Abstract
Background:
Multiple single-arm clinical trials showed promising pathologic complete response (pCR) rates with neoadjuvant immune checkpoint inhibitors (ICIs) in muscle-invasive bladder cancer. We conducted a cost-effectiveness analysis comparing neoadjuvant ICIs with cisplatin-based chemotherapy (CBC).
Methods:
We applied a decision analytic simulation model with a health care payer perspective to compare neoadjuvant ICIs vs CBC. For the primary analysis we compared pembrolizumab with ddMVAC. We performed a secondary analysis with gemcitabine/cisplatin as CBC and exploratory analyses with atezolizumab or nivolumab/ipilimumab as ICI. We input pCR rates from trials or meta-analysis and costs from average sales price. Outcomes of interest included costs, 2-year recurrence-free survival (RFS), and incremental cost-effectiveness ratio (ICER) of cost per 2-year RFS. A threshold analysis estimated a price reduction for ICI to be cost-effective and one-way and probabilistic sensitivity analyses were performed.
Results:
The incremental cost of pembrolizumab compared with ddMVAC was $8,041 resulting in an incremental improvement of 1.5% in 2-year RFS for an ICER of $522,143 per 2-year RFS. An 21% reduction in cost of pembrolizumab would render it more cost-effective with an ICER of $100,000 per 2-year RFS. GC required an 89% pembrolizumab cost reduction to achieve an ICER of $100,000 per 2-year RFS. Atezolizumab appeared to be more cost-effective than ddMVAC.
Conclusions:
ICIs were not cost-effective as neoadjuvant therapies, except when atezolizumab was compared with ddMVAC. Randomized clinical trials, larger sample sizes and longer followup are required to better understand the value of ICIs as neoadjuvant treatments.
Keywords: Muscle invasive bladder cancer, Urothelial Carcinoma, Neoadjuvant, Immunotherapy, Cost effectiveness analysis
1. Introduction:
Approximately 25% of patients with bladder cancer present with muscle-invasive bladder cancer (MIBC).1 Radical cystectomy and pelvic lymph node dissection (PLND) is the standard of care for MIBC; however, this alone results in a 5-year recurrence-free survival (RFS) rate of 68% which declines to 35% for those with lymph node involvement.2 Systemic cisplatin-based chemotherapy (CBC) prior to radical cystectomy is the standard of care for cisplatin-eligible patients with MIBC and yields pathologic complete response (pCR) in approximately a third of patients with clinically meaningful increases in overall survival (OS) compared with local therapy alone.3
Since 2016, five immune checkpoint inhibitors (ICIs) targeting programmed cell death protein 1 (PD-1) and programmed death-ligand 1 (PD-L1) have been approved by the US Food and Drug Administration to treat locally advanced (unresectable) or metastatic urothelial carcinoma.4 Pembrolizumab (PD-1 inhibitor) improved overall survival (OS) in the salvage setting after platinum-based chemotherapy5 and avelumab (PD-L1 inhibitor) improved OS after first-line platinum-based chemotherapy as switch-maintenance therapy.6 ICIs (atezolizumab or pembrolizumab) have also been approved for cisplatin-unfit patients with high PD-L1 expression or platinum (cisplatin and carboplatin)-unfit patients in the US. Moreover, pembrolizumab has been FDA-approved for BCG-unresponsive carcinoma in situ (with or without papillary tumors) in patients who cannot undergo or refuse radical cystectomy and PLND.
Multiple clinical trials have also investigated the use of ICIs in the neoadjuvant setting, either as monotherapy7,8, dual ICI blockade9–11 or in combination with chemotherapy12,13. The PURE-017 trial was an open-label, single-arm phase 2 trial that enrolled 114 patients with MIBC and treated with three cycles of neoadjuvant pembrolizumab and noted a pCR rate of 37%, which is indirectly comparable to CBC. Multiple other single-arm trials have also shown similar pCR rates ranging from 31% to 46% with single or double agent ICI in patients who were cisplatin-ineligible or refused CBC, suggesting a potential new therapeutic option (under investigation in five ongoing phase 3 trials – NIAGARA14, ENERGIZE15, KEYNOTE-86616, KEYNOTE-905/EV-30317, NCT0420911418) in this “curative-intent” setting. ICI is less toxic and better tolerated than CBC, but these agents are not standard of care as neoadjuvant therapy, as data remain limited to single-arm phase 2 trials with moderate sample sizes and short follow-up. Moreover, costs associated with these agents may increase the substantial financial burden associated with definitive treatment options for MIBC, given the expense of radical cystectomy and PLND.19,20 Acknowledging the inherent differences of patients who are eligible or ineligible for cisplatin (based on defined criteria21,22), there is a dire need to understand the cost-effectiveness and value of various neoadjuvant therapeutic strategies. We hypothesized that ICIs would not be cost-effective since they have similar pCR rates to CBC but with higher costs. To address this hypothesis, we evaluated the cost-effectiveness of neoadjuvant ICI compared with standard of care CBC for treatment of MIBC in the US, focusing on pembrolizumab, since the PURE-01 trial included cisplatin-fit patients. As an exploratory analysis, we attempted to create a data-driven context of other ICIs as a conceptual framework, with the caveat that data on other ICIs were derived from studies of cisplatin-ineligible patients (different population).
2. Materials and Methods:
2.1. Study design and scope
We compared neoadjuvant ICI with CBC as neoadjuvant treatment for patients with MIBC using a series of parallel analyses. In our primary analysis, we compared pembrolizumab to accelerated (dose dense) methotrexate, vinblastine, doxorubicin and cisplatin (ddMVAC) with G-CSF (4 cycles). We also repeated the analysis with an alternate chemotherapy regimen (gemcitabine/cisplatin [4 cycles]) and as an exploratory analysis with other ICIs (atezolizumab and nivolumab/ipilimumab).
The doses and schedules for ICIs were based on clinical trials (PURE-017 for pembrolizumab, ABACUS8 for atezolizumab and NABUCCO10 for nivolumab/ipilimumab), and for chemotherapy based on phase 2 trials.23,24 We assumed that all patients receiving ddMVAC were treated with G-CSF and 10% of patients receiving GC were treated with G-CSF. Since none of the neoadjuvant ICI trials have specifically reported RFS or OS (and we were unable to attain despite reaching out to the investigators of all those trials), we made the assumption that the RFS with ICI would be the same as with chemotherapy depending on pCR status at the time of radical cystectomy.
Outputs from the model include costs and 2-year RFS rate. These outputs are used to calculate an incremental cost-effectiveness ratio (ICER) of cost per 2-year recurrence saved. The perspective assumed for this analysis was that of a third-party payer. The time horizon was two years. Given the short time horizon, no discounting was performed. The 2-year RFS was selected as a clinically meaningful endpoint based on available retrospective data25 as well as the selection of RFS as an endpoint in many currently ongoing randomized phase 3 trials.
2.2. Analytic approach
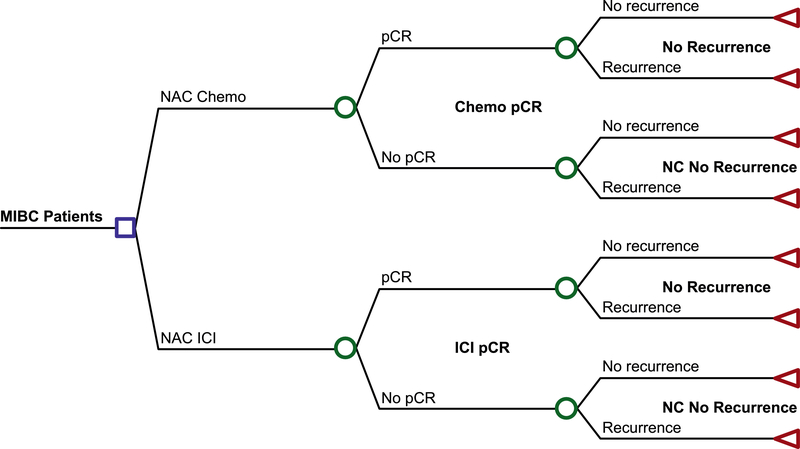
We used a decision analytic model to estimate health outcomes and costs (Figure 1). The first node of the decision tree is the presence of pCR at the time of radical cystectomy and the second node is for 2-year RFS. Table 1 shows the values and inputs for the probabilities in the decision tree. The probability of pCR with ICI was derived from the pCR rate from the respective ICI trials. For chemotherapy, the pCR rate for GC was derived from a prior meta-analysis26 comparing neoadjuvant chemotherapy regimens from MIBC and for ddMVAC based on a weighted average from two prior trials23,24. The probability for RFS was derived from a weighted average of the pCR stratified 2-year RFS reported by two ddMVAC trials with available data23,24. The study was designed and reported in accordance with the CHEERS guidelines.27
Figure 1.
Decision tree used for cost-effectiveness analysis comparing neoadjuvant ICI to neoadjuvant cisplatin-based chemotherapy
Table 1.
Model probability input parameters (with one-way sensitivity analysis range) and data source
| Parameter | Probability | Lower bound OWSA | Upper bound OWSA | Source |
|---|---|---|---|---|
| pCR with ddMVAC | 0.30 | 0.20 | 0.41 | Choueiri et al.23 and Plimack et al.24 |
| pCR with GC | 0.26 | 0.23 | 0.28 | Yin et al.26 |
| pCR with pembrolizumab (PURE-01) | 0.37 | 0.28 | 0.46 | PURE-017 |
| pCR with atezolizumab (ABACUS) | 0.31 | 0.21 | 0.41 | ABACUS8 |
| pCR with ipi/nivo (NABUCCO) | 0.46 | 0.26 | 0.67 | NABUCCO10 |
| 2-year recurrence free survival if pCR | 0.73 | 0.6 | 0.9 | Choueiri et al.23 and Plimack et al.24 |
| 2-year recurrence free survival if no pCR | 0.51 | 0.4 | 0.6 | Choueiri et al.23 and Plimack et al.24 |
2.3. Cost and resource use
Drug costs were based on the July 2020 Average Sales Price (ASP) published by the Centers for Medicare and Medicaid Services.28 Table 2 shows the estimated cost for each treatment regimen. Chemotherapy doses were calculated based on an average 70kg patient and/or body-surface of 1.86mg/m2, as required for dosing. All patients treated with ddMVAC and 10% treated with GC29 were assumed to receive treatment with granulocyte colony-stimulating factor (G-CSF). For GC, cost was based on a 21-day treatment cycle, but a 28-day cycle was factored into the one-way sensitivity range for this regimen.
Table 2.
Cost inputs for decision tree analytical model (with one-way sensitivity analysis range)
| Regimen | Number of cycles | Cost per neoadjuvant treatment course | Cost with alternate dosing | Lower bound sensitivity range | Upper bound sensitivity range |
|---|---|---|---|---|---|
| ddMVAC + G-CSF | 4 | $22,515 | $16,886 | $28,143 | |
| GC* | 4 | $910 | $765 | $574 | $1,137 |
| Pembroli zumab | 3 | $30,556 | $22,917 | $38,195 | |
| Atezolizu mab | 2 | $18,838 | $14,129 | $23,548 | |
| Nivoluma b/Ipilimu mab | 2x Ipilimumab 3mg/kg + 1x Nivolumab 1mg/kg + 1x Nivolumab 3mg/kg | $74,052 | $55,539 | $92,565 |
Alternate dosing with 3wk cycle instead of 4wk cycle
2.4. Sensitivity analysis
We performed a one-way sensitivity analysis by varying each input to appropriate lower and upper bounds and recording the effect on the model. Table 1 shows the lower and upper bounds used and the source for this range. The 95% confidence interval was used for the lower and upper bounds for the pCR rates in the ICI trials. The pCR range for chemotherapy regimens was derived from a calculated 95% confidence interval for each regimen. The range for RFS was based on the observed range in the contributing studies. Upper and lower bounds for cost were calculated based on 25% range around the calculated cost.
We also did two threshold analyses to estimate the pCR rate required or the cost of rebate required for pembrolizumab to meet a cost per 2-year recurrence of $100,000 as an extrapolation from a regularly used willingness-to-pay threshold of cost per quality adjusted life-year30. Finally, we performed a probabilistic sensitivity analysis (PSA) and the distribution and input for the PSA are reported in Supplemental Table 1.
3. Results:
The base case results are presented in Table 3. The proportion of patients with 2-year RFS was 59.1% and 57.6% with pembrolizumab and ddMVAC, respectively, for a 1.5% improved RFS with pembrolizumab. The cost of neoadjuvant treatment with pembrolizumab was estimated to be $30,556 compared with $22,515 for ddMVAC (incremental cost $8,042). This resulted in an ICER of cost per 2-year recurrence saved with pembrolizumab of $522,143. This ICER would rise to $1,225,058 if pembrolizumab was compared with gemcitabine/cisplatin (GC). Similar results were noted in exploratory analyses comparing nivolumab/ipilimumab with ddMVAC or GC and comparing atezolizumab with GC. However, atezolizumab had an incrementally lower cost of $3,677 and a 0.22% incrementally better 2-year RFS compared with ddMVAC, so it was considered dominant in for this analysis.
Table 3.
Cost-effectiveness analysis results
| Cost | Inc. Cost | Survival | Inc. Survival | ICER (per 2 yr RFS) | |
|---|---|---|---|---|---|
|
| |||||
| Chemotherapy (GC) | $910 | - | 0.5672 | - | |
| Pembrolizumab | $30,556 | $29,646 | 0.5914 | 0.024 | $1,225,058 |
| Atezolizumab | $18,838 | $17,928 | 0.5782 | 0.011 | $1,629,855 |
| Nivolumab + Ipilimumab | $74,052 | $73,142 | 0.6112 | 0.044 | $1,662,327 |
|
| |||||
| Chemo (ddMVAC) | $22,515 | 0.5760 | |||
| Pembrolizumab | $30,556 | $8,042 | 0.5914 | 0.015 | $552,143 |
| Atezolizumab | $18,838 | −$3,677 | 0.5782 | 0.002 | DOMINANT |
| Nivolumab + Ipilimumab | $74,052 | $51,537 | 0.6112 | 0.035 | $1,464,119 |
Abbreviations: GC, gemcitabine/cisplatin; ddMVAC, dose-dense methotrexate, vinblastine, doxorubicin, cisplatin
We performed a threshold analysis and estimated that an ICER of $100,000 per 2-year recurrence would require a 21% reduction in cost compared with ddMVAC and a 89% reduction in cost compared with GC. Alternatively, a pCR rate of 67% with pembrolizumab would also yield an ICER of $100,000 when compared with ddMVAC. However, even a pCR rate of 100% by pembrolizumab would not approach an ICER of $100,000 when compared with GC.
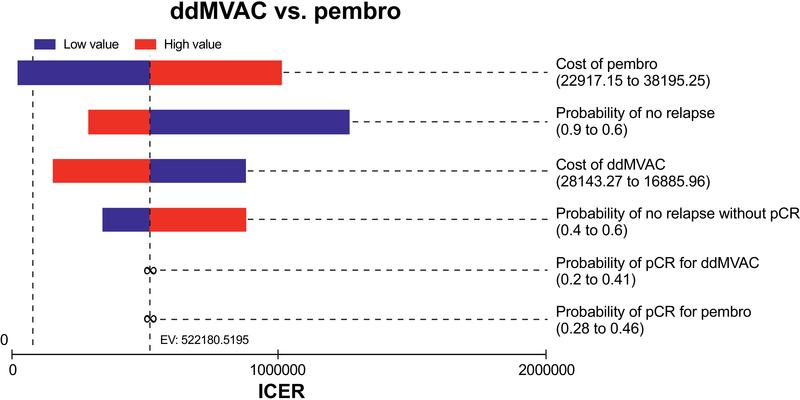
A one-way sensitivity analysis was performed by individually varying each input to the lower and upper bound and recording the output as specified above. Parameters most influential in the primary analysis model were the cost of pembrolizumab, probability of 2-year RFS and cost of ddMVAC (Figure 2). Similar one-way sensitivity analyses were performed for pembrolizumab vs. GC and for each of the other ICI regimens compared with either ddMVAC or GC (supplemental figures 1–5).
Figure 2.
Tornado diagram of the one-way sensitivity analyses for comparison of Pembrolizumab to ddMVAC.
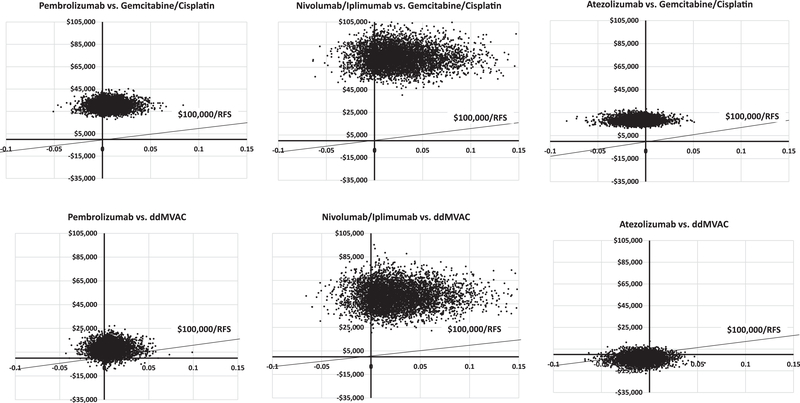
Pembrolizumab was cost-effective 11% of the time with an ICER threshold of $100,000 (Figure 3). Similar results were noted comparing each ICI with GC or ddMVAC, with the exception that—compared with ddMVAC—atezolizumab was cost-effective in 82% of iterations (Figure 3).
Figure 3.
Probabilistic sensitivity analysis incremental cost-effectiveness scatterplot for each cost-effectiveness analysis performed between neoadjuvant immune checkpoint inhibitors (pembrolizumab, atezolizumab or nivolumab/ipilimumab) and cisplatin-based chemotherapy (ddMVAC or gemcitabine/cisplatin)
4. Discussion:
As the most costly cancer from diagnosis to death on a per patient basis31, costs associated with bladder cancer care are substantial. Economic models estimate costs will be upwards of $6 billion (for all ages and all phases of bladder cancer) in 202032. However, these models do not account for the recent introduction of ICIs into the treatment landscape. We evaluated the cost-effectiveness of neoadjuvant ICI compared with standard of care CBC in patients with MIBC and found that pembrolizumab would have an ICER over $500,000 per 2-year RFS.
Despite the higher cost of ICIs relative to CBC, pembrolizumab was previously shown to be cost-effective relative to carboplatin-based chemotherapy at a willingness-to-pay threshold of €100,000 in the Swedish healthcare system for the treatment of advanced urothelial carcinoma in cisplatin-ineligible patients.33 However, pembrolizumab or other ICIs were not cost-effectiveness in our modeling in the neoadjuvant setting. A notable exception was that atezolizumab was dominant when compared with ddMVAC. Based on our modeling, the cost of atezolizumab was $3,677 lower than ddMVAC and the 2-year RFS was marginally better, suggested this could be a cost-effective treatment option. Though, atezolizumab has not been evaluated and shown to be more effective than ddMVAC in a randomized trial, a necessary step to confirm effectiveness. In addition, it also was not cost effective when compared with GC. The lower cost estimated with atezolizumab was driven by fewer doses of atezolizumab administered (two doses) in the ABACUS trial than when compared with both chemotherapy and other ICIs. Despite the fewer doses, the efficacy with atezolizumab, as measured by pCR, was similar to that observed with other agents. This may be an appealing option that can be considered in future ICI trials.
It is notable that the cost of chemotherapy with ddMVAC was substantially higher than GC. This was largely due to the high cost of G-CSF needed with ddMVAC. It is possible that biosimilar forms of G-CSF will improve the affordability, though the early data suggests these remain similarly costly.34 Currently, either GC or ddMVAC is used as neoadjuvant chemotherapy, in the absence of a definitive, adequately powered, published randomized phase III trial comparing these two regimens in the neoadjuvant setting. Most (but not all) retrospective series have not noted significant differences in pCR rates between regimens.35 A prior meta-analysis also suggested that GC and MVAC have similar results, but this study did not include ddMVAC.26 Two ongoing trials have reported mixed preliminary results. In the GETUC/AFU V05 VESPER phase III trial comparing these two regimens, ddMVAC had higher pCR rates than GC (42% vs 36%).36 However, in the SWOG 1314 COXEN trial, pCR rates were similar (32% with ddMVAC and 35% GC), though this was not the primary objective of this trial.37
An appealing feature of ICIs in the neoadjuvant setting is the favorable toxicity profile, which may enable more patients with MIBC to receive neoadjuvant therapy. Prior studies have estimated that fewer than 50% of patients are treated with neoadjuvant cisplatin-based chemotherapy prior to radical cystectomy.38 One significant barrier to receipt of neoadjuvant chemotherapy is eligibility for cisplatin.21 Therefore, ICIs may have a role in patients who either refuse (despite proper counseling) or are ineligible for cisplatin-based chemotherapy. Both the ABACUS and NABUCCO trials included in our exploratory analysis enrolled patients who were cisplatin-ineligible or refused CBC, which is a different population; this can certainly limit direct comparisons in our analysis. This is also relevant to the fact that ongoing randomized phase III trials with ICIs (alone or in combination), without chemotherapy, involve only cisplatin-ineligible patients. Moreover, ongoing randomized phase III trials in cisplatin-eligible patients are comparing CBC plus ICI to CBC alone, and do not include ICI alone. The results of our analysis further support the latter point.
Notably, our current model does not factor in toxicity related to therapy. Without this consideration, it is also possible that our model overestimates the value of CBC given the generally higher toxicity of chemotherapy relative to ICIs.39 However, while toxicity related to ICIs is less prevalent and less severe, immune-related adverse events can still cause significant morbidity and can be costly.40 Similarly, in the PURE-01 trial there were five patients (10%) initially treated with pembrolizumab who subsequently received CBC (due to toxicity or inadequate response to ICI) prior to radical cystectomy. None of these patients achieved pCR, so they are unlikely to introduce bias into our model. Despite promising preliminary results with ICIs, more mature data and randomized phase 3 trials are needed to confirm their clinical benefit in the neoadjuvant or peri-operative setting. Currently, there are five ongoing phase 3 trials investigating ICIs in cisplatin-eligible (NIAGARA14, ENERGIZE15, KEYNOTE-86616) and cisplatin-ineligible patients (KEYNOTE-905/EV-30317, NCT0420911418). As these agents continue to make their way through development and regulatory consideration, a relevant parameter is also the cost of therapy. The rising cost of cancer care continues to be a significant area of concern for patients and payers in the US and around the world. This concern has led to the development of value frameworks by the American Society of Clinical Oncology, the National Comprehensive Cancer Network, Memorial Sloan Kettering Cancer Center, European Society of Medical Oncology, and the Institute for Clinical and Economic Review.
One proposed approach to address the rising cost of cancer care is the use of value-based pricing to adjust the price of treatment to meet the benefit measured. Here, cost-effectiveness analyses can help inform the value and pricing accordingly. In our study, we used a threshold analysis to identify that pembrolizumab would potentially approach an acceptable cost-effective threshold (compared with ddMVAC) with a 21% rebate from the base price input into our model and a heftier 89% rebate compared with GC. Though, this analysis assumes that willingness-to-pay thresholds for cost per quality adjusted life year would be the same for our model output as cost per 2-year RFS.
Our findings need to be interpreted within the context of the study design. We used a model-based analysis that used early (mostly single arm phase II) clinical trial results and average sales price for drug costs without real-world data. In addition, the different comparator arms in our model are not from the same clinical trial. Thus, we made comparisons across clinical trials, with heterogeneous populations (cisplatin-eligible vs. cisplatin-ineligible), which can introduce selection and confounding biases. However, most trials enrollees were similar in many of the clinical and tumor characteristics. We also extrapolated RFS for ICI based on chemotherapy trials, as there is no mature RFS data available. We also could not analyze data from all the available trials due to absence of data granularity. Lastly, we did not include additional “micro-costs” associated with other factors, e.g. missed work, patient transportation and lodging, infusion, professional and facility fees, or diagnosis and management of therapy-related adverse events. Given the substantial costs associated with ICIs and ICERs calculated, these additional cost considerations would likely not impact our findings. Despite these inherent limitations, we present the first cost-effectiveness analysis comparing neoadjuvant ICI vs CBC for localized resectable MIBC. These data can create a relevant literature context and certainly inform discussions, among several stakeholders, about drug development and pricing, as these agents continue to be investigated in large randomized phase III trials.
5. Conclusion
In summary, although ICIs are very promising agents for neoadjuvant treatment of MIBC, they remain costly. Price rebates and/or other approaches to reduce the price may help render these agents cost-effective for future use in the neoadjuvant setting. Randomized clinical trials and “pragmatic real-world studies” can provide additional datasets for future cost-effectiveness analyses, which can be relevant in our financially challenged healthcare systems.
Supplementary Material
Supplemental figure 1. One-way sensitivity analysis for comparison of pembrolizumab to gemcitabine/cisplatin.
Supplemental figure 2. One-way sensitivity analysis for comparison of atezolizumab to ddMVAC.
Supplemental figure 3. One-way sensitivity analysis for comparison of atezolizumab to gemcitabine/cisplatin.
Supplemental figure 4. One-way sensitivity analysis for comparison of nivolumab/ipilimumab to ddMVAC.
Supplemental figure 5. One-way sensitivity analysis for comparison of nivolumab/ipilimumab to gemcitabine/cisplatin.
ACKNOWLEDGEMENTS:
ARK was supported by the National Cancer Institute under training grant, award #T32CA009515. PG acknowledges the support from Seattle Translational Tumor Research Program at the Fred Hutchinson Cancer Research Center and Kure It Cancer Research. SBW was supported by a Department of Defense Peer Reviewed Cancer Research Program (PRCRP) Career Development Award (W81XWH1710576). SK unfortunately passed away during the manuscript preparation and all authors acknowledge her valuable contributions to the present study.
FUNDING STATEMENT: No external funding was used for this study aside from that which is stated in the acknowledgements of the manuscript.
Footnotes
DISCLOSURES:
Dr. P Grivas (all unrelated in the last 3 years):
Consulting: AstraZeneca; Bayer; Bristol-Myers Squibb; Clovis Oncology; Dyania Health, Driver; EMD Serono; Exelixis; Foundation Medicine; Genentech/Roche; Genzyme; GlaxoSmithKline; Heron Therapeutics; Immunomedics, Janssen; Merck; Mirati Therapeutics; Pfizer; Seattle Genetics; QED Therapeutics.
Research Funding to Institution: AstraZeneca, Bayer; Genentech/Roche; Merck; Mirati Therapeutics; Oncogenex; Pfizer, Clovis Oncology, Bavarian Nordic, Immunomedics, Debiopharm, Bristol-Myers Squibb, QED Therapeutics, GlaxoSmithKline, Mirati Therapeutics, Kure It Cancer Research.
Dr S. Williams:
Consulting: Photocure, Digital Science
Travel: Janssen
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
6. References:
- 1.Bladder Cancer: Non-Muscle Invasive Guideline - American Urological Association. Accessed August 3, 2020. https://www.auanet.org/guidelines/bladder-cancer-non-muscle-invasive-guideline
- 2.Stein JP, Lieskovsky G, Cote R, et al. Radical Cystectomy in the Treatment of Invasive Bladder Cancer: Long-Term Results in 1,054 Patients. Journal of Clinical Oncology. 2001;19(3):666–675. doi: 10.1200/JCO.2001.19.3.666 [DOI] [PubMed] [Google Scholar]
- 3.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant Chemotherapy plus Cystectomy Compared with Cystectomy Alone for Locally Advanced Bladder Cancer. New England Journal of Medicine. 2003;349(9):859–866. doi: 10.1056/NEJMoa022148 [DOI] [PubMed] [Google Scholar]
- 4.Gopalakrishnan D, Koshkin VS, Ornstein MC, Papatsoris A, Grivas P. Immune checkpoint inhibitors in urothelial cancer: recent updates and future outlook. Ther Clin Risk Manag. 2018;14:1019–1040. doi: 10.2147/TCRM.S158753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as Second-Line Therapy for Advanced Urothelial Carcinoma. New England Journal of Medicine. 2017;376(11):1015–1026. doi: 10.1056/NEJMoa1613683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powles T, Park SH, Voog E, et al. Maintenance avelumab + best supportive care (BSC) versus BSC alone after platinum-based first-line (1L) chemotherapy in advanced urothelial carcinoma (UC): JAVELIN Bladder 100 phase III interim analysis. JCO. 2020;38(18_suppl):LBA1–LBA1. doi: 10.1200/JCO.2020.38.18_suppl.LBA1 [DOI] [Google Scholar]
- 7.Necchi A, Anichini A, Raggi D, et al. Pembrolizumab as Neoadjuvant Therapy Before Radical Cystectomy in Patients With Muscle-Invasive Urothelial Bladder Carcinoma (PURE-01): An Open-Label, Single-Arm, Phase II Study. JCO. 2018;36(34):3353–3360. doi: 10.1200/JCO.18.01148 [DOI] [PubMed] [Google Scholar]
- 8.Powles T, Kockx M, Rodriguez-Vida A, et al. Clinical efficacy and biomarker analysis of neoadjuvant atezolizumab in operable urothelial carcinoma in the ABACUS trial. Nat Med. 2019;25(11):1706–1714. doi: 10.1038/s41591-019-0628-7 [DOI] [PubMed] [Google Scholar]
- 9.Grande E, Guerrero F, Puente J, et al. DUTRENEO Trial: A phase II randomized trial of DUrvalumab and TREmelimumab as NEOadjuvant approach in muscle-invasive urothelial bladder cancer (MIBC) patients prospectively selected by immune signature scores. JCO. 2019;37(15_suppl):TPS4588–TPS4588. doi: 10.1200/JCO.2019.37.15_suppl.TPS4588 [DOI] [Google Scholar]
- 10.van Dijk N, Gil-Jimenez A, Silina K, et al. Preoperative ipilimumab plus nivolumab in locoregionally advanced urothelial cancer: the NABUCCO trial. Nature Medicine. Published online October 12, 2020:1–6. doi: 10.1038/s41591-020-1085-z [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Navai N, Alhalabi O, et al. Neoadjuvant PD-L1 plus CTLA-4 blockade in patients with cisplatin-ineligible operable high-risk urothelial carcinoma. Nature Medicine. Published online October 12, 2020:1–7. doi: 10.1038/s41591-020-1086-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta S, Sonpavde G, Weight CJ, et al. Results from BLASST-1 (Bladder Cancer Signal Seeking Trial) of nivolumab, gemcitabine, and cisplatin in muscle invasive bladder cancer (MIBC) undergoing cystectomy. JCO. 2020;38(6_suppl):439–439. doi: 10.1200/JCO.2020.38.6_suppl.439 [DOI] [Google Scholar]
- 13.Hoimes CJ, Albany C, Hoffman-Censits J, et al. LBA33A phase Ib/II study of neoadjuvant pembrolizumab (pembro) and chemotherapy for locally advanced urothelial cancer (UC). Ann Oncol. 2018;29(suppl_8). doi: 10.1093/annonc/mdy424.039 [DOI] [Google Scholar]
- 14.AstraZeneca. A Phase III, Randomized, Open-Label, Multi-Center, Global Study to Determine the Efficacy and Safety of Durvalumab in Combination With Gemcitabine+Cisplatin for Neoadjuvant Treatment Followed by Durvalumab Alone for Adjuvant Treatment in Patients With Muscle-Invasive Bladder Cancer. clinicaltrials.gov; 2021. Accessed January 19, 2021. https://clinicaltrials.gov/ct2/show/NCT03732677 [Google Scholar]
- 15.Sonpavde G, Necchi A, Gupta S, et al. ENERGIZE: a Phase III study of neoadjuvant chemotherapy alone or with nivolumab with/without linrodostat mesylate for muscle-invasive bladder cancer. Future Oncol. 2020;16(2):4359–4368. doi: 10.2217/fon-2019-0611 [DOI] [PubMed] [Google Scholar]
- 16.Merck Sharp & Dohme Corp. A Phase 3, Randomized, Double-Blind Study to Evaluate Perioperative Pembrolizumab (MK-3475) + Neoadjuvant Chemotherapy Versus Perioperative Placebo + Neoadjuvant Chemotherapy in Cisplatin-Eligible Participants With Muscle-Invasive Bladder Cancer (KEYNOTE-866). clinicaltrials.gov; 2020. Accessed January 19, 2021. https://clinicaltrials.gov/ct2/show/NCT03924856 [Google Scholar]
- 17.Merck Sharp & Dohme Corp. A Randomized Phase 3 Study Evaluating Cystectomy With Perioperative Pembrolizumab and Cystectomy With Perioperative Enfortumab Vedotin and Pembrolizumab Versus Cystectomy Alone in Cisplatin-Ineligible Participants With Muscle-Invasive Bladder Cancer (KEYNOTE-905/EV-303). clinicaltrials.gov; 2021. Accessed January 19, 2021. https://clinicaltrials.gov/ct2/show/NCT03924895 [Google Scholar]
- 18.Bristol-Myers Squibb. A Phase 3, Randomized, Study of Neoadjuvant and Adjuvant Nivolumab Plus NKTR-214, Versus Nivolumab Alone Versus Standard of Care in Participants With Muscle-Invasive Bladder Cancer (MIBC) Who Are Cisplatin Ineligible. clinicaltrials.gov; 2020. Accessed January 19, 2021. https://clinicaltrials.gov/ct2/show/NCT04209114 [Google Scholar]
- 19.Williams SB, Shan Y, Ray-Zack MD, et al. Comparison of Costs of Radical Cystectomy vs Trimodal Therapy for Patients With Localized Muscle-Invasive Bladder Cancer. JAMA Surg. 2019;154(8):e191629. doi: 10.1001/jamasurg.2019.1629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams SB, Shan Y, Jazzar U, et al. Comparing Survival Outcomes and Costs Associated With Radical Cystectomy and Trimodal Therapy for Older Adults With Muscle-Invasive Bladder Cancer. JAMA Surg. 2018;153(10):881–889. doi: 10.1001/jamasurg.2018.1680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Galsky MD, Hahn NM, Rosenberg J, et al. Treatment of Patients With Metastatic Urothelial Cancer “Unfit” for Cisplatin-Based Chemotherapy. JCO. 2011;29(17):2432–2438. doi: 10.1200/JCO.2011.34.8433 [DOI] [PubMed] [Google Scholar]
- 22.Koshkin VS, Barata PC, Rybicki LA, et al. Feasibility of Cisplatin-Based Neoadjuvant Chemotherapy in Muscle-Invasive Bladder Cancer Patients With Diminished Renal Function. Clin Genitourin Cancer. 2018;16(4):e879–e892. doi: 10.1016/j.clgc.2018.02.002 [DOI] [PubMed] [Google Scholar]
- 23.Choueiri TK, Jacobus S, Bellmunt J, et al. Neoadjuvant Dose-Dense Methotrexate, Vinblastine, Doxorubicin, and Cisplatin With Pegfilgrastim Support in Muscle-Invasive Urothelial Cancer: Pathologic, Radiologic, and Biomarker Correlates. Journal of Clinical Oncology. 2014;32(18):1889–1894. doi: 10.1200/JCO.2013.52.4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Plimack ER, Hoffman-Censits JH, Viterbo R, et al. Accelerated Methotrexate, Vinblastine, Doxorubicin, and Cisplatin Is Safe, Effective, and Efficient Neoadjuvant Treatment for Muscle-Invasive Bladder Cancer: Results of a Multicenter Phase II Study With Molecular Correlates of Response and Toxicity. JCO. 2014;32(18):1895–1901. doi: 10.1200/JCO.2013.53.2465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonpavde G, Khan MM, Lerner SP, et al. Disease-free survival at 2 or 3 years correlates with 5-year overall survival of patients undergoing radical cystectomy for muscle invasive bladder cancer. The Journal of urology. 2011;185(2):456–461. doi: 10.1016/j.juro.2010.09.110 [DOI] [PubMed] [Google Scholar]
- 26.Yin M, Joshi M, Meijer RP, et al. Neoadjuvant Chemotherapy for Muscle-Invasive Bladder Cancer: A Systematic Review and Two-Step Meta-Analysis. The Oncologist. 2016;21(6):708–715. doi: 10.1634/theoncologist.2015-0440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Husereau D, Drummond M, Petrou S, et al. Consolidated Health Economic Evaluation Reporting Standards (CHEERS) statement. Eur J Health Econ. 2013;14(3):367–372. doi: 10.1007/s10198-013-0471-6 [DOI] [PubMed] [Google Scholar]
- 28.Medicare Part B Drug Average Sales Price | CMS. Accessed August 4, 2020. https://www.cms.gov/Medicare/Medicare-Fee-for-Service-Part-B-Drugs/McrPartBDrugAvgSalesPrice
- 29.Robinson P, von der Maase H, Bhalla S, et al. Cost-utility analysis of the GC versus MVAC regimens for the treatment of locally advanced or metastatic bladder cancer. Expert Rev Pharmacoecon Outcomes Res. 2004;4(1):27–38. doi: 10.1586/14737167.4.1.27 [DOI] [PubMed] [Google Scholar]
- 30.Hirth RA, Chernew ME, Miller E, Fendrick AM, Weissert WG. Willingness to Pay for a Quality-adjusted Life Year: In Search of a Standard. Med Decis Making. 2000;20(3):332–342. doi: 10.1177/0272989X0002000310 [DOI] [PubMed] [Google Scholar]
- 31.Mossanen M, Gore JL. The burden of bladder cancer care: direct and indirect costs. Current Opinion in Urology. 2014;24(5):487–491. doi: 10.1097/MOU.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 32.Mariotto AB, Robin Yabroff K, Shao Y, Feuer EJ, Brown ML. Projections of the Cost of Cancer Care in the United States: 2010–2020. J Natl Cancer Inst. 2011;103(2):117–128. doi: 10.1093/jnci/djq495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Patterson K, Prabhu V, Xu R, et al. Cost-effectiveness of Pembrolizumab for Patients with Advanced, Unresectable, or Metastatic Urothelial Cancer Ineligible for Cisplatin-based Therapy. European Urology Oncology. 2019;2(5):565–571. doi: 10.1016/j.euo.2018.09.009 [DOI] [PubMed] [Google Scholar]
- 34.Chen X, Agiro A, Barron J, Debono D, Fisch M. Early Adoption of Biosimilar Growth Factors in Supportive Cancer Care. JAMA Oncol. 2018;4(12):1779–1781. doi: 10.1001/jamaoncol.2018.5090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patel VG, Oh WK, Galsky MD. Treatment of muscle-invasive and advanced bladder cancer in 2020. CA: A Cancer Journal for Clinicians. n/a(n/a). doi: 10.3322/caac.21631 [DOI] [PubMed] [Google Scholar]
- 36.Culine S, Gravis G, Flechon A, et al. Randomized phase III trial of dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin (dd-MVAC) or gemcitabine and cisplatin (GC) as perioperative chemotherapy for muscle invasive urothelial bladder cancer (MIUBC): Preliminary results of the GETUG/AFU V05 VESPER trial on toxicity and pathological responses. JCO. 2020;38(6_suppl):437–437. doi: 10.1200/JCO.2020.38.6_suppl.437 [DOI] [Google Scholar]
- 37.Flaig TW, Tangen CM, Daneshmand S, et al. SWOG S1314: A randomized phase II study of co-expression extrapolation (COXEN) with neoadjuvant chemotherapy for localized, muscle-invasive bladder cancer. JCO. 2019;37(15_suppl):4506–4506. doi: 10.1200/JCO.2019.37.15_suppl.4506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Booth CM, Karim S, Brennan K, Siemens DR, Peng Y, Mackillop WJ. Perioperative chemotherapy for bladder cancer in the general population: Are practice patterns finally changing? Urol Oncol. 2018;36(3):89.e13–89.e20. doi: 10.1016/j.urolonc.2017.11.015 [DOI] [PubMed] [Google Scholar]
- 39.Nishijima TF, Shachar SS, Nyrop KA, Muss HB. Safety and Tolerability of PD-1/PD-L1 Inhibitors Compared with Chemotherapy in Patients with Advanced Cancer: A Meta-Analysis. Oncologist. 2017;22(4):470–479. doi: 10.1634/theoncologist.2016-0419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grivas P, DerSarkissian M, Shenolikar R, Laliberté F, Doleh Y, Duh MS. Healthcare resource utilization and costs of adverse events among patients with metastatic urothelial cancer in USA. Future Oncology. Published online October 9, 2019. doi: 10.2217/fon-2019-0434 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental figure 1. One-way sensitivity analysis for comparison of pembrolizumab to gemcitabine/cisplatin.
Supplemental figure 2. One-way sensitivity analysis for comparison of atezolizumab to ddMVAC.
Supplemental figure 3. One-way sensitivity analysis for comparison of atezolizumab to gemcitabine/cisplatin.
Supplemental figure 4. One-way sensitivity analysis for comparison of nivolumab/ipilimumab to ddMVAC.
Supplemental figure 5. One-way sensitivity analysis for comparison of nivolumab/ipilimumab to gemcitabine/cisplatin.