Abstract
Objective:
Describe MRI susceptibility changes in progressive multifocal leukoencephalopathy (PML) and identify neuropathological correlates.
Methods:
PML cases and matched controls with primary central nervous system lymphoma (PCNL) were retrospectively identified. MRI brain at 3T and 7T were reviewed. MRI-pathology correlations in fixed brain autopsy tissue were conducted in 3 subjects with confirmed PML.
Results:
With PML (n=26 total, n = 5 multiple sclerosis natalizumab-associated), juxtacortical changes on susceptibility weighted imaging (SWI) or gradient echo (GRE) sequences were noted in 3/3 cases on 7 tesla (T) MRI and 14/22 cases (63.6%) on 1.5T or 8/22 (36.4%) 3T MRI. Similar findings were only noted in 3/25 (12.0%) of PCNSL patients (OR 12.83, 95% CI 2.9 – 56.7, p < 0.001) on 1.5T or 3T MRI. On susceptibility sequences available prior to diagnosis of PML, 7 (87.5%) had changes present on average 2.7 ± 1.8 months (mean ± SD) prior to diagnosis. Postmortem 7T MRI showed SWI changes corresponded to areas of increased iron density along the grey-white matter (GM-WM) junction predominantly in macrophages.
Conclusions:
Susceptibility changes in PML along the GM-WM junction can precede noticeable FLAIR changes and correlates with iron accumulation in macrophages.
Keywords: PML, 7T MRI, susceptibility changes, u-fiber, grey matter, pathology, immunohistochemistry, iron, progressive multifocal leukoencephalopathy, macrophage
Introduction
Reactivation of the JC polyomavirus (JCV) in patients with a compromised immune system causes progressive multifocal leukoencephalopathy (PML) leading to extensive white matter (WM) and grey matter (GM) pathology. The immunomodulatory drug natalizumab, used to treat multiple sclerosis (MS) and Crohn’s colitis, is associated with PML in 832 confirmed cases (Biogen data on file), with a global incidence of 4.08/1000 patients (765,985 patient-years) as of August 2019.1,2,3,4 PML also occurs infrequently with other MS therapies: fingolimod, dimethyl fumarate, and ocrelizumab.5
Mortality varies −24% with natalizumab, 50–60% with HIV, and up to 90% with hematological malignancies.6–8 Recognition of unifocal subtle changes on MRI in asymptomatic individuals is critical given a lack of effective treatments and poorer outcomes associated with multifocal and bihemispheric involvement. 9,10,11–13
Brain biopsy or JCV DNA in the cerebrospinal fluid (CSF) with a compatible clinical course and neuroimaging is necessary but often delayed given overlap of PML with mimics such as primary CNS lymphoma (PCNSL)15,14 Using susceptibility weighted imaging (SWI), linear juxtacortical hypointense (at the cortical-WM junction) changes are observed in association with PML lesions even prior to clinical manifestations in some individuals.16–21 This is an attractive imaging biomarker of PML in the appropriate clinical context – patients on immunomodulatory/immunosuppressive therapies or an immunocompromised state.22 PML paramagnetic changes have been interpreted as occurring in the cortex, deep cortical layers or adjacent U-fibers and its etiology has been enigmatic.18,21,23,24 Here, we evaluated the presence of juxtacortical susceptibility abnormalities in subjects with PML using 1.5 Tesla (T) and 3T MRI acquired as part of routine clinical practice, supplemented by dedicated research 7T imaging. PCNSL was selected as a comparator given its similar clinical and neuroimaging presentations.14 We then sought to determine the histopathological correlates of the susceptibility changes in a postmortem cohort of subjects with PML.
Material and methods
I. Retrospective Case Control Study
Patient selection
Patients ≥ 18-years-old with confirmed PML (CSF JCV PCR or brain biopsy) were retrospectively identified between October 2008 - February 2020. The suspected cause of PML, presence of seizures, and occurrence of immune reconstitution inflammatory syndrome (IRIS) were noted. An equal number of patients with PCNSL was selected as imaging “controls” using a random number generator. Basic demographics (age, race, and mortality) and MRI features (field strength and lesion location, characteristics, and enhancement pattern) were collected.
In vivo imaging
MRI studies either pre- or post-diagnosis of PML/PCNSL were reviewed by neuroradiologists (SEJ, JL) and neurologists (MA, KRM, DO). Conventional clinical MRIs included fluid-attenuated inversion recovery (FLAIR), diffusion weighted imaging (DWI), T2-weighted, T1 magnetization-prepared rapid acquisition with gradient echo (MPRAGE), SWI, and GRE sequences. FLAIR abnormalities in the cerebral WM, brainstem, and the cerebellum were recorded. Juxtacortical SWI/GRE hypointensities, diffusion restriction, and gadolinium enhancement were also noted. 7T MRI of three PML patients was conducted as part of a research protocol and were reviewed. Details of FLAIR, SWI, GRE, T2* sequences are provided in Supplemental Table 1A.
II. Postmortem study
Postmortem tissue selection
Brain tissue from three subjects with MS and a confirmed diagnosis of PML by JCV CSF DNA were acquired as part of the Cleveland Clinic MS Tissue Bank.25 Four hemispheric 1-cm fixed slices with grossly apparent hyperpigmentation along the cortex-WM junction were selected for each subject for 7T postmortem imaging.
Postmortem fixed-tissue 7T MRI
Slices were imaged using a Bruker Biospec 7T scanner (details in Supplemental Table 1B). Sequences included T2*-weighted multiple gradient echo (MGE), T2-Turbo-Rapid Acquisition with Relaxation Enhancement (T2), and SWI. The slice with the greatest SWI hypointensity at the junction was selected for further characterization by resecting the region of interest.
Neuropathology, iron histochemistry, and immunohistochemistry
A tissue block with the most marked SWI hypointensity at the cortical GM-WM junction was resected from each of the 3 subjects with PML and 30-μm free floating sections were cut on a sliding microtome.
For total non-heme iron staining, a diaminobenzidine (DAB)-enhanced Turnbull staining protocol to detect both total ferric and ferrous non-heme iron was used.26
DAB immunohistochemistry was done as previously described.27 For immunofluorescence, double labeling was done using antibodies against ferritin-light chain (ferritin-L, 10727–1-AP, rabbit polyclonal, Proteintech, Rosemont, IL, USA) with one of the following: MHCII, CD68 (Dako KP1 clone, ga60961–2, mouse monoclonal), or Iba-1 (mouse monoclonal, Cleveland Clinic Hybridoma Core); all dilutions 1:500. Secondary antibodies were Alexa Fluor donkey (anti-rabbit 488 [A21206], anti-rabbit 555 [A32794], anti-mouse 488 [A21202], and anti-mouse 555 [A31570]; Invitrogen, ThermoFisher Scientific, Waltham, MA, USA).
Standard protocol approvals
Both the retrospective case control study and postmortem tissue bank were separately approved by the Cleveland Clinic Institutional Review Board.
Data analysis and statistics
For in vivo clinical scans, Student’s two-sided t-test was performed to compare continuous variables and chi-squared statistic was used to analyze categorical variables; significance p < 0.05. Susceptibility changes were between patients with PML and PCNSL and ORs with 95% CI were derived. Inter-rater agreement and Cohen’s kappa (κ) are reported where applicable.
An in-house script (ImageJ version 1.52e, NIH, USA) was used to calculate the percentage area occupied by iron staining, immunohistochemistry (MHCII, GFAP), and immunofluorescence (MHCII and ferritin-L). Pairwise comparisons of different regions (GM, GM-WM junction, and WM) were done using t-tests with pooled SD and Bonferroni correction. R Studio/R was used for statistical analysis.28
Results
I. Retrospective case control study
In vivo disease characteristics of patients with PML
Twenty-six PML and an equal number of PCNSL cases were identified with demographic data and lesion distribution summarized (Table 1). PML etiologies included HIV (34.6%), lymphoproliferative disorders (26.9%; leukemia, lymphoma, and idiopathic lymphopenia), MS on natalizumab (19.2%), organ transplant (11.5%), and chronic inflammatory disorders (7.7%; polymyositis and dermatomyositis).
Table 1.
Demographics characteristics and MRI features of included patients with PML and PCNSL.
| PML | PCNSL | |
|---|---|---|
| Age (mean ± SD) | 56.8 ± 12.7 years | 69.8 ± 9.9 years |
| Sex (%) | ||
| Female | 5 (19) | 15 (58) |
| Ethnicity (%) | ||
| Caucasian | 19 (73) | 25 (96) |
| African American | 6 (23) | 0 (0) |
| Unknown | 1 (4) | 1 (4) |
| Mortality (%) | 5 (19.2) | 9 (34.6) |
| Location of lesions on MRI % | ||
| Frontal | 84.0 | 57.7 |
| Parietal | 52.0 | 26.9 |
| Brainstem | 12.0 | 7.7 |
| Cerebellum | 20.0 | 15.4 |
| Occipital | 16.0 | 15.4 |
| Temporal | 36.0 | 26.9 |
| Contrast enhancement (%) | 8 (34.8) | 26 (100) |
| Susceptibility sequence (%) | ||
| GRE | 9 (40.9) | 11 (42.3) |
| SWI | 13 (59.1) | 15 (57.7) |
| Field strength (%) | ||
| 1.16 | 0 (0) | 1 (3.8) |
| 1.5 | 13 (59.1) | 19 (73.1) |
| 3.0 | 9 (40.9) | 6 (23.1) |
PML was diagnosed by CSF JCV DNA in 20 (76.9%) and brain biopsy in six (23.1%). Four with negative CSF had typical PML biopsy findings. Seven (26.9%) patients with PML developed IRIS. Natalizumab-PML (n = 5; all with MS) had a higher risk of developing IRIS compared with other PML etiologies (OR 85.8, 95% CI 3.56–2064.2, p = 0.006). In HIV-associated PML, at the time of diagnosis of PML and MRI review, 4/9 (44.4%) were recently started on highly active antiretroviral therapy (HAART), 1 was on chronic HAART, and the remainder (4/9) were newly diagnosed with HIV (not on HAART).
Patients with MS on natalizumab developed PML in 6.6 ± 3.0 years (mean ± SD). PML in non-MS was more likely to be multifocal (OR 12.0, 95% CI 1.1 – 134.1, p = 0.044). Brain biopsies demonstrated prominent macrophages (CD68), perivascular lymphocytes (CD3+/CD20−), primary demyelination (loss of myelin with Luxol fast blue with relatively persevered neurofilament), reactive astrocytes, and viral particles/inclusions (electron microscopy in 3, and SV40 staining in 3). Eight (30.8%) experienced new onset seizures (± 1 year from date of PML diagnosis).
In vivo MRI susceptibility changes of patients with PML and PCNSL
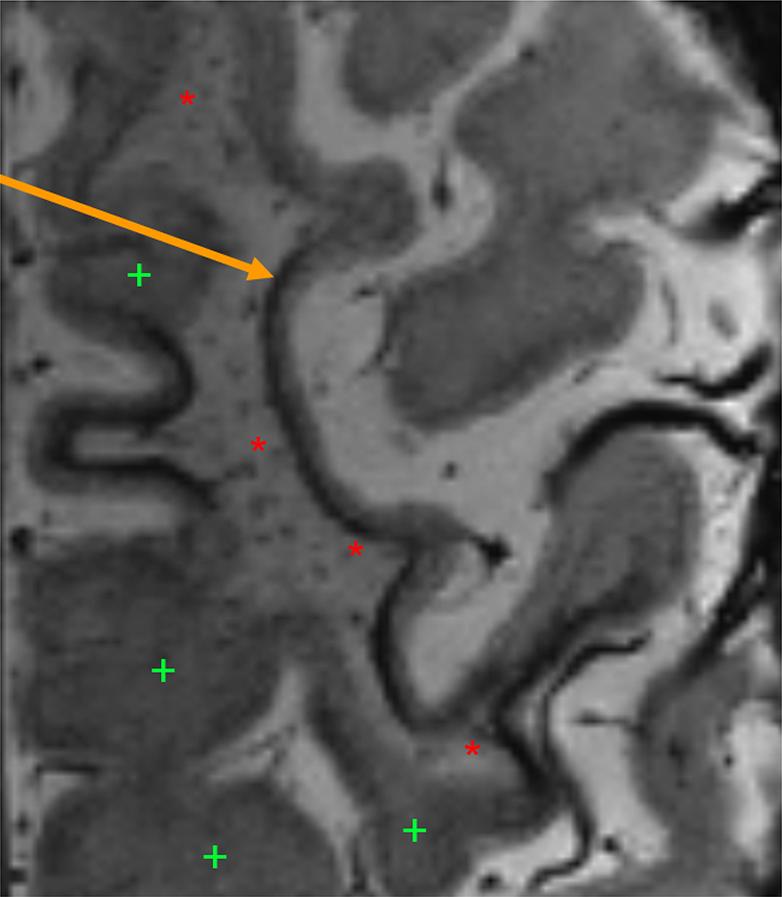
A linear juxtacortical hypointense band was apparent on 7T SWI in all cases (n = 3; Figure 1) with associated FLAIR hyperintensities (either on 1.5T, 3T, or 7T) and with evolution on follow-up imaging in 2 of the 3 cases (Figure 2). The extent of susceptibility changes, involvement of the cortex, and FLAIR hyperintensity were variable in different foci (Figure 3). Twenty-two PML cases (84.6%) had SWI/GRE available at or after the time of diagnosis on clinical imaging (1 month prior to 1 year after a confirmatory PML diagnosis). One patient was unable to have MRI (automatic implantable cardioverter defibrillator), 2 patients had MRIs with a demyelinating disease protocol lacking SWI/GRE, and 1 patient had an external MRI without SWI/GRE. Fourteen (63.6%) had 1.5T and 8 (36.4%) had 3T MRI available. Eight (36.4%) had GRE and 14 (63.6%) had SWI available. In this cohort, 14/22 (63.6%) demonstrated a band of SWI hypointensity along the cortical-WM junction as shown in Figures 1–3 (86.4% agreement, κ 0.70). Of these 14 patients with the susceptibility change, 7 (50%) were detected on 3T SWI sequences, 4 (29%) on 1.5T SWI sequences, 2 (14%) on 1.5T GRE, and 1 (7%) on 3T GRE. There was no statistically significant difference in the susceptibility change between MS vs. non-MS patients in either pre- or post-PML MRIs. Of note, 5/8 subjects with seizures had juxtacortical susceptibility changes (1 did not have a GRE/SWI available).
Figure 1.
7T SWI sequence with arrow indicating linear hypointense band along WM-cortical boundary. Red asterisks (“*”) denote WM FLAIR hyperintensities and green plus-signs (“+”) normal appearing WM.
Abbreviations: SWI – susceptibility-weighted imaging. T – tesla, WM – white matter.
Figure 2.
Progression of PML lesions (each colored arrow represents a unique lesion) on 1.5T brain MRI from left to right through time (months 0–4). Highlighted panel in yellow marking 7T SWI with a hypointense band along the cortical-WM boundary corresponding to FLAIR hyperintensity. FLAIR hyperintensities are subtle and months 0–2 preceding acquisition of the SWI sequence. The FLAIR hyperintensities associated with the SWI changes continue to evolve and become more confluent from months 3 to 4.
Figure 3.
Variability of SWI hypointensity and FLAIR hyperitensity in 3 PML regions on 3T and 7T MRI. Panel A: 7T GRE sequence, Panel B: 3T FLAIR sequence, and Panel C: 3 T SWI sequence. Variability of the SWI hypointensity and FLAIR hyperintensity are apparent in comparing 3 cortical regions. Region 1 has minimal SWI changes (C) but with marked FLAIR changes (B). Region 2 has marked SWI changed with FLAIR hyperintesity predominantly along the cortex with relatively normal appearing WM. Region 3 demonstrates both cortical SWI changes with associated WM FLAIR hyperintensity.
Abbreviations: FLAIR - fluid attenuated inversion recovery, GRE – gradient echo, PML – progressive multifocal leukoencephalopathy, SWI – susceptibility weighted imaging, T – telsa, WM – white matter.
In the PCNSL cohort, 25 patients had SWI or GRE sequence available. Similar changes were only seen in 3 (12.0%) patients with PCNSL. Two were detected in 1.5T SWI and one in 3.0T SWI sequences (100% agreement, κ 1.00).
Susceptibility changes along the cortex were more common in PML than PCNSL (OR 12.83, 95% CI 2.9 – 56.7, p < 0.001). In those with PML, 8 had SWI/GRE sequence available 1–12 months prior to diagnosis of PML. Seven (87.5%) had a cortical susceptibility hypointensity prior to diagnosis of PML (75.0% agreement, κ 0.38). Susceptibility changes occurred as early as 7 months prior to PML diagnosis (mean 2.7 months, SD 1.8 months). In all 7, FLAIR signal changes were present at the time of SWI/GRE changes. Of these 7, associated FLAIR changes were detected in 2 (28.5%) on 3T SWI, 2 (28.5%) on 1.5T SWI, 2 (28.5%) on 1.5T GRE, and 1 (14.3) on 3T GRE. One patient, who did not have early susceptibility changes, had a 1.5T GRE scan 2 months prior to diagnosis of PML. Interestingly, similar SWI hypointensity was seen on serial FLAIR imaging prior to development of more confluent hyperintensity on FLAIR (Figure 2). No statistically significant difference in the frequency of SWI changes pre- or post-PML diagnosis was found comparing natalizumab associated PML to other etiologies of PML.
In vivo MRI diffusion restriction and gadolinium enhancement in patients with PML and PCNSL
Five (22.7%) patients with PML had similar changes associated with diffusion restriction compared to 2 (7.7%) with PCNSL (100% inter-rater agreement, κ 1.00). There was no statistically significant difference in the frequency of this findings between the two groups. Diffusion restriction was not seen on any MRI prior to PML diagnosis (87.5% inter-rater agreement).
Gadolinium was used in 25/26 subjects with PML and enhancement was seen in 9 (36.0%) of those cases (100% agreement). Most of the enhancement was noted in HIV-associated PML (5/9); other cases included: natalizumab-associated PML (1/5), lymphoproliferative disorders (2/6), and chronic inflammatory disorders (1/2); organ transplant (0/3). Presence of enhancement was not significantly different in natalizumab-PML compared to other PML etiologies. The enhancement pattern was multifocal in eight (88.9%; 5 patchy, 1 nodular and patchy peripherally, 1 nodular/patchy centrally, and 1 patchy centrally) and unifocal (nodular) in one. In MRIs pre-PML diagnosis, 6 had post-gadolinium contrasted images and 2 (33.3%) had evidence of enhancement (100% agreement). Five of the nine (55.6%) subjects had enhancement directly associated with susceptibility changes and 1/9 (11.1%) had susceptibility changes independent of the focus of enhancement; the others did not have susceptibility changes.
In PCNSL, all 26 patients who received gadolinium had enhancement (100% inter-rater agreement). The enhancement patterns included: 9 homogenous, 9 patchy centrally, 3 patchy peripherally, 2 nodular/homogenous, 1 patchy centrally/peripherally, 1 patchy/nodular, and 1 nodular/leptomeningeal); fifteen (57.7%) had multifocal enhancement.
II. Postmortem study
Postmortem gross examination and 7T MRI
Subjects 1–3 in order were: 58 year old Caucasian woman with secondary progressive MS; 43 year old Caucasian man with relapsing MS; 39 year old Caucasian woman with relapsing MS. Brain tissues were evaluated for grossly apparent hyperpigmentation either in the cortex or the cortex-WM junction. Four slices with grossly evident areas of interest were imaged using T2, MGE, and SWI for each subject to identify susceptibility changes along the cortical-WM junction. Susceptibility changes were seen in all three imaging modalities along the GM-WM junction but were most apparent on SWI. The hypointensity magnitude varied between the 3 subjects and did not always correspond to a hyperpigmented line visible on gross examination in some areas. Subject 3 had the greatest SWI, T2, and MGE hypointensity. Only subjects 1 and 2 had T2-hyperintensity associated with SWI changes. SWI hypointensity and resection of the corresponding tissue is shown in Figure 4.
Figure 4.
Hemispheric 1-cm fixed coronal slices of three separate subjects with MS and confirmed PML are shown on the left 3. 7T SWI sequence is shown to the right for the respective subjects and with arrows pointing to areas of hypointensity along the GM-WM border. The block of tissue resected for sectioning and staining is shown in the far right with the corresponding enlarged MRI region. Scale bar denotes 1 cm for the gross image.
Abbreviations: GM – grey matter, MS – multiple sclerosis, PML – progressive multifocal leukoencephalopathy, SWI – susceptibility weighted imaging, T – tesla, WM – white matter
Postmortem iron histology and immunohistochemistry
Subjects 1 and 2 had cortical and WM demyelination in the characterized block of tissue while subject 3 did not (Supplemental Figure 1). Subject 3 had the greatest susceptibility changes at the GM-WM junction on MRI; representative images showing iron, microglia/macrophage, astrocyte, and myelin staining are shown in Figure 5.
Figure 5.
Representative images (5 using DAB and 1 immunofluorescence) of subject 3 are shown. Total iron is greater in the WM than GM but concentrated along the either side of the WM abutting the cortex; outlined rectangle is enlarged in the top right inset. The area of interest is not demyelinated (PLP) and the GM is clearly demarcated by the neuronal marker HuR. Astrogliosis is prevalent in the WM to a greater extent than the WM-GM junction and sparse in the GM (GFAP). The density of activated microglia/macrophages at the WM-GM junction parallels total iron staining. The WM is crowded by macrophages while the GM has activated elongated microglia. Confocal microscopy demonstrates co-localization of ferritin-L (green) predominantly with macrophages (red) at the GM-WM junction.
Abbreviations: GFAP – glial fibrillary acidic protein for astrocytes; GM – grey matter; HuR – neuronal marker; L- light chain; MHCII – major histocompatibility complex II for activated microglia/macrophages; PLP – proteolipid protein for myelin; WM – white matter.
All 3 subjects had increased iron density (using DAB) in regions along the GM-WM junction compared to the GM (p < 0.0001; Supplemental Figure 2D). Subjects 2 and 3 had greater ferritin-L in the GM-WM and WM compared to the GM (all p < 0.0001; Supplemental Figure 2C) while subject 1 showed a trend (p = 0.05). Subject 3 had the greatest SWI hypointensity observed at the GM-WM junction which corresponded to an intense band of iron staining (Figure 5, Supplemental Figure 2D, Supplemental Table). Iron was present throughout but greater in the WM than in the GM.
All three subjects had innumerous macrophages in the WM that abutted the GM junction. Although microglia were observed in the cortex, macrophages were not seen outside the WM or GM-WM junction. Subjects 1 and 2 had slightly greater microglia/ macrophage (MHCII) density in the WM compared to the GM-WM junction using DAB (p<0.001) while subject 3 did not (p = 0.06). Using immunofluorescence for MHCII, the density was comparable between the GM-WM junction and the WM in all 3 subjects (Supplemental Table and Supplemental Figure 2A and 2B).
Ferritin-L was highly expressed in the GM-WM junction and in the WM in all 3 subjects (p > 0.05); very little ferritin-L was detected in the GM (Supplemental Table and Supplemental Figure 2C). Double-labeling with ferritin-L and MHCII demonstrated co-localization in the GM-WM junction (Figure 5).
Astrogliosis was variable in the three subjects. Subjects 1 and 2 had increased density in the GM-WM junction compared to the WM (p < 0.0001). Subject 3 had extensive WM astrogliosis with a lower density of astrocytes at the GM-WM junction (p < 0.0001). Astrocyte density was lowest in the GM in all subjects compared to either the GM-WM junction or WM (p < 0.0001) (Supplemental Figure 2E). Although iron was abundantly present in myelin as well as many cell types, the greatest density of iron at the GM-WM junction co-localized with macrophages.
Discussion
Sixty-four percent of patients had a susceptibility hypointensity involving the GM-WM junction at or after the time of PML diagnosis on conventional 1.5T/3T MRI and in all patients on 7T. Susceptibility changes were only seen in 12% of subjects with PCNSL, a condition that mimics PML on conventional MRI sequences. Using postmortem MRI-pathology correlations, juxtacortical susceptibility changes correlated with a band of increased iron present in macrophages. Additional observations in our study include: 1) a possible association of seizures with cortical susceptibility changes which have been linked to iron accumulation; 2) lower likelihood of multifocal PML in the natalizumab cohort possibly due to increased monitoring.
We suspect that a threshold density of iron rich macrophages need to accumulate before SWI changes are apparent. WM T2-hyperintensities in postmortem subjects (1 and 2) occurred adjacent to GM-WM susceptibility changes (containing high density of iron in macrophages). This pattern was consistent with FLAIR hyperintensity associated with SWI/GRE changes in our in vivo study. In some regions where SWI changes preceded obvious WM FLAIR hyperintensities in vivo (Figure 2), our postmortem imaging also demonstrated SWI changes (subject 3; Figure 4 and Supplementary Figure 1) without associated T2 changes in spite of a greater density of macrophages in the apparent normal appearing WM on MRI and PLP. These findings caution against the assumption that WM FLAIR hyperintensities are requisite with an infectious process.
JCV infection may exist in the WM causing SWI changes preceeding FLAIR hyperintensity (Figure 2) –a reflection of a massive surge of macrophages that accumulate, and are trapped against the GM-WM junction. Microenvironment differences between the cortex and WM likely restricts macrophage entry into the cortex.29 GM-WM junctions with visible SWI hypointensities may reflect increased iron density from extensive WM inflammation and are harbingers for ongoing WM injury demonstrated by subsequent FLAIR changes on serial imaging.
Depending on the stage of the PML lesion, one may identify SWI changes with or without apparent FLAIR hyperintensities.
Similar to previous reports, we found 36% of PML lesions demonstrated gadolinium enhancement, but found enhancement was more frequently seen in HIV-associated PML lesions (56%) compared to natalizumab-associated PML lesions (20%).30,31 Similar rate of enhancement was noted in 33.3% of MRIs when reviewing pre-PML scans (one month pre- to one year post-PML diagnosis).” All natalizumab-associated PML patients were on natalizumab at the time of diagnosis and 4 (44.4%) of the HIV-associated PML patients were recently started on HAART Contrast enhancement could have been associated with either IRIS or PML in these subjects. Increased monitoring and earlier recognition in MS natalizumab-associated PML could explain why PML was more unifocal in this cohort compared to non-MS PML.
Limitations include the retrospective design, relatively small sample size, and in vivo characterization. As inflammatory changes can precede a clinical diagnosis of PML, absolute characterization of imaging changes “pre-diagnosis” should not be misinterpreted as preceding PML infection and inflammation. Future studies could compare these findings in MS variants (e.g. tumefactive), other demyelinating conditions (e.g. myelin-oligodendrycote glycoprotein associated disease and aquaporin-4 positive neuromyelitis optica), and other disease processes. Including longitudinal evaluation of juxtacortical susceptibility with associated FLAIR changes, multiple centers, and postmortem studies with HIV- and PCNSL-associated PML to compare with natalizumab-associated PML will be useful to further demonstrate significance of our findings.
Early identification of PML is paramount as lesion volume increases by 63% every month spent in the pre-diagnostic phase and delays in diagnosis increase mortality and morbidity due to both the extent of PML and IRIS.32,33 In patients with MS treated with natalizumab, a juxtacortical susceptibility band could serve as an early marker of PML and precede obvious FLAIR changes. Inclusion of a GRE/SWI sequence in either in higher-risk individuals or those with atypical lesions may further aid in the diagnosis of PML, particularly with serial imaging.
Supplementary Material
Acknowledgments
Funding:
K.R.M. is funded by NIH NINDS K23 Career Development Award 1K23NS109328. Postmortem program funded by NIH/NINDS R35 to B.D.T. and Sanofi Genzyme.
Disclosures
Kedar R Mahajan: Received research funding through institution from NIH/NINDS and NMSS.
Moein Amin: No relevant disclosures.
Matthew Poturalski: No relevant disclosures.
Jonathan Lee: No relevant disclosures.
Danielle Herman: No relevant disclosures.
Yufan Zheng: No relevant disclosures.
Caroline Androjna: No relevant disclosures.
Mark Howell: No relevant disclosures.
Robert Fox: Has received personal consulting fees from Actelion, Biogen, Celgene, EMD Serono, Genentech, Immunic, Novartis, Sanofi, Teva, and TG Therapeutics; has served on advisory committees for Actelion, Biogen, Immunic, and Novartis; and has received clinical trial contract and research grant funding from Biogen and Novartis.
Bruce D Trapp: Receives grant support from NIH, Sanofi Genzyme and Fast Forward; speaking fees from Sanofi Genzyme and advisory board fees from Disarm Therapeutics, Sanofi Genzyme and Therini Bio, Inc.; founder and Chief Scientific Officer of Cashel Neural.
Kunio Nakamura: Received licensing fee from Biogen; research funding through institution from NIH, DOD, NMSS, Biogen, Novartis, and Sanofi Genzyme.
Stephen Jones: Has received research support from Biogen, Siemens and St Jude Medical; consulting agreements with Monteris; advisory board of Eisai; and speaking support from Siemens, RadNet, and St Jude Hospital.
Daniel Ontaneda: Has received research support from the National Institutes of Health, National Multiple Sclerosis Society, Patient Centered Outcomes Research Institute, Race to Erase MS Foundation, Genentech, Genzyme, and Novartis. He has also received consulting fees from Biogen Idec, Janssen, Genentech/Roche, Genzyme, Novartis, and Merck.
References
- 1.Campagnolo D, Dong Q, Lee L, Ho P-R, Amarante D, Koendgen H. Statistical analysis of PML incidences of natalizumab-treated patients from 2009 to 2016: outcomes after introduction of the Stratify JCV® DxSelect™ antibody assay. J Neurovirol. 2016;22(6):880–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.MacDonald JK, McDonald JWD. Natalizumab for induction of remission in Crohn’s disease. Cochrane Database Syst Rev. 2007;(1). [DOI] [PubMed] [Google Scholar]
- 3.Vukusic S, Rollot F, Casey R, et al. Progressive multifocal leukoencephalopathy incidence and risk stratification among natalizumab users in France. JAMA Neurol. 2020;77(1):94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giovannoni G, Kappos L, Berger J, et al. Updated incidence of natalizumab-associated progressive multifocal leucoencephalopathy and its relationship with the pattern of natalizumab exposure over time. ECTRIMS. Stockholm, Sweden; 2019. [Google Scholar]
- 5.Berger JR. Classifying PML risk with disease modifying therapies. Mult Scler Relat Disord. 2017;12:59–63. [DOI] [PubMed] [Google Scholar]
- 6.Dong-Si T, Gheuens S, Gangadharan A, et al. Predictors of survival and functional outcomes in natalizumab-associated progressive multifocal leukoencephalopathy. J Neurovirol. 2015;21(6):637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pavlovic D, Patera AC, Nyberg F, Gerber M, Liu M, Consortium PML. Progressive multifocal leukoencephalopathy: current treatment options and future perspectives. Ther Adv Neurol Disord. 2015;8(6):255–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neil EC, DeAngelis LM. Progressive multifocal leukoencephalopathy and hematologic malignancies: a single cancer center retrospective review. Blood Adv. 2017;1(23):2041–2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prosperini L, De Rossi N, Scarpazza C, et al. Natalizumab-related progressive multifocal leukoencephalopathy in multiple sclerosis: findings from an Italian independent registry. PLoS One. 2016;11(12):e0168376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lindå H, von Heijne A. Presymptomatic diagnosis with MRI and adequate treatment ameliorate the outcome after natalizumab-associated progressive multifocal leukoencephalopathy. Front Neurol. 2013;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clifford DB, Nath A, Cinque P, et al. A study of mefloquine treatment for progressive multifocal leukoencephalopathy: results and exploration of predictors of PML outcomes. J Neurovirol. 2013;19(4):351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clifford DB, DeLuca A, Simpson DM, Arendt G, Giovannoni G, Nath A. Natalizumab-associated progressive multifocal leukoencephalopathy in patients with multiple sclerosis: lessons from 28 cases. Lancet Neurol. 2010;9(4):438–446. [DOI] [PubMed] [Google Scholar]
- 13.Vermersch P, Kappos L, Gold R, et al. Clinical outcomes of natalizumab-associated progressive multifocal leukoencephalopathy. Neurology. 2011;76(20):1697–1704. [DOI] [PubMed] [Google Scholar]
- 14.Berger JR, Aksamit AJ, Clifford DB, et al. PML diagnostic criteria: Consensus statement from the AAN neuroinfectious disease section. Neurology. 2013; 80(15): 1430–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wattjes MP, Richert ND, Killestein J, et al. The chameleon of neuroinflammation: magnetic resonance imaging characteristics of natalizumab-associated progressive multifocal leukoencephalopathy. Mult Scler J. 2013;19(14):1826–1840. [DOI] [PubMed] [Google Scholar]
- 16.Sinnecker T, Hadisurya J, Schneider-Hohendorf T, et al. Extensive immune reconstitution inflammatory syndrome in Fingolimod-associated PML: a case report with 7 Tesla MRI data. BMC Neurol. 2019;19(1):190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyagawa M, Maeda M, Umino M, et al. Low signal intensity in U-fiber identified by susceptibility-weighted imaging in two cases of progressive multifocal leukoencephalopathy. J Neurol Sci. 2014;344(1–2):198–202. [DOI] [PubMed] [Google Scholar]
- 18.Carra-Dalliere C, de Champfleur NM, Deverdun J, et al. Use of quantitative susceptibility mapping (QSM) in progressive multifocal leukoencephalopathy. J Neuroradiol. 2016;43(1):6–10. [DOI] [PubMed] [Google Scholar]
- 19.Hodel J, Outteryck O, Verclytte S, et al. Brain magnetic susceptibility changes in patients with natalizumab-associated progressive multifocal leukoencephalopathy. Am J Neuroradiol. 2015;36(12):2296–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ueno H, Kikumto M, Takebayashi Y, et al. Pomalidomide-associated progressive multifocal leukoencephalopathy in multiple myeloma: cortical susceptibility-weighted imaging hypointense findings prior to clinical deterioration. J Neurovirol. 2020. [DOI] [PubMed] [Google Scholar]
- 21.Pontillo G, Cocozza S, Lanzillo R, et al. Brain susceptibility changes in a patient with natalizumab-related progressive multifocal leukoencephalopathy: a longitudinal quantitative susceptibility mapping and relaxometry study. Front Neurol. 2017;8:294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papadimitropoulos GN, Lachanis S, Zompola C, et al. Laminar cortical hypointensities in susceptibility-weighted imaging in a case of progressive multifocal leukoencephalopathy. J Clin Neurol. 2017;13(2):201–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Umino M, Maeda M, Ii Y, Tomimoto H, Sakuma H. Low-signal-intensity rim on susceptibility-weighted imaging is not a specific finding to progressive multifocal leukoencephalopathy. J Neurol Sci. 2016;362:155–159. [DOI] [PubMed] [Google Scholar]
- 24.Carra-Dalliere C, Menjot de CN, Ayrignac X, Deverdun J, Labauge P. Quantitative susceptibility mapping suggests a paramagnetic effect in PML. Neurology. 2015;84(14):1501. [DOI] [PubMed] [Google Scholar]
- 25.Dutta R, Mahajan KR, Nakamura K, et al. Comprehensive autopsy program for individuals with multiple sclerosis. JoVE (Journal Vis Exp. 2019;(149):e59511. [DOI] [PubMed] [Google Scholar]
- 26.Bagnato F, Hametner S, Yao B, et al. Tracking iron in multiple sclerosis: a combined imaging and histopathological study at 7 Tesla. Brain. 2011;134(12):3602–3615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mahajan KR, Nakamura K, Cohen JA, Trapp BD, Ontaneda D. Intrinsic and extrinsic mechanisms of thalamic pathology in multiple sclerosis. Ann Neurol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.R Core Team. R: A Language and Environment for Statistical Computing. 2013. http://www.r-project.org/.
- 29.Lee J, Hamanaka G, Lo EH, Arai K. Heterogeneity of microglia and their differential roles in white matter pathology. CNS Neurosci Ther. 2019;25(12):1290–1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wijburg MT, Witte BI, Vennegoor A, et al. MRI criteria differentiating asymptomatic PML from new MS lesions during natalizumab pharmacovigilance. J Neurol Neurosurg Psychiatry. 2016; 87: 1138–45 [DOI] [PubMed] [Google Scholar]
- 31.Igra MS, Paling D, Wattjes MP, Connolly DJA and Hoggard N. Multiple sclerosis update: use of MRI for early diagnosis, disease monitoring and assessment of treatment related complications. Br J Radiol. 2017; 90: 20160721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scarpazza C, Signori A, Prosperini L, et al. Early diagnosis of progressive multifocal leucoencephalopathy: longitudinal lesion evolution. J Neurol Neurosurg Psychiatry. 2019;90(3):261–267. [DOI] [PubMed] [Google Scholar]
- 33.Dong‐Si T, Richman S, Wattjes MP, et al. Outcome and survival of asymptomatic PML in natalizumab‐treated MS patients. Ann Clin Transl Neurol. 2014;1(10):755–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.