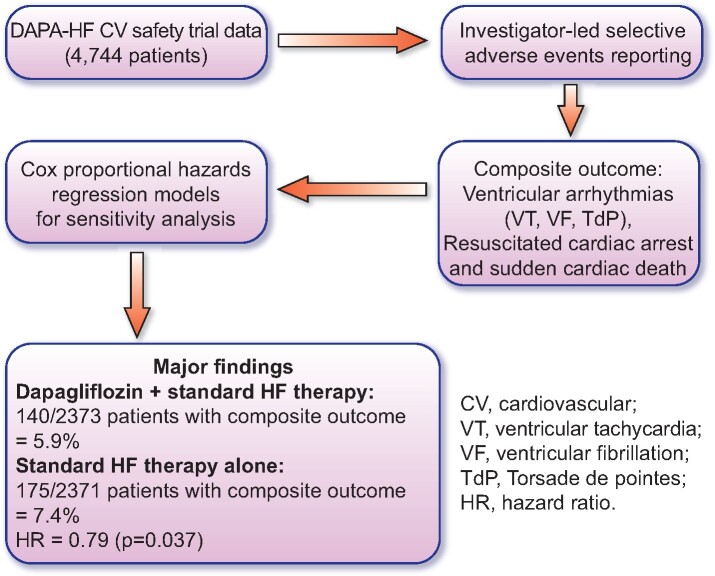
This editorial refers to ‘Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF’, by J.P. Curtain et al., doi:10.1093/eurheartj/ehab560.
Graphical Abstract.
Experimental design and major conclusions.
Sodium–glucose co-transporter 2 inhibitors (SGLT2is), such as empagliflozin and dapagliflozin, have been developed as antidiabetic drugs that promote urinary excretion of glucose to improve glycaemic control.1 Surprisingly, recent large-scale cardiovascular (CV) safety trials demonstrate that SGLT2is significantly reduce the incidence of heart failure (HF) by 30–40% in diabetes patients.2–6 Furthermore, results from the EMPEROR-Reduced and DAPA-HF trials7–9 demonstrate that the marked cardioprotective effects of the SGLT2is are preserved in the absence of diabetes, suggesting protective mechanisms that are independent of improved glycaemic control.
While the SGLT2i-mediated reduction in HF has understandably received the majority of attention, other signals such as reduction in sudden cardiac death (SCD) were also detected in these CV safety trials. These additional findings raise the possibility that SGLT2is may also possess an unexpected beneficial effect against ventricular arrhythmias which are one of the principal causes of SCD in patients with HF. Therefore, it is important to decode the trial data regarding any potential effects of the SGLTis on the incidence of ventricular arrhythmias.
In this issue of the European Heart Journal, Curtain et al. directly address this notion by performing a post-hoc analysis of the DAPA-HF trial data to examine the incidence of adverse events reporting of ventricular arrhythmias, resuscitated cardiac arrest, and sudden death in the dapagliflozin and placebo trial cohorts.10 The DAPA-HF data set8 is ideal for such an analysis on HF patients with reduced ejection fraction (HFrEF) as data from 4744 patients were available where adverse events were logged by investigators. The authors employed Cox proportional hazard regression models to perform sensitivity analyses on variables including age, sex, presence of ischaemic disease, implantable cardioverter defibrillators (ICDs), N-terminal probrain natriuretic peptide (NT-proBNP) status, and hypertension. Findings from this study indicate that dapagliflozin, when added to standard HF pharmacotherapy, reduced the composite outcome (ventricular arrhythmias, resuscitated cardiac arrest, or sudden death) to 5.9%, vs. 7.4% in the placebo group that received standard HF pharmacotherapy alone, resulting in a hazard ratio (HR) of 0.79 (P = 0.037). Furthermore, the authors also observed that this reduction in the composite outcome was further decreased in patients with below median NT-proBNP levels (HR = 0.58) when compared with patients with above-median NT-proBNP levels (HR = 0.83). Taken together, these results provide evidence for a protective effect of dapagliflozin against life-threatening electrical disturbances and that dapagliflozin may bestow additional protection in patients who were in the earlier stages of HFrEF at the time of enrolment, as defined by lower NT-proBNP levels. The strongest separation of the HR values was observed >9 months post-randomization, suggesting that the observed beneficial effects of dapagliflozin require time to develop and may involve cellular mechanisms that slow the progression of HFrEF. In this regard, there have been several pre-clinical studies that have provided insights as to precisely how the SGLT2is might afford protection against HF and cardiac arrhythmias. These include a reduction in the priming and activation of the cardiac NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome,11,12 inhibition of the late component of the cardiac sodium current,12 and the sodium–hydrogen exchanger.13 SGLT2is may also confer protection against structural (and perhaps electrical) remodelling through beneficial haemodynamic and metabolic alterations, as well as changes in sympathetic and parasympathetic tone. The use of the DAPA-HF trial data also supports the notion that the SGLT2is are of direct cardiovascular benefit for the tested composite outcome in patients without cardiac dysfunction resulting from diabetes. This study adds further evidence to the emerging concept that the SGLT2is possess a direct protective effect on the heart. Whether or not the SGLT2is reduced the composite outcome primarily as a result of a reduction in ionic disturbances that are associated with HF,14,15 and the subsequent decrease of a proarrhythmic electrophysiological substrate, or secondary to the beneficial effects of SGLT2is on structural remodelling, remains to be determined in future studies. In this respect, Curtain et al. show that the observed reduction in composite outcome was not observed in patients with ICDs, suggesting that the SGLT2is may directly influence the synchronicity of cardiac excitation. However, it may be the case that patients with ICDs are at a later stage of HF progression and are therefore unreceptive to the potential benefits afforded by SGLT2i pharmacotherapy.
There are several limitations to this study, namely the analysis was performed post-hoc and not pre-specified in the original DAPA-HF trial design, and logging of the adverse events was investigator led rather than adjudicated. However, the authors speculate that investigator-led reporting may actually result in an under-reporting of the adverse events in the composite outcome. Furthermore, the beneficial effects of the SGLT2is on CV outcomes are a class effect and have been observed in all the different SGLT2is tested to date. Curtain et al. analysed data from the DAPA-HF trial where only dapagliflozin was evaluated. Therefore, additional analysis of the datasets from other trials, such as the EMPEROR-Reduced CV safety trial data,7 is warranted and will help determine if the effects reported by Curtain et al. are specific to dapagliflozin or a generalized drug class effect.
To date there have been no reports of a direct antiarrhythmic effect of SGLT2is in humans, although a recent meta-analysis of data from 22 trials does indicate a reduction in cardiac arrhythmias in the SGLT2i treatment groups.16 Results from the study by Curtain et al. now provide the initial direct evidence that the SGLT2is may reduce the incidence of ventricular arrhythmias in patients with HFrEF.
In conclusion, the results presented by Curtain et al. advance our knowledge of this important class of drug and should be considered as proof of principle and hypothesis generating. Given the compelling evidence that has recently emerged—from clinical trials to cellular mechanisms, it seems that new trials and pre-clinical studies designed to specifically interrogate the antiarrhythmic efficacy of the SGLT2is will be key avenues to pursue in the near future. These are indeed exciting times for SGLT2i research.
Funding
This work was supported by project grants from the Canadian Institutes of Health Research, the Dr Rod Eidem Diabetes Research Fund, and the Dr Charles A. Allard Chair in Diabetes Research. P.E.L. holds a provisional US patent for the development of SGLT2i derivatives for the specific treatment of HF and associated co-morbities.
Conflict of interest: none declared.
The opinions expressed in this article are not necessarily those of the Editors of the European Heart Journal or of the European Society of Cardiology.
References
- 1.Hsia DS, Grove O, Cefalu WT.. An update on sodium–glucose co-transporter-2 inhibitors for the treatment of diabetes mellitus. Curr Opin Endocrinol Diabetes Obes 2016; 24:73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fitchett D, Inzucchi SE, Cannon CP, McGuire DK, Scirica BM, Johansen OE, Sambevski S, Kaspers S, Pfarr E, George JT, Zinman B.. Empagliflozin reduced mortality and hospitalization for heart failure across the spectrum of cardiovascular risk in the EMPA-REG OUTCOME trial. Circulation 2019;139:1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kosiborod M, Cavender MA, Fu AZ, Wilding JP, Khunti K, Holl RW, Norhammer A, Birkeland K, Jorgensen ME, Thuresson M, Arya N, Bodegård J, Hammar N, Fenici P; CVD-REAL Investigators and Study Group. The CVD-REAL Study (Comparative Effectiveness of Cardiovascular Outcomes in New Users of Sodium–Glucose Cotransporter-2 Inhibitors). Lower risk of heart failure and death in patients initiated on sodium–glucose cotransporter-2 inhibitors versus other glucose-lowering drugs. Circulation 2017;136:249–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rådholm K, Figtree G, Perkovic V, Solomon SD, Mahaffey KW, de Zeeuw D, Fulcher G, Barret TD, Shaw W, Desai M, Matthews DR, Neal B.. Canagliflozin and heart failure in type 2 diabetes mellitus. Circulation 2018;138:458–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kato ET, Silverman MG, Mosenzon O, Zelniker TA, Cahn A, Furtado RHM, Kuder J, Murphy SA, Bhatt DL, Leiter LA, McGuire DK, Wilding JPH, Bonaca MP, Ruff CT, Desai AS, Goto S, Johansson PA, Gause-Nilsson I, Johanson P, Langkilde AM, Raz I, Sabatine MS, Wiviott SD.. Effect of dapagliflozin on heart failure and mortality in type 2 diabetes mellitus. Circulation 2019;139:2528–2536. [DOI] [PubMed] [Google Scholar]
- 6.Lytvyn Y, Bjornstad P, Udell JA, Lovshin JA, Cherney DZI.. Sodium glucose cotransporter-2 inhibition in heart failure. Circulation 2017;136:1643–1658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Packer M, Anker SD, Butler J, Filippatos G, Pocock SJ, Carson P, Januzzi J, Verma S, Tsutsui H, Brueckmann M, Jamal W, Kimura K, Schnee J, Zeller C, Cotton D, Bocchi E, Böhm M, Choi DJ, Chopra V, Chuquiure E, Giannetti N, Janssens S, Zhang J, Gonzalez Juanatey JR, Kaul S, Brunner-La Rocca HP, Merkely B, Nicholls SJ, Perrone S, Pina I, Ponikowski P, Sattar N, Senni M, Seronde MF, Spinar J, Squire I, Taddei S, Wanner C, Zannad F; EMPEROR-Reduced Trial Investigators. Cardiovascular and renal outcomes with empagliflozin in heart failure. N Engl J Med 2020;383:1413–1424. [DOI] [PubMed] [Google Scholar]
- 8.McMurray JJV, Solomon SD, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Anada IS, Belohlavek J, Böhm M, Chiang CE, Chopra VK, de Boer RA, Desai AS, Diez M, Drozdz J, Dukát A, Ge J, Howlett JG, Katova T, Kitakaze M, Ljungman CEA, Merkely B, Nicolau JC, O’Meara E, Petrie MC, Vinh PN, Schou M, Tereshchenko S, Verma S, Held C, DeMets DL, Docherty KF, Jhund PS, Bengtsson O, Sjöstrand M, Langkilde AM; DAPA-HF Trial Committees and Investigators. Dapagliflozin in patients with heart failure and reduced ejection fraction. N Engl J Med 2019;381:1995–2008. [DOI] [PubMed] [Google Scholar]
- 9.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofsatd AP, Pfarr E, Jamal W, Packer M.. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet 2020;396:819–829. [DOI] [PubMed] [Google Scholar]
- 10.Curtain JP, Docherty KF, Jhund PS, Petrie MC, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, Sabatine MS, Bengtsson O, Langkilde AM, Sjöstrand M, Solomon SD, McMurray JNV. Effect of dapagliflozin on ventricular arrhythmias, resuscitated cardiac arrest, or sudden death in DAPA-HF. Eur Heart J 2021;doi:10.1093/eurheartj/ehab560. [DOI] [PMC free article] [PubMed]
- 11.Byrne NJ, Matsumura N, Maayah ZH, Ferdaoussi M, Takahara S, Darwesh AM, Levasseur JL, Jhang JWS, Vos D, Parajuli N, El-Kadi AOS, Braam B, Young ME, Verma S, Light PE, Sweeney G, Seubert JM, Dyck JRB.. Empagliflozin blunts worsening cardiac dysfunction associated with reduced NLRP3 (nucleotide-binding domain-like receptor protein 3) inflammasome activation in heart failure. Circ Hear Fail 2020;13:e006277. [DOI] [PubMed] [Google Scholar]
- 12.Philippaert K, Kalyaanamoorthy S, Fatehi M, Long W, Soni S, Byrne NJ, Barr A, Singh J, Wong J, Palechuk T, Schneider C, Darwesh AM, Maayah ZH, Seubert JM, Barakat K, Dyck JRB, Light PE.. Cardiac late sodium channel current is a molecular target for the sodium/glucose cotransporter 2 inhibitor empagliflozin. Circulation 2021;143:2188–2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uthman L, Baartscheer A, Bleijlevens B, Schumacher CA, Fiolet JWT, Koeman A, Jancev M, Hollman MW, Weber NC, Coronel R, Zuurbier CJ.. Class effects of SGLT2 inhibitors in mouse cardiomyocytes and hearts: inhibition of Na+/H+ exchanger, lowering of cytosolic Na+ and vasodilation. Diabetologia 2018;61:722–726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peana D, Domeier TL.. Cardiomyocyte Ca2+ homeostasis as a therapeutic target in heart failure with reduced and preserved ejection fraction. Curr Opin Pharmacol 2017;33:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Makielski JC.Late sodium current: a mechanism for angina, heart failure, and arrhythmia. Trends Cardiovasc Med 2016;26:115–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li HL, Lip GH, Feng Q, Fei Y, Tse YK, Wu MZ, Ren QW, Tse HF, Cheung BY, Yiu KH.. Sodium–glucose cotransporter 2 inhibitors (SGLT2i) and cardiac arrhythmias: a systematic review and meta-analysis. Cardiovasc Diabetol 2021;20:100. [DOI] [PMC free article] [PubMed] [Google Scholar]