Abstract
Throughout the world, esophageal cancer patients had a greater suicidal risk compared with ordinary people. Thus, we aimed to affirm suicide rates, standardized mortality rates, and underlying suicide-related risk factors of esophageal cancer patients. Patients suffering esophageal cancer were chosen from the Surveillance, Epidemiology, and End Results repository in 1975–2016. Suicide rates as well as standardized mortality rates in the patients were measured. Univariable and multivariable Cox regression had been adopted for establishing the latent suicide risk factors among patients suffering esophageal cancer. On multivariable Cox regression, gender (male vs. female, HR: 6.37), age of diagnosis (70–105 vs. 0–55, HR: 2.69), marital status, race (white race vs. black race, HR: 6.64; American Indian/Alaska Native, Asian/Pacific Islander vs. black race, HR: 8.60), histologic Grade (Grade III vs. Grade I, HR: 2.36), no surgery performed (no/unknown vs. yes, HR: 2.01), no chemotherapy performed were independent risk factors related to suicide in patients suffering esophageal cancer. Male sex, the older age, unmarried state, non-black race, histologic Grade III, no surgery performed, no chemotherapy performed were strongly related to suicide in patients suffering esophageal cancer.
Subject terms: Psychology, Cancer epidemiology
Introduction
Suicide has become a worldwide public health issue, or kind of sophisticated action subject to factors in physiology, psychology, society, environment and culture1. In addition, it is still the main contributor of death in people aged 15–24 globally, and also the tenth main cause of death across North America2. 817,000 people commit suicide worldwide in 2016, accounting for 1.49% in total deaths3. According to the World Health Organization (WHO), the 2016 suicide rate totaled 10.6 suicides per 100,000 persons, with 80% among middle-low income states4. Despite the decline of suicide by around 18% in 2000–2016 across most WHO areas2, the U.S. witnessed an annual increase of suicide by 1.5% after 20005.
Recently, research has discovered depression is significantly correlated with suicide, and the suicide rate in depression patients far exceeds that in ordinary people6–8. During the COVID-19 outbreak and resulting quarantine, suicidal intention and action quickly increased in high-risk groups, including unemployed9, bereaved10, smoking11, alcohol consumption12–14, or even genetic level groups15,16. Although cancer patients of both genders underwent identical stress, drastic decline of family income possibly intensified the suicidal intention and action among men17. Much evidence has suggested a stronger propensity of desperation and suicide among patients with bad prognosis illnesses (in particular cancer)18–21. Moreover, many proofs in systematic reviews have revealed the growing suicidal risk in cancer patients22–24. It is surprising that suicide rate of U.S. cancer patients almost doubled that in ordinary people25. In addition, a latest research performed by Zaorsky et al. indicates standardized mortality rate (SMR) of suicide in cancer patients is 4.44 in comparison with ordinary people26. Given that suicide can be recognized and prevented, it is imperative to identify patients at high risk of suicide27.
Throughout the world, esophageal cancer has been considered the sixth most representative cancer-related death: 572,034 new cases and 508,585 deaths were discovered in 201828. In 2019, Chelsea Anderson et al. found the SMR in esophageal cancer was 5.03 (95% Confidence Interval (CI): 4.03–6.19) in the U.S. general population (2000–2014), which might be adjusted by age, sex, as well as race29. Whereas, by far, only a limited number of reports have examined the suicide-related risk factors among esophageal cancer patients with a large sample size. Hence, the current study aims to measure suicide rates as well as SMRs in comparison with U.S. general population and recognize underlying factors associated with suicide by reference to the SEER database (1975–2016).
Methods
Data selection
Esophageal cancer patients, with diagnosis time in 1975—2016, had been chosen from the National Cancer Institute's Surveillance, Epidemiology, and End Results (SEER) Program. Data about the general U.S. population, demographic and clinical variables were gathered from the National Center for Health Statistics in 1975–2016 and acquired via the SEER Program30,31. Patients had been differentiated with primary site codes (C15.0-C15.5, C15.8, C15.9) related to esophageal cancer in line with International Classification of Diseases for Oncology codes (3rd edition) of esophageal cancer32. Main outcome was suicide-caused death, which might be recognized through the cause of death code (suicide or self-caused injury).
SEER*Stat software (version 8.3.6) was applied for establishing the patients33. Details are presented in Fig. 1.
Figure 1.
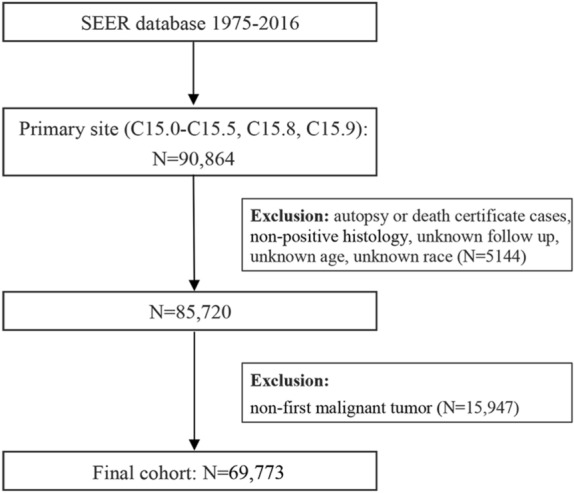
The flow diagram of patient selection (Description: There are steps of how to identify 161 suicidal patients from 90,864 esophageal cancer patients in Surveillance, Epidemiology, and End Results program during 1975–2016; SEER*Stat software, version 8.3.6, http://www.seer.cancer.gov/seerstat/; Microsoft Word software, version 16.0.14131.20296, https://www.microsoft.com/zh-cn/download/).
Statistical analysis
Suicide rates among patients suffering esophageal cancer were counted according to reported suicides per 100,000 person-years of follow-up. The U.S. population suicide rates at the National Center for Health Statistics were accessed from the SEER Program for a comparison with those of our cohort and ordinary people. Data were described using SMRs, which could be adjusted according to age, race, as well as sex in the U.S. population during the same period. Five-year age groupings were chosen in normalization34. SMRs were measured as the ratio of reported suicides in esophageal cancer patients to expected suicide counts of overall population. Expected suicide counts were calculated through multiplying overall population suicide rate by person-time of our cohort, considering the strata in age, race, and sex. Ninety-five percent of confidence interval (CI) in the SMRs was measured in the mid-P test35. In addition, between-group suicide rates were figured out by the chi-square test, and Bonferroni-corrected P value was used in multiple comparisons. Further, SMRs were evaluated in accordance with survival months (< 2 months, 2 months—11 months, 12 months—59 months, ≥ 60 months), and the initiative 2-month cutoff was chosen as the best estimation for the rational window between diagnosis and starting cancer therapy. The duration was supposed to be linked to the maximum suicide rate. To investigate interactions between different factors, we performed likelihood ratio testings to assess interactions among Sex, Age of diagnosis, SEER disease stage, Race, and Treatment performed (Surgery, Radiotherapy, and Chemotherapy). Univariable and multivariable Cox regression had been conducted to determine crude and adjusted hazard ratios (HRs) as well as 95% CI, to reveal underlying suicide-related risk factors. Merely variables satisfying P < 0.1 under univariate Cox regression model are proper for multivariate Cox regression model. In relevant analyses, patients who had 0-month follow-up were given a value of 0.5 months. Age of diagnosis was the sole continuous variable. For investigating suicide risk in patients at various age groups, X-tile software (http://tissuearray.org/) had been employed for discovering the optimal cutoffs of age (see Supplementary Fig. S1 online). Overall statistical analyses proved two-sided, and P < 0.05 demonstrated the statistical significance. SPSS (version 25.0, SPSS, Chicago, IL, USA), Microsoft Word (version 16.0.14131.20296) and Microsoft Excel (version 16.0.12730.20188, Microsoft, Redmond, State of Washington) were adopted for carrying out the statistical analyses.
Ethic declarations
The research involved no human participants or infringement of individual privacy. Thus, approval from the institutional review board was unnecessary. Informed consent was abandoned in the anonymous study.
Results
Patient baseline features
In general, 69,773 esophageal cancer patients had been determined from the SEER repository in 1975–2016, encompassing 53,665 males and 16,108 females. Of which, 161 of them (0.23%) commit suicide, 60,113 of them (86.16%) died of other reasons, whereas 9499 patients (13.61%) were alive (Table 1). The steps of choosing patients were depicted in Fig. 1. Among all patients, 38,027 (54.50%) patients had got married or mates, whereas 17,819 (25.54%) patients had once got married (divorced, widowed and separated), and 10,690 (15.32%) of them were single (never married). White race (80.73%) was the predominant race. Overall, 19,228 (27.56%) of them received cancer-directed surgery, whereas 37,400 (53.60%) patients receive chemotherapy. Regarding the patients who committed suicide, 152 (94.41%) were males, and 9 (5.59%) females. For marital status, 81 (50.31%) had got married or mates (domestic partners), whereas 41 (25.47%) were previously married, and 28 (17.39%) were single. Likewise, white (90.68%) was also a prominent race. 47 (29.19%) patients underwent cancer-directed surgery, while merely 79 (49.07%) patients had chemotherapy. Table 1 listed patient demographics as well as clinical characteristics.
Table 1.
Baseline characteristics of patients with esophageal cancer stratified by suicidal death, nonsuicidal death and alive patients.
| Variables | Overall | Suicidal death | Nonsuicidal death | Alive Patients |
|---|---|---|---|---|
| N (%) | N (%) | N (%) | N (%) | |
| Patients | 69,773 (100%) | 161 (100%) | 60,113 (100%) | 9499 (100%) |
| Year of diagnosis | ||||
| 1975–1988 | 9139 (13%) | 22 (14%) | 9076 (15%) | 41 (0%) |
| 1989–2002 | 19,512 (28%) | 51 (32%) | 18,664 (31%) | 797 (8%) |
| 2003–2016 | 41,122 (59%) | 88 (55%) | 32,373 (54%) | 8661 (91%) |
| Sex | ||||
| Male | 53,665 (77%) | 152 (94%) | 46,111 (77%) | 7402 (78%) |
| Female | 16,108 (23%) | 9 (6%) | 14,002 (23%) | 2097 (22%) |
| Age at diagnosis | ||||
| 0–55 | 12,994 (19%) | 18 (11%) | 10,787 (18%) | 2189 (23%) |
| 56–69 | 29,437 (42%) | 63 (39%) | 24,727 (41%) | 4647 (49%) |
| 70–105 | 27,342 (39%) | 80 (50%) | 24,599 (41%) | 2663 (28%) |
| Marital status | ||||
| Married/ Domestic Partner | 38,027 (55%) | 81 (50%) | 32,156 (53%) | 5790 (61%) |
| Previously Marrieda | 17,819 (26%) | 41 (25%) | 16,115 (27%) | 1663 (18%) |
| Singleb | 10,690 (15%) | 28 (17%) | 9185 (15%) | 1477 (16%) |
| Unknown | 3237 (5%) | 11 (7%) | 2657 (4%) | 569 (6%) |
| Race | ||||
| White | 56,327 (81%) | 146 (91%) | 48,011 (80%) | 8170 (86%) |
| Black | 9606 (14%) | 3 (2%) | 8898 (15%) | 705 (7%) |
| American Indian/Alaska Native, Asian/Pacific Islander | 3840 (6%) | 12 (7%) | 3204 (5%) | 624 (7%) |
| Histologic grade | ||||
| Grade I | 3621 (5%) | 6 (4%) | 2872 (5%) | 743 (8%) |
| Grade II | 22,519 (32%) | 44 (27%) | 19,027 (32%) | 3448 (36%) |
| Grade III | 28,516 (41%) | 79 (49%) | 25,343 (42%) | 3094 (33%) |
| Grade IV | 1531 (2%) | 4 (2%) | 1409 (2%) | 118 (1%) |
| Unknown | 13,586 (19%) | 28 (17%) | 11,462 (19%) | 2096 (22%) |
| Primary site | ||||
| Lower third of esophagus | 38,000 (54%) | 105 (65%) | 31,653 (53%) | 6242 (66%) |
| Middle third of esophagus | 13,460 (19%) | 19 (12%) | 12,167 (20%) | 1274 (13%) |
| Upper third of esophagus | 3967 (6%) | 8 (5%) | 3546 (6%) | 413 (4%) |
| Overlapping lesion of esophagus | 3351 (5%) | 10 (6%) | 3023 (5%) | 318 (3%) |
| Cervical esophagus | 1597 (2%) | 5 (3%) | 1414 (2%) | 178 (2%) |
| Thoracic esophagus | 2281 (3%) | 4 (2%) | 2006 (3%) | 271 (3%) |
| Abdominal esophagus | 672 (1%) | 0 (0%) | 591 (1%) | 81 (1%) |
| Esophagus, NOS | 6445 (9%) | 10 (6%) | 5713 (10%) | 722 (8%) |
| Histology recode—broad groupings | ||||
| Adenomas and adenocarcinomas | 33,797 (48%) | 88 (55%) | 27,610 (46%) | 6099 (64%) |
| Squamous cell neoplasms | 28,892 (41%) | 57 (35%) | 26,230 (44%) | 2605 (27%) |
| Others | 7084 (10%) | 16 (10%) | 6273 (10%) | 795 (8%) |
| SEER disease stage | ||||
| Localized | 15,873 (23%) | 43 (27%) | 12,341 (21%) | 3489 (37%) |
| Regional | 20,978 (30%) | 55 (34%) | 17,153 (29%) | 3770 (40%) |
| Distant | 23,622 (34%) | 40 (25%) | 22,014 (37%) | 1568 (17%) |
| Unknown/unstaged | 9300 (13%) | 23 (14%) | 8605 (14%) | 672 (7%) |
| Surgery performed | ||||
| Yes | 19,228 (28%) | 47 (29%) | 13,919 (23%) | 5262 (55%) |
| No/unknown | 50,545 (72%) | 114 (71%) | 46,194 (77%) | 4237 (45%) |
| Radiotherapy performed | ||||
| Yes | 39,423 (57%) | 88 (55%) | 33,735 (56%) | 5600 (59%) |
| No/unknown | 30,350 (43%) | 73 (45%) | 26,378 (44%) | 3899 (41%) |
| Chemotherapy performed | ||||
| Yes | 37,400 (54%) | 79 (49%) | 31,197 (52%) | 6124 (64%) |
| No/unknown | 32,373 (46%) | 82 (51%) | 28,916 (48%) | 3375 (36%) |
SEER, the surveillance, epidemiology, and end results program.
aIncluded divorced, widowed and separated.
bIncluded never married.
Difference in suicide rates and SMRs
Suicide rates
During 1975 and 2016, 161 suicide cases had been reported in 69,773 esophageal cancer patients surveyed for 128,508.08 person-years, resulting in the suicide rate of 125.28 per 100,000 person-years. Higher suicide rates in esophageal cancer patients correlated with male sex (vs. female sex, P < 0.001), white race (vs. black race, P < 0.001), as well as the middle third of the esophagus (vs. lower third of the esophagus, P < 0.01). The chi-square test of linear trend revealed growing suicide rate in esophageal cancer patients with age of diagnosis (P < 0.01) as well as survival months (P < 0.01). However, there were no significant discrepancies about suicide rates concerning year of diagnosis, marital status, histology recode-broad groupings, histologic grade, SEER disease stage, surgical procedures performed, radiotherapy performed, and chemotherapeutic options administered. Details are presented in Table 2.
Table 2.
Suicide rates and SMRs among patients with esophageal cancer by demographic and clinic characteristics (1975‐2016).
| Variables | Suicidal death | Person‐years | Suicide rate per 100,000 person‐years | P | SMRa | 95% CI | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Total | 161 | 128,508.08 | 125.28 | 5.45 | 4.66 | 6.35 | |
| Year of diagnosis | |||||||
| 1975–1988 | 22 | 13,465.71 | 163.38 | 0.389$ | 8.37 | 5.38 | 12.46 |
| 1989–2002 | 51 | 43,893.58 | 116.19 | 5.23 | 3.94 | 6.82 | |
| 2003–2016 | 88 | 71,148.79 | 123.68 | 5.13 | 4.14 | 6.29 | |
| Sex | |||||||
| Male | 152 | 98,368.04 | 154.52 | < 0.001 | 12.72 | 10.81 | 14.86 |
| Female | 9 | 30,140.04 | 29.86 | Ref | 2.47 | 1.20 | 4.53 |
| Age at diagnosis | |||||||
| 0–55 | 18 | 30,783.29 | 58.47 | < 0.01$ | 2.68 | 1.64 | 4.15 |
| 56–69 | 63 | 59,465.21 | 105.94 | 5.04 | 3.91 | 6.41 | |
| 70–105 | 80 | 38,259.58 | 209.10 | 7.76 | 6.20 | 9.61 | |
| Marital status | |||||||
| Married/domestic partner | 81 | 80,203.63 | 100.99 | Ref | 4.03 | 3.22 | 4.99 |
| Previously Marriedb | 41 | 25,983.88 | 157.79 | 0.687# | 8.67 | 6.30 | 11.64 |
| Singlec | 28 | 16,431.25 | 170.41 | 8.40 | 5.69 | 11.98 | |
| Unknown | 11 | 5,889.33 | 186.78 | 7.96 | 4.19 | 13.84 | |
| Race | |||||||
| Black | 3 | 13,759.79 | 21.80 | Ref | 1.43 | 0.36 | 3.89 |
| White | 146 | 107,948.29 | 135.25 | < 0.001# | 8.10 | 6.86 | 9.49 |
| American Indian/Alaska Native, Asian/Pacific Islander | 12 | 6,800.00 | 176.47 | 11.24 | 6.09 | 19.11 | |
| Primary site | |||||||
| Lower third of esophagus | 105 | 76,536.13 | 137.19 | Ref | 5.42 | 4.45 | 6.53 |
| Middle third of esophagus | 19 | 21,667.38 | 87.69 | < 0.01# | 4.91 | 3.05 | 7.53 |
| Upper third of esophagus | 8 | 6,392.58 | 125.15 | 6.59 | 3.06 | 12.51 | |
| Overlapping lesion of esophagus | 10 | 4,514.21 | 221.52 | 10.33 | 5.25 | 18.42 | |
| Cervical esophagus | 5 | 3,228.25 | 154.88 | 8.59 | 3.15 | 19.04 | |
| Thoracic esophagus | 4 | 3,828.54 | 104.48 | 5.67 | 1.80 | 13.67 | |
| Abdominal esophagus | 0 | 1,539.79 | 0 | 0 | - | - | |
| Esophagus, NOS | 10 | 10,801.21 | 92.58 | 4.13 | 2.10 | 7.36 | |
| Histology recode—broad groupings | |||||||
| Adenomas and adenocarcinomas | 88 | 68,622.50 | 128.24 | Ref | 4.75 | 3.83 | 5.82 |
| Squamous cell neoplasms | 57 | 47,955.46 | 118.86 | 0.101# | 7.01 | 5.36 | 9.02 |
| Others | 16 | 11,930.13 | 134.11 | 5.59 | 3.31 | 8.89 | |
| Histologic grade | |||||||
| Grade I | 6 | 9,886.42 | 60.69 | 0.660$ | 2.60 | 1.05 | 5.40 |
| Grade II | 44 | 45,318.71 | 97.09 | 4.31 | 3.17 | 5.73 | |
| Grade III | 79 | 44,038.88 | 179.39 | 7.66 | 6.11 | 9.50 | |
| Grade IV | 4 | 2,386.58 | 167.60 | 7.45 | 2.37 | 17.97 | |
| Unknown | 28 | 26,877.50 | 104.18 | 4.55 | 3.08 | 6.49 | |
| SEER disease stage | |||||||
| Localized | 43 | 51,467.67 | 83.55 | 0.151$ | 3.58 | 2.62 | 4.77 |
| Regional | 55 | 43,632.46 | 126.05 | 5.48 | 4.17 | 7.08 | |
| Distant | 40 | 19,579.63 | 204.29 | 8.92 | 6.46 | 12.02 | |
| Unknown/unstaged | 23 | 13,828.33 | 166.33 | 7.73 | 5.02 | 11.42 | |
| Surgery performed | |||||||
| Yes | 47 | 68,214.04 | 68.90 | Ref | 2.90 | 2.16 | 3.82 |
| No/unknown | 114 | 60,294.04 | 189.07 | 0.642 | 8.56 | 7.09 | 10.25 |
| Radiotherapy performed | |||||||
| Yes | 88 | 75,473.25 | 116.60 | Ref | 5.23 | 4.22 | 6.41 |
| No/unknown | 73 | 53,034.83 | 137.65 | 0.637 | 5.75 | 4.54 | 7.19 |
| Chemotherapy performed | |||||||
| Yes | 79 | 75,153.46 | 105.12 | Ref | 4.65 | 3.71 | 5.76 |
| No/unknown | 82 | 53,354.63 | 153.69 | 0.248 | 6.54 | 5.24 | 8.08 |
| Survival months | |||||||
| < 2 months | 35 | 670.50 | 5,219.99 | < 0.01$ | 216.79 | 153.36 | 298.17 |
| 2 months-11 months | 74 | 14,686.83 | 503.85 | 21.57 | 17.05 | 26.92 | |
| 12 months-59 months | 41 | 44,284.42 | 92.58 | 3.89 | 2.83 | 5.23 | |
| > = 60 months | 11 | 68,866.33 | 15.97 | 0.71 | 0.38 | 1.24 | |
SMR, standardized mortality ratio; SEER, the surveillance, epidemiology, and end results program; 95% CI, 95% confidence interval; NOS, Not Otherwise Specified.
aSMR was adjusted by age, race, and sex to the US population over the same time. Five-year age categories were used for standardization using SEER*Stat 8.3.6 and Microsoft Excel 16.0.12730.20188 (Microsoft, Redmond, Washington).
bIncluded divorced, widowed and separated.
cIncluded never married.
#The Bonferroni-corrected P value was used for multiple comparisons.
$The chi-square test for linear trend was used for ordinal multi-categorical variables.
The P values in the bold are statistically significant.
SMRs
SMRs were used for a comparison on suicide fatality rate between studied population and general population. An SMR as 5.45 (95% CI: 4.66–6.35) was reported between esophageal cancer patients and U.S. general population, with 12.72 (95% CI: 10.81–14.86) for males, 2.47 (95% CI: 1.20–4.53) for females, 8.10 (95% CI: 6.86–9.49) in white race, 1.43 (95% CI: 0.36–3.89) in black race, and 11.24 (95% CI: 6.09–19.11) in other races (American Indian/Alaska Native, Asian/Pacific Islander). Suicide rates generally declined from 1975 to 2016 (1975–1988, SMR: 8.37, CI: 5.38–12.46; 1989–2002, SMR: 5.23, CI: 3.94–6.82; 2003–2016, SMR: 5.13, CI: 4.14–6.29), regardless of the lack of any statistical pattern (P = 0.389). Remarkably elevated suicide rates in esophageal cancer patients were observed during the first five years after cancer diagnosis (< 2 months, SMR: 216.79, 95% CI: 153.36–298.17; 2 months-11 months, SMR: 21.57, 95% CI: 17.05–26.92; 12 months-59 months, SMR: 3.89, 95% CI: 2.83–5.23, P < 0.01). Details are presented in Table 2.
Risk factors
After multiple testing, no statistically significant interactions were observed among these risk factors. Details are presented as Supplementary Table S1 online. Univariable Cox regression findings confirmed a significant correlation with high suicide risk based on gender (male vs. female, HR: 5.04, 95% CI: 2.57–9.86, P < 0.001), age of diagnosis (70–105 vs. 0–55, HR: 2.81, 95% CI: 1.68–4.70, P < 0.001), race (white race vs. black, HR: 7.03, 95% CI: 2.24–22.06, P < 0.001; American Indian/Alaska Native, Asian/Pacific Islander vs. black race, HR: 8.91, 95% CI: 2.51–31.56, P < 0.001), histologic grade (grade III vs. grade I, HR: 2.30, 95% CI: 1.00–5.27, P = 0.050), surgery performed (no/unknown vs. yes, HR: 1.82, 95% CI: 1.28–2.58, P < 0.001), chemotherapy performed (no/unknown vs. yes, HR: 1.57, 95% CI: 1.15–2.14, P < 0.01) (Table 3). Multivariable Cox regression outcomes showed gender (male vs. female, HR: 6.37, 95% CI: 3.21–12.67, P < 0.001), age of diagnosis (70–105 vs. 0–55, HR: 2.69, 95% CI: 1.58–4.57, P < 0.001), marital status (previously married vs. married/mate, HR: 1.75, 95% CI: 1.19–2.57, P < 0.01; bachelor (single) vs. married/mate, HR: 2.07, 95% CI: 1.33–3.21, P < 0.01), race (white race vs. black race, HR: 6.64, 95% CI: 2.10–21.06, P < 0.01; American Indian/Alaska Native, Asian/Pacific Islander vs. black race, HR: 8.60, 95% CI: 2.41–30.66, P < 0.001), histologic grade (grade III vs. grade I, HR: 2.36, 95% CI: 1.03–5.45, P = 0.044), surgery performed (no/unknown vs. yes, HR: 2.01, 95% CI: 1.38–2.93, P < 0.001), chemotherapy performed (no/unknown vs. yes, HR: 1.72, 95% CI: 1.18–2.49, P < 0.01) might predict suicide. Table 3 described all the details linked to suicide indexes of the whole cohort.
Table 3.
Univariable and multivariable analysis for the suicide of esophageal cancer patients.
| Variables | Univariable analysis | Multivariable analysis | ||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P | HR | 95% CI | P | |||
| Lower | Upper | Lower | Upper | |||||
| Year of diagnosis | ||||||||
| 1975–1988 | 1.32 | 0.83 | 2.11 | 0.247 | ||||
| 1989–2002 | 1.14 | 0.81 | 1.62 | 0.453 | ||||
| 2003–2016 | Ref | 0.464 | ||||||
| Sex | ||||||||
| Male | 5.04 | 2.57 | 9.86 | < 0.001 | 6.37 | 3.21 | 12.67 | < 0.001 |
| Female | Ref | Ref | ||||||
| Age at diagnosis | ||||||||
| 0–55 | Ref | < 0.001 | Ref | < 0.001 | ||||
| 56–69 | 1.66 | 0.98 | 2.80 | 0.059 | 1.70 | 1.00 | 2.88 | 0.051 |
| 70–105 | 2.81 | 1.68 | 4.70 | < 0.001 | 2.69 | 1.58 | 4.57 | < 0.001 |
| Marital status | ||||||||
| Married/Domestic Partner | Ref | 0.109 | Ref | < 0.01 | ||||
| Previously Marrieda | 1.34 | 0.92 | 1.95 | 0.132 | 1.75 | 1.19 | 2.57 | < 0.01 |
| Singleb | 1.49 | 0.97 | 2.29 | 0.070 | 2.07 | 1.33 | 3.21 | < 0.01 |
| Unknown | 1.76 | 0.94 | 3.31 | 0.078 | 1.83 | 0.96 | 3.46 | 0.065 |
| Race | ||||||||
| Black | Ref | < 0.01 | Ref | < 0.01 | ||||
| White | 7.03 | 2.24 | 22.06 | < 0.001 | 6.64 | 2.10 | 21.06 | < 0.01 |
| American Indian/Alaska Native, Asian/Pacific Islander | 8.91 | 2.51 | 31.56 | < 0.001 | 8.60 | 2.41 | 30.66 | < 0.001 |
| Primary site | ||||||||
| Lower third of esophagus | Ref | 0.318 | 0.430 | |||||
| Middle third of esophagus | 0.58 | 0.36 | 0.95 | 0.029 | 0.68 | 0.41 | 1.12 | 0.131 |
| Upper third of esophagus | 0.83 | 0.40 | 1.70 | 0.612 | 0.83 | 0.40 | 1.73 | 0.621 |
| Overlapping lesion of esophagus | 1.38 | 0.72 | 2.64 | 0.330 | 1.42 | 0.74 | 2.72 | 0.295 |
| Cervical esophagus | 1.15 | 0.47 | 2.81 | 0.767 | 1.28 | 0.52 | 3.18 | 0.597 |
| Thoracic esophagus | 0.70 | 0.26 | 1.89 | 0.480 | 0.80 | 0.29 | 2.17 | 0.656 |
| Abdominal esophagus | 0 | 0 | 1.69E + 94 | 0.930 | 0 | 0 | 8.99E + 96 | 0.932 |
| Esophagus, NOS | 0.66 | 0.35 | 1.26 | 0.210 | 0.58 | 0.30 | 1.12 | 0.103 |
| Histology recode—broad groupings | ||||||||
| Adenomas and adenocarcinomas | Ref | 0.681 | ||||||
| Squamous cell neoplasms | 0.87 | 0.62 | 1.21 | 0.395 | ||||
| Others | 1.00 | 0.59 | 1.70 | 0.998 | ||||
| Histologic grade | ||||||||
| Grade I | Ref | 0.030 | 0.025 | |||||
| Grade II | 1.38 | 0.59 | 3.25 | 0.455 | 1.46 | 0.62 | 3.43 | 0.390 |
| Grade III | 2.30 | 1.00 | 5.27 | 0.050 | 2.36 | 1.03 | 5.45 | 0.044 |
| Grade IV | 2.22 | 0.63 | 7.87 | 0.217 | 2.31 | 0.65 | 8.22 | 0.195 |
| Unknown | 1.51 | 0.63 | 3.66 | 0.357 | 1.44 | 0.59 | 3.49 | 0.421 |
| SEER disease stage | ||||||||
| Localized | Ref | 0.440 | ||||||
| Regional | 1.21 | 0.81 | 1.81 | 0.357 | ||||
| Distant | 1.31 | 0.84 | 2.05 | 0.233 | ||||
| Unknown/unstaged | 1.49 | 0.89 | 2.48 | 0.126 | ||||
| Surgery performed | ||||||||
| Yes | Ref | Ref | ||||||
| No/unknown | 1.82 | 1.28 | 2.58 | < 0.001 | 2.01 | 1.38 | 2.93 | < 0.001 |
| Radiotherapy performed | ||||||||
| Yes | Ref | Ref | ||||||
| No/unknown | 1.30 | 0.95 | 1.78 | 0.096 | 1.06 | 0.73 | 1.54 | 0.761 |
| Chemotherapy performed | ||||||||
| Yes | Ref | Ref | ||||||
| No/unknown | 1.57 | 1.15 | 2.14 | < 0.01 | 1.72 | 1.18 | 2.49 | < 0.01 |
SMR, standardized mortality ratio; SEER, the surveillance, epidemiology, and end results; HR, Hazard Ratio; 95% CI, 95% confidence interval; NOS, Not Otherwise Specified.
aIncluded divorced, widowed and separated.
bIncluded never married.
#The Bonferroni-corrected P value was used for multiple comparisons.
$The chi-square test for linear trend was used for ordinal multi-categorical variables.
The P and HR values in the bold were statistically significant or considered to be analyzed in multivariate regression model.
Discussion
By reference to associated surveys, suicide risk in cancer patients across various countries has gone up24,25,36,37. To be specific, the Italian data analysis performed by Ravaioli. A. et al. verified the growing suicide risk among cancer patients (pooled SMR: 1.7; 95% CI: 1.5–1.9)24. In addition to the finding, scholars in Norway (HR: 2.5; 95% CI: 1.7–3.8)38, Lithuania (SMR:1.62; 95% CI: 1.27–2.06)39, the U.K. (SMR: 1.20, 95% CI: 1.16–1.25)36, as well as the U.S. (SMR: 2.06; 95% CI: 2.00–2.12) have also given alike reports over the past few decades25. A novel contribution of this research is that analysis on suicide-associated risk factors among esophageal cancer patients on the basis of SEER database, which has the largest sample size at present, provides an important basis for clinical prevention and intervention of esophageal cancer suicide. As indicated by the population-based research, suicide rate among esophageal cancer patients reached up to 125.28 per 100,000 person-years, while gross SMR amounted to 5.45 (95% CI: 4.66–6.35). Male sex (SMR: 12.72), diagnosed at an older age (SMR: 7.76), unmarried state, non-black race, histologic grade III (SMR: 7.66), no surgery performed (SMR: 8.56) and no chemotherapy performed (SMR: 6.54) might significantly increase suicide rate in esophageal cancer patients. Details are presented in Table 2.
The SMR results of the above risk factors suggested suicide rates among patients suffering esophageal cancer were obviously greater compared with those of the general U.S. population, especially in men, older age, patients without chemotherapy or surgery performed. According to our results in multivariate analysis, the suicide rates among patients suffering esophageal cancer were subject to multiple demographic features, histopathologic characteristics, as well as treatment therapies. Whereas, corresponding risk factors related to suicide among patients suffering esophageal cancer varied from those of the non-cancer population in the United States40–42. Therefore, it was necessary to be complemented with relevant references and present the links between suicide and cancer (e.g., common risk factors such as alcohol12–14, smoking11, risk behaviours43–45, genetics15,16, and increased stress due to cancer diagnosis26,37,46,47). Additional points to the relationship between cancer and suicide, we have elaborated on reasons for not proceeding to treatment (chemotherapy, surgery).
Gender
In Tables 2, 3, the male suicide rate (154.52 per 100,000 person-years) was almost five times larger relative to the female suicide rate (P < 0.001). Besides that, males had a higher risk of committing suicide in contrast to females, with an HR of 6.34 in our results, which was corresponding to some previous findings, such as those for the general population48, as well as patients suffering other cancer diseases, like lung cancer (SMR in males: 4.61, 95% CI: 4.34–4.90; SMR in females: 3.02, 95% CI: 2.53–3.58)49, gastric carcinomas (SMR in males: 4.85, 95% CI: 3.89–5.98; SMR in females: 3.74, 95% CI: 1.94–6.48)25,26. Although cancer patients of both genders possibly had experienced the same stress50,51, smoking11, alcohol consumption12–14, dramatic decline of family income urged men to generate growing suicidal intention and action17.
Age at diagnosis
The current research reported a significant growing trend of suicide rate in the elderly (70–105 vs. 0–55, HR: 2.69, 95% CI: 1.58–4.57, P < 0.001), as shown by Tables 2, 3. Recently, a few studies have further defined older age as a suicide-related risk factor in patients suffering from cancer diseases as well as ordinary people37,52. However, exceptions did exist. According to the research carried out by Gaitanidis A et al. and Kroenke CH et al., patients at a younger age had a greater potential of committing suicide in comparison with elder breast cancer patients53,54. A possible reason was young females showed a stronger propensity for desperation in physiology and psychology compared with middle-aged and elder counterparts following breast cancer diagnosis. This intensified their suicidal action and intention53.
Marital status
The current study found that unmarried state (previously married vs. married/domestic partner, HR: 1.75, 95% CI: 1.19–2.57, P < 0.01, single vs. married/domestic partner, HR: 2.07, 95% CI: 1.33–3.21, P < 0.01) was not protective against suicide in individuals with esophageal cancer. Additionally, a total of 36,221 patients with pancreatic adenocarcinoma was analyzed by Kiran K. Turaga al et., and its results also showed that the SMR of single and married was respectively 16.3 (95% CI: 14.3–18.6) and 6.4 (95% CI: 5.2–7.8), comparing to U.S. population aged 65 to 74 years old55. Besides, this trend was also consistent with patients suffering kidney cancers56, head and neck cancers as well as genitourinary malignancies57,58, which might be attributed to the superior physical quality, higher socioeconomic rank, and greater emotional support and social attention of the married43–45.
Race
In addition, the research continued to inspect all risk factors related to suicide of patients from the perspective of race. Research results found that the white race proved to be one risk factor, which contributed to suicide, and the suicide rate of white (vs. black race, HR: 6.64, 95% CI: 2.10–21.06, P < 0.01) was 135.25 per 100,000 person-years. The finding demonstrated that the white race was possibly a major predictor related to suicide among cancer patients. Further, white race is considered as another risk factor related to suicide in a good number of studies54,59. As to the low suicide rate in black race, the most plausible reason can be probably attributable to the influence of genetics15,16, religious beliefs, family support as well as suicide-rejection culture60–62.
Histologic grade
Regarding distinct clinical variables of esophageal cancer in Table 3, the patients with higher histologic grade (Grade III vs. Grade I, HR: 2.36, 95% CI: 1.03–5.45, P = 0.044) were considered to be at a higher suicide risk in contrast to those of lower histologic grade. It was universally recognized that low histologic grade represented cancer cells with well differentiation, denoting favorable prognosis and improved living standards63.
Treatment performed
As depicted by Table 3, a factor linked to suicide was no cancer-directed surgery conducted (HR: 2.01, 95% CI: 1.38–2.93, P < 0.001), which implied that the possibility of suicide in esophageal cancer patients with surgical indications might also be a factor that should not be ignored. Likewise, Anderson. C. et al. claimed patients suffering from cancer diseases linked to the digestive system who received surgery had a lower propensity of committing suicide, compared with those not undergoing surgical treatment (SMR: 5.20, 95% CI: 4.64–5.81)29. Besides, the results from Samawi, H. H. et al. and also proved that no surgery was an independent risk factor64. We further found that no chemotherapy performed (HR: 1.72, 95% CI: 1.18–2.49, P < 0.01) predicted higher suicide risks compared with those with chemotherapies. Fortunately, the combination chemotherapy regimen was still one of the main treatments for esophageal cancer, particularly among patients with advanced or metastatic tumors.
Findings obtained in the current research basically conformed to a former survey which discovered that maximal standardized mortality ratios (SMRs) of suicide of cancer patients could be seen from those who had more serious tumour grades as well as those who had not received therapy49. Patients having advanced cancers were likely to experience more sufferings and showed a stronger propensity to anxiety or depression in comparison with those having early tumours65. A reasonable explanation for the correlation of therapy with lower suicide risk was that post-cancer diagnosis treatment provided more comfort and further reinforced their confidence in rehabilitation. This, to some extent, relieved the suffering caused by cancers66. For radiotherapy, we speculate that it may be because the dysphagia of patients after radiotherapy would not improve immediately. In most cases, patients would have weakness, neck and shoulder pain, and other symptoms after radiotherapy, which may increase the pressure and discomfort of patients. However, the tumor size after radiotherapy may be further reduced, increasing the resectability rate of surgery, and the prognosis may be improved, thus alleviating the pessimistic mood of patients67.
Therefore, the effect of radiotherapy on suicide in patients with esophageal cancer may not be significant, but the role of chemotherapy and surgery on suicide prevention can not be underestimated.
Survival months
Survival months proved to be a main suicide risk factor in esophageal cancer patients, in particular two months following diagnosis (SMR: 216.79, 95% CI: 153.36–298.17; Table 2). In good agreement with former studies for other cancers, suicide risk among esophageal cancer patients often seemed better in the early stage following diagnosis compared with that in other stages, underscoring the necessity for social support and monitoring of esophageal cancer patients during such particular periods26,37,46,47. The government, clinicians, as well as family members are supposed to make regular evaluations on esophageal cancer patients about their suicide attempts or potential suicide risk actions, and meanwhile, use proper strategies to lower their suicide risk, particularly among patients diagnosed within two months26,68.
Additionally, examining variables not included by the SEER dataset, in particular those about perceived discrimination, as well as sentiment of estrangement from the mainstream culture seems necessary.
Limitations
There are many inevitable constraints in the current study, such as rich retrospective data in SEER. Underlying confounders, including comorbidities, cancer recurrences, socioeconomic status, health insurance, underlying psychiatric diseases, suicide attempts, as well as details about therapeutic interventions cannot be used for further analysis because of the non-availability of corresponding data sources in the SEER program. However, by far, it remains to be the most all-round investigation about the subject. Moreover, incomplete information on psychological status is a common issue among patients with physical illness (i.e., cancer). Further work should be conducted to improve the prediction ability by applying more appropriate models incorporating other potential risk factors. Due to the retrospective design of this study, it was difficult to explain some ratings. Besides, anonymization of information inhibited the verification of whether respondent descriptions had precisely figured out the events happened69.
Conclusions
To sum up, males, with older ages (70–105), bachelor, non-black race, histologic grade III, no surgical treatment or chemotherapy performed constituted remarkable indicators of suicide among esophageal cancer.
Supplementary Information
Acknowledgements
The authors appreciate the SEER program for the access to high-quality data.
Author contributions
Concept design: C.C., Y.J., H.L.; Data acquisition and summary: C.C., F.X., L.L.; Data analysis and illustration: C.C., F.Y., H.L.; Manuscript compilation: C.C., Q.C., J.L.; Manuscript approval: C.C., Y.J., H.L., F.X., J.L., Q.C., F.Y., L.L.
Data availability
Data involved in the research can be provided by the corresponding author if required.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-98260-w.
References
- 1.Turecki G, et al. Suicide and suicide risk. Nat. Rev. Dis. Primers. 2019;5:74. doi: 10.1038/s41572-019-0121-0. [DOI] [PubMed] [Google Scholar]
- 2.Fazel S, Runeson B. Suicide. New Engl. J. Med. 2020;382:266–274. doi: 10.1056/NEJMra1902944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naghavi M. Global, regional, and national burden of suicide mortality 1990 to 2016: systematic analysis for the Global Burden of Disease Study 2016. BMJ. 2019;364:l94. doi: 10.1136/bmj.l94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Age-standardized suicide rates (per 100 000 population), both sexes, 2016. Geneva: World Health Organization, 2018. Available from: https://www.who.int/gho/mental_health/suicide_rates/en/. Accessed September 3, 2020.
- 5.Stone DM, et al. Vital signs: trends in state suicide rates - United States, 1999–2016 and circumstances contributing to suicide - 27 states, 2015. MMWR Morb Mortal Wkly Rep. 2018;67:617–624. doi: 10.15585/mmwr.mm6722a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bostwick JM, Pankratz VS. Affective disorders and suicide risk: a reexamination. Am. J. Psychiatry. 2000;157:1925–1932. doi: 10.1176/appi.ajp.157.12.1925. [DOI] [PubMed] [Google Scholar]
- 7.Spijker J, Graaf RD, Have MT, Nolen WA, Speckens A. Predictors of suicidality in depressive spectrum disorders in the general population: results of the Netherlands Mental Health Survey and Incidence Study. Soc. Psychiatry Psychiatr. Epidemiol. 2010;45:513–521. doi: 10.1007/s00127-009-0093-6. [DOI] [PubMed] [Google Scholar]
- 8.Inskip HM, Harris EC, Barraclough B. Lifetime risk of suicide for affective disorder, alcoholism and schizophrenia. Br. J. Psychiatry. 1998;172:35–37. doi: 10.1192/bjp.172.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Kawohl W, Nordt C. COVID-19, unemployment, and suicide. The lancet. Psychiatry. 2020;7:389–390. doi: 10.1016/S2215-0366(20)30141-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pitman A, Osborn D, King M, Erlangsen A. Effects of suicide bereavement on mental health and suicide risk. Lancet Psychiatry. 2014;1:86–94. doi: 10.1016/s2215-0366(14)70224-x. [DOI] [PubMed] [Google Scholar]
- 11.Kessler RC, Borges G, Sampson N, Miller M, Nock MK. The association between smoking and subsequent suicide-related outcomes in the National Comorbidity Survey panel sample. Mol. Psychiatry. 2009;14:1132–1142. doi: 10.1038/mp.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.World Health Organization (WHO). Global status report on alcohol and health. Geneva: World Health Organization; 2014. (Available at: https://www.who.int/substance_abuse/publications/global_alcohol_report/gsr_2018/en/. Accessed May 26, 2020.).
- 13.Shield KD, Rylett M, Rehm J. Public health successes and missed opportunities. Trends in alcohol consumption and attributable mortality in the WHO European Region, 1990–2014. Copenhagen, Denmark: WHO European Region, 2016.
- 14.Zaridze D, Lewington S, Boroda A, et al. Alcohol and mortality in Russia: prospective observational study of 151,000 adults. Lancet. 2014;383(9927):1465–1473. doi: 10.1016/S0140-6736(13)62247-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Flory JD, et al. Gene expression associated with suicide attempts in US veterans. Transl. Psychiatry. 2017;7:e1226. doi: 10.1038/tp.2017.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.García-Gutiérrez MS, et al. Alterations in gene and protein expression of cannabinoid CB(2) and GPR55 receptors in the dorsolateral prefrontal cortex of suicide victims. Neurotherapeutics. 2018;15:796–806. doi: 10.1007/s13311-018-0610-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iemmi V, et al. Suicide and poverty in low-income and middle-income countries: a systematic review. Lancet Psychiatry. 2016;3:774–783. doi: 10.1016/s2215-0366(16)30066-9. [DOI] [PubMed] [Google Scholar]
- 18.Breitbart W, et al. Depression, hopelessness, and desire for hastened death in terminally Ill patients with cancer. JAMA. 2000;284:2907–2911. doi: 10.1001/jama.284.22.2907. [DOI] [PubMed] [Google Scholar]
- 19.Tombal B. Prostate cancer, depression, and risk of suicide: should we pay more attention? Eur. Urol. 2010;57:396–397. doi: 10.1016/j.eururo.2009.11.039. [DOI] [PubMed] [Google Scholar]
- 20.Mayor S. Patients with cancer at 20% increased risk of suicide, show figures for England. BMJ. 2018;361:k2703. doi: 10.1136/bmj.k2703. [DOI] [PubMed] [Google Scholar]
- 21.Walker J, et al. Better off dead: suicidal thoughts in cancer patients. J. Clin. Oncol. 2008;26:4725–4730. doi: 10.1200/jco.2007.11.8844. [DOI] [PubMed] [Google Scholar]
- 22.Robson A, Scrutton F, Wilkinson L, MacLeod F. The risk of suicide in cancer patients: a review of the literature. Psychooncology. 2010;19:1250–1258. doi: 10.1002/pon.1717. [DOI] [PubMed] [Google Scholar]
- 23.Anguiano L, Mayer DK, Piven ML, Rosenstein D. A literature review of suicide in cancer patients. Cancer Nurs. 2012;35:E14–26. doi: 10.1097/NCC.0b013e31822fc76c. [DOI] [PubMed] [Google Scholar]
- 24.Ravaioli A, et al. Suicide death among cancer patients: new data from northern Italy, systematic review of the last 22 years and meta-analysis. Eur. J. Cancer. 2020;125:104–113. doi: 10.1016/j.ejca.2019.08.019. [DOI] [PubMed] [Google Scholar]
- 25.Misono S, Weiss NS, Fann JR, Redman M, Yueh B. Incidence of suicide in persons with cancer. J. Clin. Oncol. 2008;26:4731–4738. doi: 10.1200/jco.2007.13.8941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zaorsky NG, et al. Suicide among cancer patients. Nat. Commun. 2019;10:207. doi: 10.1038/s41467-018-08170-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zalsman G, et al. Suicide prevention strategies revisited: 10-year systematic review. Lancet Psychiatry. 2016;3:646–659. doi: 10.1016/s2215-0366(16)30030-x. [DOI] [PubMed] [Google Scholar]
- 28.Bray F, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 29.Anderson C, Park EM, Rosenstein DL, Nichols HB. Suicide rates among patients with cancers of the digestive system. Psychooncology. 2018;27:2274–2280. doi: 10.1002/pon.4827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With County, Total U.S. (1969–2016) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2017 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released December 2018. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
- 31.Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Populations - Total U.S. (1969–2016) <Katrina/Rita Adjustment> - Linked To County Attributes - Total U.S., 1969–2016 Counties, National Cancer Institute, DCCPS, Surveillance Research Program, released November 2017.
- 32.Organization, W. WHO International Classification of Diseases for Oncology, 3rd edn (ICD-O-3). (2000).
- 33.Surveillance Research Program, National Cancer Institute SEER*Stat software version 8.3.6. 2016. Available from: www.seer.cancer.gov/seerstat. Accessed September 3, 2020.
- 34.Breslow, N. E. & Day, N. E. Statistical methods in cancer research. Volume 2-The design and analysis of cohort studies. IARC Sci Publ (1987). [PubMed]
- 35.Ury HK, Wiggins AD. Another shortcut method for calculating the confidence interval of a poisson variable (or of a standardized mortality ratio) Am. J. Epidemiol. 1985;122:197–198. doi: 10.1093/oxfordjournals.aje.a114083. [DOI] [PubMed] [Google Scholar]
- 36.Henson KE, et al. Risk of Suicide After Cancer Diagnosis in England. JAMA Psychiat. 2019;76:51–60. doi: 10.1001/jamapsychiatry.2018.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Fang F, et al. Suicide and cardiovascular death after a cancer diagnosis. New Engl. J. Med. 2012;366:1310–1318. doi: 10.1056/NEJMoa1110307. [DOI] [PubMed] [Google Scholar]
- 38.Gunnes MW, et al. Suicide and violent deaths in survivors of cancer in childhood, adolescence and young adulthood—A national cohort study. Int. J. Cancer. 2017;140:575–580. doi: 10.1002/ijc.30474. [DOI] [PubMed] [Google Scholar]
- 39.Dulskas A, Patasius A, Kaceniene A, Urbonas V, Smailyte G. Suicide risk among colorectal cancer patients in Lithuania. Int. J. Colorectal Dis. 2019;34:555–558. doi: 10.1007/s00384-018-03228-4. [DOI] [PubMed] [Google Scholar]
- 40.Romanowicz M, O'Connor S, Schak K, Swintak C, Lineberry T. Use of the Suicide Status Form-II to investigate correlates of suicide risk factors in psychiatrically hospitalized children and adolescents. J. Affect. Disord. 2013;151:467–473. doi: 10.1016/j.jad.2013.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Luoma J, Pearson J. Suicide and marital status in the United States, 1991–1996: is widowhood a risk factor? Am. J. Public Health. 2002;92:1518–1522. doi: 10.2105/ajph.92.9.1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ji YD, Robertson FC, Patel NA, Peacock ZS, Resnick CM. Assessment of risk factors for suicide among US health care professionals. JAMA Surg. 2020;155:713–721. doi: 10.1001/jamasurg.2020.1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wyke S, Ford G. Competing explanations for associations between marital status and health. Soc. Sci. Med. 1992;34:523–532. doi: 10.1016/0277-9536(92)90208-8. [DOI] [PubMed] [Google Scholar]
- 44.Goldzweig G, et al. Psychological distress among male patients and male spouses: what do oncologists need to know? Ann. Oncol. 2010;21:877–883. doi: 10.1093/annonc/mdp398. [DOI] [PubMed] [Google Scholar]
- 45.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann. Oncol. 2006;17:5–19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 46.Lu D, et al. Suicide and suicide attempt after a cancer diagnosis among young individuals. Ann. Oncol. 2013;24:3112–3117. doi: 10.1093/annonc/mdt415. [DOI] [PubMed] [Google Scholar]
- 47.Yang J, et al. Incidence and risk factors for suicide death in male patients with genital-system cancer in the United States. Eur. J. Surg. Oncol. 2019;45:1969–1976. doi: 10.1016/j.ejso.2019.03.022. [DOI] [PubMed] [Google Scholar]
- 48.Spicer RS, Miller TR. Suicide acts in 8 states: incidence and case fatality rates by demographics and method. Am. J. Public Health. 2000;90:1885–1891. doi: 10.2105/ajph.90.12.1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Urban D, et al. Suicide in lung cancer: who is at risk? Chest. 2013;144:1245–1252. doi: 10.1378/chest.12-2986. [DOI] [PubMed] [Google Scholar]
- 50.Hawton K. Sex and suicide. Br. J. Psychiatry. 2000;177:484–485. doi: 10.1192/bjp.177.6.484. [DOI] [PubMed] [Google Scholar]
- 51.Kendal WS. Suicide and cancer: a gender-comparative study. Ann. Oncol. 2007;18:381–387. doi: 10.1093/annonc/mdl385. [DOI] [PubMed] [Google Scholar]
- 52.Brower V. Suicide in survivors of childhood and young adult cancers. Lancet Oncol. 2016;17:e522. doi: 10.1016/s1470-2045(16)30554-x. [DOI] [PubMed] [Google Scholar]
- 53.Kroenke CH, et al. Functional impact of breast cancer by age at diagnosis. J. Clin. Oncol. 2004;22:1849–1856. doi: 10.1200/jco.2004.04.173. [DOI] [PubMed] [Google Scholar]
- 54.Gaitanidis A, Alevizakos M, Pitiakoudis M, Wiggins D. Trends in incidence and associated risk factors of suicide mortality among breast cancer patients. Psychooncology. 2018;27:1450–1456. doi: 10.1002/pon.4570. [DOI] [PubMed] [Google Scholar]
- 55.Turaga KK, Malafa MP, Jacobsen PB, Schell MJ, Sarr MG. Suicide in patients with pancreatic cancer. Cancer. 2011;117:642–647. doi: 10.1002/cncr.25428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Guo CY, et al. Risk factors associated with suicide among kidney cancer patients: a surveillance, epidemiology, and end results analysis. Cancer Med. 2019;8:5386–5396. doi: 10.1002/cam4.2400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Osazuwa-Peters N, et al. Suicide risk among cancer survivors: Head and neck versus other cancers. Cancer. 2018;124:4072–4079. doi: 10.1002/cncr.31675. [DOI] [PubMed] [Google Scholar]
- 58.Klaassen Z, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121:1864–1872. doi: 10.1002/cncr.29274. [DOI] [PubMed] [Google Scholar]
- 59.Zhou H, et al. Trends in incidence and associated risk factors of suicide mortality in patients with non-small cell lung cancer. Cancer Med. 2018;7:4146–4155. doi: 10.1002/cam4.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.O'Reilly D, Rosato M. Religion and the risk of suicide: longitudinal study of over 1 million people. Br. J. Psychiatry. 2015;206:466–470. doi: 10.1192/bjp.bp.113.128694. [DOI] [PubMed] [Google Scholar]
- 61.The L. Suicide prevention: creating a safer culture. The Lancet. 2016;388:1955. doi: 10.1016/S0140-6736(16)31796-2. [DOI] [PubMed] [Google Scholar]
- 62.Neeleman J, Wessely S, Lewis G. Suicide acceptability in African- and white Americans: the role of religion. J. Nerv. Ment. Dis. 1998;186:12–16. doi: 10.1097/00005053-199801000-00003. [DOI] [PubMed] [Google Scholar]
- 63.Simpson WG, et al. Analysis of suicide risk in patients with penile cancer and review of the literature. Clin. Genitourin. Cancer. 2018;16:E257–E261. doi: 10.1016/j.clgc.2017.09.011. [DOI] [PubMed] [Google Scholar]
- 64.Samawi HH, et al. Risk and predictors of suicide in colorectal cancer patients: a surveillance, epidemiology, and end results analysis. Curr. Oncol. 2017;24:e513–e517. doi: 10.3747/co.24.3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nordin, K., Berglund, G., Glimelius, B. & Sjödén, P. O. Predicting anxiety and depression among cancer patients: a clinical model. Eur. J. Cancer (Oxford, England : 1990)37, 376–384 (2001). [DOI] [PubMed]
- 66.Zhang X, Sun S, Peng P, Ma F, Tang F. Prediction of risk of suicide death among lung cancer patients after the cancer diagnosis. J. Affect. Disord. 2021;292:448–453. doi: 10.1016/j.jad.2021.05.123. [DOI] [PubMed] [Google Scholar]
- 67.Jeene P, et al. Short-course external beam radiotherapy versus brachytherapy for palliation of dysphagia in esophageal cancer: a matched comparison of two prospective trials. J. Thorac. Oncol. 2020;15:1361–1368. doi: 10.1016/j.jtho.2020.04.032. [DOI] [PubMed] [Google Scholar]
- 68.Bolton JM, Walld R, Chateau D, Finlayson G, Sareen J. Risk of suicide and suicide attempts associated with physical disorders: a population-based, balancing score-matched analysis. Psychol. Med. 2015;45:495–504. doi: 10.1017/s0033291714001639. [DOI] [PubMed] [Google Scholar]
- 69.Park HS, Lloyd S, Decker RH, Wilson LD, Yu JB. Limitations and biases of the surveillance, epidemiology, and end results database. Curr. Probl. Cancer. 2012;36:216–224. doi: 10.1016/j.currproblcancer.2012.03.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data involved in the research can be provided by the corresponding author if required.


