Abstract
RMBD (acronym of Raw Meat Based Diet) and BARF diets (acronym for Biologically Appropriate Raw Food or Bones and Raw Food) account dietary regimens based on raw ingredients (including raw meat), popular in pet feeding. Animal tissues and organs as well as other uncooked ingredients are more and more popularly used by pet owners to feed household pets. However, the increased risk of exposure to microbiological and parasitic agents poses the question as to whether such diets may be recommendable to be handled and offered to domestic cats and dogs co-living in domestic and urban environment. Above all, the threat of human and animal infections by parasites from raw meat fed to pets is not sufficiently explored and tracked, meanwhile deserving particular surveillance, instead. At this regard, raw meat feeding to pets may represent a route of exposure to the increased risk of environmental load. In fact, some parasites typically found in rural environment can be given the chance to complete their life-cycle, for the closeness between definitive and intermediate hosts. This is of particular concern, as potentially infected pets serving as definitive hosts can become a continuous source of environmental diffusion of parasites, both at domestic and urban level. The handling of raw meat requires adequate knowledge and awareness of the hygienic principles to prevent the onset of disorders related to both manipulation by pet owners and uncooked food consumption by the pet. This review aimed to shed a comprehensive overview of the hygienic aspects related to raw pet feeding, as to handling of raw meat in domestic environment, with special emphasis on parasitic agents and related zoonotic hazards.
Keywords: BARF, Diet, Parasites, Zoonosis
1. Introduction
In recent years, the trend of feeding raw meat based diets (RMBD) to domestic cats and dogs has significantly grown among pet keepers [1]. Approximately, 60% of pet owners feed their cats and dogs completely or partially raw meat based diet, and this practice is popular in several European countries [2]. RMBDs consist of raw ingredients such as organs, muscle tissues and bones of slaughtered animals which may be prepared and offered as home made diets, or purchased, either refrigerated, frozen or dried from the market likely complemented by cooked carbohydrate premix. These diets are also referred as BARF (Biologically Appropriate Raw Food or Bones and Raw Food) when feeding regimen is completely based on raw ingredients including carbohydrate part [3]. It is a common belief that raw diet is a natural and healthy way to advance pet health, because respectful of ancestral feeding habits. However, such opinions are not supported by scientific evidence of beneficial effects on pet health, most of times empirically supported by owner persuasion [4,5]. Anecdotes circulating on the web support the benefits of raw feeding, including the boost of immune system with maximization of general health and body conditions, accompanied by behavioural improvements [3,6]. However, plausible evidence indicated nutritional imbalances, as well as metabolic and gastrointestinal dysfunction in some cases, as a result of unbalanced and “self-prepared BARF diets [7].
The European legislation on pet food safety is the same for feeds destined to livestock. In particular, the legislation in force concerning animal by products (ABP) accounts Regulations of the (EC) no. 1069/2009 [8] and no. 142/2011 [9]. Health rules on the use of the different categories of ABP are a reference point for the use of ingredients of animal origin for pet food production. Raw pet food suppliers are subjected principally to abide by regulation (EC) No. 1069/2009 governing animal by-products and derivatives, which are fit but not intended for human consumption. The type of byproducts allowed in pet food is regulated in “Category 3” raw materials. This also include human food containing animal byproducts which are safe and hygienic but removed from human food chain owing to certain commercial reasons [10]. In addition, the regulation also entails those by products which are declared unfit for human consumption but permitted for use in pet diets, provided that animal was slaughtered at abattoir and approved fit for human consumption during ante mortem examination and didn't manifest any evidence of communicable diseases during post mortem inspection [1,8].
Along with this, the publication of Regulation of the European Union 2017/625 also serves to secure feed hygiene with limitation and prevention of risk to animals and humans, ruling the conduction of official controls and adoption of a series of measures across all stages of the production chain [11].
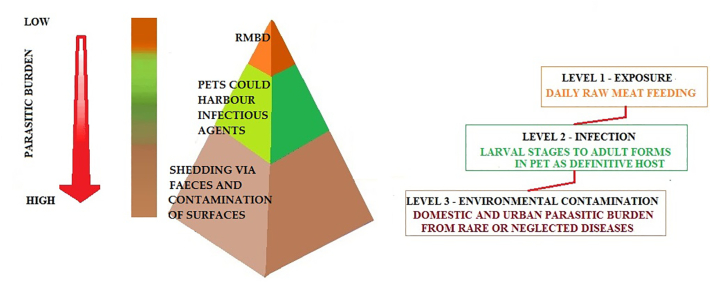
However, feeding raw ingredients to household cats and dogs may pose some concerns as to the safe handling and use at home. Investigations on hygiene of raw diets have highlighted how the occurrence of positive testing to various zoonotic bacterial pathogens, such as Escherichia coli, Clostridium spp., Salmonella spp., Listeria spp. and Campylobacter spp., could pose a concrete risk to pets and to the people handling raw meat products together with the challenge of fecal shedding by infected pets [[12], [13], [14]]. In addition, pets may also get infected after eating raw fish, with the potential presence of diverse types of parasites such as Anisakis simplex in cats [15], Dioctophyma renale (giant kidney worm), Diphyllobothrium latum (fish tapeworm), Opisthorchis tenuicollis (trematodes of the small intestine, bile duct and pancreatic ducts) and salmon infection to dogs by Nanophyetus salmincola [14]. Should those infections occur, a health issue for cats and dogs must be faced, but the parasitic load in the domestic environment is not expected to be of concern, as infection requires direct consumption or manipulations of infected raw fish. Very recently (2020), the outbreak of feline tuberculosis in England was possibly linked to BARF feeding and this fact supports the debate on a series of concerns about the safety of such feeding practice [16]. It is reasonable to consider that RMBD diets are often adopted without adequate awareness about hygienic aspects. A number of potentially dangerous parasites, bacteria and viruses (Table 1) could represent a serious threat for animal and human health when raw feeding is adopted [1,3,12,13]. The exposure to such pathogens is multifaceted, due to infected pets which can transmit pathogens to their owners by direct contact, or through contamination of surfaces at home (Fig. 2) [3].
Table 1.
Non exhaustive list of pathogens potentially present in raw meat, posing risk of infection. Among parasites, only those presenting potential zoonotic hazard are reported.
| Pathogens in raw meat posing health risk | ||
|---|---|---|
| Parasites [1,3,12,13] | Bacteria [1,3,12,13] | Viruses [1] |
|
Toxoplasma gondii Neosporum caninum Sarcocystis spp. Cryptosporidium parvum Echinococcus granulosus |
Staphylococcus spp. Enterococcus spp. Clostridia spp. Listeria spp. Brucella spp. Campylobacter jejuni Salmonella spp. Escherichia coli |
Rabies (Lysavirus) Feline and canine Calicivirus African Horse Sickness (Orbivirus) Hepatitis E (Hepatitis E Virus) |
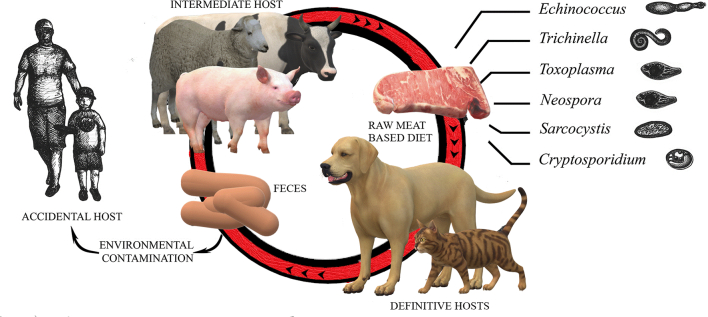
Fig. 1.
Zoonotic agents potentially present in the raw meat of intermediate hosts, fed to domestic cats and dogs.
The potential hazard of parasitic infections in pet animals is based for some parasites upon the ingestion of raw meat. Cats and dogs can act as definitive hosts (harboring the adult stage of the parasites which shed eggs via the faeces, thus leading to environmental burden) and livestock as intermediate hosts, carrying cysts in their meat (Fig. 1) [1,3,12,13] and enhancing the chance to make the human an occasional host. This scenario is of great significance in view of the one health principle which considers animal, human and environment as a whole, in a reciprocal health cycle. Though some of those parasites are very rare thanks to veterinary surveillance and inspection throughout the food chain (i.e., trichinellosis, which however may find in game meat a potential route of infection) it appears reasonable to improve the awareness of consumers/owner on the risk associated with reintroducing such rare diseases due to the increased risk of exposure of their household pets via RMBDs.
Fig. 2.
Scheme of potential magnification of parasitic burden in case of consumption of contaminated raw meat based diet by domestic cats and dogs harboring different infectious agents.
Information about the risk of parasites to either pets or owners as a result of raw feeding is scanty to date, whereas microbiological investigations have pointed to several risks from the manipulation of raw meat, above all as to some bacterial agents like Salmonella spp., E. coli and Campylobacter jejuni [13,14,17]. It appears of utmost importance for pet owners to acquire knowledge, in terms of safe handling of raw meat, meanwhile being aware of the possibility of exposure to and from environmental contamination through pet raw feeding. The control of parasitic hazards associated with meat safety is of major economic significance. For those reasons, a series of potential parasites, as infectious agents from raw meat consumption by cats and dogs, is reported according to their life cycle along with the main potential zoonotic hazards brought and potentially diffused in domestic or urban environment.
2. Life cycle of some parasites from raw meat feeding involving pets and humans
Safety issues related to raw meat based diets are of utmost importance as untreated meat products pose a risk of infection to pets as well as to humans. Several notable parasites may be present in organs and muscular tissues of carcasses, including protozoans, cestodes, trematodes and nematodes. If no thermal treatments are adopted, raw meat can harbor larval stages of parasites which may find in cat and dogs the definitive hosts, to complete the life cycle (adult stage parasite) (Table 2). Occasionally, if livestock is missing, humans can serve as intermediate host and develop the disease, presumably coming into contact with accidentally contaminated domestic environment.
Table 2.
Parasites in raw meat based diets with their potential intermediate and final hosts along with summary of literature reported.
| Parasite | Intermediate hosts | Definitive hosts | Sampling details | Literature available |
|---|---|---|---|---|
| Echinococcus granulosus | Domestic ungulates | Mostly dogs sometimes cats | N/A | N/A |
| Sarcocystis spp | Domestic ungulates | Dogs | 35 Commercial frozen RMBD's | S. cruzi in 11% of diets based on bovine meat and S.tenella in 11% of diets based on bovine or sheep meat [13] |
| Toxoplasma gondii | Many mammals and birds | Domestic Cats | Frozen commercial BARF diet | 6% samples positive for T. gondii [13] |
| Neospora caninum | Domestic ungulates as well as dogs | Dogs | Fresh raw meat | 6 (37.5%) of the 16 seropositive (16 on 218 samples; 7.33%) bitches positive of N. caninum [55] |
| Cryptosporidium spp. | Vertebrate host (dogs, cats, humans and livestock) on consumption of contaminated food and water or contact with infected animal. | – | Commercial BARF diets containing raw bovine meat or raw turkey meat in canned food | 2.11% of samples tested positive to Cryptosporidium spp. [65] |
| Trichinella. Spp | Two generations in the single vertebrate host (humans, pigs, horses acting both as definitive and potential intermediate host | – | N/A | N/A |
N/A = Not Available.
(−) = No definitive hosts.
2.1. Cestodes
2.1.1. Echinococcus granulosus
Cystic echinococcosis (CE) or hydatidosis, is a fatal cosmopolitan neglected zoonosis [18] with overwhelming health significance due to high prevalence, morbidity and mortality in humans and livestock [19]. Hydatidosis, is typically through the larval stage of canids tapeworm Echinococcus granulosus sensu lato, a species complex that has many genotypes and cryptic species [20]. The tapeworm involves synanthropic cycle with canids. In particular, the dog (definitive host) harbors the adult stage in the intestine, for this reason capable to shed parasite eggs via the faeces. Intermediate hosts, such as domestic ungulates, accidently grab infection from the environment and develop larval cysts in internal organs [21]. The lifecycle of echinococcosis, biologically perpetuate when dogs are fed on raw organs of infected domestic ungulates and dog remain asymptomatic but contain residual worm burden [22]. Epidemiological evidence, verify that humans are dead-end intermediate hosts and acquire infection through inadvertent consumption of eggs shed by the dogs or by physical contact with them [23].CE constitutes a rising public health threat, with average annual incidence in humans estimated to be as high as 7.74 cases/100,000 population in different countries, around the world [24]. High CE infection in humans is circumstantially linked to environmental dissemination of eggs on various matrices and surfaces by infected dogs [25]. To date, there are no specific research studies which detected the presence of CE in purchased RMBD's.
2.2. Protozoans
2.2.1. Sarcocystis spp.
Sarcocystis spp. is an intracellular apicomplexan protozoan, infectious to a wide range of vertebrates, including some species zoonotic to humans [26]. Sarcocystis spp., lifecycle is characterized by obligatory predator-prey interplay involving sexual and asexual multiplication in host species. In striated muscle of herbivores and omnivores it replicate asexually behaving as intermediate hosts; sexual proliferation occurs in the intestinal epithelium of cervids as definitive hosts, leading to expulsion of oocysts with faeces in the environment [27]. Frequently, domestic dogs are infected by ingesting infected muscle tissue of various intermediate hosts [28] and intermediate hosts are horizontally infected by ingesting feed and water contaminated by sporocysts, shed from the faeces of definitive hosts [29]. Most Sarcocystis, species infect distinct hosts or closely linked host species [30] and humans act as both intermediate and definitive hosts in many Sarcocystis species [31]. Sheep act as intermediate host to four species of Sarcocystis (Sarcocystis gigantea, Sarcocystis medusiformis, Sarcocystis tenella and Sarcocystis arieticanis) with felids and canids as definitive hosts [26]. Among them, S. medusiformis and S. gigantea are non-infectious, disseminated through felids and produces macroscopically visible cysts in tissues [32]. However, S. tenella and S. arieticanis are infectious, spread through canids and produce microscopic cysts [33]. In cattle, six species of Sarcocystis act as intermediate hosts, with canids (Sarcocystis cruzi), felids (Sarcocystis hirsute and Sarcocystis bovifelis, Sarcocystis bovini) and humans (Sarcocystis heydorni and Sarcocystis hominis) as definitive hosts [28,34]. The prevalence of humans Sarcocystis infection has been estimated to be 10.4% in Europe, between 0.4% to 23.2% in Asia and 0.5% in Australia [35]. A study, on 35 commercial frozen RMBD's found out S. cruzi in 4 products (11%), and S. tenella in another 4 products (11%) based on bovine or sheep meat [13].
2.2.2. Toxoplasma gondii
Toxoplasma gondii is a zoonotic apicomplexan cyst forming protozoan, known to infect all warm blooded vertebrates, and a major concern of public health worldwide [36]. The disease involve felids as only definitive host and vast range of vertebrates as intermediate hosts [37]. Domestic cats however contribute to greatest source of environmental disease burden and may shed billion of oocysts, after consuming only one cyst from infected meat [38]. Acquisition of infection in humans is by ingestion of raw meat from infected animals or by ingestion of sporulated oocysts from contaminated environment or direct contact with infected cats [39,40]. Cats after getting infected start oocyst shedding, typically for a period of 1–2 weeks in primary infections [41] and re-shedding reactivate in super infection with other feline diseases, malnourishment and during the immunosuppression [42]. The global burden of human toxoplasmosis is remarkably high and about 60% people of population approximately one to two billion population are reported to be infected with illness [43,44]. There is established evidence proving that naive cats fed with RMBD diets have higher seropositivity rates and shed a large number of oocysts of T. gondii in their faeces [3] and a study in Netherland, found out 2 products (6%) out of 35 commercial frozen RMBD's samples positive for T. gondii [13].
2.2.3. Neospora caninum
Neospora caninum is an obligate apicomplexan parasite, with broad host spectrum [45] predominantly, emerged as a serious disease in dog and cattle [46]. The lifecycle of N. caninum is facultative, heteroxenous and superimposable from T. gondii [47]. Dogs are its definitive hosts as well as intermediate hosts. Infection can occur through oral uptake of ruminant infected raw meat, while asexual proliferation occurs in intermediate hosts infected horizontally via ingestion of oocysts from contaminated food or drinking water [48]. The unsporulated oocyst, shed via the faeces of dogs, are highly tenacious and play a significant role in contamination of environment and maintenance of infection [49]. Because of its close relationship with T. gondii, it is considered that the resistance of N. caninum oocysts in environment is analogous to that of T. gondii oocysts [50]. The zoonotic potential of the disease is still unknown, however antibodies against N. caninum in humans are reported [48,51]. In Europe, prevalence of N. caninum in dogs ranges from 0.5% in Sweden [52] to as high as 15.3% in Denmark [53] and worldwide aggregated prevalence is 17.14% in dogs is estimated [54]. A research study, confirmed 6 (37.5%) of the 16 seropositive (16 on 218 samples; 7.33%) bitches were fed raw diets [55].
2.2.4. Cryptosporidium spp.
The protozoan Cryptosporidium are obligate, intracellular protozoan of veterinary and public health significance that infects, dogs, cats, humans and livestock [56] causing mild to severe gastrointestinal symptoms [57]. The transmission dynamics of Cryptosporidium is directly related to consumption of contaminated food and water, containing oocysts by single host [58] resulting in release of Cryptosporidium resistant oocyst with faeces in the environment [59]. The situation is of greater significance in case of infested pet, on account of closer association with household members and absence of personal and community safety guidelines constituting a zoonotic risk [60,61]. Molecular studies confirmed about 50 genotypes of Cryptosporidium few with broader host range such as zoonotic Cryptosporidium parvum and some highly host specific such as Cryptosporidium felis in cats and Cryptosporidium canis in dogs [62]. Dogs are regarded as one of potential reservoirs for transmitting the Cryptosporidium infection to humans [63]. The reports of the prevalence of Cryptosporidium in dogs are reported to be as high as 53% in some parts of the world [64]. In humans, 14% aggregated prevalence of Cryptosporidium infection is reported in HIV patients, and prevalence of 19.7% is reported in developing countries [63]. A study on commercial BARF diets in USA, molecularly confirmed, 2.11% of samples tested positive to Cryptosporidium spp. that had raw bovine and turkey meat as a integral component in canned diets [65].
2.3. Nematodes
2.3.1. Trichinella spp.
Trichinella spp. is a cosmopolitan food-borne parasite and a zoonotic nematode, of public health concern worldwide [66]. The lifecycle of the genus Trichinella is peculiar among all nematodes due to diverse host spectrum, lifecycles with development of two generations in the single vertebrate host, acting both as definitive and potential intermediate host [67]. In human, the occurrence of trichinellosis is reported from consumption of undercooked meat from domestic pigs, horses and wild boars [68]. Trichinella larvae, can survive in decomposed carcasses for a long time and act similar to the animals spreading larvae or eggs of nematodes [69]. Dogs and other carnivores are important reservoirs of a number of Trichinella species, such as T. britovi, T. spiralis, T. nelsoni,T. pseudospiralis and Trichinella spp. T9 [70]. Household dogs are found to be frequently infected with Trichinella spp. in many parts of the world due to their scavenging behavior [71]. There are studies verifying Trichinellosis in cats fed on infected pork scraps, during food preparation at home or during slaughtering [72]. The main risk for humans is either by consuming undercooked meat from infected animals or horizontally by cutting boards, knives and other utensils used for handling contaminated raw meat at home and afterwards employed in raw food dishes preparation, such as salads [73]. Human trichinellosis, estimated to affect a population of 11 million in 55 countries with clinical cases of about 10,000 reported each year [74]. The infection rate dropped significantly worldwide after the imposition of sanitary regulations on feeding lots of domestic pigs with veterinary control and prohibition of feeding animal waste to animals [75].
3. Methods to reduce parasite burden in meat
Different methods are available to inactivate parasites such as cooking, freezing, curing, and traditionally applied food-processing techniques, as well as high-pressure treatment and irradiation. Table 3 provides an overview of different treatments available to inactivate parasites in raw meat.
Table 3.
Summary of methods available for inactivation of parasites in meat.
| Parasite | Heating | Freezing | High pressure processing (HPP) |
Gama irradiation | Other non-thermal methods Salting, curing etc. |
|---|---|---|---|---|---|
| Sarcocysts spp. | 65 °C; 20-25 min (thigh muscles) [82,85] Or Min 70 °C for 15 min [83] |
−4 °C; 2 days [82,85] −20 °C; 1 day [83] −4 °C for 2 days [83] |
N/A | N/A | N/A |
| Echinococcus granulosus | N/A | −18 °C; 6–9 h [87] | N/A | N/A | N/A |
| Cryptosporidium spp. | ≥70 °C and above; ≥ 10 s [84] |
−20 °C for 1 h [84] |
550 MPa; ≥3 min [93] |
1–2 kGy [93] | N/A |
|
Trichinella spp Muscle Larvae |
71.1 °C (core temperature) [85] | −21 °C; 7 days [86] | >500 MPa [95] |
0.3–0.6 kGy [99] | ≥1.3% NaCl; pH 5.2; [91] |
|
Toxoplasma gondii Tissue cysts |
>61 °C; 3.6 min [80] |
−20 °C; 3 days [80] |
400 MPa; 30 s [94] |
T. gondii 0.4 to 0.7 kGy [100] | 4.2–6.2% NaCl; 64 h [89] |
N/A = Not Available.
3.1. Thermal methods
Thermal treatment is considered as robust method for the control of parasite in meat. However its efficacy depends on parasite species, developmental stage, as well as temperature and time combinations [76]. In general, it is believed that cooking at core temperature of 60–75 °C for 15–30 min and freezing at −21 °C for 1–7 days kills most of parasites in food of animal origin [77]. Among protozoans, T. gondii is an intensively studied parasite with its freezing inactivating temperature for meat ranges between −12 to −25 °C, for a variable period time (2–35 days) [78,79]. Yet, freezing at −20 °C for three days is a requisite to neutralize T. gondii in meat tissues [80]. T. gondii is vulnerable to cooking, consequently temperature range of 60–70 °C is adequate to kill T. gondii cysts in meat, provided that heat is evenly distributed in tissues [80]. Furthermore, high temperature can kill both sporulated and unsporulated T. gondii oocysts [81].
As to Sarcocystis spp., experiments reveal conflicting results. A study reported cooking buffalo meat to 65 °C and freezing at −4 °C inactivates sporocysts [82]. Another study suggested that pork containing Sarcocystis spp. requires cooking at a minimum of 70 °C for 15 min or freezing at −4 °C for 2 days or − 20 °C for 1 day [83].
In regard to Cryptosporidium spp., little evidence is available pertaining inactivation by thermal treatment. However, a single evidence in the literature reports that freezing at −20 °C for one hour or by cooking at 70 °C for one minute inactivate oocysts [84].
On the other hand, for Trichinella spp. cooking at core temperature from 70 °C to 71.1 °C inactivates Trichinella in pork meat, game birds and horse meat [85] and freezing at −21 °C for 7 days inactivates Trichinella in wild boar meat [86].
Regarding metazoan parasites, such as E. granulosus, robust guidelines are extremely low. A study demonstrated that freezing at −18 °C for 6–9 h proved to be effective in neutralizing hydatid cysts in meat. Besides that, research also ascertained that infested organs, if frozen at −18 °C for a minimum of 6 h, can potentially sanitize from hydatid cysts [87]. It is pertinent to mention, that standard indications for thermal inactivation of several meat borne parasites is still lacking e.g. N. caninum which requires attention in future for safe handling as well as harmless consumption of meat from domestic pets.
3.2. Non thermal methods
Several other conventional techniques of particular relevance, such as drying, salting curing etc. are also employed to inactivate parasitic transmission at different stages from meat [88]. Inactivation of T. gondii cysts in mutton meat, salted with 4.2–6.2% NaCl, takes at least 64 h [89]. Another study reports that T. gondii cyst are inactivated at a 2.5% NaCl in mice muscle after 24 h [88]. A trial revealed,that Parma ham dry cured for at least 12 months with 5% salt concentration doesn't infect mice when inoculated [90]. For Trichinella spp., curing conditions include >1.3% NaCl combined with fermentation at a low pH of 5.2, which results in the deactivation of a 96% of Trichinella larvae found in muscles within 7–10 days [91]. However, owing to high resistivity of Trichinella to curing and smoking, those are not recommended as single methods [92]. Some other novel approaches are also in operation now a days to eliminate the threat of parasites in meat tissues. Among them, high pressure processing (HPP) and irradiation are of highest interest and significance [77]. High pressure processing, uses liquid medium to compress at constant rate, resulting in the deactivation of parasites. Parasites like C. parvum [93], T. gondii [94] and T. spiralis [95] were observed to be inactivated at a low pressure (110–400 MPa) [96]. Irradiation, is also a spread technique at present, which uses high energy electrons [97] and gamma irradiation [98] for inactivation in tissues. The minimum effected dose (MED) for Trichinella cyst inactivation is 0.3–0.6 kGy [99], C. parvum 1–2 kGy [93] and T. gondii 0.4 to 0.7 kGy [100]. The available literature is very diverse pertaining inactivation methods in meat, calling meticulous attention for standardization to deactivate parasites in meat in future.
4. Safe use of RMBD diet
In light of the literature explored, the practice to feed RMBD diets largely overlooks the potential health-threatening consequences to household pets and owners. The spreading of selected parasites, for which cats and dogs serve as definitive hosts and could get infested by means of potentially positive raw meat, can be amplified notably on daily exposure from raw meat consumption. In fact, the opportunity offered to parasites to complete their life-cycle otherwise highly limited in the case of cooked meat, poses non negligible risks of domestic and urban parasitic burden responsible of diseases considered rare to date because limited to certain areas and condition (rural areas). A One Health approach is of relevance for mitigating and tackling parasitic infections with safeguarding human health and for safe feeding of pets. Responsible care by the pet owner feeding raw meat based diets to pets should therefore be accounted and adequate information should be provided by veterinary practitioners. As a general rule, it is recommended to adopt hygienic measures to limit potential exposure to oral-fecal route and harmful load in the household, alongside with routine coprological examination of pets in order to keep at check any zoonotic parasitic infections [101]. At this regard, it must be also reported that some bacterial pathogens are associated with higher risk of causing infection in animals fed RMDBs (i.e., Salmonella): the same risk, despite lower, exists for pets fed manufactured foods if not correctly handled both in the plants and at home by the owner [102]. To the best of our knowledge, no evidence is reported in the literature regarding the risk of parasitic agents in manufactured pet food. In different Countries, the veterinary and pet food associations (WSAVA, AVMA, AAHA, CVMA, for instance) have clearly taken a position against the use of RMBDs because of the associated microbiological risks. The potential risk of parasitic infections, environmental contamination and zoonosis from handling or feeding client-owned pet with RMBD should be considered as well, in view of the additional evidence provided in this review, involving the need of specific recommendations and public education about the hygienic requirements concerning such feeding practice.
Funding information
This research was funded by PON RI 2014/2020 and INPS- Prof. Antonio Varcasia and Prof. Maria Grazia Cappai, of the University of Sassari, Italy.
Declaration of Competing Interest
The authors declare no conflict of interest.
Contributor Information
Maria Grazia Cappai, Email: mgcappai@uniss.it.
Antonio Varcasia, Email: varcasia@uniss.it.
References
- 1.Davies R.H., Lawes J.R., Wales A.D. J. Small Anim. Pract. 2019 doi: 10.1111/jsap.13000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anturaniemi J., Barrouin-Melo S.M., Zaldivar-López S., Sinkko H., Hielm-Björkman A. Owners’ perception of acquiring infections through raw pet food: a comprehensive internet-based survey. Vet. Rec. 2019 doi: 10.1136/vr.105122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Freeman L.M., Chandler M.L., Hamper B.A., Weeth L.P. Current knowledge about the risks and benefits of raw meat-based diets for dogs and cats. J. Am. Vet. Med. Assoc. 2013 doi: 10.2460/javma.243.11.1549. [DOI] [PubMed] [Google Scholar]
- 4.Morelli G., Bastianello S., Catellani P., Ricci R. Raw meat-based diets for dogs: survey of owners’ motivations, attitudes and practices. BMC Vet. Res. 2019 doi: 10.1186/s12917-019-1824-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schlesinger D.P., Joffe D.J. Raw food diets in companion animals: A critical review. Can. Vet. J. 2011;52(1):50–54. [PMC free article] [PubMed] [Google Scholar]
- 6.Joffe D.J., Schlesinger D.P. Preliminary assessment of the risk of Salmonella infection in dogs fed raw chicken diets. Can. Vet. J. 2002;43(6):441–442. [PMC free article] [PubMed] [Google Scholar]
- 7.Lumbis R., Chan D.L. The raw deal: clarifying the nutritional and public health issues regarding raw meat-based diets. Vet. Nurse. 2015 doi: 10.12968/vetn.2015.6.6.336. [DOI] [Google Scholar]
- 8.Regulation O. Regulation (EC) no 1069/2009 of the European parliament and of the council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) no 1774/2002 animal. Off. J. Eur. Union. 2009;300:33. [Google Scholar]
- 9.O. Regulation, Regulation CE 142/2011 of 25 February (2011) Implementing Regulation (EC) No. 1069/2009 of the European Parliament and of the Council Laying Down Health Rules as Regards Animal By-Products and Derived Products Not Intended for Human Consumption.
- 10.E. Commission Guidelines for the feed use of food no longer intended for human consumption. Off. J. Eur. Union C. 2018;133:2–18. [Google Scholar]
- 11.Menditto A., Anniballi F., Auricchio B., De Medici D., Stacchini P. Regulation (EU) 2017/625 and the ‘Union Agri-Food Chain Legislation. Eur. Food Feed Law Rev. 2017;12(5):406–412. [Google Scholar]
- 12.Strohmeyer R.A., Morley P.S., Hyatt D.R., Dargatz D.A., Scorza A.V., Lappin M.R. Evaluation of bacterial and protozoal contamination of commercially available raw meat diets for dogs. J. Am. Vet. Med. Assoc. 2006 doi: 10.2460/javma.228.4.537. [DOI] [PubMed] [Google Scholar]
- 13.van Bree F.P.J. Zoonotic bacteria and parasites found in raw meat-based diets for cats and dogs. Vet. Rec. 2018;182(2):50. doi: 10.1136/vr.104535. [DOI] [PubMed] [Google Scholar]
- 14.Lejeune J.T., Hancock D.D. Public health concerns associated with feeding raw meat diets to dogs. J. Am. Vet. Med. Assoc. 2001 doi: 10.2460/javma.2001.219.1222. [DOI] [PubMed] [Google Scholar]
- 15.Huh S., Sohn W.M., Chai J.Y. Intestinal parasites of cats purchased in Seoul. Korean J. Parasitol. 1993 doi: 10.3347/kjp.1993.31.4.371. [DOI] [PubMed] [Google Scholar]
- 16.O’Halloran C. Feline tuberculosis caused by Mycobacterium bovis infection of domestic UK cats associated with feeding a commercial raw food diet. Transbound. Emerg. Dis. 2020 doi: 10.1111/tbed.13889. [DOI] [PubMed] [Google Scholar]
- 17.Weese J.S., Rousseau J., Arroyo L. Bacteriological evaluation of commercial canine and feline raw diets. Can. Vet. J. 2005;46(6):513–516. [PMC free article] [PubMed] [Google Scholar]
- 18.Berlinguer F. Help from the sky: Can vultures contribute to Cystic Echinococcosis control in endemic areas? PLoS Negl. Trop. Dis. 2021 doi: 10.1371/journal.pntd.0009615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Otero-Abad B., Torgerson P.R. A systematic review of the epidemiology of echinococcosis in domestic and wild animals. PLoS Negl. Trop. Dis. 2013 doi: 10.1371/journal.pntd.0002249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mehmood N. Genetic diversity and transmission patterns of Echinococcus granulosus sensu stricto among domestic ungulates of Sardinia, Italy. Parasitol. Res. 2021 doi: 10.1007/s00436-021-07186-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varcasia A. Cystic echinococcosis in the endemic island of Sardinia (Italy): has something changed? Parasitol. Res. 2020 doi: 10.1007/s00436-020-06717-0. [DOI] [PubMed] [Google Scholar]
- 22.Bonelli P. Cystic echinococcosis in a domestic cat (Felis catus) in Italy. Parasite. 2018 doi: 10.1051/parasite/2018027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Torgerson P.R. Source attribution of human echinococcosis: A systematic review and meta-analysis. PLoS Negl. Trop. Dis. 2020;14(6) doi: 10.1371/journal.pntd.0008382. e0008382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamarozzi F., Legnardi M., Fittipaldo A., Drigo M., Cassini R. Epidemiological distribution of Echinococcus Granulosus S.L. infection in human and domestic animal hosts in european mediterranean and balkan countries: a systematic review. PLoS Negl. Trop. Dis. 2020 doi: 10.1371/journal.pntd.0008519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tamarozzi F., Deplazes P., Casulli A. Reinventing the wheel of Echinococcus granulosus sensu lato transmission to humans. Trends Parasitol. 2020;36(5):427–434. doi: 10.1016/j.pt.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 26.Dubey J.P., Calero-Bernal R., Rosenthal B.M., Speer C.A., Fayer R. second edition. 2015. Sarcocystosis of Animals and Humans. [Google Scholar]
- 27.Dubey J.P., Moré G., Van Wilpe E., Calero-Bernal R., Verma S.K., Schares G. Sarcocystis Rommeli, nSp. (Apicomplexa: Sarcocystidae) from Cattle (Bos taurus) and its Differentiation from Sarcocystis Hominis. J. Eukaryot. Microbiol. 2016 doi: 10.1111/jeu.12248. [DOI] [PubMed] [Google Scholar]
- 28.Dubey J.P., van Wilpe E., Calero-Bernal R., Verma S.K., Fayer R. Sarcocystis Heydorni, n Sp. (Apicomplexa: Sarcocystidae) with Cattle (Bos taurus) and Human (Homo sapiens) Cycle. Parasitol. Res. 2015 doi: 10.1007/s00436-015-4645-2. [DOI] [PubMed] [Google Scholar]
- 29.Gazzonis A.L. Prevalence and Molecular Characterisation of Sarcocystis Miescheriana and Sarcocystis Suihominis in Wild Boars (Sus scrofa) in Italy. Parasitol. Res. 2019 doi: 10.1007/s00436-019-06249-2. [DOI] [PubMed] [Google Scholar]
- 30.Fayer R., Esposito D.H., Dubey J.P. Human infections with Sarcocystis Species. Clin. Microbiol. Rev. 2015 doi: 10.1128/CMR.00113-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fayer R. Sarcocystis spp. in human infections. Clin. Microbiol. Rev. 2004 doi: 10.1128/CMR.17.4.894-902.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bahari P., Salehi M., Seyedabadi M., Mohammadi A. Molecular Identification of Macroscopic and Microscopic Cysts of Sarcocystis in Sheep in North Khorasan Province, Iran. Int. J. Mol. Cell. Med. 2014;3(1):51–56. [PMC free article] [PubMed] [Google Scholar]
- 33.Heckeroth A.R., Tenter A.M. Development and validation of species-specific nested PCRs for diagnosis of acute sarcocystiosis in sheep. Int. J. Parasitol. 1999 doi: 10.1016/S0020-7519(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 34.Gjerde B. Molecular characterisation of Sarcocystis Bovifelis Sarcocystis Bovini N. Sp., Sarcocystis Hirsuta and Sarcocystis Cruzi from Cattle (Bos taurus) and Sarcocystis Sinensis from Water Buffaloes (Bubalus bubalis) Parasitol. Res. 2016 doi: 10.1007/s00436-015-4881-5. [DOI] [PubMed] [Google Scholar]
- 35.Poulsen C.S., Stensvold C.R. Current status of epidemiology and diagnosis of human sarcocystosis. J. Clin. Microbiol. 2014;52(10):3524–3530. doi: 10.1128/JCM.00955-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dubey J.P. Toxoplasmosis in sheep-The last 20 years. Vet. Parasitol. 2009 doi: 10.1016/j.vetpar.2009.02.026. [DOI] [PubMed] [Google Scholar]
- 37.Mur L., Atzeni M., Martínez-López B., Feliziani F., Rolesu S., Sanchez-Vizcaino J.M. Thirty-five-year presence of african swine fever in sardinia: history, evolution and risk factors for disease maintenance. Transbound. Emerg. Dis. 2016 doi: 10.1111/tbed.12264. [DOI] [PubMed] [Google Scholar]
- 38.Dubey J.P. Oocyst shedding by cats fed isolated bradyzoites and comparison of infectivity of bradyzoites of the veg strain toxoplasma gondii to cats and mice. J. Parasitol. 2001 doi: 10.1645/0022-3395(2001)087[0215:osbcfi]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 39.Hill D., Dubey J.P. Toxoplasma Gondii: Transmission, Diagnosis, and Prevention. Clin. Microbiol. Infect. 2002 doi: 10.1046/j.1469-0691.2002.00485.x. [DOI] [PubMed] [Google Scholar]
- 40.Maleki B. Toxoplasma oocysts in the soil of public places worldwide: a systematic review and meta-analysis. Trans. R. Soc. Trop. Med. Hyg., Nov. 2020 doi: 10.1093/trstmh/traa133. [DOI] [PubMed] [Google Scholar]
- 41.Davis S.W., Dubey J.P. Mediation of Immunity to Toxoplasma Gondii Oocyst Shedding in Cats. J. Parasitol. 1995 doi: 10.2307/3284034. [DOI] [PubMed] [Google Scholar]
- 42.Zulpo D.L. Toxoplasma Gondii: a study of oocyst re-shedding in domestic cats. Vet. Parasitol. 2018 doi: 10.1016/j.vetpar.2017.10.021. [DOI] [PubMed] [Google Scholar]
- 43.Attias M., Teixeira D.E., Benchimol M., Vommaro R.C., Crepaldi P.H., De Souza W. The life-cycle of Toxoplasma gondii reviewed using animations. Parasit. Vectors. 2020;13(1):588. doi: 10.1186/s13071-020-04445-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reiling S.J., Measures L., Feng S., Boone R., Merks H., Dixon B.R. Toxoplasma gondii, Sarcocystis sp. and Neospora caninum-like parasites in seals from northern and eastern Canada: potential risk to consumers. Food Waterborne Parasitol. 2019;17 doi: 10.1016/j.fawpar.2019.e00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dubey J.P., Schares G. Neosporosis in animals-the last five years. Vet. Parasitol. 2011 doi: 10.1016/j.vetpar.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 46.Duarte P.O. Serological and molecular detection of Neospora Caninum and Toxoplasma Gondii in human umbilical cord blood and placental tissue Samples. Sci. Rep. 2020 doi: 10.1038/s41598-020-65991-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dubey J.P., Hattel A.L., Lindsay D.S., Topper M.J. Neonatal Neospora Caninum infection in dogs: isolation of the causative agent and experimental transmission. J. Am. Vet. Med. Assoc. 1988;193(10):1259–1263. [PubMed] [Google Scholar]
- 48.Dubey J.P., Schares G., Ortega-Mora L.M. Epidemiology and control of neosporosis and Neospora caninum. Clin. Microbiol. Rev. 2007 doi: 10.1128/CMR.00031-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dubey J.P., Knickman E., Greene C.E. Neonatal Neospora Caninum Infections in Dogs. Acta Parasitol. 2005;50(2):176–179. [Google Scholar]
- 50.Dubey J.P. Toxoplasmosis - A waterborne zoonosis. Vet. Parasitol. 2004 doi: 10.1016/j.vetpar.2004.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Lobato J. Detection of Immunoglobulin G Antibodies to Neospora Caninum in Humans: High Seropositivity Rates in Patients Who Are Infected by Human Immunodeficiency Virus or Have Neurological Disorders. Clin. Vaccine Immunol. 2006 doi: 10.1128/CVI.13.1.84-89.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Björkman C., Lunden A., Uggla A. Prevalence of antibodies to Neospora caninum and toxoplasma gondii in Swedish dogs. Acta Vet. Scand. 1994;35(4):445–447. doi: 10.1186/BF03548321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rasmussen K., Jensen A.L. Some epidemiologic features of canine neosporosis in Denmark. Vet. Parasitol. 1996;62(3–4):345–349. doi: 10.1016/0304-4017(95)00867-5. [DOI] [PubMed] [Google Scholar]
- 54.Anvari D. Seroprevalence of Neospora caninum infection in dog population worldwide: a systematic review and Meta-analysis. Acta Parasitol. 2020;65(2):273–290. doi: 10.2478/s11686-019-00163-4. [DOI] [PubMed] [Google Scholar]
- 55.Villagra-blanco R. 2018. Seroprevalence of Neospora caninum- specific antibodies in German breeding bitches; pp. 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ryan U., Zahedi A., Paparini A. Cryptosporidium in humans and animals—a one health approach to prophylaxis. Parasite Immunol. 2016 doi: 10.1111/pim.12350. [DOI] [PubMed] [Google Scholar]
- 57.Firoozi Z. Prevalence and Genotyping Identification of Cryptosporidium in Adult Ruminants in Central Iran. Parasit. Vectors. 2019 doi: 10.1186/s13071-019-3759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Abe N., Sawano Y., Yamada K., Kimata I., Iseki M. Cryptosporidium Infection in Dogs in OsakaJapan. Vet. Parasitol. 2002 doi: 10.1016/S0304-4017(02)00204-2. [DOI] [PubMed] [Google Scholar]
- 59.Sotiriadou I., Pantchev N., Gassmann D., Karanis P. Molecular identification of Giardia and Cryptosporidium from dogs and cats. Parasite. 2013 doi: 10.1051/parasite/2013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khan A., Shams S., Khan S., Khan M.I., Khan S., Ali A. Evaluation of prevalence and risk factors associated with Cryptosporidium infection in rural population of District Buner, Pakistan. PLoS One. 2019 doi: 10.1371/journal.pone.0209188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Berrilli F. Giardia Duodenalis Genotypes and Cryptosporidium Species in Humans and Domestic Animals in Côte d’Ivoire: Occurrence and Evidence for Environmental Contamination. Trans. R. Soc. Trop. Med. Hyg. 2012 doi: 10.1016/j.trstmh.2011.12.005. [DOI] [PubMed] [Google Scholar]
- 62.Lucio-Forster A., Griffiths J.K., Cama V.A., Xiao L., Bowman D.D. Minimal zoonotic risk of cryptosporidiosis from pet dogs and cats. Trends Parasitol. 2010 doi: 10.1016/j.pt.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 63.Taghipour A. The global prevalence of Cryptosporidium infection in dogs: a systematic review and meta-analysis. Vet. Parasitol. 2020;281:109093. doi: 10.1016/j.vetpar.2020.109093. [DOI] [PubMed] [Google Scholar]
- 64.Raza A., Rand J., Qamar A., Jabbar A., Kopp S. Gastrointestinal parasites in shelter dogs: occurrence, pathology, treatment and risk to shelter workers. Animals. 2018;8(7):108. doi: 10.3390/ani8070108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Papadopoulos E., Sioutas G. 2020. Parasites and BARF:The raw truth; pp. 118–123. [Google Scholar]
- 66.Pozio E., Darwin Murrell K. Systematics and epidemiology of Trichinella. Adv. Parasitol. 2006 doi: 10.1016/S0065-308X(06)63005-4. [DOI] [PubMed] [Google Scholar]
- 67.Gajadhar A.A. Trichinella diagnostics and control: mandatory and best practices for ensuring food safety. Vet. Parasitol. 2009;159(3–4):197–205. doi: 10.1016/j.vetpar.2008.10.063. [DOI] [PubMed] [Google Scholar]
- 68.Troiano G., Nante N. Human Trichinellosis in Italy: an epidemiological review since 1989. J. Prev. Med. Hyg. 2019;60(2):E71–E75. doi: 10.15167/2421-4248/jpmh2019.60.2.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gottstein B., Pozio E., Nöckler K. Epidemiology, diagnosis, treatment, and control of trichinellosis. Clin. Microbiol. Rev. 2009 doi: 10.1128/CMR.00026-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Pozio E., Zarlenga D.S. New pieces of the trichinella puzzle. Int. J. Parasitol. 2013 doi: 10.1016/j.ijpara.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 71.Gómez-Morales M.A. Hunting dogs as sentinel animals for monitoring infections with Trichinella Spp. in Wildlife. Parasit. Vectors. 2016 doi: 10.1186/s13071-016-1437-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pozio E. Factors affecting the flow among domestic, synanthropic and sylvatic cycles of Trichinella. Vet. Parasitol. 2000;93(3):241–262. doi: 10.1016/S0304-4017(00)00344-7. [DOI] [PubMed] [Google Scholar]
- 73.Wilson N.O., Hall R.L., Montgomery S.P., Jones J.L. Trichinellosis surveillance—United States, 2008–2012. Morb. Mortal. Wkly. Rep. Surveill. Summ. 2015;64(1):1–8. [PubMed] [Google Scholar]
- 74.Pozio E. World distribution of Trichinella Spp. infections in animals and humans. Vet. Parasitol. 2007 doi: 10.1016/j.vetpar.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 75.Diaz J.H., Warren R.J., Oster M.J. The disease ecology, epidemiology, clinical manifestations, and Management of Trichinellosis Linked to consumption of wild animal meat. Wilderness Environ. Med. 2020;31(2):235–244. doi: 10.1016/j.wem.2019.12.003. [DOI] [PubMed] [Google Scholar]
- 76.Kijlstra A., Jongert E. Control of the risk of human toxoplasmosis transmitted by meat. Int. J. Parasitol. 2008 doi: 10.1016/j.ijpara.2008.06.002. [DOI] [PubMed] [Google Scholar]
- 77.Franssen F. Inactivation of parasite transmission stages: Efficacy of treatments on food of animal origin. Trends Food Sci. Technol. 2019 doi: 10.1016/j.tifs.2018.11.009. [DOI] [Google Scholar]
- 78.Dubey J.P., Kotula A.W., Sharar A., Andrews C.D., Lindsay D.S. Effect of high temperature on infectivity of Toxoplasma Gondii tissue cysts in pork. J. Parasitol. 1990 doi: 10.2307/3283016. [DOI] [PubMed] [Google Scholar]
- 79.Olgica D.D., Milenković V. Effect of refrigeration and freezing on survival of Toxoplasma Gondii tissue cysts. Acta Vet. Brno. 2000;50(5–6):375–380. [Google Scholar]
- 80.Mirza Alizadeh A. A review on inactivation methods of Toxoplasma gondii in foods. Path Global Health. 2018 doi: 10.1080/20477724.2018.1514137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ito S., Tsunoda K., Taki T., Nishikawa H., Matsui T. Destructive effect of heating against toxoplasma oocysts. Natl. Inst. Anim. Health Q. (Tokyo) 1975;15(3):128–130. [PubMed] [Google Scholar]
- 82.Srivastava P.S., Saha A.K., Sinha S.R.P. Effects of heating and freezing on the viability of sarcocysts of Sarcocystis Levinei from cardiac tissues of buffaloes. Vet. Parasitol. 1986 doi: 10.1016/0304-4017(86)90080-4. [DOI] [PubMed] [Google Scholar]
- 83.Saleque A., Juyal P.D., Bhatia B.B. Effect of temperature on the infectivity of Sarcocystis Miescheriana cysts in pork. Vet. Parasitol. 1990 doi: 10.1016/0304-4017(90)90047-F. [DOI] [PubMed] [Google Scholar]
- 84.Rose J.B., Slifko T.R. Giardia, Cryptosporidium, and Cyclospora and their impact on foods: A review. J. Food Prot. 1999 doi: 10.4315/0362-028X-62.9.1059. [DOI] [PubMed] [Google Scholar]
- 85.Blackburn C.D.W., McClure P.J. Second edition. 2009. Foodborne Pathogens: Hazards, Risk Analysis and Control. [Google Scholar]
- 86.Lacour S.A. Freeze-tolerance of trichinella muscle larvae in experimentally infected wild boars. Vet. Parasitol. 2013 doi: 10.1016/j.vetpar.2013.01.049. [DOI] [PubMed] [Google Scholar]
- 87.Koutsoumanis K. Public health risks associated with food-borne parasites. EFSA J. 2018 doi: 10.2903/j.efsa.2018.5495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Dorny P., Praet N., Deckers N., Gabriel S. Emerging food-borne parasites. Vet. Parasitol. 2009 doi: 10.1016/j.vetpar.2009.05.026. [DOI] [PubMed] [Google Scholar]
- 89.Lundén A., Uggla A. Infectivity of Toxoplasma Gondii in mutton following curing, smoking, freezing or microwave cooking. Int. J. Food Microbiol. 1992 doi: 10.1016/0168-1605(92)90069-F. [DOI] [PubMed] [Google Scholar]
- 90.Deng H., Swart A., Bonačić Marinović A.A., van der Giessen J.W.B., Opsteegh M. The effect of salting on toxoplasma gondii viability evaluated and implemented in a quantitative risk assessment of meat-borne human infection. Int. J. Food Microbiol. 2020 doi: 10.1016/j.ijfoodmicro.2019.108380. [DOI] [PubMed] [Google Scholar]
- 91.Hill D.E. Curing conditions to inactivate Trichinella Spiralis muscle larvae in ready-to-eat pork sausage. Food Waterborne Parasitol. 2017 doi: 10.1016/j.fawpar.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Johne A. Survival of Trichinella Spiralis in cured meat products. Vet. Parasitol. 2020 doi: 10.1016/j.vetpar.2020.109260. [DOI] [PubMed] [Google Scholar]
- 93.Collins M.V. The effect of high-pressure processing on infectivity of Cryptosporidium parvum oocysts recovered from experimentally exposed eastern oysters (Crassostrea virginica) J. Eukaryot. Microbiol. 2005 doi: 10.1111/j.1550-7408.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 94.Lindsay D.S., Collins M.V., Holliman D., Flick G.J., Dubey J.P. Effects of high-pressure processing on Toxoplasma Gondii Tissue cysts in ground pork. J. Parasitol. 2006 doi: 10.1645/GE-631R.1. [DOI] [PubMed] [Google Scholar]
- 95.Heinz V., Buckow R. Food preservation by high pressure. J. fur Verbraucherschutz und Leb. 2010 doi: 10.1007/s00003-009-0311-x. [DOI] [Google Scholar]
- 96.Rendueles E., Omer M.K., Alvseike O., Alonso-Calleja C., Capita R., Prieto M. Microbiological food safety assessment of high hydrostatic pressure processing: A review. LWT Food Sci. Technol. 2011 doi: 10.1016/j.lwt.2010.11.001. [DOI] [Google Scholar]
- 97.Murray K.A. Effects of electron Beam irradiation on the property behaviour of poly(ether-block-amide) blended with various stabilisers. Radiat. Phys. Chem. 2015 doi: 10.1016/j.radphyschem.2015.01.009. [DOI] [Google Scholar]
- 98.Duan Y.F. Effect of Gamma-Irradiation on infectivity of Clonorchis sinensis metacercariae. Zhongguo Ji Sheng Chong Xue Yu Ji Sheng Chong Bing Za Zhi. 1993;11(1):45–49. [PubMed] [Google Scholar]
- 99.Kasprzak W., Pozio E., Rauhut W., Nowosad P. Effect of low-dose irradiation on viability of Trichinella isolates. Acta Parasitol. 1994;39(4):201–207. [Google Scholar]
- 100.Dubey J.P., Brake R.J., Murrell K.D., Fayer R. Effect of irradiation on the viability of Toxoplasma Gondii cysts in tissues of mice and pigs. Am. J. Vet. Res. 1986;47(3):518–522. [PubMed] [Google Scholar]
- 101.Nguyen T., Clarke N., Jones M.K. Perception of dog owners towards canine gastrointestinal parasitism and associated human health risk in Southeast Queensland. One Hlt. 2021 doi: 10.1016/j.onehlt.2021.100226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Barton Behravesh C., Ferraro A., Deasy M. Human Salmonella infections linked to contaminated dry dog and cat food, 2006–2008. Pediatrics. 2010;126(3):477–483. doi: 10.1542/peds.2009-3273. [DOI] [PubMed] [Google Scholar]