Abstract
A 7-year-old girl was admitted to the hospital with acute lymphoblastic leukemia and was treated with allogenic cord blood transplantation. At day 30 after graft, she developed a fever and multiple nodular lesions disseminated in the liver and lungs. All bacterial cultures attempted on liver and lung biopsy specimens and blood remained sterile on standard axenic media. However, inoculation of liver and lung biopsy specimens on eukaryotic cell monolayers by the centrifugation-shell vial technique (M. Marrero and D. Raoult, Am. J. Trop. Med. Hyg. 40:197–199, 1989) led to the recovery of a strain of Legionella pneumophila serogroup 1, identified by 16S rRNA gene amplification and sequencing and serotyping. Our findings demonstrate that the centrifugation-cell culture method, which has previously been useful for the isolation of other strictly or facultatively intracellular bacteria, can also serve as a method for the recovery of L. pneumophila from clinical material.
Legionella pneumophila, the agent of Legionnaires’ disease, was first recognized during an outbreak of pneumonia among visitors to a Philadelphia, Pa., hotel in 1976 (7). As diagnosis methods have improved, this agent has been found to be a more and more common cause of community-acquired and nosocomial pneumonia (14). However, extrapulmonary legionellosis is uncommon. The most common extrapulmonary site is the heart, but Legionella sp. strains have also been implicated in cases of sinusitis, cellulitis, pancreatitis, peritonitis, and pyelonephritis (14). These disseminations apparently occur through bacteremia, and in many cases, there is no overt pneumonia (12). Specialized laboratory tests are necessary to establish a diagnosis of legionellosis and must be specifically requested, as they are not routinely performed. The definitive method for the diagnosis of legionellosis is culture of the organism, with sensitivity varying from 32 to 80% (1, 14). The other available diagnostic tests include direct fluorescent-antibody staining of respiratory tract samples, urinary antigen detection assays, and serologic testing for specific antibodies (14). The shell vial assay (6), originally devised for the isolation of viruses, has now been adapted for the isolation of rickettsiae and other intracellular bacteria from tissue biopsy specimens and blood samples. In this report, we describe the first application of this method to the clinical isolation of L. pneumophila.
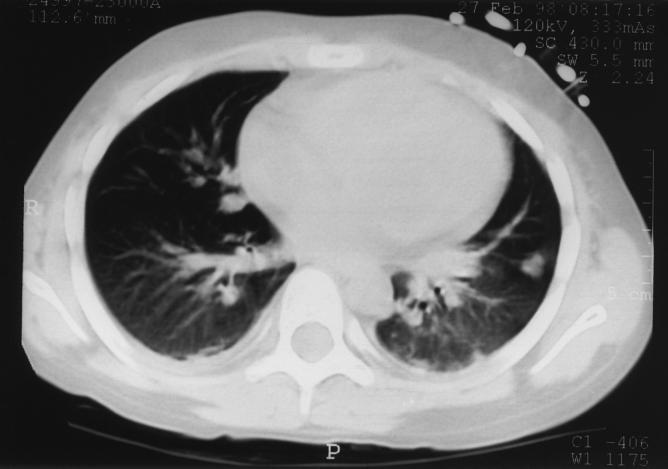
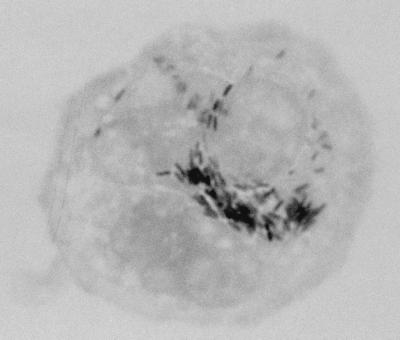
A 7-year-old girl was admitted to the hospital for an allogenic cord blood transplantation. She was suffering from acute lymphoblastic leukemia and was in complete remission of her disease at the time of transplantation. A conditioning regimen consisted of total body irradiation (12 Gy in six fractions over 3 days with lung shielding at 8 Gy), cyclophosphamide, and antithymocyte globulin. The graft was an unrelated and HLA-mismatched umbilical cord blood unit, previously frozen in a cord blood bank. This unit contained 137 × 107 nucleated cells (i.e., 4.7 × 107 per kg of body weight of recipient). Posttransplant prophylaxis of graft-versus-host disease was a combination of cyclosporine and steroids. At day 7 posttransplant, the patient presented with high fever (39.5°C), and a strain of Streptococcus mitis was isolated from her blood. This bacteremia was successfully treated with a 15-day course of vancomycin. At day 30 after transplantation, the fever recurred and was accompanied by a cough and abdominal pain. At this time, the leukocyte count was 109/liter with 64% neutrophils, 6% lymphocytes, and 30% monocytes. HLA typing of these leukocytes showed that they were of donor origin. Standard axenic culture of blood, sputum, and urine specimens remained sterile. A combination of vancomycin and imipenem was administered. The following day, the patient underwent abdominal echography and thoracic tomodensitometry (Fig. 1) which demonstrated multiple nodular lesions of 0.7 to 5 mm in diameter disseminated in the liver and lungs. As a fungal infection was suspected, liposomal amphotericin B was added to her regimen. During the next few days, the patient developed dyspnea and sever hypoxemia. Her clinical condition progressively worsened, and she died at day 40 posttransplant despite assisted ventilation. Lung and hepatic biopsy specimens were collected on the day of death. Microscopic examination of Gram- and Ziehl-Neelsen-stained preparations of lung and liver biopsy specimens did not reveal any bacteria, and no organisms were isolated with standard clinical microbiology media or special culture media for mycobacteria, fungi, and mycoplasmas. One month later, as no etiologic agent had been found, we decided to try to isolate bacteria that do not grow on the culture media initially used, including strictly intracellular bacteria such as Coxiella burnetii or facultatively intracellular bacteria such as Bartonella spp. Remaining biopsy samples were thawed and inoculated onto ECV 304 human endothelial cells and human embryonic lung (HEL) fibroblasts in shell vials by methods described previously (6). Briefly, the biopsy specimens were homogenized in 1 ml of brain heart infusion broth, and the homogenate was aspirated into a 1-ml syringe through a 27-gauge needle to disrupt coarse material. One-half of this sample was inoculated into shell vials (3.7 ml; Sterilin, Feltham, England) containing a monolayer of ECV 304 cells or HEL cells grown on a 1-cm2 coverslip. Three shell vials of each cell line were inoculated and then centrifuged for 1 h at 700 × g at 22°C. The brain heart infusion was then discarded and replaced with culture medium (RPMI medium with 10% fetal calf serum and 1 mM l-glutamine per liter for ECV 304 cells and Eagle’s minimal essential medium with 4% fetal calf serum and 1 mM l-glutamine per liter for HEL cells). After incubation for 1 week at 37°C, small vacuoles were observed in ECV 304 cells by using an inverted microscope. A coverslip from one shell vial was stained by the Gimenez method. Small extra- and intracellular bacilli were observed (Fig. 2). By the same procedure, only a few bacteria were detected on HEL monolayers. To determine whether these bacteria would grow in the cell culture medium alone, this bacterium was inoculated and cultivated under the same conditions described above but without cells. We also inoculated 5% sheep blood agar and chocolate agar plates, which were then incubated at 37°C in a 5% CO2 atmosphere. After 1 week, no growth was obtained with both these procedures. DNA extracts, suitable for use as a template in PCR assays, were prepared from one remaining shell vial by using the QIAmp tissue kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. These DNA extracts were amplified by using PCR incorporating previously described broad-range 16S rRNA gene primers fD1 and rP2 (5). The nucleotide base sequence of the amplification product was determined by linear PCRs incorporating previously described primers, and the products of these reactions were electrophoretically resolved on a 6% polyacrylamide gel (Ready Mix Gels, automated laser fluorescent grade; Pharmacia Biotech Europe, Brussels, Belgium) with an ALF DNA sequencer (Pharmacia Biotech Europe) as previously described (5). The sequences obtained from individual reactions were aligned and then combined with one another to yield an almost complete 16S rRNA gene. This sequence was compared to 16S rRNA sequences of other bacteria by a FASTA search of the GenBank database. Once ambiguities in our sequence had been removed, the sequence derived from the shell vial isolate was found to have 99.9% sequence similarity with L. pneumophila. As a result of this finding, remaining samples from liver and lung biopsies and the contents of the third shell vial were inoculated onto buffered charcoal yeast extract (BCYEα) agar plates (Biomérieux, Marcy l’Étoile, France) and incubated at 37°C in a 5% CO2 atmosphere. Four days later, small white translucent colonies were detected on all BCYEα agar plates. All isolates were submitted to the Centre National de Référence for Legionella (J. Etienne, Lyon, France) where their identity was serologically determined as L. pneumophila serogroup 1.
FIG. 1.
Thoracic tomodensitometry demonstrating multiple bilateral nodular lesions disseminated in lung parenchyma.
FIG. 2.
Gimenez staining of an ECV 304 endothelial cell from a shell vial coverslip infected with L. pneumophila (magnification, ×1,000).
The classic triad of diagnostic approaches is available for diagnosis of Legionella infections: isolation of the bacterium in culture, direct detection of bacterial antigens or nucleic acids in clinical specimens, and documentation of a serological response to the bacterium. This last approach is, however, not suitable for patients who have recently undergone bone marrow graft, as they are unable to mount a humoral response. Culture remains the method of choice (16) and, when samples are processed correctly, has a sensitivity comparable to or higher than that of other methods (about 80%). Furthermore, a 100% specificity is a significant advantage for an infection of low prevalence. The established isolation method of Legionella spp. from clinical specimens is BCYEα, to which an antibiotic may be added when specimens to be inoculated are derived from nonsterile sources (15). Media supporting growth of Legionella spp. should always be used to culture respiratory tract samples and other specimens obtained from immunocompromised patients with nosocomial pneumonia. More recently, clinical isolates of L. pneumophila have been obtained by coculture of specimens with free-living amoebae (13). Use of this procedure has led to the isolation of L. pneumophila when inoculated BCYEα agar plates have remained sterile. Nevertheless, this technique is, at present, appropriate for use only by specialized reference laboratories.
The centrifugation-shell vial system (6) is a versatile method which can be applied to many viruses as well as facultatively or strictly intracellular bacteria. As a reference center for the diagnosis and study of rickettsioses, we have used this technique routinely and successfully to isolate Rickettsia spp. (4), C. burnetii (9), and Bartonella spp. (11) from blood and tissue biopsy samples. We have also recently used this approach for the isolation of Francisella tularensis from inoculation eschar biopsy samples (2). After inoculation and incubation in shell vials, detection of bacteria can be assessed by the use of acridine orange, Gimenez, and Giemsa stainings of the shell vial supernatant or by immunofluorescence staining of the cell monolayer by using the patient serum, if suitable, as primary antibody. When bacterial growth is detected, identification can be achieved by PCR amplification and then sequencing of the 16S rRNA gene.
The development of L. pneumophila on cell culture is not unexpected, as it has already been shown to multiply well on several leukocyte-derived cell lines (10). Moreover, when first isolated in 1947, the organism was considered a rickettsia-like agent, as it did not grow on standard bacteriologic media but grew in embryonated hens’ eggs and was pathogenic for guinea pigs (8). As the use of the shell vial technique requires specialized equipment and trained personnel, it is not suitable for use in most clinical conditions. However, in its favor, this procedure provides a means for the isolation of a wide range of intracellular bacteria, even when only very little biopsy material is available.
To our knowledge, this is the first diagnosis of legionellosis made by using the shell vial culture of a patient’s specimen. Clearly, as yet we are unable to draw any meaningful conclusions regarding its clinical use compared to that of BCYEα agar plates. Nevertheless, work in our laboratory on the recovery of another facultatively intracellular gram-negative bacterium, Bartonella sp., has shown that in some instances cell culture is successful when blood agar plates are not (3). Further studies on the usefulness of the shell vial assay for isolation of L. pneumophila are therefore perhaps warranted.
REFERENCES
- 1.Edelstein P H. Legionnaires’ disease. Clin Infect Dis. 1993;16:741–747. doi: 10.1093/clind/16.6.741. [DOI] [PubMed] [Google Scholar]
- 2.Fournier P E, Bernabeu L, Shubert B, Mutillod M, Raoult D. Isolation of Francisella tularensis by centrifugation of shell vial cell culture from an inoculation eschar. J Clin Microbiol. 1998;36:2782–2783. doi: 10.1128/jcm.36.9.2782-2783.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler J E, Quinn F D, Berger T G, Leboit P E, Tappero J W. Isolation of Rochalimaea species from cutaneous and osseous lesions of bacillary angiomatosis. N Engl J Med. 1992;327:1625–1631. doi: 10.1056/NEJM199212033272303. [DOI] [PubMed] [Google Scholar]
- 4.La Scola B, Raoult D. Diagnosis of Mediterranean spotted fever by cultivation of Rickettsia conorii from blood and skin samples using the centrifugation-shell vial technique and by detection of R. conorii in circulating endothelial cells: a 6-year follow-up. J Clin Microbiol. 1996;34:2722–2727. doi: 10.1128/jcm.34.11.2722-2727.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.La Scola B, Michel G, Raoult D. Use of amplification and sequencing of the 16S rRNA gene to diagnose Mycoplasma pneumoniae osteomyelitis in a patient with hypogammaglobulinemia. Clin Infect Dis. 1997;24:1161–1163. doi: 10.1086/513631. [DOI] [PubMed] [Google Scholar]
- 6.Marrero M, Raoult D. Centrifugation-shell vial technique for rapid detection of Mediterranean spotted fever rickettsia in blood culture. Am J Trop Med Hyg. 1989;40:197–199. doi: 10.4269/ajtmh.1989.40.197. [DOI] [PubMed] [Google Scholar]
- 7.McDade J E, Shepard C C, Fraser D W, Tsai T R, Redus M A, Dowle W R Laboratory Investigation Team. Legionnaires’ disease. Isolation of a bacterium and demonstration of its role in other respiratory disease. N Engl J Med. 1997;297:1197–1203. doi: 10.1056/NEJM197712012972202. [DOI] [PubMed] [Google Scholar]
- 8.McDade J E, Brenner D J, Bozeman F M. Legionnaires’ disease bacterium isolated in 1947. Ann Intern Med. 1979;90:659–661. doi: 10.7326/0003-4819-90-4-659. [DOI] [PubMed] [Google Scholar]
- 9.Musso D, Raoult D. Coxiella burnetii blood cultures from acute and chronic Q fever patients. J Clin Microbiol. 1995;33:3129–3132. doi: 10.1128/jcm.33.12.3129-3132.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Neumeister B, Schöniger S, Faigle M, Eichner M, Dietz K. Multiplication of different Legionella species in Mono Mac 6 cells and in Acanthamoeba castellanii. Appl Environ Microbiol. 1997;63:1219–1224. doi: 10.1128/aem.63.4.1219-1224.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raoult D, Fournier P E, Drancourt M, Marrie T J, Etienne J, Cosserat J, Cacoub P, Poinsignon Y, Leclercq P, Sefton A M. Diagnosis of 22 new cases of Bartonella endocarditis. Ann Intern Med. 1996;125:646–652. doi: 10.7326/0003-4819-125-8-199610150-00004. [DOI] [PubMed] [Google Scholar]
- 12.Rihs J D, Yu V L, Zuravleff J J, Goetz A, Muder R R. Isolation of Legionella pneumophila from blood with the BACTEC system: a prospective study yielding positive results. J Clin Microbiol. 1985;22:422–424. doi: 10.1128/jcm.22.3.422-424.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rowbotham T J. Isolation of Legionella pneumophila from clinical specimens via amoebae and the interaction of those and other isolates with amoebae. J Clin Pathol. 1983;36:978–986. doi: 10.1136/jcp.36.9.978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stout J E, Yu V L. Legionellosis. N Engl J Med. 1997;337:682–687. doi: 10.1056/NEJM199709043371006. [DOI] [PubMed] [Google Scholar]
- 15.Winn W C., Jr . Legionella. In: Murray P R, Baron E J, Pfaller M A, Tenover F C, Yolken R H, editors. Manual of clinical microbiology. 6th ed. Washington, D.C: ASM Press; 1995. pp. 533–544. [Google Scholar]
- 16.Zuravleff J J, Yu V L, Shonnard J W, Davis B K, Rihs J D. Diagnosis of Legionnaires’ disease. An update of laboratory methods with new emphasis on isolation by culture. JAMA. 1983;250:1981–1985. doi: 10.1001/jama.250.15.1981. [DOI] [PubMed] [Google Scholar]