Abstract
Introduction
Pediatric exposures to cannabis edibles have been associated with serious adverse effects, such as respiratory depression. Yet, their incidence and relationship to exposure characteristics are not well defined. We attempt to describe the temporal, demographic, and clinical characteristics of pediatric patients with edible cannabis exposures and examine the relationship between these characteristics and two clinical outcomes: need for respiratory support and hospital admission.
Methods
A retrospective chart review was conducted at a single, tertiary care academic medical center covering a 28-month period. Inclusion criteria were: evaluation in the ED, age <18 years at the time of presentation, and physician documented exposure to edible cannabis. Exclusion criteria were: known or suspected co-ingestion of other substances.
Results
Thirty-two cases of edible cannabis ingestions were identified. Age <10 years was associated with bradypnea, hypertension, hospital admission, and respiratory support. Use of respiratory support was significantly associated with the presence of lethargy, bradypnea, hypercarbia, seizure, and hypertension. There was a five-fold increase in the number of pediatric edible cannabis exposures after recreational cannabis dispensaries opened in Massachusetts. Five patients (16%) required respiratory support and eleven (34%) required hospital admission.
Conclusions
There was a low incidence of need for respiratory support in our population, but hospital admission was more common. Severe symptoms (including lethargy and respiratory depression), need for respiratory support and hospital admission were more frequent in younger children. Exposures occurred with increasing frequency over time. Larger studies are needed to explore the relationship between THC dosage, age, and incidence of adverse outcomes.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13181-021-00849-0.
Keywords: Pediatrics, Cannabis, Marijuana use, Drug overdose, Toxicity, Epidemiology
Introduction
The past decade has seen an increase in the legalization of cannabis for both medicinal and recreational use across the USA. As of April 2021, 36 states allow the use of cannabis for medicinal purposes, with a number of additional states anticipated to legalize medical cannabis in the near future [1]. Since cannabis first became legal for recreational use in Colorado in 2012, fifteen additional states and the District of Columbia have followed suit. Following legalization of medical and recreational cannabis, multiple states and poison control centers have reported an increase in the incidence of pediatric cannabis exposures [2–7]. This population is particularly vulnerable to accidental exposures, with children less than the age of five being most commonly reported [4, 8].
Of particular concern are edible cannabis products, which are both visually appealing and palatable to children and adolescents, and have been implicated in the increasing numbers of accidental pediatric exposures [8–10]. Many of the cannabis-containing products marketed towards adults are naturally attractive to children, such as chocolates, baked goods, and candies. In addition, these products can be packaged and labeled in ways nearly identical to well-known commercially produced candies and other food items familiar to children, which may also lead to accidental overdose [11]. These products can contain a variable amount of delta-9 tetrahydrocannabinol (THC), with multiple suggested servings often contained in one discrete food object (i.e., multiple servings in one chocolate bar or cookie). Some products contain as much as 500 mg of THC per package [5]. Therefore, a single brownie, cookie, or other edible might still contain multiple serving units, and when consumed as a whole by a child can result in significant THC toxicity [4, 8]. Many states have attempted to limit the potential toxicity of edibles by restricting the amount of THC allowed within a serving unit [12]. However, products purchased online may not be subject to such regulations or may have inaccurate packaging, circumventing this safeguard mechanism.
There are numerous case reports, case series, and retrospective cohort studies in the literature that describe the effects of cannabis overdose in pediatric patients [2, 13–15]. Lethargy, altered mental status, and respiratory depression are the most frequently reported symptoms of unintentional cannabis exposures [2, 13–15]. Additional reported signs and symptoms include vomiting, dizziness, euphoria, ataxia, hypotonia, mydriasis, and tachycardia [13, 14, 16]. This spectrum of presenting symptoms has been fairly well established; however, the details regarding the true incidence of these findings are lacking.
Of the ten retrospective studies referenced in the 2017 systematic review on pediatric exposures to edible cannabis products by Richards et al., only four of the studies were from hospital-based data, and the remainder of the studies were based on poison center data [13]. As noted by the authors of the review, while valuable, poison center data is limited by under reporting and incomplete data collection. Of the four hospital-based studies included in the review, two were French studies that focused only on children under 3 years of age or children admitted to the PICU [17, 18]. The remaining two retrospective studies were based out of Children’s Hospital Colorado and covered the same time period from 2009 to 2014. Both studies searched through all patients presenting to the hospital system; however, Wang et al. only focused on patients less than 10 years of age [4]. The remaining study by Heizer et al. is the most comprehensive due to its inclusion of patients up to 20 years of age, its differentiation between cannabis naïve vs non-naïve patients, and its evaluation for a dose-dependent effect [19]. However, this study combined both inhalational exposures and oral exposures in their analysis of signs and symptoms incidence and was limited by a small sample size (N=8) for their dosing-based analysis. Therefore, the current body of work on this topic is limited by non-generalizable populations, incomplete data sets, selection bias, and combined routes of exposure.
Additional research on pediatric exposures to edible cannabis products is needed to fully elucidate the incidence of key signs, symptoms, and outcomes in relationship to the amount of edible product consumed. While many of the typical presenting signs and symptoms of cannabis intoxication can be distressing to patients and parents, the most concerning toxicity from the standpoint of morbidity and mortality is respiratory depression. A better understanding of the frequency of cannabis-induced respiratory depression and its relationship to dose ingested and other exposure characteristics would improve risk stratification in cases of exposure, aid clinicians in determining safe disposition, and inform policy makers when determining packaging regulations. As an initial step to fill this gap in the literature, the present study aims to: [1] understand the demographic, clinical, and temporal characteristics of patients exposed to edible cannabis products in a retrospective cohort and [2] examine the relationship between these characteristics and two clinical outcomes of interest: need for respiratory support and hospital admission.
Methods
Study Design and Data Source
This retrospective chart review was approved by the University of Massachusetts Medical School Institutional Review Board. To identify relevant cases from patient charts, we utilized an analytics function of the EPIC electronic medical record (Web Intelligence System). We collected data for a 28-month period, reflecting available data from the month our institution began using the EPIC electronic medical record (October 2017) to the month the search was conducted (January 2020). Our search algorithm was based on the work of Marx et al. and cross-referenced patient ages, dates of emergency department (ED) encounters, diagnosis codes, and chief complaints as recorded by nursing staff at triage (algorithm details in Supplementary Data) [20]. These chief complaints represent the nursing interpretation of the patient’s reported chief complaint and therefore represent an amalgamation of more specific complaints. Cases identified by this algorithm were manually reviewed by the principal investigator (PI) to determine whether they met inclusion or exclusion criteria. Cases that screened in were independently evaluated and double coded using a standardized data abstraction tool by two investigators (EK and KL, Supplementary Table 1). The co-investigator was trained by the PI (EK) on the use of the abstraction tool, and the PI was available for questions as needed throughout the abstraction process. The abstractors were not blinded to the goal of the study, and inter-rater reliability was not calculated as all cases were double coded. Any discrepancies in data abstraction were resolved by a third investigator (SC). When occurrence data was missing, it was coded as a non-occurring event. When numerical data was missing, listwise deletion was employed during analysis utilizing that data point.
Inclusion and Exclusion Criteria
Inclusion criteria were evaluation at our ED, less than 18 years of age at the time of presentation, and physician documented suspected exposure to edible cannabis. Cases with known or suspected co-ingestion of other drugs in addition to the edible cannabis exposure were excluded. A manual review of the final diagnoses of each case was performed by the PI, and cases with diagnoses signifying an exclusion criterion (i.e., alcohol intoxication) were removed. The text of the remaining cases was then screened for the presence of the keywords: “edible,” “THC,” “CBD,” “cannabis,” “marijuana,” or “pot.” Finally, cases containing any of the keywords were manually reviewed in detail by the PI to confirm that the remaining inclusion criteria were met.
Selection of Demographic, Clinical, and Outcome Variables
Demographic, historical, and clinical factors were selected as potential predictor variables based on clinical relevance to the exposure, in addition to the completeness of the available data in the electronic medical record. The patient’s highest and lowest recorded vital signs (heart rate, systolic blood pressure, respiratory rate, and oxygen saturation) were abstracted from the ED and inpatient documentation for each case, and vital sign abnormalities were identified using age appropriate normal vital sign ranges defined by Nelson Textbook of Pediatrics [21]. Other variables specific to the exposure of interest included estimated maximum potential dose of THC ingested (in mg and mg/kg), whether the ED visit occurred before or after the opening of recreational retail cannabis shops in the state of Massachusetts (November 20, 2018), whether a urine drug screen was performed, and if the result of the urine drug screen was positive for cannabinoids. The primary clinical outcome of interest was needed for respiratory support, defined as the need for supplemental oxygen, non-invasive positive pressure ventilation, endotracheal intubation, or other advanced airway maneuvers. Admission to the hospital was evaluated as a secondary outcome.
Statistical Analysis
Descriptive statistics (mean, median, standard deviation (SD), interquartile range for continuous variables, and frequency for categorical variables) were calculated for all predictor and outcome variables to understand sample characteristics and trends. Normality of variable distribution was assessed with the Shapiro-Wilk test. Differences in continuous variables based on outcomes of interest were tested using Students T test (for two groups, normally distributed with equal variance) or Wilcoxon Ranks Sum (for two groups, with skewed distributions). Differences in categorical variables based on outcomes of interest were assessed using the Chi-square test. Statistical analyses were performed using Stata/IC 14.2 (StataCorp, College Station, TX).
Results
Case Selection
The initial search using the previously described logic system yielded 1534 cases (Fig. 1). After manual review of final diagnoses, 119 cases were excluded, yielding 1415 cases. Of these 1415, 103 relevant cases with cannabis exposure were identified via a manual keyword search and review of the charts. Seventy-one cases were excluded due to alternative route of cannabis exposure (i.e., inhalation) and polysubstance use. The final sample consisted of 32 cases of confirmed or suspected cannabis edible exposures.
Fig. 1.
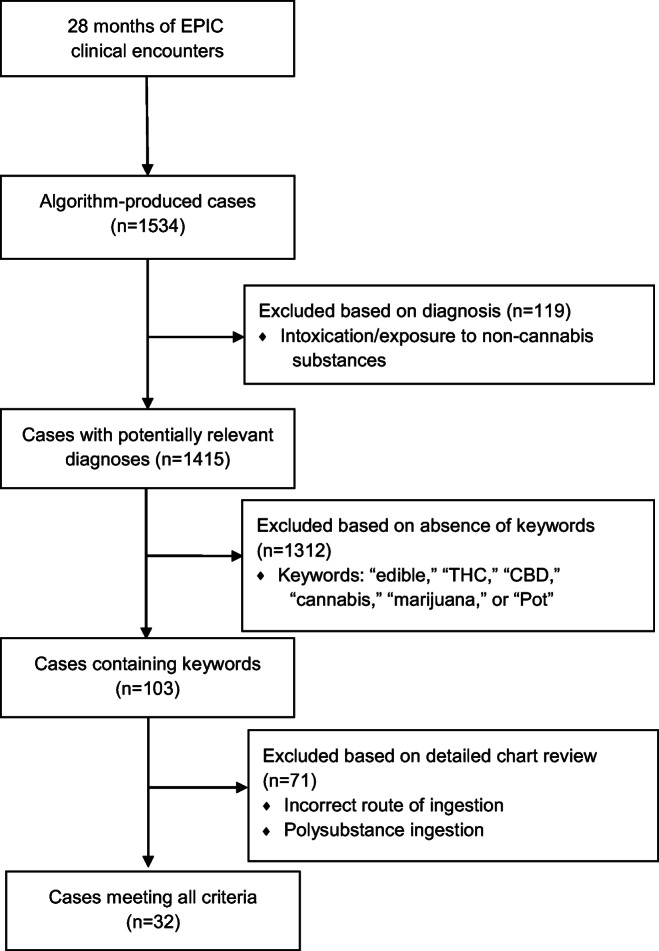
Recruitment flow diagram.
Study Population and Presentation Characteristics
The final sample of N=32 unique subjects (Table 1) was 53% female, 72% white, and had a median age of 12.5 years (range 1–17). Most common chief complaints were ingestion (69%) and altered mental status (16%). Most common symptoms were lethargy/somnolence (59%), ataxia/dizziness (50%), and confusion (34%). Most common vital sign abnormalities were tachycardia (91%) and hypotension (75%). Of note, five patients (16%) received respiratory support and seizure occurred in only one case (3%). No arrhythmias or conduction abnormalities were found on review of the patients’ electrocardiograms, with the longest QRS being 100 msec and the longest QTc being 457 msec.
Table 1.
Study population and patient characteristics.
| Factor | Level | Value |
|---|---|---|
| N | 32 | |
| Age (years), median (IQR) | 12.5 (4, 16) | |
| Sex, n (%) | Male | 15 (47%) |
| Race, n (%) | White | 23 (72%) |
| Black or African American | 4 (12%) | |
| Other | 3 (9%) | |
| Hispanic | 1 (3%) | |
| Multiracial | 1 (3%) | |
| Ethnicity, n (%) | Not Hispanic or Latino | 28 (88%) |
| Hispanic or Latino | 4 (12%) | |
| Weight (kg), median (IQR) | 32.8 (16.3, 68) | |
| ED chief complaint, n (%) | Ingestion | 22 (69%) |
| Altered mental status | 5 (16%) | |
| Drug overdose | 4 (12%) | |
| Seizure | 1 (3%) | |
| Exposures after cannabis shops open, n (%) | 27 (84%) | |
| Hospital admission, n (%) | Any | 11 (34%) |
| PICU | 8 (25%) | |
| Length of stay (min), median (IQR) | 434.5 (304, 1342) | |
| Urine drug screen | Performed, n (%) | 19 (59%) |
| Positive, n (% of performed) | 17 (89%) | |
| THC consumed (mg), median (IQR) | 100 (20, 200) | |
| THC dose (mg/kg), median (IQR) | 1.4 (1, 4.4) | |
| Intent | Accidental | 19 (59%) |
| Need for respiratory support, n (%) | Any | 5 (16%) |
| Nasal cannula | 4 (13%) | |
| HFNC | 1 (3%) | |
| BiPAP | 1 (3%) | |
| Nasal trumpet | 1 (3%) | |
| Signs/symptoms, n (%) | Lethargy/somnolence | 19 (59%) |
| Ataxia/dizziness | 16 (50%) | |
| Confusion | 11 (34%) | |
| Vomiting | 9 (28%) | |
| Seizure | 1 (3%) | |
| Vital sign abnormalities, n (%) | Tachycardia | 29 (91%) |
| Hypotension | 24 (75%) | |
| Hypertension | 12 (38%) | |
| Bradypnea | 11 (34%) | |
| Bradycardia | 2 (6%) | |
| Hypoxia | 2 (6%) | |
| Hypercarbia | 2 (6%) | |
| ECG findings | QRS (msec), median (IQR) | 80 (74, 86) |
| QTc, mean (SD) | 435.4 (12.3) |
ED emergency department, PICU pediatric intensive care unit, min minute, THC delta-9-tetrahydrocannabinol, HFNC high flow nasal cannula, BiPAP bilevel positive airway pressure, ECG electrocardiogram, msec millisecond, IQR interquartile range, SD standard deviation
The type of edible was identified and documented in 91% of cases. Chocolates (38%) and gummies (34%) were the most common (Supplementary Table 2), but other types included brownies, juice and other liquids, toaster pastries, and cookies. A specific edible product name was identified and documented in 22% of cases, with product names like TKO Edibles, Stoney Patch, Willy Wonka, Chilly Wonka, and Patriot Care Dispensary (Supplementary Table 3).
The most common treatment was intravenous (IV) fluids (59%), and no treatment was administered in 31% of cases (Supplementary Table 4). Thirty-two unique International Statistical Classification of Diseases and Health Related Problems, 10th Revision (ICD-10) codes were identified in the ED admission and hospital discharge diagnoses (Supplementary Table 5), the most common being “Altered mental status” (N=4), “Accidental marijuana overdose” (N=3), “Drug intoxication without complication” (N=3), and “Marijuana use” (N=3).
Exposures by Age Category
When patients were stratified into age groups, there were N=12 patients in the younger group (0–9 years) and N=20 in the older group (10–17 years, Table 2). This age cut off was chosen based on prior work by Wang et al. and creates a comparable study population of patients less than 10 years of age [4]. Patients in the younger age group were more likely to have a lower median weight, require hospital admission and respiratory support, have an accidental ingestion, receive a urine drug screen, and have bradypnea and/or hypertension. While not statistically significant, the older age group had larger median ingested doses of THC with a median of 2.3 mg/kg compared to the younger age group with a median of 1.4 mg/kg.
Table 2.
Patient characteristics and age.
| Factor | Age 0–9 years | Age 10–17 years | P-value |
|---|---|---|---|
| N | 12 | 20 | |
| Exposures after cannabis shops open, n (%) P | 12 (100%) | 15 (75%) | 0.059 |
| Intent (accidental), n (%) P | 12 (100%) | 7 (35%) | <0.001 |
| Sex (male), n (%) P | 4 (33%) | 11 (55%) | 0.23 |
| Weight (kg), median (IQR) W | 16.0 (14.2, 20.6) | 67.9 (44.8, 79.2) | <0.001 |
| Age (years), median (IQR) W | 3 (2, 4.5) | 15 (13, 16.5) | <0.001 |
| Need for respiratory support (any), n (%) P | 4 (33%) | 1 (5%) | 0.033 |
| Hospital admission (any), n (%) P | 10 (83%) | 1 (5%) | <0.001 |
| Hospital admission (PICU), n (%) P | 7 (58%) | 1 (5%) | <0.001 |
| Length of stay (min), median (IQR) W | 1342 (944, 2077) | 350 (259, 455) | <0.001 |
| Urine drug screen (performed), n (%) P | 11 (92%) | 8 (40%) | 0.004 |
| Urine drug screen (+), n (% of performed) P | 10 (91%) | 7 (88%) | 0.81 |
| THC consumed (mg), median (IQR) W | 20 (17.5, 150) | 102.5 (70, 300) | 0.15 |
| THC dose (mg/kg), median (IQR) W | 1.35 (1.03, 9.7) | 2.3 (0.9, 3.6) | 0.6 |
| Lethargy/somnolence, n (%) P | 11 (92%) | 8 (40%) | 0.004 |
| Ataxia, n (%) P | 4 (33%) | 12 (60%) | 0.14 |
| Seizure, n (%) P | 1 (8%) | 0 (0%) | 0.19 |
| Vomiting, n (%) P | 2 (17%) | 7 (35%) | 0.26 |
| Confusion, n (%) P | 4 (33%) | 7 (35%) | 0.92 |
| Hypercarbia, n (%) P | 2 (17%) | 0 (0%) | 0.059 |
| Bradypnea, n (%) P | 10 (83%) | 1 (5%) | <0.001 |
| Hypoxia, n (%) P | 1(8%) | 1(5%) | 0.71 |
| Bradycardia, n (%) P | 1 (8%) | 1 (5%) | 0.71 |
| Tachycardia, n (%) P | 12 (100%) | 17 (85%) | 0.16 |
| Hypotension, n (%) P | 9 (75%) | 15 (75%) | 1 |
| Hypertension, n (%) P | 9 (75%) | 3 (15%) | <0.001 |
| QRS (msec), median (IQR) W | 76 (71, 84) | 82 (78, 86) | 0.14 |
| QTc, mean (SD) T | 436.4 (11.0) | 434.8 (13.3) | 0.76 |
P= Pearson’s Chi-Square Test, T=Student’s t-test, W= Wilcoxon Ranks Sum
Intentional vs Unintentional Exposures
Intent may be a confounding factor in the outcomes of interest, and patients’ history of cannabis use prior to their ED visit was not available. Therefore, the intent of exposure was evaluated as a surrogate marker of potential tolerance. There were N=19 patients in the accidental ingestion group and N=13 in the intentional ingestion group (Table 3). The accidental exposure group was younger with a median age of 5 years (range 1–17), compared to the intentional group with a median age of 16 years (range 13–17). Accidental exposures were found to have a statistically significant association with younger age, lower weight, admission, a urine drug screen collection, bradypnea, and hypertension.
Table 3.
Patient characteristics and ingestion intent.
| Factor | Accidental ingestion | Intentional/unknown intent ingestion | P-value |
|---|---|---|---|
| N | 19 | 13 | |
| Exposures after cannabis shops open, n (%) P | 17 (89%) | 10 (77%) | 0.34 |
| Sex (male), n (%) P | 9 (47%) | 6 (46%) | 0.95 |
| Weight (kg), median (IQR) W | 21.2 (14.3, 34.2) | 72.55 (66.95, 78.4) | 0.001 |
| Age (years), median (IQR) W | 5 (3, 12) | 16 (14, 17) | <0.001 |
| Need for respiratory support (any), n (%) P | 4 (21%) | 1 (8%) | 0.31 |
| Hospital admission (Any), n (%) P | 10 (53%) | 1 (8%) | 0.009 |
| Hospital admission (PICU), n (%) P | 7 (37%) | 1 (8%) | 0.061 |
| Length of stay (min), median (IQR) W | 877 (298, 1801) | 427 (310, 523) | 0.20 |
| Urine drug screen (performed), n (%) P | 14 (74%) | 5 (38%) | 0.046 |
| Urine drug screen (+), n (% of performed) P | 12 (86%) | 5 (100%) | 0.37 |
| THC consumed (mg), median (IQR) W | 20 (16.7, 200) | 105 (70, 300) | 0.17 |
| THC dose (mg/kg), median (IQR) W | 1.4 (1, 7.1) | 1.7 (1.0, 3.4) | 0.65 |
| Lethargy/somnolence, n (%) P | 12 (63%) | 7 (54%) | 0.60 |
| Ataxia, n (%) P | 10 (53%) | 6 (46%) | 0.72 |
| Seizure, n (%) P | 1 (5%) | 0 (0%) | 0.40 |
| Vomiting, n (%) P | 5 (26%) | 4 (31%) | 0.78 |
| Confusion, n (%) P | 5 (26%) | 6 (46%) | 0.25 |
| Hypercarbia, n (%) P | 2 (11%) | 0 (0%) | 0.23 |
| Bradypnea, n (%) P | 10 (53%) | 1 (8%) | 0.009 |
| Hypoxia, n (%) P | 1 (5%) | 1 (8%) | 0.78 |
| Bradycardia, n (%) P | 1 (5%) | 1 (8%) | 0.78 |
| Tachycardia, n (%) P | 18 (95%) | 11 (85%) | 0.33 |
| Hypotension, n (%) P | 16 (84%) | 8 (62%) | 0.15 |
| Hypertension, n (%) P | 10 (53%) | 2 (15%) | 0.033 |
| QRS (msec), median (IQR) W | 79 (72, 88) | 82 (80, 84) | 0.38 |
| QTc, mean (SD) T | 437.0 (12.3) | 432.8 (12.5) | 0.43 |
P= Pearson’s Chi-Square Test, T=Student’s t-test, W= Wilcoxon Ranks Sum
Evaluation of THC Weight-Based Exposure
Data to estimate weight-based THC dose was available on less than 50% (N=15) of the sample. Of these patients, one had an unwitnessed ingestion, did not develop symptoms, and had a negative urine drug screen leaving N=14 for analysis (Supplementary Table 6). THC dose per kilogram was not significantly different based on primary or secondary outcome categories, although the dose of THC was higher in the respiratory support group (median 7.1 mg/kg) compared to the group that did not receive respiratory support (median 1.18 mg/kg) (Table 4). Among children under the age of 10 years (N=7), median dose of THC was 1.4 mg/kg (range 0.4–24.4), and in children 10 years of age and over (N=7), median dose was 2.3 mg/kg (range 0.5–4.4). All patients under 10 years of age required admission except one child who ingested 1 mg/kg of THC, whereas only one subject in the 10–17 year age range required admission after ingesting 2.3 mg/kg. Multiple patients within the older age group were able to tolerate up to 3–4 mg/kg ingestions with subsequent discharge after a period of ED observation.
Table 4.
Patient characteristics and need for respiratory support.
| Factor | (−) Respiratory support | (+) Respiratory support | P-value |
|---|---|---|---|
| N | 27 | 5 | |
| Intent (accidental), n (%) P | 15 (56%) | 4 (80%) | 0.31 |
| Exposures after cannabis shops open, n (%) P | 22 (81%) | 5 (100%) | 0.29 |
| Sex (male), n (%) P | 15 (56%) | 0 (0%) | 0.02 |
| Weight (kg), median (IQR) W | 39.5 (20, 68) | 16.3 (14.1, 25) | 0.13 |
| Age (years), median (IQR) W | 13 (5, 16) | 4 (3, 4) | 0.10 |
| Hospital admission (Any), n (%) P | 6 (22%) | 5 (100%) | <0.001 |
| Hospital admission (PICU), n (%)P | 3 (11%) | 5 (100%) | <0.001 |
| Length of stay (min), median (IQR) W | 401 (282, 877) | 2319 (1635, 2348) | 0.001 |
| Urine drug screen (performed), n (%) P | 14 (52%) | 5 (100%) | 0.044 |
| Urine drug screen (+), n (% of performed) P | 12 (86%) | 5 (100%) | 0.37 |
| THC consumed (mg), median (IQR) W | 70 (20, 300) | 175 (100, 200) | 0.31 |
| THC dose (mg/kg), median (IQR) W | 1.18 (0.95, 3.3) | 7.1 (2.3, 12.3) | 0.083 |
| Lethargy/somnolence, n (%) P | 14 (52%) | 5 (100%) | 0.044 |
| Ataxia, n (%) P | 14 (52%) | 2 (40%) | 0.63 |
| Seizure, n (%) P | 0 (0%) | 1 (20%) | 0.018 |
| Vomiting, n (%) P | 7 (26%) | 2 (40%) | 0.52 |
| Confusion, n (%) P | 8 (30%) | 3 (60%) | 0.19 |
| Hypercarbia, n (%) P | 0 (0%) | 2 (40%) | <0.001 |
| Bradypnea, n (%) P | 6 (22%) | 5 (100%) | <0.001 |
| Hypoxia, n (%) P | 0 (0%) | 2 (40%) | <0.001 |
| Bradycardia, n (%) P | 2 (7%) | 0 (0%) | 0.53 |
| Tachycardia, n (%) P | 24 (89%) | 5 (100%) | 0.43 |
| Hypotension, n (%) P | 20 (74%) | 4 (80%) | 0.78 |
| Hypertension, n (%) P | 7 (26%) | 5 (100%) | 0.002 |
| QRS (msec), median (IQR) W | 82 (78, 88) | 74 (68, 80) | 0.055 |
| QTc, mean (SD) T | 435.6 (13.3) | 435.8 (6.4) | 0.91 |
P= Pearson’s Chi-Square Test, T=Student’s t-test, W= Wilcoxon Ranks Sum
Temporal Distribution of Cases
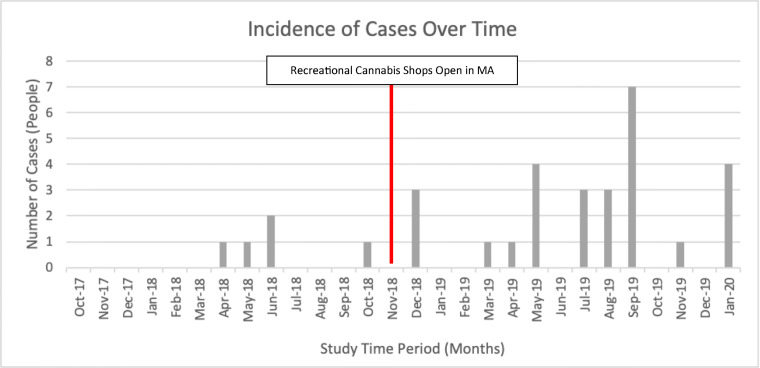
While there was no discernable pattern in the month-to-month variation of cases, there was an increase in the number of edible exposure cases in the second half of the study compared to the first half. Of note, the mid-point of our study coincides with the date that legal cannabis dispensaries opened in the state of Massachusetts (November 20, 2018). More specifically, five (16%) of our cases occurred before this date, and the remaining 27 cases (84%) occurred after (Fig. 2).
Fig. 2.
Changes in case volume over time.
Analysis of Primary Clinical Outcome—Need for Respiratory Support
Five patients (16%) demonstrated the primary outcome (need for respiratory support). The types of respiratory support used were nasal cannula (NC, N=4), high flow nasal cannula (HFNC, N=1), bilevel intermittent positive airway pressure (BiPAP, N=1), and nasal trumpet (N=1) with some patients alternating between modalities. The results of predictor variables stratified by the primary outcome are outlined in Table 4. The need for respiratory support had statistically significant associations with female sex, seizure, lethargy/somnolence, hypertension, and the collection of a urine drug screen. The outcome was also associated with clinical markers of respiratory insufficiency (bradypnea, hypoxia, and hypercarbia) and hospital admission. Median age and median weight were both lower in the group that needed respiratory support, but this difference was not statistically significant. However, when stratified by age groups (ages 0–9 and 10–17 years, Table 2), the younger age cohort was significantly associated with requirement for respiratory support.
Analysis of Secondary Clinical Outcome—Hospital Admission
The incidence of any hospital admission and pediatric intensive care unit (PICU) admission were 34% (N=11) and 25% (N=8) of the total sample, respectively. The results of predictor variables stratified by the secondary outcome are presented in Table 5. Overall admission was associated with lower weight, younger age, need for respiratory support, lethargy/somnolence, collection of a urine drug screen, bradypnea, hypercarbia, hypoxia, unintentional ingestions, and hypertension. The number of non-PICU admissions was notably low (N=3) and thus precluded meaningful comparisons of the PICU vs non-PICU admission groups.
Table 5.
Patient characteristics and the need for hospital admission.
| Factor | (−) Hospital admission | (+) Hospital admission | P-value |
|---|---|---|---|
| N | 21 | 11 | |
| Exposures after cannabis shops open, n (%) P | 16 (76%) | 11 (100%) | 0.078 |
| Intent (accidental), n (%) P | 9 (43%) | 10 (91%) | 0.009 |
| Sex (male), n (%) P | 11 (52%) | 4 (36%) | 0.39 |
| Weight (kg), median (IQR) W | 63.5 (33.5, 78.4) | 15.6 (14.1, 23.2) | <0.001 |
| Age (years), median (IQR) W | 14 (12, 16) | 3 (2, 4) | <0.001 |
| Need for respiratory support (any), n (%) P | 0 (0%) | 5 (45%) | <0.001 |
| Hospital admission (PICU), n (%) P | 0 (0%) | 8 (73%) | <0.001 |
| Length of stay (min), median (IQR) W | 344 (263, 432) | 1635 (1014, 2319) | <0.001 |
| Urine drug screen (performed), n (%) P | 8 (38%) | 11 (100%) | <0.001 |
| Urine drug screen (+), n (% of performed) P | 7 (88%) | 10 (91%) | 0.81 |
| THC consumed (mg), median (IQR) W | 100 (70, 300) | 60 (17.5, 187.5) | 0.47 |
| THC dose (mg/kg), median (IQR) W | 1 (.9, 3.6) | 1.85 (1.18, 9.7) | 0.3 |
| Lethargy/somnolence, n (%) P | 9 (43%) | 10 (91%) | 0.009 |
| Ataxia, n (%) P | 12 (57%) | 4 (36%) | 0.26 |
| Seizure, n (%) P | 0 (0%) | 1 (9%) | 0.16 |
| Vomiting, n (%) P | 7 (33%) | 2 (18%) | 0.37 |
| Confusion, n (%) P | 6 (29%) | 5 (45%) | 0.34 |
| Hypercarbia, n (%) P | 0 (0%) | 2 (18%) | 0.044 |
| Bradypnea, n (%) P | 1 (5%) | 10 (91%) | <0.001 |
| Hypoxia, n (%) P | 0 (0%) | 2 (18%) | 0.044 |
| Bradycardia, n (%) P | 1 (5%) | 1 (9%) | 0.63 |
| Tachycardia, n (%) P | 18 (86%) | 11 (100%) | 0.19 |
| Hypotension, n (%) P | 15 (71%) | 9 (82%) | 0.52 |
| Hypertension, n (%) P | 3 (14%) | 9 (82%) | <0.001 |
| QRS (msec), median (IQR) W | 82 (78, 86) | 78 (71, 85) | 0.33 |
| QTc, mean (SD) T | 434.53 (13.2) | 438.9 (11.2) | 0.66 |
P= Pearson’s Chi-Square Test, T=Student’s t-test, W= Wilcoxon Ranks Sum
Discussion
During our analysis of patient characteristics, we found that female sex, lethargy/somnolence, seizure, hypercarbia, bradypnea, hypoxia, and hypertension were associated with the need for respiratory support. Younger age, lower weight, lethargy/somnolence, hypercarbia, bradypnea, and hypertension were associated with hospital admission. While bradypnea occurred fairly often in our study N=11 (34%), it occurred mostly in children under 10 years of age (N=10, 83% of bradypnic patients) and rarely required significant respiratory interventions such as BiPAP or HFNC (N=2, 6% total and 18% of bradypnic patients). There was a single incidence of seizure (3%), which occurred in a 4-year-old girl with an unclear ingestion history, though her confirmatory urine testing was positive for cannabinoids and negative for synthetic cannabinoids. While the median mg/kg THC ingestions were lower in the younger group compared to the older group, there were several subjects in the younger age group who were exposed to markedly larger mg/kg THC ingestions, with the highest reported dose being 24.4 mg/kg compared to 4.4mg/kg in the older age group. This is likely due to a combination of their smaller weights and usually accidental nature of the ingestion. While our data clearly indicates that younger children are at increased risk for severe symptoms such as respiratory depression and lethargy due to cannabis intoxication, it is unclear whether this is due to the cases of higher mg/kg dosing exposures skewing the data or some inherit or acquired difference in underlying pharmacokinetics and pharmacodynamics leading to varying degrees of tolerance between the age groups.
Similar to studies performed in other states where recreational cannabis has been legalized, we found a nearly five-fold increase in the number of pediatric edible cannabis exposures when comparing the time periods before and after recreational cannabis dispensaries were allowed to open in our state [5, 10]. Increased pediatric exposure to cannabis has been concurrent with the rise in adult cannabis use, which has more than doubled over the past decade [6]. Use of cannabis among parents with children in the home has increased [22], which may also contribute to the risk of accidental ingestion of cannabis products by children due to increased accessibility.
The public normalization of cannabis use comes at the risk of minimalizing public perception of its potential harms. The increased in-home use and the failure of adult consumers to properly secure their cannabis products increase the potential for accidental exposures in children [6, 7, 13]. The incidence of respiratory depression requiring intervention in our study may be low (N=5, 16%); however, it was not negligible, and it is important to remain cognizant that pediatric deaths attributed to cannabis overdose have been reported [4, 6]. Of our patients that required respiratory support, there was one case with documented hypercarbia alone and one case of hypercarbia with hypoxia, treated with NC then HFNC and nasal trumpet then BiPAP, respectively. This supports the idea that a potentially life-threatening decrease in respiratory drive is possible with cannabis product ingestions. The remaining three patients received nasal cannula only, but only one case had documented hypoxia. While the hypoxia may have simply not been documented for these other two patients, it remains unclear in this retrospective study what the indication for oxygen was in these cases.
Caretakers may not immediately recognize the symptoms of cannabis ingestion and subsequent intoxication in their child given the delay in onset of symptoms. There is no standardized form of cannabis, and the concentration and formulation of cannabis ingested can impact the acute effects of intoxication, as well as the overall recovery time [23]. As opposed to inhaled THC, which reaches peak concentration within 10 minutes of use, ingested THC reaches peak concentration between 2 and 3 hours post-ingestion, with symptoms lasting upwards of 5 hours or longer in typical studied doses, and potentially much longer in overdoses [5, 24]. This delayed onset can make it difficult to determine the time of ingestion and expected duration of symptoms at the time of patient presentation. Lethargy/somnolence were present in 19 patients (59%), making them the most commonly reported symptoms in our exposed patient population. This suggests that cannabis intoxication should be considered in the differential diagnosis of altered mental status in children, particularly in those ages that are at increased risk of exploratory behavior (i.e., toddlers) and in states where there is increased access to cannabis-containing products. Awareness of the spectrum of symptoms of accidental cannabis ingestion in a pediatric patient can help guide an evaluation and may prevent a costly and invasive workup in a child who presents with altered mental status.
To our knowledge, this is the only study besides Heizer et al. that attempts to demonstrate the relationship between the symptoms and the disposition of pediatric patients exposed to edible cannabis-containing products and the total dose of THC consumed. Our study is underpowered to fully model the complex relationships between age and the weight-based dose of THC ingested and symptom incidence and hospital disposition. However, the data we were able to collect has interesting implications. The range of recorded THC dose exposures for the 10–17-year age group was 0.5–4.4 mg/kg, and only one patient in this group required admission after an ingestion of 2.3 mg/kg. However, three patients in the <10 age group required hospital admission for ingested doses lower than that (2.3 mg/kg). This discrepancy could simply be due to the fact that older children can verbalize their symptoms and make their needs known, which may make parents and providers more comfortable with outpatient observation when compared to younger children. Provider uncertainty when dealing with intoxicated younger children is supported by the higher percentage of urine drug screens performed on the <10 age group (N=11, 92%) compared to the 10–17 age group (N=8, 40%). Yet, it is also possible that older children may be able to tolerate higher weight-based doses without becoming symptomatic enough to require admission. If there is a true difference in tolerance between the age groups, the question becomes whether it may be due to prior cannabis use by older children, an underlying age-based difference in pharmacokinetic or pharmacodynamic properties, or some other unknown mechanism.
Our study has several limitations. Given its retrospective nature, identification of relevant cases relied on a search algorithm that included chief complaints, a keyword search, and final diagnosis. The chief complaints recorded in our medical record represent an abbreviated summary of the patient’s chief complaint and are not always a complete reflection of their reason for presentation. The six keywords that were used to screen case record text were thought to be high yield based on prior work, but are by no means an exhaustive list [20]. The six primary diagnosis codes used were thought to represent the most common diagnoses that would be used to describe cannabis intoxication. However, our study population included 32 unique ICD-10 codes indicating an unexpected level of heterogeneity in provider documentation. Taken together, the limitations of each aspect of the search algorithm likely led to some degree of selection bias. Our case yield may also be low due to asymptomatic and minimally symptomatic patients not having presented to the hospital for treatment, or receiving identifying descriptors that could not be picked up by the EPIC analytics function, contributing to selection bias and a reporting bias, respectively. During our analysis, the product name and potential dose of THC ingested were inconsistently documented or unknown. This led to a small sample size that could not be used to perform adjusted analyses or more sophisticated modeling. However, even when the edible packaging and dosing information was available, there is a theoretical risk that the reported THC content in each edible is inaccurate. While Massachusetts regulations stipulate that edible cannabis products produced in the state must be homogenous and have labeling with content accuracy [25], products purchased online or out of state may not follow such regulations. Since the origins of the edible products consumed by patients included in this study are unknown, one could argue that this makes the reliability of the reported THC dose questionable. Finally, our small sample size made it challenging to achieve our initial objectives, particularly to establish a dose-effect relationship.
Conclusions
Pediatric edible cannabis exposures have been occurring with increasing frequency since recreational cannabis was legalized in the state of Massachusetts, and hospital admission is a common outcome. Overall admission was associated with lower weight, younger age, need for respiratory support, lethargy/somnolence, collection of a urine drug screen, bradypnea, hypercarbia, hypoxia, unintentional ingestions, and hypertension. While younger children seem to be more at risk for significant symptoms such as respiratory depression and lethargy, larger studies are needed to explore the relationship between THC dosage, age, and incidence of adverse outcomes.
Supplementary Information
(DOCX 33.6 kb)
Acknowledgements
The authors would like to acknowledge Catherine W. Carr, MLIS, AHIP, for her contribution as education clinical services librarian.
Author Contribution
Dr. Kaczor conceptualized and designed the study, designed the data collection instruments, collected and organized the data, interpreted the results, and reviewed and revised the manuscript.
Dr. Mathews participated in study design, drafted the initial manuscript, interpreted the results, and reviewed and revised the manuscript.
Dr. LaBarge participated in study design, performed data extraction, interpreted the results, and reviewed and revised the manuscript.
Ms. Chapman participated in study design, designed the data collection instruments, interpreted the results, and reviewed and revised the manuscript.
Dr. Carreiro participated in study conceptualization and design, carried out statistical analysis of the data, interpreted the results, and reviewed and revised the manuscript.
All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Funding
Dr. Carreiro is funded by NIH/NIDA (K23DA045242).
Declarations
Conflict of Interest
None.
Footnotes
Previous Presentations: Abstract accepted to the 2021 American College of Medical Toxicology Annual Scientific Meeting
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Legislatures NCoS. State Medical Marijuana Laws [Available from: https://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx.
- 2.Thomas AA, Dickerson-Young T, Mazor S. Unintentional pediatric marijuana exposures at a Tertiary Care Children’s Hospital in Washington State: a retrospective review. Pediatr Emerg Care. 2018, Publish Ahead of Print. [DOI] [PubMed]
- 3.Whitehill JM, Harrington C, Lang CJ, Chary M, Bhutta WA, Burns MM. Incidence of pediatric cannabis exposure among children and teenagers aged 0 to 19 years before and after medical marijuana legalization in Massachusetts. JAMA Netw Open. 2019;2(8):e199456. doi: 10.1001/jamanetworkopen.2019.9456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wang GS, Le Lait MC, Deakyne SJ, Bronstein AC, Bajaj L, Roosevelt G. Unintentional pediatric exposures to marijuana in Colorado, 2009-2015. JAMA Pediatr. 2016;170(9):e160971. doi: 10.1001/jamapediatrics.2016.0971. [DOI] [PubMed] [Google Scholar]
- 5.Wang GS, Roosevelt G, Le Lait MC, Martinez EM, Bucher-Bartelson B, Bronstein AC, et al. Association of unintentional pediatric exposures with decriminalization of marijuana in the United States. Ann Emerg Med. 2014;63(6):684–689. doi: 10.1016/j.annemergmed.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Graham J, Leonard J, Banerji S, Wang GS. Illicit drug exposures in young pediatric patients reported to the National Poison Data System, 2006-2016. J Pediatr. 2020. [DOI] [PubMed]
- 7.Wang GS, Davies SD, Halmo LS, Sass A, Mistry RD. Impact of marijuana legalization in Colorado on adolescent emergency and urgent care visits. J Adolesc Health. 2018;63(2):239–241. doi: 10.1016/j.jadohealth.2017.12.010. [DOI] [PubMed] [Google Scholar]
- 8.Cao D, Srisuma S, Bronstein AC, Hoyte CO. Characterization of edible marijuana product exposures reported to United States poison centers. Clin Toxicol (Phila). 2016;54(9):840–846. doi: 10.1080/15563650.2016.1209761. [DOI] [PubMed] [Google Scholar]
- 9.Wang GS. Pediatric concerns due to expanded cannabis use: unintended consequences of legalization. J Med Toxicol. 2017;13(1):99–105. doi: 10.1007/s13181-016-0552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang GS, Banerji S, Contreras AE, Hall KE. Marijuana exposures in Colorado, reported to regional poison centre, 2000-2018. Inj Prev. 2019. [DOI] [PubMed]
- 11.Wong KU, Baum CR. Acute cannabis toxicity. Pediatr Emerg Care. 2019;35(11):799–804. doi: 10.1097/PEC.0000000000001970. [DOI] [PubMed] [Google Scholar]
- 12.Association APH. Cannabis policies in four states [Available from: https://www.apha.org/-/media/Files/PDF/topics/State_Cannabis_Policy.ashx.
- 13.Richards JR, Smith NE, Moulin AK. Unintentional cannabis ingestion in children: a systematic review. J Pediatr. 2017;190:142–152. doi: 10.1016/j.jpeds.2017.07.005. [DOI] [PubMed] [Google Scholar]
- 14.Thomas AA, Mazor S. Unintentional marijuana exposure presenting as altered mental status in the pediatric emergency department: a case series. J Emerg Med. 2017;53(6):e119–ee23. doi: 10.1016/j.jemermed.2017.08.007. [DOI] [PubMed] [Google Scholar]
- 15.Wang GS, Roosevelt G, Heard K. Pediatric marijuana exposures in a medical marijuana state. JAMA Pediatr. 2013;167(7):630–633. doi: 10.1001/jamapediatrics.2013.140. [DOI] [PubMed] [Google Scholar]
- 16.Lapoint J. Cannabinoids. In: Hoffman R, Howland, M, Lwein, N, Nelson, L, Goldfrank, L, editor. Goldfrank’s Toxicologic Emergencies. 10 ed: McGraw Hill Professional 2014.
- 17.Claudet I, Le Breton M, Brehin C, Franchitto N. A 10-year review of cannabis exposure in children under 3-years of age: do we need a more global approach? Eur J Pediatr. 2017;176(4):553–556. doi: 10.1007/s00431-017-2872-5. [DOI] [PubMed] [Google Scholar]
- 18.Le Garrec S, Dauger S, Sachs P. Cannabis poisoning in children. Intensive Care Med. 2014;40(9):1394–1395. doi: 10.1007/s00134-014-3395-4. [DOI] [PubMed] [Google Scholar]
- 19.Heizer JW, Borgelt LM, Bashqoy F, Wang GS, Reiter PD. Marijuana misadventures in children: exploration of a dose-response relationship and summary of clinical effects and outcomes. Pediatr Emerg Care. 2018;34(7):457–462. doi: 10.1097/PEC.0000000000000770. [DOI] [PubMed] [Google Scholar]
- 20.Marx GE, Chen Y, Askenazi M, Albanese BA. Syndromic surveillance of emergency department visits for acute adverse effects of marijuana, Tri-County Health Department, Colorado, 2016-2017. Public Health Rep. 2019;134(2):132–140. doi: 10.1177/0033354919826562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kliegman RM. Nelson textbook of pediatrics. 21st edition. ed. Philadelphia, MO: Elsevier; 2019. pages cm p.
- 22.Goodwin RD, Cheslack-Postava K, Santoscoy S, Bakoyiannis N, Hasin DS, Collins BN, et al. Trends in cannabis and cigarette use among parents with children at home: 2002 to 2015. Pediatrics. 2018;141(6). [DOI] [PMC free article] [PubMed]
- 23.Dharmapuri S, Miller K, Klein JD. Marijuana and the pediatric population. Pediatrics. 2020;146(2). [DOI] [PubMed]
- 24.Ashton CH. Pharmacology and effects of cannabis: a brief review. Br J Psychiatry. 2001;178:101–106. doi: 10.1192/bjp.178.2.101. [DOI] [PubMed] [Google Scholar]
- 25.Commonwealth of Massachusetts Cannabis Control Commission 935 CMR 500.150: Adult Use of Marijuana, Edibles, (2021).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 33.6 kb)