Significance Statement
Aging slowly alters kidney function, with progressive nephron reduction leading to metabolic and mechanical constraints for the remaining nephrons. Podocytes, due to their anatomic location, are particularly exposed to aging-related stress. Knowledge of biological mechanisms behind podocyte adaptation is limited. We identified REST, a repressor of neuronal genes, as a key player in nephron adaptation to aging. Innovative tools demonstrate that REST acts on the podocyte cytoskeleton, promoting resistance to mechanical stressors, and that REST regulates podocyte survival. Finally, REST expression is upregulated in human podocytes during aging, consistent with a conserved mechanism of stress resistance. This study suggests REST protects the kidney from injury and degeneration during aging, with potentially important therapeutic implications.
Keywords: podocyte, aging, REST, cytoskeleton, apoptosis
Visual Abstract
Abstract
Background
CKD is associated with the loss of functional nephr ons, leading to increased mechanical and metabolic stress in the remaining cells, particularly for cells constituting the filtration barrier, such as podocytes. The failure of podocytes to mount an adequate stress response can lead to further nephron loss and disease progression. However, the mechanisms that regulate this degenerative process in the kidney are unknown.
Methods
We combined in vitro, in vivo, and organ-on-chip approaches to identify the RE1-silencing transcription factor (REST), a repressor of neuronal genes during embryonic development, as a central regulator of podocyte adaptation to injury and aging.
Results
Mice with a specific deletion of REST in podocytes exhibit albuminuria, podocyte apoptosis, and glomerulosclerosis during aging, and exhibit increased vulnerability to renal injury. This phenotype is mediated, in part, by the effects of REST on the podocyte cytoskeleton that promote resistance to mechanical stressors and augment podocyte survival. Finally, REST expression is upregulated in human podocytes during aging, consistent with a conserved mechanism of stress resistance.
Conclusions
These results suggest REST protects the kidney from injury and degeneration during aging, with potentially important therapeutic implications.
Podocytes are postmitotic cells that are exposed to different type of stress, including arterial pulse pressure. Podocyte adaptation to stress is an important mechanism that ensure functionality over a lifetime. The podocyte plays a central role in the progression of CKD.1 Indeed, the podocyte can be affected directly (i.e., in diabetes, genetic disorders, or after exposure to toxins) or indirectly (after nephron reduction or during aging). After injury, the podocyte cytoskeleton is reorganized and the podocyte foot processes are dismantled, leading to podocyte detachment, proteinuria, and apoptosis.1 The loss of podocytes can only be compensated by hypertrophy of the remaining podocytes with a progressive decline in the number of cells able to cover a given filtration area and the constitution of synechia leading to focal and segmental glomerulosclerosis.1–3 The cellular mechanisms that are involved in the podocyte response to injury are therefore critical for preserving kidney function but are poorly understood.
The RE1-silencing transcription factor (REST) is a repressor of neuronal genes during embryonic development that is downregulated once terminal neuronal differentiation has occurred.4 Remarkably, REST is induced in the ageing human brain and regulates a network of genes that mediate cell death and stress resistance.5 The REST-controlled gene network becomes dysregulated at early stages of Alzheimer’s disease, when REST is lost from the nucleus.5,6 Conditional REST knockout mice and Caenorhabditis elegans models, and in vitro experiments in primary neuron cultures, indicate that REST protects neurons from age-related toxic insults, by regulating a neuroprotective stress response that is central to cognitive preservation during ageing.5
Podocytes and neurons are both postmitotic cells that share a number of morphologic properties.7 These cells have a comparable structural organization, and share the same highly tissue-specific proteins, such as nephrin, ion channels (i.e., BKC), or axonal guidance factors.7 Furthermore, many cytoskeleton proteins that regulate the actin dynamics of foot processes in podocytes, such as synaptopodin, nestin, dendrin, or INF2, are also expressed in neurons. Finally, several genetic diseases are known to affect both podocytes and neurons.7
Given the shared similarities between postmitotic podocytes and neurons, and their substantial burden of stress during aging, we hypothesized that these two cell types might share similar stress response mechanisms. On the basis of recent findings implicating REST in neural stress resistance, we asked whether REST might regulate stress resistance in kidney podocytes and play a role in the development of CKD. Here, we uncover a novel role for REST in podocyte survival, stress resistance, and cytoskeletal homeostasis. Moreover, we present evidence for a role of REST in podocyte homeostasis during aging, paralleling the role of REST in aging neurons.
Methods
Detailed methods are available in Supplemental Appendix 1.
Animals
Mice were housed and fed according to the regulation directed by the institutional Animal Care and Use Committee of Harvard Medical School, the Departmental Director of Services Vétérinaires de la Prefecture de Police de Paris and by the Ethical Committee of Université de Paris (2019021913194044).
Mice carrying floxed alleles of REST flanking exon 2 (previously described5) were crossed with Nestin-Cre transgenic mice (strain 003771 from the Jackson Laboratory) to generate REST conditional knock-out in podocyte (RESTΔpod). The primers used to genotype the mice were: 5′-CATGCGAGTACTGCCATACCCAAC-3′, 5′-GTGATGGGGCAGTCTTCTGGAGG-3′, and 5′-GGGCACACCTTTAATCCTAGCTTC-3′. Primers allow to identify wild-type (WT), floxed, and recombined REST alleles respectively at 220, 264, and 375 bp.
Tomato-green fluorescent protein (GFP) C57bl/6 mice11 were crossed with C57bl/6 Podocin-Cre mice (Nphs2-Cre) to activate GF P expression in podocin-expressing cells. Before 2 weeks of age, GFP-Cre+ and WT mice were euthanized, kidneys were harvested and digested by collagenase. Then, GFP+ podocytes were isolated using a flow cytometer (Sony SH800).
Balb/c mice from Charles River were also used for doxorubicin treatment. Two-month old mice were injected with a single dose of 17 µg/g of doxorubicin. At time of sacrifice, kidneys were collected for RNA, protein, and morphologic studies.
Plasma and Urine Analyses
Coomassie gel was used to explore albuminuria. Ten ml of urine were run by SDS-PAGE, followed by Coomassie blue staining. Mouse blood and urinary albumin or creatinine concentration were measured precisely using an Olympus multiparametric analyzer (Instrumentation Laboratory).
Cell Culture
HEK cells, GFP+ primary podocytes, and immortalized mouse podocytes3 were used in this study. Specific culture conditions are described in Supplemental Appendix 1.
Cell Transfection and Doxorubicin or H2O2 Treatment
For overexpression experiments, cells were transiently transfected by Addgene LCP-flag-REST-WT (Addgene plasmid ref 41903). An empty vector plasmid was used as negative control. Transfection protocols used in this study are available in Supplemental Appendix 1.
For inhibition of expression experiments, cells were transiently transfected using TriFECTa Dicer-substrate short interfering RNA (siRNA) kit (mm.Ri.Rest.13 and hs.Ri.REST.13). HEK cells/podocytes were transfected with human/mouse DsiREST RNA or with human/mouse negative control Dicer-substrate siRNAs at 20nM using INTERFERinin vitro siRNA/microRNA (Polyplus-transfection, ref 409–10) transfection reagent. Full protocols of transient transfection are shown in Supplemental Appendix 1.
Histologic Analysis
Next, 4 µm sections were stained with Masson’s trichrome, picrosirius red, hematoxylin and eosin, and periodic acid–Schiff. Slides were examined by an observer masked to sample identity. Glomerular and tubular lesions and fibrosis were quantified using a semiquantitative score methodology, as previously described.6
Immunochemistry and Immunofluorescence
Paraffin-embedded 4-µm kidney sections were used. The following antibodies were used: REST (Millipore, 07579, 1:50), nephrin (Progen, GP-N2, 1:100), podocin (Sigma-Aldrich, P0372, 1:50), WT1 (Dako, M3561, clone 6F-H2, 1:50), nestin (Santa Cruz,. SC-58813, 1:50), and cleaved caspase 3 (Cell Signaling Technology, 9664, 1:50). Nuclei were stained by the Hoechst solution (Thermo Fisher Scientific, 33342). Glomerular WT1 or cleaved caspase 3 positive cells were counted.
HEK cells or immortalized mouse podocyte or mouse primary GFP+ podocytes were stained with following antibodies: REST (Millipore, 07579, 1:50), nephrin (Progen, GP-N2, 1:100), podocin (Sigma-Aldrich, P0372, 1:50), WT1 (Dako, M3561, clone 6F-H2, 1:50), nestin (Santa Cruz, SC-58813, 1:50), cleaved caspase 3 (Cell Signaling Technology, 9664, 1:50), synaptopodin (Abcam, ab109560, 1:50), paxillin (Abcam, ab32084, 1:100), or phallodin-tetramethylrhodamine (Sigma-Aldrich).
Human kidney biopsies were processed as previously described.6 Sections were exposed to REST (Millipore, 07579, 1:50) and nephrin (Progen, GP-N2, 1:100) primary antibodies, followed by appropriated secondary antibodies.
Confocal Zeiss LSM 700 confocal microscope (magnification ×400) were used to analyze immunofluorescence studies and ImageJ software were used to perform quantifications.
Isolation of Glomeruli by Laser-capture Microdissection Assay
Kidney sections (30 µm) were fixed in absolute ethanol and stained with eosin Y as previously described.6 The Palm Microbeam laser microdissection microscope (ZEISS) was used to selectively microdissect glomeruli. A sufficient number of glomeruli were dissected from each mouse, then the appropriate buffers were used for protein extraction.
β-galactosidase Staining
Mouse primary GFP+ podocytes or human HEK cells were fixed with 4% PFA for 3 minutes at room temperature, then incubated with SA-β gal staining solution overnight at 37°C, as previously described.5
Terminal Deoxynucleotidyl Transferase–mediated Digoxigenin-deoxyuridine Nick-end Labeling–induced Apoptosis Assay
The 4-µm paraffin-embedded kidney sections were deparaffinazed and rehydrated as previously described.6 The number of apoptotic podocytes was determined as the number of terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling–positive nuclei per glomeruli, and counted using ZEISS LSM700 confocal microscope (magnification ×400).
Quantitative Real-time PCR
RNA extracts were obtained from kidney cortex, whole kidneys, or cells (see Supplemental Appendix 1).
Western Blot
Western blot analysis was performed as previously described.8 The following antibodies were used: REST (Millipore, 07–579, 1:500), β-actin (Sigma-Aldrich, A5316, 1:5000), cleaved-caspase 3 (Asp175) (5A1E) (Cell Signaling, 9664, 1:1000), poly (adp-ribose) polymerase (Cell Signaling, 9542S, 1:1000), phosphor-H2A× (Ser139) (20E3) (Cell Signaling, 9718, 1:1000), nephrin (Progen, GP-N2, 1:500), podocin (Sigma-Aldrich, P0372, 1:1000), WT1 (Thermo Fisher Scientific, PA5–16879, 1:500), α-tubulin (Sigma-Aldrich, T5168, 1:5000), and lamin AC (Cell Signaling, 4777, 1:1000), followed by the appropriate peroxidase-conjugated or fluorescence secondary antibodies (1:5000).
Organ-on-chips Cell Culture System
The device and the basic research kit used were purchased from Emulate. Experiments were conducted following the manufacturer’s instructions (see Supplemental Appendix 1).9 Once attached, cells were exposed over 6 days to a 60 µl/h flow rate and 0% of stretch. Then during the last 2 days, cells were exposed to 80 µl/h flow rate and 5% of stretch. Images were obtained using a Nikon CKX53 microscope.
Data Analysis and Statistics
The statistical analysis was performed using GraphPad Prism 6 software. Data were expressed as mean±SEM. To compare two experimental groups Mann–Whitney or t test was used. ANOVA and Tukey–Kramer test was used to compare more experimental groups. The difference between groups was significant if P<0.05.
Results
Rest Deletion Accelerates Podocyte Injury and Proteinuria
To explore the role of REST in kidney function during aging, we began by examining REST mRNA expression. REST mRNA levels in total mouse kidney showed a 2.3±0.1 increase with ageing (Figure 1A). Immunofluorescence microscopy showed that the REST protein was localized to the cytoplasm of kidney podocytes and tubular cells in young adult mice (8 weeks) (Figure 1B). In older mice (52 weeks), however, REST appeared in the nucleus of podocytes, but remained cytoplasmic in the tubular compartment (Figure 1B). We then explored REST expression in subtotal nephrectomy (subtotal Nx), a model of accelerated nephron loss. Subtotal Nx was associated with 1.37±0.3 increased REST mRNA expression (Figure 1C). At the protein level, although sham-operated mice had barely detectable REST expression, REST appeared in the nucleus of podocytes after subtotal Nx (Figure 1D). To explore the role of REST in podocytes during ageing, we isolated primary podocytes from young (8 weeks) and aged mice (52 weeks). For this purpose, we generated GFPΔpod mice by breeding Tomato/GFP with Nphs2-Cre mice, then selectively isolated GFP podocytes using flow cytometry. We confirmed that isolated podocytes expressed several important differentiation markers such as WT1, podocin, synaptopodin, nephrin, and nestin (Supplemental Figure 1A). Immunofluorescence microscopy showed REST expression only in the nucleus of aged podocytes (Figure 1E). Furthermore, cellular subfractions confirmed that REST was predominantly localized to the nucleus (Figure 1F).
Figure 1.

Rest deletion accelerates podocyte injury and proteinuria. (A) Quantitative RT-PCR (RT-qPCR) of REST mRNA in kidney extracts from young 8-week-old (n=6) and aged 52 weeks (n=5) C57bl/6 mice. (B) Double-labeling for REST and nestin in young (n=6) and aged (n=5) C57bl/6 mice. Scale bar, 10 μm. Insets show higher-magnification images of representative podocytes. (C) REST mRNA in kidney extracts of Sham-operated (Sh) (n=6) and 75% nephrectomized (Nx) (n=10) C57BL/6 mice at 2 months after surgery. (D) Immunolabeling of REST in Sh (n=6) and Nx (n=10) C57bl/6 mice at 2 months after surgery. Insets show higher-magnification images of representative podocytes. (E) Double labeling for REST and GFP in primary isolated GFP-podocytes from 8- and 52-week-old C57BL/6 mice. (F) Western blot analysis of REST in cytoplasmic (n=4) and nuclear fractions (n=4) of primary isolated GFP-podocytes from 52-week-old C57bl/6 mice. (G) Immunofluorescence of REST and Nestin in podocyte-specific Rest mutant (RESTΔpod) (n=5) and Rest floxed (RESTflox/flox) (n=5) 52-week-old C57BL/6 mice. Scale bar, 10 μm. Insets show higher-magnification image of representative podocytes. (H) Western blot of REST in laser microdissected glomerular extracts from kidneys of RESTflox/flox (n=4) and RESTΔpod (n=4) 52-week-old C57bl/6 mice. (I) Albumin to creatinine ratio (mg/mmol) in urine samples from C57bl/6 RESTflox/flox and RESTΔpod 52-week-old mice (n=5 for both conditions). (J)–(L) Morphology and lesion scores of kidneys from RESTflox/flox (n=4) and RESTΔpod (n=4) 52-week-old C57bl/6 mice. Scale bar, 10 μm. (M–O) Cleaved caspase-3 (CC3) immunolabeling (M), terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling assay (N), and WT1 immunolabeling and quantification (lower panel) of kidneys from RESTflox/flox and RESTΔpod 52-week-old C57BL/6 mice. Scale bar, 10 μm. (P) Western blot and quantitative analysis of CC3 in kidney extracts from RESTflox/flox (n=4) and RESTΔpod (n=4) 52-week-old C57bl/6 mice. Scale bar, 10 μm. All data are shown as the mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 between groups, Mann–Whitney or t test.
To better understand the role played by REST in aging podocytes, we generated Rest mutant mice (RestΔpod) by crossing mice bearing floxed Rest alleles (RESTflox/flox) with Nestin-Cre mice. Nestin expression is restricted to differentiated podocytes in the adult kidney.10 Immunofluorescence analysis and glomerular isolation using laser capture microdissection confirmed the marked reduction (90%±2%) of REST protein expression in podocytes of mutant mice at 52 weeks old (Figure 1, G and H and Supplemental Figure 1B). RestΔpod mice were born in an expected Mendelian ratio and were indistinguishable from Restflox/flox control littermate at least 6 months after birth under physiologic conditions (data not shown). However, whereas urinary albumin excretion remained stable in RESTflox/flox mice throughout the 2- to 12-month follow-up, it progressively increased in RestΔpod mice (Figure 1I). Morphologic analysis showed more severe glomerular lesions in 12-month old RestΔpod mice than in WT littermates (Figure 1J). In addition, we observed that the severity of tubular dilations and interstitial fibrosis (Figure 1, K and L) was significantly increased in RestΔpod mice 12 months after birth. Tubular dilations and fibrosis were attributed to the presence of albuminuria.11 These results suggest REST plays an essential role in the maintenance of podocyte function, enabling the kidney to adapt during aging.
Previous studies have shown that REST pathway activation inhibits apoptosis and may regulate cell proliferation and migration.5,6 We therefore monitored the functional effect of REST deletion in podocytes during ageing. Whereas the percentage of glomeruli with apoptotic cells stained for cleaved caspase 3 and terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling was slightly elevated in WT mice with ageing, it was substantially increased in RestΔpod mice (Figure 1, M and N). Glomerular apoptosis was further confirmed by Western blot analysis of glomerular extract (Figure 1P). The increased apoptosis correlated with podocyte rarefaction as assessed by the significant reduction of WT1-positive cells in RestΔpod mice compared with RESTflox/flox (Figure 1O). Thus, REST maintains podocyte survival during the ageing process.
REST Is Regulated by Stressful Conditions
To gain further insight into the regulation of REST, we analyzed REST expression in vitro, starting with HEK cells. We exposed HEK cells to increasing concentrations of hydrogen peroxide to induce a replicative senescence phenotype. Interestingly, cells exposed to 100 nM of hydrogen peroxide demonstrated senescence-associated β-galactosidase staining (Figure 2A), p16 and p21 mRNA expression, and elevated REST mRNA and protein expression (Figure 2, B and C). We then examined REST expression in primary cultures of GFP-mouse podocytes. Similar to HEK cells, cultured podocytes exposed to hydrogen peroxide showed evidence of replicative senescence (Figure 2, D and E), and an increase in REST expression (Figure 2F). Thus, REST is induced by oxidative stress.
Figure 2.
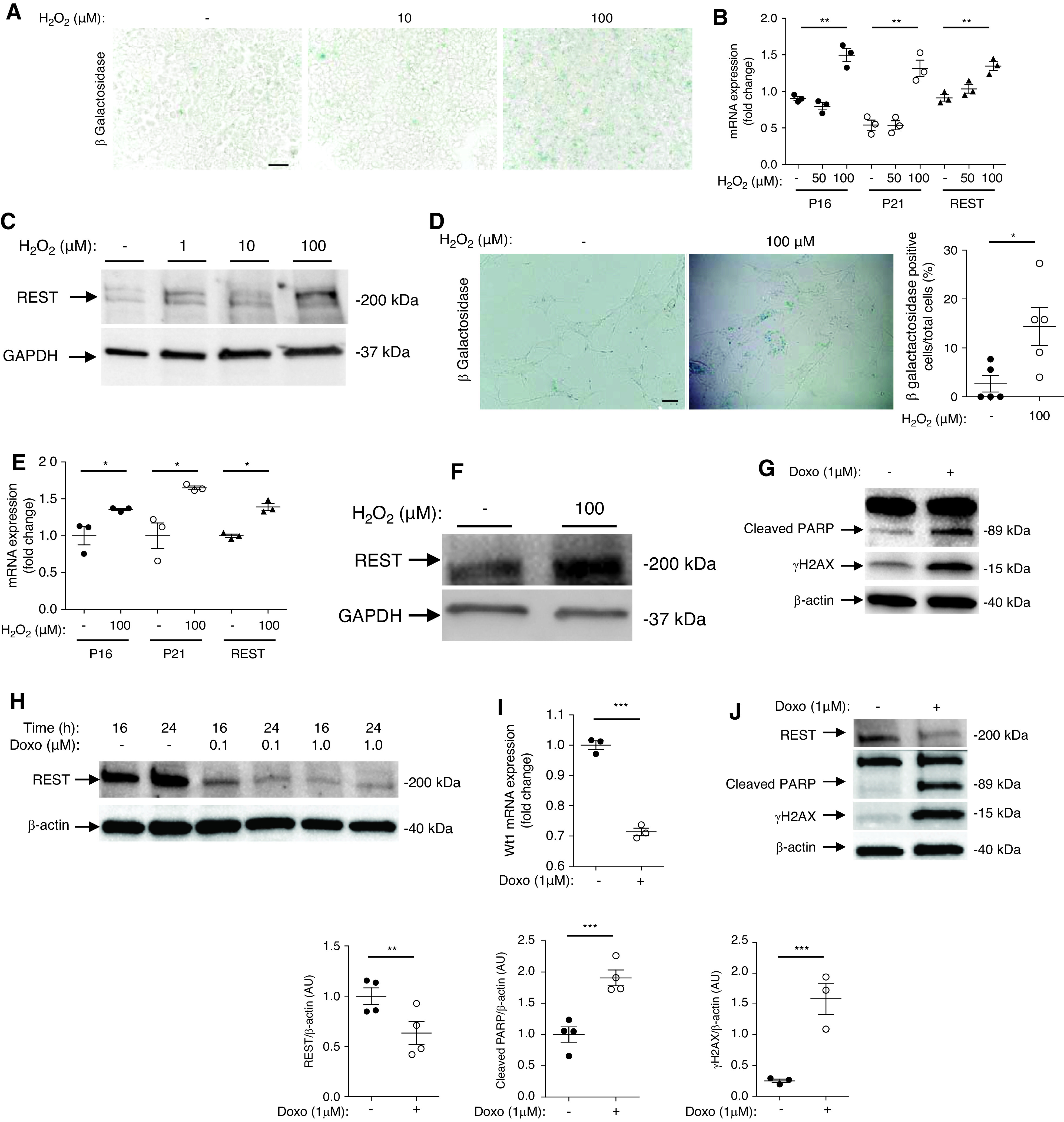
REST induction by podocyte oxidative stress and injury. (A) β-galactosidase staining of 0, 10, and 100 μM H2O2 treated HEK cells (n=3 experiments). Scale bar, 5 μm. (B) RT-qPCR of P16, P21, and REST in HEK cells treated with 0, 50, and 100 μM of H2O2 (n=3 different experiments). (C) Western blot of REST in 0, 10, and 100 μM H2O2 treated HEK cells. (D) β-galactosidase staining and quantification of primary culture of GFP-podocytes exposed to either 0 or 100 μM H2O2 (n=4 experiments). Scale bar, 5 μm. (E) RT-qPCR of P16, P21, and REST in primary cultures of isolated GFP-podocytes exposed to 0 and 100 μM of H2O2 (n=3 experiments). (F) Western blot showing REST expression in primary cultures of isolated GFP-podocytes exposed to 0 and 100 μM of H2O2. (G) Western blot showing cleaved PARP and γH2AX expression in HEK cells treated with either 0 or 1 μM of doxorubicin (n=4 experiments). (H) Western blot of REST in HEK cells exposed to 0, 0.1, and 1 μM of doxorubicin. (I) WT1 mRNA expression of primary cultures of isolated GFP-podocytes exposed to 1 μM of doxorubicin (n=3 experiments). (J) Western blot and quantification of REST, cleaved PARP, and γH2AX in primary culture of isolated GFP-podocytes exposed to1 μM of doxorubicin (n=4 experiments). All data are shown as the mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 between groups, Mann–Whitney or t test.
We next investigated the role of REST in the regulation of apoptosis. We first exposed HEK cells to increasing concentrations of doxorubicin. Doxorubicin induced DNA damage and cell apoptosis (Figure 2G) with a decreased expression of REST (Figure 2H). Next, we exposed primary culture of podocytes to doxorubicin. After doxorubicin exposure, we observed a loss of podocyte markers (Figure 2I), and reduced REST expression, together with induction of the apoptosis marker cleaved PARP (Figure 2J). To identify a potential protective role of REST, we transfected HEK cells with REST cDNA. HEK cells overexpressing REST showed reduced expression of the apoptosis markers cleaved PARP and cleaved caspase 3 after exposure to doxorubicin compared with cells transfected with the empty vector (Figure 3, A and B). Immortalized podocytes transfected with REST cDNA showed a trend for reduced expression of cleaved PARP after exposure to doxorubicin (Figure 3C). Conversely, immortalized podocytes transfected with short hairpin RNA (shRNA) lentivirus targeting REST showed significantly elevated apoptotic markers, and HEK cells showed a trend toward elevated apoptosis (Figure 3, D and E).
Figure 3.
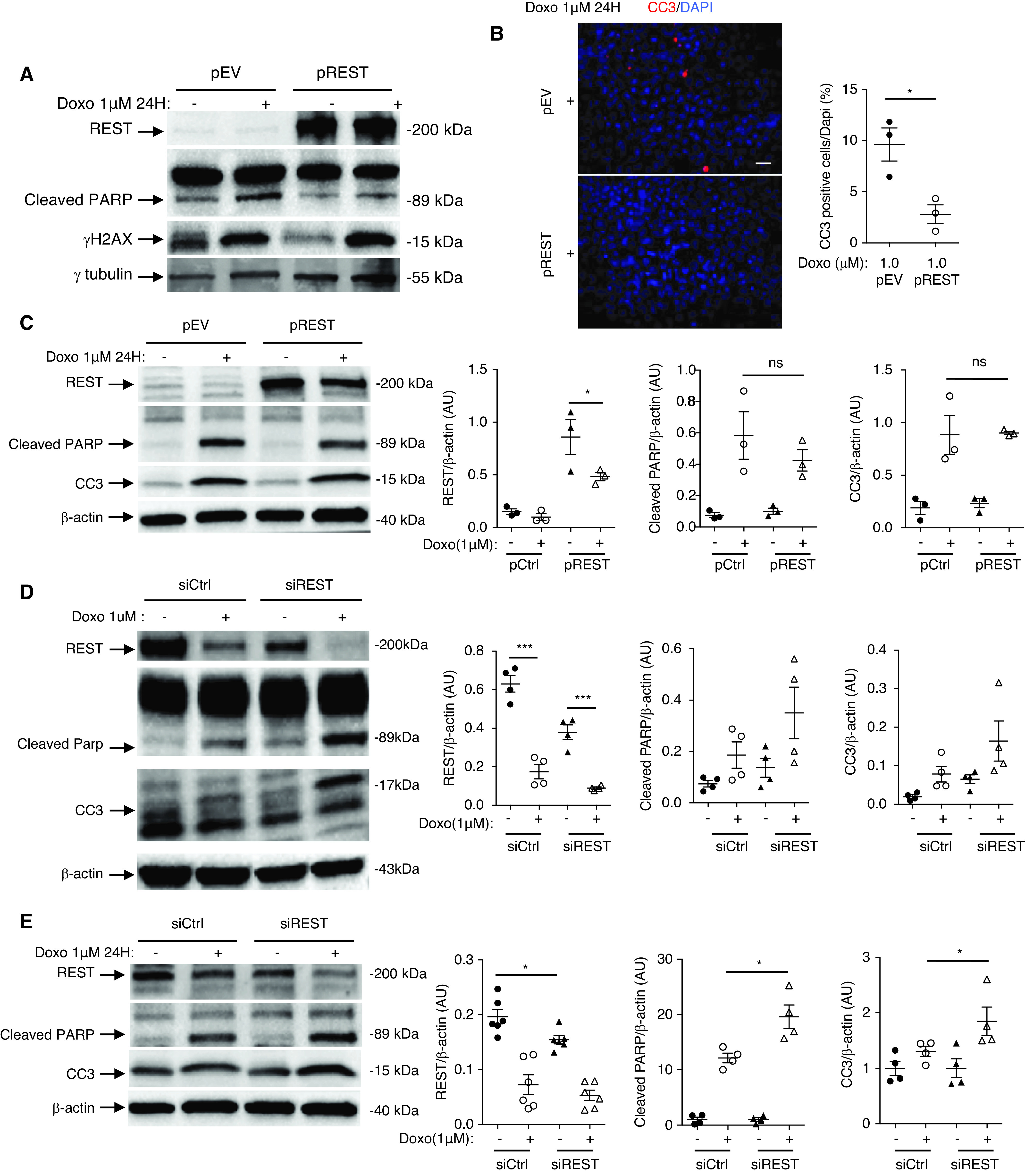
REST protects against podocyte apoptosis. (A) Western blot of REST, cleaved PARP, and γH2AX in HEK cells transfected with REST cDNA plasmid (pREST) or with empty vector (pEV), and treated with 0 or 1 μM of doxorubicin. (B) Immunofluorescence (left) and quantification (right) of cleaved caspase-3 (CC3) in pREST and pEV HEK cells exposed to either 0 or 1 μM of doxorubicin (n=3 experiments). Scale bar, 5 μm. (C) Western blot (left) and quantification (right) of REST, cleaved PARP, and CC3 in pREST or pEV transfected immortalized mouse podocytes exposed to either to 0 or 1 μM of doxorubicin (n=3 experiments). (D) Western blot and quantification of REST, cleaved PARP, and CC3 in HEK transfected cells with siREST or siCtrl and treated with 0 or 1 μM of doxorubicin (n=4 experiments). (E) Western blot (left) and quantifications (right) of REST, cleaved PARP, and CC3 in immortalized mouse podocyte transfected with siREST or siCtrl and treated with 0 or 1 μM of doxorubicin (n=4–6 experiments). All data are shown as the mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ****P<0.0001 between groups, Mann–Whitney or t test.
To further characterize the function of REST in podocytes, we examined effects on the actin cytoskeleton. An immortalized podocyte cell line labeled with paxillin and phalloidin showed that REST knockdown resulted in F-actin cytoskeleton reorganization, with a reduction in the number of focal adhesion processes, as determined by a decrease in the number of paxillin patches (Figure 4A). Such modifications correlated with an increase in motility, as revealed by the higher rate of wound closure of Rest−/− podocytes compared with controls (Figure 4B). Conversely, overexpression of REST in immortalized podocytes led to a significant increase in the number of paxillin patches (Figure 4C), but with a moderate effect on motility (Figure 4D).
Figure 4.
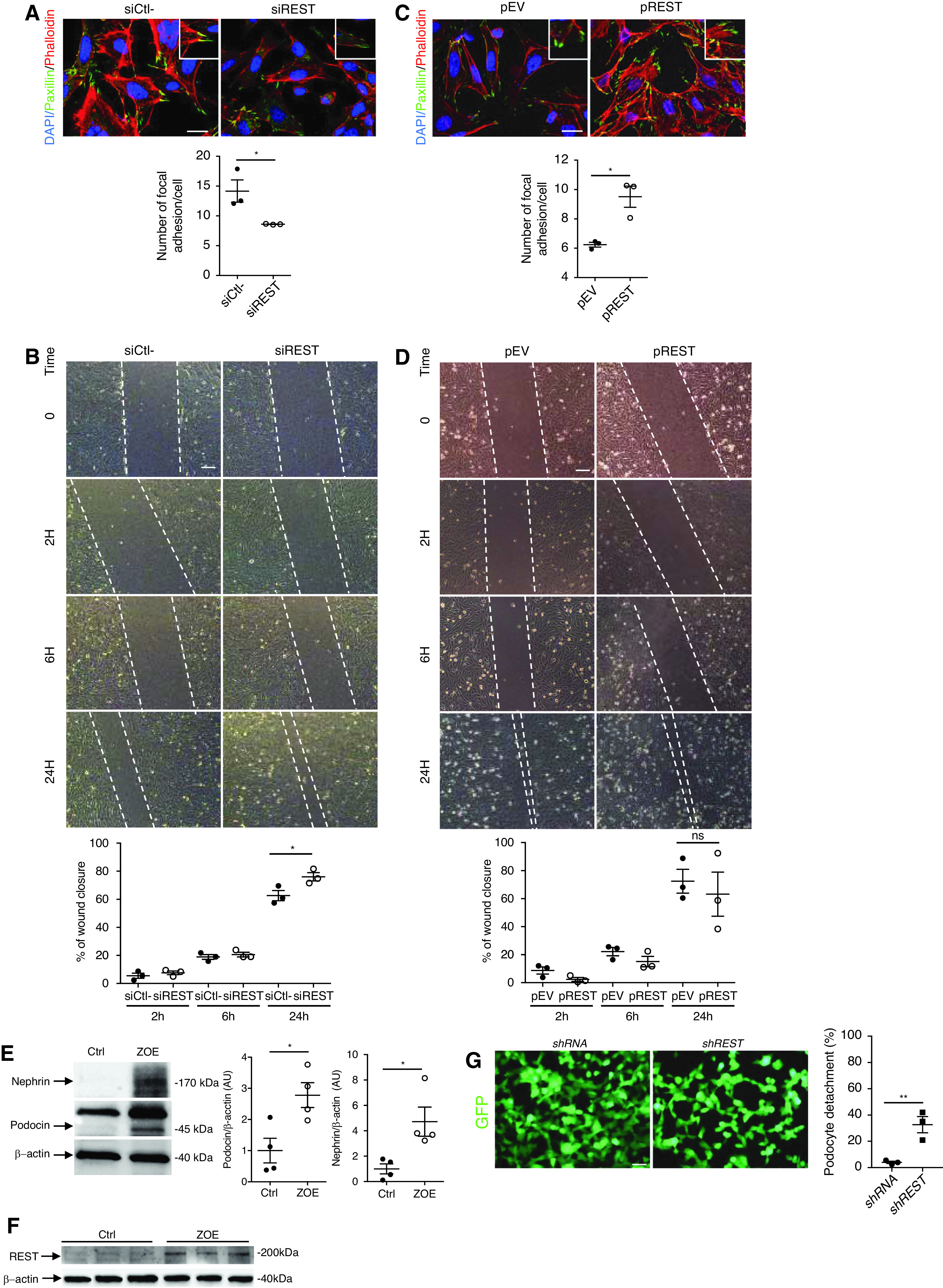
REST regulates podocyte migration and attachment to the basement membrane. (A) Coimmunostaining using phalloidin, an F-actin marker, and paxillin-specific antibodies and quantification in immortalized mouse podocyte transfected with siREST or siCtrl (n=3 experiments). Scale bars, 10 μm. The insets show higher magnification images of podocyte structures with stress fibers and focal adhesions. (B) Wound healing assay (upper left) and quantification of percentage of wound closure after 2 hours, 6 hours, and 24 hours (lower left) in immortalized mouse podocytes transfected with either siREST or siCtrl (n=3 experiments). Scale bars, 5 μm. (C) Coimmunostaining using phalloidin, an F-actin marker, and paxillin-specific antibodies in immortalized mouse podocytes transfected with either pREST or pEV (n=3 experiments). Scale bars, 10 μm. The insets show higher magnification images of podocyte structures with stress fibers and focal adhesions in pREST-transfected mouse podocytes. (D) Wound healing assay (upper right) and quantification of percentage of wound closure (lower right) after 2 hours, 6 hours, and 24 hours using immortalized mouse podocytes transfected with either pREST or pEV (n=3 experiments). Scale bars, 5 μm. Western blot and quantification of nephrin (E), podocin (E), and REST (F) in immortalized mouse podocytes exposed to mechanical stress and flow over 8 days (ZOE organ-on-a-chip) or maintained under normal static culture conditions (Ctrl). (G) Immortalized podocytes transfected with either scrambled shRNA or REST shRNA. Both shRNAs coexpress GFP. Cells transfected with REST shRNA show greater detachment under mechanical and flow constraints compared with controls (n=3). Quantification shows the percentage of detached cells before and after mechanical/flow stress. Scale bar, 5 μm. All data are shown as the mean±SEM. *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001 between groups, Mann–Whitney, or t test.
To more specifically mimic the in vivo condition of hemodynamic stress, we took advantage of a new device that allows organ-on-chip exploration.9 Immortalized mouse podocytes were cocultured with immortalized mouse endothelial cells in a chip in which the cells were separated by a polydimethylsiloxane membrane containing micropores. Podocytes were exposed to mechanical stress and flow forces. After 8 days, there was a substantial increase of the expression of differentiation markers, such as nephrin, podocin, and WT1, and increased REST expression compared with podocytes maintained in a static condition (Figure 4, E and F and Supplemental Figure 1, C and D). Cells were then transfected with either a control scrambled shRNA or REST shRNA and exposed to mechanical and flow constraints. Importantly, we observed that cells lacking REST expression were detaching from the polydimethylsiloxane membrane compared with controls (Figure 4G). These results suggest that REST promotes cytoskeletal adaption to mechanical stretch forces.
To explore the role of REST in podocyte survival in vivo, we injected mice with doxorubicin. In Balb/c mice, a single dose of intravenous doxorubicin resulted in a very high level of albuminuria (Figure 5A). Consistently, doxorubicin-injected mice had severe glomerular damage with podocyte dedifferentiation, apoptosis, and a reduction in REST expression (Figure 5, B, C, and D). Next, we injected C57bl/6 RESTflox/flox (control mice) and RestΔpod mice with doxorubicin. The C57bl/6 line of mice is relatively resistant to doxorubicin toxicity.12 Mice received a single injection of doxorubicin, 17 ug/g. Compared with vehicle-treated mice, RESTflox/flox mice did not develop significant glomerular lesions or an increase in proteinuria. In contrast, RestΔpod mice developed a more pronounced glomerular lesion score with albuminuria (Figure 5, E and F), and was characterized by dedifferentiated podocytes and increased podocyte apoptosis (Figure 5G). Collectively, these data suggest REST mediates a stress response that maintains podocyte survival and function (Supplemental Figures 2–4, Supplemental Table 1).
Figure 5.
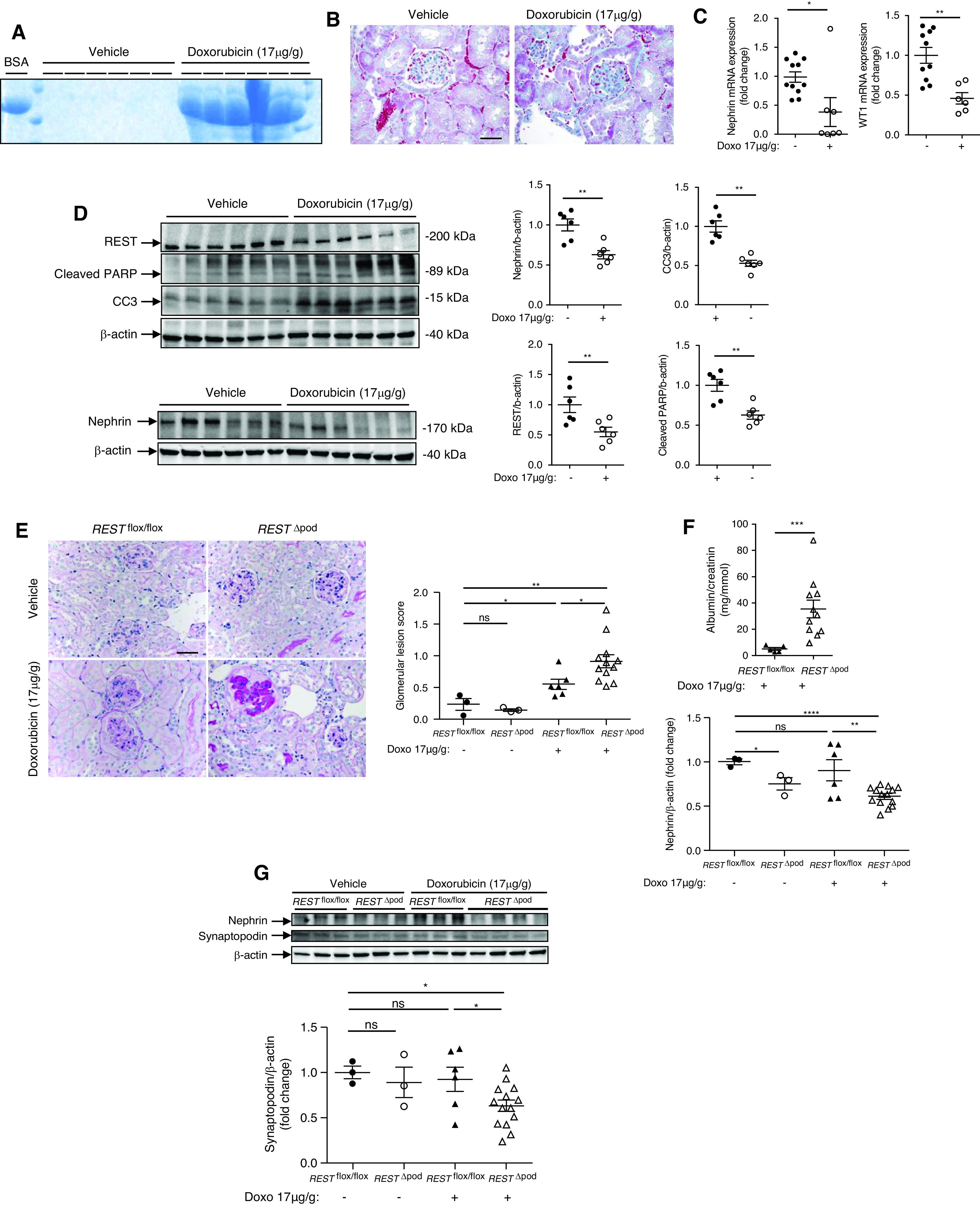
REST protects against doxorubicin-induced nephropathy. (A) Representative urine Coomassie blue staining 7 days after venous retro-orbital injection of either vehicle or a single dose of 17 μg/g of doxorubicin in BALB/c WT mice. BSA is shown as a positive control. (B) Trichrome staining of the kidneys 7 days after retro-orbital injection of either vehicle (n=6) or a single dose of 17 μg/g of doxorubicin into Balb/c WT mice. Scale bar, 10 μm. (C) Nephrin and WT1 mRNA in the kidneys of mice injected with either vehicle or a single dose of 17 μg/g of doxorubicin. (D) Western blot (left) and quantification (right) of REST cleaved PARP, cleaved caspase-3 (CC3) (top blot), and nephrin (lower) 7 days after injection of either vehicle or a single dose of 17 μg/g of doxorubicin. (E) Periodic acid–Schiff staining (left) and quantitative analysis of glomerular lesion scores (right) in kidneys of C57bl/6 RESTflox/flox and RESTΔpod mice, 3 months after injection of either vehicle or a single dose of doxorubicin (17 μg/g). At the time of sacrifice, the kidneys of vehicle-injected mice and RESTflox/flox mice injected with doxorubicin had no obvious abnormal histology, whereas RESTΔpod mice injected with doxorubicin exhibited glomerular lesions with glomerulosclerosis, tubular vacuolization and intratubular casts. Scale bar, 10 μm. (F) RESTΔpod mice injected with doxorubicin displayed albuminuria compared with doxorubicin treated RESTflox/flox mice. (G) Western blot (upper panel) and quantification of nephrin and synaptopodin (lower panels) in C57bl/6 RESTflox/flox and RESTΔpod mice, injected with either vehicle or a single dose of doxorubicin (17 μg/g). *P<0.05, **P<0.01, ***P<0.001, ***P<0.0001 between groups, ANOVA, and post-hoc Tukey–Kramer test were used.
Induction of REST in Ageing Human Podocytes
We asked if REST is also expressed in human kidney cells, and whether it is induced during aging in podocytes, as observed in mice. To address this question, we labeled for REST in preimplant biopsies from young (n=8, mean [SD] 27.8 [6.8] years old) and old adult deceased donors (n=8, mean [SD] 83 [4.2] years old), and determined the percentage of glomeruli expressing REST and nephrin (Supplemental Table 1). Interestingly, whereas REST staining was not detectable in the kidney of young donors, its expression was clearly detectable in the nucleus of podocytes from aged donors (Figure 6, A and B). These results suggest REST is induced in aging podocytes in both the human and mouse kidney.
Figure 6.

Podocyte REST expression is upregulated in the aging human kidney. (A) Representative immunofluorescence labeling of REST and nephrin in human kidney biopsies from young (n=8) and old (n=8) donors. Scale bar, 10 μm. Insets show higher-magnification images of representative podocytes. (B) Quantification of the number of glomeruli per slide containing cells expressing REST and nephrin per slides.
Discussion
The molecular pathways responsible for implementing a cellular stress response after podocyte injury, or during aging, are largely unknown. By combining experimental models of podocyte diseases with genetic modeling of REST loss-of-function in mouse podocytes in vivo, we have uncovered a novel function for the REST protein in podocytes. We showed that REST plays an important role in podocyte adaptation to stress. REST inactivation leads to podocyte dysfunction and apoptosis, resulting in proteinuria and glomerulosclerosis in response to toxins and injury, and during aging. Furthermore, we show that REST promotes podocyte stress resistance in part by maintaining cytoskeletal integrity. These results unveil a novel molecular pathway of podocyte adaptation to stress, with potentially important implications for the pathogenesis of CKD.
REST is dynamically regulated in the aging kidney podocyte, with increasing expression and nuclear translocation, that parallels the induction of REST in the aging human brain.9 Our findings suggest this epigenetic mechanism prevents podocyte dedifferentiation and apoptosis as the kidney is subjected to age-related stress. Moreover, REST was also dynamically regulated with increased expression and nuclear translocation after subtotal nephrectomy, suggesting an acute stress response mechanism. Similar to the neuroprotective function of REST in the aging brain,9 podocyte REST also prevented apoptosis and functional decline after oxidative stress, toxin exposure, and subtotal nephrectomy. The observation that nuclear REST levels are markedly upregulated in aging human podocytes suggests this mechanism of epigenetic resilience may be conserved from mice to humans across multiple organ systems. Conversely, the toxin doxorubicin led to a striking reduction in REST podocyte expression that accompanied degeneration. Importantly, we found that overexpression of REST was sufficient to improve the survival of different cell lines, including podocytes. By contrast, REST deletion, either in vitro or in vivo, was associated with spontaneous apoptosis that was increased after doxorubicin exposure. Finally, RestΔpod mutant mice exposed to doxorubicin demonstrated a higher level of proteinuria and exhibited glomerular lesions with elevated numbers of apoptotic cells, indicating REST protects podocytes from injury.
One of the most prominent structural features of podocytes is the very specific organization of the cytoskeleton that appears crucial for their proper function.4 Indeed, there is a strong correlation between podocyte cytoskeleton modifications and podocyte-enhanced migratory properties and the development of albuminuria and glomerulosclerosis.2,13 Interestingly, we observed that the ability to regulate cytoskeleton organization and migration was dependent on the level of REST expression. Indeed, cell lines, including immortalized podocytes, with reduced REST expression demonstrated enhanced migratory properties, with decreased formation of focal adhesion junctions.
Using a microfluidic organ-on-a-chip, we were able to improve the differentiation state of immortalized podocytes in vitro, in agreement with previous observations.12,13 This approach led to the observation that mechanical constraints increase the level of REST expression. Importantly, REST augmented podocyte adaptation to mechanical forces that are likely to be encountered during normal kidney function and may be exaggerated under pathologic conditions, such as hypertension, that impair kidney function. Thus, REST plays a central role in podocyte biology, and may modulate the vulnerability of the aging kidney to CKD.
REST is well known to be a repressor of neuronal genes during embryonic development that is downregulated once terminal neuronal differentiation has occurred.4 Remarkably, REST is induced in the ageing human brain and regulates a network of genes that mediate cell death and stress resistance.5 Indeed, it is possible that REST controls directly or indirectly the expression of podocyte differentiation genes. However, this will deserve further investigations.
These findings identify REST as a novel stress-response factor in podocytes that maintains cytoskeleton homeostasis protects against apoptosis and maintains kidney function during aging. Disruption of this adaptive REST-centered pathway leads to glomerular degeneration and albuminuria. Therefore, activation of REST function in podocytes may represent a novel therapeutic approach for the maintenance of glomerular function during aging and the prevention of CKD.
Disclosures
C. Legendre reports having consultancy agreements with CSL Behring and Hansa Medical; reports receiving honoraria from Alexion, Astellas, Novartis, and Sandoz; reports being a scientific advisor or member of Hansa Medical; and reports speakers bureau with Hansa Medical. F. Terzi reports Consultancy Agreements with ENYO; Research Funding from ENYO; and Scientific Advisor or Membership as Editor of Experimental Nephron, and Advisory Board of Swiss National Science Foundation. All remaining authors have nothing to disclose.
Funding
This study was partly supported by the European Research Council (STG-2015 grant 679254), the Emmanuel Boussard Foundation (London, UK), the Fondation Day Solvay (Paris, France), Institut National de la Santé et de la Recherche Médicale, Assistance Publique Hôpitaux de Paris, and Université de Paris.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2021020231/-/DCSupplemental.
Supplemental Appendix 1. Material and methods.
Supplemental Figure 1. Primary culture of podocytes characterization and REST mouse model characterization.
Supplemental Figure 2. Uncut Western blots of Figures 1 and 2.
Supplemental Figure 3. Uncut Western blots of Figures 3 and 4.
Supplemental Figure 4. Uncut Western blots of Figure 5.
Supplemental Table 1. Donor ages.
References
- 1.Hostetter TH: Progression of renal disease and renal hypertrophy. Annu Rev Physiol 57: 263–278, 1995 [DOI] [PubMed] [Google Scholar]
- 2.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, et al.: AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 3.Kriz W, Lemley KV: Potential relevance of shear stress for slit diaphragm and podocyte function. Kidney Int 91: 1283–1286, 2017 [DOI] [PubMed] [Google Scholar]
- 4.Chen ZF, Paquette AJ, Anderson DJ: NRSF/REST is required in vivo for repression of multiple neuronal target genes during embryogenesis. Nat Genet 20: 136–142, 1998 [DOI] [PubMed] [Google Scholar]
- 5.Lu T, Aron L, Zullo J, Pan Y, Kim H, Chen Y, et al.: REST and stress resistance in ageing and Alzheimer's disease. Nature 507: 448–454, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meyer K, Feldman HM, Lu T, Drake D, Lim ET, Ling K-H, et al.: REST and neural gene network dysregulation in iPSC models of Alzheimer's disease. Cell Rep 26: 1112–1127.e9. 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Weide T, Huber TB: Signaling at the slit: Podocytes chat by synaptic transmission. J Am Soc Nephrol 20: 1862–1864, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Canaud G, Bienaime F, Tabarin F, Bataillon G, Seilhean D, Nöel L-H, et al.: Inhibition of the mTORC pathway in the antiphospholipid syndrome. N Engl J Med 371: 303–312, 2014 [DOI] [PubMed] [Google Scholar]
- 9.Musah S, Dimitrakakis N, Camacho DM, Church GM, Ingber DE: Directed differentiation of human induced pluripotent stem cells into mature kidney podocytes and establishment of a Glomerulus Chip. Nat Protoc 13: 1662–1685, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen J, Boyle S, Zhao M, Su W, Takahashi K, Davis L, et al.: Differential expression of the intermediate filament protein nestin during renal development and its localization in adult podocytes. J Am Soc Nephrol 17: 1283–1291, 2006 [DOI] [PubMed] [Google Scholar]
- 11.El Karoui K, Viau A, Dellis O, Bagattin A, Nguyen C, Baron W, et al.: Endoplasmic reticulum stress drives proteinuria-induced kidney lesions via Lipocalin 2. Nat Commun 7: 10330, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hakroush S, Cebulla A, Schaldecker T, Behr D, Mundel P, Weins A: Extensive podocyte loss triggers a rapid parietal epithelial cell response. J Am Soc Nephrol 25: 927–938, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



