Abstract
In the present study, we have explored the potential of the RNAi mediated silencing of genes encoding peroxisomal biogenesis factor and β-1,3-glucanosyltransferase in Fusarium oxysporum f. sp. lycopersici (Fol) to confer resistance to Fusarium wilt in transgenic tomato plants. The partial gene fragments from these genes were utilized independently to generate hairpin RNAi constructs in appropriate silencing vectors and used for Agrobacterium-mediated transformation of tomato. The presence of gene-specific siRNAs was confirmed by stem-loop RT-PCR analysis of selected transgenic tomato lines. Transgenic lines expressing gene-specific dsRNA displayed enhanced resistance to Fol with delayed development of disease symptoms. The survival rate of transgenic tomato lines after fungal infection was higher as compared to that of the untransformed tomato plants.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13205-021-02973-8.
Keywords: Fungal resistance, Fusarium oxysporum, Gene silencing, RNA interference, Tomato
Introduction
Vascular wilt of tomato ascribed to Fusarium oxysporum f. sp. lycopersici (Fol) is responsible for annual yield losses of 30–40%. In India, adverse environmental conditions favorable to this disease may cause yield losses of up to 80% (Nirmaladevi et al. 2016; Sidharthan et al. 2018). Key conventional methods to control wilt in tomato primarily involve the deployment of resistant cultivars. However, resistance to this disease can breakdown over time because of the rapidly evolving pathogen. Strategic management of Fusarium wilt also involves the use of systemic fungicides. However, Fol evolves resistance to fungicides rendering them ineffective (Shanmugam et al. 2015). Host-induced gene silencing (HIGS) is an alternative approach that uses the host plant as a delivery system to cause gene silencing in the pathogens (Fairbairn et al. 2007). In HIGS, the host plant is transformed with a hairpin construct to express dsRNA targeting vital genes of fungal pathogens. The siRNA molecules produced in RNAi transgenic lines are taken up by the invading fungal pathogen upon infection, ultimately inducing gene silencing in the pathogen (Singh et al. 2020).
Here, efficacy of HIGS to confer resistance to Fol was tested in transgenic tomato plants through expression of dsRNA directed against the target genes of this pathogen. Two genes were selected because of their functional importance to F. oxysporum. PEX6 gene encodes peroxisome biogenesis factor 6 protein that belongs to the ATPase protein family and plays an important role in peroxisome biogenesis in all eukaryotes. Deletion of PEX6 gene results in loss of pathogenicity in Alternaria alternata (Imazaki et al. 2010; Wu et al. 2020). Importance of PEX6 gene in parasitism and conidiation has also been documented for Coniothyrium minitans (Wei et al. 2013). We have recently found that PEX6 gene is involved in pigmentation, sporulation and pathogenicity in F. oxysporum (Tetorya and Rajam 2017). GAS1 gene encodes putative β-1,3-glucanosyltransferase and plays active role in fungal cell wall biosynthesis and morphogenesis. GAS1 is required for virulence in F. oxysporm (Caracuel et al. 2005) and has also been found to affect the structure of the rice blast fungal cell wall during aspersorium-mediated host plant infection (Samalova et al. 2016). We show that silencing of these functionally important genes in Fol significantly reduces the Fusarium wilt disease symptoms in transgenic tomato.
Materials and methods
Fungal strain, plant material and culture conditions
F. oxysporum f. sp. lycopersici wild-type strain 4471 and the seeds of tomato (Solanum lycopersicum Mill. cv. Pusa Early Dwarf) were obtained from Indian Agriculture Research Institute (IARI-New Delhi). Fusarium strain was maintained on potato dextrose agar (PDA) at 28 °C in dark.
Search for potential target genes and in silico analysis for identification of potential off-targets
Wide literature survey of fungal genes was performed to identify suitable Fol target genes for silencing through HIGS. Two important Fol genes were selected, namely, PEX6 (FOXG_09292.3) and GAS1 (FOXG_05331.3). Regions of 300 bp in FoPEX6 and 280 bp in FoGAS1 were found to have maximum siRNA scores as per the criteria described by Reynolds et al. (2004). Furthermore, the stability of selected sequence was checked by S-fold program (sfold.wadsworth.org).
Construction of silencing vectors for tomato transformation and generation of transgenic lines
The pMVR-hp vector was selected for preparing the hpRNAi constructs and was generated by cloning the RNAi cassette of pFGC5941 in pCAMBIA2300 (Kumar 2011). The RNAi cassette has CaMV35S promoter, chalcone synthase (ChsA) intron and restriction sites flanking the intron for the cloning of each gene fragment in opposite orientations. Genomic DNA was isolated from F. oxysporum using CTAB method (Doyle and Doyle 1990). FoPEX6 and FoGAS1 specific primers (Sigma, USA) (Table S1) harboring the appropriate restriction sites were used for PCR amplification. PCR amplified DNA fragments were then cloned into pGEM-T easy vector (Promega, USA) and mobilized into E. coli XL1 Blue strain and putative recombinant clones were checked for the presence of respective genes by restriction digestion with EcoRI and were further confirmed by sequencing. The confirmed fragments of genes were then cloned into the pMVR RNAi vector in sense using AscI and SwaI restriction sites, while XbaI and BamHI restriction sites were used to clone fragments in antisense orientation. The RNAi constructs thus generated were transferred into Agrobacterium tumefaciens (strain LBA4404) and employed for tomato transformation. Tomato transformation was performed in accordance with a well-established lab protocol (Madhulatha et al. 2007).
Molecular characterization of the putative tomato RNAi transformants
Genomic DNA extracted from the putative transgenic tomato plants by the CTAB method was utilized for PCR analysis using primers specific to the selectable marker gene (NPT-II) and the gene of interest to determine integration of the transgene. DNA (100 ng) from the putative transgenic plants was mixed with 100 nM of forward and reverse primers, 1 × PCR buffer, 2 mM MgCl2, 100 µM dNTP mix and 0.5 U of Taq polymerase (Biotools, India). The samples were denatured initially at 94 °C for 5 min, followed by 30 cycles of denaturation for 1 min at 94 °C, primer annealing for 1 min at appropriate temperature (60 °C) and extension for 2 min at 72 °C, with a final extension for 10 min at 72 °C. The PCR products were analyzed on 1% agarose gel.
Total RNA was isolated using Trizol (Invitrogen) from excised leaves of transgenic tomato lines. First strand cDNA was synthesized using two-step RT-PCR kit according to manufacturer’s protocol (Thermo-scientific, USA). The mixture was incubated at 42 °C for 60 min, followed by 94 °C for 5 min, 28–30 cycles of 30 s denaturation at 94 °C, annealing temperature for 30 s, extension at 72 °C for 1 min and final extension at 72 °C for 10 min. Actin gene from tomato was used as internal control. The PCR product was analyzed on 1.2% agarose gel.
Stem-loop RT-PCR was performed using the protocol described by Varkonyi-Gasic et al. (2007). Briefly, sequences of targeted gene fragments were analyzed using siRNA finder software for virtual detection of potent siRNAs (www.genescript.com). Primers for the detection of siRNAs were designed and reverse transcription reactions were made including 0.05 µM stem-loop primers and 1 µg total RNA (DNase treated), which were heated to 70 °C for 10 min and chilled to 4 °C for 10 min. Then, 3 mM MgCl2, 0.25 mM of each dNTP, 200 U Revertaid (Fermentas, Canada) in appropriate buffer were added to a final volume of 20 µL. The reaction was conducted at 16 °C for 30 min, followed by 60 cycles at 30 °C for 30 s, at 42 °C for 30 s and 50 °C for 1 s, and a final step at 85 °C for 5 min to inactivate the enzyme. Final amplification was then performed in reactions with 1 µL cDNA, 1.5 mM MgCl2, 0.2 mM of dNTPs, 0.2 µM of each primer, and 1.5 U Taq DNA polymerase in appropriate buffer (Fermentas, Canada). The amplification cycle started at 94 °C for 2 min, followed by 35 cycles at 94 °C for 15 s and 60 °C for 1 min. Products were examined after separation on 4% agarose gels.
To determine the segregation of a selectable marker gene, PCR analysis was performed for the T1 transgenic lines. Initially T1 seeds (obtained by self-pollination) were germinated on MS basal medium supplemented with appropriate concentration of kanamycin but many false positives were obtained. Therefore, transgene integration was determined by PCR analysis using marker specific (NPT-II) primers and data were validated using the chi-square test.
Fusarium wilt resistance assays
Spores of F. oxysporum were harvested from 7-day-old culture grown on PDA medium and used for pathogenicity assays. The root-inoculation assays were performed as described by Singh et al. (2020). One-month-old T1 transgenic and wild-type seedlings were inoculated by immersing the roots in microconidia (106 spores/ml) for 30 min. Seedlings were then transplanted in the plastic pots containing soil: vermiculite mix (1:1) and were kept in plant growth chamber with controlled conditions, i.e., 26 °C and 90% RH in dark for 2 days and then they were maintained at the same temperature, RH and 16 photoperiod for the next 28 days. Disease severity index (DI) was scored on a standard scale at the time wild-type tomato plants had developed severe wilting and necrosis (7–15 day post-inoculation). The variation in the seedling growth and disease symptoms caused by the fungus was reflected on a five-class disease severity scale as defined (Lin and Xiao 1995). A highly susceptible plant had DI of 1.0, while the tolerant plants had DI below 1.0 approaching zero. Experiment was performed with 6 seedlings and repeated thrice.
Assays for invasive growth on tomato fruits were carried out according to the previously reported method (López-Berges et al. 2010). Briefly, the wild-type and transgenic tomato fruits were taken at red-ripe stage and surface sterilized using 70% ethanol. Epidermal layer of tomato fruit was punctured with sterile pipette tip and inoculated with 103 spores/ml of F. oxysporum and then inoculated fruits were kept in a plant growth chamber under similar conditions as described above and observed for 6 days for invasive mycelial growth.
Data analysis
All experiments were performed with three biological replications. Student’s t test (*P < 0.05; **P < 0.01) was used to analyze any significant differences between the wild-type and transgenic tomato lines.
Results
Selection of F. oxysporum genes for functional validation and analysis of siRNAs for their off-target effects
For this study, two genes of Fol were selected for HIGS. Phylogenetic analysis showed that FoPEX6 shared common ancestor during evolution with the PEX6 gene of Colletotrichum gloeosporioides (CgPEX6). FoPEX6 has 71% sequence similarity with CgPEX6 at the nucleotide level. FoGAS1 encoded GAS1 protein showed high similarity (58–89%) with three closely related fungi, namely, Fusarium graminearum, Colletotrichum gloeosporioides and Verticillium dahlia, respectively. These proteins share conserved motifs in their C-terminal part. To avoid off-target effects of the ectopic siRNAs on tomato transcripts, cDNA fragments with little or no homology with sequences of other plant and beneficial organisms were selected. Gene sequences were used for BLAST searches against the fully sequenced genome of tomato. Both low and high stringency parameters were used for the similarity searches, and it was found that 300 bp sequence of FoPEX6 and 280 bp sequence of FoGAS1 were appropriate for generation of gene silencing constructs.
Expression of FoPEX6 and FoGAS1 in transgenic tomato lines
RNAi vectors (Fig. S1) were used for stable expression of targeted genes in tomato. Several tomato transformants were obtained through Agrobacterium-mediated transformation and these putative transgenic plants were normal in plant morphology, flowering, pollen viability and fruit setting when evaluated along with wild-type control plants (Fig. S2). The transformation frequency was determined on the basis of a number of co-cultivated explants surviving and showing shoot regeneration on the selection medium among the total number of co-cultivated explants (Tables S2 and S3).
Molecular analyses of tomato transformants
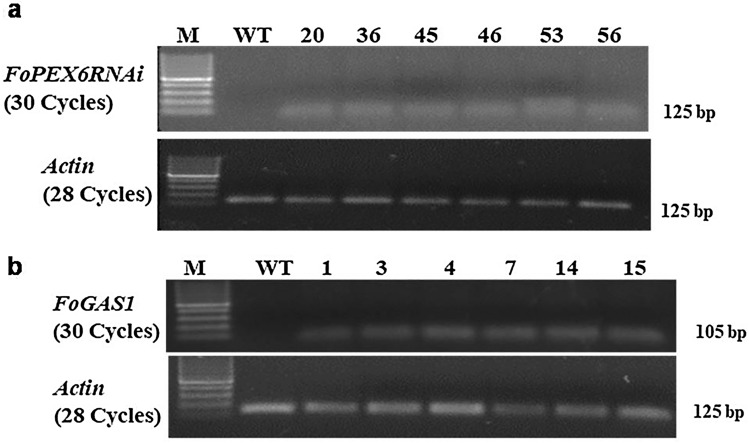
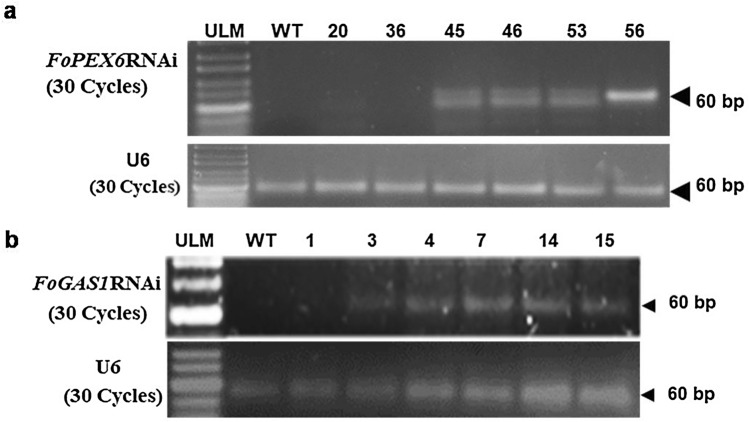
The presence of transgenes in the putative tomato transformants was verified by PCR analysis, utilizing primers specific to NPT-II coding sequence and primers specific to the FoPEX6 and FoGAS1 genes (Fig. S3). DNA from the transgenic plants produced the expected 704 bp NPT-II amplification product and the gene-specific amplification products of 300 bp and 280 bp, for FoPEX6 and FoGAS1, respectively. PCR positive transgenic lines were further validated by RT-PCR analysis (Fig. 1). Six independent transgenic lines were used for transcript analysis from FoPEX6 and FoGAS1 RNAi lines. It was observed that all six lines showed the presence of FoPEX6 and FoGAS1 transcripts, while the wild-type control tomato plants did not show any transcripts. Tomato actin gene (Mamta and Rajam 2016) was used as an internal control to confirm an equal amount of RNA used for RT-PCR. Stem-loop RT-PCR was also employed to detect small RNAs in the RT-PCR positive transgenic tomato lines. PCR amplification of small RNA showed detectable amplicons of different sizes among all RT-PCR positive transgenic lines, including FoPEX6RNAi-45, FoPEX6RNAi-46, FoPEX6RNAi-53 and FoPEX6RNAi-56 of hpFoPEX6-RNAi lines and FoGAS1RNAi-3, FoGAS1RNAi-4, FoGAS1RNAi-7, FoGAS1RNAi-14 and FoGAS1RNAi-15 of hpFoGAS1-RNAi lines. No detectable amplicons were observed in transgenic lines FoPEX6RNAi-20, FoPEX6RNAi-36, FoGAS1RNAi-1 and wild-type tomato plants (Fig. 2).
Fig. 1.
Semi-quantitative RT-PCR analysis for detection of FoPEX6-RNAi and FoGAS1-RNAi transcripts in transgenic tomato lines. Expression of transcripts was normalized with Actin gene from tomato. Semi-quantitative RT-PCR analysis was performed by utilizing total RNA from PCR positive transgenic tomato lines a FoPEX6-RNAi and b FoGAS1-RNAi, M—100 bp DNA Ladder, Lane 2—WT (wild-type) control, 3–8 RNAi tomato lines
Fig. 2.
Detection of siRNAs in transgenic tomato lines a FoPEX6-RNAi and b FoGAS1-RNAi tomato lines showing siRNAs. Lane 1-ULM (Ultra low molecular weight markers), Lane 2-WT (Wild-type) control, Lanes 3–8 transgenic tomato lines
Segregation analysis of the NPT-II marker gene
NPT-II specific primers were used for PCR screening of T1 progenies. Six lines (PEX45, PEX46, PEX56, GAS7, GAS14 and GAS15) out of nine, showed 3:1 transgene segregation, suggesting single copy transgene integration, while three lines (PEX53, GAS3 and GAS4) showed deviation from 3:1 ratio.
Testing of tomato RNAi transgenic lines for resistance to Fusarium wilt
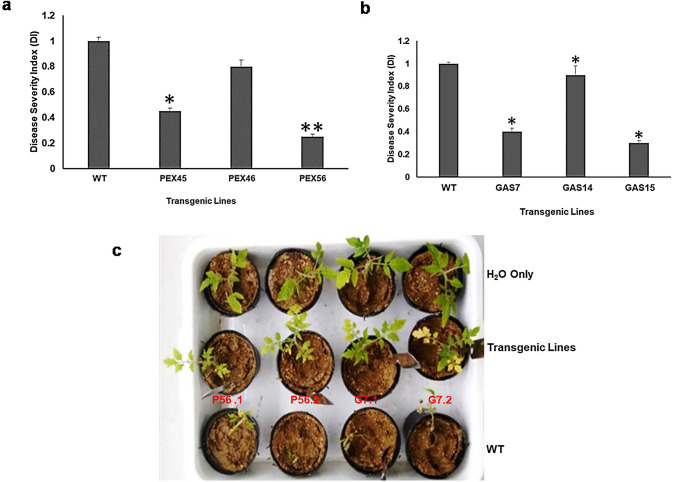
The T1 transgenic tomato lines (positive segregants) were evaluated for resistance against Fusarium wilt. Experiments were performed by inoculating roots of the transgenic lines relative to the wild-type control plants with fungal spores and disease severity index (DI) values were determined 3 week post-inoculation. Six seedlings each from the wild-type and transgenic lines were taken for fungal resistance assays. Variations in the disease severity among all transgenic seedlings were observed as supported by differences in the DI values (Fig. 3). DI values for the wild-type control plants were recorded as 1.0, whereas transgenic lines exhibited DI values ranging from 0.2 to 0.7. Lines 45 and 56 of FoPEX6RNAi with DI values ranging from 0.25 to 0.5 were found to be 80–70% resistant, on the contrary line 46 was significantly less resistant with 0.7 DI (30% resistance). Similarly, lines 7 and 15 of FoGAS1RNAi with DI values ranging from 0.3 to 0.5 were found 80–70% resistant, while line 14 showed 45–50% resistant with 0.65 DI. Wild-type seedlings developed severe wilting and necrosis and eventually died. Fruit invasion assays were also performed with fruits of selected transgenic lines, namely, FoPEX6RNAi-56 and FoGAS1RNAi-7. Tomato fruits were surface sterilized and inoculated with freshly collected micro-conidia of F. oxysporum (103 spores/ml). Fol efficiently colonized and macerated the wild-type tomato fruit tissue and produced huge aerial mycelium on the epidermal surface of tomato fruits. Relative lesion size was determined and significant difference was observed between the lesions of the wild-type and transgenic fruits (Fig. 4).
Fig.3.
Fungal resistance assay with FoPEX6-RNAi and FoGAS1-RNAi transgenic tomato lines. Experiments were performed by inoculating the roots with fungal spores and disease severity index (DI) values were determined after 3 weeks for the transgenic lines relative to the wild-type control plants. DI values for wild-type control plants were recorded as 1.0, whereas transgenic lines have shown DI values ranging from 0.2 to 0.7; a lines 45 and 56 of FoPEX6RNAi with DI values ranging from 0.25 to 0.5 were 70–80% resistant. In contrast, line 46 was significantly less resistant with DI of 0.7 (30% resistant); b similarly, lines 7 and 15 of FoGAS1RNAi with DI values ranging from 0.3 to 0.5 were 70–80% resistant, while line 14 was 45–50% resistant with DI of 0.65; c Assessment of RNAi transgenic tomato lines for resistance against Fusarium wilt. Photographs were taken 3-week post-inoculation. Tomato seedlings (6 each from the wild-type and transgenic lines) were used for fungal resistance assays
Fig. 4.
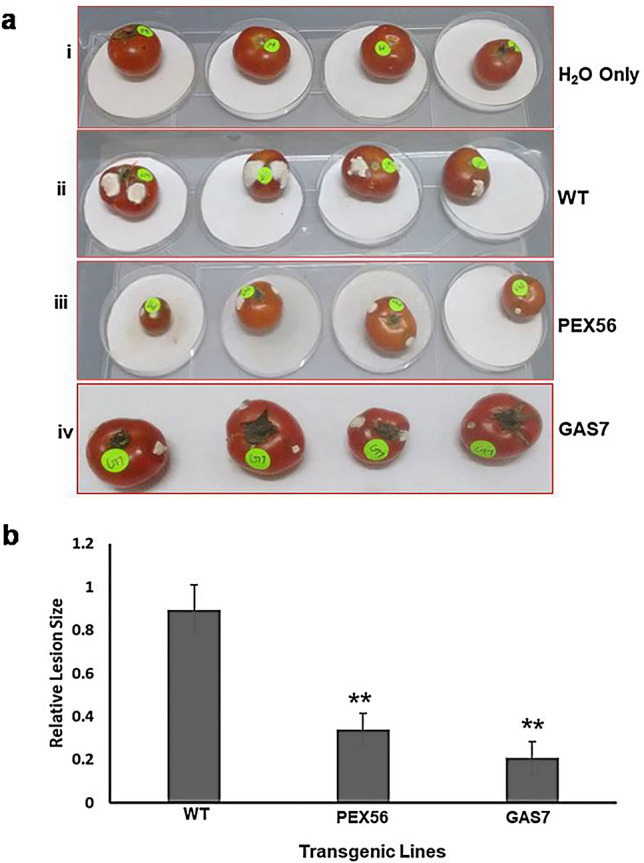
Invasive growth of F. oxysporum on transgenic tomato fruits. a Inoculation was performed by injecting 103 microconidia/ml into wild-type and RNAi lines. The photographs were taken after incubation for 6 days at 26 °C and 90% RH. (i) H2O Only, (ii) Wild-type control tomato fruits, (iii) and (iv) FoPEX6RNAi and FoGAS1RNAi tomato fruit infected with microconidia; b Relative lesion sizes of the infected transgenic tomato fruits were measured 6 day post-inoculation (dpi) using the ImageJ software. Error bars indicate the standard deviations (s.d.) of 12 samples
Discussion
HIGS has emerged as a promising strategy for improving plant resistance to nematodes, insects and fungi by targeting genes essential to these pests/pathogens (Kuo and Falk 2020; Rajam 2020; Gebremichael et al. 2021). It is based on the nuclear expression of genes in plants for dsRNA molecules to silence essential genes in a pathogen or a pest.
Fusarium wilt is a major threat to tomato production in several countries, where it is grown including India. Attempts to enhance resistance to this disease in India have met with limited success due to a lack of durable single-gene resistance. HIGS offers a viable alternative due to season-long control and enhanced trait durability it provides. In the present study, transgenic tomato plants expressing the two hpRNAi constructs for silencing the expression of PEX6 and GAS1 genes showed increased resistance to Fol infection and significant reduction in symptom development even 25–30 day post-inoculation. Different RNAi tomato lines showed different levels of resistance against Fusarium wilt, and this may be due to the position effect and copy number of the transgene (Mamta and Rajam 2016). The PEX6 and GAS1 gene sequences were retrieved from Fusarium database resource and their in silico bioinformatics analysis was performed to ascertain that these genes did not have notable sequence homology with the genome sequence of tomato. This is probably why our transgenic RNAi tomato plants looked similar to wild-type plants with no noticeable effects on their growth and development. The potential of HIGS for control of Fusarium wilt has also been demonstrated recently by targeting the ornithine decarboxylase (ODC) gene in Fol for its control (Singh et al. 2020). Our study lends strong support for the notion that pathogen genes performing essential housekeeping functions or playing critical roles during pathogenesis are potential targets for silencing via HIGS to control economically important fungal pathogens of crops. Thus, HIGS has been successfully used to control several important fungal diseases, such as Fusarium head blight in barley and Arabidopsis (Koch et al. 2013), powdery mildew and rusts in wheat (Nowara et al. 2010; Yin et al. 2011; Panwar et al. 2013; Pliego et al. 2013), Anthracnose in chilli and tomato (Mahto et al. 2020), and downy mildew in lettuce (Govindarajulu et al. 2015). The control of Fusarium wilt in transgenic banana has also been demonstrated through silencing of specific genes in F. oxysporum f. sp. cubense (Ghag et al. 2014). Success of HIGS is dependent on the selection of specific fungal genes targeted for silencing. Fortunately, there is a huge reservoir of genome sequence information available for Fol to enable selection of candidate genes via high-throughput screening (Srinivas et al. 2019).
Movement of small RNAs within an organism and between organisms across different kingdoms is a well-understood phenomenon that facilitates gene silencing in adjacent cells, surrounding cells and also to distant cells (Weiberg et al. 2013, 2015). Moreover, transfer of siRNAs via extracellular vesicles has been illustrated revealing the role of endosomal vesicle trafficking to support transfer of transgene-derived siRNAs between host cells and fungal pathogen cells (Koch et al. 2020). It remains to be shown if small RNAs targeting the PEX6 and GAS1 genes are transferred from tomato cells to Fol cells through endosomal vesicle trafficking. Although HIGS has been proven to be a very promising approach for control of the Fusarium wilt disease in transgenic tomato, plant genome-editing technologies, particularly through the CRISPR (clustered regularly interspaced short palindromic repeats)/Cas system, offer another commercially viable approach for management of Fusarium wilt disease by silencing the endogenous susceptibility gene(s), so-called “S” gene(s), in tomato. In addition, overexpression of a specific transcription factor, such as the NAC transcription factor, may also prove useful in conferring resistance to this disease in transgenic tomato (Negi et al. 2021).
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file1 Fig. S1. Schematic representation of T-DNA map of RNAi gene cassettes harboring. (a) PEX6 and (b) GAS1 genes in sense and antisense orientation, separated by a spacer sequence from chalcone synthase (ChaS) gene intron having neomycin phosphotransferase (NPT-II) gene as a plant selection marker (JPG 56 KB)
Supplementary file2 Fig. S2. Generation of (a) FoPEX6-RNAi and (b) FoGAS1-RNAi tomato transformants through Agrobacterium-mediated transformation (A) Wild-type leaf explants cultured on shoot regeneration medium (SRM- MS basal + 1 mg/l BAP + 0.1 mg/l NAA + 3% sucrose); (B) Wild-type leaf explants cultured on selection medium (SRM+ 300 mg/l augmentin + 30 mg/l kanamycin); (C) Co-cultivated explants cultured on selection medium; (D) FoPEX6-RNAi and FoGAS1-RNAi transformants on shoot proliferation medium (MS basal + 0.5 mg/l BAP + 3% sucrose + 300 mg/l augmentin + 30 mg/l kanamycin); (E) Wild-type (left); FoPEX6-RNAi and FoGAS1-RNAi transformed (middle and right) shoots on the rooting medium (½ MS basal + 3% sucrose + 300 mg/l augmentin+30 mg/l kanamycin); (F) Wild-type; FoPEX6-RNAi and FoGAS1-RNAi (right) plants in pots showing normal phenotype. Morphology of (c) FoPEX6-RNAi and (d) FoGAS1-RNAi tomato plants as compared to wild-type tomato plants (JPG 122 KB)
Supplementary file4 Fig. S3. PCR analysis for transgene integration. PCR analysis of putative transgenic tomato plants of FoPEX6RNAi-56 with (a) marker-specific (NPT-II) primers (704 bp) and (b) gene -specific primers (300 bp) using DNA from respective samples (M, 1 kb ladder; B, Blank, C, DNA from wild-type control plant; the numbers denote DNA from transgenic lines; +ve control, DNA from positive plasmid. PCR analysis of transgenic lines of FoGAS1RNAi-7 with (c) marker-specific (NPT-II) primers (704 bp) and (d) gene -specific primers (280 bp) using DNA from respective samples ( M, 1 kb ladder; B, Blank, C, DNA from wild-type control plant; the numbers denote DNA from transgenic lines; +ve control, DNA from positive plasmid (JPG 85 KB)
Acknowledgements
The authors are grateful to the Department of Biotechnology, Govt. of India (BT/PR10713/AGR/36/601/2008) for financial assistance. We also thank the Department of Science and Technology (DST) for FIST (Level 2) programme, University Grant Commission (UGC) Special Assistance Programme (DRS-III) and DU-DST PURSE grant. MVR is grateful to UGC for BSR Faculty Fellowship. MT acknowledges UGC for Senior Research Fellowship (No. F.7-50/2007, BSR).
Abbreviations
- Fol
Fusarium oxysporum f. sp. lycopersici
- GAS1
β-1,3-Glucanosyltransferase
- HIGS
Host-induced gene silencing
- PEX6
Peroxisomal biogenesis factor
- RNAi
RNA interference
- siRNAs
Small interfering RNAs
Author contributions
MVR and MT conception and design of study and MT performed the experiments and acquisition of data. MT and MVR analysis and interpretation of data and drafting of the manuscript. MVR corrected and edited the manuscript and both the authors approved the final manuscript.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent to participate
All authors participant agree to publish this work in your esteem Journal: 3 Biotech.
Consent to publish
Yes, all authors have given their consent.
References
- Caracuel Z, Martinez-Rocha AL, Di Pietro A, Madrid MP, Roncero MI. Fusarium oxysporum GAS1 encodes a putative β-1, 3- glucanosyltransferase required for virulence on tomato plants. Mol Plant Microbe Interact. 2005;18:1140–1147. doi: 10.1094/MPMI-18-1140. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. Isolation of plant DNA from fresh tissue. Focus. 1990;12:13–15. [Google Scholar]
- Fairbairn DJ, Cavallaro AS, Bernard M, Mahalinga-Iyer J, Graham MW, Botella JR. Host-delivered RNAi: an effective strategy to silence genes in plant parasitic nematodes. Planta. 2007;226:1525–1533. doi: 10.1007/s00425-007-0588-x. [DOI] [PubMed] [Google Scholar]
- Gebremichael DE, Haile ZM, Negrini F, Sabbadini S, Capriotti L, Mezzetti B, Baraldi E. RNA interference strategies for future management of plant pathogenic fungi: prospects and challenges. Plants. 2021 doi: 10.20944/preprints202103.0179.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghag SB, Shekhawat UKS, Ganapathi TR. Host induced post-transcriptional hairpin RNA mediated gene silencing of vital fungal genes confers efficient resistance against Fusarium wilt in banana. Plant Biotechnol J. 2014;12:541–553. doi: 10.1111/pbi.12158. [DOI] [PubMed] [Google Scholar]
- Govindarajulu M, Epstein L, Wroblewski T, Michelmore RW. Host-induced gene silencing inhibits the biotrophic pathogen causing downy mildew of lettuce. Plant Biotechnol J. 2015;13:875–883. doi: 10.1111/pbi.12307. [DOI] [PubMed] [Google Scholar]
- Imazaki A, Tanaka A, Harimoto Y, et al. Contribution of peroxisomes to secondary metabolism and pathogenicity in the fungal plant pathogen Alternaria alternata. Eukaryot Cell. 2010;5:684–694. doi: 10.1128/EC.00369-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Kumar N, Weber L, Imani KH, J, Karl-Heinz, K, Host-induced gene silencing of cytochrome P450 lanosterol C14 ademethylase-encoding genes confers strong resistance to Fusarium species. Proc Natl Acad Sci USA. 2013;110:19324–19329. doi: 10.1073/pnas.1306373110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A, Schlemmer T, Höfle L, Werner B et al (2020) Host-induced gene silencing involves transfer of dsRNA-derived siRNA via extracellular vesicles. bioRxiv. 10.1101/2020.02.12.945154
- Kumar M (2011) RNAi-mediated targeting of acetylcholinesterase gene of Helicoverpa armigera for insect resistance in transgenic tobacco and tomato. Ph.D Thesis. University of Delhi, Delhi
- Kuo YW, Falk BW. RNA interference approaches for plant disease control. Biotechniques. 2020;69:469–477. doi: 10.2144/btn-2020-0098. [DOI] [PubMed] [Google Scholar]
- Lin B, Xiao Y. Sources of resistance to Verticillium wilt in Solanum melongena and its affinities identified by improved root dip method. Capsicum Eggplant Newslett. 1995;14:81–84. [Google Scholar]
- Lopez-Berges MS, Rispail N, Prados-Rosales RC, Di Pietro A. A nitrogen response pathway regulates virulence functions in Fusarium oxysporum via the protein kinase TOR and the bZIP protein MeaB. Plant Cell. 2010;22:2459–2475. doi: 10.1105/tpc.110.075937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhulatha P, Pandey R, Hazarika P, Rajam MV. High Transformation frequency in Agrobacterium-mediated genetic transformation of tomato by using polyamines and maltose in shoot regeneration medium. Physiol Mol Biol Plants. 2007;13:191–198. [Google Scholar]
- Mahto BK, Singh A, Pareek M, Rajam MV, Dhar-Ray S, Reddy PM. Host-induced silencing of the Colletotrichum gloeosporioides conidial morphology 1 gene (CgCOM1) confers resistance against Anthracnose disease in chilli and tomato. Plant Mol Biol. 2020;104:381–395. doi: 10.1007/s11103-020-01046-3. [DOI] [PubMed] [Google Scholar]
- Mamta RKRK, Rajam MV. Targeting chitinase gene of Helicoverpa armigera by host-induced RNA interference confers insect resistance in tobacco and tomato. Plant Mol Biol. 2016;90:281–292. doi: 10.1007/s11103-015-0414-y. [DOI] [PubMed] [Google Scholar]
- Negi S, Tak H, Ganapathi TR. Overexpression of MusaSNAC1 improves shoot proliferation in transgenic banana lines. 3 Biotech. 2021;11:188. doi: 10.1007/s13205-021-02744-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nirmaladevi D, Venkataramana M, Srivastava RK, Uppalapati SR, Gupta VK, Yli-Mattila T, Chandra NS. Molecular phylogeny, pathogenicity and toxigenicity of Fusarium oxysporum f.sp. lycopersici. Sci Rep. 2016;6:1–30. doi: 10.1038/srep21367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowara D, Gay A, Lacomme C, Shaw J, Ridout C, Douchkov D, Hensel G, Kumlehn J, Schweizer P. HIGS: host induced gene silencing in the obligate biotrophic fungal pathogen Blumeria graminis. Plant Cell. 2010;22:3130–3141. doi: 10.1105/tpc.110.077040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panwar V, McCallum B, Bakkeren G. Endogenous silencing of Puccinia triticina pathogenicity genes through in planta-expressed sequences leads to the suppression of rust diseases on wheat. Plant J. 2013;73:521–532. doi: 10.1111/tpj.12047. [DOI] [PubMed] [Google Scholar]
- Pareek M, Rajam MV. RNAi-mediated silencing of MAP kinase signaling genes (Fmk1, Hog1 and Pbs2) in Fusarium oxysporum reduces pathogenesis on tomato plants. Fungal Biol. 2017;121:775–784. doi: 10.1016/j.funbio.2017.05.005. [DOI] [PubMed] [Google Scholar]
- Pliego C, Nowara D, Bonciani G, Gheorghe DM, Xu R, Surana P, Whigham E, Nettleton D, Bogdanove AJ, Wise RP, Schweizer P, Bindschedler LV, Spanu PD. Host-induced gene silencing in barley powdery mildew reveals a class of ribonuclease-like effectors. Mol Plant Microbe Interact. 2013;26:633–642. doi: 10.1094/MPMI-01-13-0005-R. [DOI] [PubMed] [Google Scholar]
- Rajam MV. RNA silencing technology: a boon for crop improvement. J Biosci. 2020;45:118. doi: 10.1007/s12038-020-00082-x. [DOI] [PubMed] [Google Scholar]
- Reynolds A, Leake D, Boese Q, Scaringe S, Marshall WS, Khvorova A. Rational siRNA design for RNA interference. Nat Biotechnol. 2004;22:326–330. doi: 10.1038/nbt936. [DOI] [PubMed] [Google Scholar]
- Samalova M, Mélida H, Vilaplana F, Bulone V, Soanes DM, Talbot NJ, Gurr SJ. The β-1, 3-glucanosyltransferases (gels) affect the structure of the rice blast fungal cell wall during appressorium-mediated plant infection. Cell Microbiol. 2016;19:1–14. doi: 10.1016/j.chom.2015.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugam V, Chugh P, Sharma P. Cold-tolerant Trichoderma species for the management of Fusarium wilt of tomato plants. Ann Microbiol. 2015;65:543–551. doi: 10.1007/s13213-014-0890-3. [DOI] [Google Scholar]
- Sidharthan KV, Rashmi KVA, Surenthiran N, Shanmugam V. Selection and characterization of the virulent Fusarium oxysporum f. sp. lycopersici isolate inciting vascular wilt of tomato. Int Curr Microbiol Appl Sci. 2018;7(2):1749–1756. doi: 10.20546/ijcmas.2018.702.212. [DOI] [Google Scholar]
- Singh N, Mukherjee SK, Rajam MV. Silencing of the ornithine decarboxylase gene of Fusarium oxysporum f. sp. lycopersici by host-induced RNAi confers resistance to Fusarium wilt in tomato. Plant Mol Biol Rep. 2020;38:419–429. doi: 10.1007/s11105-020-01205-2. [DOI] [Google Scholar]
- Srinivas C, Nirmala Devi D, Narasimha Murthy K, et al. Fusarium oxysporum f. sp. lycopersici causal agent of vascular wilt disease of tomato: Biology to diversity—a review. Saudi J Biol Sci. 2019;26(7):1315–1324. doi: 10.1016/j.sjbs.2019.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetorya M, Rajam MV. RNA silencing of PEX6 gene causes decrease in pigmentation, sporulation and pathogenicity of Fusarium oxysporum. Plant Pathol. 2017;67:67–75. doi: 10.1111/ppa.12712. [DOI] [Google Scholar]
- Varkonyi-Gasic E, Wu R, Wood M, Walton EF, Hellens RP. Protocol: a highly sensitive RT-PCR method for detection and quantification of microRNAs. Plant Methods. 2007;3:12–23. doi: 10.1186/1746-4811-3-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Zhu W, Cheng J, Xie J, et al. CmPEX6, a gene involved in peroxisome biogenesis, i essential for parasitism and conidiation by the sclerotial parasite Coniothyrium minitans. Appl Environ Microbiol. 2013;79:3659–3666. doi: 10.1128/AEM.00375-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Wang M, Lin FM, Zhao H, Zhang Z, Kaloshian I, Huang HD, Jin HL. Fungal small RNAs suppress plant immunity by hijacking host RNA interference pathways. Science. 2013;342:118–123. doi: 10.1126/science.1239705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiberg A, Bellinger M, Jin HL. Conversations between kingdoms: small RNAs. Curr Opin Biotechnol. 2015;32:207–215. doi: 10.1016/j.copbio.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu PC, Chen CW, Choo CYL, Chen YK, Yago JI, Chung KR. Proper functions of peroxisomes are vital for pathogenesis of citrus brown spot disease caused by Alternaria alternate. J Fungi (basel) 2020;6(4):248. doi: 10.3390/jof6040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin C, Jurgenson JE, Hulbert SH. Development of a host-induced RNAi system in the wheat stripe rust fungus Puccinia striiformis f. sp. tritici. Mol Plant-Microbe Interact. 2011;24:554–561. doi: 10.1094/MPMI-10-10-0229. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file1 Fig. S1. Schematic representation of T-DNA map of RNAi gene cassettes harboring. (a) PEX6 and (b) GAS1 genes in sense and antisense orientation, separated by a spacer sequence from chalcone synthase (ChaS) gene intron having neomycin phosphotransferase (NPT-II) gene as a plant selection marker (JPG 56 KB)
Supplementary file2 Fig. S2. Generation of (a) FoPEX6-RNAi and (b) FoGAS1-RNAi tomato transformants through Agrobacterium-mediated transformation (A) Wild-type leaf explants cultured on shoot regeneration medium (SRM- MS basal + 1 mg/l BAP + 0.1 mg/l NAA + 3% sucrose); (B) Wild-type leaf explants cultured on selection medium (SRM+ 300 mg/l augmentin + 30 mg/l kanamycin); (C) Co-cultivated explants cultured on selection medium; (D) FoPEX6-RNAi and FoGAS1-RNAi transformants on shoot proliferation medium (MS basal + 0.5 mg/l BAP + 3% sucrose + 300 mg/l augmentin + 30 mg/l kanamycin); (E) Wild-type (left); FoPEX6-RNAi and FoGAS1-RNAi transformed (middle and right) shoots on the rooting medium (½ MS basal + 3% sucrose + 300 mg/l augmentin+30 mg/l kanamycin); (F) Wild-type; FoPEX6-RNAi and FoGAS1-RNAi (right) plants in pots showing normal phenotype. Morphology of (c) FoPEX6-RNAi and (d) FoGAS1-RNAi tomato plants as compared to wild-type tomato plants (JPG 122 KB)
Supplementary file4 Fig. S3. PCR analysis for transgene integration. PCR analysis of putative transgenic tomato plants of FoPEX6RNAi-56 with (a) marker-specific (NPT-II) primers (704 bp) and (b) gene -specific primers (300 bp) using DNA from respective samples (M, 1 kb ladder; B, Blank, C, DNA from wild-type control plant; the numbers denote DNA from transgenic lines; +ve control, DNA from positive plasmid. PCR analysis of transgenic lines of FoGAS1RNAi-7 with (c) marker-specific (NPT-II) primers (704 bp) and (d) gene -specific primers (280 bp) using DNA from respective samples ( M, 1 kb ladder; B, Blank, C, DNA from wild-type control plant; the numbers denote DNA from transgenic lines; +ve control, DNA from positive plasmid (JPG 85 KB)