Abstract
We sought to demonstrate the impact of improved peak exercise oxygen consumption (V̇O2) during maximal exercise testing after cardiac rehabilitation (CR) on the incidence of arrhythmias in patients with heart failure (HF). The present study comprised of 220 patients with HF, and peak V̇O2 was examined at 2 and 5 months after CR. Of the 220 patients, 110 (50%) had a low peak V̇O2 of < 14 mL/min/kg at 2 months. The peak V̇O2 improved in 86 of these 110 (78%) patients at 5 months after CR. During a median follow-up of 6 years, the patients with improvement in peak V̇O2, compared to those without peak V̇O2 improvement, had a lower rate of mortality (4% vs. 29%, log-rank, P < 0.001) and HF hospitalization (6 vs. 17%, log-rank, P = 0.044) and a lower incidence of new-onset atrial arrhythmias (9 vs. 27%, log-rank, P = 0.013), with no difference in the incidence of ventricular arrhythmias between groups (1 vs. 4%, log-rank, P = 0.309). The majority of deaths in the patients without an improved peak V̇O2 were because of cardiovascular events (73%), particularly progressive HF (55%). Early detection and management of atrial arrhythmias may improve outcomes in patients without peak V̇O2 improvement after CR.
Subject terms: Interventional cardiology, Cardiology
Introduction
Reduced exercise capacity is a principal symptom of heart failure (HF). Determination of peak exercise oxygen consumption (V̇O2) during maximal exercise testing has been used to assess exercise capacity in patients with HF. Mancini et al. demonstrated that a low peak V̇O2 of < 14 mL/kg/min was associated with poor prognosis in patients with severe HF (higher-risk)1. Improvement in exercise capacity is one of the most important effects of exercise training in cardiac rehabilitation (CR)2–5, leading to a better outcome in patients with ischemic HF6–9. Although atrial and ventricular arrhythmias contribute to the morbidity and mortality of patients with HF3, the impact of improvement in exercise capacity after CR on the long-term incidence of atrial and ventricular arrhythmias remains uncertain. We hypothesized that improvement in peak V̇O2 after CR would reduce the risk of long-term incidence of cardiac arrhythmias and mortality in patients with HF. In the present study, we sought to determine the association between the changes in exercise capacity after CR and the incidence of new-onset atrial and ventricular arrhythmias and the rate of mortality and hospitalization due to HF exacerbation during long-term follow-up.
Methods
Study design and participants
In this retrospective single-center study, 220 consecutive patients with HF who underwent CR at the Tokai University Hospital (Kanagawa, Japan) from April 2012 to March 2016 were included in the study. Patients with acute HF or exacerbation of chronic HF participated in the CR programs during the acute phase once the patients’ condition was stabilized with pharmacological and/or non-pharmacological interventions4. Patients who were not eligible for CR included those with acute coronary syndrome before non-pharmacological interventions, refractory arrhythmias, uncontrolled HF (e.g., New York Heart Association [NYHA] Class IV), and symptomatic severe aortic stenosis before surgical interventions, as well as those unable to provide consent to CR. Patients were classified into two groups: those with a low peak V̇O2 (higher-risk patients) and those without a low peak V̇O2 (lower-risk patients) at 2 months after the start of CR. Furthermore, higher-risk patients were subdivided into two groups: higher-risk patients with an improved peak V̇O2 at 5 months and those without improvement in the peak V̇O2. Improvement in the exercise capacity was defined as the increase in the peak V̇O2 after the CR. The clinical characteristics, the incidence of new-onset atrial arrhythmias including atrial fibrillation (AF) and atrial tachycardia, and ventricular arrhythmias including ventricular tachycardia and ventricular fibrillation, and the rate of mortality and hospitalization due to HF exacerbation were determined among higher-risk patients. The present study was approved by the Institutional Review Board for Clinical Research, Tokai University (20R-058). The board waived the condition of obtaining informed consent for study participation. All methods included in this study were carried out in accordance with relevant guidelines and regulations.
Cardiopulmonary exercise testing
Patients underwent symptom-limited cardiopulmonary exercise testing (CPX) using a cycle ergometer (Lode, Groningen, Netherlands) with respiratory gas exchange analysis, when they were clinically stable, at 2 and 5 months during the CR program. The CPX protocol consisted of the following: 4-min rest period while sitting on the cycle ergometer; 2-min exercise at the free-wheel; and an incremental exercise phase with an increase of 10 W/min (ramp protocol). A 12-lead electrocardiogram was continuously monitored throughout the test (Nihon Kohden Corporation, Tokyo, Japan). Breath-by-breath expiratory gas analysis was measured, and measured minute ventilation, oxygen uptake (V̇O2), and carbon dioxide production data were stored on a computer hard disk every 6 s for off-line analysis (Minato Medical Science, Osaka, Japan). Peak V̇O2 was defined as the highest V̇O2 during exercise testing, which was expressed as a value adjusted to body weight (mL/kg/min). A low peak V̇O2 was defined as < 14 mL/min/kg1. Predicted values of V̇O2 was calculated as follows: peak VO2 pred = (Height—Age)*20 if male, = (Height—Age)*14 if female10.
Statistical analysis
Categorical variables are expressed as absolute and relative frequencies. For categorical data, Fisher’s exact test was applied. Quantitative variables were described by their mean (± standard deviation [SD]) or by the median and interquartile range (IQR), as appropriate for their distribution, and compared using the Mann–Whitney U test. Survival curves were estimated using the Kaplan–Meier method and compared using log-rank tests. We performed univariate Cox regression analysis of clinical outcomes in association with improvement in peak V̇O2 with CR in higher-risk patients. A 2-tailed p value of < 0.05 was considered to indicate statistical significance. All statistical analyses were performed using SPSS Statistics version 23.0 (IBM Corp, Armonk, NY, USA).
Results
Patient characteristics
The median age of the study group was 67 (IQR, 59–74) years, including 51 women (23%); the median plasma brain natriuretic peptide level was 215.2 (IQR, 104.1–494.7) pg/ml. The majority of patients had an NYHA class I, with optimal medical therapy including angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers and beta-blockers, at the 2-month time point of CR.
Of the 220 patients included, 110 had a low peak V̇O2 (50%) at 2 months of CR (higher-risk patients, Table 1). Compared to lower-risk patients, the proportion of female patients was higher (30% vs. 16%, P = 0.025) and the median left ventricular ejection fraction was lower (49% vs. 54%, respectively, P = 0.019). In 117 patients with preserved ejection fraction on echocardiography, the cause of heart failure was not different between the two groups: ischemic in 69% in higher-risk patients vs. 83% in lower-risk patients (P = 0.086). The mean values of peak V̇O2 at 2 months were 11.8 ± 1.8 mL/min/kg in higher-risk patients and 17.4 ± 2.7 mL/min/kg in lower-risk patients, respectively (P < 0.001). The mean values of peak V̇O2 at 5 months were 14.2 ± 3.7 mL/min/kg in higher-risk patients and 19.0 ± 4.5 mL/min/kg in lower-risk patients, respectively (P < 0.001). Peak V̇O2 percentage of predicted value was 43.2 ± 9.8% in higher-risk patients and 57.5 ± 10.7% in the lower-risk patients, respectively (P < 0.001).
Table 1.
Clinical characteristics of the enrolled patients.
| All (n = 220) | Lower-risk patients (n = 110) | Higher-risk patients (n = 110) | P value | |
|---|---|---|---|---|
| Median age (IQR), years | 67 (59–74) | 65 (58–71) | 68 (62–76) | 0.110 |
| Female sex, N (%) | 51 (23) | 18 (16) | 33 (30) | 0.025 |
| Median body mass index (IQR), kg/m2 | 23.5 (20.8–25.2) | 23.4 (20.5–25.2) | 23.5 (21.0–25.4) | 0.625 |
| Median BNP level (IQR), pg/ml | 215.2 (104.1–494.7) | 213.9 (103.6–364.8) | 217.6 (105.6–618.7) | 0.294 |
| Median left ventricular ejection fraction (IQR), % | 50 (41–60) | 54 (43–61) | 49 (40–56) | 0.019 |
| Median estimated GFR(IQR), ml/min/1.73 m2 | 63.0 (52.0–77.8) | 65.0 (56.0–75.5) | 61.0 (46.0–78.3) | 0.141 |
| Peak V̇O2 at 2 months | 14.6 ± 3.6 | 17.4 ± 2.7 | 11.8 ± 1.8 | < 0.001 |
| Peak V̇O2 at 5 months | 16.6 ± 4.7 | 19.0 ± 4.5 | 14.2 ± 3.7 | < 0.001 |
| Peak V̇O2 percentage of predicted value | 50.3 ± 12.5 | 57.5 ± 10.7 | 43.2 ± 9.8 | < 0.001 |
| NYHA class -N. (%) | 0.066 | |||
| I | 147 (67) | 80 (73) | 67 (61) | |
| II | 57 (26) | 24 (22) | 33 (30) | |
| III | 16 (7) | 6 (6) | 10 (9) | |
| Coexisting conditions, N (%) | ||||
| Hypertension | 152 (69) | 81 (74) | 71 (65) | 0.189 |
| Diabetes | 77 (35) | 39(36) | 38 (35) | 1.000 |
| Atrial fibrillation | 24 (11) | 7(6) | 17(16) | 0.050 |
| Prior thromboembolic events | 5 (2) | 2 (2) | 3 (3) | 1.000 |
| CHADS2 score (IQR) | 2 (2–3) | 2 (2–3) | 2 (2–3) | 0.756 |
| CHA2DS2–VASc (IQR) | 4 (3–4) | 4 (3–4) | 4 (3–5) | 0.942 |
| Heart failure with preserved ejection fraction, N (%) | 117 (53) | 63 (57) | 54 (49) | 0.280 |
| Cause of heart failure, N (%) | 0.878 | |||
| Ischemic | 159 (72) | 79 (72) | 80 (73) | |
| Non-ischemic | ||||
| Idiopathic | 31 (14) | 17 (16) | 14 (13) | |
| Valvular | 30 (14) | 14 (6) | 16 (7) | |
| Medications, N (%) | ||||
| ACE inhibitor or ARB | 200 (91) | 100 (91) | 100 (91) | 1.000 |
| Beta-blocker | 177 (81) | 90 (82) | 87 (79) | 0.734 |
| Amiodarone | 11 (5) | 5 (5) | 6 (6) | 1.000 |
| Preexisting pacemaker or CRT, N (%) | 8 (4) | 5 (5) | 3 (3) | 0.486 |
IQR; interquartile range, GFR; glomerular filtration rate, NYHA; New York Heart Association, ACE; angiotensin-converting enzyme, ARB; angiotensin II receptor blocker, CRT; cardiac resynchronization therapy, BNP; brain natriuretic peptide, GFR; glomerular filtration rate.
Among the 110 higher-risk patients, the peak V̇O2 improved in 86 (78%) at 5 months of CR. There were no differences in clinical characteristics between higher-risk patients with improvement in peak V̇O2 and those without (Table 2), except for the peak V̇O2 at 5 months (10.8 ± 1.8 vs. 15.1 ± 3.5 mL/min/kg, P < 0.001).
Table 2.
Clinical characteristics of the higher-risk patients.
| Patients with improvement in peak V̇O2 (n = 86) | Patients without improvement in peak V̇O2 (n = 24) | P value | |
|---|---|---|---|
| Median age (IQR), years | 67 (60–76) | 70 (65–78) | 0.192 |
| Female sex, N (%) | 28 (33) | 5 (21) | 0.322 |
| Median body-mass index (IQR), kg/m2 | 23.1 (20.9–25.1) | 24.4 (21.5–27.2) | 0.178 |
| Median BNP level (IQR), pg/ml | 208.7 (104.0–513.1) | 437.5 (124.9–751.2) | 0.147 |
| Median left ventricular ejection fraction (IQR), % | 50 (41–57) | 44 (34–53) | 0.172 |
| Median estimated GFR (IQR), ml/min/1.73 m2 | 62.5 (48.8–79.0) | 51.5 (37.5–71.8) | 0.074 |
| Peak V̇O2 at 2 months | 11.7 ± 1.9 | 12.0 ± 1.4 | 0.682 |
| Peak V̇O2 at 5 months | 15.1 ± 3.5 | 10.8 ± 1.8 | < 0.001 |
| NYHA class -N. (%) | 0.457 | ||
| I | 55 (64) | 12 (50) | |
| II | 24 (28) | 9 (38) | |
| III | 7 (8) | 3 (13) | |
| Coexisting conditions, N (%) | |||
| Hypertension | 58 (67) | 13 (54) | 0.239 |
| Diabetes | 30 (35) | 8 (33) | 1.000 |
| Atrial fibrillation | 15 (17) | 2 (8) | 0.354 |
| Prior thromboembolic events | 2 (2) | 1 (4) | 0.526 |
| CHADS2 score (IQR) | 2 (2–3) | 2 (2–3) | 0.391 |
| CHA2DS2-VASc (IQR) | 4 (3–5) | 4 (3–5) | 0.673 |
| Heart failure with preserved ejection fraction, N(%) | 45 (52) | 8 (33) | 0.112 |
| Cause of heart failure, N (%) | 1.000 | ||
| Ischemic | 63 (73) | 17 (71) | |
| Non-ischemic | 8 (9) | 6 (25) | |
| Idiopathic | 15 (17) | 1 (4) | |
| Valvular | |||
| Medications, N (%) | |||
| ACE inhibitor or ARB | 77 (90) | 23 (96) | 0.688 |
| Beta-blocker | 71 (83) | 16 (67) | 0.153 |
| Amiodarone | 5 (6) | 1 (4) | 1.000 |
| Preexisting pacemaker or CRT, N (%) | 3(4) | 0 | 1.000 |
IQR; interquartile range, GFR; glomerular filtration rate, NYHA; New York Heart Association, ACE; angiotensin-converting enzyme, ARB; angiotensin II receptor blocker, CRT; cardiac resynchronization therapy, BNP; brain natriuretic peptide.
Long-term outcomes
During a median follow-up of 6 years, higher-risk patients with improvement in peak V̇O2, compared to those without peak V̇O2 improvement, had a lower rate of mortality (4% vs. 29%; log-rank, P < 0.001, Fig. 1a) and hospitalization due to HF exacerbation (6% vs. 17%; log-rank, P = 0.044, Fig. 1b). Unadjusted hazard ratios of mortality and hospitalization for higher-risk patients with an improved peak V̇O2 with CR were 0.132 (95% confidence interval [CI] 0.034–0.511; P = 0.003) and 0.156 (95% CI 0.018–0.1373; P = 0.094), respectively. Among 196 patients without prior AF, 23 patients (12%) had atrial arrhythmias: AF in 18 patients and atrial tachycardia in five. Higher-risk patients with improvement in peak V̇O2 with CR had a lower incidence of new-onset atrial arrhythmias than did those without a peak V̇O2 improvement (9% vs. 27%; log-rank, P = 0.013, Fig. 2a). Unadjusted hazard ratio of new-onset atrial arrhythmias for patients with an improved peak V̇O with CR was 0.263 (95% CI 0.085–0.830; P = 0.021). New-onset ventricular arrhythmias occurred in four patients: ventricular tachycardia in three patients and ventricular fibrillation in one. There was no difference in the incidence of ventricular arrhythmias between higher-risk patients with and without improvement in peak V̇O2 with CR (1% vs. 4%; log-rank, P = 0.309, Fig. 2b). Unadjusted hazard ratio of new-onset ventricular arrhythmias for higher-risk patients with an improved peak V̇O2 with CR was 0.262 (95% CI 0.016–4.195; P = 0.344). Patients with peak V̇O2 percentage of predicted values of < 45% had a higher risk of mortality (log-rank, P = 0.010) and higher incidence of new-onset atrial arrhythmias than did those with peak V̇O2 percentage of predicted values of ≥ 45% (log-rank, P = 0.031), though no differences were found in hospitalization due to HF exacerbation (log-rank, P = 0.081) and new-onset ventricular arrhythmias (log-rank, P = 0.229).
Figure 1.
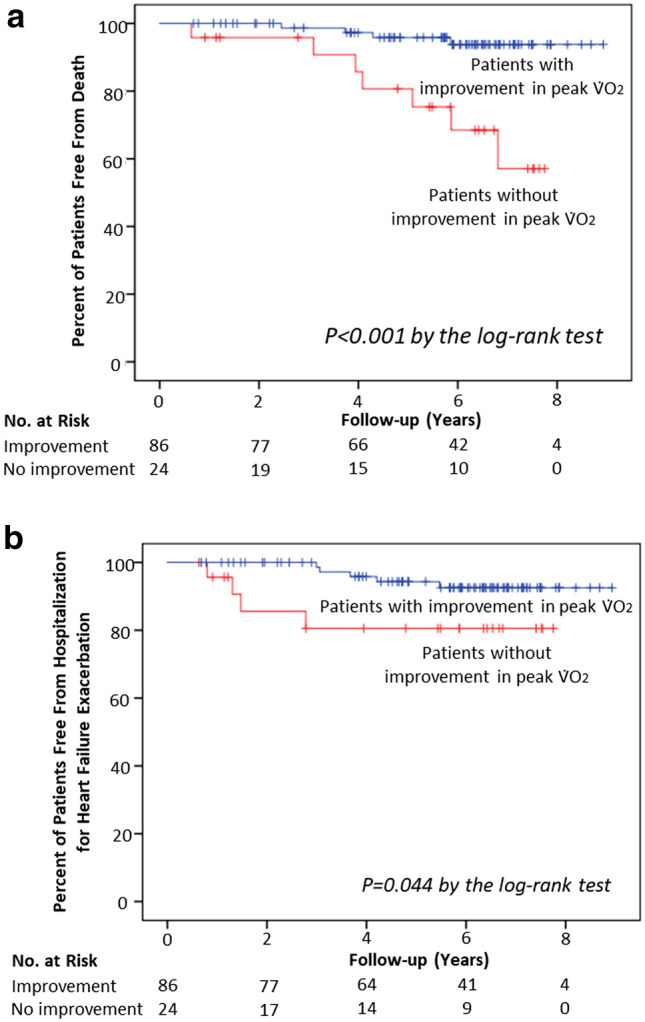
Mortality (a) and hospitalization due to heart failure exacerbation (b) over time. The Kaplan–Meier estimates of mortality and hospitalization for HF exacerbation over a median follow-up of 6 years are shown for high-risk patients with improvement in peak V̇O2, high-risk patients without improvement in peak V̇O2, and patients with a preserved V̇O2. Tick marks indicate censored data. HF, heart failure; V̇O2, peak exercise oxygen consumption.
Figure 2.
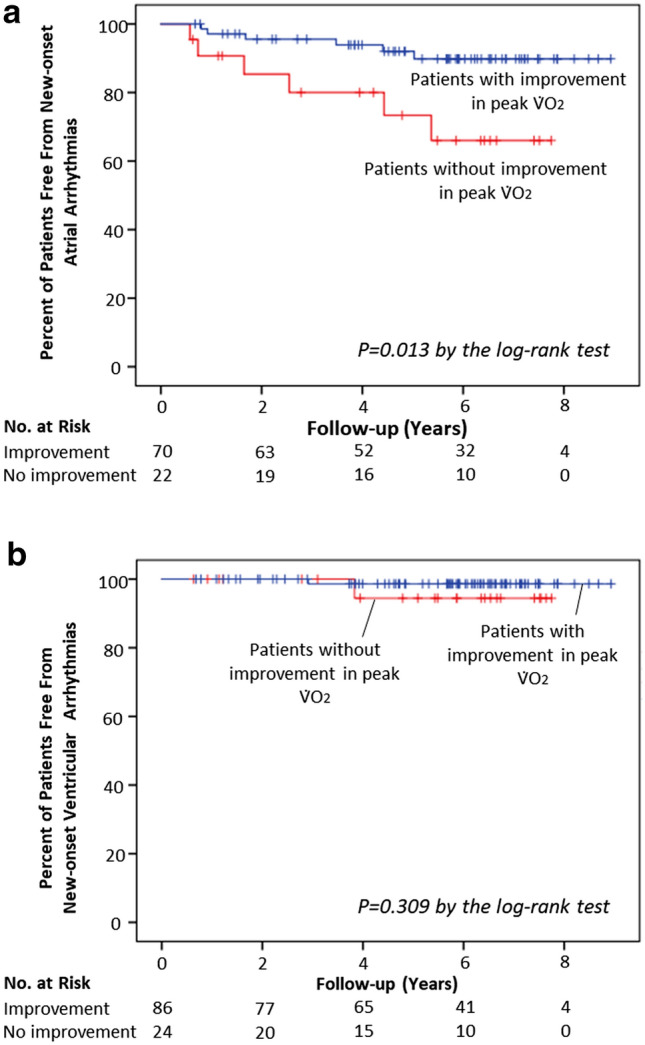
New-onset atrial (a) and ventricular (b) arrhythmias over time. The Kaplan–Meier estimates of the incidence of new-onset atrial and ventricular arrhythmias over a median follow-up of 6 years are shown for high-risk patients with improvement in peak V̇O2, high-risk patients without improvement in peak V̇O2, and patients with a preserved V̇O2. Tick marks indicate censored data. HF, heart failure; V̇O2, peak exercise oxygen consumption.
Table 3 summarizes the causes of death among the 11 patients with a low peak V̇O2. Eight of these 11 patients died from cardiovascular events (73%), with progressive HF being the predominant cause (6 of 11 patients, 55%). In these cases, there was no incidence of thromboembolic events. Of the eight patients who died from cardiovascular events, three had new-onset atrial arrhythmias (38%). None of the higher-risk patients underwent catheter ablation for new-onset atrial arrhythmias.
Table 3.
Causes of death in the higher-risk patients without improvement in peak V̇O2.
| No. (%) | |
|---|---|
| Total | 11 |
| Cardiovascular death | 8 (73) |
| Cardiac | |
| Sudden cardiac death | 2(18) |
| Progressive heart failure | 6 (55) |
| Vascular | |
| Thromboembolic events | 0 |
| Hemorrhage | 0 |
| Non-cardiovascular death | 3 (27) |
| Cancer | 1 (9) |
| Infection | 2 (18) |
Discussion
Our major findings were as follows: (1) the majority of patients with a low peak V̇O2 (higher-risk patients), defined as < 14 mL/min/kg, had improvement in peak V̇O2 at 3 months after CR; (2) higher-risk patients with an improved peak V̇O2 had a lower rate of mortality and hospitalization due to HF exacerbation, as well as a lower incidence of new-onset atrial arrhythmias than did those without improvement in peak V̇O2, but with no difference in the incidence of ventricular arrhythmias between groups; (3) patients with peak V̇O2 percentage of predicted values of < 45% had a higher risk of mortality (log-rank, P = 0.010) and higher incidence of new-onset atrial arrhythmias than did those with peak V̇O2 percentage of predicted values of ≥ 45% (log-rank, P = 0.031); and (4) the majority of deaths in higher-risk patients were cardiovascular events, with progressive HF being predominant.
Impaired exercise capacity is a major symptom in patients with HF, which is represented by low peak V̇O2 during maximal exercise testing. A previous study by Mancini et al. demonstrated that a low peak V̇O2 of < 14 mL/kg/min was associated with poor prognosis in patients with severe HF1. A comprehensive CR program includes patient evaluation, exercise training, physical activity counseling, cardiovascular risk factor management, psychosocial support, and patient education3,5. Previous studies demonstrated that CR improved peak V̇O2 and decreased all-cause mortality in patients with HF5,9,11–13. The HF-ACTION trial demonstrated the positive impact of CR on the long-term cardiovascular mortality or HF hospitalization during a median follow-up of 30 months13, which was in accordance with our findings. However, long-term outcomes of higher-risk patients without improvement in peak V̇O2 despite a comprehensive CR program have not been fully investigated, particularly regarding the long-term incidence of atrial and ventricular arrhythmias in those patients. In this study, higher-risk patients without improvement in peak V̇O2 after CR had a higher rate of mortality, HF hospitalization, and new-onset atrial arrhythmias than those with improvement. Of note, the majority of deaths were progressive HF, suggesting that most patients with severe HF, who are more prone to develop atrial arrhythmias, easily evolve towards advanced HF.
HF predisposes to the occurrence of atrial arrhythmias through various mechanisms, including an increase in left ventricular filling pressure or left atrial dilatation and fibrosis, each of which can lead to atrial structural and electrical remodeling14. Conversely, atrial arrhythmias, with an increased heart rate, can predispose to the development or worsening of HF due to impaired contractility and reduced cardiac output. Therefore, the occurrence of atrial arrhythmias in patients with HF is associated with increased adverse events, including HF progression and mortality15,16. In our study, the majority of deaths of higher-risk patients without improvement in peak V̇O2, who were at high risk for new-onset atrial arrhythmias, were cardiovascular events, mostly progressive HF, which was in accordance with previous studies. Although the treatment of atrial arrhythmias in patients with HF is of pivotal importance, previous randomized trials have failed to demonstrate that maintenance of sinus rhythm with antiarrhythmic drug therapy improves mortality in patients with coexisting HF and AF17, suggesting that side effects of antiarrhythmic drugs offset the positive effects. Recent studies have shown that catheter ablation is associated with a positive outcome in comparison with antiarrhythmic drugs18–20. The CASTLE-AF (Catheter Ablation versus Standard Conventional Therapy in Patients with Left Ventricular Dysfunction and Atrial Fibrillation) trial showed that catheter ablation was associated with lower rates of death from any cause and lower rates of hospital admission for HF in patients with HF. In a sub-analysis of the CAMERA MRA study, a regression of ventricular fibrosis in the context of reverse remodeling was observed on cardiac magnetic resonance imaging after restoration of sinus rhythm following catheter ablation of AF in patients with HF21, suggesting that timely treatment with catheter ablation may minimize irreversible ventricular remodeling. Although none of the deceased higher-risk patients with new-onset atrial arrhythmias underwent catheter ablation in this study, catheter ablation at the early stage of new-onset atrial arrhythmias may have improved the long-term outcome22.
Limitations
First, this was a retrospective single-center study with a limited number of patients since we aimed to include those who were able to be followed for a long-term period, which was possibly associated with the low incidence of new-onset ventricular arrhythmias. Furthermore, due to the limited number of patients, multivariate analysis was not performed in this study. Further multicenter investigation in a large number of patients is warranted. Second, as in many other countries, the number and duration of supervised CR sessions were limited due to a reimbursement policy in Japan. The prolonged CR may have further improved peak V̇O2, as observed in a previous study23, particularly in higher-risk patients. Third, considering that most patients enrolled in this study were on NYHA class I, with optimal therapies at the 2-month time point of CR, our data can be applied only to patients without severe HF who are refractory to medical and interventional therapies. Fourth, due to the lack of a predefined protocol in this retrospective study, systematic follow-up echocardiographic evaluation was not performed in this study to assess the relationship between the peak V̇O2 improvement and changes in echocardiographic parameters.
Conclusion
Improvement in peak V̇O2 after CR reduced the risk of new-onset atrial arrhythmias, mortality, and hospitalization due to HF exacerbation in higher-risk patients with HF. Further studies are warranted to determine whether early detection and management at the early stage of new-onset atrial arrhythmias could improve clinical outcomes in higher-risk patients without improvement in exercise capacity after CR.
Author contributions
A.Y., and T.S. designed the study, conducted data analysis, and wrote the manuscript; T.I., M.A., K.Y., and Y.I. participated in data analysis; M.M., S.S, and K.A. participated in data collection. All authors have reviewed and approved the final version of the manuscript.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Tetsuri Sakai and Atsuhiko Yagishita.
References
- 1.Mancini DM, et al. Value of peak exercise oxygen consumption for optimal timing of cardiac transplantation in ambulatory patients with heart failure. Circulation. 1991;83:778–786. doi: 10.1161/01.CIR.83.3.778. [DOI] [PubMed] [Google Scholar]
- 2.van Tol BA, Huijsmans RJ, Kroon DW, Schothorst M, Kwakkel G. Effects of exercise training on cardiac performance, exercise capacity and quality of life in patients with heart failure: A meta-analysis. Eur. J. Heart Fail. 2006;8:841–850. doi: 10.1016/j.ejheart.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Yancy CW, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62:e147–239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 4.JCS Joint Working Group Guidelines for rehabilitation in patients with cardiovascular disease (JCS 2012) Circ. J. 2014;78:2022–2093. doi: 10.1253/circj.CJ-66-0094. [DOI] [PubMed] [Google Scholar]
- 5.Bozkurt B, et al. Cardiac rehabilitation for patients with heart failure: JACC expert panel. J. Am. Coll. Cardiol. 2021;77:1454–1469. doi: 10.1016/j.jacc.2021.01.030. [DOI] [PubMed] [Google Scholar]
- 6.O'Connor GT, et al. An overview of randomized trials of rehabilitation with exercise after myocardial infarction. Circulation. 1989;80:234–244. doi: 10.1161/01.CIR.80.2.234. [DOI] [PubMed] [Google Scholar]
- 7.Froelicher V, et al. A randomized trial of exercise training in patients with coronary heart disease. JAMA. 1984;252:1291–1297. doi: 10.1001/jama.1984.03350100021023. [DOI] [PubMed] [Google Scholar]
- 8.Schuler G, et al. Myocardial perfusion and regression of coronary artery disease in patients on a regimen of intensive physical exercise and low fat diet. J. Am. Coll. Cardiol. 1992;19:34–42. doi: 10.1016/0735-1097(92)90048-R. [DOI] [PubMed] [Google Scholar]
- 9.Kitzman DW, et al. Physical rehabilitation for older patients hospitalized for heart failure. N Engl. J. Med. 2021;385:203–216. doi: 10.1056/NEJMoa2026141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Agostoni P, et al. Metabolic exercise test data combined with cardiac and kidney indexes, the MECKI score: A multiparametric approach to heart failure prognosis. Int. J. Cardiol. 2013;167:2710–2718. doi: 10.1016/j.ijcard.2012.06.113. [DOI] [PubMed] [Google Scholar]
- 11.Piepoli MF, Davos C, Francis DP, Coats AJ. ExTraMATCH collaborative exercise training meta-analysis of trials in patients with chronic heart failure (ExTraMATCH) BMJ. 2004;328:189. doi: 10.1136/bmj.328.7441.711-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carbone S, et al. Peak oxygen consumption achieved at the end of cardiac rehabilitation predicts long-term survival in patients with coronary heart disease. Eur. Heart. J. Qual. Care Clin. Outcomes. 2021 doi: 10.1093/ehjqcco/qcab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.O'Connor CM, et al. Efficacy and safety of exercise training in patients with chronic heart failure: HF-ACTION randomized controlled trial. JAMA. 2019;301:1439–1450. doi: 10.1001/jama.2009.454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Santhanakrishnan R, et al. Atrial fibrillation begets heart failure and vice versa: Temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dries DL, et al. Atrial fibrillation is associated with an increased risk for mortality and heart failure progression in patients with asymptomatic and symptomatic left ventricular systolic dysfunction: A retrospective analysis of the SOLVD trials. Studies of left ventricular dysfunction. J. Am. Coll. Cardiol. 1998;32:695–703. doi: 10.1016/S0735-1097(98)00297-6. [DOI] [PubMed] [Google Scholar]
- 16.Wang TJ, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: The Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E. [DOI] [PubMed] [Google Scholar]
- 17.Roy D, et al. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl. J. Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789. [DOI] [PubMed] [Google Scholar]
- 18.Khan MN, et al. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl. J. Med. 2018;359:1778–1785. doi: 10.1056/NEJMoa0708234. [DOI] [PubMed] [Google Scholar]
- 19.Jones DG, et al. A randomized trial to assess catheter ablation versus rate control in the management of persistent atrial fibrillation in heart failure. J. Am. Coll. Cardiol. 2013;61:1894–1903. doi: 10.1016/j.jacc.2013.01.069. [DOI] [PubMed] [Google Scholar]
- 20.Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl. J. Med. 2018;379:492. doi: 10.1056/NEJMc1806519. [DOI] [PubMed] [Google Scholar]
- 21.Prabhu S, et al. Regression of diffuse ventricular fibrosis following restoration of sinus rhythm with catheter ablation in patients with atrial fibrillation and systolic dysfunction: A substudy of the CAMERA MRI Trial. JACC Clin. Electrophysiol. 2018;4:999–1007. doi: 10.1016/j.jacep.2018.04.013. [DOI] [PubMed] [Google Scholar]
- 22.Kirchhof P, et al. Early rhythm-control therapy in patients with atrial fibrillation. N Engl. J. Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422. [DOI] [PubMed] [Google Scholar]
- 23.Reich B, et al. Efficacy of extended, comprehensive outpatient cardiac rehabilitation on cardiovascular risk factors: A nationwide registry. Eur. J. Prev. Cardiol. 2020;27:1026–1033. doi: 10.1177/2047487319898958. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.


