survivors were also at higher risk of developing MAKE, de novo CKD, and de novo hypertension throughout follow-up (Table 3). Dialysis-treated AKI survivors were at significantly increased risk of kidney failure at 0–4 years (aHR 39.75; 95% CI, 11.75 to 134.45) and >4–16 years postdischarge versus comparators (aHR 11.32; 95% CI, 4.00 to 32.04) (Table 4). Their risk of kidney failure was highest in the early period after hospital discharge and decreased over time. “Data-driven” time period data are presented in Table 4.
Table 3.
Hazard ratios of kidney outcomes and all-cause mortality, comparing dialysis-treated AKI survivors versus comparators
| Outcomea | Unadjusted Model | Adjusted Modelb | ||
|---|---|---|---|---|
| HR | 95% CI | aHR | 95% CI | |
| Primary outcomes | ||||
| Kidney failure and death composite | 3.86 | 3.10 to 4.80 | 2.96 | 2.20 to 3.97 |
| Kidney failure | 18.52c | 9.33 to 36.75 | 17.88c | 8.61 to 37.13 |
| All-cause mortality | 2.90 | 2.28 to 3.69 | 1.95 | 1.38 to 2.76 |
| Secondary outcomes | ||||
| MAKE | 5.24c | 4.42 to 6.20 | 4.97c | 4.04 to 6.10 |
| De novo CKD | 8.35c | 6.62 to 10.54 | 8.70c | 6.68 to 11.34 |
| De novo hypertension | 4.57c | 3.69 to 5.65 | 3.35c | 2.59 to 4.33 |
MAKE was defined as a composite of all-cause mortality, kidney failure, or de novo CKD. HR, unadjusted hazard ratio.
Median follow-up time was 9.1 yr for the dialysis-treated AKI cohort and 9.8 yr for the comparator cohort.
Using Cox proportional hazards models, we adjusted for the following variables: mechanical ventilation, cardiac surgery during index episode, ECMO, sepsis, stem cell transplantation, preexisting CKD, preexisting hypertension, preexisting malignancy, preexisting diabetes mellitus, and PMCA classification.
Denotes a violation of the assumption of proportionality in the associated Cox model.
Table 4.
Adjusteda hazard ratios of kidney outcomes, comparing dialysis-treated AKI survivors versus comparators
| Outcome | aHR | 95% CI | P Value | aHR | 95% CI | P Value | aHR | 95% CI | P Value |
|---|---|---|---|---|---|---|---|---|---|
| Kidney failure | 0–4 yrb | >4–16 yr | >16 yr | ||||||
| 39.75 | 11.75 to 134.45 | <0.001 | 11.32 | 4.00 to 32.04 | <0.001 | 2.20 | 0.19 to 25.37 | 0.53 | |
| MAKE | 0–0.5 yr | >0.5–5 yr | >5 yr | ||||||
| 12.72 | 8.45 to 19.14 | <0.001 | 5.15 | 3.87 to 6.87 | <0.001 | 1.84 | 1.26 to 2.70 | 0.002 | |
| De novo CKD | 0–1 yr | >1–5 yr | >5 yr | ||||||
| 34.53 | 20.39 to 58.48 | <0.001 | 7.15 | 4.77 to 10.71 | <0.001 | 2.02 | 1.23 to 3.32 | 0.006 | |
| De novo hypertension | 0–5 yr | >5 yr | |||||||
| 6.13 | 4.26 to 8.82 | <0.001 | 1.96 | 1.40 to 2.74 | <0.001 | ||||
MAKE was defined as a composite of all-cause mortality, kidney failure, or de novo CKD.
Using Cox proportional hazards models, we adjusted for the following variables: mechanical ventilation, cardiac surgery during index episode, ECMO, sepsis, stem cell transplantation, preexisting CKD, preexisting hypertension, preexisting malignancy, preexisting diabetes mellitus, and PMCA classification.
For each outcome, day zero was 104 days after hospital discharge date in both cohorts.
Dialysis-treated AKI survivors were at increased risk of MAKE at 0–0.5 years (aHR 12.35; 95% CI, 8.35 to 18.27), >0.5–5 years (aHR 5.30; 95% CI, 4.00 to 7.01), and >5 years postdischarge (aHR 1.89; 95% CI, 1.31 to 2.73). Dialysis-treated AKI survivors were at increased risk of de novo CKD at 0–1 year (aHR 34.53; 95% CI, 20.39 to 58.48), 1–5 years (aHR 7.15; 95% CI, 4.77 to 10.71), and >5 years (aHR 2.02; 95% CI, 1.23 to 3.32). Dialysis-treated AKI survivors were at increased risk of de novo hypertension at 0–5 years (aHR 6.16; 95% CI, 4.28 to 8.85), >5–10 years (aHR 2.22; 95% CI, 1.39 to 3.56), and >10 years (aHR 1.75; 95% CI, 1.11 to 2.75). “Investigator-defined” time period data are presented in Table 5.
Table 5.
Adjusteda hazard ratios of kidney outcomes, comparing dialysis-treated AKI survivors versus comparators (“investigator-defined” time periods)
| Outcome | 0–0.5 yrb | >0.5–1 yr | >1–10 yr | >10 yr | ||||
|---|---|---|---|---|---|---|---|---|
| aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | aHR | 95% CI | |
| Kidney failure | NRc | — | NR | — | 14.84 | 5.73 to 38.43 | 3.21 | 0.73 to 14.15 |
| MAKE | 12.31 | 8.32 to 18.21 | 7.88 | 4.72 to 13.14 | 3.55 | 2.70 to 4.67 | 1.89 | 1.12 to 3.22 |
| De novo CKD | 33.07 | 18.16 to 60.20 | 38.95 | 13.67 to 110.87 | 5.46 | 3.84 to 7.76 | 1.80 | 0.90 to 3.60 |
| De novo hypertension | 5.78 | 3.26 to 10.25 | 13.47 | 4.51 to 40.28 | 3.58d | 2.56 to 5.01 | 1.74 | 1.11 to 2.74 |
MAKE was defined as a composite of all-cause mortality, kidney failure, or de novo CKD. NR, not reported ; —, not applicable.
Using Cox proportional hazards models, we adjusted for the following variables: mechanical ventilation, cardiac surgery during index episode, ECMO, sepsis, stem cell transplantation, preexisting CKD, preexisting hypertension, preexisting malignancy, preexisting diabetes mellitus, and PMCA classification.
For each outcome, day zero was 104 days after hospital discharge date in both cohorts.
Hazards of kidney failure at 0–0.5 and 0.5–1 yr were not reported to avoid recalculation of small cells (< 6 events) in the nonexposed cohort.
Denotes a violation of the assumption of proportionality.
Subgroup Analysis
Among our dialysis-treated AKI cohort, we found that the incidence of kidney failure or death was similar between those that did and did not undergo cardiac surgery during their index hospitalization (Table 6). However, the incidence of kidney failure and death was higher among individuals with a history of cardiac surgery or malignancy before their index hospitalization. We found that neonates and children with dialysis-treated AKI did have an increased risk for kidney failure or death compared with comparators (neonates aHR 17.09; 95% CI, 4.6 to 63.48; children aHR 2.86; 95% CI, 2.11 to 3.88) and there was a significant difference between neonates and children (P value for interaction < 0.001).
Table 6.
Incidence rates of composite outcome of kidney failure and death, stratified by participant’s baseline characteristics
| Subgroup | Number of Patients | Incidence per 1000 pya | 95% CI |
|---|---|---|---|
| Cardiac surgery during index episode of care | |||
| No (comparators) | 6692 | 2.43 | 2.09 to 2.83 |
| No (dialysis-treated AKI) | 1120 | 9.82 | 8.2 to 11.76 |
| Yes (comparators) | 60 | 4.92 | 1.59 to 15.25 |
| Yes (dialysis-treated AKI) | 568 | 9.2 | 6.69 to 12.64 |
| Prior cardiac surgeryb | |||
| No (comparators) | 6212c | 2.45 | 2.10 to 2.86 |
| No (dialysis-treated AKI) | 1461c | 8.63 | 7.23 to 10.29 |
| Yes (comparators) | 76c | 10.84 | 5.17 to 22.74 |
| Yes (dialysis-treated AKI) | 111c | 22.75 | 14.51 to 35.67 |
| Prior malignancyb | |||
| No (comparators) | 5874c | 1.71 | 1.41 to 2.07 |
| No (dialysis-treated AKI) | 1435c | 7.30 | 6.03 to 8.84 |
| Yes (comparators) | 414c | 16.74 | 13.00 to 21.56 |
| Yes (dialysis-treated AKI) | 137c | 45.66 | 33.22 to 62.75 |
| Neonates versus childrend | |||
| Neonates (comparators) | 464 | 1.19 | 0.49 to 2.85 |
| Neonates (dialysis-treated AKI) | 116 | 13.82 | 8.03 to 23.81 |
| Children (comparators) | 6288 | 2.53 | 2.18 to 2.95 |
| Children (dialysis-treated AKI) | 1572 | 9.40 | 7.98 to 11.08 |
Median follow-up time was 9.1 yr for the dialysis-treated AKI cohort and 9.8 yr for the comparator cohort.
Prior cardiac surgery and malignancy were defined by the presence of administrative codes within 5 yr before index hospitalization (or to date of birth if age less than 5 yr at index hospitalization).
Neonates with missing data for prior cardiac surgery and malignancy (580 individuals; 116 in AKI and 464 in comparator cohorts) were excluded from this analysis.
Children included all individuals 29 days to 18 yr at index hospitalization. Neonates were defined as individuals that started dialysis between 0 and 28 days of life. Hospitalized neonates that started dialysis after 28 days of life were defined as children.
Discussion
Using provincial health administrative databases, we assembled a cohort of 1688 pediatric dialysis–treated AKI survivors who were followed for a median 9.6 years postdischarge. Dialysis-treated AKI survivors had significantly higher risks of kidney failure or death, compared with a matched hospitalized comparator cohort. The risks of kidney failure and CKD were highest in the first year postdischarge and decreased gradually over time.
These results substantiate the reported associations between severe AKI and long-term kidney sequelae in animal and human studies. In animal models, AKI is associated with parenchymal hypoxia and endothelial injury, leading to maladaptive tubular repair, medullary injury, and nephron loss.45–51 The additional functional stress placed on the remaining nephrons leads to hyperfiltration, progressive tubulointerstitial fibrosis, and a decline in kidney function.45,46,52,53 Recent adult studies have found that AKI episodes were associated with long-term CKD after adjusting for potential confounders.16,17,54,55 These studies have found dose-dependent relationships between increasing AKI severity and CKD risk.56,57 A meta-analysis of 13 cohort studies found that adult AKI survivors have a nine-times greater risk of CKD and three-times greater risk of kidney failure versus those patients without AKI.17 Adult dialysis-treated AKI survivors are at additional risk, with a 28-times higher risk of CKD stage 4–555 and a higher risk of initiating chronic dialysis (adjusted HR 15.5).14 However, the extrapolation of adult AKI data to children is problematic due to higher rates of preexisting CKD, cardiovascular disease, and diabetes among adults.
There are conflicting pediatric data on the relationship between AKI episodes and long-term kidney outcomes. Generally, pediatric AKI has been associated with adverse short-term outcomes, including increased hospital mortality,5–7,10,13 duration of mechanical ventilation, and length of ICU and hospital stay.5,6,9–12 After pediatric AKI, high rates of long-term mortality, CKD, proteinuria, and hypertension are reported.12,13,24,25,28–34,58–60 A 2014 meta-analysis of ten pediatric studies reported that the pooled incidence of kidney failure among AKI survivors was 0.4%.13 Hessey et al. studied 2235 children admitted to the pediatric ICU, finding that AKI survivors were at increased risk of CKD by 5 years postdischarge, compared with nonexposed children (multivariable HR 2.3; 95% CI, 1.3 to 4.3; P<0.05).60 Benisty et al. also found that pediatric AKI survivors had a higher risk of CKD or abnormal blood pressure by 6 years postdischarge (OR 2.2; 95% CI, 1.1 to 4.4; P<0.05), and that children with stage 2–3 were at additional risk.27 However, after cardiac surgery–associated AKI, the risk of adverse kidney outcomes is less clear. The TRIBE-AKI (n=131), FRAIL-AKI (n=51), and ASSESS-AKI (n=124) studies found no significant differences in kidney outcomes or hypertension between pediatric AKI survivors and nonexposed children at 4–7-year follow-up.24–26 Conversely, Madsen et al. (n=382) found that pediatric cardiac surgery–associated AKI survivors were at higher risk of CKD during median 4.9-year follow-up.34
In our study, there was a higher incidence of kidney failure or death among dialysis-treated AKI cases with a history of cardiac surgery, but no difference observed among those that underwent cardiac surgery during their dialysis-treated AKI admission. Dialysis-treated AKI survivors with a history of malignancy also had a higher incidence of kidney failure or death, highlighting the importance of long-term kidney surveillance among this high-risk population. When analyzed separately, we found that both neonates and children that experienced dialysis-treated AKI were at significantly increased risk of subsequent kidney failure or death. The incidence of kidney failure or death was higher among neonates than children, although this difference was not statistically significant. Our estimates were less precise for neonates given the smaller number of patients and events. However, our study may represent the largest cohort of neonates with dialysis-treated AKI (116 individuals) that has been published to date and a signal exists in our data that this population may be at higher risk of long-term adverse kidney outcomes.
In most studies, children with dialysis-treated AKI are under-represented (typically <5% of pediatric AKI cohorts) and are grouped together for analysis with other AKI survivors.24–27,34,61 Studies that have separately evaluated dialysis-treated AKI survivors have shown that they are at additional risk of adverse kidney outcomes and death.5,6,31,33 Mammen et al. studied 126 critically ill pediatric AKI survivors, finding that the incidence of CKD by 1–3 years post-AKI increased with greater AKI severity (AKI stage 1: 4.5%; stage 2: 10.6%; stage 3: 17.1%).31 CKD incidence was higher among children with dialysis-treated AKI versus those not receiving dialysis (22.7% versus 7.7%, P=0.04). In another study including 43 dialysis-treated AKI survivors, 25.6% had kidney failure requiring chronic dialysis and 9.3% had CKD at time of hospital discharge.33 In our study, dialysis-treated AKI survivors had significantly increased long-term risks of kidney failure, all-cause mortality, MAKE, de novo CKD, and de novo hypertension. Approximately half of the kidney failure and CKD diagnoses among AKI survivors occurred within the first year postdischarge and the majority within 5 years. This highlights the need for close CKD surveillance in the first 5 years after dialysis-treated AKI episodes, which should be emphasized in future AKI follow-up guidelines. Although these risks decreased gradually over time, AKI survivors remained at significantly higher risks of kidney failure, CKD, and hypertension more than 5 years postdischarge, indicating that they may also benefit from long-term CKD surveillance.
Our study has a number of strengths. To our knowledge, this represents the largest cohort of pediatric dialysis–treated AKI survivors assembled to evaluate long-term kidney outcomes. We followed participants for up to 22 years, providing a prolonged longitudinal observation period for kidney sequelae to manifest post-AKI. We leveraged unique data availability from Ontario’s universal health care system and population-based linked health administrative databases. Annual emigration from the province is low,62 minimizing loss to follow-up. Ontario’s socioeconomic and demographic diversity makes our results generalizable to other similarly developed countries. Including all pediatric patients surviving a dialysis-treated AKI episode provides additional generalizability. By including a matched comparator cohort, we compared the risk of kidney outcomes among AKI survivors with hospitalized pediatric patients without dialysis-treated AKI. This was one of the first pediatric AKI studies to include the MAKE end point, and we found that AKI survivors were at higher risk of MAKE at all time periods. The National Institute of Diabetes and Digestive and Kidney Diseases workgroup for clinical trials in AKI has recommended using MAKE as a composite end point to capture clinically important, patient-centered outcomes.63,64 Given the low incidence of kidney failure, death, and CKD observed in pediatric AKI studies, the use of MAKE as a composite primary end point may be preferred.
Our study also has a number of limitations. Use of administrative data introduces risks of miscoding for our study exposure (dialysis-treated AKI) or outcomes. However, acute dialysis is reliably captured in adults using administrative data (sensitivity 90%, specificity 94%, positive predictive value 94%),65 and accurate billing is a prerequisite for physician and facility reimbursement. Further, administrative coding for our primary outcomes (kidney failure and death) is highly accurate (sensitivity and positive predictive value >96% for both).66 In children, coding for CKD is very specific (98%–99%) and has moderate positive predictive value (52%–68%), but low sensitivity (20%–39%).37 This is similar for hypertension in adults (specificity 95%, positive predictive value 81%–87%, sensitivity 71%).38 This suggests that our results underestimate CKD and hypertension incidence after AKI. We chose not to exclude comparator individuals with administrative codes for AKI who did not receive dialysis, as this only composed 0.4% of the cohort. As a result, some comparator individuals may have experienced AKI episodes that did not require dialysis, which would attenuate the effect size of the outcomes.
In order to differentiate children that experienced an episode of dialysis-treated AKI from those that started dialysis for preexisting kidney failure or progressed directly to kidney failure after their AKI, we excluded individuals who received ongoing dialysis between 76 days post dialysis start date and 104 days postdischarge. Another limitation is the inability to determine the proportion of children that were discharged from hospital still on regular dialysis, and subsequently recovered adequate renal function to stop dialysis before the 3 months. Because laboratory and medication data were not available throughout our study period, we could not further validate CKD or hypertension diagnoses or evaluate proteinuria. We acknowledge the possibility of ascertainment bias that children with dialysis-treated AKI were more likely to visit a health care provider and thereby had an increased likelihood of the outcomes compared with the comparator cohort. We were unable to present separate secondary outcome results for neonates and children due to ICES data privacy policies limiting small cell sizes (<6 individuals). Event numbers for our primary outcome prevented us from performing additional subgroup analysis to evaluate associated risk factors. However, we had adequate event numbers to adjust for our selected covariates in primary outcome analysis. Despite matching and adjustment for relevant covariates, we cannot exclude that possible residual confounding factors and imbalances still exist between cohorts after matching. We were unable to match for additional variables such as cardiac surgery, sepsis, and mechanical ventilation due to low event numbers in comparator individuals. Instead, we adjusted for these variables in our Cox regression analyses.
Conclusion
We found that pediatric dialysis–treated AKI survivors were at significantly increased long-term risks of kidney failure and death, MAKE, CKD, and hypertension, versus hospitalized comparators. This justifies enhanced surveillance of kidney function and blood pressure after episodes of severe AKI. With CKD and hypertension being important cardiovascular risk factors, early identification and treatment of these conditions in AKI survivors could improve long-term kidney and patient survival. Further research should investigate methods of risk-stratifying AKI survivors to determine who would benefit most from specialist post-AKI clinic follow-up. Future research should also aim to determine the effects of post-AKI clinic follow-up and other tertiary prevention methods on long-term kidney outcomes.
Disclosures
D. Askenazi reports Consultancy Agreements with AKI Foundation, Baxter, Bioporto, CHF Solutions, Medtronic; and Research Funding from Baxter Renal Products and CHF Solutions. A.X. Garg reports Research Funding from Astellas; Scientific Advisor or Membership as Editorial Board member of Kidney International and American Journal of Kidney Diseases; Other Interests/Relationships as member of the Data Safety and Monitoring Board for an Investigator-Initiated Trial Program Funded by Glaxo Smith Kline, Medical Lead Role to Improve Access to Kidney Transplantation and Living Kidney Donation for the Ontario Renal Network (government-funded agency located within Ontario Health). S. Goldstein reports Consultancy Agreements with Akebia, Baxter Healthcare, Bayer, Bioporto, Inc., CHF Solutions, Fresenius, Kaneka, Inc., La Jolla Pharmaceuticals, MediBeacon, Medtronic, Otsuka, Reata, Renibus; Ownership Interest in MediBeacon; Research Funding from Baxter Healthcare, Bioporto, CHF Solutions; Honoraria from Baxter Healthcare, Fresenius; Patents and Inventions with Vigilanz; Scientific Advisor or Membership with MediBeacon; and Speakers Bureau with Baxter Healthcare and Fresenius. R. Wald reports Research Funding from Baxter; Scientific Advisor or Membership as Editorial Board member of CJASN, Kidney Medicine, and Kidney360; Other Interests/Relationships as Contributor to UpToDate. M. Zappitelli reports Consultancy Agreements with Bioporto, Inc., CytoPheryx, Inc., Eloxx Pharmaceuticals; Honoraria from Bioporto, Inc., Eloxx Pharmaceuticals; and Other Interests/Relationships with the Kidney Foundation of Canada, the Canadian Society of Nephrology, and the Canadian Pediatric Nephrologists Association. All remaining authors have nothing to disclose.
Funding
This study was funded by a New Investigator Grant from Hamilton Health Sciences (Award Number: NIF-17405) and by a Biomedical Research Grant from the Kidney Foundation of Canada (Award Number: KFOC190016). This research was undertaken, in part, thanks to funding from the Canada Research Chairs Program (to R.S. Parekh).
Supplementary Material
Acknowledgments
We acknowledge the support and guidance we received from the entire ICES Western Kidney, Dialysis and Transplantation team. This study was supported by ICES, which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). Parts of this material are based on data and information compiled and provided by MOHLTC and CIHI. The analyses, conclusions, opinions and statements expressed herein are solely those of the authors and do not reflect those of the funding or data sources; no endorsement is intended or should be inferred.
Cal H. Robinson, Rahul Chanchlani, Michael Zappitelli, Eric McArthur, Danielle Nash, and Amit X. Garg designed the study; Ron Wald, Jason Henry Greenberg, David Askenazi, Cherry Mammen, Lehana Thabane, and Stuart L. Goldstein critically appraised and revised the study protocol and initiation documents; Nivethika Jeyakumar, Bin Luo, and Eric McArthur were responsible for cohort building, data collection, cleaning and analysis; Cal H. Robinson, Nivethika Jeyakumar, and Bin Luo produced the tables and figures; Cal H. Robinson, Nivethika Jeyakumar, Bin Luo, and Rahul Chanchlani drafted the manuscript; and all authors revised the manuscript and approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020111665/-/DCSupplemental.
Supplemental Figure 1. Participant flow diagram.
Supplemental Figure 2. Cumulative probability of kidney failure among dialysis-treated AKI survivors versus comparators.
Supplemental Figure 3. Cumulative probability of all-cause mortality among dialysis-treated AKI survivors versus comparators.
Supplemental Appendix 1. Administrative codes used for cohort selection, baseline characteristics, and outcomes.
Supplemental Appendix 2. RECORD statement checklist.
References
- 1.Koralkar R, Ambalavanan N, Levitan EB, McGwin G, Goldstein S, Askenazi D: Acute kidney injury reduces survival in very low birth weight infants. Pediatr Res 69: 354–358, 2011 [DOI] [PubMed] [Google Scholar]
- 2.Gadepalli SK, Selewski DT, Drongowski RA, Mychaliska GB: Acute kidney injury in congenital diaphragmatic hernia requiring extracorporeal life support: an insidious problem. J Pediatr Surg 46: 630–635, 2011 [DOI] [PubMed] [Google Scholar]
- 3.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, et al.; Neonatal Kidney Collaborative (NKC): Incidence and outcomes of neonatal acute kidney injury (AWAKEN): a multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1: 184–194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Stojanović V, Barišić N, Radovanović T, Bjelica M, Milanović B, Doronjski A: Acute kidney injury in premature newborns—definition, etiology, and outcome. Pediatr Nephrol 32: 1963–1970, 2017 [DOI] [PubMed] [Google Scholar]
- 5.Sutherland SM, Ji J, Sheikhi FH, Widen E, Tian L, Alexander SR, et al.: AKI in hospitalized children: epidemiology and clinical associations in a national cohort. Clin J Am Soc Nephrol 8: 1661–1669, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators: Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, et al.; Acute Kidney Injury Advisory Group of the American Society of Nephrology: World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol 8: 1482–1493, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chanchlani R, Nash DM, McArthur E, Zappitelli M, Archer V, Kuwornu JP, et al.: Secular trends in incidence, modality and mortality with dialysis receiving AKI in children in Ontario: a population-based cohort study. Clin J Am Soc Nephrol 14: 1288–1296, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kari JA, Alhasan KA, Shalaby MA, Khathlan N, Safdar OY, Al Rezgan SA, et al.: Outcome of pediatric acute kidney injury: a multicenter prospective cohort study. Pediatr Nephrol 33: 335–340, 2018 [DOI] [PubMed] [Google Scholar]
- 10.Sutherland SM, Byrnes JJ, Kothari M, Longhurst CA, Dutta S, Garcia P, et al.: AKI in hospitalized children: comparing the pRIFLE, AKIN, and KDIGO definitions. Clin J Am Soc Nephrol 10: 554–561, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alkandari O, Eddington KA, Hyder A, Gauvin F, Ducruet T, Gottesman R, et al.: Acute kidney injury is an independent risk factor for pediatric intensive care unit mortality, longer length of stay and prolonged mechanical ventilation in critically ill children: a two-center retrospective cohort study. Crit Care 15: R146, 1-12, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hessey E, Morissette G, Lacroix J, Perreault S, Samuel S, Dorais M, et al.: Long-term mortality after acute kidney injury in the pediatric ICU. Hosp Pediatr 8: 260–268, 2018 [DOI] [PubMed] [Google Scholar]
- 13.Greenberg JH, Coca S, Parikh CR: Long-term risk of chronic kidney disease and mortality in children after acute kidney injury: a systematic review. BMC Nephrol 15: 184, 1-11, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wald R, Quinn RR, Luo J, Li P, Scales DC, Mamdani MM, et al.; University of Toronto Acute Kidney Injury Research Group: Chronic dialysis and death among survivors of acute kidney injury requiring dialysis. JAMA 302: 1179–1185, 2009 [DOI] [PubMed] [Google Scholar]
- 15.Hoste EAJ, Bagshaw SM, Bellomo R, Cely CM, Colman R, Cruz DN, et al.: Epidemiology of acute kidney injury in critically ill patients: the multinational AKI-EPI study. Intensive Care Med 41: 1411–1423, 2015 [DOI] [PubMed] [Google Scholar]
- 16.Ishani A, Xue JL, Himmelfarb J, Eggers PW, Kimmel PL, Molitoris BA, et al.: Acute kidney injury increases risk of ESRD among elderly. J Am Soc Nephrol 20: 223–228, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coca SG, Singanamala S, Parikh CR: Chronic kidney disease after acute kidney injury: a systematic review and meta-analysis. Kidney Int 81: 442–448, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wald R, Quinn RR, Adhikari NK, Burns KE, Friedrich JO, Garg AX, et al.; University of Toronto Acute Kidney Injury Research Group: Risk of chronic dialysis and death following acute kidney injury. Am J Med 125: 585–593, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Wu V-C, Wu C-H, Huang T-M, Wang C-Y, Lai C-F, Shiao C-C, et al.; NSARF Group: Long-term risk of coronary events after AKI. J Am Soc Nephrol 25: 595–605, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu CY, Hsu RK, Yang J, Ordonez JD, Zheng S, Go AS: Elevated BP after AKI. J Am Soc Nephrol 27: 914–923, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Odutayo A, Wong CX, Farkouh M, Altman DG, Hopewell S, Emdin CA, et al.: AKI and long-term risk for cardiovascular events and mortality. J Am Soc Nephrol 28: 377–387, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Silver SA, Goldstein SL, Harel Z, Harvey A, Rompies EJ, Adhikari NK, et al.: Ambulatory care after acute kidney injury: an opportunity to improve patient outcomes. Can J Kidney Health Dis 2: 36, 1-13 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Harel Z, Wald R, Bargman JM, Mamdani M, Etchells E, Garg AX, et al.: Nephrologist follow-up improves all-cause mortality of severe acute kidney injury survivors. Kidney Int 83: 901–908, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Greenberg JH, Zappitelli M, Devarajan P, Thiessen-Philbrook HR, Krawczeski C, Li S, et al.; TRIBE-AKI Consortium: Kidney outcomes 5 years after pediatric cardiac surgery: the TRIBE-AKI Study. JAMA Pediatr 170: 1071–1078, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cooper DS, Claes D, Goldstein SL, Bennett MR, Ma Q, Devarajan P, et al.: Follow-Up Renal Assessment of Injury Long-Term After Acute Kidney Injury (FRAIL-AKI). Clin J Am Soc Nephrol 11: 21–29, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zappitelli M, Parikh CR, Kaufman JS, Go AS, Kimmel PL, Hsu C, et al.; Assessment, Serial Evaluation, and Subsequent Sequelae in Acute Kidney Injury (ASSESS-AKI) Investigators: Acute kidney injury and risk of CKD and hypertension after pediatric cardiac surgery. Clin J Am Soc Nephrol 15: 1403-1412, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Benisty K, Morgan C, Hessey E, Huynh L, Joffe AR, Garros D, et al.: Kidney and blood pressure abnormalities 6 years after acute kidney injury in critically ill children: a prospective cohort study. Pediatr Res 88: 271–278, 2020 [DOI] [PubMed] [Google Scholar]
- 28.Hoffmeister PA, Hingorani SR, Storer BE, Baker KS, Sanders JE: Hypertension in long-term survivors of pediatric hematopoietic cell transplantation. Biol Blood Marrow Transplant 16: 515–524, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Menon S, Kirkendall ES, Nguyen H, Goldstein SL: Acute kidney injury associated with high nephrotoxic medication exposure leads to chronic kidney disease after 6 months. J Pediatr 165: 522–7.e2, 2014 [DOI] [PubMed] [Google Scholar]
- 30.Hollander SA, Montez-Rath ME, Axelrod DM, Krawczeski CD, May LJ, Maeda K, et al.: Recovery from acute kidney injury and CKD following heart transplantation in children, adolescents, and young adults: a retrospective cohort study. Am J Kidney Dis 68: 212–218, 2016 [DOI] [PubMed] [Google Scholar]
- 31.Mammen C, Al Abbas A, Skippen P, Nadel H, Levine D, Collet JP, et al.: Long-term risk of CKD in children surviving episodes of acute kidney injury in the intensive care unit: a prospective cohort study. Am J Kidney Dis 59: 523–530, 2012 [DOI] [PubMed] [Google Scholar]
- 32.Askenazi DJ, Feig DI, Graham NM, Hui-Stickle S, Goldstein SL: 3-5 year longitudinal follow-up of pediatric patients after acute renal failure. Kidney Int 69: 184–189, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Hui-Stickle S, Brewer ED, Goldstein SL: Pediatric ARF epidemiology at a tertiary care center from 1999 to 2001. Am J Kidney Dis 45: 96–101, 2005 [DOI] [PubMed] [Google Scholar]
- 34.Madsen NL, Goldstein SL, Frøslev T, Christiansen CF, Olsen M: Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 92: 751–756, 2017 [DOI] [PubMed] [Google Scholar]
- 35.Statistics Canada: Ontario [Province] and Canada [Country] (table). Census Profile. 2016 Census. Ottawa: Statistics Canada. Available at: https://www12.statcan.gc.ca/census-recensement/2016/dp-pd/prof/index.cfm?Lang=E. Accessed June 13, 2021
- 36.Benchimol EI, Smeeth L, Guttmann A, Harron K, Moher D, Petersen I, et al.; RECORD Working Committee: The Reporting of studies Conducted using Observational Routinely-collected health Data (RECORD) statement. PLoS Med 12: e1001885, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dart A, Chartier M, Komenda P, Walld R, Koseva I, Burchill C, et al.: Evaluation of administrative case definitions for chronic kidney disease in children. Pediatr Res 87: 569–575, 2020 [DOI] [PubMed] [Google Scholar]
- 38.Pace R, Peters T, Rahme E, Dasgupta K: Validity of health administrative database definitions for hypertension: a systematic review. Can J Cardiol 33: 1052–1059, 2017 [DOI] [PubMed] [Google Scholar]
- 39.D’Arienzo D, Hessey E, Ali R, Perreault S, Samuel S, Roy L, et al.: A validation study of administrative health care data to detect acute kidney injury in the pediatric intensive care unit. Can J Kidney Health Dis 6: 2054358119827525, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Canadian Institute for Health Information (CIHI): Health Indicators 2013. Canadian Institute for Health Information. Available at: https://www.cihi.ca/sites/default/files/document/health-indicators-2013-en.pdf. Accessed June 13, 2021
- 41.Bollman R: Rural Demography Update. Rural Ontario Institute. Available at: http://www.ruralontarioinstitute.ca/file.aspx?id=26acac18-6d6e-4fc5-8be6-c16d326305fe. Accessed June 13, 2021
- 42.Jenkins KJ, Gauvreau K, Newburger JW, Spray TL, Moller JH, Iezzoni LI: Consensus-based method for risk adjustment for surgery for congenital heart disease. J Thorac Cardiovasc Surg 123: 110–118, 2002 [DOI] [PubMed] [Google Scholar]
- 43.Simon TD, Haaland W, Hawley K, Lambka K, Mangione-Smith R: Development and validation of the Pediatric Medical Complexity Algorithm (PMCA) version 3.0. Acad Pediatr 18: 577–580, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Klein JP, Moeschberger ML: Survival Analysis Techniques for Censored and Truncated Data. Springer-Verlag New York, 2003. DOI: 10.1007/b97377
- 45.Chawla LS, Eggers PW, Star RA, Kimmel PL: Acute kidney injury and chronic kidney disease as interconnected syndromes. N Engl J Med 371: 58–66, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Venkatachalam MA, Weinberg JM, Kriz W, Bidani AK: Failed tubule recovery, AKI-CKD transition, and kidney disease progression. J Am Soc Nephrol 26: 1765–1776, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nath KA: Tubulointerstitial changes as a major determinant in the progression of renal damage. Am J Kidney Dis 20: 1–17, 1992 [DOI] [PubMed] [Google Scholar]
- 48.Venkatachalam MA, Griffin KA, Lan R, Geng H, Saikumar P, Bidani AK: Acute kidney injury: a springboard for progression in chronic kidney disease. Am J Physiol Renal Physiol 298: F1078–F1094, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nath KA, Croatt AJ, Haggard JJ, Grande JP: Renal response to repetitive exposure to heme proteins: chronic injury induced by an acute insult. Kidney Int 57: 2423–2433, 2000 [DOI] [PubMed] [Google Scholar]
- 50.Basile DP, Donohoe D, Roethe K, Osborn JL: Renal ischemic injury results in permanent damage to peritubular capillaries and influences long-term function. Am J Physiol Renal Physiol 281: F887–F899, 2001 [DOI] [PubMed] [Google Scholar]
- 51.Hsu CY: Yes, AKI truly leads to CKD. J Am Soc Nephrol 23: 967–969, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Polichnowski AJ, Lan R, Geng H, Griffin KA, Venkatachalam MA, Bidani AK: Severe renal mass reduction impairs recovery and promotes fibrosis after AKI. J Am Soc Nephrol 25: 1496–1507, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basile DP, Donohoe DL, Roethe K, Mattson DL: Chronic renal hypoxia after acute ischemic injury: effects of L-arginine on hypoxia and secondary damage. Am J Physiol Renal Physiol 284: F338–F348, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Chawla LS, Amdur RL, Amodeo S, Kimmel PL, Palant CE: The severity of acute kidney injury predicts progression to chronic kidney disease. Kidney Int 79: 1361–1369, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lo LJ, Go AS, Chertow GM, McCulloch CE, Fan D, Ordoñez JD, et al.: Dialysis-requiring acute renal failure increases the risk of progressive chronic kidney disease. Kidney Int 76: 893–899, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ishani A, Nelson D, Clothier B, Schult T, Nugent S, Greer N, et al.: The magnitude of acute serum creatinine increase after cardiac surgery and the risk of chronic kidney disease, progression of kidney disease, and death. Arch Intern Med 171: 226–233, 2011 [DOI] [PubMed] [Google Scholar]
- 57.Thakar CV, Christianson A, Himmelfarb J, Leonard AC: Acute kidney injury episodes and chronic kidney disease risk in diabetes mellitus. Clin J Am Soc Nephrol 6: 2567–2572, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Garg AX, Suri RS, Barrowman N, Rehman F, Matsell D, Rosas-Arellano MP, et al.: Long-term renal prognosis of diarrhea-associated hemolytic uremic syndrome: a systematic review, meta-analysis, and meta-regression. JAMA 290: 1360–1370, 2003 [DOI] [PubMed] [Google Scholar]
- 59.Hirano D, Ito A, Yamada A, Kakegawa D, Miwa S, Umeda C, et al.: Independent risk factors and 2-year outcomes of acute kidney injury after surgery for congenital heart disease. Am J Nephrol 46: 204–209, 2017 [DOI] [PubMed] [Google Scholar]
- 60.Hessey E, Perreault S, Dorais M, Roy L, Zappitelli M: Acute kidney injury in critically ill children and subsequent chronic kidney disease. Can J Kidney Health Dis 6: 2054358119880188, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chang J-W, Jeng M-J, Yang L-Y, Chen T-J, Chiang S-C, Soong W-J, et al.: The epidemiology and prognostic factors of mortality in critically ill children with acute kidney injury in Taiwan. Kidney Int 87: 632–639, 2015 [DOI] [PubMed] [Google Scholar]
- 62.Ministry of Finance: Ontario Population Projections Update, 2017–2041. Ministry of Finance. Available at: https://www.fin.gov.on.ca/en/economy/demographics/projections/#s4f. Accessed June 13, 2021
- 63.Palevsky PM, Molitoris BA, Okusa MD, Levin A, Waikar SS, Wald R, et al.: Design of clinical trials in acute kidney injury: report from an NIDDK workshop on trial methodology. Clin J Am Soc Nephrol 7: 844–850, 2012 [DOI] [PubMed] [Google Scholar]
- 64.Palevsky PM: Endpoints for clinical trials of acute kidney injury. Nephron 140: 111–115, 2018 [DOI] [PubMed] [Google Scholar]
- 65.Waikar SS, Wald R, Chertow GM, Curhan GC, Winkelmayer WC, Liangos O, et al.: Validity of International Classification of Diseases, Ninth Revision, Clinical Modification codes for acute renal failure. J Am Soc Nephrol 17: 1688–1694, 2006 [DOI] [PubMed] [Google Scholar]
- 66.Lam NN, McArthur E, Kim SJ, Knoll GA: Validation of kidney transplantation using administrative data. Can J Kidney Health Dis 2: 20: 1-5, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.