Abstract
Human Cdc25 phosphatases play important roles in cell cycle regulation by removing inhibitory phosphates from tyrosine and threonine residues of cyclin-dependent kinases. Three human Cdc25 isoforms, A, B, and C, have been discovered. Cdc25B and Cdc25C play crucial roles at the G2/M transition. In the present study, we have investigated the function of human Cdc25A phosphatase. Cell lines that express human Cdc25A in an inducible manner have been generated. Ectopic expression of Cdc25A accelerates the G1/S-phase transition, indicating that Cdc25A controls an event(s) that is rate limiting for entry into S phase. Furthermore, we carried out a detailed analysis of the expression and activation of human Cdc25A. Activation of endogenous Cdc25A occurs during late G1 phase and increases in S and G2 phases. We further demonstrate that Cdc25A is activated at the same time as cyclin E- and cyclin A-dependent kinases. In vitro, Cdc25A dephosphorylates and activates the cyclin-Cdk complexes that are active during G1. Overexpression of Cdc25A in the inducible system, however, leads to a premature activation of both cyclin E-Cdk2 and cyclin A-Cdk2 complexes, while no effect of cyclin D-dependent kinases is observed. Furthermore, Cdc25A overexpression induces a tyrosine dephosphorylation of Cdk2. These results suggest that Cdc25A is an important regulator of the G1/S-phase transition and that cyclin E- and cyclin A-dependent kinases act as direct targets.
Transitions in the cell cycle of higher eukaryotic cells are governed by a family of cyclin-dependent kinases (Cdks). Cdk activities are determined by cyclin-binding, small Cdk inhibitor proteins and both positive and negative regulatory phosphorylations (14, 26, 35). There are two families of Cdk inhibitors which negatively regulate kinase activities. The first family includes p21, p27, and p57 and acts on a variety of cyclin-Cdk complexes. The second group consists of p15, p16, and p18 and inhibits the formation of cyclin D-Cdk4 (Cdk6) complexes (13). The regulation of Cdks on the phosphorylation level is best characterized for cyclin B-Cdk1 (Cdc2) but is conserved also in Cdks that are active during G1 and S phases. A stimulatory phosphorylation event is modulated by the Cdk-activating kinase (Cak), which phosphorylates threonine-161 on Cdk1 (30, 36). Another major regulatory mechanism is governed by the wee1/mik1 and myt1 protein kinases, which phosphorylate Cdk1 on threonine-14 and tyrosine-15, thus inactivating the Cdk kinase activity (22, 24, 27).
The Cdc25 phosphatases are members of the tyrosine phosphatase family and catalyze dephosphorylation and activation of cyclins-Cdks through removal of the inhibitory phosphates. In the fission yeast, Schizosaccharomyces pombe, only one type of Cdc25 that activates Cdk1 at the G2/M-phase transition exists, thus representing a key determinant of mitotic timing. In human cells, three Cdc25 homologs called Cdc25A, Cdc25B, and Cdc25C have been identified (6, 28, 33). The three phosphatases have approximately 50% sequence identity at the amino acid sequence level. The crystal structure of the catalytic domain of human Cdc25A phosphatase has recently been determined (4). Interestingly, Cdc25A has its own topology, and only the active site loop, containing the His-Cys-(X)5-Arg motif, shows similarity to the tyrosine phosphatase family.
Cdc25B and -C are involved in the regulation of the G2/M-phase transition, while Cdc25A is needed for the G1/S-phase transition (5, 16, 19, 20, 25). Cdc25C dephosphorylates cyclin B-Cdk1, thereby activating its kinase activity. Cdc25C in turn becomes phosphorylated by cyclin B-Cdk1 at mitosis, and this further stimulates its ability to dephosphorylate Cdk1, thus creating a positive feedback loop (15, 18, 37). Recent work indicates that Cdc25B might play the role of a “starter phosphatase” by activating cyclin B-Cdk1 in order to initiate the positive feedback mechanism (20, 29).
Cdc25A and -B are putative oncogenes (8). Cdc25A is able to transform primary mouse embryonic fibroblasts in cooperation with the Ras oncogene or in a background lacking functional retinoblastoma protein (pRb). It has been reported that the proto-oncogene product c-Myc directly stimulates the expression of Cdc25A (7). Although Cdc25A seems to play a crucial role at the G1/S-phase transition, the nature of its substrate(s) remains unclear. However, likely substrates for Cdc25A are cyclin-Cdk complexes that are activated during G1 phase. Cdk2, which forms complexes with cyclins A and E, is regulated by phosphorylation on sites analogous to those found in Cdk1 (11), suggesting an activation mechanism similar to that for Cdk1. In addition, the cyclin E-Cdk2 complex has been shown to physically interact with Cdc25A in vivo (16). Moreover, cyclin E-Cdk2 phosphorylates Cdc25A, which leads to an increase of its phosphatase activity (16). Experiments where immortalized human epithelial cells (MCF-10A) were treated with transforming growth factor β (TGF-β) revealed a marked increase in tyrosine phosphorylation of Cdk4 and Cdk6 and an inhibition of their kinase activities. TGF-β treatment also leads to a repression of Cdc25A and induction of p15 (17). Upon UV treatment of rat fibroblasts, Cdk4 is phosphorylated on Tyr, and its dephosphorylation is required for S-phase entry (37). Recently, it was reported that Cdc25A also acts on substrates apart from Cdks. Cdc25A binds to and dephosphorylates the homeodomain transcription factor cut, which in turn leads to a repression of p21 promoter activity (2).
In this study, we show that Cdc25A phosphatase activity first appears during the end of G1 phase, at the same time as cyclin E-Cdk2 and cyclin A-Cdk2 kinase activities appear but after cyclin D-Cdk4 or cyclin D-Cdk6 kinase is activated. In order to better understand the role of Cdc25A in the regulation of the G1/S-transition, we have investigated the effects of conditional ectopic Cdc25A expression on cell cycle progression of rat-1 cells. Overexpression of human Cdc25A shortens the G1 phase, thereby advancing S-phase entry. In addition, high levels of Cdc25A overexpression lead to a premature activation of cyclin E-Cdk2 and cyclin A-Cdk2 kinases and dephosphorylation of Cdk2, suggesting that these kinases act as in vivo substrates of Cdc25A.
MATERIALS AND METHODS
Plasmids and site-directed mutagenesis.
A mutant of human Cdc25A, lacking phosphatase activity, was constructed by mutating the catalytic cysteine to serine (Cdc25A C430S). A single base mutation was introduced into a PCR primer (TGTTGTGTTTCACTCCGAGTTTTCTTCTGAG). The PCR product containing the mutation was used as one of the primers in a second PCR to produce a larger fragment of the Cdc25A gene. Mutagenesis was performed by PCR with Pfu DNA polymerase (Stratagene). The mutation was confirmed by DNA sequencing.
To construct the tetracycline-regulated Cdc25A and Cdc25A C430S expression vector, we subcloned the blunt-ended NcoI-XbaI fragment of Bluescript/Cdc25A, which contains the full-length human Cdc25A, into a blunt-ended EcoRI pUHD 10-3 plasmid. The pUHD 10-3 plasmid contains several repeats of the tetracycline operator linked to a cytomegalovirus minimal promoter (10). Cotransfection of pUHD 10-3 and the tetracycline repressor vector (pUHD 15-1) led to a repression of gene expression in the presence of tetracycline. Upon removal of tetracycline, gene transcription was initiated. The Tk-hygromycin plasmid contained the hygromycin resistance gene under control of the thymidine kinase promoter.
Expression and purification of recombinant Cdc25 proteins.
Human Cdc25A and Cdc25 C430S (6) were produced in the bacterial strain BL21 with the pGEX-2T expression vector. After isopropyl-β-d-thiogalactoside (IPTG) induction for 4 h (1 mM final concentration), the 95-kDa glutathione S-transferase (GST)–Cdc25A fusion protein was recovered by binding to glutathione-Sepharose beads and eluted with 20 mM glutathione in 50 mM Tris-HCl, pH 8.0.
Cell lines, transfection, and selection procedure.
HeLa cells in suspension were obtained from the Cold Spring Harbor Laboratory Tissue Culture facility (Cold Spring Harbor, N.Y.) and cultured in Dulbecco’s modified Eagle’s medium (DMEM)–5% fetal calf serum (FCS) containing 1 g of glucose per liter. Human foreskin fibroblasts (Hs68) and human lung fibroblasts (IMR-90) were purchased from the American Type Culture Collection and cultivated as described in reference 20. All media were supplemented with 100 U of penicillin-streptomycin per ml and 2 mM glutamine. rat-1 cells containing the tetracycline repressor expression vector pUHD 15-1, termed R12 cells (31), were maintained in the presence of 600 μg of G418 (Geneticin; Calbiochem) per ml. R12 cells were maintained in DMEM containing 4.5 g of glucose per liter. The pUHD 10-3 vector containing Cdc25A or Cdc25A C430S was transfected together with the hygromycin resistance vector (ratio of 1:20) into R12 cells by using the liposomal transfection reagents (DOSPER or DOTAP; Boehringer Mannheim). Cells were then cultivated in the presence of tetracycline (2 μg/ml). Clones were selected in the presence of 150 μg of hygromycin per ml. Clones showing inducible expression of Cdc25A and Cdc25A C430S after tetracycline removal for 48 h were screened for immunoblotting by using specific polyclonal antibodies raised against Cdc25A.
Elutriation and cell synchronization.
G1 HeLa cells were obtained by centrifugal elutriation with a JE-5.0 rotor (Beckman) as described previously (3). For the experiments described in the legend to Fig. 2, cell fractions enriched in early G1 were resuspended in DMEM–5% FCS at a density of 3 × 105 cells/ml. Samples were taken at the times indicated. Progression through the cell cycle in the elutriated cells was monitored by propidium iodide staining and flow cytometry. HeLa cells arrested in S phase were obtained by blocking the cells for 19 h in 10 mM hydroxyurea.
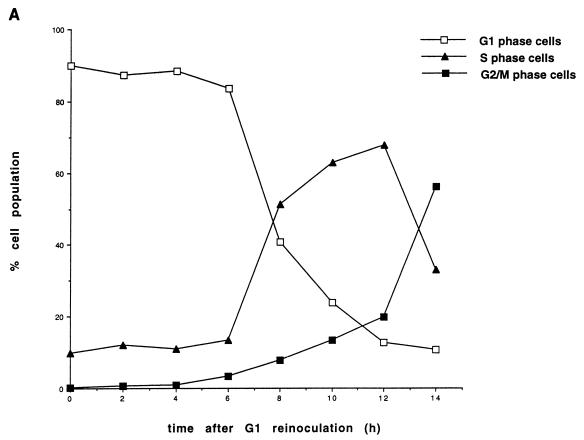
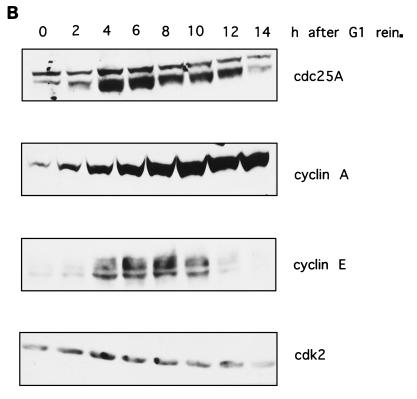
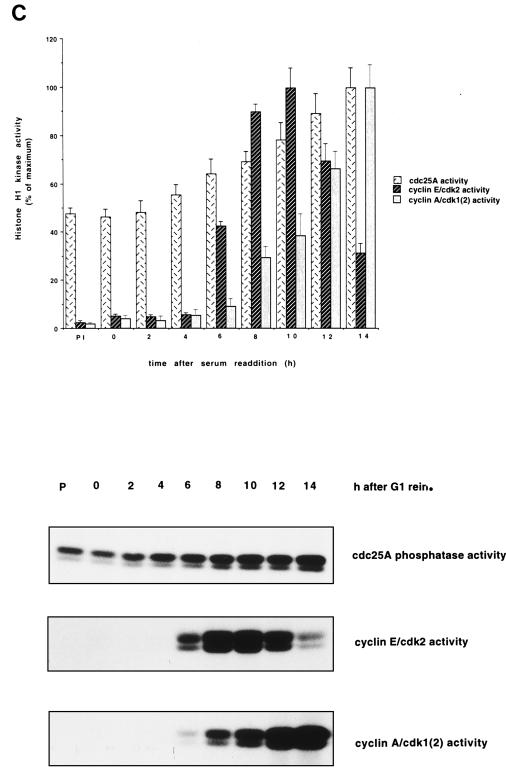
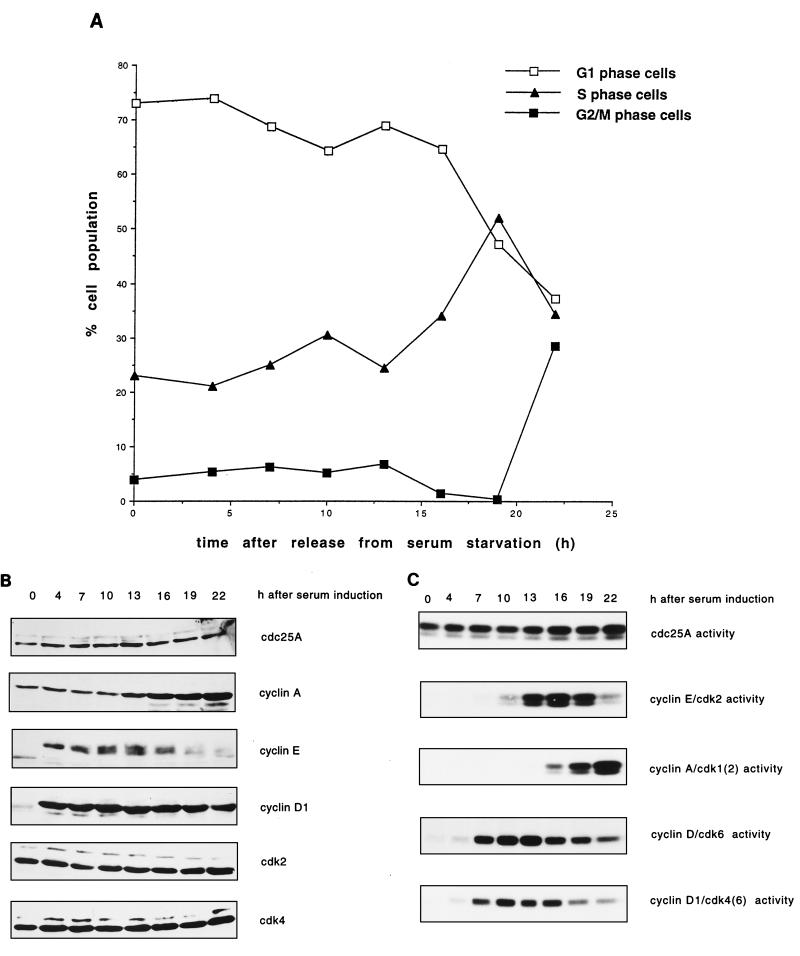
FIG. 2.
Protein levels and activity of Cdc25A and G1 cyclins-Cdks in HeLa cells. (A) Cell cycle distribution. HeLa cells grown in suspension were separated on the basis of size by centrifugal elutriation. G1-enriched cells were reinoculated in fresh medium and collected at the indicated time intervals. Cells were stained with propidium iodide and analyzed by flow cytometry. The graph shows the percentages of cells in G1, S, and G2/M phases. (B) Immunoblot analysis. Total cell extracts from the elutriated samples of panel A were analyzed by SDS-PAGE and immunoblotted with specific antibodies against Cdc25A, cyclins E and A, and Cdk2. (C) Cdc25A phosphatase activity and cyclin E- and cyclin A-associated kinase activities. Extracts were prepared at the indicated times from the elutriated samples, immunoprecipitated with Cdc25A antiserum, and incubated with tyrosine-phosphorylated inactive cyclin B-Cdk1 complex. Activation of cyclin B-Cdk1, caused by Cdc25A, was monitored on histone H1 as substrate. Cyclin E- and cyclin A-associated kinase activity was tested on histone H1 as substrate. P denotes control immunoprecipitations with a preimmune serum. The results are the means of two independent experiments. rein., reinoculation.
R12 clones were synchronized by serum starvation with DMEM containing 0.1% FCS for 48 h. The cells were released through addition of DMEM containing 10% FCS and harvested at the indicated time points. Hs68 cells were synchronized by serum starvation in DMEM without FCS for 36 h. They were then released through addition of DMEM supplemented with 20% FCS.
Cell cycle analysis.
The cell cycle distribution was assayed by propidium iodide or bromodeoxyuridine (BrdU) incorporation with flow cytometry. Adherent cells were trypsinized, and HeLa cells grown in suspension were collected by centrifugation. The cells were then washed and resuspended in phosphate-buffered saline (PBS). Ice-cold methanol was added to a final concentration of 80%. The cells were incubated for at least 20 min at 4°C and then pelleted and resuspended in 1 ml of PI mix per 106 cells (50 μg of propidium iodide per ml, 10 mM Tris-HCl [pH 7.5], 5 mM MgCl2, 10 μg of RNase A per ml). The cells were incubated for 20 min at room temperature before measurement. To assay the proportion of cells in S phase, they were incubated with 30 μM BrdU for 1 h before harvest. The cells were fixed with 70% cold ethanol. The RNA was degraded by incubation with 10 μg of RNase A per ml in PBS with 0.5% Tween 20 for 30 min at 37°C. The histones were extracted by incubation with 2 M HCl with 0.5% Tween 20 for 20 min at room temperature. The cells were labelled with 2 μg of anti-BrdU antibodies from Boehringer Mannheim and fluorescein isothiocyanate-linked anti-mouse immunoglobulin G from Jackson Immunoresearch (1:50) per ml. The cells were labelled with 40 μg of propidium iodide per ml. The analysis was performed with FACScan (Becton Dickinson) with the Cell Quest and the Mod Fit software.
Extract preparation and immunochemistry.
For cell fractionation, cell monolayers were washed twice with ice-cold PBS, and then cells were harvested by trypsinization.
Cell extracts were prepared by addition of 3 to 5 volumes of lysis buffer (50 mM Tris-HCl [pH 7.4], 0.5 M NaCl, 0.1% Triton X-100, 50 mM NaF, 1 mM dithiothreitol [DTT], 0.1 mM Na3VO4) to a cell pellet. The following protease inhibitors were added: 0.1 mM phenylmethylsulfonyl fluoride, 1 mg of leupeptin per ml, 10 mg of soybean trypsin inhibitor per ml, l-1-chlor-3-(4-tosylamido)-4-phenyl-2-butanon (TPCK), 10 mg of l-1-chlor-3-(4-tosylamido)-7-amino-2-heptanon-hydrochloride (TLCK) per ml, and 1 mg of aprotinin per ml.
To assay cyclin D-associated kinase activity, the lysates were prepared according to the method of Matsushime et al. (23). Cells were lysed in 1 ml of IP buffer (50 mM HEPES [pH 7.5], 150 mM NaCl, 1 mM EDTA, 2.5 mM EGTA, 10% glycerol, 0.1% Tween 20 1 mM DTT, 10 mM β-glycerophosphate, and the same protease and phosphatase inhibitors as in the lysis buffer) per 1 × 106 to 5 × 106 cells. The cells were frozen in dry ice, thawed, and incubated for 1 h on ice. The lysates were cleared by centrifugation for 10 min at 13,000 × g.
Total protein concentrations were determined by using the Bio-Rad protein assay system and bovine serum albumin as the calibration standard.
The Cdc25A antibodies used in the immunoprecipitation experiments were generated by injecting rabbits with a peptide coupled to keyhole limpet hemocyanin. This peptide corresponds to the C-terminal sequence of human Cdc25A protein (CKREMYSRLKKL). This sequence does not exist in either Cdc25B or Cdc25C. For use in immunoblotting experiments, an antibody against the full-length human GST-Cdc25A protein was raised in rabbits. The resulting polyclonal antiserum was affinity purified by using the GST-Cdc25A fusion protein covalently coupled to CNBr-activated Sepharose. Cyclins A, B, and D1 and Cdk2 antibodies were used as described in reference 16. Cyclin E (HE-111, HE-12, and M-20), Cdk4 (C-22), and Cdk6 (C-21) antibodies were from Santa Cruz Biotechnology, Inc. p21 (05-345) and antiphosphotyrosine antibodies (05-321) were purchased from Upstate Biotechnology Inc., Lake Placid, N.Y. For immunoblotting, 50 μg of total protein from HeLa, Hs68, or rat-1 cell lysates was loaded per lane and separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). Proteins were transferred from gels by semidry blotting as described in reference 12. As secondary antibodies, horseradish peroxidase-labelled anti-rabbit (Amersham) or horseradish peroxidase-labelled anti-mouse (Jackson Immunoresearch) antibodies were used.
Conditions for immunoprecipitation have been previously described (3).
Phosphatase and kinase assays.
To prepare the substrate for a Cdc25A phosphatase assay, cyclin B-Cdk1 was immunoprecipitated with anti-cyclin B antibodies from HeLa cells that were arrested in S phase by treatment with 10 mM hydroxyurea for 14 h. Under these conditions, Cdk1 complexed with cyclin B is phosphorylated on threonine-14 and tyrosine-15, causing an inactivation of its kinase activity. The cyclin B immunoprecipitate was incubated with recombinant or immunoprecipitated Cdc25A for 15 min at 30°C in 50 mM Tris-HCl (pH 8.0)–10 mM DTT, and the resulting activity of the kinase was measured by phosphorylation of histone H1 as described in reference 15. Briefly, after immunoprecipitations pellets were incubated at 30°C in the presence of 50 mM Tris-HCl (pH 7.5)–10 mM MgCl2–1 mM DTT–50 μM ATP–5 μCi of [γ-32P]ATP for 15 min. Samples were resolved by SDS-PAGE and analyzed by autoradiography. Cyclin E- and cyclin A-dependent kinase activities were measured on histone H1 as substrate under the same assay conditions.
For cyclin D-associated kinase assays, lysates prepared with IP buffer were used and precleared by incubation with Sepharose CL-4B beads (Pharmacia) for 1 h and centrifugation at 13,000 × g for 1 min. Protein A-Sepharose beads (Pharmacia) were precoated with antibody for 1 h and then mixed with the precleared lysates and incubated for 3 h at 4°C. The beads were washed four times with IP buffer and twice with kinase buffer (50 mM HEPES [pH 7.5], 10 mM MgCl2, 1 mM DTT). The kinase reaction was performed in kinase buffer with addition of 2.5 mM EGTA, 10 mM β-glycerophosphate, 50 μM ATP, 10 μCi of [γ-32P]ATP, and 5 μg of GST-pRb (amino acids 773 to 928). The kinase reactions were performed at 30°C for 30 min. The reactions were stopped through addition of Laemmli sample buffer, and reaction mixtures were analyzed by SDS-PAGE and autoradiography.
RESULTS
Human Cdc25A phosphatase is activated during late G1 phase.
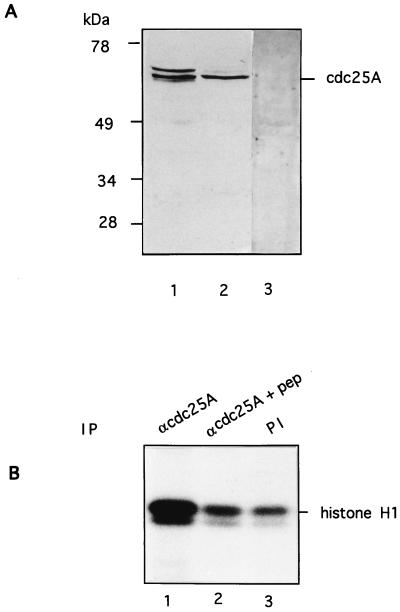
To investigate the function of human Cdc25A phosphatase, we first characterized the antibodies to be used. The polyclonal rabbit antibody raised against the full-length human GST-Cdc25A protein was affinity purified, and its specificity was tested by immunoblotting on HeLa and Hs68 cell extracts (Fig. 1A, lanes 1 and 2). The antibody specifically recognizes a doublet band at 68 to 70 kDa that was absent in the preimmune serum (Fig. 1A, lane 3). This antibody was used for immunoblotting. To be able to determine the phosphatase activity of human Cdc25A in extracts, an antibody modelled upon the Cdc25A C-terminal sequence was raised. The peptide sequence has no homology to sequences in either Cdc25B or Cdc25C phosphatase. The antibody immunoprecipitated Cdc25A that activated tyrosine-phosphorylated cyclin B-Cdk1 as determined by histone H1 phosphorylation (Fig. 1B, lane 1). Cdc25A phosphatase activity could not be immunoprecipitated in the presence of an excess of antigenic peptide (lane 2). Due to incomplete inactivation of cyclin B-Cdk1, there is a basal activity in the control immunoprecipitation by preimmune serum (lane 3). There was no detectable histone H1 kinase activity coprecipitating along with Cdc25A (data not shown). The C-terminal antibody was used for immunoprecipitations during this study.
FIG. 1.
Characterization of Cdc25A antibodies. (A) Extracts of HeLa cells (lanes 1 and 3) and HS68 cells (lane 2) were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with affinity-purified Cdc25A antibodies raised against the full-length protein (lanes 1 and 2) or preimmune serum (lane 3). (B) Cdc25A was immunoprecipitated from HeLa cell extracts with a C-terminal peptide antibody in the absence (lane 1) or presence (lane 2) of antigenic peptide and incubated with tyrosine-phosphorylated inactive cyclin B-Cdk1 complex. Activation of cyclin B-Cdk1 caused by Cdc25A was monitored on histone H1 as substrate. Lane 3 shows a control immunoprecipitation using preimmune (PI) serum. IP, immunoprecipitant.
One important unresolved issue is the nature of the Cdc25A in vivo substrate(s). To study this, we examined the exact timing of expression and activation of the Cdc25A phosphatase in comparison to the activation of potential substrates. In a first set of experiments, Cdc25A protein and phosphatase activity were analyzed at various time points during the G1 and S phases of the cell cycle in HeLa cells. HeLa cells were synchronized in early G1 according to cell size by centrifugal elutriation. The cells were recultivated, and samples were taken at the indicated time points. The relative percentages of G1-, S-, and G2- or M-phase cells in the elutriated fractions were determined for each time point by flow cytometric analysis of nuclear DNA content. Cells started to synthesize DNA between 6 and 8 h after G1 reinoculation (Fig. 2A). The amount of Cdc25A protein in the extracts was determined by immunoblotting (Fig. 2B). The Cdc25A protein reached maximal levels at the end of G1 phase and in early S phase. This is in agreement with data published by Jinno et al. (19). The discrepancy between our new data and earlier published results that showed constant Cdc25A levels (16) might be explained by the different antibodies used. Cyclin E protein is present at the end of G1 and the beginning of S phase. Cyclin A protein accumulates as the cells proceed through the G1 and S phases. The amount of Cdc25A phosphatase activity continuously increases, starting during mid-G1 phase and reaching a maximum in late S phase and early G2 phase. Cyclin E-dependent kinase activity appears simultaneously with Cdc25A phosphatase activity and decreases as the cyclin E protein disappears in S phase. The cyclin A-dependent kinase activity appears slightly later and increases in parallel with the Cdc25A phosphatase activity (Fig. 2C). Therefore, it is conceivable that the initial Cdc25A activity is necessary to activate cyclin E-Cdk2 while the rise in Cdc25A activity up to late S/G2 might be necessary to further activate cyclin A-Cdk2.
To investigate the timing of Cdc25A protein expression and activity in a cell line that has functional cyclin D-associated kinase activity, we synchronized human fibroblasts (Hs68) by serum deprivation and release. Samples were taken at the indicated time points. In Fig. 3A, the percentages of cells in G1, S, and G2 or M phase are shown. Cells started entering S phase at 16 h. The samples were then analyzed for protein levels and activities of Cdc25A and G1 cyclin-Cdk complexes. The protein levels of Cdc25A were slightly down-regulated during G0 but reappeared at 4 h after serum readdition (Fig. 3B). Cyclin A protein was present during the whole time course, but the levels increased markedly at 13 h after serum readdition. Cyclin D and E proteins were absent in G0 but were expressed again at the same time as Cdc25A. The protein levels of Cdk2 and Cdk4 remained unchanged. The overall level of Cdc25A phosphatase activity in Hs68 cells was noticeably lower than that in HeLa cells. An activation of Cdc25A phosphatase became detectable between 13 and 16 h after serum readdition (Fig. 3C). Cyclin E-Cdk2 kinase was activated slightly earlier, and cyclin A-Cdk2 underwent activation later, than Cdc25A. These results supports the finding that cyclin E-Cdk2 activates Cdc25A (16). However, cyclin D-dependent kinases and cyclin D-Cdk6 complexes were activated much earlier, at 7 h after serum readdition. From the timing of activation of cyclin E- and cyclin A-dependent kinases and the activation of Cdc25A in HeLa and Hs68 cells, we conclude that these kinases could act as physiological substrates of Cdc25A phosphatase.
FIG. 3.
Protein levels and activities of Cdc25A and G1 cyclins-Cdks in Hs68 human fibroblasts. (A) Hs68 cells were synchronized by serum starvation and released by addition of DMEM containing 20% FCS. Samples were taken for flow cytometry at the indicated times. (B) Total cell extracts were analyzed by SDS-PAGE and immunoblotted with Cdc25A-specific antibodies or with antibodies against the different G1 Cdks and cyclins. (C) Phosphatase and kinase activities from the same time points were determined as described for Fig. 2C. The activities of cyclin D immunocomplexes were measured as phosphorylation of pRb.
Cdc25A activates the G1 cyclin-Cdk complexes in vitro.
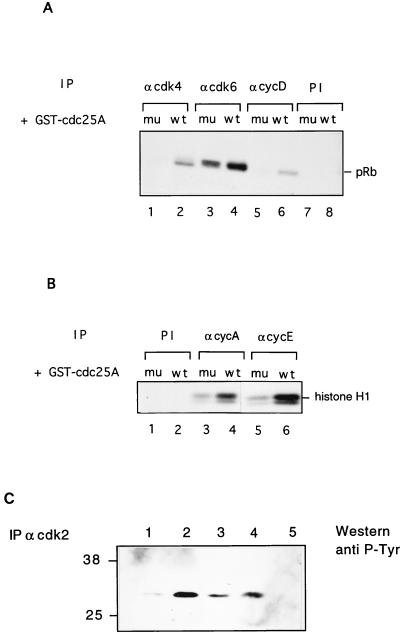
Since the Cdc25A activity appeared during the end of G1, we were interested in investigating if Cdc25A was able to activate the G1 cyclin-Cdk complexes that undergo activation at the same time as Cdc25A. In vitro, Cdc25A reveals a broad binding specificity for Cdk1 and Cdk2 complexes (39). To test the ability of GST-Cdc25A to activate G1 cyclin-Cdk complexes including cyclin D-dependent kinase, immunoprecipitates of these cyclin-Cdk complexes from exponentially growing HeLa cells or human lung fibroblasts (IMR-90) were incubated with recombinant GST-Cdc25A. To demonstrate that this activation was linked to the phosphatase activity of Cdc25A, a mutant of Cdc25A lacking phosphatase activity was constructed by changing the catalytic cysteine to a serine (Cdc25A C430S). Such mutants of Cdc25 phosphatases were previously shown to lack phosphatase activity (9). The resulting cyclin-dependent kinase activities were monitored for their ability to phosphorylate pRb (cyclin D1-Cdk4 or cyclin D1-Cdk6) or histone H1 (cyclin A-Cdk2 and cyclin E-Cdk2). Figure 4A shows that GST-Cdc25A was clearly able to activate Cdk4 and cyclin D-dependent kinase complexes (lanes 2 and 6) while the cyclin D-Cdk6 kinase could be further activated only slightly (lane 4) in comparison to the inactive GST-Cdc25A C430S protein (lanes 1, 3, and 5). Both cyclin E-Cdk2 and cyclin A-Cdk2 could be activated by recombinant GST-Cdc25A (Fig. 4B, lanes 4 and 6) compared to GST-Cdc25A C430S (lanes 3 and 5). No activating activity of Cdc25A C430S in comparison with bovine serum albumin or buffer alone was observed, indicating that the phosphatase activity of Cdc25A is critical for its activating capacity (data not shown). In this context, it should also be mentioned that Cdc25A is able to activate cyclin B-Cdk1 in vitro (Fig. 1B). Although the cyclin-Cdk complexes are activated to different extents by recombinant Cdc25A, we cannot draw conclusions about its preferred in vivo substrate.
FIG. 4.
Dephosphorylation and activation of cyclin-Cdk complexes by Cdc25A in vitro. (A) Cyclin D-Cdk4 or cyclin D-Cdk6 complexes were immunoprecipitated from exponentially growing IMR-90 cells by using antibodies against Cdk4 (lanes 1 and 2), Cdk6 (lanes 3 and 4), and cyclin D1 (lanes 5 and 6) or preimmune sera (lanes 7 and 8) and incubated with GST-Cdc25A (lanes 2, 4, 6, and 8) or GST-Cdc25A (C430S) (lanes 1, 3, 5, and 7). Activities of the complexes were determined on pRb as substrate. (B) Cyclin E-Cdk2 and cyclin A-Cdk2 complexes were immunoprecipitated from exponentially growing HeLa cells by using antibodies against cyclin A (lanes 3 and 4) and cyclin E (lanes 5 and 6) or preimmune serum (lanes 1 and 2) and incubated with GST-Cdc25A (lanes 2, 4, and 6) or GST-Cdc25A C430S (lanes 1, 3, and 5). Kinase activities were measured on histone H1 as substrate. (C) Cdk2 was immunoprecipitated from exponential HeLa cell extract and incubated with either GST-Cdc25A (lane 1), GST-Cdc25A (C430S) (lane 2), GST-Cdc25A in the presence of 1 mM sodium orthovanadate (lane 3), or buffer alone (lane 4); blotted; and incubated with antiphosphotyrosine antibodies. In lane 5, the immunoprecipitation was carried out with preimmune serum. Numbers at left indicate molecular masses in kilodaltons. IP, immunoprecipitant; PI, preimmune serum; mu, mutant; wt, wild type.
We then analyzed the phosphotyrosine levels of Cdk2 after immunoprecipitation with specific antibodies from HeLa cell extracts (Fig. 4C). Treatment of Cdk2 immunoprecipitates with recombinant GST-Cdc25A resulted in a marked reduction of phosphotyrosine while treatment with either GST-Cdc25A C430S or GST-Cdc25A in the presence of the tyrosine phosphatase inhibitor sodium orthovanadate did not reduce or only slightly reduced phosphotyrosine levels (Fig. 4C). These results clearly demonstrate that the increase in activity of G1 Cdk-cyclin complexes is concomitant with a reduced phosphotyrosine content in Cdk2, indicating that it might be a direct target of Cdc25A.
Transfectant cell lines inducibly expressing Cdc25A.
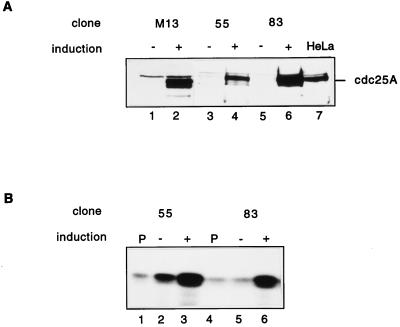
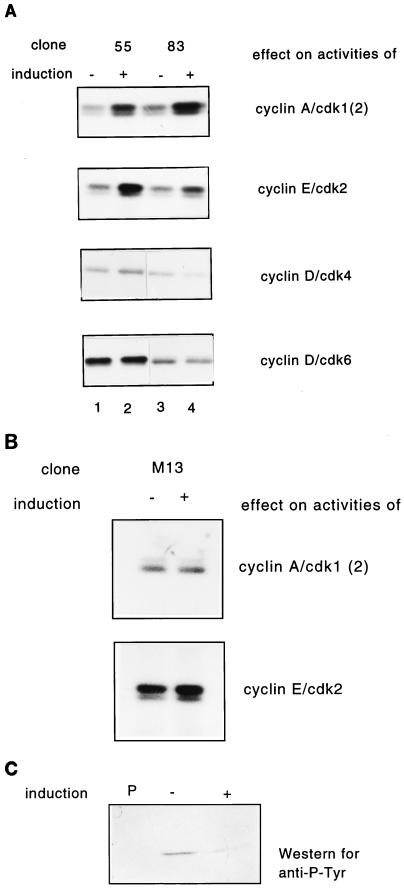
The tetracycline-inducible system takes advantage of a bacterial tetracycline resistance operator and transactivator (10). rat-1 cells stably transfected with the tetracycline transactivator were cotransfected with pUHD 10-3 vector containing Cdc25A or Cdc25A C430S and a hygromycin resistance vector. Hygromycin-resistant clones were screened by immunoblotting for inducible induction of Cdc25A after removal of tetracycline from the culture medium for 48 h. The screen resulted in 7 clones of 37 overexpressing wild-type Cdc25A and 3 clones of 20 overexpressing Cdc25A C430S. For the data presented, clone M13 expressing Cdc25A C430S and clones 55 and 83 expressing wild-type Cdc25A were used. These clones displayed highly regulated expression of Cdc25A. Removal of tetracycline from the medium led to an increase in Cdc25A protein levels in all clones (Fig. 5A, lanes 2, 4, and 6) over those in noninduced cells (Fig. 5A, lanes 1, 3, and 5). No or very weak leakiness was detected in these clones by immunoblotting. To examine whether the overexpression of Cdc25A leads to an increased Cdc25A activity, phosphatase assays on immunoprecipitated Cdc25A from induced or noninduced cells were performed (Fig. 5B). Induction of Cdc25A by tetracycline removal led to a two- to fourfold increase in Cdc25A phosphatase activity (Fig. 5B, lanes 3 and 6) over that in noninduced cells (Fig. 5B, lanes 2 and 5) or for immunoprecipitations performed with preimmune serum (Fig. 5B, lanes 1 and 3). Overexpression of Cdc25A lacking phosphatase activity did not lead to increased phosphatase activity (data not shown).
FIG. 5.
Inducible expression and activities of human Cdc25A in R12 cells. Clones M13 (lanes 1 and 2), 55 (lanes 3 and 4), and 83 (lanes 5 and 6) were grown in the presence (no induction of Cdc25A) and absence (induction of Cdc25A) of tetracycline for 48 h. Cells were then lysed as described in Materials and Methods. (A) Immunoblot analysis. Protein extracts from induced (+) and noninduced (−) cells were run on an SDS–10% polyacrylamide gel and blotted on nitrocellulose. The blot was then incubated with Cdc25A antibodies. HeLa cell lysates (lane 7) were used as controls. (B) Protein (1 mg) was used to analyze Cdc25A phosphatase activity by using inactive tyrosine-phosphorylated cyclin B-Cdk1 as substrate. Cyclin B-Cdk1 activation was determined by histone H1 phosphorylation. Lanes 1 and 4, control immunoprecipitations with preimmune serum; lanes 2 and 5, activity without induction of Cdc25A; lanes 3 and 6, activity with induction of Cdc25A.
Effects of Cdc25A overexpression on S-phase entry.
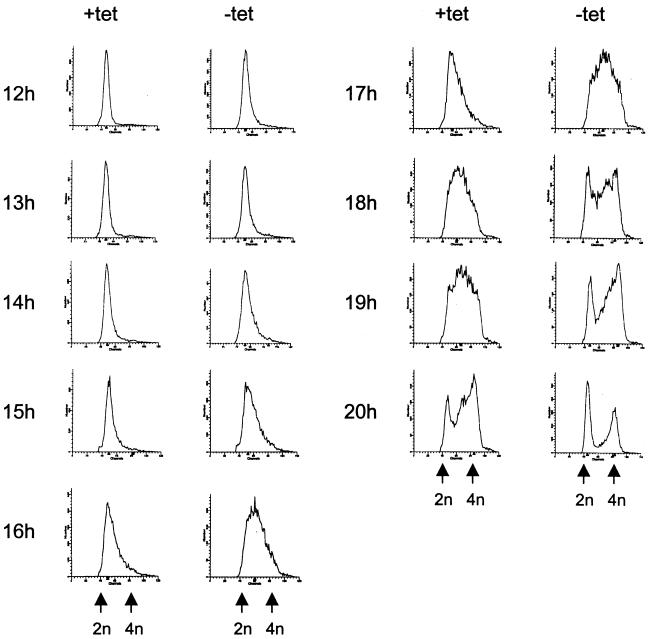
Microinjection experiments with Cdc25A-neutralizing antibodies have shown that Cdc25A is required for entry into S phase (16, 19). Therefore, we wanted to test if Cdc25A is also rate limiting in the process of S-phase entry. To study this, clones 55 and 83 were synchronized by serum starvation in the presence or absence of tetracycline for 48 h. Cells were released by serum addition (with or without tetracycline) and harvested for flow cytometry at the indicated time points after release (Fig. 6). Cdc25A overexpression was confirmed by immunoblotting (data not shown). Removal of tetracycline led to an acceleration of S-phase entry by 1.5 to 2 h in clone 55 (Fig. 6A). Similar results were obtained when the experiment was carried out with clone 83 (data not shown). These results indicate that the induced Cdc25A is functionally active and that Cdc25A performs a rate-limiting function in progression from quiescence to S phase. Tetracycline removal from synchronized R12 cells had no effect on S-phase entry (data not shown), indicating that tetracycline itself does not have any effect on S-phase entry.
FIG. 6.
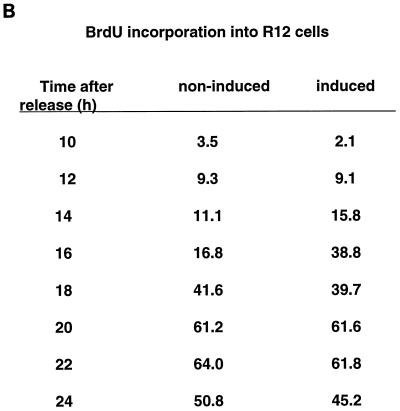
Effects of Cdc25A overexpression on cell cycle progression of rat-1 cells. Clone 55 was arrested in G0 with DMEM containing 0.1% FCS in the presence (+) (no induction of Cdc25A) or absence (−) (induction of Cdc25A) of tetracycline (tet) for 48 h. The cells were released with 10% FCS (with or without tetracycline). Cells were harvested at the indicated times after release. (A) The samples were fixed, stained with propidium iodide, and analyzed by flow cytometry. (B) One hour before harvest, 30 μM BrdU was added. Cells were double labelled with anti-BrdU antibodies and propidium iodide. BrdU staining of the cells was analyzed by flow cytometry. The figure indicates the percentage of S-phase (BrdU)-labelled cells.
To confirm the acceleration of S-phase entry, an alternative method was used to assay the number of cells in S phase. Cells were synchronized and induced as described above, but they were incubated with BrdU 1 h before harvest to allow BrdU incorporation into newly synthesized DNA. The BrdU-labelled cells were detected with an anti-BrdU antibody and a fluorescein isothiocyanate-linked secondary antibody by flow cytometry analysis. In agreement with results in Fig. 6A, we detected an entry into S phase after Cdc25A overproduction that was nearly 2 h earlier (Fig. 6B).
Effects of Cdc25A overexpression on G1 cyclin-associated kinase activities.
We have shown that Cdc25A is able to activate cyclin D1-, E-, and A-associated kinase activities in vitro (Fig. 4). We now tested whether overexpression of Cdc25A would lead to activation of any of the G1 cyclins-Cdks in vivo. Therefore, we compared the cyclin-associated kinase activities of exponentially growing clones 55 and 83 where Cdc25A was either induced or not. Cyclin-Cdk complexes were immunoprecipitated with specific antibodies, and their activity was assayed on histone H1 (for cyclin A- and cyclin E-dependent kinases) or on pRb (for cyclin D-dependent kinases). Cyclin A- as well as cyclin E-associated kinase activities were increased approximately three- to fivefold after Cdc25A overexpression (Fig. 7A). No effect on Cdk4- or Cdk6-associated kinase activities could be detected even though Cdc25A is able to activate Cdk4 complexes in vitro (Fig. 4A). Activation of cyclin A-dependent kinase activity occurs later in the cell cycle than cyclin E-Cdk2 activation (Fig. 2C). Therefore, we cannot exclude the possibility that the activation of cyclin A-Cdk2 observed after overproduction of Cdc25A is a consequence of increased cyclin E-Cdk2 activity. We did not observe any effect on cyclin A- and E-dependent kinase activities after overproduction of the inactive Cdc25A C430S in clone M13 (Fig. 7B). In a further experiment, the levels of phosphotyrosine in Cdk2 immunoprecipitates were analyzed after overproduction of wild-type Cdc25A. Figure 7C shows that the phosphotyrosine levels of Cdk2 markedly decreased after Cdc25A induction, indicating that the increase of cyclin E-Cdk2 and cyclin A-Cdk2 kinase activities is due to a direct dephosphorylation of Cdk2. Taken together, activation of cyclin A and E complexes shows not only that Cdc25A is involved in their activation but also that there is an inactive pool of cyclin E and A complexes in exponentially growing cells that can be activated after Cdc25A overexpression.
FIG. 7.
Effect of Cdc25A overproduction on cyclin-dependent kinase activities in asynchronously growing HeLa cells. (A) Wild-type Cdc25A was not induced (lanes 1 and 3) or induced (lanes 2 and 4) by tetracycline addition or removal, respectively, for 24 h. The histone H1 kinase activities associated with cyclin A and cyclin E or pRb kinase activities associated with Cdk4 and Cdk6 immunoprecipitates were analyzed. The experiment was performed with clones 55 (lanes 1 and 2) and 83 (lanes 3 and 4). (B) Mutant Cdc25A was either noninduced (−) or induced (+) as described for panel A, and the histone H1 kinase activities associated with cyclin A and cyclin E were analyzed. (C) Wild-type Cdc25A (clone 83) was either noninduced (−) or induced (+) as described for panel A. Cdk2 was immunoprecipitated and analyzed by immunoblotting with antiphosphotyrosine antibodies. P denotes a control precipitation with preimmune serum.
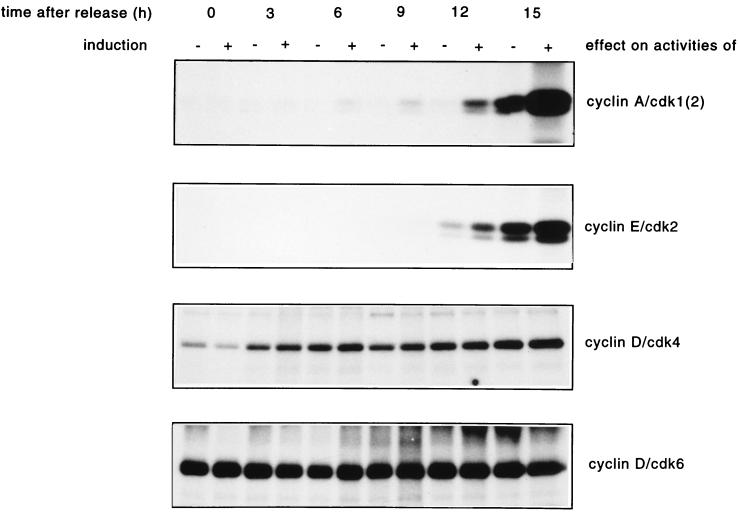
Since Cdc25A overexpression causes an acceleration of entry into S phase, it might be speculated that this is mediated by a premature activation of the kinases promoting G1- to S-phase progression. In addition, the activation of cyclin E- and cyclin A-dependent kinases in exponentially growing cells could be due to a decrease of the G1 fraction rather than a direct effect of Cdc25A overproduction. Therefore, we performed a time course assay of G1 Cdk-cyclin activation in cells released from G0 and after overproduction of Cdc25A. As shown in Fig. 8, the activity of cyclin A-Cdk complexes appeared already at 6 h in Cdc25A-overproducing cells, while in noninduced cells the activity first appeared after 12 h. The cyclin E-Cdk2 activity appears almost 3 h earlier in cells overexpressing Cdc25A than in noninduced cells. Cyclin D-Cdk4 and cyclin D-Cdk6 kinase activities are not affected by Cdc25A overproduction (Fig. 8). After Cdc25A overexpression, cyclin A-associated kinase starts to get activated 6 h earlier than in nonoverproducing cells, indicating that Cdc25A is a rate-limiting factor for the activation of cyclin A-associated kinases. Although less striking, cyclin E-associated kinase activity appears at least as much earlier as the premature S-phase entry occurs (Fig. 6) after Cdc25A overexpression.
FIG. 8.
Effect of Cdc25A overproduction on the activities of G1 Cdks-cyclins in synchronized R12 cells. Cells were synchronized and induced as described in the legend to Fig. 6. Extracts were prepared after the times indicated and used to determine G1 Cdk-cyclin activities. The kinases were immunoprecipitated with cyclin A, cyclin E, Cdk4, and Cdk6 antibodies. Cyclin A- and cyclin E-associated kinase activities were measured on histone H1 as substrate, and Cdk4 and Cdk6 activities were measured on pRb as substrate.
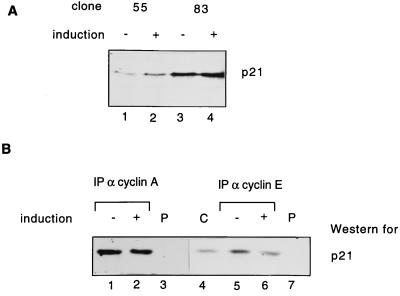
While this work was in progress, it was reported that Cdc25A modulates p21 expression through dephosphorylation of the transcription factor cut, which then leads to a down-regulation of p21 promoter activity (2). Therefore, we tested whether the effects of Cdc25A overexpression on cyclin E- and A-dependent kinase activities could be due to a decrease of p21 levels in the cell. Figure 9A shows that equal amounts of p21 protein were detected in cells with and without Cdc25A overproduction. Our results suggest that Cdc25A overproduction does not lead to a down-regulation of p21 protein levels in this particular system. It has previously been reported that Cdc25A competes with p21 for cyclin binding (34). We therefore investigated if Cdc25A overproduction might cause a decrease of the amount of p21 bound to Cdk2. This was tested by immunoprecipitation of cyclin E- or A-dependent kinases from induced or noninduced clone 83, followed by SDS-PAGE and immunoblotting with p21-specific antibodies. We did not observe any difference in p21 binding to cyclin A-Cdk2 in Cdc25A-overproducing cells (Fig. 9B), while a slight difference was observed in the amounts of p21 binding to cyclin E-Cdk2 (Fig. 9B). However, this small difference in binding cannot account for the strong activation of cyclin E-dependent kinases that we observed. Taken together, our data show that dephosphorylation of inhibitory phosphates on Cdk2 catalyzed by the Cdc25A phosphatase rather than displacement of p21 binding is critical for the observed activation of cyclin-Cdk2 kinases.
FIG. 9.
Expression and cyclin binding of p21 in Cdc25A-overproducing cells. (A) Clones 55 and 83 were induced (+) or noninduced (−) by tetracycline removal and addition for 24 h. Total levels of p21 after immunoblotting with p21 antibodies without induction (lanes 1 and 3) and with induction of Cdc25A (lanes 2 and 4) are shown. (B) Clone 83 was induced as described above. Amounts of p21 bound to immunoprecipitated cyclin A-Cdk2 (lanes 1 and 2) or cyclin E-Cdk2 (lanes 5 and 6) complexes or to control immunoprecipitations (with preimmune [P] serum) (lanes 3 and 7) were determined by immunoblotting with p21 antibodies. Lane 4 shows the migration and levels of p21 in whole rat-1 cell (C) lysates. IP, immunoprecipitant.
DISCUSSION
Our results suggest that human Cdc25A phosphatase plays an important role in the control of cell cycle progression at the G1/S-phase transition. We show that induced expression of Cdc25A, utilizing a tetracycline conditional expression system, in a serum starvation-stimulation experiment resulted in an accelerated entry into S phase by shortening the G1 phase by 10 to 15%, in comparison with that for noninduced cells. A decrease of the length of the G1 phase has also been observed by overproduction of the G1 cyclins D1, E, and A (31, 32). It is conceivable that additive effects of G1 cyclin expression would have a stronger impact on shortening the G1 phase. Induction of a Cdc25A protein lacking phosphatase activity (Cdc25A C430S [data not shown]) resulted in a modest retardation of S-phase entry. The slight effect observed with the inactive Cdc25A mutant protein suggests that a possible dominant-negative effect might be titrated out by the presence of endogenous Cdc25A protein in the R12 cell lines. Taken together, these results show that the phosphatase activity of Cdc25A is a critical regulator of entry into S phase. A similar role for Cdc25A has previously been proposed based on microinjection studies with specific antibodies against Cdc25A. Ablation of Cdc25A function blocks entry into S phase (16, 19). Our data are in good agreement with these previous findings and further suggest that Cdc25A is a rate-limiting, positive regulator of the G1- to S-phase progression.
The physiological target(s) of the Cdc25A phosphatase remained unclear for a long time. In NRK cells, treated with UV light, an increased tyrosine phosphorylation of Cdk4 (on Tyr-17) was observed, resulting in a G1 arrest (38). Microinjection of Cdc25A antibodies blocked the wild-type cells but not cells expressing the Cdk4Y15F mutant, suggesting that Cdk4 might be a target for Cdc25A. After TGF-β treatment, down-regulation of Cdc25A might be involved in an event causing the phosphorylation of Cdk4 and Cdk6 on Tyr-17 and Tyr-24, respectively, leading to cell cycle arrest in G1 (17). Induction of a nonphosphorylatable mutant of Cdk6 is not able to rescue the cell from a TGF-β-induced arrest. In contrast, induction of Cdc25A partly allows the cells to circumvent the arrest, indicating that Cdc25A also acts on other targets. However, the increase in phosphorylation(s) in Cdk4 and Cdk6 could also be caused by an activation of the inhibiting kinase. To date, the nature of the inhibiting kinase(s) acting on G1 Cdks is unclear. Cdc25A is transcriptionally regulated by c-Myc (7). Coexpression of c-Myc and Ras in quiescent cells causes a cell cycle progression (21). While Ras alone leads to an activation of the cyclin D-Cdk4 (or Cdk6)/pRB/E2F pathway, c-Myc is needed for cyclin E-Cdk2 activation, possibly via Cdc25A induction (21). In order to assess the in vivo role of Cdc25A, we studied the exact timing of activation of Cdc25A during G1 and S phase after serum stimulation of G0-arrested human foreskin fibroblasts (Hs68) and in synchronized HeLa cells. In both cell lines, activation of Cdc25A occurred simultaneously with the activation of cyclin E-Cdk2 but before cyclin A-Cdk2 and, as shown in Hs68 cells, after cyclin D-Cdk4 and cyclin D-Cdk6 activation. In addition, inducible overexpression of Cdc25A leads to activation of cyclin E-Cdk2 and cyclin A-Cdk2. No activation of cyclin D-Cdk4 or cyclin D-Cdk6 could be detected after Cdc25A overexpression. This observation is supported by the fact that neither Cdk4 nor Cdk6 is phosphorylated on Tyr-17 or Tyr-24, respectively, in an exponentially growing immortalized epithelial cell line (MCF-10A) and NRK cells. On the other hand, Cdk2 is phosphorylated on Tyr-15 in exponentially growing cells, indicating that Cdc25A is necessary for full activation of Cdk2 (11, 38). Moreover, Cdc25A has a transforming activity in an Rb−/− background (8). Since pRb is directly inactivated by cyclin D-dependent kinases, this would suggest that Cdc25A plays a role different from that of dephosphorylation of cyclin D-Cdk4 or cyclin D-Cdk6. Our results suggest that cyclin E-Cdk2 and cyclin A-Cdk2 act as critical targets for Cdc25A in both cycling cells and serum-induced cells. We can, however, not exclude the possibility that, as a consequence of external factors, Cdc25A functions on different targets.
Recent work indicates that Cdc25A also acts on substrates apart from Cdks. Cdc25A dephosphorylates the homeodomain transcription factor cut, leading to a decrease in p21 promoter activity (2). According to our data, overexpression of Cdc25A in exponentially growing cells does not affect the p21 protein levels, but we cannot exclude the possibility that Cdc25A is involved in regulation of p21 promoter activity. Cdc25A and p21 have also been shown to counterbalance each other by competing for the same binding site on the cyclin-Cdk complexes in vitro (34). p21 disrupts the interaction of Cdc25A with the cyclin-Cdk complex, thus blocking the removal of inhibitory phosphate groups on Cdk2. In our in vivo system, Cdc25A overexpression causes only a minor displacement of p21 from cyclin E-Cdk2.
Cdk2 is phosphorylated on Thr-14 and Tyr-15, and both phosphorylations lead to an inactivation of Cdk2 kinase activity since replacement of Thr-14 and Tyr-15 on Cdk2 with nonphosphorylatable residues results in elevated Cdk2 kinase activity (11). This suggests that Cdk2 is regulated by a Cdc25 phosphatase family member, similar to the regulation of Cdk1 by Cdc25C. From the timing of activation, the Cdc25A phosphatase would be a good candidate for regulating Cdk2 kinase activity. Furthermore, our results show that both cyclin E-Cdk2 and cyclin A-Cdk2 immunoprecipitates can be dephosphorylated and activated by treatment with Cdc25A but not by treatment with a Cdc25A mutant lacking phosphatase activity (Cdc25A C430A). This indicates that Cdc25A expression directly leads to an increase in activity of cyclin E-Cdk2 and/or cyclin A-Cdk2. The inactive mutant Cdc25A C430S protein strongly interacts with both cyclin A-Cdk2 and cyclin E-Cdk2 but does not lead to an activation of Cdk2 kinase activity (39). This indicates that although p21 can be competed out from cyclin E-Cdk2 complexes, this step is not sufficient to activate the kinase activity. The phosphatase activity of Cdc25A is necessary for Cdk2 activation, most likely due to dephosphorylation on Tyr-15 and Thr-14 of Cdk2. Since p21 levels do not change dramatically during the cell cycle (1), our favorite hypothesis is that when Cdc25A protein levels and activity rise, Cdc25A overcomes the inhibition of Cdk2 by p21, leading to G1 progression and entry into S phase. This would explain the observed acceleration of S-phase entry after Cdc25A overexpression.
ACKNOWLEDGMENTS
We are grateful to Harald zur Hausen for his support, to Manfred Gossen and Herrman Bujard for pUHD 10-3, to Dalia Resnitzky and Steve Reed for providing the R12 cell line, and to Carol Murphy for the hygromycin resistance vector. We thank Tareg Bashir for technical advice and many discussions. Christiane Lammer is thanked for excellent technical assistance. We thank Martin Scheffner and the members of our lab for critically reading the manuscript.
This work was supported by a grant from the Deutsche Forschungsgemeinschaft (Ho1299/3-3).
REFERENCES
- 1.Cai G, Dynlacht B. Activity and nature of p21 WAF1 complexes during the cell cycle. Proc Natl Acad Sci USA. 1998;95:12254–12259. doi: 10.1073/pnas.95.21.12254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Coqueret O, Bérubé G, Nepveu A. The mammalian Cut homeodomain protein functions as a cell-cycle-dependent transcriptional repressor which downmodulates p21WAF1/CIP/SDI1 in S phase. EMBO J. 1998;17:4680–4694. doi: 10.1093/emboj/17.16.4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Draetta G, Beach D. Activation of cdc2 protein kinase during mitosis in human cells: cell cycle-dependent phosphorylation and subunit rearrangement. Cell. 1988;54:17–26. doi: 10.1016/0092-8674(88)90175-4. [DOI] [PubMed] [Google Scholar]
- 4.Fauman E, Cogswell J, Lovejoy B, Rocque W, Holmes W, Montana V, Piwnica-Worms H, Rink M, Saper M. Crystal structure of the catalytic domain of the human cell cycle control phosphatase, Cdc25A. Cell. 1998;93:617–625. doi: 10.1016/s0092-8674(00)81190-3. [DOI] [PubMed] [Google Scholar]
- 5.Gabrielli B, De Souza C, Tonks I, Clark J, Hayward N, Ellem K. Cytoplasmic accumulation of Cdc25B phosphatase in mitosis triggers centrosomal microtubule nucleation in HeLa cells. J Cell Sci. 1996;109:1081–1093. doi: 10.1242/jcs.109.5.1081. [DOI] [PubMed] [Google Scholar]
- 6.Galaktionov K, Beach D. Specific activation of Cdc25 tyrosine phosphatase by B-type cyclins: evidence for multiple roles of mitotic cyclins. Cell. 1991;67:1181–1194. doi: 10.1016/0092-8674(91)90294-9. [DOI] [PubMed] [Google Scholar]
- 7.Galaktionov K, Beach D. Cdc25 cell-cycle phosphatase as a target of c-Myc. Nature. 1996;382:511–517. doi: 10.1038/382511a0. [DOI] [PubMed] [Google Scholar]
- 8.Galaktionov K, Lee A, Eckstein J, Draetta G, Meckler J, Loda M, Beach D. Cdc25 phosphatases as potential human oncogenes. Science. 1995;269:1575–1577. doi: 10.1126/science.7667636. [DOI] [PubMed] [Google Scholar]
- 9.Gautier J, Solomon M J, Booher R N, Bazan J F, Kirschner M W. Cdc25 is a specific tyrosine phosphatase that directly activates p34cdc2. Cell. 1991;67:197–211. doi: 10.1016/0092-8674(91)90583-k. [DOI] [PubMed] [Google Scholar]
- 10.Gossen M, Bujard H. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gu Y, Rosenblatt J, Morgan D. Cell cycle regulation of Cdk2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992;11:3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harlow E, Lane D. Antibodies: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1988. [Google Scholar]
- 13.Harper J. Cyclin dependent kinase inhibitors. Cancer Surv. 1997;29:91–107. [PubMed] [Google Scholar]
- 14.Hoffmann I. Cyclin-dependent protein kinases and the regulation of the eukaryotic cell cycle. In: Marks F, editor. Protein phosphorylation. Weinheim, Germany: Verlag Chemie; 1996. pp. 179–201. [Google Scholar]
- 15.Hoffmann I, Clarke P R, Marcote M J, Karsenti E, Draetta G. Phosphorylation and activation of human Cdc25-C by cdc2-cyclin B and its involvement in the self-amplification of MPF at mitosis. EMBO J. 1993;12:53–63. doi: 10.1002/j.1460-2075.1993.tb05631.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann I, Draetta G, Karsenti E. The tyrosine phosphatase activity of human Cdc25A is activated by cyclin E/Cdk2 during the G1/S transition. EMBO J. 1994;13:4302–4310. doi: 10.1002/j.1460-2075.1994.tb06750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iavarone A, Massague J. Repression of the CDK activator Cdc25A and cell-cycle arrest by cytokine TGF-β in cells lacking the CDK inhibitor p15. Nature. 1997;387:417–422. doi: 10.1038/387417a0. [DOI] [PubMed] [Google Scholar]
- 18.Izumi T, Maller J. Elimination of cdc2 phosphorylation sites in the Cdc25 phosphatase blocks initiation of M-phase. Mol Biol Cell. 1993;4:1337–1350. doi: 10.1091/mbc.4.12.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jinno S, Suto K, Nagata A, Igarashi M, Kanaoka Y, Nojima H, Okayama H. Cdc25A is a novel phosphatase functioning early in the cell cycle. EMBO J. 1994;13:1549–1556. doi: 10.1002/j.1460-2075.1994.tb06417.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lammer C, Wagerer S, Saffrich R, Mertens D, Ansorge W, Hoffmann I. The Cdc25B phosphatase is essential for the G2/M phase transition in human cells. J Cell Sci. 1998;111:2445–2453. doi: 10.1242/jcs.111.16.2445. [DOI] [PubMed] [Google Scholar]
- 21.Leone G, DeGregori J, Sears R, Jakoi L, Nevins J. Myc and Ras collaborate in inducing accumulation of active cyclin E/CDK2 and E2F. Nature. 1997;387:422–426. doi: 10.1038/387422a0. [DOI] [PubMed] [Google Scholar]
- 22.Liu F, Stanton J, Wu Z, Piwnica-Worms H. The human Myt1 kinase preferentially phosphorylates Cdc2 on threonine 14 and localizes to the endoplasmic reticulum and Golgi complex. Mol Cell Biol. 1997;17:571–583. doi: 10.1128/mcb.17.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Matsushime H, Quelle D, Shurtleff S, Shibuya M, Kato J. D-type cyclin-dependent kinase activity in mammalian cells. Mol Cell Biol. 1994;14:2066–2076. doi: 10.1128/mcb.14.3.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McGowan C H, Russell P. Human wee1 kinase inhibits cell division by phosphorylating p34cdc2 exclusively on Tyr15. EMBO J. 1993;12:75–85. doi: 10.1002/j.1460-2075.1993.tb05633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Millar J B A, Blevitt J, Gerace L, Sadhu K, Featherstone C, Russell P. p55Cdc25 is a nuclear protein required for initiation of mitosis in human cells. Proc Natl Acad Sci USA. 1991;88:10500–10504. doi: 10.1073/pnas.88.23.10500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Morgan D. Cyclin-dependent kinases: engines, clocks, and microprocessors. Annu Rev Cell Dev Biol. 1997;13:261–291. doi: 10.1146/annurev.cellbio.13.1.261. [DOI] [PubMed] [Google Scholar]
- 27.Mueller P R, Coleman T, Kumagai A, Dunphy W. Myt1: a membrane-associated inhibitory kinase that phosphorylates cdc2 on both threonine-14 and tyrosine-15. Science. 1995;270:86–90. doi: 10.1126/science.270.5233.86. [DOI] [PubMed] [Google Scholar]
- 28.Nagata A, Igarashi M, Jinno S, Suto K, Okayama H. An additional homolog of the fission yeast Cdc25 gene occurs in humans and is highly expressed in some cancer cells. New Biol. 1991;3:959–967. [PubMed] [Google Scholar]
- 29.Nishijima H, Nishitani H, Seki T, Nishimototo T. A dual-specificity phosphatase Cdc25B is an unstable protein and triggers p34cdc2/cyclin B activation in hamster BHK21 cells arrested with hydroxyurea. J Cell Biol. 1997;138:1105–1116. doi: 10.1083/jcb.138.5.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Poon R, Yamashita K, Adamczewski J, Hunt T, Shuttleworth J. The cdc2-related protein p40Mo15 is the catalytic subunit of a protein kinase that can activate p33Cdk2 and p34Cdc2. EMBO J. 1993;12:3123–3132. doi: 10.1002/j.1460-2075.1993.tb05981.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Resnitzky D, Gossen M, Bujard H, Reed S. Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol. 1994;14:1669–1679. doi: 10.1128/mcb.14.3.1669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Resnitzky D, Hengst L, Reed S. Cyclin A-associated kinase activity is rate limiting for entrance into S phase and is negatively regulated in G1 by p27Kip1. Mol Cell Biol. 1995;15:4347–4352. doi: 10.1128/mcb.15.8.4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sadhu K, Reed S, Richardson H, Russell P. Human homolog of fission yeast Cdc25 is predominantly expressed in G1. Proc Natl Acad Sci USA. 1990;87:5139–5143. doi: 10.1073/pnas.87.13.5139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Saha P, Eichbaum Q, Silberman E, Mayer B, Dutta A. p21 and Cdc25A: competition between an inhibitor and an activator of cyclin-dependent kinases. Mol Cell Biol. 1997;17:4338–4345. doi: 10.1128/mcb.17.8.4338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sherr C, Roberts J. Inhibitors of mammalian G1 cyclin-dependent kinases. Genes Dev. 1995;9:1149–1163. doi: 10.1101/gad.9.10.1149. [DOI] [PubMed] [Google Scholar]
- 36.Solomon M, Harper J, Shuttleworth J. CAK, the p34cdc2 activating kinase, contains a protein identical or closely related to p40MO15. EMBO J. 1993;12:3133–3142. doi: 10.1002/j.1460-2075.1993.tb05982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Strausfeld U, Fernandez A, Capony J-P, Girard F, Lautredou N, Derancourt J, C. L J, Lamb N. Activation of p34cdc2 protein kinase by microinjection of human Cdc25C into mammalian cells. J Biol Chem. 1994;269:5989–6000. [PubMed] [Google Scholar]
- 38.Terada Y, Tatsuka M, Jinno S, Okayama H. Requirement for tyrosine phosphorylation of Cdk4 in G1 arrest induced by ultraviolet irradiation. Nature. 1995;376:358–362. doi: 10.1038/376358a0. [DOI] [PubMed] [Google Scholar]
- 39.Xu X, Burke S. Roles of active site residues and the NH2-terminal domain in the catalysis and substrate binding of human Cdc25. J Biol Chem. 1996;271:5118–5124. doi: 10.1074/jbc.271.9.5118. [DOI] [PubMed] [Google Scholar]