Abstract
Autonomic dysfunction is implicated from clinical, neuroimaging and experimental studies in sudden and unexpected death in epilepsy (SUDEP). Neuropathological analysis in SUDEP series enable exploration of acquired, seizure-related cellular adaptations in autonomic and brainstem autonomic centers of relevance to dysfunction in the peri-ictal period. Alterations in SUDEP compared to control groups have been identified in the ventrolateral medulla, amygdala, hippocampus and central autonomic regions. These involve neuropeptidergic, serotonergic and adenosine systems, as well as specific regional astroglial and microglial populations, as potential neuronal modulators, orchestrating autonomic dysfunction. Future research studies need to extend to clinically and genetically characterised epilepsies, to explore if common or distinct pathways of autonomic dysfunction mediate SUDEP. The ultimate objective of SUDEP research is the identification of disease biomarkers for at risk patients, to improve post-mortem recognition and disease categorisation, but ultimately, for exposing potential treatment targets of pharmacologically modifiable and reversible cellular alterations.
Keywords: SUDEP, Brainstem, respiratory nuclei, amygdala, neuropathology
1. Introduction
In the last decade there have been considerable advances made in our understanding of sudden and unexpected death in epilepsy (SUDEP), from neurogenetics (Goldman et al., 2016), functional and structural neuroimaging (Allen et al., 2019a), clinical physiological studies (Lhatoo et al., 2015; Ryvlin et al., 2013), and experimental modelling (Noebels Jl Md, 2019). This has been accompanied by better recognition that this is the leading cause of epilepsy-related death in young adults with epilepsy (Devinsky et al., 2016; Middleton et al., 2018). Through ongoing cross-disciplinary studies, disease mechanisms can be interrogated, and risk-factors and disease biomarkers discovered with the future goal of implementing prevention strategies. Neuropathology can contribute to this process. Although examination of the brain in SUDEP may appear initially unremarkable, systematic analysis of large series has the potential to shed light on common patterns of seizure-related cellular alterations that may have reduced the brain’s resilience to the physiological challenges of seizures.
SUDEP has been defined as an unexpected and non-accidental death in patients with epilepsy (excluding status epilepticus), where no cause of death is identified following post-mortem examination including neuropathology (Nashef et al., 2012). The worldwide incidence is 1 to 2 per 1000 people with epilepsy per year (Harden et al., 2017; Sveinsson et al., 2017; Thurman et al., 2014). SUDEP affects all age groups, but primarily young, otherwise healthy adults, peaking at 20–40 years and in epilepsy with diverse causes (Devinsky et al., 2016). Deaths mainly occur in the peri-ictal period, although most are nocturnal and unwitnessed. Although the mechanisms and pathophysiology are still uncertain, there is accumulating evidence from clinical, imaging and experimental studies to indicate a failure of central autonomic regulation and, in particular, of respiratory dysfunction (Devinsky et al., 2016; Jefferys et al., 2019; Ryvlin et al., 2013).
The focus of this review is on recent neuropathological findings in the investigations of respiratory and autonomic brain regions in SUDEP, in the context of the emerging clinical and experimental data, and future directions for research.
2. Clinical, evidence for autonomic dysfunction in seizures and SUDEP
The primary risk factor, based on meta-analysis of SUDEP series, is poor control of generalised tonic-clonic seizures (DeGiorgio et al., 2017; Harden et al., 2017). During and following seizures autonomic phenomena have long been reported including prolonged periods of irregular, shallow breathing (Simon, 2010). Patients with documented central apnoea with associated hypoxaemia occurring around the time of a seizure (the peri-ictal period) are regarded at greater risk for SUDEP (Bruno et al., 2018; Lacuey et al., 2018; Vilella et al., 2018). Sympathetic outflow dominates after a seizure (‘sympathetic storm’) with effects on blood pressure, heart rate (Hampel et al., 2016) and even baroreflex sensitivity (Esmaeili et al., 2018; Hampel et al., 2017). Simultaneous parasympathetic and sympathetic stimulation or autonomic dysynchrony has also been proposed as a SUDEP risk factor, as reflected by an abnormal heart rate variability in some epilepsy patients’(DeGiorgio et al., 2010; Page et al., 2018). As SUDEP often occurs nocturnally or during sleep (Sveinsson et al., 2018) the convergence of brainstem arousal systems interacting with respiratory control during seizures may be an additional critical factor. Most SUDEP deaths are unwitnessed but documentation of the rare seizure deaths occurring while patients are on epilepsy monitoring units, where anti-seizure medications are withdrawn under video, EEG and cardio-respiratory monitoring, have provided valuable insight. In the MORTEMUS study (Ryvlin et al., 2013), SUDEP followed a generalized seizure with depressed arousal and respiration, non-tachyarrhythmic cardiac dysfunction, profound depression of cortical EEG activity with terminal apnoea within 11 mins of the seizure and preceding terminal asystole (Ryvlin et al., 2013). This was interpreted as early, centrally mediated, respiratory and cardiac dysfunction, also referred to as a ‘postictal neurovegetative breakdown’.
3. Insights from post-mortem neuropathology
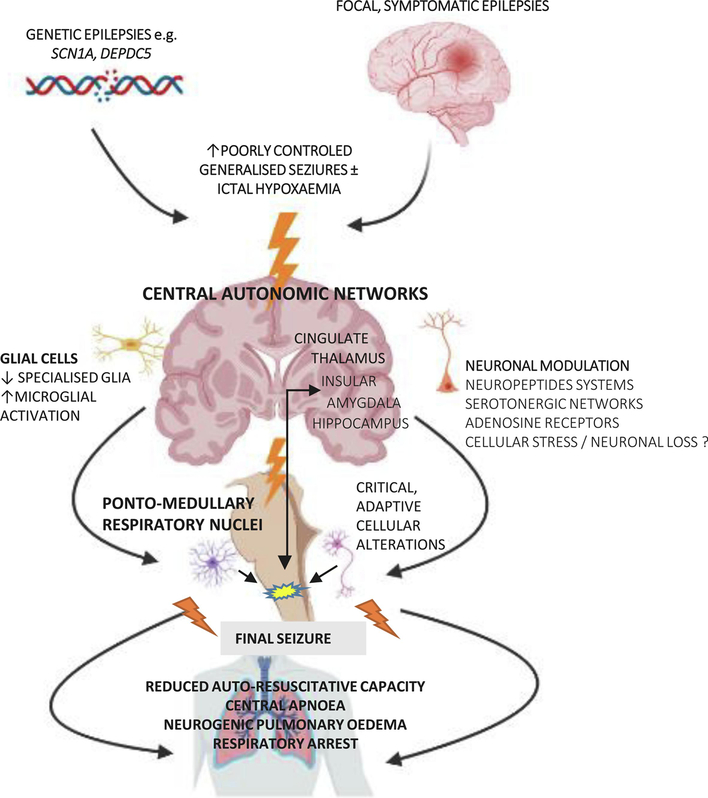
Neuropathologists performing autopsies have recognised for centuries that patients with epilepsy can die suddenly with no anatomical or toxicological cause of death found, i.e. a ‘negative’ post-mortem examination (Krohn, 1963; Sommer, 1880). In studies of post-mortem SUDEP series, common neuropathology findings include mild degrees of cerebral oedema (gyral effacement, uncal grooving) and congestion; in a larger series, macroscopic lesions were identified in 52% (old traumatic lesions 17%, hippocampal sclerosis HS 21%, cortical malformations 15%) (Thom et al., 2016). From the published series to date however (Thom et al., 2018), there is no evidence that any specific lesional pathology carries a greater burden of risk for SUDEP. It is also recognized that epilepsy can induce widespread cellular changes, recognized in the post-mortem brain, likely representing secondary or ‘acquired’ neuropathology. These alterations encompass diverse processes from neuronal or synaptic loss, neuronal hypertrophy, axonal sprouting to glial and inflammatory activation (Blanc et al., 2011; Sinjab et al., 2013; Thom et al., 2012; Thom et al., 2005). One hypothesis proposes that repeated generalised seizures leads to accumulative brain changes and increased susceptibility to SUDEP (Figure 1).
Figure 1.
Hypothesis for a common pathway of acquired seizure-related neuropathology involving autonomic regions that could culminate in increased susceptibility to SUDEP.
(The figure made using icons from Biorender).
Neuropeptidergic systems, for example, show significant dysregulation in epilepsy (Mazarati, 2004) and represent molecular candidates for exploration in SUDEP for several reasons. Neuropeptide Y, galanin, somatostatin, substance P, and dynorphin have widespread CNS distribution, particular in autonomic and brainstem centres, and are powerful modulators of neurotransmitter activity (Chi et al., 2018; Clynen et al., 2014; Kovac et al., 2013). Stored in large dense vesicles in interneurons and released on high frequency firing, they have a long half-life with long-lasting effects, interact with monoaminergic systems and exert both pre- and post-synaptic actions. Many also have anti-seizure effects (Mazarati, 2004) but are degraded by peptidases and can be transiently depleted following seizures or status epilepticus (Mazarati, 2004). Extensive experimental and human studies demonstrate alterations of neuropeptidergic neurones, neuropeptide release, axonal networks and neuropeptide receptors in limbic and cortical regions and the malleability of these systems in response to seizures and epilepsy (Chi et al., 2018; Clynen et al., 2014; Kovac et al., 2013; Mazarati, 2004; Thom, 2014).
Seizure-related neuropathology likely reflects the direct effects of cell injury (reversible or irreversible) and adaptive or re-organisational plasticity, possibly as neuroprotective phenomena, but which can further modulate neuronal function and both potentiate or dampen pro-epileptogenic networks. If such plasticity involves CNS autonomic regulatory regions there is a theoretical vulnerability for defective or inappropriate autonomic responses during seizures and therefore to SUDEP (Figure 1). In brainstem respiratory networks there is a recognized, remarkable capacity for compensatory adaptation or ‘fine tuning’ to respond to physiological challenges, including intermittent or sustained episodes of increased pCO2 or reduced O2 (Clayson et al., 2020; Dereli et al., 2019; Doi et al., 2010; Kang et al., 2017; Mitchell et al., 2001; Reeves et al., 2006; Smith et al., 2013). For example, a reduction in medullary serotonergic neurones occurs in an experimental conditions of induced hypercapnia (Burgraff et al., 2019). Such ‘respiratory neuroplasticity’ may conceivably occur in patients in response to frequent generalized seizures and associated ictal hypoxaemias (Lacuey et al., 2018). Indeed, in a clinical study of epilepsy patents on monitoring units, reduced ventilatory responses to increased pCO2 was observed in some patients and associated with the severity of postictal CO2 levels as well as SUDEP (Sainju et al., 2019). Postictal hypoperfusion of brainstem respiratory centres was observed in all patients following generalised but not focal seizures (Liu et al., 2020) and a neuroimaging study noted more severe brainstem volume loss in epilepsy patients with severe ictal hypoxia (Allen et al., 2020). Furthermore, brainstem volume reduction on MRI in TLE correlated with reduced heart rate variability (Mueller et al., 2018). In summary, brainstem pathology may be a consequence of seizures but in turn can mediate autonomic dysfunction in subsequent seizures.
4. Neuropathology studies of brain stem autonomic centres in SUDEP.
4. 1. Pre-Bötzinger complex
Brainstem respiratory rhythm generating circuitry comprises interacting nuclear groups in the medulla and pons, the Bötzinger and pre-Bötzinger complex (pre-BötC) and retrotrapezoid nucleus (RTN), under modulation by interconnecting nuclei (Smith et al., 2013) (Figure 2). The pre-Bötzinger complex is the principal kernel of inspiratory rhythm generation with pacemaker activity (reviewed in (Ghali, 2019)), multi-transmitter systems, complex interconnections and high modulation to accommodate adaptation under normal physiological conditions (Ramirez et al., 2012). Although extensively studied in animals, its anatomical location in the human medulla was only outlined in 2011 (Schwarzacher et al., 2011). It is composed of pacemaker-like somatostatin and excitatory neurokinin 1 receptor (NK1R) positive cells (Guyenet et al., 2001) as well as glycinergic and GABAergic interneurons; these form an ill-defined region in the reticular zone of the ventro-lateral human medulla (VLM) extending from obex level 6mm to 12mm. In animals there a rostro-caudal organisation of the ventral respiratory groups in the VLM; the Bötzinger complex (expiratory rhythms) and pre-BötC are more rostral than ventral respiratory groups that co-ordinate output to the phrenic and spinal motor neurones (Smith et al., 2013).
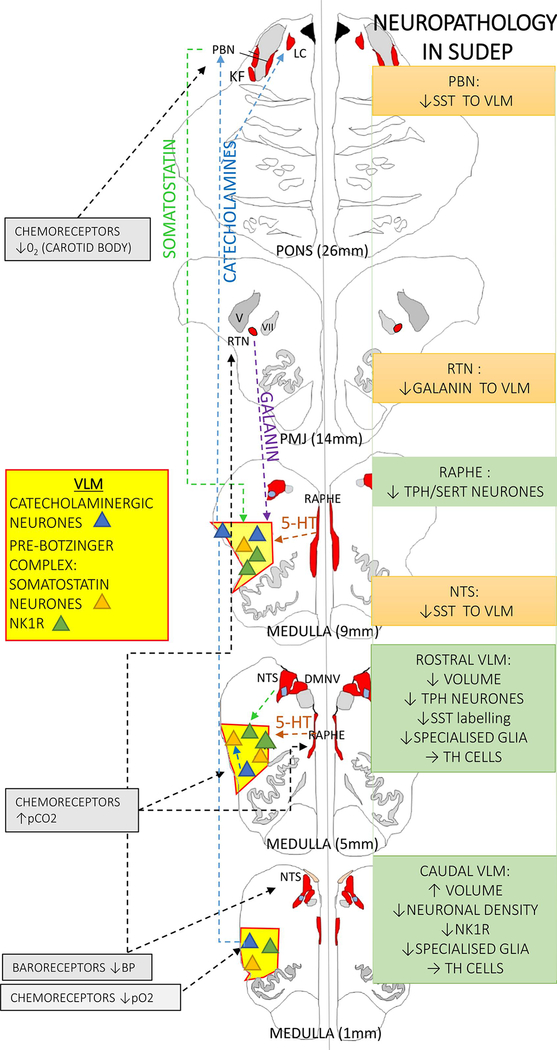
Figure 2. Medullary respiratory regulatory pathway and evidence for involvement in SUDEP.
The autonomic nuclei under study (as detailed in the text) have been highlighted only for simplicity (the autonomic and respiratory nuclei are shown in red and the ventrolateral medulla (VLM) region in yellow) and neuronal groups in VLM depicted as triangles; blue for catecholaminergic, orange for somatostatin neurones and green for NK1R positive neurones (neurokinin 1 receptor). The dashed lines indicate some of the known modulatory pathways shown on the left hand side. On the right hand side a summary of the cellular findings in SUDEP is detailed; green boxes are observations (see main text for detail) and orange boxes are changes inferred or hypothesised from observations but requiring substantiation in further studies. TH= tyrosine hydroxylase, SST= somatostatin, PBN= parabrachial nucleus, NTS= nucleus of tracus solitarius, RTN= retrotrapezoid nucleus
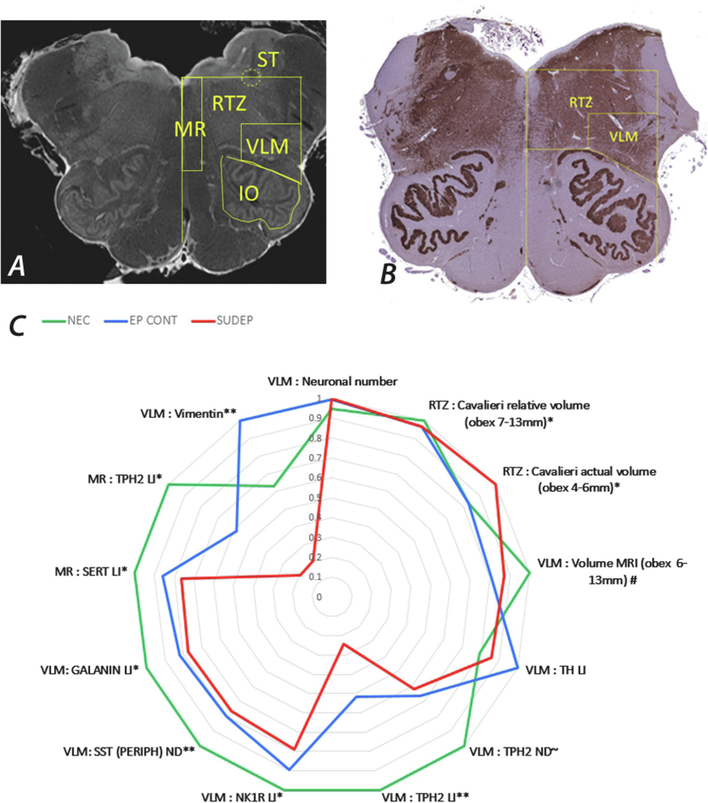
In-vivo quantitative MRI studies in SUDEP have shown volume loss in the medulla (Mueller et al., 2014; Mueller et al., 2018) although not in all studies (Allen et al., 2019b). This was recently further investigated in post-mortem brainstem samples using high field 9.4 Tesla MRI (Figure 3a,b) in addition to the Cavalieri stereological method on tissue sections for regional medullary volume estimations. Defined autonomic regions of the reticular zone and the VLM in SUDEP groups were compared to controls (Patodia et al., 2020b). Controls included non-epilepsy sudden deaths and epilepsy controls without a SUDEP. With these two methods, decreased volumes, specifically in the VLM region (but not in other brainstem regions) were identified in the rostral medulla in epilepsy compared to non-epilepsy cases, with evidence for greater volume reduction in SUDEP. In contrast, in the caudal medulla greater reticular zone volumes were observed in the SUDEP group. Our observation of lower volumes in SUDEP cases in the rostral medulla could be relevant to acquired pathology localising with the pre-BötC region.
Figure 3. Summary of Brainstem quantitative studies.
A. MRI of post-mortem brainstem (axial slice) using 9.4T imaging and the regions for volume estimation show; MR = medullary raphe, VLM = ventrolateral medulla, IO = inferior olive, RTZ = reticular zone and ST = solitary tract region. B. Tissue section immunolabelled with synaptophysin and showing some of the similar regions of interest outlined with anatomical co-ordinates that were used for volume estimations and cell density measurements. C. A radar plot to illustrated the mean measurements in the post-mortem group studies (red = SUDEP, blue = epilepsy (non-SUDEP) controls EP CONT and green = non- epilepsy controls). These studies are detailed in the main text. TH= tyrosine hydroxylase, TPH2 = tryptophan hydroxylase, NK1R = neurokinin 1 receptor, SST = somatostatin, SERT = serotonin transporter. ** signifies significant differences between SUDEP group and both NEC and EP CONT, *differences between SUDEP and NEC, ~ differences in SUDEP cases only noted at specific medullary obex levels (see text), # epilepsy group (blue) includes is all epilepsy cases (EP CONT + SUDEP) with significant differences shown from NEC. For the data on the MR TPH2 labelling index, the definite SUDEP cases only are shown.
In parallel studies neuronal subtypes in the VLM were quantified. A reduction in somatostatin neuronal labelling in the VLM, most significantly in the rostral medulla (obex 7–9mm) was found in the SUDEP group compared to normal controls (Patodia et al., 2018), therefore aligning with the previously defined level of the pre-BötC (Schwarzacher et al., 2011) (Figure 2 and 3). Somatostatin is a neuropeptide which acts as an inhibitory respiratory modulator (Cui et al., 2016; Kaczynska et al., 2018) with endogenous release in the pre-BötC stabilising breathing rhythmicity during normoxia (Kaczynska et al., 2018). Subgrouping somatostatin neurones, a specific reduction in cells with a peripheral rim of labelling was observed, in keeping with synaptic terminals, but not in neurones with diffuse cytoplasmic labelling, interpreted as the endogenously somatostatin synthesising pre-BötC neurones (Figure 4d–f). Animal studies show that somatostatin projections to the pre-BötC neurones arise mainly from the parabrachial nucleus, solitary tract nucleus as well as other regions (Bou Farah et al., 2016), modulating respiratory activity (Cui et al., 2016; Epelbaum et al., 1994) (Figure 2). Our findings therefore supported a reduction in modulating input, rather than a reduction of pre-BötC somatostatin synthesising capacity in SUDEP. Neurokinin 1 receptor (NK1R) expression (Patodia et al., 2018), present in different regions of the ventral respiratory complex (Schwarzacher et al., 2011; Wei et al., 2012) was also investigated as a second pre-BötC neuronal marker, co-localising with somatostatin neurones (Patodia et al., 2018). NK1R is preferentially activated by the neurokinin substance P, which has functionally diverse roles in respiratory reflexes (Szereda-Przestaszewska et al., 2020). Quantification of NK1R labelling in SUDEP (Figure 4b) showed a reduction in the caudal medulla VLM only, at obex level 3–4mm compared to control groups. Interestingly, stereological quantification of total VLM neurones with cresyl violet stain (Figure 4a) also identified a reduction in neuronal number at this obex level only (Patodia et al., 2018). These findings together with the MRI study of higher reticular zone volumes in SUDEP in the caudal medulla (Patodia et al., 2020b) implicate additional pathology in the ventral respiratory groups that determine the motor control of respiration (Figure 2, 3).
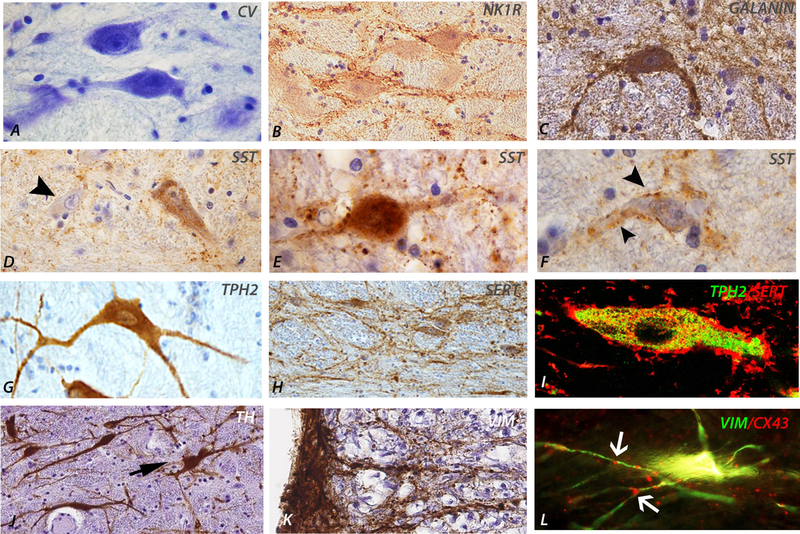
Figure 4. Medullary neuronal glial cell types and immunomarkers.
A. Neurones in the ventrolateral medulla (VLM) region of medium size and stained with cresyl violet B. Neurokinin 1 receptor labelled neurones showing a peripheral rim of labelling. C. Galanin labelled neurone in the VLM region in addition to diffuse synaptic processes. D. Somatostatin (SST) labelling of neuronal populations in the VLM showing also a negatively labelled neurone (arrowed) and in E. intensely labelled neurones. F. In addition some neurones in the VLM also displayed a peripheral rim of STT labelling in keeping with synaptic input. G. Tryptophan hydroxylase (TPH2) labelling of serotonergic medullary neurones showing dense labelling of the perikarya. H. Serotonin transporter (SERT) showed a more membranous pattern of cellular labelling in the medullary raphe region. I. Double labelling of SERT and TPH2 showed cellular co-localisation in the raphe nucleus. J. Tyrosine hydroxylase (TH) labelled catecholaminergic neurones in the VLM region. K. Vimentin labelling of distinct astroglial subsets in the brainstem, including on the lateral medullary surface, extending processes in to the VLM. L. Double labelling of vimentin with connexin 43 showed distinct glial cell populations in the medullary raphe and VLM region with punctate hemichannel aggregates (arrows).
4.2. Serotonergic medullary networks in SUDEP
Serotonin (5-HT), synthesised in the medullary raphe nuclei, acts as an excitatory modulator of inspiration, stimulating respiratory centres, including the pre-BötC neurones in response to hypercapnia (Richerson, 2013; Szereda-Przestaszewska et al., 2020) and has been a focus of recent SUDEP research (Murugesan et al., 2018; Petrucci et al., 2021; Richerson, 2013; Richerson et al., 2016; Tupal et al., 2006; Uteshev et al., 2010; Zhan et al., 2016). Rostral brainstem serotonergic monoaminergic networks from the dorsal raphe also modulate arousal and myriad physiological brain functions through their widespread projection to other brain regions (Beliveau et al., 2017). Serum serotonin levels are raised following generalized but not focal seizures (Murugesan et al., 2018) and abnormal serotonergic neuronal firing during seizures has been observed experimentally (Zhan et al., 2016). 5-HT is known to inhibit seizures, reduce seizure susceptibility and 5-HT availability contributes to the anticonvulsant action of several common anti-epilepsy drugs (Petrucci et al., 2020). A recent study also showed a lower incidence of ictal central apnoea in patients receiving serotonin reuptake inhibitors (SRIs) that act on the pre-synaptic serotonin transporter (SERT) (Lacuey et al., 2019), highlighting potential therapeutic applications.
The 5-HT synthesising neurones (tryptophan hydroxylase (TPH2) expressing) in the medulla are primarily located in the midline raphe nuclei with smaller numbers in the VLM region (Benarroch, 2014) (Figure 4g). There is extensive post-mortem literature implicating alterations of serotonergic neuronal populations in sudden infant death (Kinney et al., 2019), with fewer reports in adult studies of neurodegenerative conditions with autonomic dysfunction and sudden death (Presti et al., 2014; Schwarzacher et al., 2011; Tada et al., 2009). Quantifying TPH2 and SERT expressing neurones in the VLM and medullary raphe (Figure 4h,i) showed a reduction in SUDEP compared to controls groups (Patodia et al., 2018); specifically, this was noted for TPH2 expressing cells in the rostral VLM at obex level 7–9 mm and for SERT in the raphe nucleus at obex level 7–8mm (Figure 2,3). This suggests reduced 5-HT synthesising capacity in the VLM region as well as re-uptake by serotonergic neurones in the Raphe which may manifest as an overall reduction in availability in the pre-BötC region, of relevance in the vulnerable hypercapneic post-ictal period in terms of diminished reserves for augmenting ventilatory responses.
4. 3. Catecholaminergic medullary neurones
The above findings support alteration of specific neuronal groups in the medulla in SUDEP, more likely to be adaptive and potentially reversible. The question remains if alterations involve the brainstem systems more widely or if there is specific vulnerability of respiratory nuclear groups in epilepsy. The catecholaminergic neurones of the medulla (Tyrosine hydroxylase (TH) expressing, the rate-limiting enzyme in the synthesis of DOPA), include neurones within the VLM (C1 adrenergic neurones) that form the putative vasomotor centre regulating blood pressure but also influencing respiratory drive (Guyenet et al., 2013). VLM catecholaminergic neurones project to the pre-BötC, stimulating breathing in hypoxic conditions (Kang et al., 2017) and sleep-state dependent cardio-respiratory arousal (Abbott et al., 2013), of relevance to SUDEP as many deaths occur nocturnally. Experimental depletion of catecholaminergic neurones in the VLM impairs respiratory responses (Malheiros-Lima et al., 2017). Other groups of TH-expressing neurones are located in the dorsal medial medulla near the nucleus tractus solitarius, but their physiological function is less understood (Sevigny et al., 2012). Experimental studies have shown that both seizures (Silveira et al., 2000) and hypoxia (Kanter et al., 1996) increase VLM catecholaminergic neuronal activity which may underlie the increased blood pressure and sympathetic activity observed following some seizures (Simon, 2010).
Using similar cohorts (SUDEP, epilepsy-control groups and non-epilepsy controls) as in the study of Pre-BötC neurones, quantification of TH-expressing neurones in the VLM and dorsal medulla (Figure 4j) did not reveal any differences between cause of death groups. This contrasts to studies in Sudden infant deaths (SIDS), where a reduction of medullary TH-IR neurones was identified in some (Obonai et al., 1998; Ozawa et al., 2002) although not all series (Sawaguchi et al., 2003) and also in adults with Multiple System Atrophy associated with sudden death (Benarroch et al., 2005; Tada et al., 2009). Preservation of TH neurones in SUDEP also contrasts with the reduction of TPH2 neurones in the VLM, in support of a selective vulnerability.
However, neuronal preservation does not exclude catecholaminergic neuronal dysfunction. C-fos, an early/immediate gene and marker of recent neuronal and synaptic activation has been used experimentally to identify acute seizure-related brain injury (Barros et al., 2015; Herrera et al., 1996; Mraovitch et al., 1999) as well as monitoring respiratory neuronal activation (Wang et al., 2015). c-fos in SUDEP appeared distributed primarily in medullary autonomic regions, including TH and TPH cells (Patodia et al., 2020c) but with fewer c-fos neurones in SUDEP compared to non-epilepsy sudden death controls. Although this could indicate diminished activation in VLM neurones prior to death, due to the limitations of post-mortem analysis and ante-mortem factors, other measures of neuronal activation are needed to validate this finding (Hudson, 2018; Sauvage et al., 2019) (Herrera et al., 1996).
Furthermore, in a case of severe childhood epilepsy following perinatal hypoxic-ischaemic brain injury and subsequent sudden death, intracellular polyglucosan bodies were observed and primarily located in the TH neurones of the medulla (Patodia et al., 2021). Polyglucosan bodies represent sequestered aggregates of ubiquitinated and p62 enriched insoluble glucose polymers and likely represent a cellular protective mechanism in response to a pathogenic insult (Brewer et al., 2020). Interestingly, a predilection for p62 inclusions in the dendrites of brainstem catecholaminergic neurones has been observed with aging, suggesting an intrinsic susceptibility of these neurones to cellular metabolic stresses (Braak et al., 2013). Accelerated ‘aging’ of TH medullary populations in epilepsy could conceivably reduce their functional capacity without neuronal loss which needs to be a future focus of research.
4.4. Galaninergic medullary networks
Galanin is a bioactive peptide, with overall inhibitory action, that modulates noradrenergic and serotonergic networks and the pre-BötC (Medel-Matus et al., 2017). Brainstem galaninergic neurones are primarily located in the nucleus of the solitary tract, VLM, retrotrapezoid nucleus (RTN) and locus coeruleus (Bochorishvili et al., 2012; Spirovski et al., 2012). The neurones of the RTN, critical for central respiratory chemoreflexes and responses to pC02, synapse with NK1R neurons of the pre-BotC and activate breathing (Bochorishvili et al., 2012). In SUDEP cases, a decrease in galanin immunolabelling in the VLM region, but not in the medullary raphe, was shown compared to control groups (Patodia et al., 2018) (Figure 4c). In rodents, there is no projection from the RTN galaninergic neurons to the medullary raphe (Bochorishvili et al., 2012) (Figure 2); these observations could therefore indicate dysfunction of the RTN nucleus in SUDEP. A recent experimental study, showed reduced galanin mRNA in the RTN following a short exposure of six hours to 5% pCO2 yet with the opposite effect following long-term exposure (Dereli et al., 2019). In a paediatric study, depressed CSF galanin was noted in epileptic encephalopathy but elevated during periods of status epilepticus (Tekgul et al., 2020). Further in-depth investigation of the human galaninergic brainstem networks, its modulation by seizures or seizure-related apnoea and impact on SUDEP mechanism is needed.
4. 5. Medullary glia in SUDEP
Specific subsets of glial cells in the brain stem also act as chemoreceptors to pC02 levels, modulating neuronal function via gliotransmitters, such as adenosine (Falquetto et al., 2018) through connexin hemichannels (Huckstepp et al., 2010). They have been identified on the medullary surface (Huckstepp et al., 2010; Sobrinho et al., 2017), in the RTN (Sobrinho et al., 2017) and in the pre-BötC (Ikeda et al., 2017) and considered an integral component of respiratory homeostasis systems (Czeisler et al., 2019), including during physiological challenges (Sheikhbahaei et al., 2018b). Reduction in these specialised glia has been associated with respiratory dysfunction experimentally (Fernandes-Junior et al., 2018). In SUDEP post-mortem cases, lower densities of regionally specific and morphologically distinct glial cell types, labelled with vimentin, connexin43 and adenosine receptor A1R (Figure 4k,l), were observed in the VLM, along the medullary surface (Figure 4k) and in the medullary raphe (Figure 4l) compared to epilepsy and non-epilepsy control groups (Patodia et al., 2019). Although functional studies are not feasible in post-mortem tissue samples, their morphology, distribution and immunophenotype are in keeping with specialised glia with respiratory modulatory roles (SheikhBahaei et al., 2018a). Alteration in their distribution and density could also be of relevance to the concerted re-organisation of medullary respiratory networks in epilepsy.
5. High-risk genes for SUDEP and neuropathology findings
Recent genetic studies have demonstrated heterogeneity, complexity and potentially oligogenicity (interactive influence of a small number of genes) in SUDEP (Goldman et al., 2016). SUDEP risk genes identified so far mainly align with known epilepsy genes e.g. SCNI1A, associated with Dravet syndrome (Shmuely et al., 2016) and DEPDC5 associated epilepsy (Ribierre et al., 2018), or cardiac-epilepsy genes, for example epilepsy associated with long-QT syndrome, which arguably represent ‘cardiac-SUDEP’; there is less evidence for SUDEP genes independently influencing respiratory or autonomic systems (Friedman et al., 2018). Future research exploring if SUDEP genetic risk factors predict distinct mechanisms of death, and therefore personalized prevention strategies, is essential. For example, in a depdc5 mouse model, SUDEP was prevented through inhibition of MTORC1 (Klofas et al., 2020).
An important goal of future neuropathology, neuroimaging and clinical research is to stratify SUDEP cases according to genetic risk factors. Dravet syndrome with SCN1A mutations is associated with the highest rate of SUDEP (Cooper et al., 2016). In the above discussed studies, a cohort of seven Dravet cases was compared to all other non-genetically characterized SUDEP cases (Patodia et al., 2019; Patodia et al., 2018; Patodia et al., 2020b; Patodia et al., 2020c); the only difference noted was a higher TH neuronal density in the medullary raphe in Dravet cases (Patodia et al., 2020c). Clearly, larger genetically characterized SUDEP cohorts are required to dissect any differences in brainstem neuronal modulation.
6. Central autonomic networks connecting to the brainstem
The central autonomic network (CAN) refers to the supratentorial brain regions involved in autonomic modulation with connection to brainstem centres (Beissner et al., 2013) (Valenza et al., 2020; Valenza et al., 2019) (Figure 1). These regions orchestrate physiological and volitional modulation of cardio-respiratory output e.g. during exercise, speech, emotional responses and singing (Urfy et al., 2014). This complex network and their reciprocal inter-connectivity with the brainstem, as well as regionally specific anatomical functions, are still a focus of clinical investigation. For example, recent electrical stimulation studies of the human insular cortex subregions showed direct effects on cardiac function (Sanchez-Larsen et al., 2021). The hypothesis in SUDEP is that abnormal activity in CAN regions, either during a seizure or spreading depolarisation following a seizure, is propagated or descends to the brainstem, as has been shown experimentally (Loonen et al., 2019). Functional MRI studies in SUDEP, or epilepsy patients considered at higher risk, reveal altered connectivity between autonomic cortical and subcortical cardiac and respiratory regulatory regions, suggesting reorganisation of these networks (Allen et al., 2017; Allen et al., 2019a; La et al., 2019; Tang et al., 2014). Structural MRI studies have also revealed distinct regional patterns of increased or decreased MRI grey matter volumes in SUDEP, some of which coincide with autonomic brain regions such as the amygdala and thalamus (Allen et al., 2019a; Allen et al., 2020; Ogren et al., 2018; Wandschneider et al., 2015).
6. 1. Studies of the amygdala in SUDEP
The amygdala has been a major region of research in SUDEP. The amygdala is a nuclear complex with direct and indirect connections with the brainstem respiratory nuclei, including the pre-BötC (Bzdok et al., 2013; Swanson et al., 1998; Yang et al., 2020). The lateral nucleus of the amygdala can generate spontaneous inter-ictal activity (Graebenitz et al., 2011) and seizure propagation to the amygdala is associated with apnoea in some (Nobis et al., 2019) but not all case studies (Park et al., 2020). Electrode stimulation of the amygdala region in patients undergoing investigations for epilepsy (Dlouhy et al., 2015; Lacuey et al., 2017; Nobis et al., 2018) resulted in apnoeic episodes. These occurred following stimulation of the medial amygdala (Nobis et al., 2018) and lateral and basolateral nuclei (Dlouhy et al., 2015). More recently, a study in paediatric epilepsy patients has localised a specific amygdala region, overlapping the basolateral, basomedial, cortical medical nuclei and intercelated nuclei, associated with inhibiting respiratory activity (Rhone et al., 2020).
An earlier post-mortem study investigated pathology of the central nucleus in SUDEP, in view of the greater evidence for its direct connections with respiratory brainstem nuclei (Yang et al., 2020). Lower neuronal densities and increased astrocytic densities were noted in lateral nucleus of amygdala but not the central nucleus compared to normal controls (Thom et al., 1999). However as there was no epilepsy control group the specificity of this sclerosis pattern for SUDEP was uncertain. In a further immunohistochemistry study, Michalak et al., reported no significant difference between SUDEP, epilepsy controls and normal controls in amygdala astroglial populations as well as microglial densities (using CD163 and HLA-DR immunolabelling) and also no evidence of blood brain barrier breakdown as markers of acute neuropathology in SUDEP (Michalak et al., 2017). The amygdala is enriched in galanin (Gentleman et al., 1989; Perez et al., 2001), NPY (Adrian et al., 1983) and SST (Geola et al., 1981) compared to other brain regions. A quantitative immunohistochemistry study showed a reduction in galanin in the lateral nucleus in SUDEP cohorts compared to normal controls (Somani et al., 2020). Interestingly, all neuropeptides studied (galanin, NPY and somatostatin) were higher in epilepsy-controls than both SUDEP cases and normal controls indicating depletion in SUDEP compared to relative augmentation of networks in chronic epilepsy (Somani et al., 2020). One interpretation is that neuropeptide consumption or “exhaustion” occurs in SUDEP, resulting in neuronal fatigue with consequent effects on amydgala-brainstem networks.
More recently the mesial temporal lobe structures and amygdala have been investigated in SUDEP for adenosine receptors and its clearance mechanisms (Patodia et al., 2020a). Adenosine is a suppressor of seizure activity and candidate molecule in SUDEP (Tescarollo et al., 2020). The adenosine hypothesis of SUDEP proposes that fatal respiratory depression and impaired arousal are mediated through the adenosine receptors in autonomic and brainstem centres in the post-ictal period (Boison, 2012; Erlichman et al., 2010; Scislo et al., 2005; Shen et al., 2010; Zhang et al., 2013). Astrocytes regulate adenosine levels during and after seizures with astroglial adenosine kinase (ADK), the major metabolic clearance route (Weltha et al., 2018). Surgical tissues from patients who had undergone resective surgery for temporal lobe epilepsy with hippocampus sclerosis were used, and the cases risk stratified for SUDEP according to the frequency of generalised seizures pre-operatively. Quantitative immunohistochemistry for ADK in the amygdala revealed similar findings across SUDEP risk groups. However, significantly increased adenosine receptor A1R in the amygdala but lower A2AR in the epilepsy patients regarded as high risk for SUDEP compared to low risk was seen (Patodia et al., 2020a). Of relevance, tolerance to hypoxia is mediated through A1R (Fredholm et al., 2011) and patients with apnoea associated with amygdala stimulation characteristically show no dyspnoeal symptoms (Dlouhy et al., 2015; Rhone et al., 2020). Furthermore, adenosine is implicated in the prolonged depression of synaptic transmission after spreading depolarization via A1R receptor activation (Lindquist et al., 2012). Spreading depolarisation to the brainstem has been shown to mediate irreversible respiratory collapse in experimental SUDEP genetic models (Aiba et al., 2015; Loonen et al., 2019) although not in wild-type animals (Jefferys et al., 2019). In some experimental SUDEP models depolarisation spreads through the amygdala prior to brainstem progression (Loonen et al., 2019); observed alterations in adenosine receptor levels and distribution in both the amygdala and brainstem may therefore be of relevance.
The amygdala is also highly enriched in serotonin networks from the midbrain raphe (Beliveau et al., 2017) and SERT immunohistochemistry has been used as specific tissue marker to demonstrated and quantify serotonergic afferent networks (Asan et al., 2013). Serotonergic axons have been shown to both regenerate following injury in addition to compensatory sprouting from non-injured axons therefore demonstrating a unique potential for repair and remodelling (Kajstura et al., 2018). In a post-mortem series, increased SERT immunolabelling in the basal and accessory basal nuclei of the amygdala and peri-amygdala cortex in SUDEP compared to epilepsy controls as well as higher hippocampal SERT in TLE patients at higher risk for SUDEP has been observed (Patodia et al., Submitted). Localised increased SERT in the amygdala in SUDEP may have again have functional implications through reduced availability of 5-HT in the vulnerable post-ictal period, deregulating critical intra and inter-amygdala connections.
6. 2. The hippocampus in SUDEP
The hippocampus is recognised to have autonomic regulatory functions and connections with the brainstem (Arrigo et al., 2017; Edlow et al., 2016) and experimental stimulation confirms hippocampal modulation of respiratory and cardiovascular activity (Ajayi et al., 2018) (Lacuey et al., 2017). Sudden unexplained deaths in infancy and childhood, share some circumstantial similarities with SUDEP, and developmental anomalies of the hippocampus have been reported by several groups, including malrotational and granule cell layer broadening as a potential disease biomarker (Kinney et al., 2009; Kinney et al., 2015; Kinney et al., 2016; Kon et al., 2020). This was a basis for a similar study in SUDEP, particularly as similar hippocampal cytopathological alterations occur in temporal lobe epilepsy (Blumcke et al., 2013). In a series of 187 adult post-mortems, morphometric evaluation of the hippocampus shape and size showed that, although malrotational abnormalities of the hippocampus were more frequent in SUDEP, these were not significantly increased compared to control groups (Somani et al., 2019). In addition, the granule cell layer thickness (excluding cases with hippocampal sclerosis) was increased in the epilepsy control group but not in SUDEP, indicating a lack of an association of this developmental or ‘neo-migratory’ abnormality with SUDEP (Somani et al., 2019). A significantly increased parahippocampal gyrus length was however noted in SUDEP (Somani et al., 2019), in keeping with MRI observations showing increased grey matter volumes of this region (Wandschneider et al., 2015) (Allen et al., 2019b).
6. 3. Neocortical and subcortical regions in SUDEP
Although dysfunction of higher central autonomic networks has been proposed in SUDEP from structural and functional imaging studies (Allen et al., 2019a) there is, yet limited understanding of the underlying cellular pathology. Recently investigations of Iba1-expressing microglia as a marker of seizure activity in fourteen cortical and subcortical brain regions from post-mortem cases noted significantly regionally increased microglia, including the parahippocampal gyrus, superior temporal gyrus and pulvinar in SUDEP (Somani et al., 2021). These brain regions have recognised roles in cardio-respiratory regulation (Arrigo et al., 2017; Edlow et al., 2016) (Beissner et al., 2013; Harper et al., 2005; Koos et al., 2004; Macey et al., 2005) (Yang et al., 2018). Therefore the identification of inflammatory pathways activation is of potential relevance to both regional cortical dysfunction as well understanding the cellular basis of atrophy observed on MRI (Wandschneider et al., 2015) and warranting further investigation.
7. Future directions and conclusions
The limitations and challenges of post-mortem studies in epilepsy and SUDEP, as well as the importance of investing in future systematic state of the art biobanking to optimise sample collection and stratification for future research studies in epilepsy, has been previously reviewed (Thom et al., 2017). Epilepsy of many causes can result in SUDEP but whether there are common or distinct pathways of autonomic dysfunction leading to SUDEP, is as yet unproven. Quantitative regional immunohistochemistry studies, as described above, are an exploratory starting point that need to be further corroborated in further study designs to identify candidate cellular mechanisms. Single cell approaches, eg for somatic mutations, in addition to germline mutations (eg for DEPDC5 epilepsies (Ribierre et al., 2018)) in critical autonomic regions as the brainstem may be insightful. Fuller genetic characterization of study groups is essential to correlate with neuropathological findings. Regional ‘omics’ studies, including proteomics and transcriptomics, for metabolic pathways elucidation in autonomic regions are needed to validate immunohistochemistry findings. Indeed, such studies using proteomics methods are underway (Leitner et al., 2021) and will become more feasible in the future with advancing multiplexing techniques. Larger, clinically and neuropathology defined SUDEP cohorts need to be investigated, stratified according to known ictal-autonomic dysfunction during life or if a competing cause of death or neuropathology lesion is identified at post-mortem and factoring for the circumstances and findings at death, for example if found in a compromised prone position and the level of pulmonary and cerebral oedema. Comparisons with different clinical control groups could provide insight, for example cases with chronic respiratory diseases in addition to clinically well-characterised epilepsies with similar seizure severity but without SUDEP, to enable comparison of brainstem hypoxic versus seizure-related neuroplasticity. Extending similar examination to interconnected brainstem and cortical autonomic regions, as well as non-autonomic regulatory regions, is also essential to evaluate the extent and specificity of any pathological changes.
The ultimate goal is the further identification of specific disease biomarkers for SUDEP, the clinical screening of at risk patient groups, and for exposing potential treatment targets of pharmacologically modifiable and reversible pathological alterations. Future tissue-based studies, if invested in, can have a valuable impact in this endeavour.
Acknowledgments
The neuropathology research detailed in this review was undertaken as part of the Centre for SUDEP Research (CSR) collaboration, supported through the National Institute of Neurological Disorders And Stroke of the National Institutes of Health (Award Numbers, neuropathology of SUDEP: 5U01NS090415 and SUDEP admin core grant: U01-NS090405). Epilepsy Society through the Katy Baggott Foundation, supports the UCL Epilepsy Society Brain and Tissue Bank and the ERUK supports the Corsellis Epilepsy brain collection at UCL. This work was undertaken at UCLH/UCL who received a proportion of funding from the Department of Health’s NIHR Biomedical Research Centres funding scheme.
Footnotes
Disclosure statement
None of the authors has any conflict of interest to disclose. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abbott SB, Coates MB, Stornetta RL, Guyenet PG 2013. Optogenetic stimulation of c1 and retrotrapezoid nucleus neurons causes sleep state-dependent cardiorespiratory stimulation and arousal in rats. Hypertension 61, 835–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adrian TE, Allen JM, Bloom SR, Ghatei MA, Rossor MN, Roberts GW, Crow TJ, Tatemoto K, Polak JM 1983. Neuropeptide Y distribution in human brain. Nature 306, 584–586. [DOI] [PubMed] [Google Scholar]
- Aiba I, Noebels JL 2015. Spreading depolarization in the brainstem mediates sudden cardiorespiratory arrest in mouse SUDEP models. Science translational medicine 7, 282ra246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ajayi IE, McGovern AE, Driessen AK, Kerr NF, Mills PC, Mazzone SB 2018. Hippocampal modulation of cardiorespiratory function. Respiratory physiology & neurobiology 252–253, 18–27. [DOI] [PubMed] [Google Scholar]
- Allen LA, Harper RM, Kumar R, Guye M, Ogren JA, Lhatoo SD, Lemieux L, Scott CA, Vos SB, Rani S, Diehl B 2017. Dysfunctional Brain Networking among Autonomic Regulatory Structures in Temporal Lobe Epilepsy Patients at High Risk of Sudden Unexpected Death in Epilepsy. Frontiers in neurology 8, 544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Harper RM, Lhatoo S, Lemieux L, Diehl B 2019a. Neuroimaging of Sudden Unexpected Death in Epilepsy (SUDEP): Insights From Structural and Resting-State Functional MRI Studies. Frontiers in neurology 10, 185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Harper RM, Vos SB, Scott CA, Lacuey N, Vilella L, Winston JS, Whatley BP, Kumar R, Ogren J, Hampson JS, Rani S, Winston GP, Lemieux L, Lhatoo SD, Diehl B 2020. Peri-ictal hypoxia is related to extent of regional brain volume loss accompanying generalized tonic-clonic seizures. Epilepsia. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen LA, Vos SB, Kumar R, Ogren JA, Harper RK, Winston GP, Balestrini S, Wandschneider B, Scott CA, Ourselin S, Duncan JS, Lhatoo SD, Harper RM, Diehl B 2019b. Cerebellar, limbic, and midbrain volume alterations in sudden unexpected death in epilepsy. Epilepsia 60, 718–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrigo A, Mormina E, Calamuneri A, Gaeta M, Marino S, Milardi D, Anastasi GP, Quartarone A 2017. Amygdalar and hippocampal connections with brainstem and spinal cord: A diffusion MRI study in human brain. Neuroscience 343, 346–354. [DOI] [PubMed] [Google Scholar]
- Asan E, Steinke M, Lesch KP 2013. Serotonergic innervation of the amygdala: targets, receptors, and implications for stress and anxiety. Histochem Cell Biol 139, 785–813. [DOI] [PubMed] [Google Scholar]
- Barros VN, Mundim M, Galindo LT, Bittencourt S, Porcionatto M, Mello LE 2015. The pattern of c-Fos expression and its refractory period in the brain of rats and monkeys. Front Cell Neurosci 9, 72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beissner F, Meissner K, Bar KJ, Napadow V 2013. The autonomic brain: an activation likelihood estimation meta-analysis for central processing of autonomic function. The Journal of neuroscience : the official journal of the Society for Neuroscience 33, 10503–10511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beliveau V, Ganz M, Feng L, Ozenne B, Hojgaard L, Fisher PM, Svarer C, Greve DN, Knudsen GM 2017. A High-Resolution In Vivo Atlas of the Human Brain’s Serotonin System. The Journal of neuroscience : the official journal of the Society for Neuroscience 37, 120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE 2014. Medullary serotonergic system: organization, effects, and clinical correlations. Neurology 83, 1104–1111. [DOI] [PubMed] [Google Scholar]
- Benarroch EE, Schmeichel AM, Low PA, Boeve BF, Sandroni P, Parisi JE 2005. Involvement of medullary regions controlling sympathetic output in Lewy body disease. Brain 128, 338–344. [DOI] [PubMed] [Google Scholar]
- Blanc F, Martinian L, Liagkouras I, Catarino C, Sisodiya SM, Thom M 2011. Investigation of widespread neocortical pathology associated with hippocampal sclerosis in epilepsy: a postmortem study. Epilepsia 52, 10–21. [DOI] [PubMed] [Google Scholar]
- Blumcke I, Thom M, Aronica E, Armstrong DD, Bartolomei F, Bernasconi A, Bernasconi N, Bien CG, Cendes F, Coras R, Cross JH, Jacques TS, Kahane P, Mathern GW, Miyata H, Moshe SL, Oz B, Ozkara C, Perucca E, Sisodiya S, Wiebe S, Spreafico R 2013. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a Task Force report from the ILAE Commission on Diagnostic Methods. Epilepsia 54, 1315–1329. [DOI] [PubMed] [Google Scholar]
- Bochorishvili G, Stornetta RL, Coates MB, Guyenet PG 2012. Pre-Botzinger complex receives glutamatergic innervation from galaninergic and other retrotrapezoid nucleus neurons. The Journal of comparative neurology 520, 1047–1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boison D 2012. Adenosine dysfunction in epilepsy. Glia 60, 1234–1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bou Farah L, Bowman BR, Bokiniec P, Karim S, Le S, Goodchild AK, McMullan S 2016. Somatostatin in the rat rostral ventrolateral medulla: Origins and mechanism of action. The Journal of comparative neurology 524, 323–342. [DOI] [PubMed] [Google Scholar]
- Braak H, Thal DR, Matschke J, Ghebremedhin E, Del Tredici K 2013. Age-related appearance of dendritic inclusions in catecholaminergic brainstem neurons. Neurobiology of aging 34, 286–297. [DOI] [PubMed] [Google Scholar]
- Brewer MK, Putaux JL, Rondon A, Uittenbogaard A, Sullivan MA, Gentry MS 2020. Polyglucosan body structure in Lafora disease. Carbohydr Polym 240, 116260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruno E, Maira G, Biondi A, Richardson MP 2018. Ictal hypoxemia: A systematic review and meta-analysis. Seizure : the journal of the British Epilepsy Association 63, 7–13. [DOI] [PubMed] [Google Scholar]
- Burgraff NJ, Neumueller SE, Buchholz KJ, LeClaire J, Hodges MR, Pan L, Forster HV 2019. Brainstem serotonergic, catecholaminergic, and inflammatory adaptations during chronic hypercapnia in goats. FASEB J 33, 14491–14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bzdok D, Laird AR, Zilles K, Fox PT, Eickhoff SB 2013. An investigation of the structural, connectional, and functional subspecialization in the human amygdala. Human brain mapping 34, 3247–3266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi G, Huang Z, Li X, Zhang K, Li G 2018. Substance P Regulation in Epilepsy. Curr Neuropharmacol 16, 43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayson MS, Devereaux MEM, Pamenter ME 2020. Neurokinin-1 receptor activation is sufficient to restore the hypercapnic ventilatory response in the Substance P-deficient naked mole-rat. American journal of physiology. Regulatory, integrative and comparative physiology 318, R712–R721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clynen E, Swijsen A, Raijmakers M, Hoogland G, Rigo JM 2014. Neuropeptides as targets for the development of anticonvulsant drugs. Mol Neurobiol 50, 626–646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MS, McIntosh A, Crompton DE, McMahon JM, Schneider A, Farrell K, Ganesan V, Gill D, Kivity S, Lerman-Sagie T, McLellan A, Pelekanos J, Ramesh V, Sadleir L, Wirrell E, Scheffer IE 2016. Mortality in Dravet syndrome. Epilepsy research 128, 43–47. [DOI] [PubMed] [Google Scholar]
- Cui Y, Kam K, Sherman D, Janczewski WA, Zheng Y, Feldman JL 2016. Defining preBotzinger Complex Rhythm- and Pattern-Generating Neural Microcircuits In Vivo. Neuron 91, 602–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czeisler CM, Silva TM, Fair SR, Liu J, Tupal S, Kaya B, Cowgill A, Mahajan S, Silva PE, Wang Y, Blissett AR, Goksel M, Borniger JC, Zhang N, Fernandes-Junior SA, Catacutan F, Alves MJ, Nelson RJ, Sundaresean V, Rekling J, Takakura AC, Moreira TS, Otero JJ 2019. The role of PHOX2B-derived astrocytes in chemosensory control of breathing and sleep homeostasis. The Journal of physiology 597, 2225–2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio CM, Markovic D, Mazumder R, Moseley BD 2017. Ranking the Leading Risk Factors for Sudden Unexpected Death in Epilepsy. Frontiers in neurology 8, 473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeGiorgio CM, Miller P, Meymandi S, Chin A, Epps J, Gordon S, Gornbein J, Harper RM 2010. RMSSD, a measure of vagus-mediated heart rate variability, is associated with risk factors for SUDEP: the SUDEP-7 Inventory. Epilepsy & behavior : E&B 19, 78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereli AS, Yaseen Z, Carrive P, Kumar NN 2019. Adaptation of Respiratory-Related Brain Regions to Long-Term Hypercapnia: Focus on Neuropeptides in the RTN. Front Neurosci 13, 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devinsky O, Hesdorffer DC, Thurman DJ, Lhatoo S, Richerson G 2016. Sudden unexpected death in epilepsy: epidemiology, mechanisms, and prevention. Lancet neurology 15, 1075–1088. [DOI] [PubMed] [Google Scholar]
- Dlouhy BJ, Gehlbach BK, Kreple CJ, Kawasaki H, Oya H, Buzza C, Granner MA, Welsh MJ, Howard MA, Wemmie JA, Richerson GB 2015. Breathing Inhibited When Seizures Spread to the Amygdala and upon Amygdala Stimulation. The Journal of neuroscience : the official journal of the Society for Neuroscience 35, 10281–10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Ramirez JM 2010. State-dependent interactions between excitatory neuromodulators in the neuronal control of breathing. The Journal of neuroscience : the official journal of the Society for Neuroscience 30, 8251–8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlow BL, McNab JA, Witzel T, Kinney HC 2016. The Structural Connectome of the Human Central Homeostatic Network. Brain connectivity 6, 187–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epelbaum J, Dournaud P, Fodor M, Viollet C 1994. The neurobiology of somatostatin. Critical reviews in neurobiology 8, 25–44. [PubMed] [Google Scholar]
- Erlichman JS, Leiter JC, Gourine AV 2010. ATP, glia and central respiratory control. Respiratory physiology & neurobiology 173, 305–311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esmaeili B, Kaffashi F, Theeranaew W, Dabir A, Lhatoo SD, Loparo KA 2018. Post-ictal Modulation of Baroreflex Sensitivity in Patients With Intractable Epilepsy. Frontiers in neurology 9, 793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falquetto B, Oliveira LM, Takakura AC, Mulkey DK, Moreira TS 2018. Inhibition of the hypercapnic ventilatory response by adenosine in the retrotrapezoid nucleus in awake rats. Neuropharmacology 138, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandes-Junior SA, Carvalho KS, Moreira TS, Takakura AC 2018. Correlation between neuroanatomical and functional respiratory changes observed in an experimental model of Parkinson’s disease. Experimental physiology. [DOI] [PubMed] [Google Scholar]
- Fredholm BB, AP IJ, Jacobson KA, Linden J, Muller CE 2011. International Union of Basic and Clinical Pharmacology. LXXXI. Nomenclature and classification of adenosine receptors--an update. Pharmacol Rev 63, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman D, Kannan K, Faustin A, Shroff S, Thomas C, Heguy A, Serrano J, Snuderl M, Devinsky O 2018. Cardiac arrhythmia and neuroexcitability gene variants in resected brain tissue from patients with sudden unexpected death in epilepsy (SUDEP). NPJ Genom Med 3, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman SM, Falkai P, Bogerts B, Herrero MT, Polak JM, Roberts GW 1989. Distribution of galanin-like immunoreactivity in the human brain. Brain research 505, 311–315. [DOI] [PubMed] [Google Scholar]
- Geola FL, Yamada T, Warwick RJ, Tourtelotte WW, Hershman JM 1981. Regional distribution of somatostatin-like immunoreactivity in the human brain. Brain research 229, 35–42. [DOI] [PubMed] [Google Scholar]
- Ghali MGZ 2019. Respiratory rhythm generation and pattern formation: oscillators and network mechanisms. J Integr Neurosci 18, 481–517. [DOI] [PubMed] [Google Scholar]
- Goldman AM, Behr ER, Semsarian C, Bagnall RD, Sisodiya S, Cooper PN 2016. Sudden unexpected death in epilepsy genetics: Molecular diagnostics and prevention. Epilepsia 57 Suppl 1, 17–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graebenitz S, Kedo O, Speckmann EJ, Gorji A, Panneck H, Hans V, Palomero-Gallagher N, Schleicher A, Zilles K, Pape HC 2011. Interictal-like network activity and receptor expression in the epileptic human lateral amygdala. Brain 134, 2929–2947. [DOI] [PubMed] [Google Scholar]
- Guyenet PG, Stornetta RL, Bochorishvili G, Depuy SD, Burke PG, Abbott SB 2013. C1 neurons: the body’s EMTs. American journal of physiology. Regulatory, integrative and comparative physiology 305, R187–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guyenet PG, Wang H 2001. Pre-Botzinger neurons with preinspiratory discharges “in vivo” express NK1 receptors in the rat. J Neurophysiol 86, 438–446. [DOI] [PubMed] [Google Scholar]
- Hampel KG, Elger CE, Surges R 2017. Impaired Baroreflex Sensitivity after Bilateral Convulsive Seizures in Patients with Focal Epilepsy. Frontiers in neurology 8, 210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampel KG, Jahanbekam A, Elger CE, Surges R 2016. Seizure-related modulation of systemic arterial blood pressure in focal epilepsy. Epilepsia 57, 1709–1718. [DOI] [PubMed] [Google Scholar]
- Harden C, Tomson T, Gloss D, Buchhalter J, Cross JH, Donner E, French JA, Gil-Nagel A, Hesdorffer DC, Smithson WH, Spitz MC, Walczak TS, Sander JW, Ryvlin P 2017. Practice guideline summary: Sudden unexpected death in epilepsy incidence rates and risk factors: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology and the American Epilepsy Society. Neurology 88, 1674–1680. [DOI] [PubMed] [Google Scholar]
- Harper RM, Macey PM, Woo MA, Macey KE, Keens TG, Gozal D, Alger JR 2005. Hypercapnic exposure in congenital central hypoventilation syndrome reveals CNS respiratory control mechanisms. J Neurophysiol 93, 1647–1658. [DOI] [PubMed] [Google Scholar]
- Herrera DG, Robertson HA 1996. Activation of c-fos in the brain. Prog Neurobiol 50, 83–107. [DOI] [PubMed] [Google Scholar]
- Huckstepp RT, id Bihi R, Eason R, Spyer KM, Dicke N, Willecke K, Marina N, Gourine AV, Dale N 2010. Connexin hemichannel-mediated CO2-dependent release of ATP in the medulla oblongata contributes to central respiratory chemosensitivity. The Journal of physiology 588, 3901–3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson AE 2018. Genetic Reporters of Neuronal Activity: c-Fos and G-CaMP6. Methods Enzymol 603, 197–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda K, Kawakami K, Onimaru H, Okada Y, Yokota S, Koshiya N, Oku Y, Iizuka M, Koizumi H 2017. The respiratory control mechanisms in the brainstem and spinal cord: integrative views of the neuroanatomy and neurophysiology. The journal of physiological sciences : JPS 67, 45–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferys JGR, Arafat MA, Irazoqui PP, Lovick TA 2019. Brainstem activity, apnea, and death during seizures induced by intrahippocampal kainic acid in anaesthetized rats. Epilepsia 60, 2346–2358. [DOI] [PubMed] [Google Scholar]
- Kaczynska K, Zajac D, Wojciechowski P, Kogut E, Szereda-Przestaszewska M 2018. Neuropeptides and breathing in health and disease. Pulm Pharmacol Ther 48, 217–224. [DOI] [PubMed] [Google Scholar]
- Kajstura TJ, Dougherty SE, Linden DJ 2018. Serotonin axons in the neocortex of the adult female mouse regrow after traumatic brain injury. Journal of neuroscience research 96, 512–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JJ, Liang WH, Lam CS, Huang XF, Yang SJ, Wong-Riley MT, Fung ML, Liu YY 2017. Catecholaminergic neurons in synaptic connections with pre-Botzinger complex neurons in the rostral ventrolateral medulla in normoxic and daily acute intermittent hypoxic rats. Experimental neurology 287, 165–175. [DOI] [PubMed] [Google Scholar]
- Kanter RK, Strauss JA, Sauro MD 1996. Comparison of neurons in rat medulla oblongata with fos immunoreactivity evoked by seizures, chemoreceptor, or baroreceptor stimulation. Neuroscience 73, 807–816. [DOI] [PubMed] [Google Scholar]
- Kinney HC, Chadwick AE, Crandall LA, Grafe M, Armstrong DL, Kupsky WJ, Trachtenberg FL, Krous HF 2009. Sudden death, febrile seizures, and hippocampal and temporal lobe maldevelopment in toddlers: a new entity. Pediatric and developmental pathology : the official journal of the Society for Pediatric Pathology and the Paediatric Pathology Society 12, 455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Cryan JB, Haynes RL, Paterson DS, Haas EA, Mena OJ, Minter M, Journey KW, Trachtenberg FL, Goldstein RD, Armstrong DD 2015. Dentate gyrus abnormalities in sudden unexplained death in infants: morphological marker of underlying brain vulnerability. Acta neuropathologica 129, 65–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Haynes RL 2019. The Serotonin Brainstem Hypothesis for the Sudden Infant Death Syndrome. J Neuropathol Exp Neurol 78, 765–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinney HC, Poduri AH, Cryan JB, Haynes RL, Teot L, Sleeper LA, Holm IA, Berry GT, Prabhu SP, Warfield SK, Brownstein C, Abram HS, Kruer M, Kemp WL, Hargitai B, Gastrang J, Mena OJ, Haas EA, Dastjerdi R, Armstrong DD, Goldstein RD 2016. Hippocampal Formation Maldevelopment and Sudden Unexpected Death across the Pediatric Age Spectrum. J Neuropathol Exp Neurol 75, 981–997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klofas LK, Short BP, Zhou C, Carson RP 2020. Prevention of premature death and seizures in a Depdc5 mouse epilepsy model through inhibition of mTORC1. Hum Mol Genet 29, 1365–1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kon FC, Vazquez RZ, Lang A, Cohen MC 2020. Hippocampal abnormalities and seizures: a 16-year single center review of sudden unexpected death in childhood, sudden unexpected death in epilepsy and SIDS. Forensic science, medicine, and pathology 16, 423–434. [DOI] [PubMed] [Google Scholar]
- Koos BJ, Kawasaki Y, Hari A, Bohorquez F, Jan C, Roostaeian J, Wilson CL, Kruger L 2004. Electrical stimulation of the posteromedial thalamus modulates breathing in unanesthetized fetal sheep. J Appl Physiol (1985) 96, 115–123. [DOI] [PubMed] [Google Scholar]
- Kovac S, Walker MC 2013. Neuropeptides in epilepsy. Neuropeptides 47, 467–475. [DOI] [PubMed] [Google Scholar]
- Krohn W 1963. Causes of Death among Epileptics. Epilepsia 4, 315–322. [DOI] [PubMed] [Google Scholar]
- La A, Rm H, M, G., R, K., Ja O, Sb V, S, O., Ca S, Sd L, L, L., B, D. 2019. Altered brain connectivity in sudden unexpected death in epilepsy (SUDEP) revealed using resting-state fMRI. NeuroImage. Clinical 24, 102060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Martins R, Vilella L, Hampson JP, Rani MRS, Strohl K, Zaremba A, Hampson JS, Sainju RK, Friedman D, Nei M, Scott C, Gehlbach BK, Hupp NJ, Schuele S, Ogren J, Harper RM, Allen L, Diehl B, Bateman LM, Devinsky O, Richerson GB, Lhatoo S 2019. The association of serotonin reuptake inhibitors and benzodiazepines with ictal central apnea. Epilepsy & behavior : E&B 98, 73–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Hampson JP, Rani MRS, Zaremba A, Sainju RK, Gehlbach BK, Schuele S, Friedman D, Devinsky O, Nei M, Harper RM, Allen L, Diehl B, Millichap JJ, Bateman L, Granner MA, Dragon DN, Richerson GB, Lhatoo SD 2018. The incidence and significance of periictal apnea in epileptic seizures. Epilepsia 59, 573–582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacuey N, Zonjy B, Londono L, Lhatoo SD 2017. Amygdala and hippocampus are symptomatogenic zones for central apneic seizures. Neurology 88, 701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitner DF, Mills JD, Pires G, Faustin A, Drummond E, Kanshin E, Nayak S, Askenazi M, Verducci C, Chen BJ, Janitz M, Anink JJ, Baayen JC, Idema S, van Vliet EA, Devore S, Friedman D, Diehl B, Scott C, Thijs R, Wisniewski T, Ueberheide B, Thom M, Aronica E, Devinsky O 2021. Proteomics and Transcriptomics of the Hippocampus and Cortex in SUDEP and High-Risk SUDEP Patients. Neurology 96, e2639–e2652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhatoo S, Noebels J, Whittemore V, Research, N.C.f.S. 2015. Sudden unexpected death in epilepsy: Identifying risk and preventing mortality. Epilepsia 56, 1700–1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist BE, Shuttleworth CW 2012. Adenosine receptor activation is responsible for prolonged depression of synaptic transmission after spreading depolarization in brain slices. Neuroscience 223, 365–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Peedicail JS, Gaxiola-Valdez I, Li E, Mosher V, Wilson W, Perera T, Singh S, Teskey GC, Federico P, Calgary Comprehensive Epilepsy Program, C. 2020. Postictal brainstem hypoperfusion and risk factors for sudden unexpected death in epilepsy. Neurology 95, e1694–e1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loonen ICM, Jansen NA, Cain SM, Schenke M, Voskuyl RA, Yung AC, Bohnet B, Kozlowski P, Thijs RD, Ferrari MD, Snutch TP, van den Maagdenberg A, Tolner EA 2019. Brainstem spreading depolarization and cortical dynamics during fatal seizures in Cacna1a S218L mice. Brain 142, 412–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macey PM, Woo MA, Macey KE, Keens TG, Saeed MM, Alger JR, Harper RM 2005. Hypoxia reveals posterior thalamic, cerebellar, midbrain, and limbic deficits in congenital central hypoventilation syndrome. J Appl Physiol (1985) 98, 958–969. [DOI] [PubMed] [Google Scholar]
- Malheiros-Lima MR, Takakura AC, Moreira TS 2017. Depletion of rostral ventrolateral medullary catecholaminergic neurons impairs the hypoxic ventilatory response in conscious rats. Neuroscience 351, 1–14. [DOI] [PubMed] [Google Scholar]
- Mazarati AM 2004. Galanin and galanin receptors in epilepsy. Neuropeptides 38, 331–343. [DOI] [PubMed] [Google Scholar]
- Medel-Matus JS, Shin D, Sankar R, Mazarati A 2017. Galanin contributes to monoaminergic dysfunction and to dependent neurobehavioral comorbidities of epilepsy. Experimental neurology 289, 64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak Z, Obari D, Ellis M, Thom M, Sisodiya SM 2017. Neuropathology of SUDEP: Role of inflammation, blood-brain barrier impairment, and hypoxia. Neurology 88, 551–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton O, Atherton D, Bundock E, Donner E, Friedman D, Hesdorffer D, Jarrell H, McCrillis A, Mena OJ, Morey M, Thurman D, Tian N, Tomson T, Tseng Z, White S, Wright C, Devinsky O 2018. National Association of Medical Examiners position paper: Recommendations for the investigation and certification of deaths in people with epilepsy. Epilepsia 59, 530–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GS, Baker TL, Nanda SA, Fuller DD, Zabka AG, Hodgeman BA, Bavis RW, Mack KJ, Olson EB Jr. 2001. Invited review: Intermittent hypoxia and respiratory plasticity. J Appl Physiol (1985) 90, 2466–2475. [DOI] [PubMed] [Google Scholar]
- Mraovitch S, Calando Y 1999. Interactions between limbic, thalamo-striatal-cortical, and central autonomic pathways during epileptic seizure progression. The Journal of comparative neurology 411, 145–161. [PubMed] [Google Scholar]
- Mueller SG, Bateman LM, Laxer KD 2014. Evidence for brainstem network disruption in temporal lobe epilepsy and sudden unexplained death in epilepsy. NeuroImage. Clinical 5, 208–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller SG, Nei M, Bateman LM, Knowlton R, Laxer KD, Friedman D, Devinsky O, Goldman AM 2018. Brainstem network disruption: A pathway to sudden unexplained death in epilepsy? Human brain mapping. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugesan A, Rani MRS, Hampson J, Zonjy B, Lacuey N, Faingold CL, Friedman D, Devinsky O, Sainju RK, Schuele S, Diehl B, Nei M, Harper RM, Bateman LM, Richerson G, Lhatoo SD 2018. Serum serotonin levels in patients with epileptic seizures. Epilepsia 59, e91–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nashef L, So EL, Ryvlin P, Tomson T 2012. Unifying the definitions of sudden unexpected death in epilepsy. Epilepsia 53, 227–233. [DOI] [PubMed] [Google Scholar]
- Nobis WP, Gonzalez Otarula KA, Templer JW, Gerard EE, VanHaerents S, Lane G, Zhou G, Rosenow JM, Zelano C, Schuele S 2019. The effect of seizure spread to the amygdala on respiration and onset of ictal central apnea. Journal of neurosurgery, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobis WP, Schuele S, Templer JW, Zhou G, Lane G, Rosenow JM, Zelano C 2018. Amygdala-stimulation-induced apnea is attention and nasal-breathing dependent. Annals of neurology 83, 460–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noebels Jl Md P 2019. Brainstem spreading depolarization: rapid descent into the shadow of SUDEP. Brain 142, 231–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obonai T, Yasuhara M, Nakamura T, Takashima S 1998. Catecholamine neurons alteration in the brainstem of sudden infant death syndrome victims. Pediatrics 101, 285–288. [DOI] [PubMed] [Google Scholar]
- Ogren JA, Tripathi R, Macey PM, Kumar R, Stern JM, Eliashiv DS, Allen LA, Diehl B, Engel J Jr., Rani MRS, Lhatoo SD, Harper RM 2018. Regional cortical thickness changes accompanying generalized tonic-clonic seizures. NeuroImage. Clinical 20, 205–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa Y, Takashima S 2002. Developmental neurotransmitter pathology in the brainstem of sudden infant death syndrome: a review and sleep position. Forensic science international 130 Suppl, S53–59. [DOI] [PubMed] [Google Scholar]
- Page T, Rugg-Gunn FJ 2018. Bitemporal seizure spread and its effect on autonomic dysfunction. Epilepsy & behavior : E&B 84, 166–172. [DOI] [PubMed] [Google Scholar]
- Park K, Kanth K, Bajwa S, Girgis F, Shahlaie K, Seyal M 2020. Seizure-related apneas have an inconsistent linkage to amygdala seizure spread. Epilepsia 61, 1253–1260. [DOI] [PubMed] [Google Scholar]
- Patodia S, Paradiso B, Ellis M, Somani A, Sisodiya SM, Devinsky O, Thom M 2019. Characterisation of medullary astrocytic populations in respiratory nuclei and alterations in sudden unexpected death in epilepsy. Epilepsy research 157, 106213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Paradiso B, Garcia M, Ellis M, Diehl B, Thom M, Devinsky O 2020a. Adenosine kinase and adenosine receptors A1 R and A2A R in temporal lobe epilepsy and hippocampal sclerosis and association with risk factors for SUDEP. Epilepsia 61, 787–797. [DOI] [PubMed] [Google Scholar]
- Patodia S, Somani A, O’Hare M, Venkateswaran R, Liu J, Michalak Z, Ellis M, Scheffer IE, Diehl B, Sisodiya SM, Thom M 2018. The ventrolateral medulla and medullary raphe in sudden unexpected death in epilepsy. Brain 141, 1719–1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Somani A, Thom M 2021. Polyglucosan bodies in medullary catecholaminergic neurones in SUDEP. Epilepsy Behav Rep 15, 100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Tachrount M, Somani A, Scheffer I, Yousry T, Golay X, Sisodiya SM, Thom M 2020b. MRI and pathology correlations in the medulla in sudden unexpected death in epilepsy (SUDEP): a postmortem study. Neuropathology and applied neurobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patodia S, Tan I, Ellis M, Somani A, Scheffer IE, Sisodiya S, Thom M 2020c. Medullary tyrosine hydroxylase catecholaminergic neuronal populations in sudden unexpected death in epilepsy. Brain Pathol. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez SE, Wynick D, Steiner RA, Mufson EJ 2001. Distribution of galaninergic immunoreactivity in the brain of the mouse. The Journal of comparative neurology 434, 158–185. [DOI] [PubMed] [Google Scholar]
- Petrucci AN, Joyal KG, Chou JW, Li R, Vencer KM, Buchanan GF 2021. Post-ictal Generalized EEG Suppression is reduced by Enhancing Dorsal Raphe Serotonergic Neurotransmission. Neuroscience 453, 206–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrucci AN, Joyal KG, Purnell BS, Buchanan GF 2020. Serotonin and sudden unexpected death in epilepsy. Experimental neurology 325, 113145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti MF, Schmeichel AM, Low PA, Parisi JE, Benarroch EE 2014. Degeneration of brainstem respiratory neurons in dementia with Lewy bodies. Sleep 37, 373–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez JM, Doi A, Garcia AJ 3rd, Elsen FP, Koch H, Wei AD 2012. The cellular building blocks of breathing. Comprehensive Physiology 2, 2683–2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeves SR, Guo SZ, Brittian KR, Row BW, Gozal D 2006. Anatomical changes in selected cardio-respiratory brainstem nuclei following early post-natal chronic intermittent hypoxia. Neuroscience letters 402, 233–237. [DOI] [PubMed] [Google Scholar]
- Rhone AE, Kovach CK, Harmata GI, Sullivan AW, Tranel D, Ciliberto MA, Howard MA, Richerson GB, Steinschneider M, Wemmie JA, Dlouhy BJ 2020. A human amygdala site that inhibits respiration and elicits apnea in pediatric epilepsy. JCI Insight 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribierre T, Deleuze C, Bacq A, Baldassari S, Marsan E, Chipaux M, Muraca G, Roussel D, Navarro V, Leguern E, Miles R, Baulac S 2018. Second-hit mosaic mutation in mTORC1 repressor DEPDC5 causes focal cortical dysplasia-associated epilepsy. The Journal of clinical investigation 128, 2452–2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB 2013. Serotonin: The Anti-SuddenDeathAmine? Epilepsy currents / American Epilepsy Society 13, 241–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richerson GB, Boison D, Faingold CL, Ryvlin P 2016. From unwitnessed fatality to witnessed rescue: Pharmacologic intervention in sudden unexpected death in epilepsy. Epilepsia 57 Suppl 1, 35–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryvlin P, Nashef L, Lhatoo SD, Bateman LM, Bird J, Bleasel A, Boon P, Crespel A, Dworetzky BA, Hogenhaven H, Lerche H, Maillard L, Malter MP, Marchal C, Murthy JM, Nitsche M, Pataraia E, Rabben T, Rheims S, Sadzot B, Schulze-Bonhage A, Seyal M, So EL, Spitz M, Szucs A, Tan M, Tao JX, Tomson T 2013. Incidence and mechanisms of cardiorespiratory arrests in epilepsy monitoring units (MORTEMUS): a retrospective study. Lancet neurology 12, 966–977. [DOI] [PubMed] [Google Scholar]
- Sainju RK, Dragon DN, Winnike HB, Nashelsky MB, Granner MA, Gehlbach BK, Richerson GB 2019. Ventilatory response to CO2 in patients with epilepsy. Epilepsia 60, 508–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Larsen A, Principe A, Ley M, Navarro-Cuartero J, Rocamora R 2021. Characterization of the Insular Role in Cardiac Function through Intracranial Electrical Stimulation of the Human Insula. Annals of neurology 89, 1172–1180. [DOI] [PubMed] [Google Scholar]
- Sauvage M, Kitsukawa T, Atucha E 2019. Single-cell memory trace imaging with immediate-early genes. J Neurosci Methods 326, 108368. [DOI] [PubMed] [Google Scholar]
- Sawaguchi T, Ozawa Y, Patricia F, Kadhim H, Groswasser J, Sottiaux M, Takashima S, Nishida H, Kahn A 2003. Catecholaminergic neurons in the brain-stem and sleep apnea in SIDS victims. Early Hum Dev 75 Suppl, S41–50. [DOI] [PubMed] [Google Scholar]
- Schwarzacher SW, Rub U, Deller T 2011. Neuroanatomical characteristics of the human pre-Botzinger complex and its involvement in neurodegenerative brainstem diseases. Brain 134, 24–35. [DOI] [PubMed] [Google Scholar]
- Scislo TJ, O’Leary DS 2005. Purinergic mechanisms of the nucleus of the solitary tract and neural cardiovascular control. Neurological research 27, 182–194. [DOI] [PubMed] [Google Scholar]
- Sevigny CP, Bassi J, Williams DA, Anderson CR, Thomas WG, Allen AM 2012. Efferent projections of C3 adrenergic neurons in the rat central nervous system. The Journal of comparative neurology 520, 2352–2368. [DOI] [PubMed] [Google Scholar]
- SheikhBahaei S, Morris B, Collina J, Anjum S, Znati S, Gamarra J, Zhang R, Gourine AV, Smith JC 2018a. Morphometric analysis of astrocytes in brainstem respiratory regions. The Journal of comparative neurology 526, 2032–2047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheikhbahaei S, Turovsky EA, Hosford PS, Hadjihambi A, Theparambil SM, Liu B, Marina N, Teschemacher AG, Kasparov S, Smith JC, Gourine AV 2018b. Astrocytes modulate brainstem respiratory rhythm-generating circuits and determine exercise capacity. Nature communications 9, 370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen HY, Li T, Boison D 2010. A novel mouse model for sudden unexpected death in epilepsy (SUDEP): role of impaired adenosine clearance. Epilepsia 51, 465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmuely S, Sisodiya SM, Gunning WB, Sander JW, Thijs RD 2016. Mortality in Dravet syndrome: A review. Epilepsy & behavior : E&B 64, 69–74. [DOI] [PubMed] [Google Scholar]
- Silveira DC, Schachter SC, Schomer DL, Holmes GL 2000. Flurothyl-induced seizures in rats activate Fos in brainstem catecholaminergic neurons. Epilepsy research 39, 1–12. [DOI] [PubMed] [Google Scholar]
- Simon RP 2010. Heart and lung in the postictal state. Epilepsy & behavior : E&B 19, 167–171. [DOI] [PubMed] [Google Scholar]
- Sinjab B, Martinian L, Sisodiya SM, Thom M 2013. Regional thalamic neuropathology in patients with hippocampal sclerosis and epilepsy: a postmortem study. Epilepsia 54, 2125–2133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Abdala AP, Borgmann A, Rybak IA, Paton JF 2013. Brainstem respiratory networks: building blocks and microcircuits. Trends in neurosciences 36, 152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobrinho CR, Goncalves CM, Takakura AC, Mulkey DK, Moreira TS 2017. Fluorocitrate-mediated depolarization of astrocytes in the retrotrapezoid nucleus stimulates breathing. J Neurophysiol 118, 1690–1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somani A, El-Hachami H, Patodia S, Sisodiya S, Thom M 2021. Regional microglial populations in central autonomic brain regions in SUDEP. Epilepsia 62, 1318–1328. [DOI] [PubMed] [Google Scholar]
- Somani A, Perry C, Patodia S, Michalak Z, Ellis M, Sisodiya SM, Thom M 2020. Neuropeptide depletion in the amygdala in sudden unexpected death in epilepsy: A postmortem study. Epilepsia 61, 310–318. [DOI] [PubMed] [Google Scholar]
- Somani A, Zborovschi AB, Liu Y, Patodia S, Michalak Z, Sisodiya SM, Thom M 2019. Hippocampal morphometry in sudden and unexpected death in epilepsy. Neurology 93, e804–e814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer W 1880. Erkrankung des Ammonshorns als aetiologisches Moment der Epilepsie. Archiv für Psychiatrie und Nervenkrankheiten 10, 631–675. [Google Scholar]
- Spirovski D, Li Q, Pilowsky PM 2012. Brainstem galanin-synthesizing neurons are differentially activated by chemoreceptor stimuli and represent a subpopulation of respiratory neurons. The Journal of comparative neurology 520, 154–173. [DOI] [PubMed] [Google Scholar]
- Sveinsson O, Andersson T, Carlsson S, Tomson T 2017. The incidence of SUDEP: A nationwide population-based cohort study. Neurology. [DOI] [PubMed] [Google Scholar]
- Sveinsson O, Andersson T, Carlsson S, Tomson T 2018. Circumstances of SUDEP: A nationwide population-based case series. Epilepsia 59, 1074–1082. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Petrovich GD 1998. What is the amygdala? Trends in neurosciences 21, 323–331. [DOI] [PubMed] [Google Scholar]
- Szereda-Przestaszewska M, Kaczynska K 2020. Serotonin and substance P: Synergy or competition in the control of breathing. Autonomic neuroscience : basic & clinical 225, 102658. [DOI] [PubMed] [Google Scholar]
- Tada M, Kakita A, Toyoshima Y, Onodera O, Ozawa T, Morita T, Nishizawa M, Takahashi H 2009. Depletion of medullary serotonergic neurons in patients with multiple system atrophy who succumbed to sudden death. Brain 132, 1810–1819. [DOI] [PubMed] [Google Scholar]
- Tang Y, Chen Q, Yu X, Xia W, Luo C, Huang X, Tang H, Gong Q, Zhou D 2014. A resting-state functional connectivity study in patients at high risk for sudden unexpected death in epilepsy. Epilepsy & behavior : E&B 41, 33–38. [DOI] [PubMed] [Google Scholar]
- Tekgul H, Serin HM, Simsek E, Kanmaz S, Gazeteci H, Azarsiz E, Ozgur S, Yilmaz S, Aktan G, Gokben S 2020. CSF levels of a set of neurotrophic factors (brain-derived neurotrophic factor, nerve growth factor) and neuropeptides (neuropeptide Y, galanin) in epileptic children. Journal of clinical neuroscience : official journal of the Neurosurgical Society of Australasia 76, 41–45. [DOI] [PubMed] [Google Scholar]
- Tescarollo FC, Rombo DM, DeLiberto LK, Fedele DE, Alharfoush E, Tome AR, Cunha RA, Sebastiao AM, Boison D 2020. Role of Adenosine in Epilepsy and Seizures. J Caffeine Adenosine Res 10, 45–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M 2014. Review: Hippocampal sclerosis in epilepsy: a neuropathology review. Neuropathology and applied neurobiology 40, 520–543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Boldrini M, Bundock E, Sheppard MN, Devinsky O 2017. The past, present and future challenges in epilepsy related and sudden deaths and biobanking. Neuropathology and applied neurobiology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Boldrini M, Bundock E, Sheppard MN, Devinsky O 2018. Review: The past, present and future challenges in epilepsy-related and sudden deaths and biobanking. Neuropathology and applied neurobiology 44, 32–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Griffin B, Sander JW, Scaravilli F 1999. Amygdala sclerosis in sudden and unexpected death in epilepsy. Epilepsy research 37, 53–62. [DOI] [PubMed] [Google Scholar]
- Thom M, Liagkouras I, Martinian L, Liu J, Catarino CB, Sisodiya SM 2012. Variability of sclerosis along the longitudinal hippocampal axis in epilepsy: a post mortem study. Epilepsy research 102, 45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Michalak Z, Wright G, Dawson T, Hilton D, Joshi A, Diehl B, Koepp M, Lhatoo S, Sander JW, Sisodiya SM 2016. Audit of practice in sudden unexpected death in epilepsy (SUDEP) post mortems and neuropathological findings. Neuropathology and applied neurobiology 42, 463–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thom M, Zhou J, Martinian L, Sisodiya S 2005. Quantitative post-mortem study of the hippocampus in chronic epilepsy: seizures do not inevitably cause neuronal loss. Brain 128, 1344–1357. [DOI] [PubMed] [Google Scholar]
- Thurman DJ, Hesdorffer DC, French JA 2014. Sudden unexpected death in epilepsy: assessing the public health burden. Epilepsia 55, 1479–1485. [DOI] [PubMed] [Google Scholar]
- Tupal S, Faingold CL 2006. Evidence supporting a role of serotonin in modulation of sudden death induced by seizures in DBA/2 mice. Epilepsia 47, 21–26. [DOI] [PubMed] [Google Scholar]
- Urfy MZ, Suarez JI 2014. Breathing and the nervous system. Handbook of clinical neurology 119, 241–250. [DOI] [PubMed] [Google Scholar]
- Uteshev VV, Tupal S, Mhaskar Y, Faingold CL 2010. Abnormal serotonin receptor expression in DBA/2 mice associated with susceptibility to sudden death due to respiratory arrest. Epilepsy research 88, 183–188. [DOI] [PubMed] [Google Scholar]
- Valenza G, Passamonti L, Duggento A, Toschi N, Barbieri R 2020. Uncovering complex central autonomic networks at rest: a functional magnetic resonance imaging study on complex cardiovascular oscillations. J R Soc Interface 17, 20190878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenza G, Sclocco R, Duggento A, Passamonti L, Napadow V, Barbieri R, Toschi N 2019. The central autonomic network at rest: Uncovering functional MRI correlates of time-varying autonomic outflow. Neuroimage 197, 383–390. [DOI] [PubMed] [Google Scholar]
- Vilella L, Lacuey N, Hampson JP, Rani MRS, Sainju RK, Friedman D, Nei M, Strohl K, Scott C, Gehlbach BK, Zonjy B, Hupp NJ, Zaremba A, Shafiabadi N, Zhao X, Reick-Mitrisin V, Schuele S, Ogren J, Harper RM, Diehl B, Bateman L, Devinsky O, Richerson GB, Ryvlin P, Lhatoo SD 2018. Postconvulsive central apnea as a biomarker for sudden unexpected death in epilepsy (SUDEP). Neurology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wandschneider B, Koepp M, Scott C, Micallef C, Balestrini S, Sisodiya SM, Thom M, Harper RM, Sander JW, Vos SB, Duncan JS, Lhatoo S, Diehl B 2015. Structural imaging biomarkers of sudden unexpected death in epilepsy. Brain 138, 2907–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Guo R, Zhao W 2015. Distribution of Fos-Like Immunoreactivity, Catecholaminergic and Serotoninergic Neurons Activated by the Laryngeal Chemoreflex in the Medulla Oblongata of Rats. PloS one 10, e0130822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei XY, Zhao Y, Wong-Riley MT, Ju G, Liu YY 2012. Synaptic relationship between somatostatin- and neurokinin-1 receptor-immunoreactive neurons in the pre-Botzinger complex of rats. Journal of neurochemistry 122, 923–933. [DOI] [PubMed] [Google Scholar]
- Weltha L, Reemmer J, Boison D 2018. The role of adenosine in epilepsy. Brain Res Bull. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Feldman JL 2018. Efferent projections of excitatory and inhibitory preBotzinger Complex neurons. The Journal of comparative neurology 526, 1389–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CF, Kim EJ, Callaway EM, Feldman JL 2020. Monosynaptic Projections to Excitatory and Inhibitory preBotzinger Complex Neurons. Frontiers in neuroanatomy 14, 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Q, Buchanan GF, Motelow JE, Andrews J, Vitkovskiy P, Chen WC, Serout F, Gummadavelli A, Kundishora A, Furman M, Li W, Bo X, Richerson GB, Blumenfeld H 2016. Impaired Serotonergic Brainstem Function during and after Seizures. The Journal of neuroscience : the official journal of the Society for Neuroscience 36, 2711–2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Yin D, Wu F, Zhang G, Jiang C, Li Z, Wang L, Wang K 2013. Microinjection of adenosine into the hypothalamic ventrolateral preoptic area enhances wakefulness via the A1 receptor in rats. Neurochemical research 38, 1616–1623. [DOI] [PubMed] [Google Scholar]