Medical centers nationwide are considering race-free equations to improve the reporting of eGFR.1 Numerous alternatives2 have been proposed and implemented—including removal of the coefficient for Black race—with substantial implications for guideline-recommended care.3
Race-free alternatives are typically modified from equations developed by the CKD Epidemiology Consortium (CKD-EPI) and Modification of Diet in Renal Disease Study (MDRD). The most common current equations use serum creatinine and require race (MDRD eGFRcr and CKD-EPI eGFRcr). CKD-EPI eGFRcr-cys, computed from creatinine and cystatin C, has been suggested as the most accurate equation for measured GFR (mGFR) but also requires race. CKD-EPI eGFRcys is the only guideline-recommended equation that does not use race.
Removal of race from eGFRcr may be achieved by “blending” (averaging) race-specific outputs equally (50% “if White/Other”, 50% “if Black”), blending by population proportions (88% “if White/Other”, 12% “if Black”), or direct removal of race coefficients (100% “if White/Other”, 0% “if Black”, or vice versa). Despite the abundance of race-free alternatives, it is unclear how their eGFR distributions compare with those of eGFRcr and eGFRcr-cys. Population-level differences estimated from a representative national sample may indicate shifts in health care access and utilization to consider alongside accuracy comparisons on the basis of mGFR.
Methods
Using data from the National Health and Nutrition Examination Survey (NHANES), we compared eGFR distributions from proposed and implemented race-free equations to those of equations recommended by guidelines.4 NHANES is a cross-sectional probability sample designed to be representative of the US population and study participants provided written informed consent. Our sample comprised 4434 nonpregnant adults, from 1999 to 2002, the only years with serum measurements for both creatinine and cystatin C. Both creatinine and cystatin C were calibrated to appropriate reference materials.5,6
We computed eGFRcys and eGFRcr with race-removed and race-blended modifications and compared their distributions to that of eGFRcr-cys in terms of median eGFR difference and proportion meeting GFR thresholds for stages 3a (<60), 3b (<45), and 4 (<30) of CKD.4 Distributions and summary statistics were computed using NHANES survey weights and design. All analyses were performed using R version 4.0.0.
Results
Relative to eGFRcr-cys, eGFRcys resulted in the smallest change in CKD prevalence among Black adults (0.7%) compared with race-blended or race-removed equations (1.4%–4.5%) (Figure 1A). Similarly, eGFRcys yielded the smallest median change in eGFR among Black adults (−0.8; 95% confidence interval [95% CI], −2.6 to 0.2 ml/min per 1.73 m2) compared with other race-free alternatives, ranging from 11.3 (95% CI, 9.8 to 13.1) for race-blended (50% “if Black”) CKD-EPI eGFRcr to 29.0 (95% CI, 27.0 to 30.7) for race-removed (0% “if Black”) MDRD eGFRcr (Figure 1B). eGFRcys also produced the smallest gap in CKD prevalence between Black and White/Other patients (3.7%) relative to other race-free equations (6.1% to 7.4%).
Figure 1.
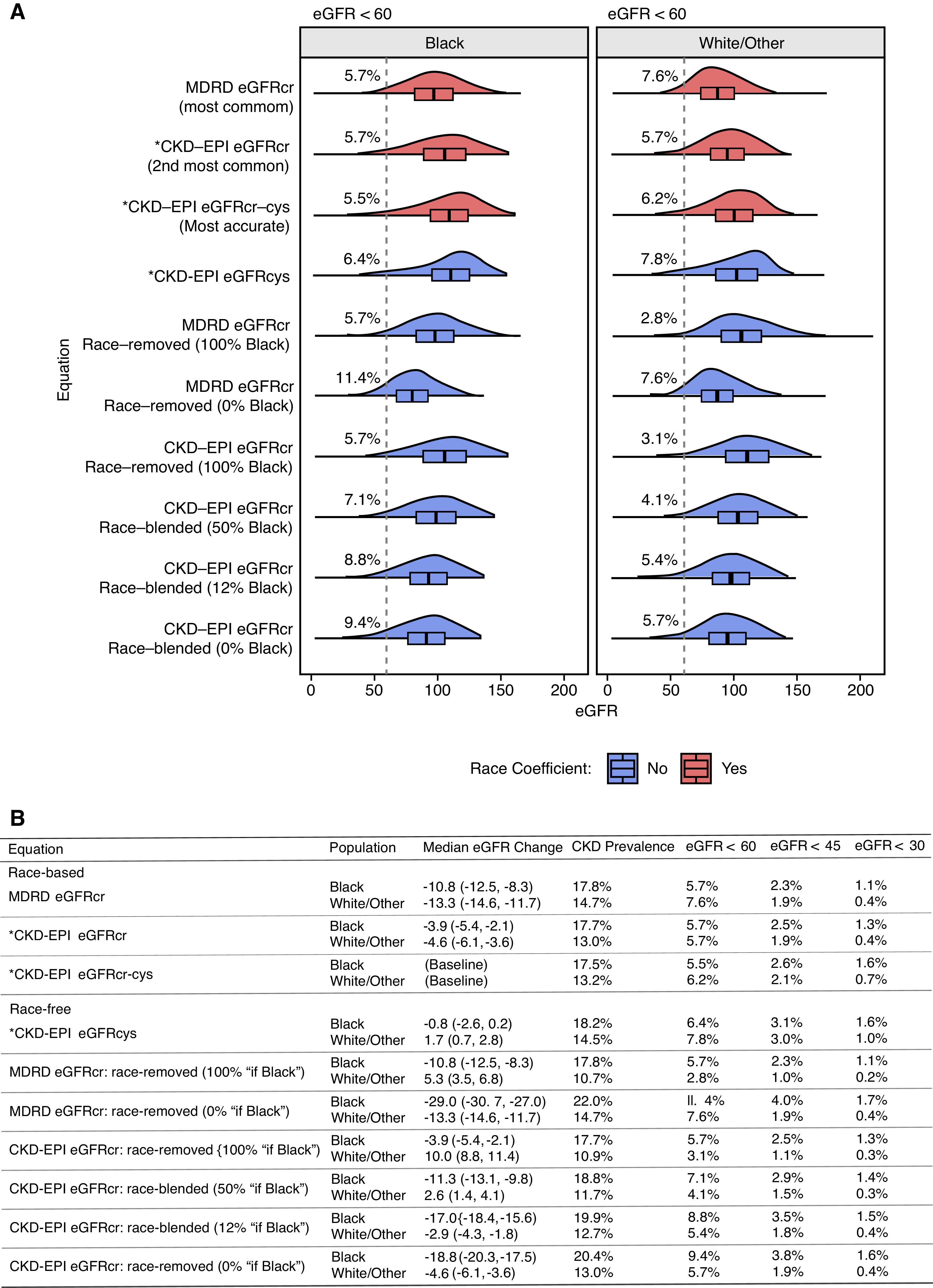
eGFR distributions for guideline-recommended equations and proposed race-free modifications. (A) Density plots for reported values across eGFR equations. Equations recommended by Kidney Disease: Improving Global Outcomes (KDIGO) are marked with asterisks (*). Percentage labels indicate the proportion falling below the dotted line (eGFR=60), a threshold recommended by KDIGO as one component for the diagnosis of CKD. The sample comprises 861 Black subjects and 3573 White/Other subjects from the NHANES. Distributions and summary statistics were computed using the NHANES weights and survey structure to be representative of the 1999–2002 US population. (B) Summary statistics and proportions under relevant eGFR thresholds recommended by KDIGO for CKD diagnosis (eGFR<60 or urinary albumin-creatinine ratio >30 mg/g) and stage reclassification (eGFR<30, 45, or 60) of CKD. Median change is evaluated relative to eGFRcr-cys, the eGFR equation with the highest reported accuracy compared with measured GFR.
The most common equations return lower eGFR values compared with eGFRcr-cys in all populations: CKD-EPI eGFRcr by 3.9 (95% CI, 2.1 to 5.4) and 4.6 (95% CI, 3.6 to 6.1) ml/min per 1.73 m2 in Black and White/Other adults, and MDRD eGFRcr by 10.8 (95% CI, 8.3 to 12.5) and 13.3 (95% CI, 11.7 to 14.6) ml/min per 1.73 m2. Attenuating the race coefficient from 100% “if Black” to 0% “if Black” in CKD-EPI eGFR increased the proportion of Black adults with CKD from 17.7% to 20.4% and the proportion with eGFR<60 from 5.7% to 9.4%. Similar increases were observed for eGFR<45 (2.5% to 3.8%) and eGFR<30 (1.3% to 1.6%). This change, equivalent to assigning Black individuals the “if White/Other” value, results in a larger median change from eGFRcr-cys for Black individuals (−18.8; 95% CI, −20.3 to −17.5 ml/min per 1.73 m2) than for White/Other individuals from assigning them the “if Black” value (10.0; 95% CI, 8.8 to 11.4). The same effect was observed for MDRD eGFRcr, amounting to −29.0 (95% CI, −30.7 to −27.0) and 5.3 (95% CI, 3.5 to 6.8) ml/min per 1.73 m2, respectively. Attenuation by blending or removal had a greater effect on MDRD eGFRcr than CKD-EPI eGFRcr due to adjustment of a larger race coefficient (1.21 versus 1.16).
Discussion
Our data indicate that among race-free alternatives to recommended equations, eGFRcys would likely result in the smallest changes to eGFR values for Black patients and associated clinical decisions. Increased attenuation of the race coefficient by blending or removal resulted in decreased eGFR estimates and increased CKD stage reclassifications. These changes may increase the proportion of patients diagnosed with and managed for CKD in all populations, which is noteworthy given potential underestimation from MDRD eGFRcr and CKD-EPI eGFRcr relative to eGFRcr-cys.
Limitations include potential nonrepresentativeness of earlier cohorts and lack of data on mGFR that precludes subject-level evaluation against the gold standard. Population-level shifts in eGFR must be evaluated while bearing in mind the paucity of Black subjects represented in validation data for eGFRcys and eGFRcr-cys,7 and the effects of structural racism resulting in underdiagnosis, undermanagement, and poorer outcomes for Black patients.8 Beyond social determinants of kidney disease, improved race-free equations may also consider genetic factors9 (e.g., APOL1), although their clinical utility is unclear.10 In the meantime, further studies should investigate and compare accuracies of currently available race-free equations using mGFR collected from diverse populations.
Disclosures
N. Powe reports receiving honoraria from, and being a scientific advisor or member of, the Patient Centered Outcomes Research Institute, Robert Wood Johnson Foundation, Vanderbilt University, University of Washington, and Yale University. All remaining authors have nothing to disclose.
Funding
This work was supported by the National Heart, Lung, and Blood Institute grant 5K01HL138259.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Eneanya ND, Yang W, Reese PP: Reconsidering the consequences of using race to estimate kidney function. JAMA 322: 113–114, 2019 [DOI] [PubMed] [Google Scholar]
- 2.Diao JA, Inker LA, Levey AS, Tighiouart H, Powe NR, Manrai AK: In search of a better equation: Performance and equity in estimates of kidney function. N Engl J Med 384: 396–399, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Diao JA, Wu GJ, Taylor HA, Tucker JK, Powe NR, Kohane IS, et al.: Clinical implications of removing race from estimates of kidney function. JAMA 325: 184–186, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group . KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter, Suppl. 2013; 3: 1–150. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO_2012_CKD_GL.pdf. Accessed November 22, 2021 [Google Scholar]
- 5.Selvin E, Manzi J, Stevens LA, Van Lente F, Lacher DA, Levey AS, et al.: Calibration of serum creatinine in the National Health and Nutrition Examination Surveys (NHANES) 1988–1994, 1999-2004. Am J Kidney Dis 50: 918–926, 2007 [DOI] [PubMed] [Google Scholar]
- 6.Selvin E, Juraschek SP, Eckfeldt J, Levey AS, Inker LA, Coresh J: Calibration of cystatin C in the National Health and Nutrition Examination Surveys (NHANES). Am J Kidney Dis 61: 353–354, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inker LA, Schmid CH, Tighiouart H, Eckfeldt JH, Feldman HI, Greene T, et al. ; CKD-EPI Investigators : Estimating glomerular filtration rate from serum creatinine and cystatin C. N Engl J Med 367: 20–29, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Powe NR: Let’s get serious about racial and ethnic disparities. J Am Soc Nephrol 19: 1271–1275, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Udler MS, Nadkarni GN, Belbin G, Lotay V, Wyatt C, Gottesman O, et al.: Effect of genetic African ancestry on eGFR and kidney disease. J Am Soc Nephrol 26: 1682–1692, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cerdeña JP, Tsai J, Grubbs V: APOL1, Black race, and kidney disease: Turning attention to structural racism [published online ahead of print January 22, 2021]. Am J Kidney Dis S0272–6386(21)00055-X, 2021 [DOI] [PubMed] [Google Scholar]


