Abstract
Despite the prominence of self-efficacy as a predictor of antiretroviral therapy (ART) adherence, relatively little work has examined domain-specific associations with steps in the care continuum or the possibility that substance use may have domain-specific associations with self-efficacy. This study analyzed data from a sample of 174 people living with HIV recruited through three clinics in the New York City metro area. Consistent with hypotheses, path analysis showed that appointments kept and viral load were each predicted only by their respective domain-specific self-efficacy components (i.e., self-efficacy for keeping appointments, B = 0.01, p = .04; and self-efficacy for taking ART medications, B = −0.02, p <.01). Path models also indicated domain-specific associations with substance use. Self-efficacy for keeping appointments was negatively associated with severity of drug use (B = −1.81, p < .01); meanwhile, self-efficacy for taking ART medications was negatively associated with severity of alcohol use (B = −0.52, p < .01). Accordingly, studies assessing barriers to retention in the HIV care continuum should conduct multi-domain assessments of self-efficacy for differential associations with specific behaviors. Furthermore, HIV care providers might consider screening for domain-specific self-efficacy to identify patients at risk of drop-out and tailoring interventions to various care continuum domains.
Keywords: self-efficacy, substance use, HIV care cascade, retention in care, viral suppression
Introduction
A range of socio-structural and individual-level factors have been linked to declines in engagement across the HIV care continuum, from diagnosis through linkage to care (CDC, 2018), provision of antiretroviral therapy (ART), adherence to an ART regimen, and ultimately viral suppression (Gardner et al., 2011). Rates of engagement decline at each stage (Nachega et al., 2014). In the U.S. in 2015, an estimated 86% of people living with HIV (PLWH) were diagnosed, 63% connected to care, 43% retained in care, and 51% virally suppressed (CDC, 2018), with markedly lower rates of engagement among younger, Black, Latinx, and female PLWH (Colasanti et al., 2016; Gant et al., 2014; Sangaramoorthy, et al., 2019).
This study ultimately focuses on two individual-level factors (self-efficacy and substance use) associated with the care continuum. These are situated within socio-structural factors that can disrupt retention, such as access and proximity to care, experiences of stigma, discrimination within health care settings and in wider society, other life demands, and neighborhood characteristics (Flores et al., 2016; Saha et al., 2013; Tieu et al., 2018). For example, Black men who reported more experiences of racism, HIV-related discrimination, and sexual orientation-based discrimination had poorer HIV-related outcomes (Bogart et al., 2010). Further, in a Canadian study of transgender women, factors such as transphobia, HIV-related stigma, and housing insecurity were prominent in accounts of lower engagement in care (Lacombe-Duncan et al., 2019).
Self-efficacy features prominently in health behavior theories (Bandura, 1977). The construct describes individuals’ expectations concerning the likelihood of their successfully enacting a chosen course of action (Maddux, 2016). The importance of self-efficacy in HIV care is illustrated in a meta-analysis of 207 studies reporting on 103,836 PLWH, in which adherence self-efficacy was the variable most strongly associated with ART adherence (Langebeek, 2014).
While self-efficacy has been widely studied, it is often examined as an aggregate or global construct, which may weaken associations with specific outcomes (Strecher, 1986). Domain-specific aspects of self-efficacy may be more informative for considering specific behaviors relating to discrete stages of the care continuum. Self-efficacy has shown reliable associations with treatment adherence independently (Brown, et al., 2013; Colbert et al., 2013; Lee et al., 2016) and alongside socio-cognitive variables such as social support (Cha, 2008; Turan et al., 2016). In one of the few studies to examine domain specific self-efficacy, provider cultural competence was linked to racial and ethnic disparities in self-efficacy for ART adherence among PLWH (Saha, 2013).
Substance use (i.e., drugs and alcohol) is prevalent among PLWH in developed nations and is the primary predictor of dropout from the care continuum (Bulsara et al., 2018). (Hartzler et al., 2017). Habitual use of illegal substances is associated with poor treatment retention among PLWH (Giordano et al., 2009; Rebeiro et al., 2013; Tobias et al., 2007). Alcohol use is similarly detrimental to outcomes for PLWH (Barai et al., 2017; Williams et al., 2019). One meta-analysis estimated that those PLWH who reported regular heavy alcohol use were 45% less likely to be classified as adherent compared to non-users (Hendershot et al., 2009). As in studies of self-efficacy, alcohol’s effects have been investigated primarily in relation to ART adherence (Vagenas et al., 2015).
The few studies examining both substance use and self-efficacy as they relate to continuity of HIV care have tended to examine their effects independently. A recent longitudinal study found that self-efficacy did not differentiate patients retained versus unretained over a 6-year period, while substance use in any quantity was linked to attrition (Colasanti et al., 2016). In contrast, there is evidence to suggest that the quantity of drinking, regardless of the severity of personal problems attributable to alcohol misuse, may undermine adherence (Parsons, Rosof, & Mustanski, 2007). To date, no study has examined the role of substance use on care retention through the mechanism of self-efficacy.
The purpose of the current study was to test associations between domain-specific self-efficacy and two key components of the HIV care continuum (retention in care and viral suppression). We also tested the possibility that domain specific self-efficacy may be involved in indirect pathways linking substance use to cascade outcomes. We hypothesized that domain-relevant aspects of self-efficacy (i.e., confidence in ability to keep appointments for HIV care; and confidence in ART adherence) would predict medical visits and HIV viral load. We further hypothesized that severity of substance use would be negatively associated with both domains of self-efficacy. Finally, it was hypothesized that substance use severity would be indirectly linked to HIV care continuum outcomes through self-efficacy.
Methods
Participants and procedure
The Positive Living through Understanding and Support (PLUS) study recruited participants at three hospital-based HIV outpatient clinics in the New York City area between March 2015 and May 2017. The larger goal of the study was to evaluate the effectiveness of a cognitive behavioral and motivational interviewing intervention to reduce substance use and improve HIV-related outcomes for PLWH (Parsons et al., 2007). The present analyses utilized baseline data.
Potential participants were identified through a prescreening review of patient EMR, which indicated patients with scheduled clinic visits who had at least one detectable viral load result within the past year. Study staff approached these patients during clinic visits to introduce the study. Participants provided verbal consent for screening and provided written informed consent if eligible and interested in enrolling. They also granted permission for the study team to access their EMR data.
Participants were eligible if they were: (1) at least 18 years old, (2) living with HIV, (3) English-speaking, (4) active patients at the clinic, (5) currently prescribed an ART regimen, but (6) had a viral load documented in the medical record of more than 200 copies/mL within the past 12 months, (7) endorsed drinking at hazardous levels (exceeding 14 standard drinks per week for men or 7 per week for women within the past 3 months), and/or (8) reported use of illicit drugs exclusive of marijuana or illicit use of prescription opioids within the past 3 months. The full protocol was approved by the institutional review boards at Hunter College and Mt. Sinai.
Measures
Demographic information.
This questionnaire captured information on age, education level, employment status, race and ethnicity, relationship status, sexual orientation, and years since HIV diagnosis.
Alcohol Use Disorders Identification Test (AUDIT).
The AUDIT is a 10-item, widely-used screening questionnaire consisting of three questions related to drinking frequency, three questions on dependence, and four on problems caused by alcohol over the past three months (Saunders et al., 1993). Scores range from 0 to 40, with higher scores indicating greater alcohol use. The AUDIT has strong psychometric properties (de Meneses-Gaya et al., 2009), and in the current study displayed strong internal consistency, Cronbach’s α = 0.88.
Drug Abuse Screening Test (DAST)-10.
This measure was used to characterize a participant’s drug use and consequences in the past three months (Skinner, 1982), a widely-used and validated measure (Yudko et al., 2007). Participants responded yes or no to items such as “Are you always able to stop using drugs when you want to?”. Scores on the DAST-10 range from 1 to 10 with higher scores indicating greater drug use and associated problems. In the current study, the scale displayed acceptable internal consistency, Cronbach’s α = 0.78.
Biological indicators of immune functioning.
Standard of care HIV-1 viral load were run using local laboratories and results were abstracted from EMR data, indicating the participant’s most recent viral load result.
Primary Care Appointment Data.
In addition to viral load data gathered through the EMR, the number of kept appointments for Primary Care over the past 12 months were gathered through the sites’ scheduling system.
Domain-Specific Self-Efficacy.
Two items utilizing a visual analogue rating scale (VAS; Aitken, 1969) were used by participants to rate their self-efficacy regarding specific domains on a scale from 0 to 100%, with higher scores indicating greater self-efficacy. Participants used a slider on a horizontal line to “… show how much you are in agreement with the statement: I feel confident in my ability to…” “take my HIV medications as prescribed” and “attend my doctor visits as scheduled.” VAS-style items have been used to measure mood (Aitken, 1969), distress (Celia & Perry, 1986), and pain (Huskisson, 1974), as well as behaviors such as adherence (e.g. HIV medications; Johnson et al., 2007), beliefs (e.g., perceptions of complexity of medication regimens; Erlen et al., 2010), and alcohol-related attitudes (Spiller et al., 2006). Measurement of self-efficacy by a single-item VAS has occurred for a range of behaviors: self-efficacy for exercising (Bergström et al., 2015), self-efficacy for performing resuscitation skills (Turner et al., 2008), self-efficacy in doctors for their ability to recognize and treat exacerbations in patients (Simpson & Jones, 2013).
Analytic Plan
Path analyses were conducted using Mplus v8.0 (Muthén & Muthén, 2017). Separate models were specified for each outcome. In each, the care continuum outcome was predicted by self-efficacy measures (for keeping HIV care appointments and for ART adherence). Self-efficacy measures were then predicted by measures of substance use severity (DAST and AUDIT). Models for each endogenous variable also adjusted for demographic characteristics including age, gender and sexual orientation, and race and ethnicity.
Following log transformation, viral load was normally distributed. Indirect effects in models predicting log viral load were therefore tested using bootstrapping estimation of standard errors and 95% confidence intervals. The number of appointments kept in the previous year was a negative binomially distributed count variable. This precluded the use of bootstrapping. Instead, a model constraint–in which the product of the two constituent direct effects was constrained to be zero–was tested. This test compares the fit of a model with the constraint to one without it. A significant Wald χ2 test indicates that the constraint significantly diminished model fit and represents evidence that the indirect effect is statistically significant.
Results
Staff completed 555 study screenings with 529 unique clinic patients. Of those screened, 191 patients were found to be eligible and, of these, 174 enrolled. As summarized in Table 1, the majority of participants identified as either heterosexual male (27.6%) or sexual-minority male (53.4%) and Black (54%), and roughly half reported having some college education or more (51.7%). The average age was 44.7 years (SD = 11.5), and participants reported an average of 15.1 (SD = 9.1) years living with HIV. Average AUDIT and DAST scores were 10.84 (SD = 9.61), and 3.74 (SD = 2.65), respectively.
Table 1.
Demographic Characteristics
| Overall |
|
|---|---|
| n (%) | |
| Total | 174 (100.0) |
| Race and Ethnicity | |
| Black | 94 (54.0) |
| Latino | 41 (23.6) |
| White | 18 (10.3) |
| Other | 21 (12.1) |
| Education | |
| High School Degree/GED or less | 84 (48.3) |
| Some College or more | 90 (51.7) |
| Gender and Sexual Identity | |
| Heterosexual males | 48 (27.6) |
| Gay or bisexual males | 93 (53.4) |
| Cisgender women | 28 (16.1) |
| Transgender women | 5 (2.9) |
| M (SD) |
|
| Age | 44.7 (11.5) |
As displayed in Table 2, Pearson’s r indicated that self-efficacy for medication adherence was positively associated with self-efficacy for keeping appointments, and DAST scores were positively associated with AUDIT scores. DAST scores were negatively associated self-efficacy for keeping appointments but not self-efficacy for medication adherence. DAST scores were also negatively associated with log viral load and unrelated to appointments kept. AUDIT scores were negatively associated with self-efficacy for medication adherence, but not with self-efficacy for keeping appointments. Kendell’s T indicated the count of actual appointments kept was positively associated with self-efficacy for keeping appointments and was negatively associated with log viral load.
Table 2.
Bivariate correlations among constructs of primary interest
| 1 | 2 | 3 | 4 | 5 | M (SD) | |
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1. DAST | – | – | – | – | – | 3.7 (2.65) |
| 2. AUDIT | .18* | – | – | – | – | 10.8 (9.61) |
| 3. Medication self-efficacy | −.08 | −.24** | – | – | – | 83.7 (22.46) |
| 4. Appointment self-efficacy | −.23** | −.11 | .55** | – | – | 85.2 (21.14) |
| 5. Viral load | −.26** | −.05 | −.22** | −.08 | – | 2.7 (1.39) |
| 6. Appointments kept | .05 | .10 | .03 | .12* | −.27** | 4.6 (2.81) |
Note. AUDIT = Alcohol Use Disorders Identification Test; DAST = Drug Abuse Screening Test. Pearson’s r is displayed for associations between continuous variables. Kendalls’ T is displayed for bivariate associations with appointments kept.
p < .05
p < .01
Viral load, self-efficacy, and substance use
Table 3 contains the results of the path model predicting log viral load. Associations among constructs of primary interest are depicted in Figure 1. Consistent with hypotheses, self-efficacy for medication adherence was negatively associated with log viral load, while self-efficacy for keeping appointments was non-significant in the prediction of log viral load. Contrary to expectations, the direct effect of DAST scores on log viral load was significant and negative (i.e., higher DAST scores were associated with lower log viral load). The direct effect of AUDIT scores on log viral load was non-significant. The association between AUDIT scores and adherence self-efficacy was statistically significant and negative, consistent with hypotheses. Contrary to hypotheses, DAST scores were not significantly associated with adherence self-efficacy.
Table 3.
Viral load path model results
| Viral load | Adherence Self-Efficacy | Appointment Self-Efficacy | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
|||||||
| R2 = 0.15** | R2 = 0.14** | R2 = 0.12** | |||||||
|
|
|
|
|||||||
| B | 95% CI | β | B | 95% CI | β | B | 95% CI | β | |
|
|
|
|
|||||||
| Intercept | |||||||||
| Adherence Self-efficacy | −0.02** | (−0.03, −0.01) | −.28 | -- | -- | -- | -- | -- | -- |
| Appointment Self-efficacy | −0.001 | (−0.01, 0.01) | −.01 | -- | -- | -- | -- | -- | -- |
| AUDIT | −0.01 | (−0.03, 0.01) | −.06 | −0.52** | (−0.91, −0.14) | −.22 | −0.12 | (−0.46, 0.21) | −.06 |
| DAST | −0.15** | (−0.23, −0.07) | −.29 | −0.54 | (−1.80, 0.72) | −.06 | −1.81** | (−2.96, −0.66) | −.23 |
| Age | −0.01 | (−0.03, 0.02) | −.05 | −0.07 | (−0.43, 0.30) | −.04 | 0.31* | (0.01, 0.62) | .17 |
| Gender and Sexual Identity (ref = Heterosexual men) | |||||||||
| Gay/bisexual men | −0.22 | (−0.78, 0.54) | −.08 | −10.30* | (−19.14, −1.46) | −.23 | 1.08 | (−6.50, 8.66) | .03 |
| Cisgender women | −0.62 | (−1.25, 0.41) | −.17 | −13.37* | (−23.79, −2.94) | −.22 | −8.36 | (−19.26, 2.54) | −.15 |
| Transgender women | −0.58 | (−1.85, 0.69) | −.07 | −21.39** | (−35.30, −7.48) | −.16 | −2.58 | (−19.27, 14.11) | −.02 |
| Race/Ethnicity (ref = White) | |||||||||
| Black | −0.12 | (−0.78, 0.54) | −.04 | 6.56 | (−4.79, 17.90) | .15 | 5.72 | (−2.24, 13.68) | .14 |
| Latino | −0.30 | (−1.02, 0.41) | −.02 | 5.34 | (−7.30, 17.98) | .10 | 6.20 | (−3.37, 15.78) | .13 |
| Other | −0.07 | (−0.89, 0.75) | −.05 | 0.08 | (−14.65, 14.81) | .00 | −1.00 | (−13.59, 11.60) | −.02 |
Note. AUDIT = Alcohol Use Disorders Identification Test; DAST = Drug Abuse Screening Test.
p < .05
p < .01
Figure 1.
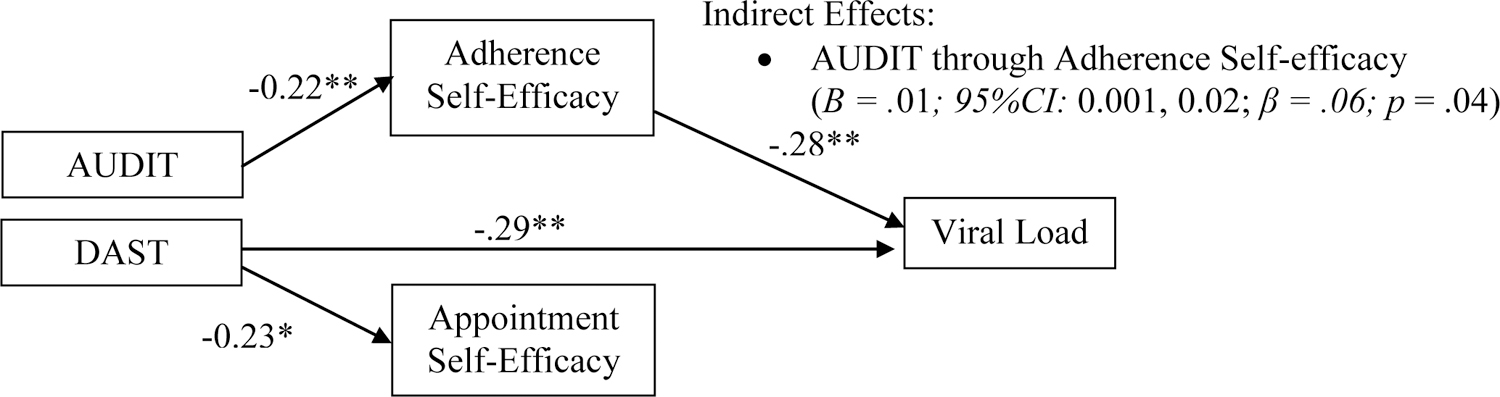
Substance use and self-efficacy: associations with viral load.
The pattern of direct effects indicated one possible indirect path of interest linking AUDIT scores to log viral load through adherence self-efficacy. The composition of effects indicated that higher AUDIT scores were indirectly associated with higher log viral loads through the association with lower adherence self-efficacy.
Appointments kept, self-efficacy, and substance use
Table 4 contains the results of the path model predicting number of appointments kept. Associations among constructs of primary interest are depicted in Figure 2. Note, the prediction of endogenous self-efficacy variables in this model is redundant with the log viral load model and so these regression coefficients are omitted from Table 4 (but can be referenced in Table 3). Consistent with hypotheses, the self-efficacy for keeping appointments was positively associated with the number of appointments kept, while the association with adherence self-efficacy was non-significant. Contrary to expectations, the direct effect of AUDIT scores on appointments kept was significant and positive (i.e., higher AUDIT scores were associated with more appointments kept). While DAST scores were negatively associated with self-efficacy for keeping appointments, the direct effect of DAST scores on appointments kept was non-significant.
Table 4.
Appointments kept path model results
| Number of appointments kept | ||||
|---|---|---|---|---|
|
| ||||
| B | 95% CI | expB | β | |
|
|
||||
| Intercept | ||||
| Adherence Self-efficacy | 0.00 | (−0.01, 0.01) | 1.0 | −.03 |
| Appointment Self-efficacy | 0.01* | (0.00, 0.01) | 1.01 | .56 |
| AUDIT | 0.01* | (0.002, 0.02) | 1.01 | .52 |
| DAST | 0.03 | (−0.001, 0.06) | 1.03 | .36 |
| Age | 0.01 | (0.00, 0.02) | 1.01 | .52 |
| Gender and Sexual Identity (ref = Heterosexual men) | ||||
| Gay/bisexual men | 0.20 | (−0.04, 0.44) | 1.22 | .46 |
| Cisgender women | 0.20 | (−0.06, 0.45) | 1.22 | .33 |
| Transgender women | 0.08 | (−0.68, 0.84) | 1.08 | .06 |
| Race/Ethnicity (ref = White) | ||||
| Black | 0.13 | (−0.10, 0.32) | 1.14 | .29 |
| Latino | 0.18 | (−0.07, 0.44) | 1.20 | .36 |
| Other | −0.03 | (−0.37, 0.32) | 0.97 | −.04 |
Note. AUDIT = Alcohol Use Disorders Identification Test; DAST = Drug Abuse Screening Test.
p < .05
Figure 2.
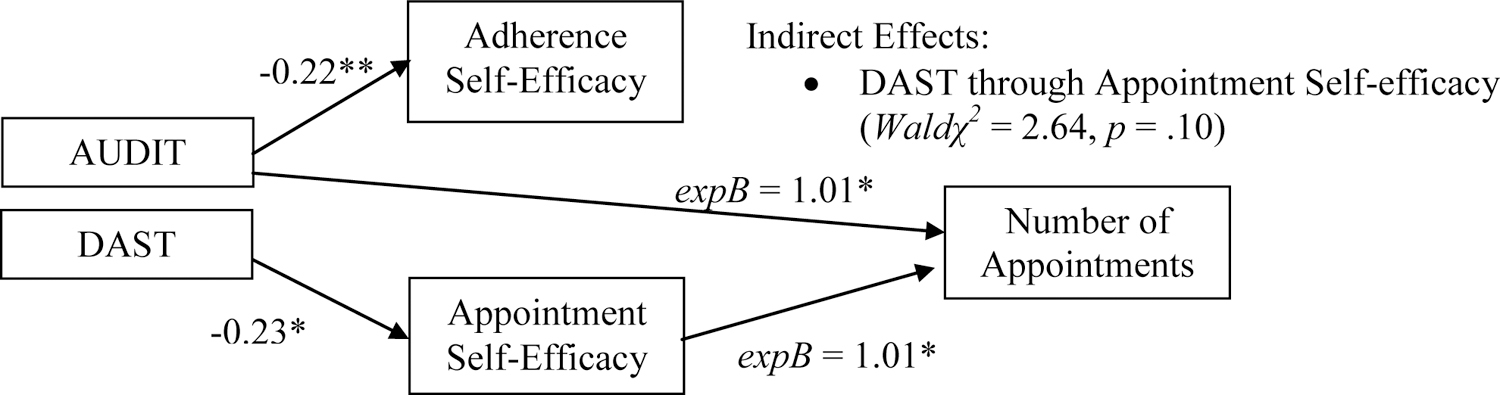
Substance use and self-efficacy: associations with number of HIV primary care appointments kept.
The pattern of direct effects indicated one possible indirect path of interest, linking DAST scores to appointments kept through appointment self-efficacy. The combination of main effects indicated that DAST might be associated with fewer appointments kept through its association with lower appointment-related self-efficacy; however, a Wald χ2 test of the model constraint (which specified that the product of the constituent direct effects was zero) was non-significant.
Discussion
These results illustrate the domain-specific nature of the associations between self-efficacy and engagement in the HIV care continuum. The two continuum outcomes modeled were each predicted only by the corresponding self-efficacy domain. Furthermore, results point to domain-specific mechanisms linking alcohol and drug use to the care continuum. These observations suggest that studies assessing global self-efficacy should be cautious in interpreting findings related to care cascade retention. Generalizing explanations for dropout from single-domain measures of self-efficacy may obscure consequential domain-specific associations (Gill & Krentz, 2009; Rebeiro et al., 2013).
Racial and ethnic disparities commonly observed in the care continuum were not evident in these data. Consistent with the study’s larger focus on implementation, participants were recruited directly through clinics, indicating that these participants were already engaged in care, despite the numerous socio-structural barriers that contribute to such disparities. Studies that recruit patients without any recent clinic contact may have greater power to detect racial and ethnic disparities. Additionally, this study drew participants from three clinics in one large urban health-care network. Suboptimal provider cultural competence has been linked to racial and ethnic disparities in HIV medication self-efficacy specifically (Saha 2013) and HIV cascade outcomes generally (Gaston, 2013). Studies that reach patients with a wider array of clinic experiences – and which have access to data on provider cultural competence – would be better equipped to identify racial and ethnic disparities.
These findings have the potential to inform the development of substance use and ART adherence interventions. A number of integrated interventions drawing upon Motivational Interviewing and Cognitive Behavioral techniques have been developed (Carrico, 2006; DiIorio et al., 2008; Golin et al., 2006; Hill & Kavookjian, 2012; Murphy et al., 2012; Parsons et al., 2018; Samet et al., 2005). While results have been mixed, our findings broadly support continued work in this area. One plausible explanation for equivocal findings is that these interventions may vary in the extent to which they addressed domain-specific self-efficacy relevant to respective outcomes.
In addition, these findings suggest that the broader literature on mastery and self-efficacy may inform future intervention work. Self-efficacy interventions have improved outcomes for patients with a wide range of chronic illnesses (Marks et al., 2005); however, their application to HIV care outcomes is limited. Formative research suggests that mastery may be an essential component of resilience for PLWH (De Santis et al., 2013). This could be incorporated into HIV care continuum interventions—and brief self-efficacy interventions could be integrated into routine care with relatively modest modifications to existing practice.
The role of appointment-keeping self-efficacy points to an under-examined intervention target. This is important given robust evidence showing that missed appointments contribute significantly to observed disparities in virologic rebound among Black and injection-drug-using PLWH in primary care (Zinski et al., 2015). Bolstering self-efficacy for keeping appointments could yield tangible benefits in improved engagement, and efforts to do so should be undertaken in conjunction with robust efforts to identify and dismantle the numerous socio-structural barriers that systemically make engagement more challenging for people with marginalized identities. Future studies should examine the role of these socio-structural barriers, such as HIV-related stigma, racism, and discrimination. This kind of integrated attention to individual and structural determinants of health care engagement may also bolster the inconsistent effects of standard supports, such as transit compensation and telephone reminders, which are typically used to address some of the structural barriers to appointment-keeping (Henry et al., 2012).
The domain-specific nature of associations between substance use-related problems and self-efficacy was unanticipated. These findings may potentially reflect unique and substance-specific barriers to retention in the care cascade. For example, given the nature of the AUDIT, high scores are much more likely to be produced by participants who have daily or near-daily alcohol use. This is not necessarily the case for the DAST because frequency is not directly assessed in item content. The regularity of use captured in high AUDIT scores may specifically diminish participants’ confidence in their ability to successfully complete a daily medication regime. Additionally, drug use is associated with more social stigma than alcohol use (Paquette et al., 2018). It is plausible that PLWH who use drugs anticipate being stigmatized by medical providers – and subsequently internalize this stigma in the form of diminished self-efficacy for appointment keeping specifically – in ways that those experiencing problematic alcohol use do not.
Several limitations to these findings are noteworthy. Our participants were New York –based and already engaged in care. As enrollment was limited to people with both detectable viral load and substance use, results cannot be generalized to the wider population of PLWH. While the study benefitted from access to EMR data, cross-sectional analyses should not be construed as evidence of causality. Future studies could employ longitudinal data to explore changes in self-efficacy and outcomes over time.
In sum, among PLWH recruited at an HIV outpatient clinic, medication adherence and appointments kept were predicted by participants’ perceived self-efficacy in each domain. These domain-specific pathways through self-efficacy explained, in part, associations between substance use and retention in the HIV care cascade. Findings suggest that, in addition to larger systemic changes which are needed to address and remove socio-structural barriers to care, it may be helpful to screen for domain-specific self-efficacy, as well as substance use, in order to identify patients at risk of poorer outcomes who may benefit from referral to personalized supportive services such as medical case management, care coordination, or counseling.
Acknowledgements:
This study was funded by a grant from the National Institute for Alcohol Abuse (R01 AA022302, PI – Starks). The authors acknowledge the contributions of the PLUS Project Team, particularly Dr. Juline Koken, Laurie Spacek, Doug Keeler, Elizabeth Savarese, Juan Castiblanco, Theresa Navalta, Joseph Carter, Evie Arroyo, Chloe Mirzayi, Tina Koo, Ruben Jimenez, and our research coordinators, Sylviah Nyamu and Kelly Reilly. We also thank our staff, recruiters, interns, and our participants who volunteered their time.
Source of Funding
This study was funded by a grant from the National Institute for Alcohol Abuse and Alcoholism (R01 AA022302, PI – Starks).
Footnotes
Conflicts of Interest
The authors declare they have no conflicts of interest.
References
- Aitken RC (1969) Measurement of feelings using visual analogue scales. Proceedings of the Royal Society of Medicine, 62, 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barai N, Monroe A, Lesko C, Lau B, Hutton H, Yang C, … Chander G (2017). The association between changes in alcohol use and changes in antiretroviral therapy adherence and viral suppression among women living with HIV. AIDS & Behavior, 21(7), 1836–1845. doi: 10.1007/s10461-016-1580-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergström G, Börjesson M, & Schmidt C (2015). Self-efficacy regarding physical activity is superior to self-assessed activity level, in long-term prediction of cardiovascular events in middle-aged men. BMC Public Health, 15(1), 820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogart LM, Wagner GJ, Galvan FH, Klein DJ (2010). Longitudinal relationships between antiretroviral treatment adherence and discrimination due to HIV-serostatus, race, and sexual orientation among African-American men with HIV. Annals of Behavioral Medicine, 40(2),184–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JL, Littlewood RA, & Vanable PA (2013). Social-cognitive correlates of antiretroviral therapy adherence among HIV-infected individuals receiving infectious disease care in a medium-sized northeastern US city. AIDS Care, 25(9), 1149–1158. doi: 10.1080/09540121.2012.752566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulsara SM, Wainberg ML, & Newton-John TRO (2018). Predictors of adult retention in HIV Care: A systematic review. AIDS & Behavior, 22(3), 752–764. doi: 10.1007/s10461-016-1644-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrico AW, Antoni MH, Durán RE, Ironson G, Penedo F, Fletcher MA, … & Schneiderman N (2006). Reductions in depressed mood and denial coping during cognitive behavioral stress management with HIV-positive gay men treated with HAART. Annals of Behavioral Medicine, 31(2), 155–164. [DOI] [PubMed] [Google Scholar]
- Cella DF, & Perry SW (1986). Reliability and concurrent validity of three visual-analogue mood scales. Psychological Reports, 59(2), 827–833. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. (2018). Understanding the HIV care continuum. Retrieved April 1, 2020.
- Cha E, Erlen JA, Kim KH, Sereika SM, & Caruthers D (2008). Mediating roles of medication–taking self-efficacy and depressive symptoms on self-reported medication adherence in persons with HIV: A questionnaire survey. International journal of nursing studies, 45(8), 1175–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colasanti J, Kelly J, Pennisi E, Hu YJ, Root C, Hughes D, … & Armstrong WS (2016). Continuous retention and viral suppression provide further insights into the HIV care continuum compared to the cross-sectional HIV care cascade. Clinical Infectious Diseases, 62(5), 648–654. doi: 10.1093/cid/civ941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert AM, Sereika SM, & Erlen JA (2013). Functional health literacy, medication-taking self-efficacy and adherence to antiretroviral therapy. Journal of Advanced Nursing, 69(2), 295–304. doi: 10.1111/j.1365-2648.2012.06007.x [DOI] [PubMed] [Google Scholar]
- de Meneses-Gaya C, Zuardi AW, Loureiro SR, & Crippa JAS (2009). Alcohol Use Disorders Identification Test (AUDIT): An updated systematic review of psychometric properties. Psychology & Neuroscience, 2(1), 83. [Google Scholar]
- De Santis JP, Florom-Smith A, Vermeesch A, Barroso S, & DeLeon DA (2013). Motivation, management, and mastery: a theory of resilience in the context of HIV infection. Journal of the American Psychiatric Nurses Association, 19(1), 36–46. doi: 10.1177/1078390312474096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erlen JA, Cha ES, Kim KH, Caruthers D, & Sereika SM (2010). The HIV Medication Taking Self‐efficacy Scale: Psychometric evaluation. Journal of Advanced Nursing, 66(11), 2560–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flickinger TE, Saha S, Roter D, Korthuis PT, Sharp V, Cohn J, … Beach MC (2016). Clinician empathy is associated with differences in patient-clinician communication behaviors and higher medication self-efficacy in HIV care. Patient Education and Counseling, 99(2), 220–226. doi: 10.1016/j.pec.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flores D, Leblanc N, & Barroso J (2016). Enroling and retaining human immunodeficiency virus (HIV) patients in their care: A metasynthesis of qualitative studies. International journal of nursing studies, 62, 126–136. doi: 10.1016/j.ijnurstu.2016.07.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gant Z, Bradley H, Hu X, Skarbinski J, Hall HI, & Lansky A (2014). Hispanics or Latinos living with diagnosed HIV: Progress along the continuum of HIV care—United States, 2010. MMWR. Morbidity and mortality weekly report, 63(40), 886. [PMC free article] [PubMed] [Google Scholar]
- Gardner EM, McLees MP, Steiner JF, del Rio C, & Burman WJ (2011). The spectrum of engagement in HIV care and its relevance to test-and-treat strategies for prevention of HIV infection. Clinical infectious diseases, 52(6), 793–800. doi: 10.1093/cid/ciq243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaston GB (2013). African-Americans’ perceptions of health care provider cultural competence that promote HIV medical self-care and antiretroviral medication adherence. AIDS Care, 25(9), 1159–1165. [DOI] [PubMed] [Google Scholar]
- Gause NK, Elliott JC, Delker E, Stohl M, Hasin D, & Aharonovich E (2018). Association between change in self-efficacy to resist drinking and drinking behaviors among an HIV-infected sample: Results from a large randomized controlled trial. Journal of Health Psychology, 23(6), 829–839. doi: 10.1177/1359105316664127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill MJ, & Krentz HB (2009). Unappreciated epidemiology: the churn effect in a regional HIV care programme. International journal of STD & AIDS, 20(8), 540–544. doi: 10.1258/ijsa.2008.008422 [DOI] [PubMed] [Google Scholar]
- Giordano TP, Hartman C, Gifford AL, Backus LI, & Morgan RO (2009). Predictors of retention in HIV care among a national cohort of US veterans. HIV Clinical Trials, 10(5), 299–305. doi: 10.1310/hct1005-299 [DOI] [PubMed] [Google Scholar]
- Golin CE, Earp J, Tien HC, Stewart P, Porter C, & Howie L (2006). A 2-arm, randomized, controlled trial of a motivational interviewing-based intervention to improve adherence to antiretroviral therapy (ART) among patients failing or initiating ART. JAIDS, 42(1), 42–51. doi: 10.1097/01.qai.0000219771.97303.0a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzler B, Dombrowski JC, Crane HM, Eron JJ, Geng EH, Christopher Mathews W, … Donovan DM (2017). Prevalence and Predictors of Substance Use Disorders Among HIV Care Enrollees in the United States. AIDS & Behavior, 21(4), 1138–1148. doi: 10.1007/s10461-016-1584-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendershot CS, Stoner SA, Pantalone DW, & Simoni JM (2009). Alcohol use and antiretroviral adherence: review and meta-analysis. JAIDS, 52(2), 180–202. doi: 10.1097/QAI.0b013e3181b18b6e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry SR, Goetz MB, & Asch SM (2012). The effect of automated telephone appointment reminders on HIV primary care no-shows by veterans. Journal of the Association of Nurses in AIDS Care, 23(5), 409–418. [DOI] [PubMed] [Google Scholar]
- Hill S, & Kavookjian J (2012). Motivational interviewing as a behavioral intervention to increase HAART adherence in patients who are HIV-positive: a systematic review of the literature. AIDS Care, 24(5), 583–592. doi: 10.1080/09540121.2011.630354 [DOI] [PubMed] [Google Scholar]
- Huskisson EC (1974). Measurement of pain. Lancet, 304(7889), 1127–1131. [DOI] [PubMed] [Google Scholar]
- Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, & Chesney MA (2007). The role of self-efficacy in HIV treatment adherence: validation of the HIV Treatment Adherence Self-Efficacy Scale (HIV-ASES). Journal of Behavioral Medicine, 30(5), 359–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacombe-Duncan A, Bauer GR, Logie CH, Newman PA, Shokoohi M, Kay ES, … & Loutfy M (2019). The HIV care cascade among transgender women with HIV in Canada: a mixed-methods study. AIDS Patient Care and STDs, 33(7), 308–322. [DOI] [PubMed] [Google Scholar]
- Langebeek N, Gisolf EH, Reiss P, Vervoort SC, Hafsteinsdóttir TB, Richter C, … & Nieuwkerk PT (2014). Predictors and correlates of adherence to combination antiretroviral therapy (ART) for chronic HIV infection: a meta-analysis. BMC medicine, 12(1), 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee WK, Milloy MJ, Walsh J, Nguyen P, Wood E, & Kerr T (2016). Psychosocial factors in adherence to antiretroviral therapy among HIV-positive people who use drugs. Health Psychology, 35(3), 290–297. doi: 10.1037/hea0000310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddux JE (2016). Self-efficacy. In Snyder CRF, D. R. (Ed.), Handbook of social and clinical psychology: The health perspective (pp. 57–78). New York: Pergamon Press. [Google Scholar]
- Marks R, Allegrante JP, & Lorig K (2005). A review and synthesis of research evidence for self-efficacy-enhancing interventions for reducing chronic disability: implications for health education practice (part II). Health Promotion and Practice, 6(2), 148–156. doi: 10.1177/1524839904266792 [DOI] [PubMed] [Google Scholar]
- Monroe AK, Lau B, Mugavero MJ, Mathews WC, Mayer KH, Napravnik S, … Chander G (2016). Heavy alcohol use is associated with worse retention in HIV Care. JAIDS, 73(4), 419–425. doi: 10.1097/QAI.0000000000001083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy DA, Chen X, Naar-King S, Parsons JT, & ATN (2012). Alcohol and marijuana use outcomes in the Healthy Choices motivational interviewing intervention for HIV-positive youth. AIDS Patient Care & STDS, 26(2), 95–100. doi: 10.1089/apc.2011.0157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthén LKM, & Muthen BO (2017). Mplus v8. 0 [statistical software]. Los Angeles: Muthén & Muthén. [Google Scholar]
- Nachega JB, Uthman OA, del Rio C, Mugavero MJ, Rees H, & Mills EJ (2014). Addressing the Achilles’ heel in the HIV care continuum for the success of a test-and-treat strategy to achieve an AIDS-free generation. Clinical infectious diseases, 59 Suppl 1, S21–27. doi: 10.1093/cid/ciu299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, John SA, Millar BM, & Starks TJ (2018). Testing the Efficacy of Combined Motivational Interviewing and Cognitive Behavioral Skills Training to Reduce Methamphetamine Use and Improve HIV Medication Adherence Among HIV-Positive Gay and Bisexual Men. AIDS & Behavior, 22(8), 2674–2686. doi: 10.1007/s10461-018-2086-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Rosof E, & Mustanski B (2007). Patient-related factors predicting HIV medication adherence among men and women with alcohol problems. Journal of Health Psychology, 12(2), 357–370. doi: 10.1177/1359105307074298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons JT, Golub SA, Rosof E, & Holder C (2007). Motivational Interviewing and Cognitive-Behavioral Intervention to Improve HIV Medication Adherence Among Hazardous Drinkers: A Randomized Controlled Trial. JAIDS, 46(4), 443–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeiro P, Althoff KN, Buchacz K, Gill J, Horberg M, Krentz H, … Design. (2013). Retention among North American HIV-infected persons in clinical care, 2000–2008. JAIDS, 62(3), 356–362. doi: 10.1097/QAI.0b013e31827f578a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saha S, Korthuis PT, Cohn JA, Sharp VL, Moore RD, & Beach MC (2013). Primary care provider cultural competence and racial disparities in HIV care and outcomes. Journal of General Internal Medicine, 28(5), 622–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samet JHH, N. J.; Meli S; Dukes K; Tripp T; Sullian L; Freedberg KA (2005). A randomized controlled trial to enhance antiretroviral therapy adherence in patients with a history of alcohol problems. Antiviral Therapy, 10, 83–93. [DOI] [PubMed] [Google Scholar]
- Sangaramoorthy T, Jamison A, & Dyer T (2019). Older African Americans and the HIV Care Continuum: A Systematic Review of the Literature, 2003–2018. AIDS & Behavior, 23(4), 973–983. doi: 10.1007/s10461-018-2354-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders JB, Asland OG, Babor TF, De La Fuente JR, & Grant M (1993). Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption—II. Addiction, 88, 791–804. [DOI] [PubMed] [Google Scholar]
- Simpson E, & Jones MC (2013). An exploration of self-efficacy and self-management in COPD patients. British Journal of Nursing, 22(19), 1105–1109. [DOI] [PubMed] [Google Scholar]
- Skinner HA (1982). The Drug Abuse Screening Test. Addictive Behaviors, 7, 363–371. [DOI] [PubMed] [Google Scholar]
- Spiller V, Zavan V, & Guelfi GP (2006). Assessing motivation for change in subjects with alcohol problems: the MAC2-A questionnaire. Alcohol and Alcoholism, 41(6), 616–623. [DOI] [PubMed] [Google Scholar]
- Strecher VJD, B. M.; Becker MH; Rosenstock IM (1986). The role of self-efficacy in achieving health behavior change. Health Education Q, 13(1), 73–91. [DOI] [PubMed] [Google Scholar]
- Tieu HV, Koblin BA, Latkin C, Curriero FC, Greene ER, Rundle A, & Frye V (2018). Neighborhood and network characteristics and the HIV care continuum among gay, bisexual, and other men who have sex with men. Journal of Urban Health, 1–17. doi: 10.1007/s11524-018-0266-2 [DOI] [PMC free article] [PubMed]
- Tobias CR, Cunningham W, Cabral HD, Cunningham CO, Eldred L, Naar-King S, … Drainoni ML (2007). Living with HIV but without medical care: barriers to engagement. AIDS Patient Care & STDS, 21(6), 426–434. doi: 10.1089/apc.2006.0138 [DOI] [PubMed] [Google Scholar]
- Turan B, Fazeli PL, Raper JL, Mugavero MJ, & Johnson MO (2016). Social support and moment-to-moment changes in treatment self-efficacy in men living with HIV: Psychosocial moderators and clinical outcomes. Health Psychology, 35(10), 1126–1134. doi: 10.1037/hea0000356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner NM, Van De Leemput AJ, Draaisma JM, Oosterveld P, & Ten Cate OTJ (2008). Validity of the visual analogue scale as an instrument to measure self‐efficacy in resuscitation skills. Medical Education, 42(5), 503–511. [DOI] [PubMed] [Google Scholar]
- Vagenas P, Azar MM, Copenhaver MM, Springer SA, Molina PE, & Altice FL (2015). The Impact of Alcohol Use and Related Disorders on the HIV Continuum of Care: a Systematic Review: Alcohol and the HIV Continuum of Care. Current HIV/AIDS Reports, 12(4), 421–436. doi: 10.1007/s11904-015-0285-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams EC, McGinnis KA, Edelman EJ, Matson TE, Gordon AJ, Marshall BDL, … Bradley KA (2019). Level of Alcohol Use Associated with HIV Care Continuum Targets in a National U.S. Sample of Persons Living with HIV Receiving Healthcare. AIDS & Behavior, 23(1), 140–151. doi: 10.1007/s10461-018-2210-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf MS, Davis TC, Osborn CY, Skripkauskas S, Bennett CL, & Makoul G (2007). Literacy, self-efficacy, and HIV medication adherence. Patient Education and Counseling, 65(2), 253–260. doi: 10.1016/j.pec.2006.08.006 [DOI] [PubMed] [Google Scholar]
- Yudko E, Lozhkina O, & Fouts A (2007). A comprehensive review of the psychometric properties of the Drug Abuse Screening Test. Journal of Substance Abuse Treatment, 32(2), 189–198. [DOI] [PubMed] [Google Scholar]
- Zinski A, Westfall AO, Gardner LI, Giordano TP, Wilson TE, Drainoni ML, … & Mugavero MJ (2015). The contribution of missed clinic visits to disparities in HIV viral load outcomes. American Journal of Public Health, 105(10), 2068–2075. doi: 10.2105/AJPH.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]


