Abstract
Chronic sympathetic nervous system (SNS) overactivity, characteristic of heart failure (HF) with reduced ejection fraction (HFrEF), is associated with poor prognosis and contributes to increased mortality risk. Sacubitril-valsartan is a recently approved, first-in-class, angiotensin receptor neprilysin inhibitor (ARNI) drug that markedly reduces the risks of death from cardiovascular causes and hospitalization for HF in patients with HFrEF, but the physiologic mechanisms underlying these benefits are not fully understood. This single-arm, open-label, prospective study sought to test the hypothesis that short-term treatment with sacubitril-valsartan reduces SNS activity, measured directly via muscle sympathetic nerve system activity (MSNA), in patients with HFrEF. MSNA, heart rate (HR), and arterial blood pressure (BP) were assessed in stable Class II and III patients with HFrEF (n = 9, 69 ± 8 yrs; 28.6 ± 3.6 kg/m2) on contemporary, guideline-directed medical treatment who were subsequently started on sacubitril-valsartan. These measurements were repeated after two months of treatment with sacubitril-valsartan. Sacubitril-valsartan reduced MSNA burst frequency (baseline: 43 ± 10 bursts/min; 2-month: 36 ± 10 bursts/min, p=0.05) and burst incidence (baseline: 68 ± 16 bursts/100 heartbeats; 2-month: 55 ± 16 bursts/100 heartbeats, p=0.02), while HR and BP were unchanged following of treatment (p>0.05). These preliminary findings provide new evidence regarding the ability of sacubitril-valsartan to rapidly reduce SNS activity in patients with HFrEF, suggesting the presence of a novel sympathoinhibitory effect of this new drug class.
Keywords: heart failure, muscle sympathetic nerve activity, sympathetic nervous system, Entresto
INTRODUCTION
Chronic elevation of sympathetic nervous system (SNS) activity is a hallmark feature of patients with heart failure (HF) with reduced ejection fraction (HFrEF) (Grassi et al., 2019; Seravalle et al., 2019). The initial reflex increase in SNS activity supports the failing myocardium, serving as a compensatory response to help maintain perfusion pressure and cardiac output. However, sustained SNS overactivity represents a maladaptive process, resulting in end-organ damage (Lambert et al., 2019) and increased risk of fatal arrhythmias (Franciosi et al., 2017), ultimately contributing to the progression and severity of HF (Grassi et al., 2020). Importantly, despite being optimized on current pharmacotherapy including beta (β)-adrenergic receptor blockade and inhibition of the renin-angiotensin-aldosterone system (RAAS) (De Matos et al., 2004; Kawamura et al., 2009; Mancia et al., 1997; Ruzicka et al., 2013), patients with HFrEF still exhibit elevations in muscle sympathetic nerve activity (MSNA) (Grassi et al., 2019). Given that sympathetic overdrive independently predicts the mortality risk in patients with HFrEF (Barretto et al., 2009), targeting the SNS may represent one of the potential treatment options, as a reduction in MSNA may confer an improvement in clinical prognosis for these patients (Grassi et al., 2020; van Bilsen et al., 2017).
Recently, guidelines for the management of HFrEF have recommended the use of sacubitril-valsartan, a first-in-class combined angiotensin receptor and neprilysin inhibitor (ARNI), in place of an angiotensin-II receptor blocker or an angiotensin-converting enzyme inhibitor (ACEi) (Yancy et al., 2017). This change in HF pharmacotherapy guidance stems from results of the PARADIGM-HF (Prospective Comparison of ARNI with an ACE-Inhibitor to Determine Impact on Global Mortality and Morbidity in HF) trial, which demonstrated the superiority of sacubitril-valsartan to enalapril (an ACEi) in reducing the risks of death from cardiovascular causes and hospitalization for HF in patients with HFrEF (McMurray et al., 2014). The physiologic mechanisms underpinning these clear benefits of sacubitril-valsartan are currently not well understood. While it has been speculated that sacubitril-valsartan may possess sympathomodulatory properties (Fabris et al., 2019; Liu, 2018), to our knowledge, no studies have directly examined the short-term effect of treatment with sacubitril-valsartan on SNS activity in patients with HFrEF. Thus, in this single-arm, open-label, prospective study, we sought to test the hypothesis that short-term treatment with sacubitril-valsartan reduces SNS activity, measured directly via MSNA, in patients with HFrEF.
METHODS
Study Population
We recruited nine male patients with NYHA Class II-III HFrEF from the HF clinic at the University of Utah. The inclusion criteria were a diagnosis of chronic HFrEF with left ventricular ejection fraction ≤40%, ongoing guideline-directed medical treatment for HF for at least 6 months, and a clinical decision to initiate treatment with sacubitril-valsartan. Exclusion criteria included frequent arrhythmias that would interfere with MSNA signals, uncorrected primary valvular disease, current smokers, severe chronic obstructive pulmonary disease, peripheral artery disease, stroke, uncontrolled hypertension, severe renal insufficiency, or end-stage malignancy. This study was approved by the University of Utah and the Salt Lake City Veteran Affairs Institutional Review Board (IRB_00084575) and conformed to the standards set by the Declaration of Helsinki, except for registration in a database. Written informed consent was obtained from all patients before study participation.
Experimental Design
All data collection took place with patients in the supine position in a thermoneutral environment (22-24°C). Patients reported to the laboratory at least 4 hours postprandial, having consumed a low-fat, standardized breakfast and abstained from caffeine, alcohol, and exercise for 24 hours. Data collection included venous blood samples, the assessment of heart rate (HR), arterial blood pressure, and MSNA. Following completion of baseline data collection, the patients taking ACEI were instructed to withhold their ACEI medication for 36 hours prior to initiating treatment with sacubitril-valsartan, but were maintained on all other prescribed medication. Concomitant use of an ACEI and sacubitril-valsartan is contraindicated due to an increased risk of angioedema (Sauer et al., 2019). Based on a clinical decision, three patients were treated with 24 mg/26 mg BID and one patient with 49 mg/51 mg BID for two months, while five patients were started with 24 mg/26 mg BID in the first month and up-titrated to 49 mg/51 mg BID for the second month. The clinical team closely monitored patient safety and treatment tolerability in accordance with established guidelines (Sauer et al., 2019). Patients were re-evaluated after two months of the treatment.
Experimental Measurements
Multiunit recordings of postganglionic MSNA were obtained by inserting a unipolar tungsten microelectrode (Frederick Haer, Bowdoin, ME, USA) percutaneously through the intact, unanaesthetized skin and positioned in the muscle nerve fascicles of the peroneal nerve near the fibular head. A reference electrode was inserted, subcutaneously, 2-3 cm from the microneurography electrode. Both electrodes were connected to a differential preamplifier and then to an amplifier (Dept. of Bioengineering, University of Iowa, Iowa City, IA, USA), band passed filtered (bandwidth 700-2,000 Hz), rectified, and integrated (time constant, 0.1 s) to obtain a mean voltage neurogram. Acceptable recordings of MSNA were identified by their characteristic spontaneous, pulse-synchronous bursts that increased in response to an end-expiratory apnea and remained unchanged during auditory stimulation or stroking of the skin. After a satisfactory MSNA recording was obtained, there was a minimum of 10 min of quiet rest before 10 min of continuous data collection. HR was monitored using a standard three-lead ECG interfaced with a data acquisition device (Biopac, Goleta, CA, USA). Resting brachial systolic and diastolic blood pressure were measured on the left arm using an automated blood pressure monitor (Tango+, Suntech, Morrisville, NC, USA). Respiratory movements were monitored using a strain-gauge pneumograph placed in a stable position over the abdomen (Biopac, Goleta, CA, USA). Venous blood samples were processed using standard procedures at the Salt Lake City Veteran Affairs Medical Center.
Data Analysis
ECG and MSNA signals were sampled at 1,000 Hz and stored for off-line analysis (Biopac, Acknowledge). Data were imported and analyzed in the WinCPRS software (Absolute Aliens, Turku, Finland). R-waves were detected and marked in the time series. Muscle sympathetic nerve bursts were automatically detected on the basis of amplitude using a signal-to-noise of 3:1 within a 0.5-sec search window centered on a 1.3-sec expected burst peak latency from the previous R-wave. Potential bursts were displayed and edited by a trained investigator who was blinded to the condition to avoid bias. Data were calculated as mean values over a 10-min steady-state period. MSNA was quantified using standard measures as burst frequency (bursts/min) and burst incidence (bursts/100 heartbeats), the latter informed of any potential effects of treatment with sacubitril-valsartan on HR and the inherent cardiac synchronicity of MSNA (Delius et al., 1972).
Statistical Analyses
Statistical analyses were performed using a commercially available software (SigmaPlot 13, Systat Software Inc., Point Richmond, CA, USA). Data were assessed for normality with the Shapiro-Wilk test. For normally distributed data, a paired sample t-test was used to identify the effects of treatment with sacubitril-valsartan on the variables of interest, whereas non-normally distributed data were analyzed using the Wilcoxon paired signed rank test. The One Sample t-test was used to identify whether the change values, assessed as the difference between two months after treatment and baseline, were statistically different from zero. All data were expressed as mean ± SD. Significance was established at p<0.05.
RESULTS
Disease Characteristics and Sacubitril-Valsartan Tolerance
Six patients were being transitioned to sacubitril-valsartan from ACEi, and three patients from angiotensin receptor blocker (ARB). Patient characteristics and clinical variables at baseline and in response to the treatment are documented in Table 1. No changes were observed for any of these patient characteristics and clinical variables as a consequence of the treatment (p>0.05). Disease-related characteristics and pharmacologic information for the patients with HFrEF are documented in Table 2. Treatment with sacubitril-valsartan for two months was welltolerated in all patients.
Table 1.
Patient characteristics and clinical variables at baseline and after two months of treatment with sacubitril-valsartan.
| Baseline | 2-month | p value | |
|---|---|---|---|
| Age (years) | 69 ± 8 | - | |
| Height (cm) | 176.9 ± 9.2 | - | |
| Weight (kg) | 89.6 ± 12.8 | 90.6 ± 13.1 | 0.25 |
| Body mass index (kg/m2) | 28.6 ± 3.6 | 28.9 ± 3.7 | 0.21 |
| Heart rate (beats/min) | 66 ± 9 | 66 ± 6 | 0.81 |
| Total cholesterol (mg/dL) | 143 ± 50 | 149 ± 45 | 0.41 |
| HDL cholesterol (mg/dL) | 44 ± 13 | 40 ± 7 | 0.23 |
| LDL cholesterol (mg/dL) | 77 ± 36 | 80 ± 28 | 0.49 |
| Triglycerides (mg/dL) | 131 ± 75 | 154 ± 92 | 0.41 |
| Glucose (mg/dL) | 118 ± 45 | 104 ± 23 | 0.15 |
| Hemoglobin (g/dL) | 14.6 ± 1.1 | 14.7 ± 1.0 | 0.92 |
| Hematocrit (%) | 44.7 ± 3.1 | 44.4 ± 3.5 | 0.71 |
| Erythrocytes (M/μL) | 4.77 ± 0.40 | 4.74 ± 0.34 | 0.78 |
| Leukocytes (K/ μL) | 6.39 ± 2.12 | 5.89 ± 1.45 | 0.34 |
| Potassium (mmol/L) | 4.5 ± 0.4 | 4.2 ± 0.4 | 0.08 |
| Creatinine (mg/dL) | 1.45 ± 0.43 | 1.33 ± 0.41 | 0.17 |
| Albumin (g/dL) | 4.39 ± 0.21 | 4.31 ± 0.20 | 0.09 |
| eGFR (mL/min/1.73m2) | 54 ± 22 | 60 ± 22 | 0.22 |
| Total bilirubin (mg/dL) | 0.93 ± 0.33 | 0.89 ± 0.27 | 0.69 |
| Aspartate aminotransferase (IU/L) | 24.8 ± 7.5 | 22.1 ± 6.5 | 0.21 |
| Alanine aminotransferase (IU/L) | 24.0 ± 7.7 | 22.4 ± 6.4 | 0.48 |
| NT-proBNP (pg/mL) | 1542 ± 1143 | 1319 ± 1175 | 0.26 |
| BNP (pg/mL) | 138 ± 122 | 139 ± 153 | 0.95 |
| NT-proANP (ng/mL) | 27.5 ± 9.8 | 29.9 ± 12.7 | 0.43 |
| Troponin T (pg/mL) | 323 ± 313 | 276 ± 194 | 0.95 |
Data are mean ± SD (n = 9 for all, except NT-proBNP, BNP, and Troponin T (n = 8). HDL, high density lipoprotein; LDL, low density lipoprotein; eGFR, estimated glomerular filtration rate; NT-proBNP, N-terminal pro-brain natriuretic peptide; BNP, B-type natriuretic peptide; NT-proANP, N-terminal pro-atrial natriuretic peptide.
Table 2.
Patient disease-specific characteristics and medications
| HFrEF | |
|---|---|
| Disease-specific characteristics | |
| Left ventricular ejection fraction (%) | 29 ± 8 |
| Left ventricular end-diastolic diameter (cm) | 6.4 ± 1.1 |
| Left ventricular end-systolic diameter (cm) | 5.6 ± 1.4 |
| Left ventricular fractional shortening (%) | 13 ± 8 |
| B-type natriuretic peptide (pg/mL) | 138 ± 122 |
| Diagnosis | |
| Ischemic cardiomyopathy | 4 / 9 |
| Non-ischemic cardiomyopathy | 5 / 9 |
| NYHA class II | 7 / 9 |
| NYHA class III | 2 / 9 |
| Type 2 diabetes | 2 / 9 |
| Coronary artery disease | 6 / 9 |
| Medications | |
| β-blocker | 8 / 9 |
| Angiotensin-converting enzyme inhibitor | 7 / 9 |
| Angiotensin receptor inhibitor | 2 / 9 |
| Statin | 8 / 9 |
| Diuretic | 8 / 9 |
| Aldosterone antagonist | 9 / 9 |
| Anticoagulant | 3 / 9 |
| Metformin | 2 / 9 |
Data are mean ± SD or number of all cases (n = 9). HFrEF, heart failure with reduced ejection fraction; NYHA, New York Heart Association.
Muscle Sympathetic Nerve Activity and Arterial Blood Pressure
An example of a neurogram from one patient before and after treatment is presented in Figure 1. Treatment with sacubitril-valsartan reduced MSNA burst frequency from 43 ± 10 bursts/min at baseline to 36 ± 10 bursts/min after two months (Δ value of −7±10 bursts/min) (p=0.05, Figure 2A). Likewise, treatment with sacubitril-valsartan reduced MSNA burst incidence from 68 ± 16 bursts/100 heartbeats at baseline to 55 ± 16 bursts/100 heart beats after two months (Δ value of −13±13 bursts/100 heartbeats) (p<0.05, Figure 2B). No changes were observed for systolic blood pressure (baseline: 110 ± 20 mmHg; 2 months: 102 ± 17 mmHg; Δ value of −8±15 mmHg) or diastolic blood pressure (baseline: 69 ± 8 mmHg; 2 months: 66 ± 8 mmHg; Δ value of −3±8 mmHg) (p>0.05, Figure 2C & 2D, respectively).
Figure 1.
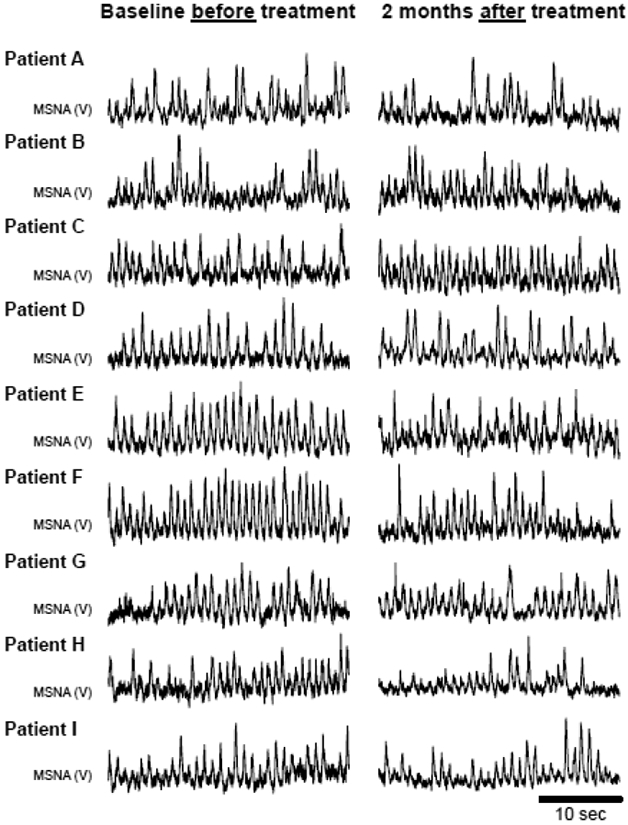
Original recordings of resting muscle sympathetic nerve activity (MSNA) at baseline and after two months of treatment with sacubitril-valsartan in all patients with heart failure with reduced ejection fraction (n = 9).
Figure 2.
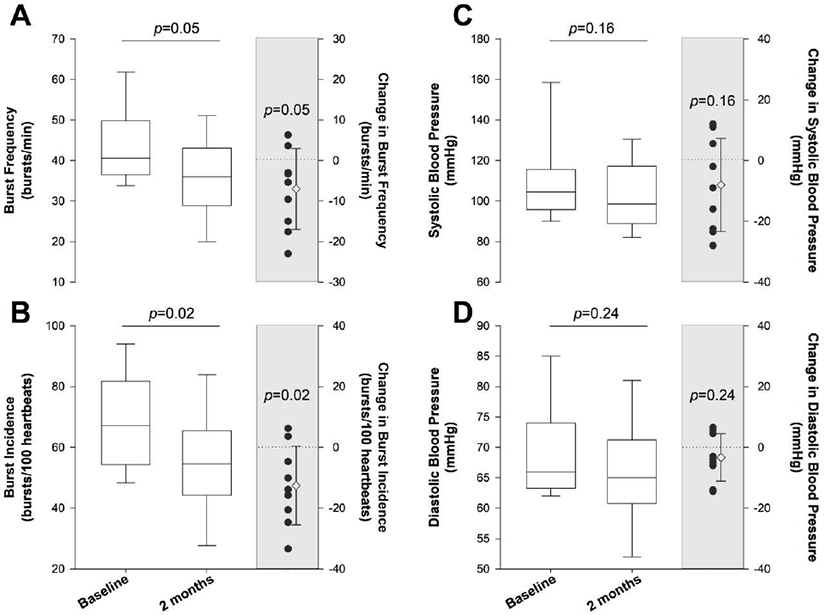
Muscle sympathetic nerve activity (MSNA) burst frequency (Panel A) and burst incidence (Panel B), as well as systolic blood pressure (Panel C) and diastolic blood pressure (Panel D) at baseline and after 2 months of treatment with sacubitril-valsartan in male patients with heart failure with reduced ejection fraction (n = 9). For all panels, the left and right boxplots represent baseline and after two months of treatment with sacubitril-valsartan. The solid line in the box plot represents the median, whereas the upper and lower limits of the box plot represent the 75th percentile and 25th percentile, respectively. The whiskers represent the range of data (minimum and maximum). The scatterplot (shaded region) represents the individual change values as well as the group mean and standard deviation.
DISCUSSION
Using a single-arm, open-label approach, we sought to determine the impact of sacubitril-valsartan, a first-in-class ARNI drug, on directly measured SNS activity in patients with HFrEF. Following two months of sacubitril-valsartan treatment, we observed a reduction in resting MSNA burst frequency (≈17%) and burst incidence (≈19%) in patients with HFrEF. To our knowledge, this is the first study to identify the rapid sympathoinhibitory effect of this drug class, thereby providing preliminary, new information regarding the potential mechanism by which sacubitril-valsartan therapy may improve outcomes in patients with HFrEF.
SNS Activity in Patients with HFrEF.
The presence of elevated SNS activity is a well-known maladaptive state in patients suffering from HFrEF (Grassi et al., 2019; Seravalle et al., 2019). Chronic elevation of cardiac SNA is known to initiate cardiac β-adrenergic receptor damage and attenuation of neurotransmitter reuptake (Delehanty et al., 1994), while peripheral overexpression of SNS activity contributes to the development of left ventricular dysfunction (de Souza et al., 2013) and renal failure (Klein et al., 2003), though its contribution to arterial hypertension is unequivocal (Keir et al., 2020; Seravalle et al., 2015). In the present study, patients with HFrEF had a resting MSNA burst frequency of 43 ± 10 bursts/min (range: 34-62 bursts/min) and burst incidence of 68 ± 16 bursts/100 heartbeats (range: 48-94 bursts/100 heartbeats) at baseline before treatment initiation, which is consistent with those of patients with HFrEF with a similar NYHA Class II-III (ranges: ~64-61 bursts/min or ~57-80 bursts/100 heartbeats) (Azevedo et al., 2001; De Matos et al., 2004; Grassi et al., 2019; Grassi et al., 1995). Given these unwanted complications of chronic sympathoexcitation and the strong association between MSNA and increased cardiac mortality risks in this patient group (Barretto et al., 2009), SNS overactivity has become one of the primary therapeutic targets for the treatment of HF (Grassi et al., 2020; van Bilsen et al., 2017).
Indeed, drugs that inhibit cardiac β-adrenergic receptors have proven effective in mitigating the damaging effects of a disease-related increase in cardiac SNS activity, providing a clear mortality benefit for patients with HFrEF in sinus rhythm (Cohen-Solal et al., 2005; Yamazaki et al., 2001). Azevedo et al. (Azevedo et al., 2001) observed reductions in systemic and cardiac norepinephrine spillover following four months of treatment with carvedilol (non-selective β-adrenergic receptor blockade), but not metoprolol (selective β-adrenergic receptor blockade), without any changes in MSNA, demonstrating the sympathomodulatory effect of carvedilol on pre-junctional β-adrenergic receptors that facilitate noradrenergic neurotransmission. Using similar dosage as those of Azevedo et al. (Azevedo et al., 2001), De Matos et al. (De Matos et al., 2004) identified a reduction in MSNA following six months of carvedilol therapy in patients with HFrEF, demonstrating the efficacy of this therapeutic approach to reduce SNS expression. While the potential for a β-adrenergic receptor blockade to reduce central sympathetic outflow is less clear (Azevedo et al., 2001; De Matos et al., 2004), sympathoinhibition via pharmacologic targeting of the renin-angiotensin-aldosterone system (RAAS) has been proven effective, with evidence for the efficacy of both ACEi (Mancia et al., 1997) and ARB (Ruzicka et al., 2013), as well as combined ACEi and ARB therapy (Kawamura et al., 2009) to reduce MSNA in patients with HFrEF. There is also recent evidence for the sympathoinhibitory effect of simvastatin treatment in patients with HFrEF (Deo et al., 2012). However, despite the favorable sympathomodulatory effects of these medications that constitute standard optimized HFrEF pharmacotherapy, pathologic elevations in SNS activity remain a hallmark characteristic of the HFrEF clinical syndrome (Grassi et al., 2019; Seravalle et al., 2019), suggesting that additional treatment strategies aimed at reducing MSNA are warranted.
Sympathoinhibitory Properties of Sacubitril-Valsartan.
Until now, there has not been a single study that has evaluated the early effects of sacubitril-valsartan, a first-in-class ARNI drug, on SNS activity in human HF. In this study, we observed a ≈17% reduction in MSNA burst frequency (Figure 2A) and a ≈19% reduction in MSNA burst incidence (Figure 2B) following two months of treatment with sacubitril-valsartan. This robust reduction in SNS activity is particularly noteworthy considering patients were evaluated while on contemporary, guideline-directed pharmacotherapy that included β-blockade, ACEi/ARB, aldosterone blockade, and statins (Table 1). As noted above, each of these drug classes promote sympathoinhibition, and, thus, the observed further reduction in MSNA, following the addition of an ARNI drug to the standard HFrEF pharmacologic regimen, provides further insight into its physiological effects in HFrEF. The reduction in MSNA was not accompanied by statistically significant changes in systolic or diastolic blood pressures (Figure 2C & 2D, respectively), likely due to small sample size and high variability in the responses. Though sympathoinhibition is often accompanied by a reduction in arterial blood pressure, a parallel change in these two outcomes is not always observed. Indeed, in a recent study by Dell’Oro et al. (Dell'Oro et al., 2017), patients with HFrEF exhibit reductions in MSNA, coupled with an improvement in spontaneous sympathetic vascular baroreflex sensitivity, without any changes in resting arterial blood pressure, following an average of 47 months of baroreflex activation therapy. Thus, in the present study, the reduction in SNS activity in an absence of changes in arterial blood pressure may be attributed to an improvement in sympathetic vascular baroreflex sensitivity and/or altered sympathetic neurovascular transduction, although the possibility of a longer duration of treatment is needed to achieve significant arterial blood pressure reductions cannot be ruled out.
While this is the first report of the potential of short-term treatment with sacubitril-valsartan to reduce SNS activity in human HF, preclinical studies offer additional evidence in support of the current findings. Indeed, Kusaka et al. (Kusaka et al., 2015) demonstrated that sacubitril-valsartan administration reduces low frequency blood pressure variability, an index of vascular sympathetic modulation, in spontaneously hypertensive rats more so than in animals treated with valsartan alone. The current findings extended this previous work by demonstrating the sympathoinhibitory effect of sacubitril-valsartan in patients with HFrEF, providing new insight regarding the benefits of this drug for the treatment of HF.
Potential Mechanisms of Sympathoinhibition following Treatment with Sacubitril-Valsartan.
The mechanisms by which treatment with sacubitril-valsartan might further reduce SNS activity in optimally medicated patients with HFrEF remain unclear, but may be related to the ability of this new drug class to concomitantly affect both the RAAS and natriuretic peptide (NP) pathways. RAAS activation promotes the release of angiotensin II (ANGII), which contributes to sustained vasoconstriction (Cody, 1997; Kobalava et al., 2016), and intravenous infusion of ANGII has been demonstrated to increase MSNA in healthy individuals (Matsukawa et al., 1991). In patients with HFrEF, high doses of the ARB losartan (200 mg per day) reduced MSNA and plasma catecholamines, while conventional, low dose (50 mg per day) losartan did not exert a sympathoinhibitory effect (Ruzicka et al., 2013). In the present study, the ARNI drug contains as little as 26 mg of valsartan and could be up-titrated to 51 mg, a dosage closer to the minimum effective dose that may be better tolerated in patients with HFrEF. However, our observation of the sympathoinhibitory benefit at a low-dose valsartan is likely due to the additive effect with neprilysin. In contrast to the sympathoexcitatory properties of RAAS pathway activation, the NP system promotes the release of atrial (ANP) and brain (BNP) natriuretic peptides, both of which promote sympathoinhibition (Kobalava et al., 2016). Using either an intravenous infusion of ANP or an oral administration of candoxatril (a natriuretic endopeptidase inhibitor to reduce metabolism of ANP), Ando et al. (Ando et al., 1995) observed similar reductions in preload, assessed via central venous pressure, without any reflex increases in MSNA in young men, demonstrating the sympathomodulatory effects of the NP system. In patients with HFrEF, intravenous infusion of BNP has been documented to reduce regional sympathetic activity, assessed via renal and cardiac norepinephrine spillover (Brunner-La Rocca et al., 2001). Furthermore, intravenous infusion of ANP also results in a reduction of MSNA (Abramson et al., 1999) and improved modulation of MSNA (Kubo et al., 2001) in patients with HFrEF, together providing evidence that NP agonists may be beneficial in terms of both sympathoinhibition and neurogenic circulatory control.
Given this evidence supporting the individual contribution of both the RAAS and NP pathways to sympathoexcitation in HF, concomitant targeting of these two pathways via ARNI therapy appears to exert a synergistic effect on SNS activity in patients with HFrEF, as supported by a reduction in MSNA following treatment with sacubitril-valsartan in this study. The efficacy of this drug combination is consistent with clinical trials that ultimately led to the development and approval of the ARNI drug class. Indeed, while the majority of clinical trials evaluating the efficacy of neprilysin inhibition provided neutral results (Cleland et al., 1998), the combination of neprilysin inhibitor with an ARB has proven highly effective in the treatment of both HTN (Williams et al., 2017) and HFrEF (McMurray et al., 2014). In addition, we have recently documented reductions in plasma concentrations of pro-inflammatory cytokines tumor necrosis factor-alpha and interleukin-18 following short-term treatment sacubitril-valsartan in patients with HFrEF (Bunsawat et al., 2020), findings that are consistent with preclinical work in animals with diabetic cardiomyopathy demonstrating the anti-inflammatory potential of this drug class (Ge et al., 2019). Given the interplay between inflammation and the SNS (Janig, 2014), it is possible that the reduction in MSNA may be mediated, in part, by an improvement in inflammatory status in the present study. Furthermore, it is tempting to speculate that, given the association between MSNA and mortality in patients with HFrEF (Barretto et al., 2009), the reduction in MSNA observed in the present study may, in part, serve as a potential mechanism explaining the clear benefits of treatment with sacubitril-valsartan in the PARADIGN-HF trial (McMurray et al., 2014), though additional studies are required to evaluate this interesting possibility.
Experimental Limitations.
The current study utilized a single-arm, open-label, prospective study design, which is an efficient approach for evaluating the efficacy of an intervention using a small number of patients and repeated measurements for pre- vs post-treatment comparisons (Evans, 2010). Using this approach, we only studied patients with HFrEF who were instructed to initiate treatment with sacubitril-valsartan based on their cardiologist’s clinical decision, which enabled the opportunity to study these patients in a real-world setting. Importantly, we acknowledge the limitations of this study design with no control group and blinding of participants, assessors, and analysts, which may allow for potential introduction of considerable conscious and unconscious bias that can significantly inflate positive findings. Thus, our findings may not be definitive and would benefit by confirming using a large patient cohort. Despite this limitation, this type of study design in not uncommon in clinical studies seeking to evaluate the efficacy of an intervention prior to large-scale trials (Sciascia et al., 2020; Zeng et al., 2019), including our recent work evaluating the impact of sacubitril-valsartan on conduit vessel function, functional capacity, and inflammation in patients with HFrEF (Bunsawat et al., 2020). It is also acknowledged that this study includes a relatively small sample size with only male patients, which may limit generalizability of our findings. We did not observe any changes in plasma concentrations NT-proBNP, BNP, NT-proANP, and Troponin T, or endothelin-1 across the 3 months of treatment with sacubitril-valsartan (Table 3), which may be due to differences in sample size, drug dosing, and duration of treatment between the prior and current studies. Likewise, serial assessments of cardiac function are beyond the scope of the present study; however, findings from long-term studies (12 months) have reported the beneficial effects of ARNI therapy on cardiac function and remodeling in patients with HFrEF (Hsiao et al., 2020; Januzzi et al., 2019; Polito et al., 2020). In addition, despite evidence demonstrating the clear benefits of ARNI therapy over previous standard of care, only a minority of eligible patients are currently prescribed sacubitril-valsartan, likely attributable to barriers including practitioner’s unfamiliarity with ARNIs, safety concerns, and payer reimbursement issues that prevent the prescription of sacubitril-valsartan in all eligible patients (Sauer et al., 2019). These novel, preliminary findings may not only provide insight into the sympathoinhibitory benefits of this drug class, but also encourage clinicians to prescribe sacubitril-valsartan in place of conventional ARB therapy for the treatment of eligible patients with HFrEF (Grassi et al., 2020). Additional studies are needed to confirm our findings using a longer treatment duration and a placebo-controlled group, as well as to determine the relationship between drug dose and drug’s sympathoinhibitory effect.
Conclusions.
The present study demonstrates that sacubitril-valsartan lowered resting sympathetic activity in patients with HFrEF after a brief duration of treatment, providing preliminary, new insight regarding the therapeutic potential for this drug class to target the sympathetic nervous system in HF.
ACKNOWLEDGMENTS AND FUNDING
The authors thank the participants for their time and effort. This work was funded in part by the National Institutes of Health (R01 HL118313, D.W.W.; R56 AG057584, R.S.R; T32 HL139451, K.B.), the U.S. Department of Veterans Affairs (RX001311, D.W.W.; E6910-R, E1697-R, E3207-R, E1572-P, and E9275-L, R.S.R) and the American Heart Association (18POST33960192; K.B.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT ON INTEREST
The authors have no other funding, financial relationships, or conflicts of interest to disclose.
REFERENCES
- Abramson BL, Ando S, Notarius CF, Rongen GA, Floras JS 1999. Effect of atrial natriuretic peptide on muscle sympathetic activity and its reflex control in human heart failure. Circulation 99, 1810–1815. [DOI] [PubMed] [Google Scholar]
- Ando S, Rahman MA, Butler GC, Senn BL, Floras JS 1995. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension 26, 1160–1166. [DOI] [PubMed] [Google Scholar]
- Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD 2001. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation 104, 2194–2199. [DOI] [PubMed] [Google Scholar]
- Barretto AC, Santos AC, Munhoz R, Rondon MU, Franco FG, Trombetta IC, Roveda F, de Matos LN, Braga AM, Middlekauff HR, Negrao CE 2009. Increased muscle sympathetic nerve activity predicts mortality in heart failure patients. International journal of cardiology 135, 302–307. [DOI] [PubMed] [Google Scholar]
- Brunner-La Rocca HP, Kaye DM, Woods RL, Hastings J, Esler MD 2001. Effects of intravenous brain natriuretic peptide on regional sympathetic activity in patients with chronic heart failure as compared with healthy control subjects. Journal of the American College of Cardiology 37, 1221–1227. [DOI] [PubMed] [Google Scholar]
- Bunsawat K, Ratchford SM, Alpenglow JK, Park SH, Jarrett CL, Stehlik J, Smith AS, Richardson RS, Wray DW 2020. Sacubitril-Valsartan Improves Conduit Vessel Function and Functional Capacity, and Reduces Inflammation in Heart Failure with Reduced Ejection Fraction. J Appl Physiol (1985). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleland JG, Swedberg K 1998. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet 351, 1657–1658. [DOI] [PubMed] [Google Scholar]
- Cody RJ 1997. The sympathetic nervous system and the renin-angiotensin-aldosterone system in cardiovascular disease. The American journal of cardiology 80, 9J–14J. [DOI] [PubMed] [Google Scholar]
- Cohen-Solal A, Rouzet F, Berdeaux A, Le Guludec D, Abergel E, Syrota A, Merlet P 2005. Effects of carvedilol on myocardial sympathetic innervation in patients with chronic heart failure. J Nucl Med 46, 1796–1803. [PubMed] [Google Scholar]
- De Matos LD, Gardenghi G, Rondon MU, Soufen HN, Tirone AP, Barretto AC, Brum PC, Middlekauff HR, Negrao CE 2004. Impact of 6 months of therapy with carvedilol on muscle sympathetic nerve activity in heart failure patients. J Card Fail 10, 496–502. [DOI] [PubMed] [Google Scholar]
- de Souza SB, Rocha JA, Cuoco MA, Guerra GM, Ferreira-Filho JC, Borile S, Krieger EM, Bortolotto LA, Consolim-Colombo FM 2013. High muscle sympathetic nerve activity is associated with left ventricular dysfunction in treated hypertensive patients. American journal of hypertension 26, 912–917. [DOI] [PubMed] [Google Scholar]
- Delehanty JM, Himura Y, Elam H, Hood WB Jr., Liang CS 1994. Beta-adrenoceptor downregulation in pacing-induced heart failure is associated with increased interstitial NE content. The American journal of physiology 266, H930–935. [DOI] [PubMed] [Google Scholar]
- Delius W, Hagbarth KE, Hongell A, Wallin BG 1972. General characteristics of sympathetic activity in human muscle nerves. Acta physiologica Scandinavica 84, 65–81. [DOI] [PubMed] [Google Scholar]
- Dell'Oro R, Gronda E, Seravalle G, Costantino G, Alberti L, Baronio B, Staine T, Vanoli E, Mancia G, Grassi G 2017. Restoration of normal sympathetic neural function in heart failure following baroreflex activation therapy: final 43-month study report. Journal of hypertension 35, 2532–2536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deo SH, Fisher JP, Vianna LC, Kim A, Chockalingam A, Zimmerman MC, Zucker IH, Fadel PJ 2012. Statin therapy lowers muscle sympathetic nerve activity and oxidative stress in patients with heart failure. Am J Physiol Heart Circ Physiol 303, H377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans SR 2010. Clinical trial structures. J Exp Stroke Transl Med 3, 8–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabris E, Merlo M, Rapezzi C, Ferrari R, Metra M, Frigerio M, Sinagra G 2019. Sacubitril/Valsartan: Updates and Clinical Evidence for a Disease-Modifying Approach. Drugs 79, 1543–1556. [DOI] [PubMed] [Google Scholar]
- Franciosi S, Perry FKG, Roston TM, Armstrong KR, Claydon VE, Sanatani S 2017. The role of the autonomic nervous system in arrhythmias and sudden cardiac death. Auton Neurosci 205, 1–11. [DOI] [PubMed] [Google Scholar]
- Ge Q, Zhao L, Ren XM, Ye P, Hu ZY 2019. LCZ696, an angiotensin receptor-neprilysin inhibitor, ameliorates diabetic cardiomyopathy by inhibiting inflammation, oxidative stress and apoptosis. Exp Biol Med (Maywood) 244, 1028–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grassi G, D'Arrigo G, Pisano A, Bolignano D, Mallamaci F, Dell'Oro R, Quarti-Trevano F, Seravalle G, Mancia G, Zoccali C 2019. Sympathetic neural overdrive in congestive heart failure and its correlates: systematic reviews and meta-analysis. J Hypertens 37, 1746–1756. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Cattaneo BM, Lanfranchi A, Vailati S, Giannattasio C, Del Bo A, Sala C, Bolla GB, Pozzi M 1995. Sympathetic activation and loss of reflex sympathetic control in mild congestive heart failure. Circulation 92, 3206–3211. [DOI] [PubMed] [Google Scholar]
- Grassi G, Seravalle G, Esler M 2020. Sympathomodulation in congestive heart failure: From drugs to devices. International journal of cardiology. [DOI] [PubMed] [Google Scholar]
- Hsiao FC, Wang CL, Chang PC, Lu YY, Huang CY, Chu PH 2020. Angiotensin Receptor Neprilysin Inhibitor for Patients With Heart Failure and Reduced Ejection Fraction: Real-World Experience From Taiwan. J Cardiovasc Pharmacol Ther 25, 152–157. [DOI] [PubMed] [Google Scholar]
- Janig W 2014. Sympathetic nervous system and inflammation: a conceptual view. Auton Neurosci 182, 4–14. [DOI] [PubMed] [Google Scholar]
- Januzzi JL Jr., Prescott MF, Butler J, Felker GM, Maisel AS, McCague K, Camacho A, Pina IL, Rocha RA, Shah AM, Williamson KM, Solomon SD, Investigators P-H 2019. Association of Change in N-Terminal Pro-B-Type Natriuretic Peptide Following Initiation of Sacubitril-Valsartan Treatment With Cardiac Structure and Function in Patients With Heart Failure With Reduced Ejection Fraction. Jama 322, 1085–1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura A, Yuasa F, Yokoe H, Masue Y, Sugiura T, Iwasaka T 2009. Augmented sympathoinhibitory effect of valsartan when added to angiotensin-converting enzyme inhibitor in patients with left ventricular dysfunction. J Cardiol 53, 171–178. [DOI] [PubMed] [Google Scholar]
- Keir DA, Badrov MB, Tomlinson G, Notarius CF, Kimmerly DS, Millar PJ, Shoemaker JK, Floras JS 2020. Influence of Sex and Age on Muscle Sympathetic Nerve Activity of Healthy Normotensive Adults. Hypertension 76, 997–1005. [DOI] [PubMed] [Google Scholar]
- Klein IH, Ligtenberg G, Neumann J, Oey PL, Koomans HA, Blankestijn PJ 2003. Sympathetic nerve activity is inappropriately increased in chronic renal disease. Journal of the American Society of Nephrology : JASN 14, 3239–3244. [DOI] [PubMed] [Google Scholar]
- Kobalava Z, Kotovskaya Y, Averkov O, Pavlikova E, Moiseev V, Albrecht D, Chandra P, Ayalasomayajula S, Prescott MF, Pal P, Langenickel TH, Jordaan P, Rajman I 2016. Pharmacodynamic and Pharmacokinetic Profiles of Sacubitril/Valsartan (LCZ696) in Patients with Heart Failure and Reduced Ejection Fraction. Cardiovascular therapeutics 34, 191–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T, Ando S, Picton P, Atchison DJ, Notarius CF, Pollard MJ, Abramson BL, Floras JS 2001. Atrial natriuretic peptide augments the variability of sympathetic nerve activity in human heart failure. Journal of hypertension 19, 619–626. [DOI] [PubMed] [Google Scholar]
- Kusaka H, Sueta D, Koibuchi N, Hasegawa Y, Nakagawa T, Lin B, Ogawa H, Kim-Mitsuyama S 2015. LCZ696, Angiotensin II Receptor-Neprilysin Inhibitor, Ameliorates High-Salt-Induced Hypertension and Cardiovascular Injury More Than Valsartan Alone. American journal of hypertension 28, 1409–1417. [DOI] [PubMed] [Google Scholar]
- Lambert EA, Esler MD, Schlaich MP, Dixon J, Eikelis N, Lambert GW 2019. Obesity-Associated Organ Damage and Sympathetic Nervous Activity. Hypertension 73, 1150–1159. [DOI] [PubMed] [Google Scholar]
- Liu RC 2018. Focused Treatment of Heart Failure with Reduced Ejection Fraction Using Sacubitril/Valsartan. American journal of cardiovascular drugs : drugs, devices, and other interventions 18, 473–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancia G, Giannattasio C, Grassi G 1997. Treatment of heart failure with fosinopril: an angiotensin converting enzyme inhibitor with a dual and compensatory route of excretion. Am J Hypertens 10, 236S–241S. [DOI] [PubMed] [Google Scholar]
- Matsukawa T, Gotoh E, Minamisawa K, Kihara M, Ueda S, Shionoiri H, Ishii M 1991. Effects of intravenous infusions of angiotensin II on muscle sympathetic nerve activity in humans. The American journal of physiology 261, R690–696. [DOI] [PubMed] [Google Scholar]
- McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR, Investigators, P.-H., Committees. 2014. Angiotensin-neprilysin inhibition versus enalapril in heart failure. The New England journal of medicine 371, 993–1004. [DOI] [PubMed] [Google Scholar]
- Polito MV, Silverio A, Rispoli A, Vitulano G, Auria F, De Angelis E, Loria F, Gigantino A, Bonadies D, Citro R, Carrizzo A, Galasso G, Iaccarino G, Vecchione C, Ciccarelli M 2020. Clinical and echocardiographic benefit of Sacubitril/Valsartan in a real-world population with HF with reduced ejection fraction. Sci Rep 10, 6665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka M, Floras JS, McReynolds AJ, Coletta E, Haddad H, Davies R, Leenen FH 2013. Do high doses of AT(1)-receptor blockers attenuate central sympathetic outflow in humans with chronic heart failure? Clinical science 124, 589–595. [DOI] [PubMed] [Google Scholar]
- Sauer AJ, Cole R, Jensen BC, Pal J, Sharma N, Yehya A, Vader J 2019. Practical guidance on the use of sacubitril/valsartan for heart failure. Heart Fail Rev 24, 167–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sciascia S, Apra F, Baffa A, Baldovino S, Boaro D, Boero R, Bonora S, Calcagno A, Cecchi I, Cinnirella G, Converso M, Cozzi M, Crosasso P, De Iaco F, Di Perri G, Eandi M, Fenoglio R, Giusti M, Imperiale D, Imperiale G, Livigni S, Manno E, Massara C, Milone V, Natale G, Navarra M, Oddone V, Osella S, Piccioni P, Radin M, Roccatello D, Rossi D 2020. Pilot prospective open, single-arm multicentre study on off-label use of tocilizumab in patients with severe COVID-19. Clin Exp Rheumatol 38, 529–532. [PubMed] [Google Scholar]
- Seravalle G, Lonati L, Buzzi S, Cairo M, Quarti Trevano F, Dell'Oro R, Facchetti R, Mancia G, Grassi G 2015. Sympathetic nerve traffic and baroreflex function in optimal, normal, and high-normal blood pressure states. Journal of hypertension 33, 1411–1417. [DOI] [PubMed] [Google Scholar]
- Seravalle G, Quarti-Trevano F, Dell'Oro R, Gronda E, Spaziani D, Facchetti R, Cuspidi C, Mancia G, Grassi G 2019. Sympathetic and baroreflex alterations in congestive heart failure with preserved, midrange and reduced ejection fraction. J Hypertens 37, 443–448. [DOI] [PubMed] [Google Scholar]
- van Bilsen M, Patel HC, Bauersachs J, Bohm M, Borggrefe M, Brutsaert D, Coats AJS, de Boer RA, de Keulenaer GW, Filippatos GS, Floras J, Grassi G, Jankowska EA, Kornet L, Lunde IG, Maack C, Mahfoud F, Pollesello P, Ponikowski P, Ruschitzka F, Sabbah HN, Schultz HD, Seferovic P, Slart R, Taggart P, Tocchetti CG, Van Laake LW, Zannad F, Heymans S, Lyon AR 2017. The autonomic nervous system as a therapeutic target in heart failure: a scientific position statement from the Translational Research Committee of the Heart Failure Association of the European Society of Cardiology. Eur J Heart Fail 19, 1361–1378. [DOI] [PubMed] [Google Scholar]
- Williams B, Cockcroft JR, Kario K, Zappe DH, Brunel PC, Wang Q, Guo W 2017. Effects of Sacubitril/Valsartan Versus Olmesartan on Central Hemodynamics in the Elderly With Systolic Hypertension: The PARAMETER Study. Hypertension 69, 411–420. [DOI] [PubMed] [Google Scholar]
- Yamazaki J, Muto H, Kabano T, Yamashina S, Nanjo S, Inoue A 2001. Evaluation of beta-blocker therapy in patients with dilated cardiomyopathy--Clinical meaning of iodine 123-metaiodobenzylguanidine myocardial single-photon emission computed tomography. American heart journal 141, 645–652. [DOI] [PubMed] [Google Scholar]
- Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr., Colvin MM, Drazner MH, Filippatos GS, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C 2017. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. Journal of the American College of Cardiology 70, 776–803. [DOI] [PubMed] [Google Scholar]
- Zeng Y, Moscicki AB, Sahasrabuddhe VV, Garcia F, Woo H, Hsu CH, Szabo E, Dimond E, Vanzzini S, Mondragon A, Butler V, DeRose H, Chow HS 2019. A prospective, single-arm, open-label, non-randomized, phase IIa trial of a nonavalent prophylactic HPV vaccine to assess immunogenicity of a prime and deferred-booster dosing schedule among 9-11 year-old girls and boys - clinical protocol. BMC Cancer 19, 290. [DOI] [PMC free article] [PubMed] [Google Scholar]


