Infection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) elicits cellular and humoral immune responses. In patients with coronavirus disease 2019 (COVID-19), IgM, IgA, and IgG antibodies against SARS-CoV-2 emerge within a few days,1 and can be detected in serum and oral fluids.2 Although IgA and IgM antibodies vanish within about 2 months, IgG levels persist for at least 8 months in the general population.3 SARS-CoV-2 seropositivity in patients on dialysis has been demonstrated,4,5 but data on SARS-CoV-2 antibody dynamics are limited.
Although repeated blood draws in patients on hemodialysis pose no major medical or logistic problems, the situation is different in patients on peritoneal dialysis (PD) who lack permanent vascular access. Therefore, we explored the presence or absence of SARS-CoV-2 antibodies in 96 spent PD dialysate samples from 58 patients as a complement for blood samples. Patients are classified into four groups: patients on chronic PD, prepandemic (Group 1; collected between May 2019 and February 2020); patients who were COVID-19 negative and on chronic PD (Group 2; collected between March and September 2020); patients with COVID-19 and on chronic PD (Group 3; collected between March and September 2020); and patients with Acute Kidney Injury (AKI), COVID-19, and treated with acute PD (Group 4; from April 2020). All patients in Group 3 and Group 4 were confirmed by SARS-CoV-2 RT-PCR using nasopharyngeal swab. None of the patients in Group 3 and Group 4 received convalescent plasma treatment. Sample collection procedures, patient characteristics, and institutional review board protocol details are provided in the Supplemental Methods and Supplemental Table 1. PD dialysate samples in Group 3 and Group 4 were collected on average 39 days and 26 days, respectively, after symptom onset. As reported previously, all PD dialysate samples in Group 3 and Group 4 were tested negative for SARS-CoV-2 viral RNA.6,7 We had no access to serum SARS-CoV-2 antibody levels in any patient.
SARS-CoV-2 IgG in spent PD dialysate was measured by enzyme-linked immunosorbent assay (ELISA), targeting nucleocapsid protein of SARS-CoV-2 virus (MPBIO cat 08440100). IgG concentration (expressed in ng/ml) was calculated using the IgG standard provided with the ELISA kit. Figure 1A illustrates IgG levels in the four groups. Average antibody levels of each group are listed in the Supplemental Methods and Supplemental Table 1. Receiver operating characteristic (ROC) analysis revealed an excellent discrimination between patients with and without COVID-19 (area under the curve, 0.944; Figure 1B).
Figure 1.
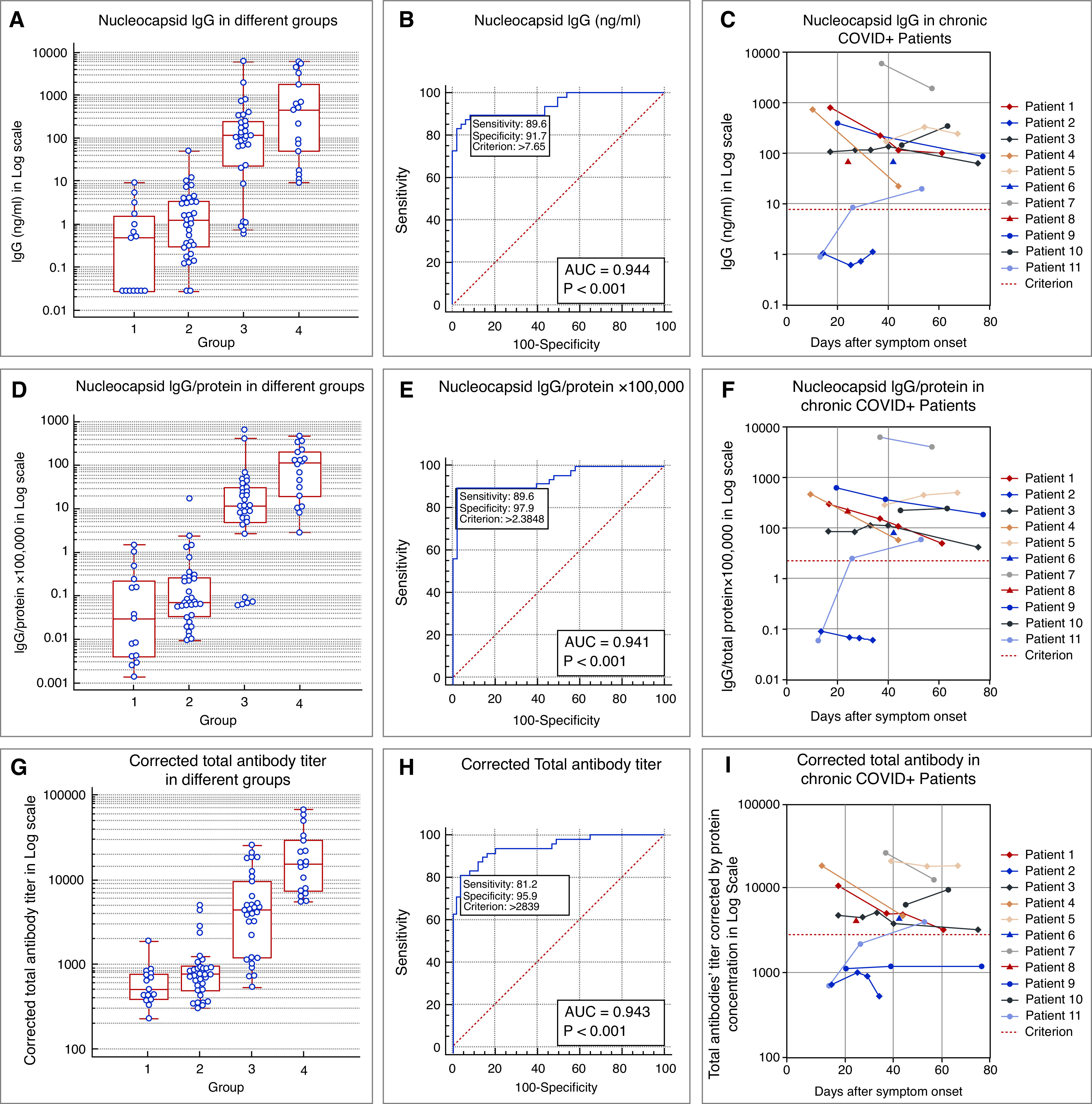
SARS-CoV-2 antibodies in spent peritoneal dialysate. Box and whisker plots of (A) nucleocapsid IgG concentration, (D) IgG-to-protein ratio, and (G) corrected total SARS-CoV-2 antibody titer in spent PD dialysate. Samples were collected in the prepandemic era (Group 1; patients on chronic PD); patients without COVID-19 and on chronic PD during the pandemic (Group 2); patients on chronic PD with confirmed COVID-19 (Group 3); and patients with confirmed COVID-19 and AKI treated with acute PD (Group 4). ROC curves and optimal cut-off threshold with the associated sensitivity and specificity for (B) nucleocapsid IgG, (E) IgG-to-protein ratio, and (H) corrected total SARS-CoV-2 antibodies. We report area under the ROC curve (AUC ROC) and a P value to test the null hypothesis of AUC ROC equals 0.50. Sensitivity and specificity are presented for discrimination points with maximum Youden index (Youden index = sensitivity + specificity – 1). Longitudinal evolution of antibodies in spent dialysate from symptom onset. (C) Nucleocapsid IgG, (F) IgG-to-protein ratio, and (I) corrected total SARS-CoV-2 antibodies. Note that repeated samples were available for nine out of 11 patients. Patients 6 and 8 provided only one sample and the respective results are indicated by a single triangle in the figure.
To account for the variability of protein loss in spent PD dialysate, we normalized IgG levels to the dialysate protein concentration.8 The latter was determined by Bradford protein assay (Thermo Fisher Scientific, cat 23200). IgG-to-protein ratio and the respective ROC analysis are shown in Figure 1D and 1E. Compared with IgG levels, the IgG-to-protein ratio has similar sensitivity (89.6%) but an increased specificity (91.7% versus 97.9%) at optimal thresholds. The arithmetic means of IgG-to-protein ratio in Group 3 and Group 4 are 87-fold (P=0.02) and 222-fold (P=0.0003), respectively, above the mean of Groups 1 and 2 combined.
Using the threshold determined by ROC analysis, nine out of 11 patients in Group 3 had positive IgG in all samples (Figure 1C, 1F). Repeated samples were available in nine patients from Group 3. In five patients, IgG levels decreased. In patient 11, IgG was not detected on day 14 but increased after day 26. In patient 2, IgG was not detected in any sample (collected on days 14, 25, 29, and 34). Of note, IgG was present in all spent PD dialysate samples in Group 4.
We next used ELISA targeting both nucleocapsid protein and spike protein to measure total SARS-CoV-2 antibodies in spent dialysate (AFFYPRO cat EA821). To calculate antibody titer, we first created a dilution series of a COVID-19–negative sample. At 640-fold dilution, the optical density (OD) approached the ELISA’s detection limit; therefore, the titer of this sample was determined as 640. This sample was used as standard to calculate the titers of the other samples. We also report titers corrected for the sample protein concentration as follows:
The geometric means of corrected total antibody titers in Group 3 and Group 4 are 5.6-fold and 22.5-fold, respectively, above those of Groups 1 and 2 combined (both P<0.001). Distribution pattern, ROC analysis, and dynamic change over time of total SARS-CoV-2 antibodies are comparable to those of IgG (Figure 1G–I).
To our knowledge, this is the first report on SARS-CoV-2 antibodies in spent PD dialysate. COVID-19 serology tests provide valuable information regarding infection status, immune response after infection and vaccination, and seroprevalence surveys. Currently, most serology tests require blood draws. Measurement of SARS-CoV-2 antibodies in spent PD dialysate provides a simple, novel way to repeatedly and noninvasively assess the antibody response of patients on PD to infection with SARS-CoV-2 and, possibly, vaccination. Although the area under the curve ROC analyses are comparable between the three tests reported here (between 0.941 and 0.944), the IgG-to-protein ratio outperforms the other tests regarding specificity (97.9%) combined with very good sensitivity (89.6%). PD effluent antibody testing could complement serum serology testing and inform patients on PD and their care providers about a possible infection with SARS-CoV-2. Depending on the clinical situation nasopharyngeal swab and RT-PCR testing may be considered. Of note, a negative PD dialysate antibody test does not rule out current or past COVID-19 infection. As shown in our study, one out of 11 patients on PD with documented COVID-19 had no detectable antibodies in the spent dialysate. Whether antibodies in PD effluent post-COVID-19 or after vaccination indicate development of immunity is a topic for ongoing research.
Disclosures
N. Grobe, P. Kotanko, A. Patel, and X. Wang, are employees of the Renal Research Institute, a wholly owned subsidiary of Fresenius Medical Care North America. J. Uribarri and S. Sharma are employees of Icahn School of Medicine at Mount Sinai, New York. J. Uribarri reports receiving Honoraria from Baxter. P. Kotanko holds stock and reports receiving research funding from Fresenius Medical Care; reports receiving honoraria from HSTalks and UpToDate; reports being a scientific advisor or member of the Editorial Board of Blood Purification, Editorial Board of Kidney and Blood Pressure Research; and reports having other interests/relationships in Fresenius Medical Care. All remaining authors have nothing to disclose.
Funding
This study was supported in part by the National Institutes of Health National Institute of Diabetes and Digestive and Kidney Diseases grant R01DK130067.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021020161/-/DCSupplemental
Supplemental Methods. Patient cohorts and spent PD dialysate collection.
Supplemental Table 1. Patient characteristics and antibody test results.
References
- 1.Long Q-X, Liu B-Z, Deng H-J, Wu G-C, Deng K, Chen Y-K, et al.: Antibody responses to SARS-CoV-2 in patients with COVID-19. Nat Med 26: 845–848, 2020 [DOI] [PubMed] [Google Scholar]
- 2.MacMullan MA, Chellamuthu P, Mades A, Das S, Turner F, Slepnev VI, et al.: Detection of SARS-CoV-2 antibodies in oral fluid obtained using a rapid collection device. J Clin Microbiol 59: e02510-20, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dan JM, Mateus J, Kato Y, Hastie KM, Yu ED, Faliti CE, et al.: Immunological memory to SARS-CoV-2 assessed for up to 8 months after infection. Science 371: eabf4063, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anand S, Montez-Rath M, Han J, Bozeman J, Kerschmann R, Beyer P, et al.: Prevalence of SARS-CoV-2 antibodies in a large nationwide sample of patients on dialysis in the USA: A cross-sectional study. Lancet 396: 1335–1344, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clarke C, Prendecki M, Dhutia A, Ali MA, Sajjad H, Shivakumar O, et al.: High prevalence of asymptomatic COVID-19 infection in hemodialysis patients detected using serologic screening. J Am Soc Nephrol 31: 1969–1975, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang X, Patel A, Tisdale L, Haq Z, Ye X, Lasky R, et al.: SARS-CoV-2 in spent dialysate from chronic peritoneal dialysis patients with COVID-19. Kidney360 2: 86–89, 2020. Dec;10.34067/KID.0006102020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.El Shamy O, Vassalotti JA, Sharma S, Aydillo-Gomez T, Marjanovic N, Ramos I, et al.: Coronavirus disease 2019 (COVID-19) hospitalized patients with acute kidney injury treated with acute peritoneal dialysis do not have infectious peritoneal dialysis effluent. Kidney Int 98: 782, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenkrantz MJ, Gahl GM, Kopple JD, Kamdar AV, Jones MR, Kessel M, et al.: Protein losses during peritoneal dialysis. Kidney Int 19: 593–602, 1981 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


