Sir,
Infections due to multidrug-resistant bacteria pose an increasing therapeutic challenge, often requiring the use of newly introduced antibiotics. A further difficulty is now represented by the overwhelming clinical and epidemiological challenge of COVID-19 (coronavirus disease 2019). With regard to infections caused by Gram-negative bacteria, new potentially active drugs (e.g. cefiderocol, ceftolozane/tazobactam, ceftazidime/avibactam and meropenem/vaborbactam) have enriched the antimicrobial armamentarium, but some concerns remain about the treatment of infections caused by isolates having specific resistance mechanisms such as metallo-β-lactamase production, porin loss and increased efflux pump activity. In the case of Pseudomonas aeruginosa, all of these resistance mechanisms may be expressed, even simultaneously, thus resulting in difficult-to-treat infections.
Here we report the case of a 64-year-old man suffering from a chronic left pleural empyema caused by a carbapenem-resistant P. aeruginosa [imipenem and meropenem minimum inhibitory concentrations (MICs) >8 mg/L] following pleuropneumonectomy surgery carried out in June 2019 after which a mesothelioma was diagnosed. In this context, thoracostomy drainage was maintained in place for a long period to allow pleural discharge.
In March 2020, the patient noticed an increase in discharge from the pleural drainage. At this time, carbapenem-resistant P. aeruginosa was still detected from the purulent fluid, associated with low-grade fever. The isolate was also resistant to all β-lactams and quinolones but was susceptible to ceftolozane/tazobactam (MIC = 4 mg/L), gentamicin (MIC = 4 mg/L), amikacin (MIC = 8 mg/L) and colistin (MIC = 2 mg/L).
The patient was then admitted to the emergency department of A. Manzoni Hospital (Lecco, Italy). On admission, a nasopharyngeal swab was performed according to local guidelines implemented during the first phase of the COVID-19 pandemic. Real-time RT-PCR (ELITe InGenius® Platform; ELITechGroup) revealed the presence of SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) RNA. In addition, a computed tomography (CT) scan of the thorax was suggestive of interstitial pneumonia (Supplementary Fig. S1), thus requiring a specific treatment for COVID-19 including hydroxychloroquine 200 mg twice daily and low-molecular-weight heparin at a prophylactic dosage.
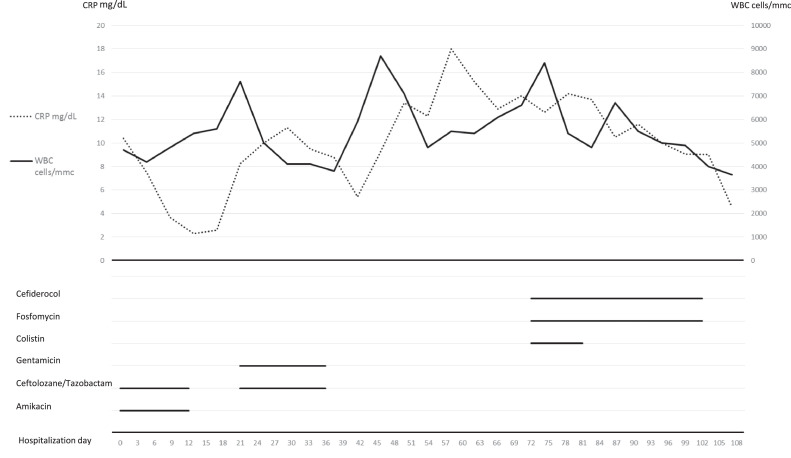
Based on previous microbiological results, antimicrobial treatment with ceftolozane/tazobactam 3 g three times daily (t.i.d.) and amikacin 1 g once daily (q.d.) was started. This treatment was then changed to ceftolozane/tazobactam 3 g t.i.d. and gentamicin 320 mg q.d., but without a significant resolution of fever. Unfortunately, shortly after administration of these antibiotics, a P. aeruginosa isolate also resistant to ceftolozane/tazobactam (MIC > 32 mg/L) was isolated from the drainage. Of note, the investigational antibiotic cefiderocol showed high activity (MIC = 0.5 mg/L). Bacterial identification was performed using matrix-assisted laser desorption/ionisation time-of-flight mass spectrometry (MALDI-TOF/MS) (VITEK® MS; bioMérieux, Marcy-l’Étoile, France). Antimicrobial susceptibility was determined by broth microdilution using Sensititre panels (DKMGN and CMP1SHIH; Thermo Fisher Scientific). Since fever and inflammatory parameters worsened, pleural washing with urokinase 200 000 IU was applied daily. Surgical debridement of the empyema was suggested but only if associated with a concomitant active antibiotic treatment and a negative nasopharyngeal swab for SARS-CoV-2. The latter condition was achieved 45 days from admission. The surgical debridement procedure was then scheduled and cefiderocol was administered as compassionate use at a dosage of 3 g t.i.d. in association with colistin 3 MU t.i.d. (after a loading dose of 9 MU), and fosfomycin 4 g four times a day. A decrease in inflammatory biomarkers and body temperature was promptly observed in the following days. A week after the introduction of the novel antibiotic therapy, the patient underwent a new intervention of thoracostomy repackaging with surgical debridement of the empyema at the Surgery Department of Spedali Civili Hospital (Brescia, Italy), where the previous procedures had been performed. Cultures from the pleural material obtained during the surgical procedure were negative. Thus, the thoracostomy was closed and colistin was stopped. Cefiderocol and fosfomycin were interrupted after 3 weeks from the surgical procedure. One month after discharge the patient did not show any adverse event or relapse of the pleural infection or mesothelioma. Clinical and laboratory data are summarised in Fig. 1 .
Fig. 1.
Trends in C-reactive protein (CRP) and white blood cell (WBC) levels and antibiotic treatments during the patient's hospital stay.
Cefiderocol is a novel parental siderophore cephalosporin with high stability against hydrolysis by all carbapenemases and a peculiar entry into bacterial cells through active iron transporters, independently of porin channels and efflux pumps [1]. Some clinical trials have shown that cefiderocol may be effective against severe and difficult-to-treat infections. A few case reports have previously described the successful use of cefiderocol for treating P. aeruginosa infections in different settings such as osteomyelitis [2] and surgical site infections [3], but no conclusive results are available regarding some questions. For instance, it remains largely unclear whether cefiderocol should be used alone or in combination with other antimicrobials [4].
Empyema has been described as a possible sanctuary for resistant bacteria, since antibiotics have been proven to reach the site of infection at subtherapeutic concentrations, thus increasing the risk of resistance emergence [5]. Our experience shows that a combined antibiotic treatment including cefiderocol may be effective for eradicating infection in this setting even though the role of a dedicated surgical procedure remains crucial for treating pleural empyema. It is also noted that in our case prolonged cefiderocol treatment (4 weeks) was not associated with the presence of significant adverse events.
Funding
None.
Ethical approval
Written consent was obtained from the patient for all off-label treatments carried out during the stay. For the compassionate use of cefiderocol, the approval of the Hospital Ethics Committee was also received.
Declaration of Competing Interest
None declared.
Editor: Dr Michele Bartoletti
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jgar.2021.09.005.
Appendix. Supplementary materials
References
- 1.Bonomo RA. Cefiderocol: a novel siderophore cephalosporin defeating carbapenem-resistant pathogens. Clin Infect Dis. 2019;69(Suppl 7):S519–S520. doi: 10.1093/cid/ciz823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alamarat ZI, Babic J, Tran TT, Wootton SH, Dinh AQ, Miller WR, et al. Long-term compassionate use of cefiderocol to treat chronic osteomyelitis caused by extensively drug-resistant Pseudomonas aeruginosa and extended-spectrum-β-lactamase-producing Klebsiella pneumoniae in a pediatric patient. Antimicrob Agents Chemother. 2020;64:e01872–e01919. doi: 10.1128/AAC.01872-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bavaro DF, Romanelli F, Stolfa S, Belati A, Diella L, Ronga L, et al. Recurrent neurosurgical site infection by extensively drug-resistant P. aeruginosa treated with cefiderocol: a case report and literature review. Infect Dis (Lond) 2021;53:206–211. doi: 10.1080/23744235.2020.1856921. [DOI] [PubMed] [Google Scholar]
- 4.Giacobbe DR, Ciacco E, Girmenia C, Pea F, Rossolini GM, Sotgiu G, et al. Evaluating cefiderocol in the treatment of multidrug-resistant Gram-negative bacilli: a review of the emerging data. Infect Drug Resist. 2020;13:4697–4711. doi: 10.2147/IDR.S205309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kufel WD, Steele JM, Riddell SW, Jones Z, Shakeraneh P, Endy TP. Cefiderocol for treatment of an empyema due to extensively drug-resistant Pseudomonas aeruginosa: clinical observations and susceptibility testing considerations. IDCases. 2020;21:e00863. doi: 10.1016/j.idcr.2020.e00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.