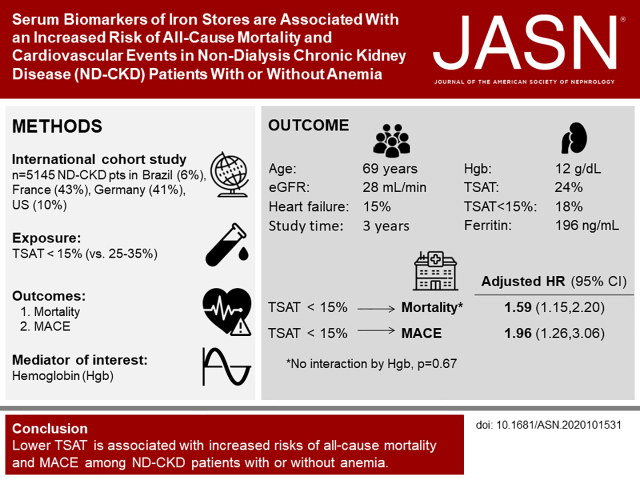
Significance Statement
Management of iron deficiency in patients with nondialysis CKD focuses on improving erythropoiesis. Studies in patients with heart failure with similar iron deficiency pathogenesis found that treating iron deficiency improves cardiovascular outcomes, regardless of anemia. To evaluate a possible anemia-independent association of iron stores with outcomes in individuals with nondialysis CKD, the authors studied patients in nephrology-based clinics from a multinational cohort. They show that iron deficiency, as reflected by transferrin saturation index, is associated with higher risk of mortality and cardiovascular events in patients with CKD, with or without anemia. Intervention studies addressing the effects of treating iron deficiency beyond effects on erythropoiesis are necessary to challenge the current anemia-focused paradigm of iron deficiency management in nondialysis CKD, and potentially foster better strategies for improving patient outcomes.
Keywords: chronic kidney disease, anemia, mortality
Visual Abstract
Abstract
Background
Approximately 30%–45% of patients with nondialysis CKD have iron deficiency. Iron therapy in CKD has focused primarily on supporting erythropoiesis. In patients with or without anemia, there has not been a comprehensive approach to estimating the association between serum biomarkers of iron stores, and mortality and cardiovascular event risks.
Methods
The study included 5145 patients from Brazil, France, the United States, and Germany enrolled in the Chronic Kidney Disease Outcomes and Practice Patterns Study, with first available transferrin saturation (TSAT) and ferritin levels as exposure variables. We used Cox models to estimate hazard ratios (HRs) for all-cause mortality and major adverse cardiovascular events (MACE), with progressive adjustment for potentially confounding variables. We also used linear spline models to further evaluate functional forms of the exposure-outcome associations.
Results
Compared with patients with a TSAT of 26%–35%, those with a TSAT ≤15% had the highest adjusted risks for all-cause mortality and MACE. Spline analysis found the lowest risk at TSAT 40% for all-cause mortality and MACE. Risk of all-cause mortality, but not MACE, was also elevated at TSAT ≥46%. Effect estimates were similar after adjustment for hemoglobin. For ferritin, no directional associations were apparent, except for elevated all-cause mortality at ferritin ≥300 ng/ml.
Conclusions
Iron deficiency, as captured by TSAT, is associated with higher risk of all-cause mortality and MACE in patients with nondialysis CKD, with or without anemia. Interventional studies evaluating the effect on clinical outcomes of iron supplementation and therapies for alternative targets are needed to better inform strategies for administering exogenous iron.
Iron deficiency (ID), defined as a reduction in bone marrow iron stores, is a common condition in CKD, occurring in 30%–45% of patients across CKD stages.1,2 ID is further classified as absolute or functional, with the latter defined by the absence of iron stores, whereas the former reflects insufficient iron availability to ensure adequate erythropoiesis.3 Kidney Disease Improving Global Outcomes guidelines of anemia management in patients with nondialysis CKD (ND-CKD) suggest screening and monitoring of iron stores, a recommendation that is restricted to patients with anemia who are being considered to start or are in maintenance therapy with erythropoiesis stimulating agents (ESA).4 The guidelines go on to state that transferrin saturation (TSAT) ≤30% and ferritin ≤500 ng/ml targets should be pursued to ensure sufficient absolute and functional iron stores, enabling effective erythropoiesis with ESA doses as low as possible to achieve recommended targets.4 Despite these evidence-based recommendations published almost a decade ago, our recent real-world multinational study reported that patients with ND-CKD and clear indications for iron replacement therapy remain considerably undertreated.5
The aforementioned approach of managing ID with the single focus of providing effective erythropoiesis has been recently challenged in a randomized control trial (RCT) among patients on hemodialysis.6 In this study, a proactive strategy using intravenous (IV) iron sucrose resulted in a 15% lower risk of the primary composite outcome of all-cause mortality plus cardiovascular events, in spite of similar achieved hemoglobin (Hgb) over the follow-up period.6 Because tissue ID globally affects energy metabolism and muscle function, an anemia-independent effect of ID on cardiovascular outcomes, particularly those related to heart failure (HF), is biologically plausible.7 In fact, RCTs in patients with HF show the correction of ID has a positive effect on heart function, measured by echocardiogram, and yields a lower risk of HF hospitalizations and cardiovascular mortality.8–10 This evidence, mainly for IV iron, supports the guidelines’ recommendations for treatment of ID in patients with symptomatic HF.11,12
Given the spectrum of cardio-renal anemia syndromes and the underlying mechanisms linking ID to cardiovascular health, the assessment of the anemia-independent effect of ID on clinical outcomes in patients with ND-CKD is overdue, considering the potential for a paradigm shift in iron management in CKD. Although previous observational studies of patients with ND-CKD have shown that ID is associated with higher mortality and cardiovascular hospitalization, the restricted population enrolled in those studies, and the absence of assessment of effect among patients without anemia, limits the assessment of iron stores in terms of clinical outcomes beyond its erythropoietic effects.13,14 Therefore, we have assessed the anemia-independent association of iron stores with all-cause mortality and cardiovascular events in the Chronic Kidney Disease Outcomes and Practice Patterns Study (CKDopps), an ongoing multinational cohort study of adult ND-CKD individuals followed in nephrology clinics.
Methods
Patient Sample
The study sample consisted of patients with stage 3–5 ND-CKD enrolled in the CKDopps between 2013 and 2017 from Brazil, France, Germany, and the United States.15 In each country, a national list of nephrologist-run clinics was assembled to serve as the sampling frame. Random selection of clinics was stratified by geographic region and key clinic characteristics (e.g., clinic size and public versus private) to be as nationally representative as possible. Within each stratum, randomly selected clinics were sequentially approached for participation. Included patients were at least 18 years of age (without an upper age limit), receiving care for CKD at the clinic, and had an eGFR <60 ml/min per 1.73m2 at the time of screening. Individuals with a prior kidney transplant or receiving maintenance dialysis were excluded. Patients were included in our analyses according to availability of TSAT or ferritin laboratory measurements.
Exposure Definition
Iron stores were assessed using the first available TSAT and ferritin measurements from enrollment. When available, TSAT and ferritin samples collected on the same day were used. We used multiple imputation if one of these two was not reported; all patients were required to have at least one of the two samples for inclusion in our analysis. Hgb was collected on the same day as TSAT and ferritin.
Outcomes
Primary Outcome
The primary outcome was all-cause mortality pre-KRT, defined as death occurring before reaching dialysis or transplant. For this outcome, all four countries were included in the sample.
Secondary Outcome
The secondary outcome was the pre-KRT composite of major adverse cardiovascular events (MACE). For this study, MACE was defined as cardiovascular death, which was any cardiovascular event leading to death, nonfatal acute myocardial infarction (AMI) events, nonfatal stroke, and nonfatal HF hospitalization. Cardiovascular deaths and nonfatal AMI events in the French sample were formally adjudicated by two cardiologists, following the framework proposed by the SCTI Guidelines, whereas nonfatal stroke and non-fatal HF hospitalization were coded by a physician on the basis of SCTI Guideline criteria.16 For CKDopps Brazil and United States, events were defined on the basis of clinical site-reported causes of death and hospitalization data. Two study investigators independently evaluated the reports to determine the cause of hospitalization or death, considering SCTI recommendations. All events in the Brazil and US data were validated by a cardiologist. All German patients were excluded from MACE analysis due to the absence of reports on cause-specific mortality.
Statistical Analysis
To assess associations between iron stores and time-to-event outcomes (all-cause mortality and MACE), we used cause-specific Cox models to account for competing risks.17,18 Time at risk started at the date of the measurement of iron parameters and ended at the event of interest (all-cause mortality for primary outcome or MACE for secondary outcome), patient departure (e.g., transfer to another facility), end of study data collection, or a competing event. For all-cause mortality, the competing event was the start of KRT; for MACE, the competing events were the start of KRT and death from noncardiovascular causes. The proportional hazards assumption of the Cox models was checked by Kaplan-Meier plots and formally tested by interaction between time and exposure terms in the models.
We used categorical TSAT exposure levels of ≤15%, 16%–20%, 21%–25%, 26%–35% (reference group), 36%–45%, and ≥46%; ferritin level categories were <50 ng/ml, 50–99 ng/ml, 100–299 ng/ml (reference group), and ≥300 ng/ml. To explore the functional form between the exposure-outcomes associations, TSAT was also modeled as a linear spline with a single knot at 40%; this model was chosen after examining both the categorical model results and a cubic spline TSAT model with five knots. In an exploratory analysis, we used a flexible yet parsimonious interaction model with continuous TSAT and the log of ferritin, along with their squares and interactions; the results of this analysis are conveyed visually in a contour plot. In this continuous model, the reference values of TSAT 25% and ferritin 130 ng/ml were chosen to correspond roughly to the median values.
Analyses were adjusted for baseline confounders including country, age (linear), sex, Black race, body mass index (linear), eGFR (calculated using the Modification of Diet in Renal Disease Study Group Equation19; linear), albuminuria (categorical; see Table 1), serum albumin (linear), white blood cell count (linear), ESA use, 11 summary comorbidities (diabetes, hypertension, coronary artery disease, cerebrovascular disease, peripheral vascular disease, HF, other cardiovascular disease, gastrointestinal bleeding, lung disease, cancer, and ulcers/gangrene), and Hgb (linear). Models with TSAT as the main exposure were adjusted for ferritin, and vice versa. We included the covariates in stages, in particular fitting models with and without Hgb as a covariate. Subgroup analyses were performed by testing exposure interactions with anemia status (defined as Hgb <12 g/dl) and with CKD stage (stage 3 vs 4 and 5), sex, diabetic status, albuminuria (defined as ≥30 mg/g creatinine), and HF.
Table 1.
Baseline sample characteristics across TSAT categories
| Characteristics | TSAT (%) | All | |||||
|---|---|---|---|---|---|---|---|
| ≤15 | 16–20 | 21–25 | 26–35 | 36–45 | ≥46 | ||
| N patients (row %) | 926 (18) | 1067 (21) | 1178 (23) | 1408 (27) | 428 (8) | 138 (3) | 5145 |
| Age, yr | 71±13 | 69±13 | 70±13 | 69±14 | 68±14 | 65±16 | 69±13 |
| % male | 53 | 56 | 58 | 64 | 69 | 62 | 59 |
| % Black | 6 | 6 | 4 | 5 | 6 | 5 | 5 |
| BMI, kg/m2 | 30±7 | 29±6 | 29±6 | 28±6 | 28±5 | 27±5 | 29±6 |
| Comorbidities (%) | |||||||
| Diabetes | 56 | 50 | 46 | 38 | 36 | 27 | 45 |
| Hypertension | 89 | 89 | 90 | 88 | 86 | 81 | 88 |
| Coronary artery disease | 33 | 32 | 29 | 24 | 23 | 24 | 28 |
| HF | 21 | 17 | 13 | 12 | 11 | 9 | 15 |
| Cerebrovascular disease | 13 | 12 | 11 | 10 | 8 | 8 | 11 |
| Peripheral vascular disease | 22 | 21 | 19 | 19 | 16 | 12 | 20 |
| Other cardiovascular disease | 31 | 25 | 25 | 23 | 20 | 17 | 25 |
| GI bleeding in past year | 3 | 1 | 1 | 1 | 1 | 0 | 1 |
| Lung disease | 13 | 11 | 9 | 8 | 7 | 10 | 10 |
| Cancer | 18 | 17 | 18 | 18 | 19 | 18 | 18 |
| Ulcers/gangrene of extremity | 2 | 2 | 3 | 2 | 2 | 0 | 2 |
| Neurologic disease | 3 | 3 | 3 | 4 | 4 | 4 | 3 |
| Psychiatric disorder | 9 | 10 | 8 | 7 | 8 | 8 | 8 |
| Prescriptions | |||||||
| ESA, % | 16 | 12 | 11 | 13 | 13 | 26 | 13 |
| Iron, IV or oral, % | 32 | 24 | 18 | 15 | 15 | 15 | 21 |
| Labs | |||||||
| TSAT, % | 12±3 | 18±1 | 23±1 | 29±3 | 39±3 | 55±12 | 24±10 |
| Ferritin, ng/ml | 122±160 | 160±178 | 189±177 | 235±219 | 257±221 | 444±502 | 196±214 |
| Hgb, g/dl | 11.5±1.8 | 12.1±1.7 | 12.3±1.8 | 12.6±1.8 | 12.8±1.9 | 12.4±2.1 | 12.3±1.8 |
| eGFR, ml/min per 1.73m2 | 27±10 | 28±10 | 28±11 | 29±11 | 29±12 | 30±12 | 28±11 |
| Albuminuria (%) | |||||||
| A1 (normal) | 28 | 29 | 28 | 28 | 28 | 30 | 28 |
| A2 (high) | 34 | 33 | 33 | 32 | 32 | 34 | 33 |
| A3 (very high) | 28 | 28 | 30 | 30 | 29 | 25 | 29 |
| A3 (nephrotic) | 10 | 10 | 9 | 10 | 11 | 11 | 10 |
| Serum albumin, g/dl | 3.9±0.5 | 4.0±0.5 | 4.0±0.4 | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 |
| White blood cells, 109/L | 7.7±2.4 | 7.3±2.0 | 7.2±1.9 | 6.9±2.0 | 6.8±1.9 | 6.8±2.5 | 7.2±2.1 |
Results reported as % or mean±SD. Albuminuria categories: A1: <30 mg/g creatinine, A2: 30–300 mg/g creatinine, A3 (very high): >300–2000 mg/g creatinine, and A3 (nephrotic): >2000 mg/g creatinine.
Multiple imputation, implemented by IVEware20 with the chained equations method, was used in the analyses to impute missing exposure and covariate values, with the assumption of missingness at random. The exposures, outcomes, and all covariates were included in the imputation model. Missingness was 18% for TSAT and 12% for ferritin; per our inclusion criteria, all patients had at least one of these measurements. Missingness was <5% for all other covariates except white blood cell count (8%), Hgb (9%), albuminuria (17%), and serum albumin (33%). In total, 20 complete datasets were imputed, all analyses were performed with each imputed dataset, and results were combined using Rubin’s rules.21
All analyses used SAS software, version 9.4 (SAS institute, Cary, NC). Confidence intervals are reported with 95% confidence level.
Results
Of 8212 patients on CKDopps, 2953 (36%) were excluded due to missing TSAT and ferritin, 19 (0.2%) had eGFR >60 ml/min per 1.73m2 at the time of the TSAT/ferritin measurement, and 95 (1%) had no reported outcome data. Our final analysis included 5145 patients: 2228 from France (43%), 2129 from Germany (41%), 494 from the United States (10%), and 294 from Brazil (6%). Overall, patients had a mean age of 69 years, were predominantly male (59%), with a mean eGFR 28 ml/min per 1.73 m2 (Table 1). In total, 45% of patients had diabetes, 28% had coronary artery disease, and 15% had HF. Mean TSAT and ferritin were 24% and 196 ng/ml, respectively, whereas mean Hgb was 12.3 g/dl. ESAs were prescribed at baseline for 13% of the sample, and 21% were prescribed iron (either IV or oral).
The proportion of patients with TSAT categories of ≥15%, 16%–20%, 21%–25%, 26%–35%, 36%–45%, and ≥46% were 18%, 21%, 23%, 27%, 8%, and 3%, respectively. Baseline characteristics stratified by TSAT levels are summarized in Table 1. Patients with lower TSAT tended to be female, diabetic, have higher body mass index, and had more cardiovascular comorbidities, such as coronary artery disease and HF. eGFR and Hgb levels varied positively with TSAT, whereas iron interventions were more common among those with lower TSAT. For ferritin, considering the categories of <50 ng/ml, 50–99 ng/ml, 100–299 ng/ml, and ≥300 ng/ml, the sample proportions were 15%, 23%, 43%, and 18%, respectively. Patient characteristics across ferritin levels are shown in Table 2.
Table 2.
Baseline sample characteristics across ferritin categories
| Characteristics | Ferritin (ng/ml) | All | |||
|---|---|---|---|---|---|
| <50 | 50–99 | 100–299 | ≥300 | ||
| N patients (row %) | 777 (15) | 1201 (23) | 2236 (43) | 931 (18) | 5145 |
| Age, yr | 69±14 | 69±13 | 70±13 | 69±13 | 69±13 |
| % male | 49 | 56 | 63 | 64 | 59 |
| % Black | 4 | 5 | 5 | 8 | 5 |
| Body mass index, kg/m2 | 29±6 | 29±6 | 29±6 | 29±6 | 29±6 |
| Comorbidities, % | |||||
| Diabetes | 51 | 47 | 45 | 41 | 45 |
| Hypertension | 86 | 88 | 89 | 89 | 88 |
| Coronary artery disease | 31 | 30 | 27 | 27 | 28 |
| HF | 15 | 15 | 14 | 15 | 15 |
| Cerebrovascular disease | 12 | 12 | 11 | 10 | 11 |
| Peripheral vascular disease | 18 | 21 | 19 | 20 | 20 |
| Other cardiovascular disease | 26 | 25 | 24 | 24 | 25 |
| GI bleeding in past year | 2 | 1 | 1 | 1 | 1 |
| Lung disease | 10 | 10 | 9 | 10 | 10 |
| Cancer | 17 | 18 | 18 | 18 | 18 |
| Ulcers/gangrene of extremity | 2 | 2 | 2 | 2 | 2 |
| Neurologic disease | 2 | 3 | 3 | 4 | 3 |
| Psychiatric disorder | 8 | 9 | 8 | 8 | 8 |
| Prescriptions (%) | |||||
| ESA | 9 | 10 | 12 | 25 | 13 |
| Iron, IV or oral | 21 | 18 | 19 | 26 | 21 |
| Labs | |||||
| TSAT, % | 18±9 | 22±9 | 25±9 | 28±12 | 24±10 |
| Ferritin, ng/ml | 31±11 | 74±15 | 177±55 | 537±295 | 196±214 |
| Hgb, g/dl | 12.2±1.8 | 12.4±1.8 | 12.4±1.8 | 11.8±1.9 | 12.3±1.8 |
| eGFR, ml/min per 1.73m2 | 30±11 | 29±11 | 28±11 | 27±11 | 28±11 |
| Albuminuria, % | |||||
| A1 (normal) | 31 | 28 | 28 | 27 | 28 |
| A2 (high) | 35 | 32 | 33 | 32 | 33 |
| A3 (very high) | 26 | 31 | 29 | 30 | 29 |
| A3 (nephrotic) | 9 | 10 | 10 | 11 | 10 |
| Serum albumin, g/dl | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 | 4.0±0.5 |
| White blood cells, 109/L | 7.3±2.1 | 7.2±2.1 | 7.2±2.0 | 7.2±2.2 | 7.2±2.1 |
Results reported as % or mean±SD. Albuminuria categories: A1: <30 mg/g creatinine, A2: 30–300 mg/g creatinine, A3 (very high): >300–2000 mg/g creatinine, and A3 (nephrotic): >2000 mg/g creatinine.
The overall pre-KRT mortality rate was 4.7 per 100 patient-years; median follow-up was 3.0 years. For patients with TSAT categories of ≥15%, 16%–20%, 21%–25%, 26%–35%, 36%–45%, and ≥46%, mortality rates were 7.1, 4.7, 5.0, 3.6, 2.7, and 5.0 out of 100 patient-years, respectively. The number of patients who died or who were censored due to KRT or other causes are given in Supplemental Table 1.
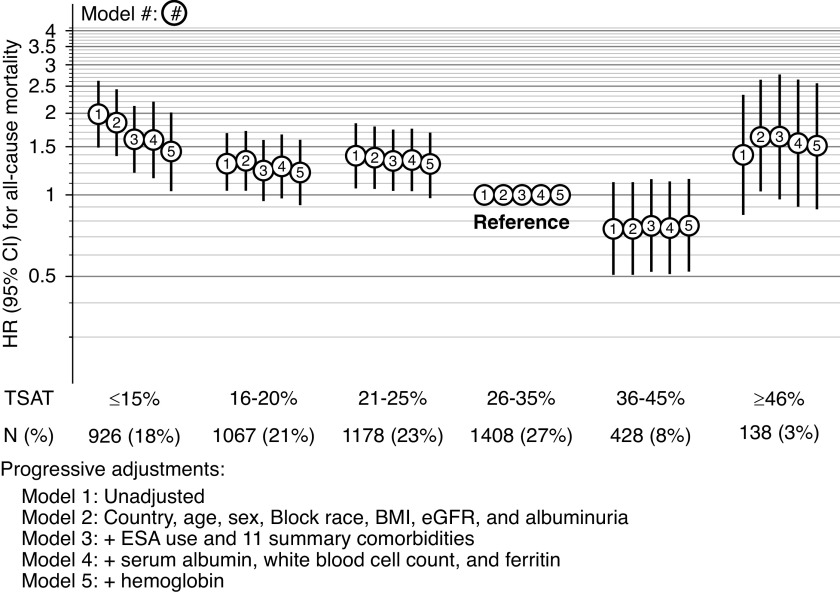
TSAT and pre-KRT mortality tended to follow U-shaped associations, with highest risks at the extremes of TSAT ≥15% and ≥46%, whereas lowest for those with TSAT 36%–45%. The unadjusted hazard ratio (HR) for TSAT was ≥15% compared with 26%–35% was 1.98 (95 confidence interval [95% CI], 1.49 to 2.61). Progressive covariate adjustment attenuated the results somewhat, with an HR of 1.59 (95% CI, 1.15 to 2.20) in the adjusted model without Hgb. Even after adjustment for Hgb, lower TSAT levels were associated with higher mortality risk (Figure 1, model 4 versus model 5 adjustments). In the linear spline analysis, for those with TSAT levels ≤40%, each five-unit decrease in TSAT was associated with a 10% (HR, 1.10; 95% CI, 1.02 to 1.19) higher risk of pre-KRT all-cause mortality (Supplemental Figure 1); results from the five-knot cubic spline analysis were very similar but with wider confidence intervals. In subgroup analyses, the effect estimates for TSAT categories and mortality were not modified by Hgb level ≥ versus <12 (interaction P=0.67; see Supplemental Table 2 for HRs within each anemia subgroup), CKD stage (interaction P=0.27), sex (interaction P=0.55), diabetic status (interaction P=0.55), albuminuria ≥ versus <30 mg/g creatinine (interaction P=0.67), or HF (interaction P=0.99). Ferritin levels ≥300 ng/ml were consistently associated with increased risks of pre-KRT mortality even after adjustment for Hgb, although the association did attenuate with adjustment (Table 3).
Figure 1.
HR for all-cause mortality by TSAT categories, with progressive covariate adjustment.
Table 3.
HR for all-cause mortality and MACE by ferritin level, with progressive covariate adjustment
| Outcome | Ferritin (ng/ml) | N (%) | Hazard Ratios (95% CIs) | ||||
|---|---|---|---|---|---|---|---|
| Model 1 | Model 2 | Model 3 | Model 4 | Model 5 | |||
| All-cause mortality | <50 | 777 (15) | 1.17 (0.94 to 1.45) | 1.21 (0.96 to 1.52) | 1.14 (0.91 to 1.43) | 0.97 (0.75 to 1.26) | 0.99 (0.76 to 1.28) |
| 50–99 | 1201 (23) | 1.00 (0.80 to 1.24) | 1.02 (0.81 to 1.28) | 1.01 (0.80 to 1.28) | 0.96 (0.75 to 1.22) | 0.97 (0.76 to 1.24) | |
| 100–299 | 2236 (43) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| ≥300 | 931 (18) | 1.37 (1.10 to 1.70) | 1.29 (1.03 to 1.61) | 1.22 (0.98 to 1.53) | 1.26 (1.00 to 1.59) | 1.21 (0.96 to 1.53) | |
| MACE | <50 | 431 (14) | 1.19 (0.83 to 1.68) | 1.18 (0.82 to 1.70) | 1.10 (0.78 to 1.56) | 0.92 (0.63 to 1.34) | 0.94 (0.65 to 1.36) |
| 50–99 | 707 (23) | 1.01 (0.76 to 1.33) | 1.03 (0.77 to 1.39) | 0.97 (0.72 to 1.30) | 0.91 (0.67 to 1.26) | 0.94 (0.70 to 1.27) | |
| 100–299 | 1346 (45) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | 1 (ref) | |
| ≥300 | 532 (18) | 1.13 (0.85 to 1.49) | 1.12 (0.85 to 1.48) | 1.01 (0.75 to 1.36) | 1.11 (0.84 to 1.47) | 1.06 (0.80 to 1.42) | |
For all-cause death, the sample includes patients from Brazil, France, Germany, and the United States. For MACE, the sample includes patients from Brazil, France, and the United States. Progressive adjustments: Model 1: Unadjusted. Model 2: Country, age, sex, Black race, body mass index (BMI), eGFR and albuminuria. Model 3: + ESA use and 11 summary comorbidities. Model 4: + serum albumin, white blood cell count, and TSAT. Model 5: + Hgb.
The analysis of TSAT/ferritin interaction on all-cause mortality had an overall interaction P value of 0.04. Figure 2A shows the contour plot of the HR for combinations of TSAT and ferritin on all-cause mortality. In general, patients with the combination of lower TSAT and higher ferritin had higher risks for mortality, particularly among those with TSAT lower than 20% with a simultaneous ferritin higher than 100 ng/ml. Figure 2B shows the P values for the estimates for each range of TSAT and ferritin. When a separate Cox model was fit with categorical, cross-classified TSAT and ferritin (Supplemental Table 3), the results were similar to those in the contour plot.
Figure 2.
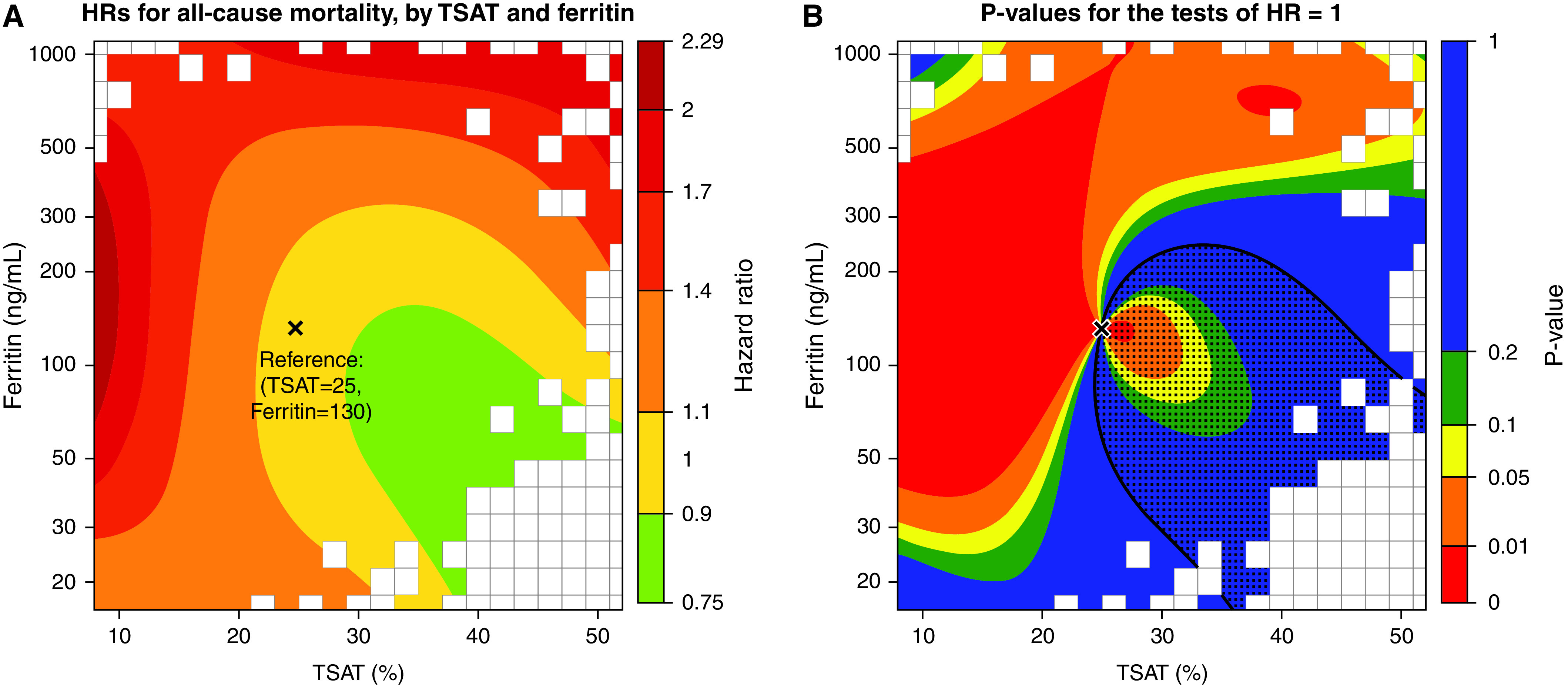
Contour plot of HR for all-cause mortality by continuous TSAT and ferritin, relative to reference values of TSAT 25% and ferritin 130 ng/ml. (A) HRs for each combination of TSAT and ferritin values compared with the reference values. (B) P values for each comparison, testing if the HR at a point is 1; the dotted region denotes where the estimated HR <1. The gray rectangles denote regions where no patients had data. Results on the basis of an adjusted Cox model with the following main effects and interaction terms for TSAT and ferritin: TSAT, TSAT2, log(ferritin), log(ferritin)2, TSAT×log(ferritin), TSAT2×log(ferritin), TSAT×log(ferritin)2, and TSAT2×log(ferritin)2. P value for the joint test of all four interaction terms was 0.04. Adjustments were country, age, sex, Black race, body mass index, eGFR, albuminuria, ESA use, 11 summary comorbidities, serum albumin, white blood cell count, and Hgb. The TSAT and ferritin reference values were chosen to correspond roughly to the median values. Values on the y-axis are ferritin values in ng/ml, but spaced out according to the log scale.
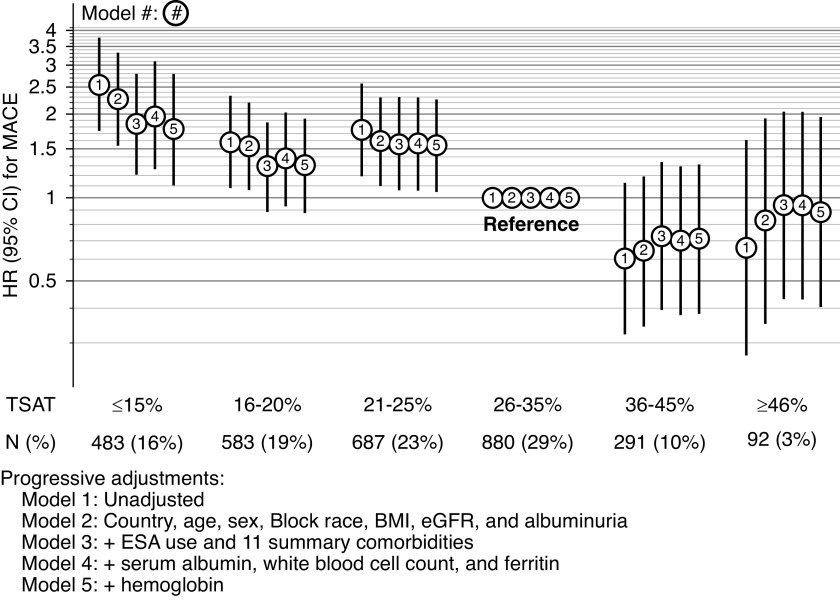
For the MACE outcome, overall event rate was 4.8 per 100 patient-years. For TSAT categories of ≥15%, 16%–20%, 21%–25%, 26%–35%, 36%–45%, and ≥46%, MACE rates were 8.6, 5.3, 5.8, 3.3, 2.0, and 2.2 per 100 patient-years, respectively. As opposed to pre-KRT mortality, the associations between TSAT and MACE followed a linear shape, also with highest risks for those with levels ≥15%, but without increased risks for those with TSAT ≥46%. Compared with patients with TSAT 26%–35%, those with TSAT ≥15% had a crude HR estimate for MACE of 2.55 (95% CI, 1.73 to 3.76). Progressive adjustment for confounders slightly decreased effects sizes, with an HR of 1.96 (95% CI, 1.26 to 3.06) in the fully adjusted model without Hgb. Further adjustment for Hgb only slightly attenuated the effects (Figure 3, model 4 versus model 5 adjustments). Consistent with all-cause mortality, for patients with TSAT ≤40%, each five-unit decrease in TSAT was associated with a 16% increase in the risk of MACE (HR, 1.16; 95% CI, 1.05 to 1.28) (Supplemental Figure 2); results were again very similar with a five-knot cubic spline. Contrary to all-cause mortality, higher ferritin levels were not associated with risk of MACE after adjustment for confounders and Hgb (Table 3).
Figure 3.
HR for MACE by TSAT categories, with progressive covariate adjustment.
Discussion
In this CKDopps analysis using data from France, Brazil, Germany, and the United States, we observed low TSAT levels are associated with an increase in the risk of all-cause mortality and incidence of major cardiovascular events in patients with moderate to advanced CKD, an effect that was not modified by the presence of anemia. Patients with TSAT ≥46% or ferritin ≥300 ng/ml tended to have higher risks of all-cause mortality, but not MACE. Additionally, patients with low TSAT simultaneous to high ferritin displayed the highest risk of adverse clinical events.
The most compelling evidence for the effects of correcting ID on clinical outcomes independently from Hgb levels comes from RCTs in patients with HF. In this population, administration of iron treatment, in particular IV iron formulations, even among nonanemic patients, leads not only to improvements in measures of cardiac function, such as ejection fraction, but also for patient symptoms and, importantly, yields benefits in terms of HF hospitalization and mortality.8,9,22 In an individual patient data metanalysis of four RCTs assessing the efficacy of ferric carboxymaltose in patients with stable, ambulatory HF, iron treatment decreased the composite outcome of HF hospitalizations/cardiovascular mortality in 49% as compared with placebos.10 In these aggregate data, about 45% of patients had Hgb <12 g/dl and about the same proportion had eGFR <60 ml/min. In the recently published A Randomized, Double-Blind Placebo-Controlled Trial Comparing the Effect of Intravenous Ferric Carboxymaltose on Hospitalizations and Mortality in Iron Deficient Subjects Admitted for Acute Heart Failure, patients who were hospitalized with HF and who were randomized to receive ferric carboxymaltose after initial stabilization had a lower risk of cardiovascular hospitalizations. Consistently, in this trial, about half of the population had anemia at baseline, whereas 52% had CKD.23 These results highlight the overlap between CKD and HF and establish the role of iron therapy beyond anemia correction in the HF population.
In contrast with HF studies, clinical trials in NDD-CKD have mainly targeted the erythropoietic response prompted by iron administration, as opposed to clinical or patient-reported outcomes. Although proactive strategies for iron supplementation targeting ferritin levels up to 400–600 µg/L seem to be safe and yield lower ESA doses,24 the extent to which such achievements lead to improved clinical outcomes is unknown. Recently published, a RCT in patients on hemodialysis provided the first evidence that such a proactive strategy may be beneficial in terms of all-cause mortality and MACE outcomes.6 In ND-CKD, a recent RCT comparing distinct IV iron formulations (iron isomaltoside 1000/ferric derisomaltose versus iron sucrose) provided estimates on cardiovascular events as a secondary safety endpoint. However, this study was not designed to assess the effect of iron correction, or distinct iron stores targets, on cardiovascular outcomes.25
In the absence of RCTs comparing distinct iron targets on nonsurrogate endpoints among patients with ND-CKD, the association between ID and increased risk of adverse clinical events has been described in previous observational analyses. In a propensity score matched analysis of the Veteran Affairs (VA) System, patients with early CKD (>80% with CKD stage 3) with abnormal iron parameters had an increased risk of all-cause mortality.13,14 Generalizability of these findings is restricted due to limitations such as broad exclusion of patients from the analysis (<3% of the original sample was included), and distribution-based cutoffs for TSAT and ferritin for defining ID subtypes, which further compromises clinical interpretability. In another study from the VA System, patients—mostly having CKD stage 3A—with functional and absolute ID had increased risks of cardiovascular hospitalization, whereas only those with the former subtype had increased risks for all-cause mortality.14 Neither of these studies provide estimates on MACE; they also do not approach the potential role of ID independent of Hgb levels. A further analysis of the VA cohort included 453 patients with ND-CKD and provided trending results for TSAT consistent with ours for all-cause mortality. In this study, although the analysis adjusted for Hgb, there was no description of study design or analytical assumptions permitting interpretations of TSAT estimates not mediated by Hgb.26 Finally, in an analysis adjusted for a restricted set of potential confounders, a smaller cohort of patients with early-stage CKD, mostly stage 2, showed that lower TSAT, but not ferritin, was associated with a higher risk of adverse events.27
In our study, we found TSAT levels of 36%–45% were associated with lowest risks of both all-cause mortality and MACE. Given the iron demand for patients with late stages of CKD, such relatively high TSAT levels may match iron delivery not only for maintaining adequate Hgb levels, but also providing tissue iron for cellular functions. Indeed, compared with previous trials in ND-CKD, such as the Ferinject Assessment in Patients with Iron Deficiency Anaemia and Non-Dialysis-Dependent-CKD study, in which achieved mean TSAT levels were around 31%, our study provides, albeit limited by its observational nature, further insights into potential new strategies to be tested aiming at higher TSAT levels compared with recommended guideline-based targets. Importantly, iron demand varies considerably according to ESA treatment in patients with CKD. Therefore, ensuring adequate iron-store testing in the context of ESA treatment is essential to correct ID, which, albeit reiterated by many guidelines, has been shown to be poorly followed in clinical practice. In patients with ND-CKD, iron-store targets should probably vary according to ESA treatment utilization, which has never been tested in well-designed RCTs to this date.
Of note, patients with TSAT levels >45% were found to be at increased risk for all-cause mortality, but not MACE, although reduced sample size in this subgroup implies results should be cautiously interpreted. It may be hypothesized that these patients are at increased risk of infectious disease events, which may explain these results, although this conclusion would be out of the scope of our objectives in this study. A recent prespecified secondary analysis from the Proactive IV Iron Therapy in Haemodialysis Patients study raised doubts on the causal link between higher iron stores driven by iron therapy and risk of infections in hemodialysis patients.28 Additionally, TSAT levels >45% may be indicative of iron overload and iron-mediated oxidative stress, which may cause organ dysfunction and increased risks of adverse clinical events.29,30 Important to note, in the aforementioned Proactive IV Iron Therapy in Haemodialysis Patients study,28 IV iron infusions were limited if patients achieved TSAT levels >40%, which led to mean achieved TSAT between 26% and 28% in the high-dose iron arm. The effects of driving TSAT levels >40% with proactive iron treatment remains unknown in patients with CKD, although such levels are well established predictors of iron overload in the general population.29,31
We found a strong interaction between TSAT and ferritin for all-cause mortality. Being a marker of both iron stores and an acute-phase reactant, higher ferritin levels, for any given TSAT, may represent underlying inflammation and restricted iron availability to tissues.32 Our contour plots illustrate the complexity that underlies the definition of single cutoffs for targets for TSAT and ferritin. As CKD progresses, the more active role of inflammation and therefore restricted iron availability may magnify the importance of such interactions in the clinical interpretation of TSAT and ferritin for improving tissue iron delivery.
Consistently, the mechanisms by which ID may affect outcomes independently from Hgb levels are supported by the concept of tissue ID.7 Iron plays an essential role in many biologic functions, including the synthesis of proteins necessary for mitochondrial function.33 Tissues involved in high metabolic demand, such as muscles, are particularly affected by such tissue ID. This framework may explain improvements in functional status followed by iron administration in patients with HF.7 In fact, a previous CKDopps analysis showed that patients with ND-CKD and ID had a lower overall physical health-related quality of life, even after adjustment for multiple confounders and Hgb, which further reinforces the concept that tissue ID results in lower functionality (Guedes et al., in press, NDT, 2021). Another potential implication of this concept for cardiovascular events is a relative predominance of nonatherosclerotic events compared with atherosclerotic ones, mainly due to cardiac contractility dysfunction leading to HF decompensation. This model is consistent with the particular relevance of HF events in iron trials.6
This study has some limitations. Due to the observational nature, we cannot rule out residual confounding, despite our broad inclusion of potential confounders in stepwise models. This implies that results provided in this study should be confirmed in a well-designed RCT. Although clinics were chosen randomly in the CKDopps sampling mechanism, clinics could still refuse to participate, which may introduce selection bias. Additionally, due to the absence of cause-specific mortality for all countries included in this study, the MACE outcomes were analyzed only in a subset of our sample. Particularly, the exclusion of data from Germany for this outcome may have reduced the power to detect associations, particularly for ferritin. Of note, 36% of patients in CKDopps were excluded for missing both TSAT and ferritin, possibly resulting in selection bias. Among included patients, multiple imputation was required for 18% of TSAT and 12% of ferritin values. Moreover, our exposure variable was defined as a single-measurement of serum biomarkers of iron stores. This may introduce information bias, because iron tests tend to change according to clinical and laboratory variables. Because CKDopps was designed to capture real-world practice, availability of iron tests in our cohort followed monitoring on the basis of clinicians’ indication. As recently reported in a CKDopps publication, TSAT and ferritin are not monitored as frequently as recommended by guidelines, and, surprisingly, even among patients with CKD and with Hgb <10 g/dl,34 roughly one third of patients had at least one iron test (TSAT or ferritin) within 3 months of Hgb measurement. Also, patients with more advanced CKD tend to be monitored more frequently. Therefore, a time-varying approach to serum biomarkers of iron tests could considerably reduce study generalizability. Finally, another recently published CKDopps analysis has shown that iron treatment remains lower than expected among patients with ND-CKD with ID and the rate of treatment discontinuation over time is high, which would imply that variations of iron stores due to treatment in this cohort should be low.35 Study strengths include that we provide a unique cohort of patients with advanced CKD, which reflects real-world nephrology practice. To our knowledge, this is the first observational study to evaluate MACE outcomes in patients with ND-CKD exposed to ID. Our events were formally adjudicated in patients from the French cohort, and additional events from United States and Brazil were independently validated by coauthors. Our models included a comprehensive set of key confounders, ensuring a separate inclusion of Hgb for assessing nonmediated associations.
This study provides observational support to the hypothesis that ID, regardless of anemia status, may affect clinical outcomes in patients with ND-CKD. Long overdue in the ND-CKD setting, well-designed RCTs are needed to assess the clinical benefits of iron supplementation for patients with ID, with or without anemia, and therefore confirm the results provided in this study. Proactive iron interventions, particularly IV iron, may lead to better tissue iron delivery and therefore better outcomes. With the shift in the paradigm in the anemia treatment provided by the approval of the novel class of hypoxia-inducible factor prolyl hydroxylase inhibitors, which, among other effects, improve iron availability, an integrative approach considering both iron status and Hgb may yield important benefits for ND-CKD individuals. Such an integrative approach may tackle particularly challenging subpopulations, such as those with cardio-renal ID anemia syndromes, ESA-resistant individuals, or those at higher risk of adverse events due to ESAs.36
In conclusion, our study suggests that serum biomarkers of iron stores are associated with all-cause mortality and cardiovascular events in patients living with ND-CKD. Patients with isolated low TSAT levels or high ferritin simultaneous with low TSAT are at highest risk for mortality, independently from Hgb levels. RCTs are needed to confirm these results and to establish the role of iron treatment, even in the absence of anemia, in the management of patients with ND-CKD.
Disclosures
B. Bieber, D.G. Muenz, J. Zee, M. Guedes, R. Pecoits-Filho, and R.L. Pisoni are employees of Arbor Research Collaborative for Health, which administers the DOPPS Programs; they received research funding from the DOPPS Program, which is supported by Amgen, Baxter Healthcare, and Kyowa Hakko Kirin; additional commercial support was provided by AstraZeneca, FMC Asia-Pacific Ltd., FMC Canada Ltd., Janssen, Keryx, MEDICE Arzneimittel Pütter, Proteon, and Vifor Fresenius Medical Care Renal Pharma. All support is provided without restrictions on publications, all grants are made to Arbor Research Collaborative for Health and not to these authors. B. M. Robinson reports receiving consultancy fees or travel reimbursement since 2018 from AstraZeneca, GlaxoSmithKline, and Kyowa Kirin Co., all paid directly to his institution of employment; he is an employee of Arbor Research Collaborative for Health, which administers the DOPPS Programs. B. Stengel reports receiving support from CKD-Renal Epidemiology and Information Network (CKD-REIN), which is supported by a public-private partnership with funding from nine pharmaceutical companies including Amgen, AstraZeneca since 2018, Baxter and Merck Sharp & Dohme-Chibret (MSD France) from 2012 to 2017, Fresenius Medical Care, and GlaxoSmithKline (GSK), since 2012, Lilly France since 2013, Otsuka Pharmaceutical since 2015, Sanofi-Genzyme from 2012 to 2015, and Vifor Fresenius. D. Charytan reports receiving personal fees from Allena Pharmaceuticals (DSMB), Amgen, AstraZeneca, Eli Lilly/Boehringer Ingelheim, Fresenius, Gilead, GSK, Janssen, Medtronic, Merck, NovoNordisk, PLC Medical, and Zoll; reports receiving research support from Amgen, Bioporto, Gilead, Medtronic, and NovoNordisk; reports scientific advisor or membership with CJASN; and other interests/relationships via Expert Witness Fees related to Proton Pump Inhibitors. H. Reichel reports having consultancy agreements with Amgen and Vifor; and reports receiving honoraria from Vifor. J. Zee reports receiving honoraria from Booz Allen Hamilton. M. Wong is a former consultant for Arbor Research Collaborative for Health; and reports other interests/relationships with Michael Smith Foundation for Health Research. R. Pecoits-Filho reports having consultancy agreements with Akebia, AstraZeneca, Bayer, Fresenius Medical Care, and Rethrophin; reports receiving research funding from Fresenius Medical Care; reports receiving honoraria from AstraZeneca and Novo Nordisk; reports being a scientific advisor or member of SONG Initiative Executive Committee and the Editorial board of AJKD, Blood Purification, Hemodialysis International, Peritoneal Dialysis International, and Nephrology; and reports speakers bureau from AstraZeneca, Bayer, and Novo Nordisk. R. Pisoni reports a membership with the Editorial Board for Kidney360. S. Wachter is an employee of Vifor Pharma Ltd., market leader in IV iron products. Z. Massy reports receiving grants and other from Amgen, Baxter, and Sanofi-Genzyme; reports receiving grants from the FMC, French Government, GSK, Lilly, MSD, Outsuka; reports receiving other from Astellas and Daichi, outside the submitted work; reports receiving honoraria to the charities or for travel from Baxter and Genzyme-Sanofi; and reports being a scientific advisor or members with Journal of Renal Nutrition, Journal of Nephrology, KI, NDT, and Toxins. The remaining author has nothing to disclose. Global support for the ongoing DOPPS Programs is provided without restriction on publications by a variety of funders. For details see https://www.dopps.org/AboutUs/Support.aspx.
Funding
This work was supported by Vifor-Fresenius Medical Care Renal Pharma Ltd. In France, CKDopps is part of the CKD-REIN cohort, which is funded by the Agence Nationale de la Recherche through the 2010 Cohortes-Investissements d’Avenir program and by the 2010 National Programme Hospitalier de Recherche Clinique. CKD-REIN is also supported through a public-private partnership with Amgen, Fresenius Medical Care, and GlaxoSmithKline since 2012, Baxter and Merck Sharp & Dohme Chibret (MSD France), from 2012 to 2017, Lilly France since 2013, and Otsuka Pharmaceutical since 2015, Vifor Fresenius since 2017, and Sanofi-Genzyme from 2012 to 2015. In Germany, funding support for participation of German CKD clinics in CKDopps is provided by Wissenschaftliches Institut für Nephrologie of the Verband Deutsche Nierenzentren. In the United States and Brazil support for the CKDopps Coordinating Center has been provided by Keryx.
Supplementary Material
Acknowledgments
B. Beiber, D. Charytan, M. Guedes, N. Mansencal, Z. Massey, D. Muenz, R. Pecoits-Filho, R. Pisoni, H. Reichel, B. Robinson, S. Waechter, M. Wong, and J. Zee were responsible for substantial contributions to conception and design, acquisition of data, and analysis and interpretation of data; B. Beiber, D. Charytan, M. Guedes, N. Mansencal, Z. Massey, D. Muenz, R. Pecoits-Filho, R. Pisoni, H. Reichel, B. Robinson, S. Waechter, M. Wong, and J. Zee wrote the manuscript and revised it critically for important intellectual content; B. Beiber, D. Charytan, M. Guedes, N. Mansencal, Z. Massey, D. Muenz, R. Pecoits-Filho, R. Pisoni, H. Reichel, B. Robinson, S. Waechter, M. Wong, and J. Zee approved the final version to be published; B. Beiber, M. Guedes, N. Mansencal, Z. Massey, D. Muenz, R. Pecoits-Filho, R. Pisoni, H. Reichel, B. Robinson, and J. Zee agreed to be accountable for all aspects of the work. Ms. Jennifer McCready-Maynes, an employee of Arbor Research Collaborative for Health, provided editorial support.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2020101531/-/DCSupplemental.
Supplemental Figure 1. Hazard ratio for all-cause mortality by TSAT, with TSAT as a linear spline with one knot at 40%.
Supplemental Figure 2. Hazard ratio for MACE by TSAT, with TSAT as a linear spline with one knot at 40%.
Supplemental Table 1. Number (%) of patients who died (all-cause) before KRT, or who were censored due to KRT or other causes (patient departure or end of study data collection).
Supplemental Table 2. HR for all-cause mortality and MACE by TSAT and ferritin levels, in subgroups with and without anemia (Hgb <12 versus ≥12 g/dl).
Supplemental Table 3. HR for all-cause mortality by categorical, cross-classified TSAT and ferritin. Reference group is TSAT 26%–35% and ferritin 100–299 ng/ml.
References
- 1.Fishbane S, Spinowitz B: Update on anemia in ESRD and earlier stages of CKD: Core curriculum 2018. Am J Kidney Dis 71: 423–435, 2018 [DOI] [PubMed] [Google Scholar]
- 2.Stancu S, Stanciu A, Zugravu A, Bârsan L, Dumitru D, Lipan M, et al. Bone marrow iron, iron indices, and the response to intravenous iron in patients with non-dialysis-dependent CKD. Am J Kidney Dis 55: 639–647, 2010 [DOI] [PubMed] [Google Scholar]
- 3.Babitt JL, Lin HY: Mechanisms of anemia in CKD. J Am Soc Nephrol 23: 1631–1634, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Group KDIGOKAW: KDIGO clinical practice guideline for anemia in chronic kidney disease. Kidney Int Suppl 2: 279–335, 2012. Available at: https://kdigo.org/wp-content/uploads/2016/10/KDIGO-2012-Anemia-Guideline-English.pdf. Accessed June 4, 2021 [Google Scholar]
- 5.Wong MMY, Tu C, Li Y, Perlman RL, Pecoits-Filho R, Lopes AA, et al. CKDopps Investigators: Anemia and iron deficiency among chronic kidney disease Stages 3-5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: Often unmeasured, variably treated. Clin Kidney J 13: 613–624, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macdougall IC, White C, Anker SD, Bhandari S, Farrington K, Kalra PA, et al. PIVOTAL Investigators and Committees: Intravenous iron in patients undergoing maintenance hemodialysis. N Engl J Med 380: 447–458, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Beard JL: Iron biology in immune function, muscle metabolism and neuronal functioning. J Nutr 131: 568S–579S, discussion 580S, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Zhou X, Xu W, Xu Y, Qian Z: Iron supplementation improves cardiovascular outcomes in patients with heart failure. Am J Med 132: 955–963, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Anker SD, Comin Colet J, Filippatos G, Willenheimer R, Dickstein K, Drexler H, et al. FAIR-HF Trial Investigators: Ferric carboxymaltose in patients with heart failure and iron deficiency. N Engl J Med 361: 2436–2448, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Anker SD, Kirwan BA, van Veldhuisen DJ, Filippatos G, Comin-Colet J, Ruschitzka F, et al. Effects of ferric carboxymaltose on hospitalisations and mortality rates in iron-deficient heart failure patients: an individual patient data meta-analysis. Eur J Heart Fail 20: 125–133, 2018 [DOI] [PubMed] [Google Scholar]
- 11.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Am Coll Cardiol 70: 776–803, 2017 [DOI] [PubMed] [Google Scholar]
- 12.Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. ESC Scientific Document Group: 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 37: 2129–2200, 2016 [DOI] [PubMed] [Google Scholar]
- 13.Cho ME, Hansen JL, Peters CB, Cheung AK, Greene T, Sauer BC: An increased mortality risk is associated with abnormal iron status in diabetic and non-diabetic Veterans with predialysis chronic kidney disease. Kidney Int 96: 750–760, 2019 [DOI] [PubMed] [Google Scholar]
- 14.Awan AA, Walther CP, Richardson PA, Shah M, Winkelmayer WC, Navaneethan SD: Prevalence, correlates and outcomes of absolute and functional iron deficiency anemia in nondialysis-dependent chronic kidney disease. Nephrol Dial Transplant 36: 129–136, 2021 [DOI] [PubMed] [Google Scholar]
- 15.Mariani L, Stengel B, Combe C, Massy ZA, Reichel H, Fliser D, et al. The CKD Outcomes and Practice Patterns Study (CKDopps): Rationale and methods. Am J Kidney Dis 68: 402–413, 2016 [DOI] [PubMed] [Google Scholar]
- 16.Hicks KA, Mahaffey KW, Mehran R, Nissen SE, Wiviott SD, Dunn B, et al. Standardized Data Collection for Cardiovascular Trials Initiative (SCTI): 2017 cardiovascular and stroke endpoint definitions for clinical trials. Circulation 137: 961–972, 2018 [DOI] [PubMed] [Google Scholar]
- 17.Austin PC, Lee DS, Fine JP: Introduction to the analysis of survival data in the presence of competing risks. Circulation 133: 601–609, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li L, Yang W, Astor BC, Greene T: Competing risk modeling: Time to put it in our standard analytical toolbox. J Am Soc Nephrol 30: 2284–2286, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D; Modification of Diet in Renal Disease Study Group: A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 20.Raghunathan TE, Solenberger PW, Berglund J, Van Hoewyk J: IVEware: Imputation and variance estimation software. Survey Methodology Program, Survey Research Center, Institute for Social Research, University of Michigan. Available at: https://www.src.isr.umich.edu/software/. Accessed June 4, 2021
- 21.Rubin DB: Multiple imputation for nonresponse in surveys, New York, John Wiley and Sons, 1987 [Google Scholar]
- 22.Beck-da-Silva L, Piardi D, Soder S, Rohde LE, Pereira-Barretto AC, de Albuquerque D, et al. IRON-HF study: A randomized trial to assess the effects of iron in heart failure patients with anemia. Int J Cardiol 168: 3439–3442, 2013 [DOI] [PubMed] [Google Scholar]
- 23.Ponikowski P, Kirwan B-A, Anker SD, McDonagh T, Dorobantu M, Drozdz J, et al. AFFIRM-AHF investigators: Ferric carboxymaltose for iron deficiency at discharge after acute heart failure: A multicentre, double-blind, randomised, controlled trial. Lancet 396: 1895–1904, 2020 [DOI] [PubMed] [Google Scholar]
- 24.Macdougall IC, Bock AH, Carrera F, Eckardt KU, Gaillard C, Van Wyck D, et al. FIND-CKD Study Investigators: FIND-CKD: A randomized trial of intravenous ferric carboxymaltose versus oral iron in patients with chronic kidney disease and iron deficiency anaemia. Nephrol Dial Transplant 29: 2075–2084, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bhandari S, Kalra PA, Berkowitz M, Belo D, Thomsen LL, Wolf M: Safety and efficacy of iron isomaltoside 1000/ferric derisomaltose versus iron sucrose in patients with chronic kidney disease: The FERWON-NEPHRO randomized, open-label, comparative trial. Nephrol Dial Transplant 36: 111–120, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kovesdy CP, Estrada W, Ahmadzadeh S, Kalantar-Zadeh K: Association of markers of iron stores with outcomes in patients with nondialysis-dependent chronic kidney disease. Clin J Am Soc Nephrol 4: 435–441, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenga MF, Nolte IM, van der Meer P, Bakker SJL, Gaillard CAJM: Association of different iron deficiency cutoffs with adverse outcomes in chronic kidney disease. BMC Nephrol 19: 225, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Macdougall IC, Bhandari S, White C, Anker SD, Farrington K, Kalra PA, et al. PIVOTAL Investigators and Committees: Intravenous iron dosing and infection risk in patients on hemodialysis: A prespecified secondary analysis of the PIVOTAL trial. J Am Soc Nephrol 31: 1118–1127, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Macdougall IC, Bircher AJ, Eckardt K-U, Obrador GT, Pollock CA, Stenvinkel P, et al. Conference Participants: Iron management in chronic kidney disease: Conclusions from a “Kidney Disease: Improving Global Outcomes” (KDIGO) Controversies Conference. Kidney Int 89: 28–39, 2016. Available at: https://kdigo.org/wp-content/uploads/2017/02/KDIGO-Iron-Management-in-CKD-Conf-Full-Report.pdf. Accessed June 4, 2021 [DOI] [PubMed] [Google Scholar]
- 30.Rostoker G, Vaziri ND, Fishbane S: Iatrogenic iron overload in dialysis patients at the beginning of the 21st century. Drugs 76: 741–757, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cullis JO, Fitzsimons EJ, Griffiths WJ, Tsochatzis E, Thomas DW; British Society for Haematology: Investigation and management of a raised serum ferritin. Br J Haematol 181: 331–340, 2018 [DOI] [PubMed] [Google Scholar]
- 32.Wish JB, Aronoff GR, Bacon BR, Brugnara C, Eckardt KU, Ganz T, et al. Positive iron balance in chronic kidney disease: How much is too much and how to tell? Am J Nephrol 47: 72–83, 2018 [DOI] [PubMed] [Google Scholar]
- 33.Hentze MW, Muckenthaler MU, Galy B, Camaschella C: Two to tango: Regulation of Mammalian iron metabolism. Cell 142: 24–38, 2010 [DOI] [PubMed] [Google Scholar]
- 34.Wong MMY, Tu C, Li Y, Perlman RL, Pecoits-Filho R, Lopes AA, et al.: Anemia and iron deficiency among chronic kidney disease Stages 3–5ND patients in the Chronic Kidney Disease Outcomes and Practice Patterns Study: Often unmeasured, variably treated. Clin Kidney J 13: 613–624, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopes MB, Tu C, Zee J, Guedes M, Pisoni RL, Robinson BM, et al. A real-world longitudinal study of anemia management in non-dialysis-dependent chronic kidney disease patients: a multinational analysis of CKDopps. Sci Rep 11: 1784, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Macdougall IC, Canaud B, de Francisco AL, Filippatos G, Ponikowski P, Silverberg D, et al. Beyond the cardiorenal anaemia syndrome: recognizing the role of iron deficiency. Eur J Heart Fail 14: 882–886, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.