Abstract
Uterine leiomyomas or fibroids are the most common tumors of the female reproductive tract. Estrogen (E2), a steroid-derived hormone, and its receptors (ERs), particularly ER-α, are important drivers for the development and growth of leiomyomas. We previously demonstrated that simvastatin, a drug used for hyperlipidemia, also possesses anti-leiomyoma properties. The aim of this work is to investigate the impact of simvastatin on ER-α signaling in leiomyoma cells, including its expression, downstream signaling, transcriptional activity, post-translational modification, trafficking and degradation. Primary and immortalized human uterine leiomyoma (HuLM) cells were used for in vitro experiments. Immunodeficient mice xenografted with human leiomyoma tissue explants were used for in vivo studies. Leiomyoma samples were obtained from patients enrolled in an ongoing double-blinded, phase II, randomized controlled trial. Here, we found that simvastatin significantly reduced E2-induced proliferation and PCNA expression. In addition, simvastatin reduced total ER-α expression in leiomyoma cells and altered its subcellular localization by inhibiting its trafficking to the plasma membrane and nucleus. Simvastatin also inhibited E2 downstream signaling, including ERK and AKT pathways, E2/ER transcriptional activity and E2-responsive genes. To explain simvastatin effects on ER-α level and trafficking, we examined its effects on ER-α post-translational processing. We noticed that simvastatin reduced ER-α palmitoylation; a required modification for its stability, trafficking to plasma membrane, and signaling. We also observed an increase in ubiquitin-mediated ER-α degradation. Importantly, we found that the effects of simvastatin on ER-α expression were recapitulated in the xenograft leiomyoma mouse model and human tissues. Thus, our data suggest that simvastatin modulates several E2/ER signaling targets with potential implications in leiomyoma therapy and beyond.
Keywords: uterine leiomyoma, estrogen signaling, palmitoylation, receptor trafficking, receptor degradation, simvastatin
Graphical Abstract

1. Introduction
Uterine leiomyomas, also called fibroids or myomas, are the most common benign tumors of the female reproductive tract, affecting an estimated 70% of women and are associated with heavy and prolonged menstrual bleeding, pelvic pain, and infertility [1, 2]. Despite their heavy clinical burden, current medications have limited efficacy and mostly offer only short-term benefits. Estrogen (E2) and its receptors (ER), particularly ER-α, are known to be essential for the development of leiomyomas, and several estrogen-related aberrations have been identified in these tumors, including overexpression of its receptors, higher estrogen tissue levels, as well as epigenetic alterations of its responsive genes [3–5]. Aromatase inhibitors, which suppress the formation of E2, were shown to significantly reduce the size of fibroids and improve leiomyoma symptoms [6]. However, aromatase inhibitors cannot be given for an extended period due to their side effects. In addition, estrogen induces the expression of progesterone receptors and promotes the action of progesterone, which is necessary for the growth of these tumors [7]. The E2/ER complex acts via genomic and nongenomic mechanisms to regulate gene expression, activate various protein kinase cascades, and modulate target transcription factors. ER-α undergoes palmitoylation, a post-translational modification mediated by palmitoyl acyltransferases DHHC7 and DHHC21 [8]. Palmitoylation was previously shown to regulate ER-α transcriptional activities and proteasome-mediated degradation [8].
Simvastatin is an anti-hyperlipidemic drug with pleiotropic effects. Previous in vitro, in vivo, and epidemiologic studies have shown the beneficial effects of simvastatin on uterine leiomyomas by inhibiting cellular proliferation, inducing calcium-dependent apoptosis, reducing extracellular matrix deposition, and restoring the altered state of mechanotransduction signaling [9–13]. Here, we showed that simvastatin modulates ER-α expression; downstream signaling; transcriptional activity; and alters its trafficking and degradation via modulating its palmitoylation and ubiquitination.
2. Materials and methods
2.1. Cell culture
Immortalized human uterine leiomyoma (HuLM) cells were maintained in a smooth muscle cell growth medium (SmGM, Lonza, Walkersville, MD), containing smooth muscle basal medium (SmBM), 5% fetal bovine serum (FBS), 0.1% insulin, 0.2% recombinant human fibroblast growth factor B, 0.1% of a mixture of gentamicin sulfate and amphotericin B, and 0.1% human epidermal growth factor in 5% CO2 at 37 °C. Primary leiomyoma cells were isolated from five different human leiomyoma tissue samples approved by the Institutional Review Board of Johns Hopkins University, and informed consents were obtained from all subjects. Primary leiomyoma cells were isolated a previously published [13]. Cells were cultured in Dulbecco’s modified Eagle’s medium/nutrient mixture F-12 (DMEM/F-12) (Thermo Fisher Scientific, Waltham, MA) supplemented with HEPES, L-glutamine, 10% FBS, and 1% antibiotic–antimycotic in 5% CO2 at 37 °C.
2.2. Reagents
Simvastatin (Cayman Chemical, Ann Arbor, MI) stock solution (10 mM) was prepared in dimethyl sulfoxide (DMSO; Sigma-Aldrich, St. Louis, MO) and stored at −20 °C until use. Estrogen was purchased from Sigma-Aldrich, and the stock solution (10 mM) was prepared in ethanol (Sigma-Aldrich). The rest of the chemicals and reagents used in the experiments were purchased from Thermo Fisher Scientific and Sigma-Aldrich.
2.3. Cell viability assays
HuLM cells were plated onto 96-well plates and grown overnight. Prior to treatment with E2, the media were changed to phenol red-free SmGMTM-2 supplemented with 10% charcoal-stripped FBS (HyClone, Logan, UT). The cells were then treated with simvastatin (0.001, 0.01, 0.1, and 1 μM) and E2 (10 nM), alone or in combination, for 48 h. DMSO was used as a vehicle control. The concentrations were chosen based on previous publications and clinical relevance. Cell viability was determined by an MTT assay as previously reported [10]. Based on cell viability data, we chose 1 μM simvastatin for subsequent experiments.
2.4. Real-time PCR analyses
HuLM cells were plated onto 6-well plates and maintained in SmGM media until reaching 60 to 70% confluence. After overnight serum starvation, cells were treated with 0.001, 0.01, 0.1, and 1 μM simvastatin or DMSO (control) for 48 h. Total RNA was extracted using RNeasy Mini Kit (Qiagen, Gaithersburg, MD) and reverse-transcribed into cDNA using iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA) in a Bio-Rad Thermocycler according to the manufacturer’s instructions. The prepared cDNA was then subjected to a quantitative real-time polymerase chain reaction (RT-qPCR) using LightCycler 96 System (Roche Diagnostics, Mannheim, Germany) and FastStart Essential DNA Green Master (Roche Diagnostics). The human primer sequences were as follows: ESR1: sense, 5′-GCATTCTACAGGCCAAATTCA-3′, and antisense, 5′-TCCTTGGCAGATTCCATAGC-3′; RPLP0: sense, 5’-GCGACCTGGAAGTCCAACT-3’, and antisense, 5’-GGTCCTCCTTGGTGAACAC-3’. The relative mRNA expression was expressed as a fold change and calculated using the 2ΔΔCT method.
2.5. Western blot analyses
Cells were harvested after treatment with simvastatin (0.001, 0.01, 0.1, and 1 μM) and E2 (10 nM), alone or in combination, at the specified time points. Cells were lysed in a lysis buffer (radioimmunoprecipitation assay buffer, Sigma-Aldrich) containing a protease and phosphatase inhibitor cocktail (Sigma-Aldrich). An equal amount of protein lysates was resolved using 4 to 12% Bis-Tris protein gradient gels (Thermo Fisher Scientific) and transferred to a nitrocellulose membrane (Thermo Fisher Scientific). Membranes were blocked using 5% nonfat milk in Tris-buffered saline with 0.1% Tween-20 (TBST, Thermo Fisher Scientific) for 1 h at room temperature and incubated with PCNA (CST, #13110), anti- ER-α (Invitrogen, PA5–16440), pERK1/2 (CST, #4695S), ERK1/2 (CST, #4370), pAKT (Santa Cruz; sc-7985), AKT (Santa Cruz; sc-8312), anti-collagen 1 (Thermo Fisher Scientific, PA5–29569), anti-β-actin (Sigma, A3854), anti-Na,K-ATPase (CST, 23565), or anti-lamin B1 (Invitrogen, PA5–19468) at 4 °C overnight on a rocker. Membranes were washed with TBST, co-incubated with appropriate horseradish peroxidase (HRP)-conjugated secondary antibodies (GE Healthcare, Chicago, IL) for 1 h at room temperature and visualized using an Azure Imager c300 (Azure Biosystems, Dublin, CA). Band signals were quantified using the NIH ImageJ software (version 1.52r, USA).
2.6. Immunocytochemistry
Primary leiomyoma and HuLM cells were grown on 8-well chamber slides. Cells were treated with simvastatin (0.001, 0.01, 0.1, and 1 μM) for 48 h, fixed in 4% formaldehyde, and incubated in a blocking solution (1× PBS, 5% normal goat serum (CST) and 0.3% Triton X-100 (Sigma-Aldrich)) for 1 h at room temperature. Afterward, the cells were incubated overnight at 4 °C with primary antibodies against anti- ER-α, followed by anti-rabbit Alexa 488 (Invitrogen, #A11034) and anti-mouse Alexa 546 (Invitrogen, #A11030) conjugated secondary antibody for 1 h at room temperature in the dark. The slide was fixed with a ProLong Gold Antifade Mountant with DAPI (Thermo Fisher Scientific) overnight at room temperature. The samples were examined using a Leica SP8 Microsystems (Wetzlar, Germany) confocal microscope. The time of acquaintance was 1.9 microseconds per pixel, and each image had 4 megapixels; therefore, the total scanning time was 7.6 seconds. The time of acquaintance was analogous for each collection. Anti- ER-α staining images were taken at 63× magnification.
2.7. Transient transfection and luciferase reporter gene assay
HuLM cells were plated on a 24-well plate in a charcoal-stripped, phenol red-free medium and grown until reaching 60% confluence. The cells were serum-starved overnight. Plasmid pERE-TK-Luc (expression plasmid) and pEGFP-C1-ER alpha (expression vector) were acquired from Addgene (Watertown, Massachusetts, USA). The cells were transfected with pERE-TK-Luc (500 ng/well) and pEGFP-C1-ER alpha (100 ng/well) using Lipofectamine 2000 (Thermo Fisher Scientific), according to the manufacturers’ instructions. After transfection for 4 h, the cells were treated with simvastatin and E2, alone and/or in combination, for 48 h. Luciferase assays were carried out using a luciferase assay kit (Promega, Madison, WI), and activity was measured using a CLARIOstar Microplate Reader (BMG LABTECH, Cary, NC). The luciferase values were normalized against protein concentrations using Precision Red Advanced Protein Assay Reagent (Cytoskeleton, Denver, CO).
2.8. RT2 profiler PCR array analysis
Total RNA was extracted from HuLM cells treated with simvastatin (1 μM) and vehicle (DMSO) for 48 h using RNeasy Mini Kit. Equal amounts of RNA were then reverse transcribed to the cDNA using an RT2 First Strand kit (Qiagen), and RT-qPCR was performed using SYBR Green Master mix with a human estrogen receptor signaling RT2 profiler PCR array (PAHS-005ZA, Qiagen) on a LightCycler 96 System (Roche Diagnostics) as recommended by the manufacturer. Each array was composed of 84 different genes related to human estrogen receptor signaling, 6 housekeeping genes, 3 reverse transcription controls, and 3 positive PCR controls. Fold changes in the expression of mRNA transcripts in the simvastatin-treated compared to vehicle-treated groups were analyzed using specific Qiagen® software.
2.9. Subcellular fractionation
HuLM cells were treated with 0.001, 0.01, 0.1, and 1 μM simvastatin or DMSO (control) for 48 h. The membrane, cytoplasmic, and nuclear fractions were separated using a subcellular fractionation kit for cultured cells (Thermo Fisher Scientific) according to the manufacturer’s instructions. The purity of the fraction in the input was confirmed by immunoblot analysis of cytoplasm (β-actin), membrane (Na,KATPase), and nucleus (lamin B1) markers.
2.10. Palmitoylation assay
HuLM cells were plated onto 10 cm dishes and maintained with SmGM media until reaching 60 to 70% confluence. After overnight serum starvation, the cells were treated with 0.1 μM simvastatin or DMSO (control) for 48 h. Acyl resin-assisted capture (Acyl-RAC) was used to assess the status of proteins S-acylation as previously described [14]. The cells were lysed in a buffer containing 1% of n-dodecyl β-D-maltoside detergent (DDM) in a Dulbecco’s phosphate-buffered saline (DPBS), 10 μM ML211, phosphatase, and protease inhibitor cocktail, and 10 mM phenylmethanesulfonyl fluoride (PMSF). The lysate was precipitated in chloroform-methanol (CM) by adding methanol (CH3OH) and chloroform (CHCl3) to the lysate at a mixture ratio of 2:2:1, vigorously shaking, to create a homogeneous suspension. A pellet was formed at the interphase between aqueous and organic phases by spinning the tube at 10,000 × g for 5 minutes. The solvent excess was aspirated, and the protein pellet was dissolved in the 2SHB buffer (2% SDS; 5 mM EDTA; 100 mM Hepes; pH 7.4) by vortexing at 42 °C/1500 rpm in a thermal shaker. Next, free thiol groups were blocked by adding 200 ml of 0.2% MMTS (S-methyl methanethiosulfonate) in 2SHB buffer, resulting in a final concentration of 0.1% MMTS. MMTS was removed by three rounds of chloroform-methanol precipitation, followed by the dissolution of protein pellets in 2SHB buffer. We retained 10% of the sample as input, and the remaining samples were divided into two equal volumes, + HAM and −HAM. To promote thioester bond cleavage, 400 mM hydroxylamine (HAM, pH 7–7.2, freshly prepared) was added to the + HAM group, and 400 mM sodium chloride was added to the - HAM group. Afterward, both groups were incubated at RT for 1 h with 30 mL of streptavidin agarose beads. After incubation, beads were collected, and proteins were eluted with 4x SDS sample buffer supplemented with 5 mM DTT by Western blotting.
2.11. ER-α degradation
To examine the effects of simvastatin on ER-α degradation, HuLM cells were treated with cycloheximide (CHX) (protein synthesis inhibitor) and MG132 (proteasomal inhibitor). The cells were treated with 50 μg/mL CHX for 16 h and with 0.1 μM simvastatin for 32 h; the cells were treated in the presence of CHX in the simvastatin group for an additional 16 h. Similarly, the cells were treated with MG132 10 μM for 2 h and 0.1 μM simvastatin for 46 h. For the simvastatin treatment, the cells were treated for an additional 2 h in the presence of MG132. DMSO was used as vehicle control in both experiments. Cell lysates were then collected and subjected to Western blot analysis for ER-α degradation.
2.12. Ubiquitination pull-down assay
The effect of simvastatin on ER-α ubiquitination was examined using the Signal-Seeker Ubiquitination Detection Kit (BK161, Cytoskeleton, Denver, CO) according to the manufacturer’s instructions. HuLM cells were grown to 70% confluency and treated with MG132 (10 μM/2 h) prior to the treatment of 0.1 μM simvastatin or DMSO (control) for 48 h. Cells were washed once in ice-cold PBS and collected in BlastR lysis buffer supplemented with de-ubiquitination inhibitor and protease inhibitor cocktail. Lysates were precleared with a BlastR filter and diluted further with BlastR dilution buffer. We aliquoted 20 uL of lysate as an input lysate control, and the remaining samples were incubated for 2 h at 4 °C with gentle shaking with either control beads or ubiquitination affinity beads. Beads were washed three times with BlastR-2 wash buffer for 5 min for each sample at 4 °C; the pellets were centrifuged and eluted with bead elution buffer for 5 min at RT. After collecting the samples on the spin columns, 2-mercaptoethanol was added, which was then boiled for 5 min before SDS-PAGE and Western blot analysis was conducted. The membrane was probed with anti- ER-α and re-probed with an anti-ubiquitin-HRP antibody.
2.13. Animal model study and tissue preparation
Six-week-old female immunodeficient NOG (NOD/Shi-scid/IL-2Rgnull) mice were purchased from Taconic, and 17β-estradiol (0.05 mg)/progesterone (50 mg) 60-day pellets (Innovative Research of America) were placed subcutaneously at least 5 days before leiomyoma xenograft placement. For the leiomyoma xenograft mouse model, leiomyoma tumors were obtained from patients. Uniform 2 mm × 2 mm × 3 mm sterile tumor xenografts were dipped into a matrigel basement membrane matrix and inserted subcutaneously into 20 mice via an incision in their flanks (2 tumors per mouse). The incision site was closed with sterile surgical staples. Following implantation, the animals were closely observed for pain and infection. The staples were removed after one week of transplantation, and the treatment was initiated. The animals were randomly divided into two groups and treated with the vehicle control (n = 10) or 20 μg/gm body weight of simvastatin (n = 10) subcutaneously for 28 days. Subsequently, the tumor volumes were measured with a caliper and calculated using the following formula: 0.52 × length × width × height (this is a simplification of tumor volume = 4/3 × π × length/2 × width/2 × height/2). A high-resolution ultrasound system was used to measure tumor sizes in 3 dimensions (18–38 MHz probe, Vevo 2100; VisualSonics, Toronto, Canada) and was done at the University of Texas Medical Branch. Animal euthanasia was achieved using isoflurane overdose followed by cervical dislocation, and tumors were removed following skin incisions. Tumor tissues were fixed with a 10% buffered formalin solution and kept at 4 °C until immunohistochemistry.
2.14. Clinical leiomyoma tissue collection
Human leiomyoma tissue samples were obtained from a double-blinded, phase II, randomized controlled trial (https://clinicaltrials.gov/ct2/show/NCT03400826) to evaluate the effects of simvastatin in patients with uterine leiomyoma. Patients between the ages of 18 and 55 with uterine leiomyomas were recruited for treatment with simvastatin (40 mg daily) or placebo (starch 1500 encapsulated) for a total of 12 weeks. Patients recruited in the study could have any number of fibroids, but at least one fibroid should have a diameter of >3cm. Patients were excluded if they were pregnant, had a diagnosis of reproductive cancer, had a recent rapid growth of their tumors, were post-menopausal, had a suspicion of leiomyosarcoma, or if they urgently needed the surgery. Patients did not take any estrogen or progesterone hormonal treatment (birth control pills, contraceptive injection, or hormonal intrauterine device) during the duration of the simvastatin treatment, and those who were taking a hormonal treatment prior to the study had to wait at least 1 month as a washout period. Patients were randomized based on race. Patients enrolled in the trial underwent hysterectomy or myomectomy at the end of the treatment, and leiomyoma samples were collected after surgery to evaluate the effects of simvastatin on leiomyoma tissue.
2.15. Immunohistochemical staining
Immunohistochemical staining of clinical leiomyomas was performed at the Oncology Tissue Services Core facility of the Johns Hopkins University School of Medicine. Immunolabeling for ER-α was performed on formalin‐fixed, paraffin-embedded sections on a Ventana Discovery Ultra autostainer (Roche Diagnostics). Briefly, following dewaxing and rehydration on board, epitope retrieval was performed using Ventana Ultra CC1 buffer (catalog# 6414575001, Roche Diagnostics) at 96 °C for 64 minutes. The primary antibody, anti‐ER-α (RTU; catalog# 790–4324, Roche), was applied at 36 °C for 20 minutes. Primary antibodies were detected using an anti-rabbit HQ detection system (catalog# 7017936001 and 7017812001, Roche Diagnostics) and a Chromomap DAB IHC detection kit (catalog # 5266645001, Roche Diagnostics), counterstaining with Mayer’s hematoxylin, dehydration, and mounting. The optical density (OD) of ER-α was analyzed via automated macroinstructions written in ImageJ software. Stained areas (in pixels2) were identified and validated on >15 random fields in each slide. The ratio between stained and total areas was calculated for each sample. The area of ER-α coverage was determined by the ratio between DAB-labeled and total areas for each sample with the use of automated macro instruction on ImageJ. Animal tissues were fixed in formalin, embedded with paraffin, and sectioned. Hematoxylin and eosin staining was performed to examine tissue morphology. Immunohistochemistry for ER-α was performed using the immunoperoxidase method with DAB as the chromogen. Immunostaining was then quantitated using Image-Pro Plus software (Media Cybernetics, Rockville, MD), as described previously [11].
2.16. Statistical analysis
Statistical analyses were performed on GraphPad Prism version 6.01 for Windows. The mRNA expression was calculated using the ΔΔCT method and is presented as fold changes. Protein expression was quantified by measuring the area and intensity of specific antibody staining using Image J software (National Institutes of Health). For immunohistochemistry, analysis was performed using automated macro instruction written in Image J and Image-Pro Plus software (Media Cybernetics, Rockville, MD). To test for data distribution, the D’Agostino–Pearson omnibus normality test was used. Unpaired Student’s t-test was performed when comparing between two groups. Multiple comparisons were accomplished using one-way ANOVA with an appropriate Tukey’s post hoc test. The nonparametric tests Kruskal–Wallis followed by Dunn’s test and Mann–Whitney U were used for protein expression and mRNA level, respectively, whenever normality could not be assumed. Differences at P < 0.05 were considered statistically significant. All experiments were performed in duplicates, repeated three independent times, and the results were expressed as the mean ± standard error of the mean (SEM).
2.17. Study approval
All animal experiments were performed according to the protocols approved by the Institutional Animal Care and Use Committee at the University of Texas Medical Branch. Human leiomyoma tissue samples were obtained from a double-blind, phase II, randomized control trial to evaluate the effects of simvastatin in patients with uterine leiomyoma (https://clinicaltrials.gov/ct2/show/NCT03400826). Informed written consents were obtained from participants prior to enrollment.
3. RESULTS
3.1. Simvastatin inhibits estrogen-induced proliferation in uterine leiomyoma cells
Since estrogen (E2) and its receptors are known to be essential for the development of leiomyomas, we sought to evaluate the effect of simvastatin on E2-induced cell proliferation. We treated cells with both E2 and simvastatin. Simvastatin treatment for 48 h resulted in a dose-dependent decrease in cell proliferation compared to the vehicle control (DMSO, Fig. 1A). Treatment with E2 alone for 48 h increased proliferation by 20%, while simvastatin treatment resulted in decreased E2-induced cell proliferation at all tested concentrations (Fig. 1B). Cell proliferation was also assessed through proliferating cell nuclear antigen (PCNA) expression. Treatment with E2 for 30 mins to 48 h resulted in a time-dependent increase in PCNA levels in HuLM cells (Fig. 1C). In agreement with the MTT results, simvastatin treatment showed a reduction in PCNA expression in a dose-dependent manner after 48 h of treatment (Fig. 1D). Next, we observed the effects of simvastatin and E2 combinations on PCNA expression. Simvastatin treatment significantly suppressed the PCNA expression compared to E2 treatment alone (p< 0.05) (Fig. 1E). These findings indicate that simvastatin can inhibit E2-induced proliferation in leiomyoma cells.
Fig. 1. Reduction of estrogen (E2)-induced proliferation in simvastatin-treated leiomyoma cells.

A, Human leiomyoma cells (HuLM) were treated with the indicated amounts of simvastatin for 48 h. B, HuLM cells were treated with E2 (10 nM) alone or in combination with different simvastatin doses for 48 h. Dimethyl sulfoxide (DMSO) was used as a vehicle control. Cell viability was assessed by an MTT assay. C, HuLM cells were treated with E2 (10 nM) at the indicated time points and PCNA protein expression levels were detected by Western blotting, and β-actin was used as a loading control. D, HuLM cells were treated with the indicated amounts of simvastatin for 48 h and PCNA protein expression levels were detected by Western blotting, and β-actin was used as a loading control. E, HuLM cells were treated with E2 (10 nM) and simvastatin (1 μM), alone and in combination, for 48 h and PCNA protein expression levels were detected by Western blotting, and β-actin was used as a loading control. The data are presented as the mean ± SEM from three independent experiments. *, p < 0.05; **, p < 0.01; ***, p < 0.001 vs. the control; #, p < 0.05 vs. E2.
3.2. Simvastatin reduces ER-α level and alters ER-α cellular localization
We next examined the effects of simvastatin on ER-α expression level and its cellular localization. We found that simvastatin significantly decreases ESR1 mRNA levels (as assayed by qRT-PCR) by up to 44% (p<0.001) and protein levels (as assayed by Western blotting) by 29–40% (p<0.01) compared to untreated cells (Fig. 2A and 2B). Of note, the simvastatin inhibitory effect on the ESR1 transcript levels at the lower concentrations was not evident. This suggests that the effect of simvastatin on ER-α expression is mediated at the post-translational level. In accordance with mRNA and Western blotting findings, immunocytochemistry showed that simvastatin suppresses ER-α staining intensity, both in primary leiomyoma and HuLM cells (Fig. 2C and 2D). Since ER-α is known to be differentially localized in the cell with effects mostly exerted in the nucleus and plasma membranes [15], we looked at the effect of simvastatin on the subcellular localization of the protein. Using a subcellular protein fractionation kit, the membrane, cytoplasmic, and nuclear fractions were prepared. Simvastatin treatment reduced ER-α expression in the membrane and nuclear fractions (p<0.05) but not the cytoplasm (Fig. 2E, 2F and 2G). This means that simvastatin inhibited ER-α from cytoplasm to the plasma membrane and nucleus.
Fig. 2. ER-α expression and cellular localization after simvastatin treatment.
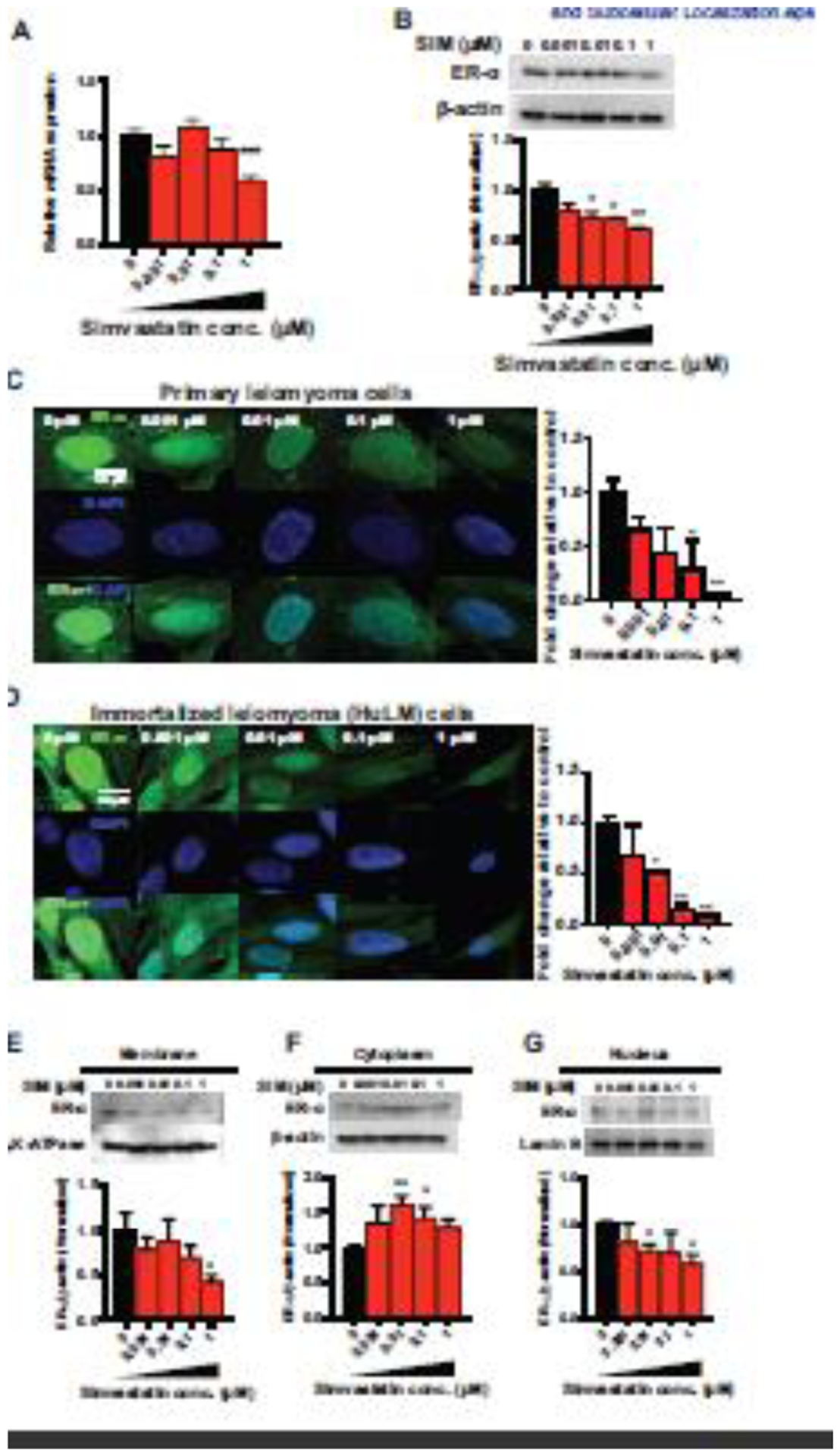
HuLM cells were treated with the indicated amounts of simvastatin for 48 h. DMSO was used as a vehicle control. A, mRNA expression levels of ESR1 were quantitated by RT-qPCR. RPLP0 was amplified under the same RT-qPCR conditions used for normalizing data. B, The ER-α protein expression levels were detected by Western blotting, and β-actin was used as a loading control. Cellular localization was assessed in the membrane, cytoplasm, and nucleus. C, D, Immunocytochemistry staining was performed to confirm the cellular localization of ER-α (green fluorescence) and DAPI (blue fluorescence). All images were captured with same time exposure using a confocal microscope (63× magnification). Scale bar, 50 μm. ER-α expression in the membrane (E), cytoplasm (F), and nuclear (G) cellular fractions was determined using Western blot analysis. β-Actin, Na,K-ATPase, and Lamin B were used as a loading control for whole cells, membrane and nucleus fractions. The data are presented as the mean ± SEM of three independent experiments. *, p < 0.05; **, p < 0.01 vs. the control.
3.3. Simvastatin inhibits estrogen downstream signaling and transcriptional activity
To determine if simvastatin modulates ER-α activity, we examined its effects on downstream estrogen signaling. After binding to ER-α, E2 acts through genomic (canonical) or nongenomic (non-canonical or extranuclear) signaling pathways [9, 11, 16, 17]. ERK1/2 and AKT are major signaling pathways activated by E2/ER complexes in the nongenomic pathway. According to our previous reports, simvastatin treatment suppressed the basal level of p-ERK1/2/ERK-1/2 and p-AKT/AKT in leiomyoma cells [10, 11]. In the present work, we focused on examining the simvastatin effects on E2-induced changes in these pathways. As expected, treatment of HuLM cells with E2 for 2 h resulted in a rapid increase in the levels of phospho-ERK1/2 (p-ERK1/2) (Fig. 3A). Treatment with simvastatin significantly suppressed ERK1/2 phosphorylation (p<0.05) (Fig. 3B), and pretreatment with simvastatin prevented the E2-induced increase in p-ERK1/2 (p<0.05) (Fig. 3C). Similarly, treatment with E2 for 2 h resulted in a rapid increase in the levels of phospho-AKT (p-AKT, Fig. 3D). Treatment with simvastatin significantly reduced its activation (p<0.05) (Fig. 3E), and simvastatin pretreatment prevented the E2-induced activation of p-AKT protein (p<0.05) (Fig. 3F).
Fig. 3. Suppression of E2-induced ER signaling after simvastatin treatment.

A, D, G, HuLM cells were treated with E2 (10 nM) at the indicated time points. A, phosphorylated to total ERK1/2 ratio (pERK1/2/ERK1/2), (D) phosphorylated to total AKT ratio (pAKT/AKT), and (G) COL1A1 protein expression levels were detected by Western blotting, and β-actin was used as a loading control. B and E, HuLM cells were treated with the indicated amounts of simvastatin for 48 h. The phosphorylated to total ERK1/2 ratio (pERK1/2/ERK1/2) (B) and phosphorylated to total AKT ratio (pAKT/AKT) (E) of the protein expression levels were determined by Western blotting, and β-actin was used as a loading control. C, F, and H, HuLM cells were treated with E2 (10 nM) and simvastatin (1 μM) alone and in combination for 48 h. C, pERK1/2/ERK1/2, F, pAKT/AKT, and H, COL1A1 protein expression levels were detected by Western blotting, and β-actin was used as a loading control. I, The transcriptional activity of E2/ER was reduced by simvastatin treatment. HuLM cells were transfected with plasmids expressing the ERETK-LUC reporter and human ER-α. Cells were treated with the vehicle, simvastatin, E2, and E2 + simvastatin, as indicated, for 48 h. Luciferase activity was determined by a luminometer and normalized by protein concentration. The results are expressed as the fold change relative to the vehicle control. The data are presented as the mean ± SEM of three independent experiments. *, p < 0.05; **, p < 0.01 vs. the control; #, p < 0.05 vs. E2; §, p < 0.05 vs. SIM; ns, non-significant.
Another important downstream target of E2 in leiomyoma cells is type 1 collagen (COL1A1), which is overexpressed in leiomyoma [18, 19]. We found that E2 increases the expression of COL1A1 protein in HuLM cells in a time-dependent manner, with its expression peaking at 24 h (Fig. 3G). Simvastatin treatment suppressed the basal COL1A1 expression [13] and pretreatment with simvastatin prevented E2-induced COL1A1 expression (p<0.05) in HuLM cells (Fig. 3H).
To investigate the effect of simvastatin on ER-α transcriptional activities, HuLM cells were transfected with ER-mediated Estrogen Response Element (ERE)-Luc, and the cells were treated with simvastatin. There was a significant, 4.2-fold, estrogen-dependent induction of ERE-Luc activity (Fig. 3I). Treatment with simvastatin (0.1 and 1 μM) significantly decreased (p<0.05) reporter transcriptional activity up to 2-fold compared with E2-induced cells, whereas the effect was not noted at the lower dosage (0.001 and 0.01 μM).
The effects of simvastatin on the transcription of genes involved in estrogen signaling were also investigated at the molecular level by analyzing the expression of a panel of estrogen signaling-related genes including ER co-regulators, interacting proteins and downstream target genes. For this purpose, we used the estrogen receptor signaling RT2 Profiler PCR Array system in HuLM cells treated with simvastatin, which were compared to untreated cells (Supplementary Fig. 1A, 1B and 1C). Results showed a significant change of several genes. Here, we highlight the more relevant genes in leiomyoma biology and highest fold change by simvastatin treatment. The most notable changes include suppression of caveolin 1 (CAV1, 2.73-fold), cyclin D1 (CCND1, 1.64-fold), connective tissue growth factor (CTGF, 1.86-fold), V-erb-b2 erythroblastic leukemia viral oncogene homolog 2 (ERBB2, 2.17-fold), estrogen receptor 1 (ESR1, 1.31-fold), G protein-coupled estrogen receptor 1 (GPER1, 3.07-fold), proline, glutamate and leucine-rich protein 1 (PELP1, 1.59-fold), suppressor of cytokine signaling 3 (SOCS3, 2.22-fold), thrombospondin 1 (THBS1, 2-fold), and wingless-type MMTV integration site family, member 4 (Wnt4, 2.26-fold). There is evidence of increased expression of some genes, including AHR, BDNF2, CCL2, CKB, CTSD, CYP19A1, G6PD, HSP90AA1, IGFBP, LTBP1, MED1, MMP9, NAB2, NCOAs, NRIP1, PTGS2, S100A6, TGFβ3, WSP2, WNT5A, XBP1, VEGFA and B2M. A study of the effects of simvastatin in the presence of E2 would be interesting, and we will consider in future work.
3.4. Simvastatin reduces palmitoylation of ER-α
As palmitoylation is a key regulator of ER-α signaling and its intracellular localization and trafficking, it is possible that simvastatin effects on ER-α are mediated via regulating its palmitoylation. Thus, we sought to examine the effects of simvastatin on this modification. Palmitoylation, aka acylation, is a reversible, post-translational modification in which saturated 16 carbon fatty acids are attached predominantly to the cysteine (S-palmitoylation) residue of proteins. The addition of palmitate alters how proteins interact with the lipid and protein components of a membrane, consequently altering protein–protein interactions, protein stability, membrane anchoring, receptor trafficking, and signaling efficacy [20]. The palmitoylation process is illustrated in Fig. 4A. Palmitoylation of ER-α by DHHC7 and DHHC21 at the cysteine 447 site regulates its nongenomic signaling via altering its plasma membrane localization and activity [20, 21]. The Acyl-Resin-Assisted Capture (Acyl-RAC) assay was used to measure the S-acylation of ER-α in HuLM cells. Simvastatin-treated cells demonstrated remarkably reduced ER-α S-acylation, observed in hydroxylamine treated sample (+ HAM lane) (Fig. 4B). A negative control consists of a sample treated with sodium chloride (− HAM lane) (Fig. 4B). Input served as a loading control.
Fig. 4. Effect of simvastatin on ER-α palmitoylation and ubiquitination.

A, In autopalmitoylation, the DHHC domain is acylated at cysteine residues using palmitate from palmitoyl-CoA, and in protein S-palmitoylation, palmitoyl is transferred to the thiol of a cysteine in a protein substrate. B, HuLM cells were treated with simvastatin (0.1 μM) for 48 h. DMSO was used as a vehicle control. An acyl-RAC assay was used to detect S-acylation of the ER-α proteins. Western blotting of ER-α -specific antibodies shows S-acylation of these proteins in the hydroxylamine-treated sample (+HAM lane). A sample treated with sodium chloride (−HAM lane) served as the negative control. Untreated lysates are shown in the input lane, act as a loading control. C, Cells were treated with 50 μg/mL cycloheximide (CHX) for 16 h in CHX group, 0.1 μM simvastatin for 48 h in SIM group, and 0.1 μM simvastatin for 32 h and were treated for an additional 16 h in the presence of CHX in the CHX+SIM group. ER-α protein expression levels were detected in the whole cells by Western blotting, and β-actin was used as a loading control. D, Cells were treated with MG132 (10 μM) for 2 h in MG132 group, 0.1 μM simvastatin for 48 h in SIM group, and 0.1 μM simvastatin for 46 h and cells were treated for an additional 2 h in the presence of MG132 in MG132+SIM group. ER-α protein expression levels were detected in whole cells by Western blotting, and β-actin was used as a loading control. Ubiquitination of ER-α was identified using the Signal-Seeker Ubiquitination Detection Kit. HuLM cells were grown to 70% confluency and treated with MG132 (10 μM/ 2h) prior to treatment with 0.1 μM simvastatin or DMSO (control) for 48 h. Western blot analysis was performed to detect ER-α ubiquitination. The membrane was probed with anti- ER-α (E, left panel) and re-probed with anti-ubiquitin-HRP antibodies (E, right panel). Input lane served as a marker for the unmodified protein band. *, p < 0.05; **, p < 0.01 vs. the control.
3.5. Simvastatin induces ER-α ubiquitination and degradation
Since we found that simvastatin reduces ER-α palmitoylation, and palmitoylation is known to regulate ER-α degradation, we sought to examine the effects of simvastatin on ER-α degradation. We used the protein synthesis inhibitor cycloheximide (CHX) alone or in combination with simvastatin in HuLM cells to see simvastatin’s effect on ER-α protein levels. As expected, treatment with CHX decreased ER-α protein levels. The addition of simvastatin after CHX further lowered ER-α level (Fig. 4C), which suggests that simvastatin reduces ER-α level via an additional mechanism other than synthesis. To examine if this effect is mediated via inducing ER-α degradation, we used the proteasome inhibitor MG132. As expected, MG132 treatment increased ER-α level in HuLM cells (Fig. 4D) as it inhibits its degradation. Interestingly, treatment with MG132 abrogated the effect of simvastatin on ER-α level which suggests that these effects are mediated via inducing proteasomal degradation.
Next, we sought to investigate how simvastatin induces ER-α degradation. Since ubiquitination is known to be a key regulator of ER-α degradation, we examined the effect of simvastatin on ER-α ubiquitination. Ubiquitination is a post-translational modification process where ubiquitin covalently attaches to a substrate protein at its lysine residues via a series of enzymatic reactions, resulting in tagging the target protein for proteasomal degradation. ER-α is a target of mono- and poly-ubiquitination [22]. Three enzymes, E1, a ubiquitin-activating enzyme, E2, a ubiquitin-conjugating enzyme, and E3, a ubiquitin ligase, orchestrate ubiquitination in a series of reactions resulting in the mono- and polyubiquitination of the protein substrate [23]. It was shown that ER-α protein undergoes ubiquitin-dependent protein degradation by targeting lysines K302 and K303 for polyubiquitination [24]. HuLM cells were pretreated with MG132 before exposure to simvastatin. This was done to block proteasomal degradation and let ubiquitinated ER-α accumulate so the level of ubiquitinated ER-α can be measured. Then, cell lysates were precipitated with ubiquitin affinity beads in the presence of an anti-ER-α antibody. The results showed that simvastatin treatment induces greater ER-α ubiquitination compared to the control (Fig. 4E, left panel). Input lane served as a marker for the unmodified protein band. The membrane was again re-probed with anti-ubiquitin-HRP antibodies for checking the immunoprecipitation of total ubiquitinated species (Fig. 4E, right panel). These results indicate that simvastatin increases ER-α degradation through inducing ER-α ubiquitination.
3.6. Simvastatin reduces ER-α expression in the leiomyoma xenograft animal model
Previously, we demonstrated that simvastatin treatment decreases tumor growth and suppresses the expression of the proliferation marker Ki67 in leiomyoma xenograft mouse model [11]. To see if the simvastatin effects on ER-α expression that were observed in vitro are recapitulated in vivo, we evaluated ER-α expression in leiomyoma xenograft mouse model (Fig. 5A). We harvested and processed tissues from simvastatin-treated and control mice. Immunohistochemical analysis showed that simvastatin treatment significantly reduces ER-α levels (p<0.05) compared to the control (Fig. 5B).
Fig. 5. Immunohistochemical (IHC) staining showing decreased expression of ER-α in simvastatin-treated xenograft mouse tissue.

A, Diagram illustrating the experiment’s design. The mice were injected with estrogen and progesterone pellets after adjusting to their environment, followed five days later by leiomyoma xenografts. Treated animals (n = 10) received simvastatin (20 μg/g body weight/d) subcutaneously, and the control animals (n =10) received the vehicle for 28 days. After the animals were sacrificed, the tissues were collected and placed in 10% buffered formalin and then processed to prepare the tissue sections. B, The tissues were stained with an ER-α antibody. Expression was then graded using an arbitrary grading system that was generated by the image analysis software and was correlated with the percentage of positively stained cells and the intensity of the staining in 10 separate high-power fields (20×) per slide. The expression is presented as arbitrary numbers. Scale bars, 50 μm. Data are presented of 20 independent slides from the treatment group and 20 slides from the control group. Representative images are at 40x magnification. Data are presented as the means ± SEM. * p < 0.05 vs. the control.
3.7. Simvastatin reduces ER-α expression in human tissues obtained from phase II, randomized controlled trial
To see if these effects are also recapitulated in humans, we used leiomyoma tissues from a double-blinded, phase II, randomized controlled trial (https://clinicaltrials.gov/ct2/show/NCT03400826) (Fig. 6A). Patients in this trial were randomized into two groups, placebo, or simvastatin 40mg. Patients received the drug daily for 12 weeks before undergoing surgical removal of the leiomyoma. During the study, patients were not receiving estrogen or any hormonal contraception. At the end of the treatment, leiomyoma samples were collected during their surgeries to evaluate the effects of simvastatin. Immunohistochemical analysis showed that leiomyoma tissue from patients treated with simvastatin contained significantly lower ER-α levels (p=0.015) compared to placebo (P = 0.015) (Fig. 6B and 6C).
Fig. 6. Immunohistochemical (IHC) staining showing decreased expression of ER-α in simvastatin-treated clinical tissue.

A, Patients with uterine fibroids were recruited for treatment with simvastatin (40 mg daily) or a placebo (starch 1500 encapsulated) for a total of 12 weeks. At the end of the treatment, fibroid samples were collected after the surgery to evaluate the effects of simvastatin. The tissues were fixed with a 10% buffered formalin solution for 24 h and kept in 70% ethanol at 4 °C. Tissues were then stained with ER-α antibody. B, Representative images of IHC staining of the ER-α antibody. Scale bars, 50 μm. Optical density (OD) was measured and quantified in 20× images using ImageJ software with automated macro instructions. C, Fifteen histologically similar fields were randomly selected from each slide to analyze OD. * p < 0.05 vs. the placebo.
4. DISCUSSION
Previous work suggested promising therapeutic effects of simvastatin on leiomyoma, both in vitro and in vivo [9–13]. In this study, we showed that simvastatin decreases E2-induced cell proliferation in leiomyoma cells, inhibits genomic and nongenomic estrogen signaling, and decreases ER-α expression. The specific reduction of the ER-α level in the plasma membrane and nucleus led us to discover that simvastatin reduces ER-α palmitoylation, a necessary post-translational modification, and increases degradation through increasing ER-α ubiquitination. Fig. 7 illustrates the ER-α signaling pathways and post-transcriptional modifications and highlights the simvastatin effects outlined in this study.
Fig. 7. Summary of results.

This figure represents a summary of the ER-α palmitoylation, ubiquitination, and signaling pathways, all of which alter ER-α downstream regulation. After binding to its receptor, estrogen initiates downstream signaling pathways that include the genomic, where the E2/ER complex translocates to the nucleus to induce the transcription of estrogen responsive genes, and nongenomic signaling pathways, which includes ERK and AKT phosphorylation. ER-α can undergo palmitoylation, and this post-translational modification step was previously shown to be necessary for ER-α membrane localization, its downstream signaling, and protection against degradation. Simvastatin is implicated in several steps with red, stunted arrows denoting simvastatin suppression and blue arrows indicating simvastatin induction. ER-α: estrogen receptor α, E2: estrogen, Palm: palmitate, Hsp90: heat shock protein 90, ERE: estrogen response element, CoR: coregulators, and TF: transcription factors.
E2 and its receptor facilitate pathobiological processes crucial for leiomyoma growth. E2 induces mature leiomyoma cells to release mitogenic stimuli to the surrounding immature cells, thereby maintaining leiomyoma growth. We previously showed that simvastatin decreases tumor growth and suppresses expression of the proliferation marker in a patient-derived xenograft animal model [11]. In the present study, the results from phase II clinical trial (NCT03400826) samples showed that ER-α expression was significantly suppressed by simvastatin treatment.
At a molecular level, E2 binds to either ER-α and acts through genomic or nongenomic signaling pathways to exert its effect on various protein kinase cascades and target transcription factors to ultimately exert its effects. A growing number of experimental evidence indicates that E2 promotes uterine leiomyoma growth through ER-α, which is expressed in larger quantities in uterine leiomyoma compared to a normal myometrium [3–5]. In the present study, we found that simvastatin significantly reduces the expression of ER-α in leiomyoma cells, consequently altering its associated pathways.
E2/ER complexes are known to trigger certain protein kinase cascades, including the ERK1/2 component of the MAPK family and the AKT kinase in the PI3K axis, to activate nongenomic signaling. These pathways are implicated in cellular proliferation, survival, and apoptosis [15]. Several studies have demonstrated that an aberrantly overexpressed MAPK pathway and a dysregulated PI3K-Akt-mTOR have a significant role in leiomyoma pathobiology [25, 26]. Here, we demonstrated that simvastatin treatment suppresses E2-stimulated pERK1/2 and p-AKT activation in uterine leiomyoma cells, suggesting the potential role of simvastatin as an ERK1/2 and AKT inhibitor. Additionally, in our previous report, we demonstrated the inhibitory effects of simvastatin on COL1A1 production [13]. Type 1 collagen is a major component of extracellular matrix, which is both excessive and disordered in uterine leiomyoma. Type 1 collagen is overexpressed in leiomyoma and induces integrin activation. In the present study, we found that simvastatin treatment suppresses E2-induced COL1A1 expression in leiomyoma cells. When it comes to the genomic pathway, E2/ER/DNA complex regulates cellular function via the transcription of target genes or through protein–protein interactions involving other transcription factors (AP1, SP1, and NF-кB). In our study, E2-induced ERE-Luc activity was reduced after simvastatin treatment. These results suggest that simvastatin targets both genomic and nongenomic signaling in leiomyoma cells.
The downregulation of CAV1, CCND1, CTGF, ERBB2, GPER1, PELP1, SOCS-3, THBS1, WNT4, and other ER co-regulator, interacting, and down-stream target genes, allows us to have an overall view of the changes that take place following simvastatin treatment. CAV1 potentiates ER-α mediated gene expression through direct interactions with ER-α and/or altering the E2-mediated translocation and ER-α activation [27]. It has already been reported that high CCND1 levels are correlated with leiomyoma growth and CCND1 acts as a downstream target of ER [28]. CTGF interacts with ER DNA binding domain [29], and CTGF act as an essential transcription factor of tissue remodeling and fibrosis [30]. Similarly, upregulated CTGF was observed in leiomyoma cells compared to myometrium [31]. There was a transcriptional link between tumors driven by ER and ERBB2 [32], and a higher level of ERBB2 was identified in leiomyoma compared to the myometrium, which suggests an enhancement of their involvement in tumorigenesis [33]. E2 binds to GPER1 in the membrane and rapidly activates the main signaling pathways of ER in leiomyoma [15]. PELP1 functions as a transcriptional coactivator for ER, and it interacts with receptors in a ligand-dependent manner in endometrial cancer cells [34]. ER-α directly influences SOCS-3 promoter activity by modulates cytokine production, while E2 treatment increased SOCS-3 in breast cancer cells [35]. Upregulation of THBS1 plays a role in modulating angiogenesis and tumor growth in E2 dependent tumors. The high expression level of this gene was observed in leiomyoma compared to matched myometrium in African Americans and Caucasians [36]. WNT mediates paracrine signals from ER-rich differentiated leiomyoma cells and leiomyoma stem cells to support tumorigenesis by activating the β-catenin pathway [37]. Higher expression of WNT4 was observed in uterine leiomyoma with MED12 mutations compared to those without mutations [38].
Palmitoylation facilitates ER-α localization in the plasma membrane, a necessary step for estrogen rapid nongenomic signaling. Notably, the palmitoylation of a single amino acid regulates ER-α trafficking, and this residue is well preserved across species. This process is necessary for the activation of downstream signaling pathways such as ERK/MAPK, PI3K/AKT, and PKC signaling, thereby contributing to E2 effect on proliferation [39]. We demonstrated that simvastatin reduces ER-α palmitoylation in leiomyoma cells, maybe through acting on the shared DHHC acyltransferases [21]. Reduced palmitoylation could explain the finding that simvastatin reduces the localization of ER-α in the plasma membrane.
E2 reduces the half-life of ER by increasing its ubiquitination and degradation [40]. Ubiquitination is a tremendously critical post-translational modification that governs a myriad of complex cellular processing beyond protein degradation as it was shown to modulate protein interactions, activity, and localization [41]. Treatment with E2 decreases ER-α mono-ubiquitination and increases degradative polyubiquitination [24]. Factors governing ligand activated ER-α protein degradation are not fully understood. It has been reported that E2-bound ER-α is more susceptible to degradation when it is not palmitoylated [42], and that ER-α is transported to the nuclear matrix for proteasomal degradation [43]. In this study, we showed that simvastatin increased ubiquitination of ER-α, reducing the availability of this receptor to bind to its ligand, thus affecting its ability to induce its transcriptional changes. Increased ubiquitination could explain the finding that simvastatin reduces the expression of ER-α in the nucleus compared to the cytoplasm.
Collectively, our results suggest a novel connection between simvastatin and uterine leiomyoma as they highlight possible mechanisms by which simvastatin can inhibit the growth of leiomyomas that are hyperresponsive to estrogen.
Supplementary Material
Highlights.
Simvastatin, a commonly available anti-hyperlipidemic drug, can prevent the growth of uterine leiomyoma in vitro and in vivo.
Simvastatin reduces E2-mediated downstream signaling, including ERK and AKT pathways, and ESR1 transcriptional activity.
Simvastatin affects the estrogen receptor trafficking by reducing ER-α palmitoylation, and increasing ubiquitin-mediated ER-α degradation.
Simvastatin suppresses ER-α expression in xenograft leiomyoma mouse model and clinical samples, promising possible therapeutic potential in uterine leiomyoma.
Acknowledgments:
We are grateful to George McNamara, PhD; Manager, Ross Fluorescent Imaging Center, Johns Hopkins School of Medicine; Barbara Smith, Electron Microscopy Specialist, Cell Bio Imaging Facility, Johns Hopkins School of Medicine; Sujayita Roy, Senior Research Specialist, Oncology Tissue Services, Johns Hopkins School of Medicine.
Funding:
This work was supported, in part, by NIH grant 1R01HD094380.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure summary: The authors have nothing to disclose.
Competing interests: The authors declare no conflict of interest.
Data and materials availability:
All data associated with this study are present in the paper.
References
- [1].Cramer SF, Patel A, The frequency of uterine leiomyomas, Am. J. Clin. Pathol 94(4) (1990) 435–438. [DOI] [PubMed] [Google Scholar]
- [2].Baird DD, Dunson DB, Hill MC, Cousins D, Schectman JM, High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence, Am. J. Obstet. Gynecol 188(1) (2003) 100–107. [DOI] [PubMed] [Google Scholar]
- [3].Kasai T, Shozu M, Murakami K, Segawa T, Shinohara K, Nomura K, Inoue M, Increased Expression of Type I 17β-Hydroxysteroid Dehydrogenase Enhances in Situ Production of Estradiol in Uterine Leiomyoma, J. Clin. Endocrinol. Metab 89(11) (2004) 5661–5668. [DOI] [PubMed] [Google Scholar]
- [4].Walker CL, Stewart EA, Uterine fibroids: the elephant in the room, Science 308(5728) (2005) 1589–1592. [DOI] [PubMed] [Google Scholar]
- [5].Yang Q, Mas A, Diamond MP, Al-Hendy A, The Mechanism and Function of Epigenetics in Uterine Leiomyoma Development, Reprod. Sci 23(2) (2016) 163–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Varelas FK, Papanicolaou AN, Vavatsi-Christaki N, Makedos GA, Vlassis GD, The effect of anastrazole on symptomatic uterine leiomyomata, Obstet. Gynecol 110(3) (2007) 643–649. [DOI] [PubMed] [Google Scholar]
- [7].Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T, Progesterone is essential for maintenance and growth of uterine leiomyoma, Endocrinology 151(6) (2010) 2433–2442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Pedram A, Razandi M, Deschenes RJ, Levin ER, DHHC-7 and-21 are palmitoylacyltransferases for sex steroid receptors, Mol. Biol. Cell 23(1) (2012) 188–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Borahay MA, Fang X, Baillargeon JG, Kilic GS, Boehning DF, Kuo YF, Statin use and uterine fibroid risk in hyperlipidemia patients: a nested case-control study, Am. J. Obstet. Gynecol 215(6) (2016) 750.e1–750.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Borahay MA, Kilic GS, Yallampalli C, Snyder RR, Hankins GDV, Al-Hendy A, Boehning D, Simvastatin potently induces calcium-dependent apoptosis of human leiomyoma cells, J. Biol. Chem 289(51) (2014) 35075–35086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Borahay MA, Vincent K, Motamedi M, Sbrana E, Kilic GS, Al-Hendy A, Boehning D, Novel Effects of Simvastatin on Uterine Fibroids: In vitro and Patient-Derived Xenograft Mouse Model Study, Am. J. Obstet. Gynecol 213(2) (2015) 196.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Malik M, Britten J, Borahay M, Segars J, Catherino WH, Simvastatin, at clinically relevant concentrations, affects human uterine leiomyoma growth and extracellular matrix production, Fertil. Steril 110(7) (2018) 1398–1407 e1. [DOI] [PubMed] [Google Scholar]
- [13].Afrin S, Islam MS, Patzkowsky K, Malik M, Catherino WH, Segars JH, Borahay MA, Simvastatin ameliorates altered mechanotransduction in uterine leiomyoma cells, Am. J. Obstet. Gynecol 223(5) (2020) 733.e1–733.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Tewari R, West SJ, Shayahati B, Akimzhanov AM, Detection of protein s-acylation using acyl-resin assisted capture, J. Vis. Exp (158) (2020) e61016 DOI: 10.3791/61016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Borahay MA, Asoglu MR, Mas A, Adam S, Kilic GS, Al-Hendy A, Estrogen receptors and signaling in fibroids: Role in pathobiology and therapeutic implications, Reprod. Sci 24(9) (2017) 1235–1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].O’Lone R, Frith MC, Karlsson EK, Hansen U, Genomic targets of nuclear estrogen receptors, Mol. Endocrinol 18(8) (2004) 1859–1875. [DOI] [PubMed] [Google Scholar]
- [17].Lösel R, Wehling M, Nongenomic actions of steroid hormones, Nat. Rev. Mol. Cell Biol 4(1) (2003) 46–55. [DOI] [PubMed] [Google Scholar]
- [18].Clark AL, Slayden OD, Hettrich K, Brenner RM, Estrogen increases collagen I and III mRNA expression in the pelvic support tissues of the rhesus macaque, Am. J. Obstet. Gynecol 192(5) (2005) 1523–9. [DOI] [PubMed] [Google Scholar]
- [19].Stewart EA, Friedman AJ, Peck K, Nowak RA, Relative overexpression of collagen type I and collagen type III messenger ribonucleic acids by uterine leiomyomas during the proliferative phase of the menstrual cycle, J. Clin. Endocrinol. Metab 79(3) (1994) 900–906. [DOI] [PubMed] [Google Scholar]
- [20].Anderson AM, Ragan MA, Palmitoylation: a protein S-acylation with implications for breast cancer, NPJ Breast Cancer 2(1) (2016) 16028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tonn Eisinger KR, Woolfrey KM, Swanson SP, Schnell SA, Meitzen J, Dell’Acqua M, Mermelstein PG, Palmitoylation of caveolin-1 is regulated by the same DHHC acyltransferases that modify steroid hormone receptors, J. Biol. Chem 293(41) (2018) 15901–15911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tecalco-Cruz AC, Ramírez-Jarquín JO, Polyubiquitination inhibition of estrogen receptor alpha and its implications in breast cancer, World J. Clin. Oncol 9(4) (2018) 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dikic I, Wakatsuki S, Walters KJ, Ubiquitin-binding domains - from structures to functions, Nat. Rev. Mol. Cell Biol 10(10) (2009) 659–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berry NB, Fan M, Nephew KP, Estrogen receptor-alpha hinge-region lysines 302 and 303 regulate receptor degradation by the proteasome, Mol. Endocrinol 22(7) (2008) 1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Nierth-Simpson EN, Martin MM, Chiang T-C, Melnik LI, Rhodes LV, Muir SE, Burow ME, McLachlan JA, Human uterine smooth muscle and leiomyoma cells differ in their rapid 17beta-estradiol signaling: implications for proliferation, Endocrinology 150(5) (2009) 2436–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Sefton EC, Qiang W, Serna V, Kurita T, Wei J-J, Chakravarti D, Kim JJ, MK-2206, an AKT inhibitor, promotes caspase-independent cell death and inhibits leiomyoma growth, Endocrinology 154(11) (2013) 4046–4057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Schlegel A, Wang C, Katzenellenbogen BS, Pestell RG, Lisanti MP, Caveolin-1 potentiates estrogen receptor α (ERα) signaling: caveolin-1 drives ligand-independent nuclear translocation and activation of ERα, J. Biol. Chem 274 (47) (1999) 33551–6. [DOI] [PubMed] [Google Scholar]
- [28].Kovács KA, Oszter A, Göcze PM, Környei JL, Szabó I, Comparative analysis of cyclin D1 and oestrogen receptor (α and β) levels in human leiomyoma and adjacent myometrium, Hum. Reprod. Update 7(11) (2001) 1085–1091. [DOI] [PubMed] [Google Scholar]
- [29].Cheng L, Yang Z, Wang X, Jiao Y, Xie X, Lin J, Zhang H, Han J, Jiang K, Ye Q, Suppression of estrogen receptor transcriptional activity by connective tissue growth factor, PLoS One 6(5) (2011) e20028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Lipson KE, Wong C, Teng Y, Spong S, CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis, Fibrogenesis Tissue Repair, BioMed Central, 2012, pp. 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Islam MS, Afrin S, Singh B, Jayes FL, Brennan JT, Borahay MA, Leppert PC, Segars JH, Extracellular matrix and Hippo signaling as therapeutic targets of antifibrotic compounds for uterine fibroids, Clin. Transl. Med 11(7) (2021) e475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Hurtado A, Holmes KA, Geistlinger TR, Hutcheson IR, Nicholson RI, Brown M, Jiang J, Howat WJ, Ali S, Carroll JS, ERBB2 regulation by estrogen receptor-Pax2 determines tamoxifen response, Nature 456(7222) (2008) 663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Davis BJ, Risinger JI, Chandramouli GV, Bushel PR, Baird DD, Peddada SD, Gene expression in uterine leiomyoma from tumors likely to be growing (from black women over 35) and tumors likely to be non-growing (from white women over 35), PloS one 8(6) (2013) e63909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Vadlamudi RK, Balasenthil S, Broaddus RR, Gustafsson J-A, Kumar R, Deregulation of estrogen receptor coactivator proline-, glutamic acid-, and leucine-rich protein-1/modulator of nongenomic activity of estrogen receptor in human endometrial tumors, J. Clin. Endocrinol. Metab 89(12) (2004) 6130–6138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Matthews J, Almlöf T, Kietz S, Leers J, Gustafsson J-Å, Estrogen receptor-α regulates SOCS-3 expression in human breast cancer cells, Biochem. Biophys. Res. Commun 335(1) (2005) 168–174. [DOI] [PubMed] [Google Scholar]
- [36].Pan Q, Luo X, Chegini N, Genomic and proteomic profiling I: leiomyomas in African Americans and Caucasians, Reprod. Biol. Endocrinol 5(1) (2007) 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Ono M, Yin P, Navarro A, Moravek MB, Coon JS, Druschitz SA, Serna VA, Qiang W, Brooks DC, Malpani SS, Paracrine activation of WNT/β-catenin pathway in uterine leiomyoma stem cells promotes tumor growth, Proc. Natl. Acad. Sci 110(42) (2013) 17053–17058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Markowski DN, Bartnitzke S, Löning T, Drieschner N, Helmke BM, Bullerdiek J, MED12 mutations in uterine fibroids—their relationship to cytogenetic subgroups, Int. J. Cancer 131(7) (2012) 1528–1536. [DOI] [PubMed] [Google Scholar]
- [39].Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M, Palmitoylation-dependent Estrogen Receptor α Membrane Localization: Regulation by 17β-Estradiol, Mol. Biol. Cell 16(1) (2005) 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Nirmala PB, Thampan RV, Ubiquitination of the rat uterine estrogen receptor: dependence on estradiol, Biochem.Biophys.Res.Comm 213(1) (1995) 24–31. [DOI] [PubMed] [Google Scholar]
- [41].Komander D, Rape M, The ubiquitin code, Annu. Rev. Biochem 81 (2012) 203–229. [DOI] [PubMed] [Google Scholar]
- [42].La Rosa P, Pesiri V, Leclercq G, Marino M, Acconcia F, Palmitoylation regulates 17β-estradiol-induced estrogen receptor-α degradation and transcriptional activity, Mol. Endocrinol 26(5) (2012) 762–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Reid G, Hubner MR, Metivier R, Brand H, Denger S, Manu D, Beaudouin J, Ellenberg J, Gannon F, Cyclic, proteasome-mediated turnover of unliganded and liganded ERα on responsive promoters is an integral feature of estrogen signaling, Mol. Cell 11(3) (2003) 695–707. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data associated with this study are present in the paper.


