Abstract
Background
Over the past decade, U.S. FDA has approved 10 opioid analgesics in abuse-deterrent formulations (ADFs). ADFs are intended to reduce abuse of a prescription opioid through manipulation of the product to use one or more routes of abuse. Although it is critically needed for evaluation of the abuse deterrent properties of an opioid product, the relationship between systemic exposure and likelihood of abuse of the opioid has not been fully characterized. To fill the current knowledge gap, we have evaluated the association of subjective measures predictive of abuse potential (e.g., scores of “drug liking,” “take drug again”), which are referred to as ‘pharmacodynamic (PD)’ responses for measuring abuse potential, with systemic exposure of the opioid using the data from all the clinical abuse potential trials submitted to FDA in support of the approval of innovator ADFs.
Methods
Extensive pharmacokinetic (PK) and subjective response data from 11 clinical abuse potential trials in recreational opioid users following oral and nasal administration of intact and manipulated oxycodone, hydrocodone and morphine products from the FDA internal database were utilized for the present analysis. This retrospective study used data collected from January 11th, 2010 until March 25th, 2015. The potential relationship between PK metrics, especially those for early exposure measures, and the subjective measures of drug liking and take drug again as PD metrics of abuse potential were explored using linear and logistic regression analyses. Heterogeneity analysis was conducted to assess study-to-study variation and multi-level logistic regression analysis was used to affirm the identified PK-PD relationship based on pooled data.
Findings
Following oral and nasal administration of intact and manipulated opioids, the maximum visual analogue scale (VAS) for Drug Liking was generally achieved no later than the time to peak plasma drug concentration. Both heterogeneity analysis and multi-level logistic regression indicated insignificant inter study variability for the evaluated PK-PD relationships. Duration of Drug Liking response (i.e., VAS ≥ 65) lasted for 2 to 4 h after drug administration. The early portion of the systemic area under the plasma concentration-time curve (AUC), e.g., partial AUCs in the first 3 h and 4 h were found to be associated with abuse potential measures including maximum Drug Liking VAS and maximum Taking Drug Again VAS. Neither a formulation factor (e.g., immediate-release vs. extended-release, intact vs. manipulated) nor a route of administration was identified as a significant factor together with early partial AUCs to predict the probability of maximum Drug Liking or maximum Take Drug Again responses being greater than or equal to 65.
Interpretation
Our assessment indicates that the measure of early systemic drug exposure of opioids is the best predictor of the abuse potential response in recreational opioid users following oral or nasal administration of a single dose of an intact or manipulated abuse deterrent opioids. Our findings support FDA's recommendation of comparative PK studies with early partial AUCs as a supportive PK metric for the assessment of abuse deterrent properties of generic opioid drug products in the general and product-specific guidance's of ADFs.
Funding
The study was partially funded by Fiscal Year 2017 Critical Path of the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration.
Keywords: Opioid analgesics, Abuse potential, Partial AUC, Drug Liking Visual Analogue Scale (VAS), Take Drug Again VAS
Research in context.
Evidence before this study
Despite its importance in evaluation of the abuse-deterrent formulations (ADFs), the relationship between systemic drug exposure and likelihood of abuse of opioids has not been fully characterized. Neither the conventional PK metrics, including peak plasma opioid concentration (Cmax), time to Cmax (Tmax), and area under the plasma concentration-time curve (AUC), nor the measure of the initial rise of drug concentrations expressed as abuse quotient (AQ) is deemed to be a good predictor of abuse potential. Due to the subjective nature of the measures of abuse potential (e.g., VASs for Drug Liking, Taking Drug Again, Drug High, and Overall Drug Liking), which are referred to as PD responses in our study, as well as the wide range of abuse deterrent technologies employed in opioid analgesics, the relationship between systemic drug exposure in terms of PK metrics and abuse potential in terms of PD response measures have been viewed as complex and inconsistent.
Added value of this study
Our study reports the association of early partial AUCs with the likelihood of abuse potential based on the analyses of the premarket abuse potential clinical trials submitted to FDA. Our finding is that the correlation between plasma opioid exposure and opioid drug liking effect would be strongest if we consider the onset of action, highest drug liking, offset of action and duration of action. Specifically, early partial AUCs of PK profile is tightly correlated with the partial area under curve of Drug Liking VAS score versus time profile.
Our findings shed light on the controversial view on the most predictive PK driver of clinically relevant PD measures of drug liking or taking drug again based on the PK- clinical response relationship for opioid products. Therefore, our analysis supports the inclusion of the early partial AUC metrics in addition to the conventional PK metrics (i.e., Cmax and AUC) for the assessment of abuse deterrent properties of the opioid products containing a single active ingredient in ADFs.
Implications of all the available evidence
FDA considers the development of abuse deterrent opioid analgesics as an important public health priority. Our study findings have an impact on the regulatory guidance and assessment of opioid ADFs. It supports the agency's current thinking on the recommendation of the comparative PK studies to demonstrate abuse deterrent property for an opioid product submitted as a New Drug Application (NDA) and an Abbreviated New Drug Application (ANDA).
Alt-text: Unlabelled box
1. Introduction
While opioid therapy is effective for the management of acute and chronic pain, the addiction, abuse, and misuse of prescription opioid analgesics continue to be a major public health challenge in the United States. Prescription opioid analgesics have been one of the most common drugs involved in overdose deaths in the United States. In 2016, an estimated 48.5 million persons reported use of illicit drugs or misuse of prescription drugs in the past year and more than 17,087 fatal overdoses involved prescription opioids.1
As an effort to mitigate the harm associated with prescription opioid analgesics while maintaining legitimate access for patients who need them, the U.S. Food and Drug Administration (FDA) has supported the development of abuse deterrent formulations (ADFs). ADFs are reformulation of opioids intending to make abuse of opioid products more difficult or less rewarding. Some ADFs are based on physical/chemical barriers such as increased mechanical strength, lower opioid extraction rate, and/or reduced syringeability compared to non-AD products, whereas others use opioid agonists/antagonists combinations (e.g., naltrexone or naloxone).2 As of August 2020 FDA has approved 10 opioid analgesics with physical, chemical, and/or pharmacological properties that are expected to deter intravenous, intranasal, and/or oral abuse of the prescription opioids.3 Current FDA approval of an AD opioid product depends on the totality of the evidence from the studies appropriate for evaluating the AD properties. The new drug guidance, Guidance for Industry: Abuse-Deterrent Opioids, intends to assist industry in developing opioid drug products with potentially abuse-deterrent properties.4 It explains the FDA's current thinking about the studies that should be conducted to demonstrate that a given formulation has abuse-deterrent properties, makes recommendations about how those studies should be performed and evaluated, and discusses what labeling claims may be approved based on the results of those studies.
Those studies generally include in vitro manipulation and extraction studies (Category 1), PK studies (Category 2), and clinical abuse potential studies (Category 3). These three categories of studies have been conducted for all the FDA approved opioid products with AD labeling claims.3
A better understanding of the relationship between systemic drug exposure and likelihood of abuse of opioids will provide regulatory clarity on the evaluation of ADFs. In addition to using clinical abuse potential measures, especially given the challenges for patient recruitment, clinical study operation, and the subjective nature of abuse potential measures, using systemic drug exposure in term of PK metrics presents an alternative as a supportive measure for comparison and evaluation of abuse deterrence potential of the opioid ADFs. For the Category 2 studies, traditional PK metrics such as Cmax, time to Cmax (Tmax), and AUC have been used to assess the rate and extent of drug absorption into systemic circulation. As to the Category 3 studies, Visual Analogue Scale (VAS) for Drug Liking, Take Drug Again, Drug High, and Overall Drug Liking are among the list of subjective PD measures of abuse potential typically used for assessment of abuse deterrence of a given ADF.
Better characterization of the quantitative link between PK metrics and abuse potential measures or scores will critically support the evaluation of abuse deterrence potential of the ADFs. Some studies reported correlations between conventional PK parameters and measures of abuse potential. For example, strong correlations were established between oxycodone Cmax and maximum VAS for Drug Liking in recreational opioid users following a single dose of immediate-release/extended-release oxycodone/acetaminophen in an intact or manipulated condition.5 Several studies reported that the abuse potential of opioids are correlated with the rate of rise of drug plasma concentration.[6], [7], [8], [9] However, concerns remain for unraveling the relationships between PK exposure and clinical abuse potential scores and claims have been made such relationships may be complex and inconsistent considering the subjective nature of PD measures and the wide range of AD technologies. For instance, the Branded Industry Working Group compared chewing of oxycodone capsules under fed state (A) with chewing under fasting state (B) and noted that a higher Cmax value of oxycodone concentrations was observed under fed state (A) but a lower maximum visual analogue scale score for Take Drug Again was noted in subjects.10 Similarly, the rate of increase in blood concentration as represented by Cmax/Tmax, or referred as abuse quotient, does not take into account the blood concentrations or drug exposure before reaching Cmax. As such, including conventional PK metrics alone such as Cmax and total AUC in in vivo comparative PK studies might not be adequate or sufficient for abuse deterrence assessment of opioid products.
Therefore, identifying appropriate PK metric(s) that is predictive of abuse potential of an opioid is warranted. The current study intended to fill the knowledge gap and evaluate the association between PK metrics and abuse potential measures. We conducted retrospective and systematic analyses of the clinical PK and PD data from the eleven clinical abuse potential trials which supported the AD labeling claims for FDA-approved opioid products to date. In particular, we researched on using partial AUC (pAUC) as a PK metric, which represents the early systemic drug exposure right after drug administration, and systemically compared it with other PK metrics aforementioned to predict clinical abuse potential of an ADF. The results of our study provide the scientific basis for the recommendations of comparative PK studies in the general and product-specific guidance (PSGs) for opioid analgesics in ADFs. For instance, the results of our study is reflected in the generic drug guidance, General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products,11 which recommends studies, including comparative in vitro and pharmacokinetic studies, that the potential abbreviated new drug application (ANDA) applicant should conduct and submit to FDA in an ANDA to demonstrate that a generic solid oral opioid drug product is no less abuse-deterrent than its reference listed drug with respect to all potential routes of abuse.
2. Methods
2.1. Ethics
All relevant study protocols and their modifications were approved by the corresponding Institutional Review Boards before study conducts.
2.2. Study design and participants
Among the 10 FDA-approved AD opioid analgesics, three are agonist/antagonist combination products and are excluded from this analysis to avoid the antagonist's known effect on subjective responses. Eleven clinical abuse potential trials were available for the remaining AD opioid analgesics which include 3 oxycodone products, 2 hydrocodone products and 2 morphine products. Each of the 11 clinical trials was a randomized, double-blind, placebo-controlled and positive controlled crossover clinical abuse potential trial in recreational opioid users following an oral or nasal administration of a single dose opioid in an intact or manipulated condition. The selected 11 clinical trials used data collected from January 11th, 2010 until March 25th, 2015. As defined in the guidance, a positive control is an opioid drug product or drug substance expected to result in a predictable opioid drug liking effect and has a known potential for, or history of, abuse.12 Specifically, positive control in abuse liability and abuse-deterrence evaluation involves use of an immediate-release opioid that is of known drug liking liability. Table 1 summarizes the treatment (e.g., dose, route) and the number of subjects in each trial. Subject level demographic, PK and clinical abuse potential data from all the eleven trials were combined for data analysis.
Table 1.
Summary of randomized, double-blind, placebo-controlled crossover clinical abuse potential trials.
| Trial | Opioid | Dose, mg* | Route | Last sampling time, hr | No. of PK points | No. of PD points |
No. of subjects |
||
|---|---|---|---|---|---|---|---|---|---|
| DL | TDA | PK | PD | ||||||
| 01 | Oxycodone | 30 | IN | 24 | 10 | 8 | 2 | 29 | 30 |
| 02 | Oxycodone | 40 | IN, PO | 36 | 14 | 12 | 2 | 36 | 36 |
| 03 | Oxycodone | 40 | PO | 36 | 13 | 12 | 2 | 47 | 38 |
| 04 | Oxycodone | 30 | IN, PO | 24 | 15 | 13 | 2 | 31 | 29 |
| 05 | Hydrocodone | 60 | PO | 36 | 15 | 15 | 2 | 39 | 35 |
| 06 | Hydrocodone | 60 | IN | 36 | 16 | 15 | 2 | 27 | 25 |
| 07 | Hydrocodone | 45 | IN, PO | 48 | 20 | 19 | 2 | 41 | 34 |
| 08 | Hydrocodone | 45 | PO | 72 | 18 | 17 | 1 | 41 | 42 |
| 09 | Morphine | 60 | IN, PO | 24 | 13 | 11 | 2 | 27 | 25 |
| 10 | Morphine | 60 | PO | 24 | 12 | 11 | 2 | 39 | 38 |
| 11 | Morphine | 60 | IN, PO | 24 | 16 | 13 | 2 | 46 | 46 |
Dose is presented as the equivalent amount of oxycodone hydrochloride, hydrocodone bitartrate, and morphine sulfate for corresponding opioids. IN: intranasal administration; PO: Per Os, oral administration; DL: VAS for Drug Liking; TDA: VAS for Take Drug Again; PK: pharmacokinetic; PD: pharmaco-dynamic.
2.3. Study procedures
Each of the 11 clinical abuse potential trials used a qualification phase in addition to the eligibility screening. As a common enrichment strategy, the qualification phase identified subjects who could distinguish an active opioid drug (such as a conventional immediate-release formulation of the studied opioid) from placebo in terms of the response of VAS for Drug Liking. In general, a study subject should be able to discriminate the test opioid from placebo on a bipolar Drug Liking scale, where a score of 50 points represented neither like or dislike and 100 points resented strong like. A placebo response is defined as ≥ 40 and ≤ 60 points, a minimum of maximum Drug Liking VAS is set as 65 points in response to the active treatment and a ≥ 15-point difference is needed between the active and placebo treatments.
During the double-blind treatment phase, all subjects received each of the manipulated oral/nasal and/or intact oral treatments (including study drugs and placebo) in a random order. Each study reported a detailed description of the manipulation procedure (such as cutting, mortar and pestle crushing, grating or grinding). In general, all study products were administrated under supervision of the study personal, Nasal insufflation and oral ingestion were verified by visual inspection of the nasal cavity and mouth immediately following the dosing.
Each abuse potential evaluation cycle included a series of questions on drug effects. For Drug Liking, subjects were asked whether they liked the drug effect at this moment; for Taking Drug Again, subjects scored their desire to take the drug again at least 8 h after drug administration. Generally, each subject provided multiple Drug Liking data points and up to 2 data points for Taking Drug Again (Table 1). Serial blood samples were collected from all the subjects to measure plasma drug concentrations during the indicated time periods (Table 1).
2.4. PK and PD measures
The PK metrics included Cmax, Tmax, and AUC from time zero to last time point with measurable concentration (AUC0-t), abuse quotient (AQ), and early pAUCs. The AQ is defined as the ratio of Cmax to Tmax, Cmax/Tmax. The AQ has been considered as a measure of the rate of rise in plasma concentration over the interval between time zero and Tmax.13 The PAUC is a measure of the truncated partial AUC in a given time interval and represents the systemic drug exposure at the specified time interval. For instance, PAUC3 and PAUC4 represent the PAUC from time zero to three hours and four hours post-dosing, respectively.
While all the clinical trials used in the analyses had multiple PD endpoints (e.g., VAS for Drug High, Overall Drug Liking etc.), this analysis included only VAS for Drug Liking and Taking Drug Again metrics because maximum VAS for Drug Liking and maximum VAS for Take Drug Again appear to correlate directly with the likelihood of abuse and have been listed as key clinical findings in support of AD labeling claims.3 Other exploratory PD metrics were also used including partial area under the Drug Liking-time curve (PAUEC) from time zero to 3 or 4 h (PAUEC3 or PAUEC4, respectively).
2.5. Statistical analyses
Normal linear regression for continuous outcomes was used to assess the relationship between the PD response metrics and PK exposure metrics. The PD response metrics used in the analyses included maximum Drug Liking VAS, maximum Taking drug Again VAS, PAUEC3 and PAUEC4. The PK exposure metrics included AUC0-t, Cmax, AQ, and PAUCs (i.e., PAUC3, PAUC4).
In order to facilitate statistical analysis, the analysis dataset for a given opioid included PK/PD-evaluable subjects from oral and intranasal human abuse liability studies. Table 2 summarizes the sample size for each opioid/route/manipulation group in the analysis dataset. Heterogeneity analysis was conducted to assess inter-study variabilities (ISVs) for the observed PK-PD correlations. Logistic regression models for binary outcomes were used to analyze the probability of maximum Drug Liking VAS or maximum Taking Drug Again VAS exceeding a value (e.g., 65) as a function of PK exposure metrics. In addition, ISV was accounted in a multi-level logistic regression analysis, either as a fixed or random effect to the coefficient of covariate, to assess potential effect of study heterogeneity on the regression outcomes (NONMEM®, version 7.3). The likelihood ratio test is used to evaluate the selection of covariates including ISV in a stepwise manner. Specifically, reductions of objective function values (OFVs) in the NONMEM outputs by 6.64 and 10.83 indicate significance levels of 0.01 and 0.001 (α = 0.01 and 0.001, df = 1 for χ2 test), respectively. These values were referenced for covariate forward selection and backward elimination, respectively, in this analysis.
Table 2.
Summary of time to peak concentration and time to maximum VAS for drug liking.
| Opioid | AD | Route | Manipulation# | Numer of Subjects (N) | Tmax (h) * | Tmaxdl (h) * | Subjects with DL ≥ 65 (%) | Tonset (h) * | Toffset (h) * |
|---|---|---|---|---|---|---|---|---|---|
| hydrocodone | no | IN | no⁎⁎ | 65 | 1.25 (0.5, 7) | 1.25 (0.25, 48) | 57 (88%) | 0.5 (0.25, 2) | 6 (0.5, 48) |
| hydrocodone | no | IN | yes | 40 | 1 (0.5, 6) | 1.5 (0.5, 48) | 37 (93%) | 0.5 (0.25, 1.5) | 4 (1, 48) |
| hydrocodone | no | PO | no | 80 | 0.75 (0.25, 6) | 1.125 (0.25, 6) | 75 (94%) | 0.75 (0.5, 3) | 3 (0.75, 72) |
| hydrocodone | yes | IN | yes | 97 | 3 (0, 13) | 1.5 (0.25, 48) | 53 (55%) | 1 (0.25, 13) | 4 (0.5, 48) |
| hydrocodone | yes | PO | no | 153 | 9 (1, 36) | 2 (0.25, 72) | 43 (28%) | 1.75 (0.25, 36) | 4 (0.25, 36) |
| hydrocodone | yes | PO | yes | 79 | 3 (0.5, 7) | 1.75 (0.25, 60) | 53 (67%) | 1 (0.25, 6) | 4 (1, 72) |
| morphine | no | IN | yes | 74 | 1 (0.25, 2.5) | 1 (0.25, 8) | 66 (89%) | 0.5 (0.25, 6) | 3 (0.5, 24) |
| morphine | no | PO | yes | 39 | 0.75 (0.5, 4) | 1 (0.5, 4) | 34 (87%) | 0.75 (0.5, 3) | 2 (0.5, 12) |
| morphine | yes | IN | yes | 123 | 2 (0.75, 6) | 1.5 (0.25, 24) | 54 (44%) | 1 (0.25, 6) | 3 (0.25, 24) |
| morphine | yes | PO | no | 113 | 3 (0.5, 6) | 2 (0.25, 24) | 58 (51%) | 1.5 (0.25, 6) | 3.5 (0.25, 12) |
| morphine | yes | PO | yes | 38 | 2 (0.75, 4) | 2 (0.5, 8) | 22 (58%) | 1.5 (0.5, 4) | 3 (1, 12) |
| oxycodone | no | IN | yes | 131 | 1.25 (0.25, 6) | 0.5 (0.083, 10) | 119 (91%) | 0.5 (0.25, 24) | 4 (0.25, 24) |
| oxycodone | no | PO | yes | 65 | 0.5 (0.25, 5) | 1 (0.25, 12) | 61 (94%) | 0.5 (0.25, 8) | 4 (0.5, 24) |
| oxycodone | yes | IN | yes | 129 | 3 (0.25, 12) | 2 (0.083, 24) | 80 (62%) | 1 (0.25, 6) | 3 (0.5, 24) |
| oxycodone | yes | PO | no | 335 | 5 (0.5, 36) | 3 (0.083, 24) | 240 (72%) | 1.5 (0.25, 12) | 6 (0.25, 24) |
AD: abuse deterrent; DL: VAS for drug liking; IN: intranasal administration; PO: Per Os, oral administration; Tmax: time to reach peak plasma opioid concentration; Tmaxdl: time to maximum Drug Liking; Tonset: the time-to-onset of Drug Liking response; Toffset: the time-to-offset of Drug Liking response.
median (range).
active ingredient powder.
indicates manipulation to the original formulations or not. Both abuse deterrent and non-abuse deterrent formulations were subject to manipulation.
As AQ is calculated as Tmax as the denominator, it showed a wide range, from 0.01 to 480 ng/mL/hour. Therefore, log transformed AQ values and untransformed Cmax and PAUCs values were used in the logistic regression analysis.
2.6. Role of the funding source
The study was partially funded by Fiscal Year 2017 Critical Path of the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration. The funder had no role in study design, data collection, data analysis, data interpretation, writing of the report, or the decision to submit the paper for publication.
3. Results
3.1. Time profile of opioid plasma concentration and abuse potential response
All the opioid ADFs had a prolonged Tmax compared to the respective opioid non-ADFs (Table 2). For instance, median Tmax for intact hydrocodone ADFs and non-ADFs after oral ingestion was 9 h and 0.75 hour, respectively. In addition, physically manipulated ADFs still showed a longer Tmax than the corresponding non-ADFs following nasal insufflation (hydrocodone: 3 h vs. 1 hour; morphine: 2 h vs. 1 hour; oxycodone: 3 h vs. 1.25 h). Overall, these results suggested that opioids in ADFs exhibited a slower absorption following oral and nasal administration compared to those in non-ADFs following the same route of administration even with manipulation of original formulations. This implicates that abuse deterrence properties can be evaluated based on the PK parameters supporting the recommendation of comparative PK studies for approval of new or generic opioid in ADFs.
Although median Tmax had a wide range from 0.5 hour to 9 h, median time to maximum Drug Liking was consistently ≤ 3 h (Table 2). Most of the median time to maximum Drug Liking values were less than or equal to the median Tmax values, indicating that subjects achieved maximum Drug Liking effect regardless of presence of Cmax. Our results suggest that there may be a threshold in drug exposure for Drug Liking effect to be elicited, which may explain why Tmax or Cmax alone may not be a good predictor of abuse potential.
3.2. Association of traditional PK metrics (AUC0-t and cmax) and PD metrics
Traditional PK metrics such as AUC0-t and Cmax are reflective of the extent and rate of drug absorption, respectively. AUC0-t and Cmax were plotted by maximum Drug Liking VAS or maximum Taking Drug Again VAS groups in 0–50, 51–60, 61–70, 71–80, 81–90, and 91–100 for hydrocodone, morphine, and oxycodone, where 50 represents a neutral response of neither liking nor disliking. Figure S1 shows similar median AUC0-t among different maximum Drug Liking VAS or maximum Taking Drug Again VAS groups, indicating AUC0-t was not a significant covariate for either of the abuse potential metrics. In comparison, higher median Cmax values were noticed in groups with higher maximum Drug Liking VAS or maximum Taking Drug Again VAS (Fig. 1a). In the logistic regression analyses (Fig. 1b), Cmax was significantly associated with the probability of maximum Drug Liking VAS ≥ 65 as well as the probability of maximum Taking Drug Again VAS ≥ 65.
Fig. 1.
Association of Cmax and PD metrics. (a) Boxplot diagram showing the Cmax values in subgroups with various PD measurement values. The data were grouped per opioid for analysis. MAXDL or MAXTDA values were grouped on a bipolar scale, where a score of 50 represents neutral, i.e., neither like nor dislike. (b) logistic regression analysis showing the association of Cmax and the probability of MAXDL ≥ 65 (top panel) and MAXTDA ≥ 65 (bottom panel). MAXDL: maximum VAS for drug liking; MAXTDA: maximum VAS for take drug again. Each symbol with the error bars represents the observed probability and the associated 90% CIs per each exposure quartile for the given opioid. The line represents the logistic regression fit and the gray shaded area represents 5th–95th percentiles of the regression fit.
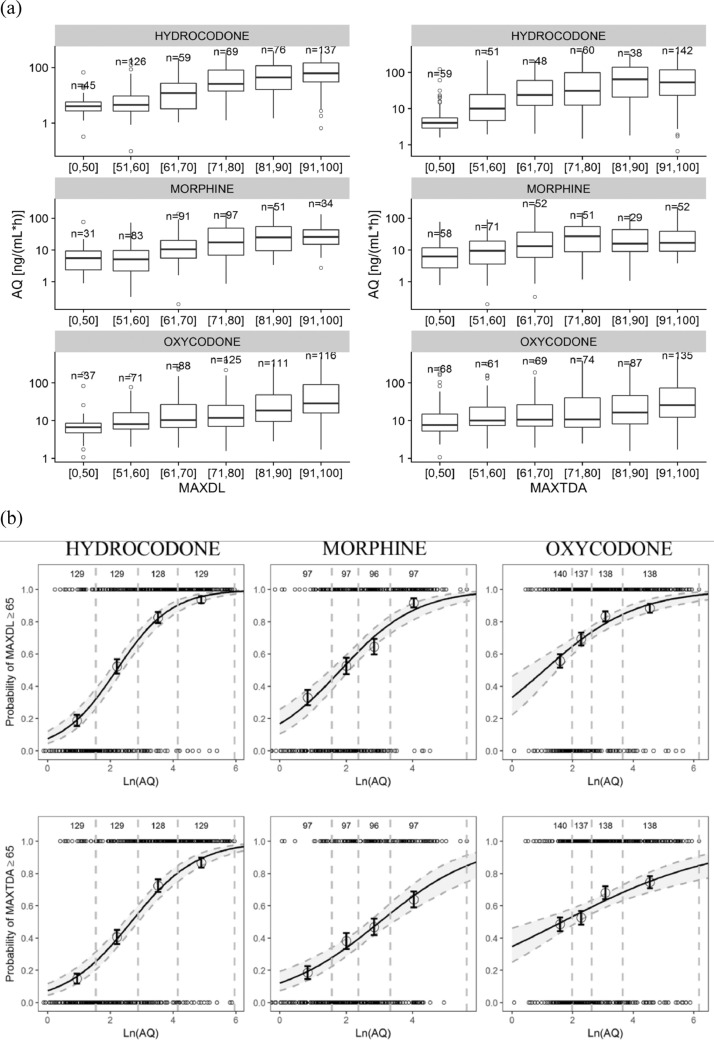
3.3. Association of abuse quotient (AQ) and PD metrics
Because literature reports suggested AQ as a PK metric reflective of likelihood of opioid abuse,[14], [15], [16], [17] the association between AQ and PD metrics were analyzed. Boxplots in Fig. 2a showed higher median AQ values in groups with higher maximum Drug Liking VAS and maximum Taking Drug Again VAS. Furthermore, as shown in Fig. 2b, natural log-transformed AQ was associated with the probability of maximum Drug Liking≥65 as well as the probability of maximum Taking Drug Again VAS≥65.
Fig. 2.
Association of AQ and PD metrics. (a) Boxplot diagram showing AQ values in subgroups with various PD measurement values. The data were grouped per opioid for analysis. MAXDL or MAXTDA values were grouped on a bipolar scale, where a score of 50 represents neutral, i.e., neither like nor dislike. (b) logistic regression analysis showing the association of natural log-transformed AQ and the probability of MAXDL ≥ 65 (top panel) and MAXTDA ≥ 65 (bottom panel). AQ: abuse quotient (Cmax/Tmax); MAXDL: maximum VAS for drug liking; MAXTDA: maximum VAS for take drug again. Each symbol with the error bars represents the observed probability and the associated 90% CIs per each exposure quartile for the given opioid. The line represents the logistic regression fit and the gray shaded area represents 5th–95th percentiles of the regression fit.
3.4. Consideration of PAUC3 and PAUC4 as an additional exposure metric to bridge innovator and generic products
In a Category 3 type study, subjects first go through screening (naloxone challenge), drug discrimination (qualification phase) and are finally recruited into treatment phase. During qualification phase of an abuse liability study, it is important to strike a balance in selecting subjects that can differentiate between placebo and positive control. The positive control is an opioid drug product or drug substance expected to result in a predictable opioid drug liking effect and has a known potential for, or history of, abuse, such as immediate release oxycodone/morphine/hydrocodone, etc. For this purpose, a value of 65 is used as cut-off on 100-point bipolar scale, that is subjects marking equal or above 65 when receiving opioid are considered to have the ability to successfully discriminate between placebo and opioid. Since the cut-off of 65 is selected in qualifying subjects into treatment phase, the same was used to identify pharmacological onset of action (drug liking, etc.) following administration of a treatment (positive control or manipulated modified release formulation).4
Using 65 as a cutoff value for Drug Liking response, the time-to-onset of Drug Liking response (Tonset) and the time-to-offset of Drug Liking response (Toffset) were determined and summarized in Table 2. Percentage of subjects who are with Drug Liking VAS≥65, i.e., who showed an onset of Drug Liking response, was significantly lower with ADFs than with non-ADFs even with manipulation indicating that ADFs were effective. Among those subjects with an onset of Drug Liking response, median Tonset values ranged from 0.5 to 1.75 h and all the median Toffset were ≤4 h except for the treatment group of hydrocodone powder in nasal administration route and AD oxycodone in oral route. Therefore, an early systemic exposure within the first 4 h may be relevant for the Drug Liking response. It should be noted that the timing of maximum Drug Liking response may occur before or after Tmax of systemic exposure supporting that Tmax or Cmax alone may not be predictive of abuse potential.
PAUC is a PK metric commonly used to assess early exposure. Starting time of the time interval for PAUC was chosen at 0 hour because of the rapid onset of Drug Liking response. It also made the implementation of this metric easy. To determine the relevant time interval, PAUEC and PAUC from 0 h to all sampling time points were analyzed for correlation using the linear regression analysis. Among all the time intervals that were analyzed, PAUEC and PAUC within the first 3 or 4 h post-dosing showed the highest R2 values for all three opioids evaluated, hydrocodone, morphine, and oxycodone.
Linear regression analysis was further performed to assess the association of abuse potential PD metrics and PK metrics using the data points grouped by clinical trials for each opioid with either oral or nasal route of administration (Fig. 3). First, the analysis showed that the two PD metrics were associated with each other, with the highest association shown for hydrocodone and the lowest for morphine. Second, it is noted that early partial AUCs, as compared to AUC0-t, Cmax and AQ metrics, explained a larger variation (R2) in maximum Drug Liking VAS and maximum Taking Drug Again using the linear regression model. As such, when compared to AUC0-t, Cmax and AQ metrics, early exposure metrics such as PAUC3 or PAUC4 were more associated with abuse potential PD metrics. We acknowledge that there is a lack of standard to inform the extent of significance of R2 in a clinical setting. In order to infer a meaningful R2 value, we examined the correlations between two closely related clinical endpoints, i.e., Drug Liking VAS and Take Drug Again VAS, which can potentially serve as benchmarks or internal reference standards for R2 values. The first row in each panel of Fig. 3 indicates the extent of correlation in terms of R2 value between maximum Take Drug Again VAS and maximum Drug Liking VAS. It shows that R2 values between the identified PAUECs and PAUCs are comparable to the ones between the two clinical endpoint measures for each of the products, demonstrating clinically meaningful correlations between early partial AUCs and drug liking potential.
Fig. 3.
Association between PK and PD metrics. R2 for Y∼X represents the variations in Y that can be explained by X using a linear regression model. MAXTDA: maximum VAS for take drug again; MAXDL: maximum VAS for drug liking; AQ: abuse quotient; AUC: area under the drug concentration-time curve; Cmax: peak plasma opioid concentration; PAUC3: partial area under the drug concentration-time curve from 0 to 3 h post-dose; PAUC4: partial area under the drug concentration-time curve from 0 to 4 h post-dose; PAUEC3: partial area under the VAS for drug liking-time curve from 0 to 3 h post-dose; PAUEC3: partial area under the VAS for drug liking-time curve from 0 to 4 h post-dose.
3.5. Association of early PAUCs and PD metrics
Given that the assumption of linear regression was not supported by the nature of PK metrics and PD metrics (i.e., PK metrics are continuous variables without a theoretical limit, while PD metrics of maximum Drug Liking and maximum Taking Drug Again can only range from 0 to 100), the greater association of early partial AUCs with PD metrics prompted us to conduct logistic regression analysis to evaluate the relationship between early exposure measures and abuse potential. Boxplots in Fig. 4a showed higher median PAUC3 values in subject groups with higher maximum Drug Liking VAS and maximum Taking Drug Again VAS.
Fig. 4.
Association of PAUC3 and PD metrics. (a) Boxplot diagram showing the PAUC3 values in subgroups with various PD measurement values. The data were grouped per opioid for analysis. MAXDL or MAXTDA values were grouped on a bipolar scale, where a score of 50 represents neutral, i.e., neither like nor dislike. (b). Example scatterplot for slopes without ISV vs with ISV for the logistic regression model. The diagonal solid line represents the identify line. Slopes without ISV indicated by X axis have three values for hydrocodone, oxycodone, and morphine, respectively, and slopes with ISV indicated by Y axis have eleven values for each of the pooled studies. (c) Logistic regression analysis showing the association of PAUC3 and the probability of MAXDL ≥ 65 (top panel) and MAXTDA ≥ 65 (bottom panel). MAXDL: maximum VAS for drug liking; MAXTDA: maximum VAS for take drug again; PAUC3: partial area under the drug concentration-time curve from 0 to 3 h post-dose. Each symbol with the error bars represents the observed probability and the associated 90% CIs per each exposure quartile for the given opioid. The line represents the logistic regression fit and the gray shaded area represents 5th – 95th percentiles of the regression fit.
Of note, the observed cross-study PK metrics in terms of Cmax, AUC0-t, AQ, PAUCs have overlapping distributions for the maximum Drug Liking VAS or maximum Taking Drug Again VAS groups in 0–50, 51–60, 61–70, 71–80, 81–90, and 91–100 for hydrocodone, morphine, and oxycodone across studies, and the. Statistical test of heterogeneity showed no significance for the relationships between the PD measures and PAUCs in all the VAS groups. In the multi-level logistic regression analysis, ISV has only demonstrated marginal effect on the regression outcomes. As shown in Fig. 4b, the scatterplot of the slope parameter values with ISV vs. without ISV shows that the individual study slopes for PAUC3 follow the identify line with acceptable variability for hydrocodone, oxycodone and morphine, indicating a consistent cross-study finding in terms of the identified PK-PD relationships.
Multivariate logistic regression analysis against available demographic, formulation related, route of administration related covariates (i.e., age, weight, BMI, sex, immediate-release vs. extended-release formulations, formulations with/without manipulation, oral vs intranasal) only showed that male had a higher probability of having MAXDL ≥ 65 than female upon the same early systemic exposure following oral or intranasal administration of morphine. The odds of having MAXDL ≥ 65 in response to early morphine exposure were greater for male than female. The sex difference was not observed in subjects taking hydrocodone or oxycodone and the identified PK-PD relationship stays to be positive in both sex groups for morphine.
As shown in Fig. 4c with the logistic regression analysis based on the pooled data, greater PAUC3 values was associated with greater probability of maximum Drug Liking VAS ≥ 65 and the probability of maximum Taking Drug Again VAS ≥ 80. When the probability of maximum Drug Liking VAS or maximum Taking Drug Again VAS ≥ 80 was assessed as the outcome in the logistic regression, PAUC3 was similarly associated with the probability of maximum Drug Liking VAS ≥ 80 and the probability of maximum Taking Drug Again VAS ≥ (Figure S2). A significant association was also found between PAUC4 and the probability of maximum Drug Liking VAS or maximum Taking Drug Again VAS ≥ 65 or 80. We observed that for the investigated opioid products, the correlation between plasma opioid exposure and opioid drug liking effect would be strongest if we take into account the onset of action, highest drug liking, offset of action and duration of action. Specifically, partial area under the curve of plasma concentration profile (onset to offset) correlates well with the partial area under the curve of drug liking VAS profile (onset to offset).
4. Discussion
Our study explored the relationships between systemic exposure and abuse potential response using the data from 11 clinical abuse potential trials supported the AD labeling claims of the 7 FDA approved opioid analgesics. No significant study heterogeneity was found that can affect the analysis outcomes for hydrocodone, morphine, and oxycodone. Our results supported the association between the early systemic exposure and abuse potential responses. In particular, early partial AUCs within the first 3 h and 4 h after dosing were associated with maximum Drug Liking and maximum Taking Drug Again responses for these opioid products following oral and nasal administrations in their intact formulation as well as after manipulation.
Many factors, both subject-related (e.g., age and sex) and formulation-related (e.g., immediate-release vs. extended-release, intact vs. manipulated), might impact whether a particular subject will respond to questionnaires about the Drug Liking and Taking Drug Again. It is of note that opioid agonists (such as oxycodone, hydrocodone and morphine) share the same mechanism of action in reducing the perception of pain while producing the feelings of pleasure.18 The binding of these opioids to opioid receptors in the brain cells blocks the transmission of pain signals from the brain and triggers a release of dopamine which rewards people with feelings of pleasure. A more rapid delivery seems to be associated with greater abuse potential. In other words, a rapid rise in plasma concentration or systemic exposure seems more likely to result in drug liking effect than a slower rise in plasma.19 Therefore, it is rational to explore the potential association between the systemic exposure and subjective abuse potential responses.
To our knowledge, this study is the first comprehensive analysis of the association of early partial AUCs with the likelihood of abuse responses based on the multiple premarket abuse potential clinical trials submitted to FDA supporting the abuse deterrent labeling. The data for seven opioid products in ADFs in intact formulation as well as with manipulation were used for our analysis. In summary, our comprehensive data analysis showed the median peak time for Drug Liking VAS was consistently ≤3 h, regardless of the administration routes (Table 2). Our findings support the notion that early drug exposure, such as the exposure within the first 3 h, may potentially be predictive of the PD response of abuse potential.
Further details of our findings are as follows. First, we observed that Cmax and maximum Drug Liking response were generally achieved within 3 h post-dosing for both intact and manipulated opioid ADFs (Table 2). This observation is congruent with the current pharmacological understanding of opioid-induced drug liking, i.e., rate of absorption is an important determinant of the abuse potential.20 It is important to note that the maximum Drug Liking response occurred regardless if the peak exposure occurred or not, implying a possible threshold of systemic exposure for eliciting Drug Liking response. Our analysis results provide insights into why conventional PK metrics such as total AUC and Cmax, or derived AQ alone may not be a good predictor of abuse potential. Secondly, the data showed that duration of Drug Liking response generally lasted for 2 to 4 h, with the median Toffset about 4 h following oral and nasal administration of manipulated opioids (Table 2). Linear regression analysis showed PAUEC and PAUC had the highest R2 value within the first 3 h or 4 h post-dosing indicating the association between the early partial AUCs as exposure metrics and PD measures of abuse potential (Fig. 3). Logistic regression analysis further suggested that the early partial AUCs are associated with abuse potential as indicated by the probability of maximum Drug Liking VAS ≥ 65 being significantly associated with PAUC3 and PAUC4 (Fig. 4). Additional supports for the association of early exposure metrics represented by PAUC3 and PAUC4 and abuse potential came from the observed association between the early partial AUCs and maximum Taking Drug Again response, another key abuse potential response metric (Fig. 4).
It is important to note that there was an association between abuse potential response metrics with two other PK metrics, namely Cmax and natural log-transformed AQ (Fig. 1, Fig. 2). This association may be cofounded by the observation in the data that Cmax was generally achieved within 3 h. However, use of Cmax and AQ as a predictor of abuse potential does not take into consideration the systemic drug concentrations before reaching Cmax. In particular, a major drawback of AQ metric for abuse potential prediction is due to that it cannot take into account the drug plasma concentrations before reaching Cmax. In addition, neither a formulation factor nor the route of administration was identified as a significant factor together with PAUCs in the logistic regression analysis, implying that changes in early systemic exposure can reflect any abuse potential difference resulting from a formulation factor or a route of administration. This supports the recommendation of comparative PK studies with PK metrics reflective of early exposure for evaluating and comparing abuse deterrence properties of the ADFs.
Our findings can be used to address the concern regarding the complexity and inconsistency of the PK/PD relationship for opioid products. Reflecting such a controversy, a comparative PK study conducted with manipulated oxycodone capsules with chewing under fasting vs. fed conditions. Chewing of oxycodone capsules under fed condition showed a higher Cmax, but maximum Drug Liking VAS response was lower compared to the results obtained under fasting condition.10,21 Applying the newly identified PAUC metrics, the geometric mean ratio of fed/fasting for PAUC3 and PAUC4 is 0.66 (90% CI: 56.49%–76.48%) and 0.76 (90% CI: 66.71%–87.50%), respectively. The PAUCs are congruent with the differential degree of responses in maximum Drug Liking VAS between fed and fasting conditions. These results also support the inclusion of the early partial AUC metrics as an additional PK metric for the assessment of AD properties for opioid products containing a single active ingredient. While PAUC3 and PAUC4 values appear reasonably associated with maximum Drug Liking VAS in the studies evaluated so far, there may be a different PAUC metric that is better associated with abuse potential measures such as maximum Drug Liking response for new innovator ADFs.
Our study findings have an impact on the regulatory guidance and assessment of opioid ADFs. FDA considers the development of these AD opioid analgesics as an important public health priority and has published two general guidance for industry, one focuses on providing regulatory clarity on demonstrating abuse-deterrent properties and the other specifically focuses on assisting ANDA applicants seeking approval of a generic version of a solid oral opioid drug product that references an opioid drug product with abuse-deterrent properties described in its labeling .4,11 In addition, product specific guidances22 are published to explain the agency's current thinking about the studies that should be conducted to demonstrate AD property of an opioid product submitted as a New Drug Application (NDA) and an ANDA. It should be emphasized that the current analysis lays the ground for the recommended comparative PK studies to support the abuse deterrence evaluation both for new and generic ADF of an opioid. Our analysis provides the scientific rationale for the relevance of the recommended PK endpoints in such comparative PK studies, in particular the early partial AUCs, to provide supportive evidence of abuse deterrence properties of an ADF.
Of note, some potential factors for abuse potential are not considered in our analyses because these premarket abuse potential trials were randomized and well-controlled in accordance with applicable regulations and guidances.4,12 For instance, the abuse potential response data in our analyses do not take into consideration any effort used by the abusers to manipulate the prescription opioid analgesics. In addition, the heterogeneity among all 11 clinical trials has not been explored considering similar study design and inclusion/exclusion criteria: all trials evaluate the abuse potential in recreational opioid users following a single dose of intact or manipulated innovator opioid product. It remains unknown if the identified early systemic exposure metrics correlate with the abuse potential after multiple doses of opioid analgesics. Finally, our analyses are limited to innovator opioid drug products which consist of a single active ingredient and do not contain any aversive agent or antagonist. Further evaluation of the exposure-response relationship would be needed for opioid products containing an aversive agent or antagonist.
In summary, we explored the association between early partial exposure metrics and abuse potential response while taking into account study heterogeneity using data from multiple clinical trials conducted in subjects with recreational opioid use following a single dose of intact or manipulated opioid products administered orally or nasally. Our findings on the relationship between the systemic exposure and abuse potential response, particularly supporting the use of early exposure as a predictor of abuse potential, are congruent with the current pharmacological understanding of opioid-induced feelings of pleasure and drug liking. Furthermore, the established association between PK and PD of abuse potential supports the agency's recommendation of comparative PK studies with early partial AUCs as additional PK metrics in the abuse deterrence assessment for generic opioid drug products and as supportive evidence for innovator opioid ADF products.
Supplementary Material
Figure S1. Boxplot diagram showing the AUC values in subgroups with various PD measurement values. MAXDL: maximum VAS for drug liking; MAXTDA: maximum VAS for take drug again.
Figure S2. Logistic regression analysis showing the association of PAUC3 and the probability of MAXDL ≥ 80 (top panel) and MAXTDA ≥ 80 (bottom panel). MAXDL: maximum VAS for drug liking; MAXTDA: maximum VAS for take drug again; PAUC3: partial area under the drug concentration-time curve from 0 to 3 h post-dose.
Funding
The study was partially funded by Fiscal Year 2017 Critical Path of the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration.
Authors’ contributions
L.Z., Z.L., L.F., MJ.K., and R.L. conceptualized the study. L.Z., Z. L., K.F., and L.F. collected and analyzed the data. SC.N., CG.S., SN.C, RA.R, C.S., K.F., and I.Z. contributed to interpretation of the results and writing the manuscript. L.Z., Z.L., and L.F. took the lead in writing the first draft. L.Z., Z. L., K.F., and L.F. had full access to all the data in the study. All authors critically reviewed, edited, and approved the final manuscript.
Data sharing
Data for all included studies are discussed in the corresponding FDA NDA reviews (https://www.accessdata.fda.gov/scripts/cder/daf/). All figures and statistical outputs are available in online.
Declaration of Competing Interest
The authors declared no competing interests for this work.
Acknowledgement
The study was partially funded by Fiscal Year 2017 Critical Path of the Center for Drug Evaluation and Research at the U.S. Food and Drug Administration. The authors would like to thank James Tolliver from Controlled Substance Staff, and Yun Xu from Office of Clinical Pharmacology at the U.S. Food and Drug Administration for their valuable suggestions on this project. The authors thank Dr. Miyoung Yoon for insightful discussions and manuscript editing assistance. Views expressed in this article do not necessarily reflect the official views or policies of the U.S. Food and Drug Administration or the U.S. Department of Health and Human Services, nor does any mention of trade names, commercial practices, or organization imply endorsement by the United States Government.
References
- 1.Jones C.M., Einstein E.B., Compton W.M. Changes in synthetic opioid involvement in drug overdose deaths in the United States, 2010-2016. JAMA. 2018;319(17):1819–1821. doi: 10.1001/jama.2018.2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Becker W.C., Fiellin D.A. Abuse-deterrent opioid formulations - putting the potential benefits into perspective. N Engl J Med. 2017;376(22):2103–2105. doi: 10.1056/NEJMp1701553. [DOI] [PubMed] [Google Scholar]
- 3.U.S. Food and Drug Administration. Drugs@FDA: FDA Approved Drug Products. https://www.accessdata.fda.gov/scripts/cder/daf/ (accessed 04/29/2021.
- 4.U.S. Food and Drug Administration . 2015. Guidance for Industry Abuse-Deterrent Opioids - Evaluation and Labeling.https://www.fda.gov/media/84819/download (accessed 04/29/2021 [Google Scholar]
- 5.Morton T.L., Devarakonda K., Kostenbader K., Montgomery J., Barrett T., Webster L. Correlation of subjective effects with systemic opioid exposure from fixed-dose combinations of Oxycodone/Acetaminophen in recreational users of prescription drugs. Pain Med. 2016;17(3):539–550. doi: 10.1111/pme.12884. [DOI] [PubMed] [Google Scholar]
- 6.Marsch L.A., Bickel W.K., Badger G.J. Effects of infusion rate of intravenously administered morphine on physiological, psychomotor, and self-reported measures in humans. J Pharmacol Exp Ther. 2001;299(3):1056–1065. [PubMed] [Google Scholar]
- 7.de Wit H., Bodker B., Ambre J. Rate of increase of plasma drug level influences subjective response in humans. Psychopharmacology (Berl) 1992;107(2–3):352–358. doi: 10.1007/BF02245161. [DOI] [PubMed] [Google Scholar]
- 8.Abreu M.E., Bigelow G.E., Fleisher L., Walsh S.L. Effect of intravenous injection speed on responses to cocaine and hydromorphone in humans. Psychopharmacology (Berl) 2001;154(1):76–84. doi: 10.1007/s002130000624. [DOI] [PubMed] [Google Scholar]
- 9.Schoedel K.A., Gillespie M., Levy-Cooperman N., Shram M.J. Rabinovich-Guilatt L. Pharmacokinetic and Pharmacodynamic correlations from 2 studies evaluating abuse potential of hydrocodone extended-release tablets. Clin Pharmacol Drug Dev. 2019;8(1):32–39. doi: 10.1002/cpdd.468. [DOI] [PubMed] [Google Scholar]
- 10.Branded Industry Working Group . Branded Industry Perspective on the Generics ADF Guidance. FDA Public Meeting on Pre-Market Evaluation of Abuse-Deterrent Properties of Opioid Drug Products. XXX; Hyattsville, MD: 2016. https://fda.report/media/100967/Branded-Industry-Perspective-on-the-Generics-ADF-Guidance--Dayno--J.pdf [Google Scholar]
- 11.U.S. Food and Drug Administration . 2017. Guidance for Industry General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products.https://www.fda.gov/media/96643/download (accessed 04/29/2021 [Google Scholar]
- 12.U.S. Food and Drug Administration . 2017. Guidance for Industry Assessment of Abuse Potential of Drugs.https://www.fda.gov/media/116739/download (accessed 04/29/2021 [Google Scholar]
- 13.Webster L.R. The question of opioid euphoria. 2009 https://www.dddmag.com/article/2009/07/question-opioid-euphoria July 30, 2009 2009. (accessed February 6, 2018. [Google Scholar]
- 14.Perrino P.J., Colucci S.V., Apseloff G., Harris S.C. Pharmacokinetics, tolerability, and safety of intranasal administration of reformulated OxyContin((R)) tablets compared with original OxyContin ((R)) tablets in healthy adults. Clin Drug Investig. 2013;33(6):441–449. doi: 10.1007/s40261-013-0085-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smith M.D., Webster L.R., Lawler J., Lindhardt K., Dayno J.M. Human Abuse Potential of an Abuse-Deterrent (AD), Extended-Release (ER) Morphine Product Candidate (Morphine-ADER Injection-Molded Tablets) versus extended-release morphine administered orally in nondependent recreational opioid users. Pain Med. 2017;18(5):898–907. doi: 10.1093/pm/pnw174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Webster L.R., Kopecky E.A., Smith M.D., Randomized Fleming AB.A. Double-blind, double-dummy study to evaluate the intranasal human abuse potential and pharmacokinetics of a novel extended-release abuse-deterrent formulation of Oxycodone. Pain Med. 2016;17(6):1112–1130. doi: 10.1093/pm/pnv020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Webster L.R., Smith M.D., Lawler J., Lindhardt K., Dayno J.M., Human Abuse Potential of an Abuse-Deterrent (AD) Extended-Release (ER) Morphine Product Candidate (Morphine-ADER Injection-Molded Tablets) vs extended-release morphine administered intra-nasally in nondependent recreational opioid users. Pain Med. 2017;18(9):1695–1705. doi: 10.1093/pm/pnw219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pasternak G.W., Pan Y.X. Mu opioids and their receptors: evolution of a concept. Pharmacol Rev. 2013;65(4):1257–1317. doi: 10.1124/pr.112.007138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Budman S.H., Grimes Serrano J.M., Butler S.F. Can abuse deterrent formulations make a difference? Expectation and speculation. Harm Reduct J. 2009;6:8. doi: 10.1186/1477-7517-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Webster L., Henningfield J., Buchhalter A.R. Human abuse potential of the new opioid analgesic molecule NKTR-181 compared with Oxycodone. Pain Med. 2018;19(2):307–318. doi: 10.1093/pm/pnw344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kopecky E.A., Fleming A.B., Levy-Cooperman N., O'Connor M., E M.S. Oral Human Abuse Potential of Oxycodone DETERx(R) (Xtampza(R) ER) J Clin Pharmacol. 2017;57(4):500–512. doi: 10.1002/jcph.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Product-Specific Guidances for Generic Drug Development. https://www.fda.gov/drugs/guidances-drugs/product-specific-guidances-generic-drug-development.