Abstract
Background
Current standard management of diabetic foot ulcers (DFUs) consists of surgical debridement followed by soak NaCl 0.9% gauzes tight infection and glycaemic control. Nowadays the use of advanced platelet-rich fibrin (A-PRF) has emerged as an adjunctive method for treating DFUs. This study was conducted to demonstrate the ability of combine A-PRF + HA as a complementary therapy in DFUs healing related with angiogenesis,inflammation and granulation index process.
Methods
This open label randomized controlled trial was conducted in Koja District Hospital and Gatot Soebroto Hospital Jakarta, Indonesia on July 2019–April 2020. DFUs patients with wound duration of three months, Wagner-2, with size of ulcer less than 40 cm2 were included in the study. The number of subjects was calculated based on the rule of thumb and allocated randomly into three groups, namely topical A-PRF + HA, A-PRF and Sodium Chloride 0.9% as a control, for each of 10 subjects. A-PRF made by 10 mL venous blood, centrifuge 200 G in 10 min, meanwhile A-PRF + HA though mix both them with vertex machine around 5 min. Biomarker such as VEGF, PDGF and IL-6 examined from DFU taken by cotton swab and analysis using ELISA. Granulation Index was measured using ImageJ. Biomarkers and granulation index were evaluated on day 0, 3, 7 and 14. Data were analysed using SPSS version 20 with Anova and Kruskal Wallis test to compare the angiogenesis and inflammation effect between the three groups.
Result
In topical dressing A-PRF + HA, there is an increase in delta VEGF on day-3 (43.1 pg/mg protein) and day-7 (275,8 pg/mg protein) compared to A-PRF on day-3 (1.8 pg/mg protein) and day-7 (104.7 pg/mg protein), also NaCl (control) on day-3 (-4.9 pg/mg protein) and day-7 (28.3 pg/mg protein). So that the delta VEGF of A-PRF + HA group increase significantly compared with others on day-3 (p = 0.003) and day- 7 (p < 0.001). Meanwhile A-PRF + AH group, there is also a decrease in delta IL-6 after therapy on day-3 (-10.9 pg/mg protein) and day-7 (-18.3 pg/mg protein) compared to A-PRF in delta IL-6 on day- 3 (-3.7 pg/mg protein) and on day-7 (-7.8 pg/mg protein). In NaCl (control) group there is a increase delta IL-6 on day-3 (4.3 pg/mg protein) and on day-7 (35.5 pg/mg protein). So that the delta IL-6 of A-PRF + HA group decrease significantly compared with others only on day- 7 (p = 0.015). In PDGF le level analysis, A-PRF + HA group increase significantly (p = 0.012) only in day -7 compare with other group (5.5 pg/mg protein).
Conclusion
The study shows the superior role of combined A-PRF + HA in the treatment DFU though increase angiogenesis and decrease inflammation pathway. The advantage of using A-PRF + HA is that it accelerates wound healing by increasing granulation tissue compared to A-PRF alone.
Keywords: Diabetic foot ulcer, Platelet-rich fibrin, Hyaluronic acid
Diabetic foot ulcer; Platelet-rich fibrin; Hyaluronic acid.
1. Introduction
Diabetic foot ulcer (DFU) is challenging to health care professionals because only few effective topical therapeutic interventions were available. Among of those existing topical treatments, growth factor has shown to be beneficial for healing of DFU in conjunction with extensive surgical debridement [1].
The complete process of DFU healing has inhibition due to lack of growth factor levels and prolonged inflammation. There is chronic inflammation that shows the levels of matrix metalloproteinase (MMPs) and tissue inhibitor of metalloproteinase (TIMPs) in which contributes wound healing [3]. These inflammatory cells release cytokines, including interleukins (IL1, IL6) and tumor necrosis factor-α (TNF-α) [4].
Neuro-ischemic disturbances in DFU will reduce oxygen and nutrients which will disrupt the cells on the wound surface such as macrophages, keratinocyte, mast cells, and fibroblasts that contribute to produce some growth factor in wound healing [5].
Growth factors has a roles in wound healing especially in inflammation and proliferative phase. At present, only topical application of recombinant human growth factor has been approved by U.S. Food and Drug Administration and European authorities for the treatment of diabetic neuropathic ulcers [6].
Autologous Platelet Rich Plasma (PRP) has been used n knee osteoarthritis case by remodeling and increases the synthetic capacity of chondrocytes and matrix production, also inhibits the apoptosis process of chondrocytes. through increased collagen type 2 deposition. In addition, cytokines that cause inflammation and pain are also inhibited by PRP so that treatment can also reduce symptoms and complaints of pain. Platelet rich fibrin (PRF), second generation of PRP, has been used in dermatology and plastics surgery for wound healing [7].
Autologous PRF gel consists of higher platelet concentration, chemokines, cytokines, any growth factors, and fibrin scaffold which stimulate proliferation and differentiation cell to form a new tissue [8]. It stimulates the molecular and cellular induction of normal wound healing responses. In the injured model, the ratio of growth factor released PRF (with a higher platelet content compared to PRP), had a higher amount of TGF-β1 which would induce stronger cell migration in vitro [36].
In the last decade, PRF has been developed to advanced-PRF (A-PRF) which has more growth factors resulting faster and better wound healing.9 although in autologous PRF from diabetic patient, the amount of growth factor was not as much as non-diabetic patient. In chronic diabetes, the systemic accumulation of glycation end products irreversibly destroys the entire physiology of fibroblasts, proliferating endothelial cells which will inhibit the production of granulation tissue [38].
Previous studies reported the use of PRF in the prevention and treatment of osteonecrosis of the jaw and gave good results and might user-friendly technique with an excellent cost-benefit ratio in oral surgery [35].
The DFU patient has also low growth factor and prolonged inflammation, thus to optimize growth factor release and control the inflammation, hyaluronic acid (HA) is added to A-PRF [10]. Hyaluronic Acid is a glycosaminoglycan that could enhance angiogenesis, wound healing and reduces chronic inflammation in DFU [11]. However, combination of A-PRF + HA has not been used in the treatment of DFU, which make it is necessary to do research to find out the effect of combined of A-PRF and HA in the treatment of DFU.
The combination of HA and Platelet concentrate has been known for the treatment of skin aging, and in the case of osteoarthritis [12] Tissue technique as a scaffold provide templates to improve GF retain in HA gel [13]. Otherwise there has not been reported yet about the combined HA and PRF that could increase diabetic foot healing. This study was conducted to demonstrate the ability of combine A-PRF + HA as a complementary therapy in DFUs healing and explain these mechanism related with angiogenesis, inflammation and granulation index.
2. Methods
We conducted an open label randomized controlled trial on July 2019 to April 2020. Informed consent was obtained from subject were willing to follow the research. The study has been approved by The Ethics Committee of the Faculty of Medicine Universitas Indonesia ID 0855/UN2.F1/ETIK/2018.
The study was conducted in Koja District Hospital and Gatot Soebroto Hospital, Jakarta. Diabetic patients with DFU, with an average wound duration of three months, categorized as Wagner 2 and ulcer less than 40 cm2 were included in the study. All subject had control normal platelet count (150.000–450.000/μL), normal blood glucose and underwent oral antidiabetic. The following criteria were excluded i.e. platelet dysfunction syndrome, unstable hemodynamic, critical thrombocytopenia and pregnancy. This is open randomized control trial, all inclusion criteria subject randomly divided depend of the group (A-PRF + HA, A-PRF and control group). Each group is 10 subject.
2.1. A-PRF + HA preparation
The A-PRF preparation protocol is very simple and the armamentarium required is the same as for PRP. Approximately 10–20 ml of whole venous blood was collected in each of the two sterile 6 ml capacity vacutainer tubes without anticoagulant. The vacutainer tube was then placed in a centrifuge (Figure 1) at 200G (708 RPM) for 8 min. We use low speed and low time concept for PRF production due to this concept will increase in growth factor release from PRF clots. The low-speed concept (A-PRF) demonstrated a significant increase in growth factor release of platelet-derived growth factor (PDGF), transforming growth factor (TGF)-β1, epidermal growth factor, and insulin-like growth factor It alsodemonstrated significantly higher messenger RNA (mRNA) levels of PDGF, TGF-β, and collagen1 at either 3 or 7 days [43].
Figure 1.
The Process to make A-PRF gel.
It will result fibrin, buffy coat and erythrocytes. Erythrocytes layer was carefully separated from the buffy coat use a sterile scissor and transferred to a new sterile tube. A-PRF was formed by fibrin and buffy coat, in which platelet (rich growth factor and cytokine) was entrapped in the fibrin. The mechanism followed here is that, fibrinogen initially concentrated at the top of the tube, combines with circulating thrombin by centrifugation, to form fibrin. A fibrin clot is then obtained in the center of the tube, just between the red blood cells at the bottom and the acellular plasma at the top. Platelets are massively trapped in the fibrin web [42].
To make homogenous A-PRF + HA gel, 1 mL A-PRF was mixed with 0,6 mL HA 0,2% using a vortex machine around 20 s. Hyaluronic acid, which is a glycosaminoglycan compound mixed with vaseline with a ratio of 1: 0.25. Each 1 g of this contained hyaluronic acid sodium salt 0.2%. Gel A-PRF + HA placed in a clean cup and is ready to be applied to the wound surface (Figure 2).
Figure 2.
The architecture and density of A-PRF + HA fibrin gel.
2.2. Wound treatment and monitoring
In this study, the subject was randomly allocated into 3 groups, i. e A-PRF + HA, A-PRF and sodium chloride (NaCl) 0,9% as control. The A-PRF + HA gel was used in a single application to the surface of the wound and covered by a protective bandage (Figure 3). The same condition was also done for A-PRF alone group. In control just use moist NaCl 0.9% gauze covered with plaster. The wounds were photographed before and after treatment on day-0, day-3, day-7 and day-14 using a digital camera. The granulation area was identified by ImageJ assessment software as an indicator of granulation growth on wound healing process.
Figure 3.
Application of A-PRF + HA gel in Diabetic Foot Ulcer.
2.3. Biomarker of wound healing
To identify the process of angiogenesis and inflammation in DFU healing, samples were taken by cotton swab from DFU surface before providing topical therapy, then the level of VEGF and IL-6 were measured using ELISA.
2.4. Statistical analysis
The data were analysed using SPSS version 20 in which association between variables was tested by Anova and Kruskal Wallis, after evaluating the normality of data distributions.
3. Results
The subjects collected in this study were 35 DM patients with DFUs Wagner 2. Base line characteristic of subject shows in Table 1. Only 30 patient included in inclusion criteria and randomly allocated into three groups (A-PRF + HA, A-PRF and control). The average age was 63.5 years old, with duration of DM more than 5 years and body mass index (BMI) around 28.3 kg/m2 (obese). At the start of this study, the baseline data for all groups showed insignificant differences even though in the A-PRF HA group increase in blood glucose and HbA1C compare with others. In addition, the A-PRF group also had the highest cholesterol levels compared to the other groups. Control of blood glucosa and cholesterol levels affected the healing of DFU.
Table 1.
Characteristic subject base on intervention.
| Characteristic | A-PRF + AH (n = 10) | A-PRF (n = 10) | Control (n = 10) | p |
|---|---|---|---|---|
| Age (year)a | 59.8 (SD 12.7) | 64.7 (SD 12.0) | 59.3 (SD 12.6) | 0.626a |
| Sex. n (%) | ||||
| Male | 5/10 | 4 (40) | 3 (30) | |
| Female | 5/10 | 6 (60) | 7 (70) | |
| BMIa | 28.9 (SD 2.7) | 27.3 (SD 2.08) | 28.4 (SD 2.5) | 0.337a |
| Hemoglobin (g/dL) | 12.7 (27.4–39.0) | 12.8 (10.1–15.8) | 12.05 (10.1–16.5) | 0.224b |
| Hematocrite (%) | 36.3 (29.2–42.9) | 35.4 (27.4–44.6) | 33.8 (24.4–40.8) | 0.145b |
| Leukocytes (103/μl)a | 13.30 (SD 1.08)∗ | 11.08 (SD1.33)∗ | 9.23 (SD 1.66)∗ | 0.985a |
| Platelet (103/μl)a | 354.9 (SD 167.5) | 338.8 (SD 164.5) | 319.9 (SD 128.4) | 0.880a |
| Random Blood Glucosa. mg/dLb | 286.0 (35.1)# | 243.8 (SD 47.4) | 254.7 (SD 58.6) | 0.104b |
| HbA1C (%)a | 11.34 (SD 1.30) | 9.0 (SD 0.68) | 8.5 (SD 0.72) | 0.950a |
| Cholesterol total mg/dL | 214.5 (SD16.9)∗ | 249.3 (SD 16.1)# | 202.3 (SD 38.6)∗ | 0.096a |
| Albumin mg/dL | 3.3 (2.8–4.2) | 3.1 (2.8–4.2) | 3.2 (2.8–4.0) | 0.662b |
∗Delta VEGF of A-PRF+ AH group compare with A-PRF group.
#Delta VEGF of A-PRF+ AH group compare with A-NaCl ( control ) group.
Mean (SD), anova test.
Median (min-max), Kruskal Wallis test.
3.1. Biomarker output
In these study we analyze of biomarker by swab i. e VEGF swab to describe wound angiogenesis and Interleukin -6 to describe wound inflammation proses.
3.2. Biomarker VEGF (angiogenesis)
In Table 2 shows in the baseline, the VEGF levels were not significantly difference among the three groups (p = 0,568), meaning that the data was homogenous. Evaluation on day-3 and day-7 days shows the VEGF level increase in the A-PRF + HA group (232.8 pg/mg to 320.6 pg/mg) and on day-7 (232.8 pg/mg to 544.5 pg/mg). In PRF group, there is a decrease of VEGF level on day-3 (185.7 pg/mg to 180.4 pg/mg) and but an increase day-7 (185.7 pg/mg to 272,8 pg/mg). In control group there is also a decrease of VEGF level on day-3 (183.7 pg/mg to 144.8 pg/mg) and day-7 (183.7 pg/mg to 167.4 pg/mg). There is significant escalation A-PRF + HA compare with others on day-3 (p = 0,007) and day-7 (p < 0,001.)
Table 2.
VEGF level in DFU swab Base on Intervention.
| Intervention | A-PRF + AH (n = 10) | A-PRF (n = 10) | control (n = 10) | p-value |
|---|---|---|---|---|
| Before∗ | 232,8 (SD 125,7) | 185,7(SD 100,8) | 183,7 (SD 127,2) | 0,568 |
| After day-3∗ | 320,6 (SD 165,8) | 180,4 (SD 87,4) | 144,8 (SD 87,7) | 0,007 |
| After day-7∗ | 544,5 (SD 266,8) | 272,8 (SD 97,7) | 167,4 (SD 98,8) | <0,001 |
Post-hoc anova anova:
A-PRF + HA group increase significantly compare with control on day -3 (p = 0,008) and day -7 (p < 0,001).
A-PRF + HA group increase significantly compare with A-PRF on day-3 (p = 0,042) and day-7 (p = 0,005).
A-PRF group increase not significantly compare with control on day-3 (p = 1.000) and day -7 (p = 0,559).
Data mean (SD), anova tes.
Figure 4 shows the ΔVEGF based on the interventions. In A-PRF + HA group, there is a increase in Δ VEGF on day 0–3 and on day 0–7 compare with A-PRF and NaCl group.
Figure 4.
The Increase of Δ VEGF based on intervention.
In sub group analysis of Δ VEGF use Mann Whitney test, on day 0–3, A-PRF + AH increase significantly compare with A-PRF group (p = 0.002∗) and control group (p = 0.005∗∗). Meanwhile Δ VEGF on day 0–7, A-PRF + AH increase significantly compare with A-PRF group (p = 0.002∗) and NaCl group (p < 0.001∗∗)
3.3. Biomarker PDGF (fibrogenesis)
Table 3 shows at baseline, the PDGF levels were not significantly difference among the three groups (p = 0.337) meaning the fibrogenesis proses in the DFU was homogenous.
Table 3.
PDGF level in DFU swab Base on Intervention.
| Intervention | A-PRF + AH (n = 10) | A-PRF (n = 10) | control (n = 10) | p-value∗ |
|---|---|---|---|---|
| Before∗ | 3.4 (SD 2.9) | 6.5(SD 2.1) | 5.2 (SD 2.5) | 0.271 |
| After day-3∗ | 5.3 (SD 3.1) | 7.3 (SD 5.5) | 4.9 (SD 4.4) | 0.436 |
| After day-7∗ | 8.9 (SD 2.2) | 5.6 (SD 2.5) | 5.3 (SD 4.7) | 0.049 |
Post-hoc anova:
A-PRF + HA group increase significantly compare with control on day -7 (p = 0.007).
A-PRF + HA group increase significantly compare with A-PRF on day -7 (p = 0,047).
Mean (SD), anova test.
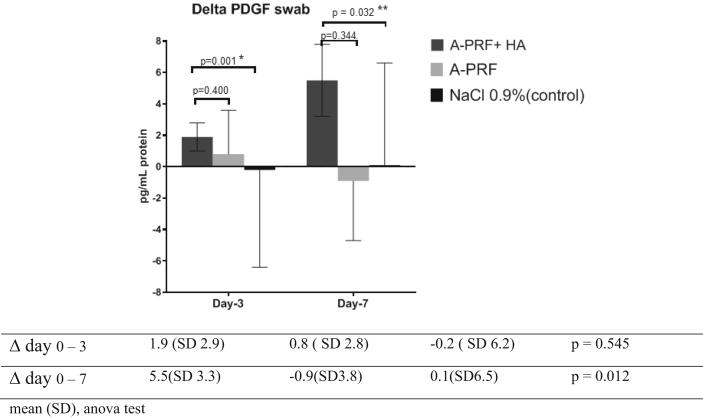
After interventions, there was a increase in PDGF for the A-PRF + HA group on day-7 (3.4 pg/mg to 8.9 pg/mg) meanwhile In the PRF group, PDGF levels decrease on day-7 (6.5 pg/mg to 5.6 pg/mg). In the control group, PDGF levels increase on day-7 (5.2 pg/mg to 5.3 pg/mg).
There is significant increase in PDGF level of A-PRF + HA group compare with others on day-7 (p = 0,049).
Figure 5 shows the analysis of PDGF by independent t-test, there is significant increase in Δ PDGF on day-3 (p = 0.001) and day 0–7 (p = 0.032) compare with NaCl, other wise the increase of Δ PDGF in group A-PRF + HA was not significant compare with A-PRF on day 0–3 (p = 0.400) and day 0–7 (p = 0.344). Thus, in A-PRF + HA group increase Δ PDGF significantly compare with A-PRF and NaCl on day-7 (p = 0.012).
Figure 5.
The Increase of Δ PDGF based on intervention. 
3.4. Biomarker IL-6 (inflammation)
Table 4 shows at baseline, the IL-6 levels were not significantly difference among the three groups (p = 0.337) meaning the inflammation condition in the DFU was homogenous.
Table 4.
IL-6 level in DFU swab base on intervention.
| Intervention | A-PRF + HA (n = 10) | A-PRF (n = 10) | Control (n = 10) | p-value |
|---|---|---|---|---|
| Before∗ | 106.4 (83.1–407.6) | 91.9 (38.6–151.6) | 125.3 (20.3–287.0) | 0.337 |
| After day-3∗ | 99.5 (76.3–302.2) | 72.8 (27.1–148.9) | 131.1(5.3–337.5) | 0.119 |
| After day-7∗ | 88.7 (44.3–217.9) | 48.8 (27.7–156.2) | 167.9 (27.7–156.2) | 0.041 |
IL-6 in A-PRF + AH compare with A-PRF decrease not significant on day-3 (p = 0.226), but significant decrease on day-7 (Mann Whitney test, p = 0,023).
IL-6 in A-PRF + AH decrease significantly compare with control on day-3 (Mann Whitney test, p = 0,049) and day -7 (Mann Whitney test, p = 0,041).
IL-6 in A-PRF decrease not significant compare with control on day-3 (Mann Whitney test, p = 0,326) and day -7 (Mann Whitney test, p = 0,545).
Data median (min-max), Kruskal Wallis test.
After interventions, there was a decrease in IL-6 for the A-PRF + HA group on day-3 (106.4 pg/mg to 99.5 pg/mg) and day-7 (106.4 pg/mg to 88.7 pg/mg). In the PRF group, IL-6 levels decrease on day-3 (91.9 pg/mg to 72.8 pg/mg) and on day-7 (91.9 pg/mg to 48.8 pg/mg). Meanwhile in the control group, IL-6 levels increase on day-3 (125.3 pg/mg to 131.1 pg/mg) and on day-7 (125.3 pg/mg to 167.9 pg/mg).
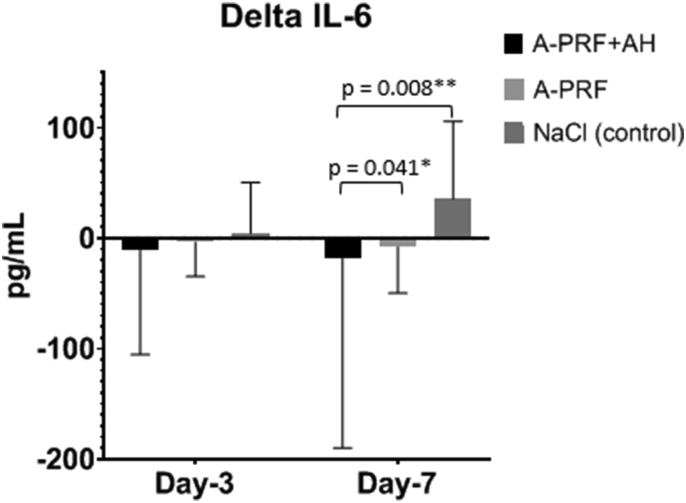
There is significant decrease in IL-6 level of A-PRF + HA group compare with others on day-7 (p = 0,041).
Figure 6 shows the Δ IL-6 based on the interventions. In A-PRF + HA group, there is a decrease in Δ IL-6 on day 0–7 compare with A-PRF and NaCl.
Figure 6.
The Decreasing of Δ IL-6 based on intervention.
Analysis of delta IL-6 by Mann Whitney test, there is significant decrease IL-6 level in A-PRF + HA group compare with A-PRF (p = 0,041∗) and NaCl (p = 0,008∗∗) on day-7.
3.5. Clinical outcomes
Table 5 shows that in A-PRF + HA group the granulation index increase significantly compare with A-PRF and control on day-3 and day-7.
Table 5.
Mean of granulation index base on intervention.
| Intervention | A-PRF + AH (n = 10) | A-PRF (n = 10) | Kontrol (n = 10) | p-value |
|---|---|---|---|---|
| Δ Day- 0−3 | 26.0 (SD 8.4) | 12.5 (SD 6.2) | 12.7 (SD 5.1) | <0.001 |
| Δ Day- 0−7 | 41.7 (SD 13.8) | 29.0 (SD 9.2) | 24.6 (SD 8.8) | 0.004 |
| Δ Day- 0−14 | 57.7 (SD 14.1) | 50.9 (SD 17.6) | 39.9 (SD 14.6) | 0.049 |
Data mean (SD), Anova test.
Post Hock Anova test.
Δ GI of, A-PRF + HA increase significantly compare with A-PRF on day -3 (p < 0.001) and day -7 (p = 0,042).
Δ GI of, A-PRF + HA increase significantly compare with control on day -3 (p < 0.001, day -7 p = 0.005) and day -14 (p = 0,048).
Further analysis by comparing the sub groups between A-PRF + HA, A-PRF and control use Post Hoc Anova test was shown in Figure 7.
Figure 7.
Granulation Index (GI) base on different intervention.
4. Discussion
One of the additional DFU therapies is to provide topical growth factors, where in chronic diabetes patients has a decrease growth factors and prolonged inflammation in which will inhibit healing. Provide growth factor from services to serve granulation tissue orders. PRF is biocompatible and has significantly improved soft tissue healing. Bone regeneration and increase in bone density in extraction sockets [37].
The use of topical A-PRF + HA induced the formation of healthy granulation tissue and allowed successful granulation area of the wound. Improvement of granulation tissue because A-PRF works synergistically with HA in increasing the release of growth factors and angiogenesis. In addition, the combination A-PRF + HA decreases the inflammation which is marked by a decrease in IL-6 [14].
4.1. Platelet Rich Fibrin release growth factors
DMT2 diabetes is often associated with chronic hyperglycaemia which can lead to inhibition of wound healing. On the other hand, there is a relative deficit of growth factors that will contribute to the mechanism of granulation tissue formation through angiogenesis, fibrogenesis and persistent inflammatory processes [15].
In diabetic patients showed decreased growth factors due to microvascular complications such as; retinopathy, diabetic foot ulcer or periodontitis. Vascular endothelial growth factor (VEGF) is a potent angiogenic and vascular permeability factor and is implicated in both of these complications in diabetes. Decrease in the production of transforming growth factor-β1 (TGF-β1) and VEGF has been associated with diabetic nephropathy, diabetic foot ulcer and retinopathy [40]. There is lack of expression of IGF1 within the basal layer and fibroblasts may contribute to retarded wound healing in diabetes mellitus. Decrease of growth factor due high glucose and intolerant insulin in diabetic foot ulcer tissue. However, in wounds of diabetic mice there was a delay in the appearance of IGF1 and IGF2 compared with non-diabetic mice [46].
Patients with chronic diabetes, there is also a decrease in platelet function. Platelets in type 2 diabetic individuals adhere to the vascular endothelium and aggregate more easily than in healthy individuals. Loss of sensitivity to normal restraint exerted by prostacyclin (PGI2) and nitric oxide (NO) produced by the vascular endothelium will decrease platelet function.
Platelet Rich Fibrin containing Platelets trapped in fibrin and will release some growth factors from platelet alpha granules. Although platelets in T2DM patients have decreased function compared to healthy people, the concentration of growth factors in PRF can still help in healing Diabetic Foot Ulcer (DFU) [41].
In platelet concentrate the amount of growth factor influence by aging, DM and antiplatelet drugs. They might decrease the concentration of growth factor, which weakens the regenerative capacity and anti-aging effects of PRP and reduces the quality of PRP [51].
According Marx 2001, The platelet count is a quantity but has been accepted as one of the major indexes for ensuring the quality of platelet concentrates However, in this study, the inclusion criteria of participants, the number of platelets must be ≥150 .103 u/L, so that the number of growth factors in PRF will certainly be homogeneous [49].
In DFU healing, when new granulation tissue occurs formed (around day-4), new blood vessels will form to provide oxygen and nutritional support for the new tissue. The angiogenesis process is stimulated by VEGF, bFGF, and TGF-β. In Platelet Rich Fibrin (PRF), granule α of platelets trapped in fibrin of PRF will release growth factors including VEGF, PDGF-BB, TGFB1 etc. It has the highest growth factor released from platelet concentrates, that is PDGF-AA followed by PDGF-BB, TGFB1, VEGF, and PDGF-AB [17]. In an in vitro study, several growth factors (PDGF, VEGF, TGF β) incubated in DMEM media were able to survive 14 days of observation with a peak on day 7 [18].
The low speed and low time concept use centrifuge 280 G, 10 min, is an important role in the revascularization of the graft by supporting angiogenesis [44].
PRF clots produced utilizing the low-speed centrifugation speeds (∼200 g for 8 min) produce clots that contained a higher concentration of evenly distributed platelets, secreted higher concentrations of growth factors over a 10 day period, and were smaller in size [45].
Low speed centrifugation concept (LSCC) selectively enriches leukocytes, platelets and growth factors within fluid PRF-based matrices [48].
The autologous Platelet Concentrate also has high growth factor in granules alpha that trap in fibrin of PRF. It used on endodontic healing [50].
4.2. Hyaluronic acid in inflammation and tissue regeneration
Hyaluronic acid (HA), a glycosaminoglycan (GAG) has an unique capacity to bind and retain water molecules and belongs to the extracellular matrix (ECM) molecules Hyaluronic acid as a lower molecular weight molecules will stimulate macrophages to have a pro-inflammatory response, one of the first steps to wound healing. In contrast, high molecular weight hyaluronic acid is demonstrated to stimulate an anti-inflammatory response from macrophages [19] To make balance of pro-inflammation and anti-inflammation, some field in medicine try to mix HA with platelet concentrate preparation. In therapeutic options for osteoarthritis and chronic tendinopathy, PRP or HA injection has used in this cases. Although several studies on the two have been published, the effects of mixing PRF and HA are not fully understood. PRF can stimulate the healing process of different tissues by delivering various growth factors and cytokines that are released by platelets, meanwhile PRF is not effective for treat DFU [23, 24]. In the present study, adding HA to the PRF increase the concentration of VEGF released on day-3 and day-7. Another report by regenerative medicine, the combination of HA + PRP can synergistically promote cartilage regeneration and inhibit OA inflammation [47].
4.3. Combine hyaluronic acid and PRF promote DFU healing by increasing angiogenesis
Effect of Hyaluronic Acid on angiogenesis by binding to receptor surfaces, activating signal transducers and mitogenesis26. Hyaluronic Acid will induce CD44 and RHAMM for angiogenesis transduction in vascular endothelial cells27 Savani et al28 showed that RHAMM-ligand interaction of endothelial cells will increase endothelial cell motility, and CD44-ligand interaction increases endothelial cell proliferation. Both these receptors work in tandem to facilitate formation of new blood vessels. Hyaluronic Acid also activates several CD44-dependent isoforms such as PKC, Raf-1 kinase, MEK-1, and ERK1/2 so that it will increase endothelial cell proliferation. 29 In addition HA binds to the RHAMM receptor and induces tyrosine phosphorylation of p125FAK, paxillin, p42/44, and extracellular ERK1/2, resulting in cell proliferation. 27 Galerno et al. 30 reported that HA leads to upregulation of eNOS and procollagen-1 and downregulation of MMP-9 and MMP-13, thereby increasing angiogenesis and influencing the production of angiogenetic-related cytokines in wounds.29 Other mechanisms for promoting EC proliferation by HA are likely related to expression and activation of ezrin, an important linkage protein that interacts with cellular HA. CD44 receptors and the cytoskeletal protein F-actin. Ezrin is an essential molecule that stimulates the proliferation and migration of EC. 21 The combination HA and A-PRF, will cause the release of growth factors slowly because of the hygroscopic nature of retain growth factors from HA itself. While the addition of HA did not enhance all the cellular responses to Vibronectin: Growth factor (VN:GF) complexes examined, it was not inhibitory, and may confer other advantages related to enhanced absorption and transport that could be beneficial in delivery of the VN:GF complexes to wounds [39].
4.4. Combine hyaluronic acid and PRF promote DFU healing by reduce inflammation
In this study, we found that A-PRF alone also increase VEGF swab, but is not optimal, compare with control. Meanwhile combine of A-PRF + HA administration increases VEGF swab, endothelial cell proliferation, migration, and tube formation that promotes wound healing in diabetic foot by increasing granulation tissue formed (Figure 3).
The addition of HA to PRF increases the rate of VEGF release because VEGF released from the PRF will stick to the HA surface and release slowly on the DFU surface. Attachment of VEGF to the DFU will stimulate endothelial cells to occur angiogenesis and stimulate the formation of granulation tissue [20]. Furthermore, it was observed that addition HA in A-PRF cause released growth factor in a significantly higher amount when compared to PRF alone. Combine A-PRF + AH will increase VEGF and endothelial cell proliferation, migration, and new vessels formation, Topical application of Hyaluronic Acid and PRF has affects angiogenetic-related cytokine production in the wound [21]. In these study, adding HA in PRF also decrease an inflammation progress in DFU, showed by decrease of IL-6 level in A-PRF + HA group on day-3 and day-7 compare with others group. These findings that stimulatory effect of HA on cytokine in PRF will decrease the inflammation progression in DFU. Roney et al [18] in cystitis patients that has damaged surface of urothelium cell. It has decreased of glycosaminoglycans (GAGs) so make higher permeability caused leading to inflammation. The additional HA in repair of the damaged urothelium through GAG replacement therapy make anti-inflammatory effect and has an ability to repair epithelial permeability. HA treatment increases sulphated GAG secretion by urothelial cells and restorate of endogenous production of GAG. The HA therapy could decrease permeability, so the tissue edema will decrease [22].
A-PRF + HA has anti-inflammation effects on immune response-related reduced pro-inflammatory M1 phenotype of macrophages and activated dendritic cells. Moreover, in vitro experiments showed PRF deleted inflammatory response related NF-κB signal pathway [23].
A-PRF + HA also affects increase polarization M1 to M2 as effect of anti-inflammation. The topical application of A-PRF + HA exerts a protective effect by decreasing the release of cytokines (IL-1β, IL-6, and TNF-α) during initial inflammation thus allowing the acceleration of the inflammatory phase previously prolonged inflammation due to hyper-glycaemia in DFU [22].
Topical application of A-PRF + HA will bind to the ICAM-1 and VCAM-1 receptors where the binding will inhibit the prolonged response of leukocytes (lymphocytes and monocytes) so that chronic inflammation can be prevented [23]. Chen et al [24] in an in vitro study, showed a synergistic effect o PRP and HA on cartilage regeneration in OA. In those report, the combination of PRP and HA reduced cytokines pro-inflammatory and increased articular chondrocyte proliferation, differentiation through Erk1/2 and Smad2/3 pathway [24, 25].
4.5. Combine hyaluronic acid and PRF promote DFU healing by increase fibrogenosis
Plateletrich plasma (PRP) and hyaluronic acid (HA) injection are both therapeutic options for osteoarthritis and chronic tendinopathy. The amounts of transforming growth factor β1 (TGF-β1) and platelet derived growth factor (PDGF-AA) released form PRF + HA on day 5. Thus, a mixture of PRP and HA may result in an enhanced the healing effect on certain tissues through increase fibrosis tissue [14, 31].
Diabetes in chronic hyperglycaemia has reduced capacity in the proliferation and synthesis of collagen because it is unresponsive to transformations of growth factor-β1 stimulation (TGF-β1) [32]. Either plateletrich fibrin (PRF) -lysate or hyaluronic acid (HA) can restore the TGF-β1 signaling pathway. Improving TGF-β1 signaling was measured by the cellular proliferation index and collagen deposition. The addition of HA to PRF-lysate resulted in a significant increase in the proliferation index and collagen deposition index rather than PRF-lysate alone. The best HA levels for this mixture ranged from 20.83 mM to 41.67 mM. The HA in PRF lysate is an excellent candidate material for treating clinical signs associated with decreased function skin fibroblasts in wound healing [33].
4.6. Propose mechanism combine of A-PRF + HA in DFU healing
To visualize This is the propose mechanism of A-PRF + HA in increasing the granulation in diabetic foot healing is shown in Figure 8.
Figure 8.
Propose mechanism of A-PRF + HA increase Granulation in DFU healing.
The wound healing theory with angiogenesis mechanisms through the role of platelet-derived growth factor (PDGF), transforming growth factor-beta (TGF-β), and vascular endothelial growth factor (VEGF) have been extensively studied in animals [31]. However, the use of combine of A-PRF-HA in increasing VEGF through the angiogenesis process has not been widely discussed. In DFU patients, IL-6 is certainly elevated due to prolonged in inflammation, but the one who get combine therapy can decrease IL-6 levels and accelerate the formation of granulation tissue. In our sturdy, the added of HA in A-PRF will increase VEGF swab through retain of GF in Hyaluronic Acid gel as a VEGF exogen [32, 33]. Hyaluronic Acid also will decrease inflammation (decrease of Interleukin -6 level) that affect increase polarization Macrophage-1 (M-1) to Macrophage-2 (M-2) M2. These polarization will induced keratinocyte and fibroblast produce VEGF endogen in the surface of the wound [33]. The other pathway of granulation increasing is repair of permeability and increase of endothelial cell function [34]. Fundamentally, the role of combine A-PRF + AH heals wounds through induce VEGF release and can suppress inflammatory reactions with decreased IL-6 [34].
So the method of making A-PRF + HA is very young and has a solid architecture so it's easy to apply on wounds due to density form of A-PRF + HA gel (Figure 6).
The application method of A-PRF + HA gel is very simple and does not require too much money, so I use this treatment cost-effectively (Figure 7).
5. Conclusion
Combination of A-PRF + HA increase the release of VEGF level on day-3 and day-7 significantly compare others group, meanwhile A-PRF + HA increase of PDGF level just on day-7. It also increase granulation index on day-3, day-7 and day-14, significantly compare others group. Topical administration of A-PRF + HA could promotes wound healing process in DFU by increasing angiogenesis and fibrogenosis. This would provide a new simple and cheap modality treatment for diabetic wounds in clinical practice.
Declarations
Author contribution statement
Ronald W Kartika: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Idrus Alwi, Franciscus D. Suyatna, Em Yunir, Todung Silalahi, Saleha Sungkar: Conceived and designed the experiments; Performed the experiments; Wrote the paper.
Sarwono Waspadji, Saptawati Bardosono: Conceived and designed the experiments; Analyzed and interpreted the data; Wrote the paper.
Suzzana Immanuel, Jusuf Rachmat: Conceived and designed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Mirta Hediyati Reksodiputro: Contributed reagents, materials, analysis tools or data; Wrote the paper.
Funding statement
This work was supported by Universitas Indonesia, Jakarta.
Data availability statement
Data associated with this study has been deposited at The Ethics Committee of the Faculty of Medicine Universitas Indonesia ID 0855/UN2.F1/ETIK/2018.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgement
This study was a dissertation of Program Doctoral Medical Science Universitas Indonesia.
REFERENCES
- 1.Carvajal Am, Gluud C., Nicola S., Racines D.S., Reveiz L., Olivia P. Growth factors for treating diabetic foot ulcers. Cochrane Database Syst. Rev. 2015;28(10):548–555. doi: 10.1002/14651858.CD008548.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Muller m, Trocme C., Lardy Morel F., Halimi S., Venhamou P.Y. Matrix metalloproteinases and diabetic foot ulcers: the ratio of MMP-1 to TIMP-1 is a predictor of wound healing. Diabet. Med. 2008 Apr 1;25(4):419–426. doi: 10.1111/j.1464-5491.2008.02414.x. Kany S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tilman K., Vollrath J.T., Relja B. Cytokines in inflammatory disease. Int. J. Mol. Sci. 2019 dec;20(23):6008. doi: 10.3390/ijms20236008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zubair m, jamal a. Role of growth factors and cytokines in diabetic foot ulcer healing. Rev. Endocr. Metab. Disord. 2019 apr 20;(8):1–11. doi: 10.1007/s11154-019-09492-1. [DOI] [PubMed] [Google Scholar]
- 6.Papanas n, maltezos e. Vol. 6. 2007. Growth factors in the treatment of diabetic foot ulcers: new technologies, any promises? Lower extremity wounds; pp. 37–53. (1) [DOI] [PubMed] [Google Scholar]
- 7.Vy Moraes, Lenza M., Tamaoki Mj, Faloppa F., Jc Belloti. Platelet-rich therapies for musculoskeletal soft tissue Injuries. Cochrane Database Syst. Rev. 2014;29:71–79. doi: 10.1002/14651858.CD010071.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blair P., Flaument R. Platelet α– granules: basic biology and clinical correlates. Blood Rev. 2009 Jul;23(4):77–189. doi: 10.1016/j.blre.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Raeissadat Sa, Rayegani Sm, Hassanabadi H. Knee osteoarthritis injection choices: platelet- rich plasma (PRP) versus hyaluronic acid (A one-year randomized clinical trial) Clin. Med. Insights Arthritis Musculoskelet. Disord. 2015;8:1–8. doi: 10.4137/CMAMD.S17894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramos-Torrecillas J., García-Martínez O., De Luna-Bertos E., OcãNa-Peinado Fm, Ruiz C. Effectiveness of platelet-rich plasma and hyaluronic acid for the treatment and care of pressure ulcers. Biol. Res. Nurs. 2015;17:152–158. doi: 10.1177/1099800414535840. [DOI] [PubMed] [Google Scholar]
- 12.Pardue El, Ibrahim S., Ramamurthi A. Role of hyaluronan in angiogenesis and its utility to angiogenic tissue engineering. Organogenesis. 2008;4(4):203–214. doi: 10.4161/org.4.4.6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Anderegg U., Simon J.C., Averbeck M. More than just a filler – the role of hyaluronan for skin homeostasis. Exp. Dermatol. 2014;23(5):295–303. doi: 10.1111/exd.12370. [DOI] [PubMed] [Google Scholar]
- 14.Drela E., Kulwas A., Wieslaw J., Goralczyk B., Boinska J., Drewniak W. VEGF-a and PDGF-BB -angiogenic factors and the stage of diabetic foot syndrome advancement. Endokrinologie. 2014;65(4):306–312. doi: 10.5603/EP.2014.0042. [DOI] [PubMed] [Google Scholar]
- 15.Amoli Mm, Ranjbar Sh, Roohipour N., Fa Sayahpour, Amiri P., Zahedi P. VEGF Gene Polymorphism association with diabetic foot ulcer. Diabetes Res. Clin. 2011;93:215–219. doi: 10.1016/j.diabres.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 17.Perez A.G., Rodrigues A.A., Luzo A.C., Lana J.F. Fibrin network architectures in pure platelet- rich plasma a characterized by fiber radius and correlated with clotting time. J. Mater. Sci. Mater. Med. 2014;25:1967–1977. doi: 10.1007/s10856-014-5235-z. [DOI] [PubMed] [Google Scholar]
- 18.Rooney Srivastava P., Watson A., Quinlan L., Leo R., Abhay P. Hyaluronic acid decreases Il-6 and Il-8 secretion and permeability in an inflammatory model of interstitial cystitis. Acta Biomater. 2015;19:66–75. doi: 10.1016/j.actbio.2015.02.030. [DOI] [PubMed] [Google Scholar]
- 19.Rayahin High and low molecular weight hyaluronic acid differentially influence macrophage activation. ACS Biomater. Sci. Eng. 2015;1(7):481–493. doi: 10.1021/acsbiomaterials.5b00181. 2015 Jul 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garbin L.C., Olver C.S. Platelet-rich products and their application to osteoarthritis. J. Equine Vet. Sci. March 2020;86 doi: 10.1016/j.jevs.2019.102820. [DOI] [PubMed] [Google Scholar]
- 21.Ito T., Williams, Fraser D.J., Philips A.O. Hyaluronan regulates transforming growth factor β1 receptor compartmentalization. J. Biol. Chem. 2004 Jun 11;279(24):25326–25332. doi: 10.1074/jbc.M403135200. [DOI] [PubMed] [Google Scholar]
- 22.Oertly B., Schimmer B.B., Wuthrich R.P. Mechanisms of hyaluronan-induced up-regulation of ICAM-1 and VCAM-1 expression by murine kidney tubular epithelial cells: hyaluronan triggers cell adhesion molecule expression through a mechanism involving activation of nuclear factor-kappa B and activating protein-1. J. Immunol. 1998;161(7):3431–3437. Oct 1. [PubMed] [Google Scholar]
- 23.Zhang Jinglun, Yin Chengcheng, Qin Zhao, Zhao Zifan, Wang Jinyang, Miron Richard J., Zhang Yufeng. Anti-inflammation effects of injectable platelet-rich fibrin via macrophages and dendritic cells. J. Biomed. Mater. Res. 2019;2(3):1–8. doi: 10.1002/jbm.a.36792. [DOI] [PubMed] [Google Scholar]
- 24.Chen W.H., Lo W.C., Hsu W.C. Synergistic anabolic actions of hyaluronic acid and platelet- rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014;35:9599–9607. doi: 10.1016/j.biomaterials.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 25.Mo Wei, Yang Cuixia, Liu Yiwen, He Yiqing, Wang Yingzhi, Gao Feng. The influence of hyaluronic acid on vascular endothelial cell proliferation and the relationship with ezrin/Merlin expression. Acta Biochim. Biophys. Sin. 2011;12(43):930–939. doi: 10.1093/abbs/gmr094. [DOI] [PubMed] [Google Scholar]
- 31.Ilio K. Hyaluronic acid induces the release of growth factors from platelet-rich plasma. Asia Pac. J. Sports Med. Arthrosc. Rehabil. Technol. 2016;4:27–32. doi: 10.1016/j.asmart.2016.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hu Y., Wu Y., Cai J., Ma S., Wang X. The procoagulant properties of hyaluronic acid- collagen (I)/chitosan complex film. J. Biomater Sci. Plym. Ed. 2009;20:1111–1118. doi: 10.1163/156856209X444457. [DOI] [PubMed] [Google Scholar]
- 33.Weigek Ph, Gf Fuller, Leboeuf Rd. A model for the role of hyaluronic acid and fibrin in the early events during the inflammatory response and wound healing. J. Theor. Biol. 1986 Mar 21;119(2):219–234. doi: 10.1016/s0022-5193(86)80076-5. [DOI] [PubMed] [Google Scholar]
- 34.Kannan Sridharan, Sivaramakrishnan G. Growth factors for diabetic foot ulcers: mixed treatment comparison analysis of randomized clinical trials. J. Theor. Biol. 1986;119:219–2123.4. doi: 10.1111/bcp.13470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Miranda M., GoanfredaF, Raffone C., Antonacci D., Pistli V., Bollero P. The role of platelet-rich fibrin (PRF) in the prevention of medication-related osteonecrosis of the jaw ( MRONJ) BioMed Res. Int. 2021;2021 doi: 10.1155/2021/4948139. Article ID 4948139, 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schar M.O., Romero J.D., Sandr Kohl, Zumstein M.A., Nesic D. Platelet-rich concentrates Differentially release growth factor and induce cell migration in vitro. Clin. Orthop. Relat. Res. 2015;473(5):1635–1643. doi: 10.1007/s11999-015-4192-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Singh A., Kohli M., Gupta N. Platelet rich fibrin:a novel approach for osseous regeneration. J. Maxollofac. Oral Surg. 2012;11(4):430–434. doi: 10.1007/s12663-012-0351-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Acosta J.B., Schultz G.S., Mola E.L., Nieto G.G., Siverio M.G., Martíne L.H. Glucose toxic effects on granulation tissue productive cells: the diabetics’ impaired healing. BioMed Res. Int. 2013 doi: 10.1155/2013/256043. Article ID 256043, 15 pages. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xie Y., Upton Z., Richards S., Rizzi Simone C., Leavesley D.I. Hyaluronic acid: evaluation as a potential delivery vehicle for vitronectin:growth factor complexes in wound healing applications. J. Contr. Release. 2011;153(3):225–232. doi: 10.1016/j.jconrel.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 40.Shi G.J., Shi G.F., Zhou J.Y., Zhan W.J., Gao C.Y., Jiang Y.P. Involvement of growth factors in diabetes mellitus and its complications: a general review. Biomed. Pharacother. 2018;101:510–527. doi: 10.1016/j.biopha.2018.02.105. [DOI] [PubMed] [Google Scholar]
- 41.Vinik A.I., Erbas T., Park R.S., Nolan R., Pitternger G. Platelet dysfunction in type 2 diabetes. Diabetes Care. 2001;24(8):1476–1485. doi: 10.2337/diacare.24.8.1476. [DOI] [PubMed] [Google Scholar]
- 42.Saluja H., Dehane V., Mahindra U. Platelet-Rich fibrin: a second generation platelet concentrate and a new friend of oral and maxillofacial surgeons. Ann. Maxillofac. Surg. 2011;1(1):53–57. doi: 10.4103/2231-0746.83158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fujioka-Kobayashi Masako, Miron Richard J., Hermandez Maria, Kandalam Umadevi, Zhang Yufeng, Joseph Choukroun. Optimized platelet-rich fibrin with the low-speed concept: growth factor release, biocompatibility, and cellular response. J. Periodontol. 2017 Jan;88(1):112–121. doi: 10.1902/jop.2016.160443. publish in. [DOI] [PubMed] [Google Scholar]
- 44.Choukroun J., Diss A., Simonpieri A. Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2006;101:299–303. doi: 10.1016/j.tripleo.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 45.Miron Richard J., Xu Hudi, Chai Jihya, Wang Jialin, Zheng Shihang, Feng Merngge. Comparison of platelet-rich fibrin (PRF) produced using three commercially available centrifuges at both high (∼ 700 g) and low (∼ 200 g) relative centrifugation forces. Clin. Oral Invest. 2020;24(3):1171–1182. doi: 10.1007/s00784-019-02981-2. [DOI] [PubMed] [Google Scholar]
- 46.Blakytny Robert, Jude Edward, Gibson Martin, Ferguson Mark. Lack of insulin-like growth factor I (IGFI) in the basal keratinocyte layer of diabetic skin and diabetic foot ulcers. J. Pathol. 2000;190(5):589–594. doi: 10.1002/(SICI)1096-9896(200004)190:5<589::AID-PATH553>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 47.lee Chiang- Her. Synergistic anabolic actions of hyaluronic acid and platelet-rich plasma on cartilage regeneration in osteoarthritis therapy. Biomaterials. 2014 Dec;35(36):9599–9607. doi: 10.1016/j.biomaterials.2014.07.058. [DOI] [PubMed] [Google Scholar]
- 48.Choukroun J., Ghanaati S. Reduction of relative centrifugation force within injectable platelet-rich-fibrin (PRF) concentrates advances patients' own inflammatory cells, platelets and growth factors: the first introduction to the low speed centrifugation concept. Eur. J. Trauma Emerg. Surg.: Off. Publ. Eur. Trauma Soc. 2018;44(1):87–95. doi: 10.1007/s00068-017-0767-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marx R.E. Platelet-rich plasma: evidence to support its use. J. Oral Maxillofac. Surg. 2004;62:489–496. doi: 10.1016/j.joms.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 50.Meschi N., Castro A.B., Vandamme K., Quirynen M., Lambrechts P. The impact of autologous platelet concentrates on endodontic healing: a systematic review. Platelets. 2016;27:613–633. doi: 10.1080/09537104.2016.1226497. [DOI] [PubMed] [Google Scholar]
- 51.Tian Ju, Lei Xuan, Li Xuan, Tang J.B., Cheng Biao. The effects of aging, diabetes mellitus, and antiplatelet drugs on growth factors and anti-aging proteins in platelet-rich plasma. Platelets (Abingdon) 2019;30(6):773–792. doi: 10.1080/09537104.2018.1514110. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data associated with this study has been deposited at The Ethics Committee of the Faculty of Medicine Universitas Indonesia ID 0855/UN2.F1/ETIK/2018.