Highlights
-
•
Anhedonia is present in many different psychiatric disorders.
-
•
Anhedonia has been associated with abnormal reward-related striatal dopamine functioning.
-
•
This study tested whether transdiagnostic anhedonia expression mapped onto striatal volume.
-
•
Our findings suggest volumetric abnormalities in the putamen and cerebellum as a common neural substrate of anhedonia severity that cut across psychiatric entities.
Keywords: Anhedonia, Transdiagnostic, Neuroimaging, Neural correlate, Putamen, Cerebellum
Abstract
Anhedonia has been associated with abnormal reward-related striatal dopamine functioning in patients with different psychiatric disorders. Here, we tested whether anhedonia expression mapped onto striatal volume across several psychiatric diagnoses.
T1-weighted images from 313 participants including 89 healthy controls (HC), 22 patients with opioid use disorder (OUD), 50 patients with major depressive disorder (MDD), 45 patients with borderline personality disorder (BPD), 49 patients with first-episode psychosis (FEP), 43 patients with cocaine use disorder (CUD) and 15 patients with schizophrenia (SZ) were included. Anhedonia was assessed with subscores of the Beck Depression Inventory (BDI) and/or the Scale for the Assessment of Negative Symptoms (SANS). Voxel-based morphometry (VBM) was conducted for identifying dimensional symptom-structure associations using region of interest (ROI, dorsal and ventral striatum) and whole-brain analyses, as well as for group comparisons of striatal volume.
ROI analyses revealed significant negative relationships between putamen volume and BDI and SANS anhedonia scores across OUD, MDD, BPD, CUD and SZ patients (n = 175) and MDD, FEP and SZ patients (n = 114), respectively. Whole-brain VBM analyses confirmed these associations and further showed negative relationships between anhedonia severity and volume of the bilateral cerebellum. There were group differences in right accumbens volume, which however were not related to anhedonia expression across the different diagnoses.
Our findings indicate volumetric abnormalities in the putamen and cerebellum as a common neural substrate of anhedonia severity that cut across psychiatric entities.
1. Introduction
Dimensional approaches in psychiatry aim to identify neurobiological correlates of core psychological domainsrelated to symptom dimensions across psychiatric disorders (Insel et al., 2010). Mapping neuronal patterns onto dimensional dysfunction of core psychological functions instead of nosological categories may help to detect targets for transdiagnostic treatment development (Whitton et al., 2015). Reward processing deficits are increasingly being conceptualised to understand abnormalities in motivational and goal-directed behaviour that are associated with depressed mood across psychiatric disorders (Whitton et al., 2015). As such anhedonia, defined as a markedly diminished interest or pleasure in previously rewarding activities (Treadway and Zald, 2011) and/or reduced ability to modulate behaviour as a function of rewards (Pizzagalli et al., 2008, Vrieze et al., 2013), has been considered as an important transdiagnostic phenotype of the Research Domain Criterion (RDoC) (Cuthbert and Insel, 2013). Anhedonia is highly prevalent in major depressive disorder (MDD) (Treadway and Zald, 2011), schizophrenia (SZ) (Horan et al., 2006), substance use disorder (SUD) (Garfield et al., 2014) and borderline personality disorder (BPD) (Marissen et al., 2012), to name a few. Despite its occurrence across several psychiatric disorders, there is no approved treatment for anhedonia (Lally et al., 2015).
Given anhedonia’s definition of diminished interest in previously rewarding activities (Treadway and Zald, 2011), brain regions involved in reward learning are especially relevant for detecting (transdiagnostic) anhedonia biomarkers (Nusslock and Alloy, 2017). The mesolimbic dopamine system is essential for reward learning (Cox and Witten, 2019) and dysregulationin this system has been linked to anhedonia (Nestler and Carlezon, 2006). A seminal work by Wise et al. (1978) showed that blocking dopamine receptors by pimozide blunted the rewarding impact of hedonic stimuli. Accordingly, anhedonia and its putative striatal dopaminergic correlates have been conceptualized as a pathological dimension transcending traditional disease categories (Heinz et al., 1994). However, Robinson and Berridge (1998) showed that dopamine dysfunction is associated with reward wanting rather than hedonic liking in animals, a finding that could be replicated in patients with depression, schizophrenia, and opiate and alcohol dependence (Schmidt et al., 2001). While mu-opioid systems in the shell of the nucleus accumbens contribute to liking or hedonic experiences (Kelley et al., 2002, Peciña and Berridge, 2005), wanting, a form of motivation, is generated by large and integrative neural systems consisting of the ventral and dorsal striatum, as well as prefrontal regions (Everitt and Robbins, 2013, Haber, 2016, Lally et al., 2014). With its limbic, associative and sensorimotor functional subdivision the striatum is critical for integrating information between cortical and subcortical reward-processing areas. While first considered as parallel operating loops (Alexander and Crutcher, 1990), subsequent studies suggest a functional interplay to enable information funnelling from the ventral to the dorsal striatum (Haber, 2003). Imaging studies in humans support the division of the striatum into functional subdivisions (Marquand et al., 2017, Tziortzi et al., 2014). Compelling evidence indicates that reward processing involves interactive striatal networks, beginning with the coding of hedonic mechanisms in the ventral striatum to strategic action planning and habit formation in the dorsal striatum (Everitt and Robbins, 2013, Haber, 2016).
Previous studies observed that anhedonia severity, but not depression per se, negatively correlated with both ventral and dorsal (putamen) striatum activity in response to pleasant stimuli in patients with MDD (Keedwell et al., 2005) and SZ (Harvey et al., 2010). Structural neuroimaging studies in contrast reported that anhedonia expression exclusively correlated with dorsal striatum (caudate and putamen) volume in non-clinical individuals (Enneking et al., 2019, Harvey et al., 2007) and MDD patients (Enneking et al., 2019, Pizzagalli et al., 2009). However, whether anhedonia severity across traditional disease boundaries maps onto ventral and/or dorsal striatum volume remains unknown.
The aim of this study was to test whether striatal grey matter volume was dimensionally related to anhedonia severity across patients with several different psychiatric diagnoses. We were interested in testing whether transdiagnostic anhedonia expression would be specifically related to ventral or dorsal striatum volume. This would provide evidence to disentangle, from a neuroscientific perspective, whether transdiagnostic anhedonia expression results from deficits in reward liking (consummatory anhedonia) and/or wanting (anticipatory anhedonia). Previous behavioural studies in SZ (Gard et al., 2007, Strauss et al., 2011) and MDD (Sherdell et al., 2012, Yang et al., 2014) provide inconsistent results. Whereas an exclusive relationship between anhedonia and volume in the ventral striatum would rather point toward deficits in reward liking (consummatory anhedonia), an involvement of dorsal striatal regions would suggest a deficit in reward wanting (anticipatory anhedonia). Given the key role of dopamine in reward wanting (and not liking) (Berridge and Robinson, 1998, Berridge and Robinson, 2016) and dysfunction of the dopamine system in psychiatric disorders including MDD, SZ, SUD and BPD (Friedel, 2004, Grace, 2016, Volkow et al., 2017), we predicted that higher transdiagnostic anhedonia severity would be related to reduced grey matter volume in dorsal striatal regions. Further, as anhedonic behaviour is also mediated by the medial prefrontal cortex, which exerts top-down control over midbrain dopaminergic interactions with the striatum (Ferenczi et al., 2016), striatal region of interest (ROI) approaches were complemented by whole-brain correlation analyses. We hypothesized that whole-brain analyses would confirm the inverse relationship between dorsal striatum volume and anhedonia expression but would additionally uncover the contribution of the medial prefrontal cortex to dimensional anhedonia expression. Finally, to characterize group differences beyond the dimensional relationship between striatal volume and anhedonia, we conducted group comparisons based on nosological disease categories, and then specifically tested for correlations with anhedonia expression within those regions, showing differences to a healthy control group. While this more classical approach to identifying symptom-related brain correlates has the advantage of establishing a direct link between brain anomalies and clinical psychopathology, it also decreases the likelihood of spurious associations with symptoms (Goghari et al., 2010). In other words, in addition to testing for brain-symptom associations irrespective of group differences in brain volume, we also tested with this later approach, whether a relation to anhedonia expression is found especially in those regions, which showed deviations from a healthy control group.
2. Materials and methods
Disorder-specific analyses in OUD (Schmidt et al., 2020), BPD (Wrege et al., 2019), FEP (Dukart et al., 2017), CUD (Engeli et al., 2020, Kirschner et al., 2018) and SZ (Stepien et al., 2018) have previously been published. Here, we report a post-hoc analysis with combined samples.
2.1. Participants
Three hundred thirteen participants were included in this analysis. Samples from two different centres were included: healthy controls (HC, n = 29), outpatients with opioid use disorder (OUD, n = 22), borderline personality disorder (BPD, n = 45) and first-episode psychosis (FEP, n = 49), and inpatients with MDD (n = 50) were recruited by clinicians from the Department of Psychiatry (Universitäre Psychiatrische Kliniken, UPK), University of Basel, Switzerland, and a sample of HCs (n = 60) outpatients with cocaine use disorder (CUD, n = 43) and chronic SZ (n = 15) were recruited from the Department of Psychiatry, Psychotherapy and Psychosomatics, University of Zurich, Switzerland. All participants provided written informed consent, and the study was approved by the respective local ethics committee.
With the exception of MDD, patients were diagnosed according to DSM-IV criteria (American Psychiatric Association, 2000) as assessed with the German Version of the Structured Clinical Interview for DSM-IV (SCID I and II) (Wittchen et al., 1997). MDD patients were recruited based on diagnoses made in the clinic according to ICD-10 criteria (World Health Organisation, 1992). Diagnoses were additionally confirmed with the Mini-International Neuropsychiatric Interview (M.I.N.I.) (Sheehan et al., 1998) according to DSM-IV criteria. Except for nicotine dependence, all patients were without current neurological or severe medical disorders and history of head injury and were above 18 and below 65 years old.
HCs (total n = 89) were recruited by advertisement and screened for any neuropsychiatric disorder using the M.I.N.I (Lecrubier et al., 1997) to ensure that they had no previous or present psychiatric illness. All participants were required to have no personal lifetime psychiatric disorder and no family history of any psychiatric disorder, head trauma, neurological illness, serious medical or surgical illness, or substance abuse. Healthy participants were further screened to exclude insufficient German language fluency. A detailed description of the study sample including medication is summarized in Table 1.
Table 1.
Sociodemographic and clinical characteristics of study participants.
| HC (n = 89) | OUDa (n = 22) | MDDb (n = 50) | BPDc (n = 45) | FEPd (n = 49) | CUDe (n = 43) | SZf (n = 15) | Between-group statistics | |
|---|---|---|---|---|---|---|---|---|
| Site | 20 Basel, 69 Zurich | Basel | Basel | Basel | Zurich | Zurich | Zurich | |
| Sex; female/male | 50/39 | 6/16 | 29/21 | 35/10 | 14/35 | 13/30 | 2/13 | χ2 = 44.24, p < 0.001 |
| Age in years, mean (SD) | 27.79 (6.27) | 50.77 (5.84) | 36.88 (10.49) | 27.51 (8.03) | 28.29 (7.26) | 30.53 (7.18) | 32.33 (9.44) | F(6, 312) = 33.687, p < 0.001 |
| Education in years, mean (SD) | 13.64 (3.47) | 10.00 (1.11) | 14.72 (2.94) | 13.01 (2.47) | 11.88 (2.93) | 11.44 (3.24) | 11.90 (1.85) | F(6, 307) = 11.55, p < 0.001 |
| Smoking, yes/no | 43/46 | 20/2 | 24/26 | 36/9 | 32/17 | 34/9 | 11/4 | χ2 = 31.78, p < 0.001 |
| Smoking, cigarettes per day, mean (SD) | 4.08 (6.02) | 17.32 (8.32) | 6.16 (8.00) | 11.93 (10.72) | 11.41 (10.86) | 11.63 (10.09) | 20.60 (24.12) | F(6, 307) = 10.01, p < 0.001 |
| BDI sum score, mean (SD) | NA | 14.05 (8.13) | 23.10 (8.92) | 26.55 (12.16) | NA | 7.93 (7.20) | 9.13 (8.35) | F(4, 174) = 29.80, p < 0.001 |
| BDI anhedonia score, mean (SD) | NA | 3.77 (2.49) | 5.22 (2.44) | 4.64 (2.83) | NA | 1.72 (1.76) | 1.87 (1.30) | F(4, 174) = 17.12, p < 0.001 |
| SANS anhedonia score, mean (SD) | NA | NA | 10.45 (5.37) | NA | 7.92 (5.36) | NA | 11.29 (7.70) | F(2, 113) = 3.25, p = 0.042 |
OUD, opioid use disorder; MDD, major depressive disorder; BPD, borderline personality disorder; FEP, first-episode psychosis; CUD, cocaine use disorder; SZ, schizophrenia; SD, standard deviation; BDI, Beck Depression Inventory; SANS, Scale for the Assessment of Negative Symptoms; NA, Not applicable;
Patients with OUD were actively enrolled in a heroin-assisted therapy for at least 6 months (mean 7.295 ± 4.74 years) with an unchanged dose of diacetylmorphine (DAM) during the previous 3 months (mean dose: 341.82 ± 126.52 mg). Duration of opioid use was 21.82 ± 5.82 years with an age of onset of 19.09 ± 3.41 years.
with the exception of one patient, all MDD patients received standard antidepressant medication (mean (SD) fluoxetine equivalence dose: 31.53 ± 20.29 mg; mean (SD) duration: 15.94 ± 9.04 days). 23 patients were additionally treated with antipsychotics (mean (SD) chlorpromazine equivalence dose: 83.04 ± 80.65 mg).
25 BPD patients were medication-free. 20 BPD patients were treated with antidepressants (mean (SD) fluoxetine equivalence dose: 44.00 ± 31.90 mg), of whom 10 were additionally treated with antipsychotics (mean (SD) chlorpromazine equivalence dose: 209.4 ± 190.55 mg) and 3 with antiepileptics (mean (SD) dose: 350 ± 132.29 mg). 2 patients exclusively received antipsychotics (mean (SD) chlorpromazine equivalence dose: 159.75 ± 175.72 mg).
20 FEP patients received antipsychotics: 5 × 5 mg olanzapine, 3 × 5 mg aripiprazole, 10 × quetiapine (3 × 25 mg, 1 × 40 mg, 1 × 50 mg, 2 x1 00 mg, 1 × 300 mg, 1 × 600 mg, 1 × 1000 mg), 1 × 6 mg risperidone, 1 × 50 mg amisulpride (mean (SD) chlorpromazine equivalence dose: 250 ± 285.49 mg).
CUD were not medicated.
14 (out of 15) schizophrenia patients were treated with antipsychotics: 4 × clozapine (1 × 50 mg, 1 × 75 mg, 1 × 175 mg, 1 × 200 mg), 3 × aripiprazole (1 × 5 mg, 1 × 10 mg, 1 × 15 mg), 1 × 80 mg lurasidone, 3 × olanzapine (2 × 15 mg, 1 × 20 mg), 2 × paliperidone (1 × 100 mg, 1 × 150 mg), 1 × 200 mg quetiapine (mean (SD) chlorpromazine equivalence dose: 1146.53 ± 2412.78 mg).
2.2. Assessment of anhedonia symptoms
In the OUD, MDD, BPD, CUD and SZ group, depressive symptoms were assessed with the Beck Depression Inventory (BDI) total score (Beck et al., 1961). As in previous studies (Pizzagalli et al., 2009, Pizzagalli et al., 2005), a ‘BDI anhedonic subscore’ was calculated with a total score on BDI items associated with anhedonic symptoms: loss of pleasure, loss of interest, loss of energy, and loss of interest in sex. Internal consistency of this subscore was acceptable (Cronbachs alpha α = 0.78, CI 0.73–0.83). In the MDD, FEP and SZ group, anhedonia was (additionally) assessed with the Anhedonia-Asociality subscale of the Scale for the Assessment of Negative Symptoms (SANS (Andreasen, 1982), recreational interest and activities, sexual interest and activity, ability to feel intimacy and closeness, relationship with friends and peers, global rating of anhedonia-asociality). Internal consistency was good (α = 0.89, CI 0.87–0.92). SANS assessments were conducted by trained psychiatrists (MK, NS, EE, SB, MW, JW).
2.3. MRI data acquisition
The Basel sample was scanned using a 3T MRI system (Siemens Magnetom Prisma, Erlangen, Germany) and a 20-channel phased-array radio frequency head coil. Head movement was minimized by foam padding across the forehead. A whole brain 3-dimensional T1-weighted magnetization prepared rapid acquisition gradient (MPRAGE) sequence was applied. 176 slices were acquired in 4:08 min with a field of view of 256 mm2, voxels size 1 mm3 isotropic spatial resolution, inversion time of 1000 ms, repetition time of 2000 ms, echo time of 3.37 ms, flip angle of 8° and bandwidth of 200 Hz/pixel. The Zurich sample was scanned using a Philips Achieva 3T whole-body scanner equipped with a 32-channel receive-only phased-array head coil (Philips Healthcare, Best, The Netherlands). Whole brain 3-dimensional T1-weighted anatomical data were obtained by using a MPRAGE with the following parameters: The MPRAGE sequence acquired 160 slices in 7:32 min with a field of view of 240 mm2, voxels size 1 mm3 isotropic spatial resolution, inversion time of 1008 ms, repetition time of 2987 ms, echo time of 3.7 ms, flip angle of 8° and bandwidth of 192 Hz/pixel. Raw images in both centres were assessed by trained neuroradiologists for radiological abnormalities.
2.4. Voxel-based morphometry (VBM)
MRI data were analysed with the standard automated processing stream of FSL-VBM (Douaud et al., 2007) (http://fsl.fmrib.ox.ac.uk/fsl/fslwiki/FSLVBM), an optimized VBM protocol (Good et al., 2001b) performed with FSL tools (Smith et al., 2004). The standard and optimized VBM protocol has been validated with highly reproducible segmentation results (Douaud et al., 2007, Good et al., 2001a, Good et al., 2001b, Good et al., 2002, Maguire et al., 2000, Voets et al., 2008). First, structural images were brain extracted and grey matter segmented before being registered to the 2 mm MNI 152 standard space using nonlinear registration. The resulting images were averaged and flipped along the x-axis to create a left–right symmetric, study-specific grey matter template. Second, all native grey matter images were nonlinearly registered to this study-specific template and “modulated” to correct for local expansion (or contraction) due to the nonlinear component of the spatial transformation. The modulated grey matter images were then smoothed with an isotropic Gaussian kernel with a sigma of 3 mm. The outputs of each VBM step were visually checked by authors (ACS, AS). In practice, all VBM steps (except for brain extraction) did not require any manual interventions. The brain extraction (FSL BET (Smith, 2002)) sometimes resulted in inaccurate brain extraction, where part of the neck was sometimes included. In these cases (n = 6), the parameters were manually tuned until the results were satisfactory.
3. Statistical analyses
3.1. Symptom-structure correlation analyses
Based on previous evidence emphasising a relationship between anhedonia expression and striatal abnormalities (Husain and Roiser, 2018, Whitton et al., 2015), dimensional symptom-structure analyses were first conducted using a region of interest (ROI) approach by restricting the analysis to the bilateral nucleus accumbens, caudate and putamen. The Harvard-Oxford subcortical structural atlas as implemented in FSL was used to create the anatomical ROI mask (Supplementary Fig. 1A). In case of significant ROI findings, we counted the number of voxels that overlapped between the significant ROI clusters and the functional subdivisions of the striatum according to studies by Mawlawi, Martinez and colleagues (Martinez et al., 2003, Mawlawi et al., 2001) (Supplementary Fig. 1B): Associative striatum: precommissural dorsal caudate, postcommissural caudate, and precommissural dorsal putamen; limbic striatum: equivalent to the ventral striatum; sensorimotor striatum: postcommissural putamen. The ROI approach was completed by whole-brain analyses, bearing in mind that cortical-subcortical interactions regulate reward processing and anhedonia behaviour (Ferenczi et al., 2016).
A voxel-wise general linear model (GLM) was applied with nonparametric permutation (5000) tests (randomize (Nichols and Holmes, 2002)) using a single-group average design with additional covariates to test the relationship between grey matter volume and anhedonic symptoms across the diagnostic groups. Positive and negative associations between striatal / whole-brain grey matter and anhedonia scores were tested by controlling for age, gender (dummy variable), smoking (number of cigarettes per day), diagnosis (dummy variable), intracranial volume and scanner (dummy variable). Analyses with BDI anhedonia scores were also controlled for general depressive symptoms (BDI sum score without anhedonia items). Finally, medication was also added as a categorical (dummy) variable, with ‘0′ for ‘no medication’, ‘1′ for ‘diacetylmorphine’, ‘2′ for ‘antidepressants’, ‘3′ for ‘antipsychotics’ and ‘4′ for ‘antidepressants + antipsychotics’ in the BDI analyses, and with ‘0′ for ‘no medication’, ‘1′ for ‘antidepressants’, ‘2′ for ‘antipsychotics’ and ‘3′ for ‘antidepressants + antipsychotics’ in the SANS analyses.
The statistical maps were thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparison using the threshold-free cluster enhancement (TFCE) technique (Smith and Nichols, 2009). Analysis was first conducted across patients with BDI scores (OUD, MDD, BPD, CUD, SZ, n = 175) and then across patients with available SANS scores (MDD, FEP, SZ, n = 114). Although scanner was included as a covariate in these multi-site analyses, we also conducted site-specific VBM correlation analyses. Given their effect on brain volume (Ho et al., 2011), two further subanalyses (one for BDI and one for SANS anhedonia) using chlorpromazine equivalent dose as covariate were conducted only in patients receiving antipsychotics.
3.2. Group differences in striatal volume and relationship to anhedonia
To compare striatal grey matter volume between groups including a sample of HC (total sample, n = 313), a GLM with 5000 permutations was performed using analysis of variance (ANOVA). Given the low number of SZ patients, FEP and SZ patients were considered together as one group with psychotic disorders for this analysis. Group comparisons were controlled for age, gender, smoking and scanner, demeaned across the included subjects. The statistical maps were thresholded at p < 0.05, family-wise error (FWE) corrected for multiple comparison using the TFCE technique (Smith and Nichols, 2009). Pairwise group comparisons (t-tests) were only conducted in case of a significant F test. A mask consisting of regions showing significant group effects was created for subsequent post-hoc comparisons. To test potential scanner effects, we further compared striatal volume between the HCs from Basel (n = 29) and Zurich (n = 60) adjusted for age, gender and smoking.
Finally, in case of significant group differences in striatal volume, partial correlation analysis (adjusted for age, gender, smoking, diagnosis, general depressive expression and scanner) between individual volume scores in these regions and anhedonia scores was performed across all patients.
4. Results
4.1. Diagnosis-specific anhedonia expression
BDI anhedonia scores differed between patients with OUD, MDD, BPD, CUD and SZ (Table 1, Supplementary Fig. 2A). Tukey post-hoc testing showed higher scores in MDD and BPD relative to SZ and CUD patients (p’s < 0.001). OUD patients also had higher BDI anhedonia scores than CUD patients (p = 0.009).
SANS anhedonia scores also differed between MDD, SZ and FEP patients (Table 1, Supplementary Fig. 2B). Post-hoc testing revealed a trend towards higher scores in MDD relative to FEP patients (p = 0.076) but no significant difference between SZ and FEP (p = 0.13), and MDD patients (p = 0.879), likely due to the large standard error in the SZ group.
Notably, across patients having both scores available (MDD and SZ, n = 65), BDI anhedonia and SANS anhedonia scores correlated positively (r = 0.254, p = 0.044).
4.2. Symptom-structure correlation analyses
4.2.1. BDI anhedonia
Restricting the correlation analysis to the striatum (ROI analysis), we found a significant negative relationship between grey matter volume in the left and right putamen and BDI anhedonia scores across patients with OUD, MDD, BPD, CUD and SZ (Fig. 1 A). In other words, the less volume in the putamen, the higher the transdiagnostic expression of anhedonia. Whole-brain correlation analysis confirmed the negative relationship between BDI anhedonia scores and left putamen volume and further revealed a significant negative association with volume in the bilateral cerebellum (subsuming the lateral occipital cortex and fusiform gyrus) (Fig. 1B and C).
Fig. 1.
A) Significant negative relationship between left putamen (voxel size = 300, pFWE = 0.001, center of mass: x = −28, y = −8, z = 1) and right putamen (voxel size = 108, pFWE = 0.014, center of mass: x = 29, y = −11, z = 3) volume and BDI anhedonia severity across OUD, MDD, BPD, CU and SZ patients (n = 175) using a region of interest (ROI) approach. There was an overlap of 139 and 33 voxels, respectively, between the left/right putamen cluster and masks of the left/right sensorimotor striatum (cf. Supplementary Fig. 1B). B) Whole-brain correlation analysis showing significant negative associations between BDI anhedonia scores and volume in the left putamen (voxel size = 32, pFWE = 0.036, centre of mass: x = −30, y = −11, z = 0) and bilateral cerebellum (subsuming the lateral occipital cortex and fusiform gyrus) (voxel size = 6679, pFWE = 0.001, centre of mass: x = 4, y = −58, z = −36). There was an overlap of 29 voxels between the left putamen cluster and mask of the left sensorimotor striatum. C) Scatterplot showing negative relationship between left putamen volume (mm3, values extracted from the significant whole-brain result) and anhedonia severity across OUD, MDD, BPD, CUD and SZ patients (r = −0.325). All correlations were controlled for age, gender, smoking, general depression (BDI sum score without anhedonia items), medication, diagnosis and scanner. Left hemisphere is displayed on the right.
Repeating the analysis for each centre separately, the relationship between BDI anhedonia scores and left putamen volume was significant in the Basel sample (OUD, MDD, BPD, n = 117) for both the ROI and whole-brain analysis (Supplementary Fig. 3) but not in the Zurich sample (CUD, SZ, n = 58) (ROI pFWE = 0.14), probably due to the small number of patients.
ROI and whole-brain analyses including only antipsychotic-treated patients (23 MDD, 10 BPD, 14 SZ) further confirmed the negative relationship between left putamen volume and transdiagnostic BDI anhedonia scores (Supplementary Figure 4).
4.2.2. SANS anhedonia
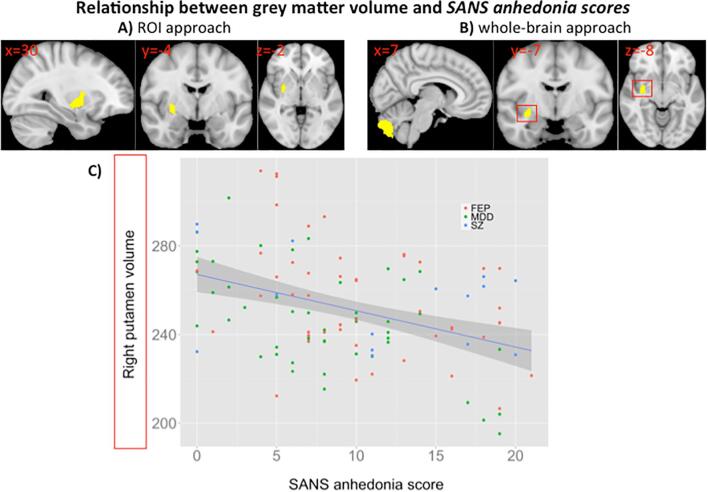
There was also a significant negative correlation between SANS anhedonia scores and right putamen volume across MDD, FEP and SZ patients (n = 114) using the ROI approach (Fig. 2A). Whole-brain analysis yielded a negative relationship between SANS anhedonia scores and right putamen and cerebellum volume (Fig. 2B and C). The negative relationship between SANS anhedonia scores and right putamen volume was also found when the analysis was restricted to MDD and FEP patients from Basel only (n = 99, Supplementary Fig. 5). Further, ROI but not whole-brain analysis (pFWE right = 0.06) in antipsychotic-treated patients (23 MDD, 20 FEP, 14 SZ) confirmed the negative relationship between right putamen volume and SANS anhedonia scores (Supplementary Fig. 6). Notably, we found no relationships between grey matter volume and SANS Avolition-Apathy scores across MDD, FEP and SZ patients.
Fig. 2.
A) Significant negative relationship between right putamen volume (voxel size = 140, pFWE = 0.002, centre of mass: x = 30, y = −4, z = −2) and SANS anhedonia severity across MDD, FEP and SZ patients (n = 114) using a region of interest (ROI) approach. There was an overlap of 57 voxels between the right putamen cluster and mask of the right sensorimotor striatum. B) Whole-brain correlation analysis showing a significant association between SANS anhedonia scores and volume in the right putamen (voxel size = 88, pFWE = 0.017, centre of mass: x = 33, y = −7, z = −8) and cerebellum (voxel size = 887, pFWE = 0.006, centre of mass: x = 4, y = −79, z = −40). There was an overlap of 11 voxels between the right putamen cluster and mask of the right sensorimotor striatum. C) Scatterplot showing relationship between right putamen volume (mm3, values extracted from significant whole-brain result) and SANS anhedonia severity across MDD, FEP and SZ patients (r = −0.453). All correlations were controlled for age, gender, smoking, medication, diagnosis and scanner. Left hemisphere is displayed on the right.
4.3. Group differences in striatal volume and relationship to anhedonia
A significant group effect in the right accumbens was observed (Fig. 3A). Compared with HC, post-hoc testing revealed lower volume in the right accumbens in patients with OUD (voxel size = 20, pFWE = 0.025) and psychotic disorders (FEP + SZ) (voxel size = 21, pFWE = 0.003) (Fig. 3B). CUD individuals had higher accumbens volume than patients with psychotic disorders (voxel size = 19, pFWE = 0.043) and OUD (voxel size = 9, pFWE = 0.040), while OUD patients also exhibited lower volume than MDD (voxel size = 21, pFWE = 0.002) and BPD patients (voxel size = 16, pFWE = 0.034) in the right accumbens (Fig. 3B). Furthermore, both MDD (voxel size = 21, pFWE = 0.001) and BPD patients (voxel size = 21, pFWE = 0.006) exhibited higher volume in the right accumbens than psychotic patients (Fig. 3B). Notably, there were no significant relationships between right accumbens volume (values extracted from main effect of group) and BDI anhedonia scores (r = −0.059, p = 0.446) across OUD, MDD, BPD, CUD and SZ patients and SANS anhedonia scores across MDD, FEP and SZ patients (r = 0.155, p = 0.112).
Fig. 3.
A) Group effect in the right accumbens (voxel size = 21, pFWE = 0.025, centre of mass: x = 7, y = 13, z = −5). B) Volume (mm3) of right accumbens for each group. HC, healthy controls; CUD, cocaine use disorder; OUD, opioid use disorder; MDD, major depressive disorder; BPD, borderline personality disorder. * and ** indicate group differences at p < 0.05 and p < 0.01, respectively.
Finally, no difference in striatal volume between HC samples from Basel and Zurich was found, indicating comparability of scanner data.
5. Discussion
The main finding of this study indicates volumetric abnormalities in the putamen and cerebellum as a neural substrate of transdiagnostic anhedonia severity. This finding was evident when assessing anhedonia with two different scales across patients with six different psychiatric diagnoses from two different centres. Our main finding extends previous studies in MDD patients showing a relationship between high anhedonia expression and low putamen volume (Enneking et al., 2019, Sachs-Ericsson et al., 2018) and activity during reward anticipation (Pizzagalli et al., 2009). Given that reduced bilateral putamen volume was further predictive for anhedonia severity in a recent study with MDD patients (Auerbach et al., 2017), our finding may have implications for transdiagnostic anhedonia prognosis and treatment.
Anhedonia subsumes a consummatory (hedonic capacity) and motivational (anticipation of and drive towards rewarding stimuli) aspect (Der-Avakian and Markou, 2012). A previous meta-analysis revealed that both consummatory and anticipatory anhedonia was associated with reduced putamen activity in MDD and SZ patients compared to healthy volunteers (Zhang et al., 2016). Results from a positron emission tomography study further showed that the fast-acting antidepressant ketamine decreased anticipatory anhedonia in patients with treatment-resistant depression along with increased glucose metabolism in the putamen and cerebellum, and that this in turn may reflect increased motivation towards pleasurable experiences (Lally et al., 2014). Notably, no relationship between anhedonia scores and ventral striatum metabolism was observed as originally hypothesized (Lally et al., 2014). In the present study, there was also no relationship between volume in the accumbens and transdiagnostic anhedonia expression, although significant group differences in this region were observed. This result indicates that etiologically relevant relationships between symptom dimensions (here anhedonia) and brain structure might not be detected by employing correlation analysis that relies on the presence of categorical group differences. Possible reasons for this result are that the differential accumbens volume between groups may reflect other disease-related features than anhedonia, or that the nucleus accumbens shell may rather mediate ‘liking’ or consummatory pleasure behaviours (Peciña and Berridge, 2005), whereas motivational hedonic behaviours (‘wanting’) may involve a wider network consisting of the accumbens but also other neural structures such as the caudate, pallidus and putamen (Berridge, 1996, Schultz et al., 2000). Therefore, the relationships between putamen volume and anhedonia expression found here may suggest that the obtained BDI and SANS anhedonia scores may reflect low anticipatory pleasure rather than decreased consummatory liking. This is supported by evidence showing that the BDI and SANS anhedonia scale is negatively related to the anticipatory but not consummatory component of the Temporal Experience of Pleasure Scale (TEPS) (Gard et al., 2006, Gard et al., 2007).
The putamen is highly connected to sensory-motor-related areas (Alexander et al., 1990, Gerardin et al., 2003) and is involved in the acquisition of stimulus-action-reward association (Haruno and Kawato, 2006). In particular, it integrates information on the expectation of reward with processes that mediate the actions leading to the reward (Haruno and Kawato, 2006, O'Doherty et al., 2004). It has further been shown that decreased functional coupling between prefrontal motor areas and the putamen was inversely related to psychomotor retardation in MDD patients (Liberg et al., 2014). Reduced putamen activation is also associated with anticipatory reward deficits (Kumar et al., 2014), and inhibitory dysfunction of the ventrolateral putamen in monkeys reduced the frequency of self-initiated actions to collect reward (Worbe et al., 2009).
Besides putamen-anhedonia associations, we also found negative relationships between the cerebellum volume and transdiagnostic anhedonia expression. Regions outside the basal ganglia, including the cerebellum, have also been associated with rewards and reward prediction errors (Garrison et al., 2013), suggesting that the cerebellum functions along with the basal ganglia to provide the neural substrate for reward coding and reward-based learning (Bostan and Strick, 2018) and are implicated in the development of psychosis and depression (Bogoian et al., 2020, Moberget and Ivry, 2019). Enhanced effective connectivity from the cerebellum to the sensorimotor putamen has been associated with improved learning of motor sequence acquisition (Tzvi et al., 2015). Within such an integrated network perspective, motivation-related signals from the basal ganglia may drive the cerebellum to optimize goal-directed movement parameters (Bostan and Strick, 2018) and are putatively altered in patients with anhedonia. Intriguingly, the cerebellum can also modulate the reward circuitry besides adjustment of goal-related movement; it has recently been shown that the cerebellum can activate the ventral tegmental area (VTA) in mice and thereby contribute to place preference (Carta et al., 2019). This finding suggests the involvement of the cerebellum in reward-related functions beyond movement refinement such as social exploration and behaviour. It is possible that the cerebellar projections to the VTA also modulate neurons that project to the prefrontal cortex, indicating a pathway how the cerebellum influences prefrontal dopamine levels and possibly negative symptoms (Brady et al., 2019). Taken together, our findings may suggest that transdiagnostic anhedonia expression might be related to volumetric alterations within a putamen-cerebellum network that mediates reward-related goal-directed behaviour. However, although we found that transdiagnostic anhedonia severity was associated with both putamen and cerebellum volume, whether abnormal structural putamen-cerebellum connectivity underlines transdiagnostic anhedonia expression must be confirmed explicitly by covariance network analyses.
This study has several limitations that need to be addressed by future research. Anhedonia has been conceptualized as comprising deficit across three partially separable subtypes of reward processing: reward liking (consummatory phase), reward wanting (selection between options and initiating and sustaining approach behaviour as well as the anticipation and preparation phase) and reward learning (learning from outcomes to optimize future decisions) (Admon and Pizzagalli, 2015, Husain and Roiser, 2018, Treadway and Zald, 2011). Disentangling whether abnormalities in the neural circuitry of these reward processes are specifically related to the clinical manifestation of social and physical anhedonia possibly provides implications for treatment regimes. In the current post-hoc investigation we used rather broad measures of anhedonia as expressed by the BDI and SANS anhedonia subscales, which do not allow a differentiation between the different subtypes of reward processing that may contribute to physical and/or social anhedonia. Behavioural paradigms providing readouts for the specific subtypes of reward processing are warranted. Related to this point, the BDI and SANS were developed for different disorders (i.e. MDD and SZ), raising the question of whether they are measuring the same construct. However, a certain degree of construct validity seems evident as we found a significant positive correlation between the two scales across MDD and SZ patients and both scales were negatively related to putamen and cerebellum volume across the disorders. Also, given the post-hoc nature of this study, measures of other (negative) symptoms were not consistently available for all participants to test whether putamen-cerebellum volumes were specifically associated with anhedonia. Finally, although we controlled our analyses for the different types of medications and especially addressed the impact of antipsychotics, we cannot completely rule out that our findings were confounded by different medication types – our findings should be interpreted with this caveat in mind.
In conclusion, our findings suggest that volumetric abnormalities in the putamen and cerebellum are related to anhedonia expression across several psychiatric entities. Mapping putamen-cerebellum aberrations to dimensional symptom expressions that are related to the different subcomponents of reward processing holds promise for developing targeted interventions across psychiatric nosology.
CRediT authorship contribution statement
Anna-Chiara Schaub: Data curation, Formal analysis, Writing - original draft, Writing - review & editing. Matthias Kirschner: Data curation, Investigation, Writing - review & editing. Nina Schweinfurth: Data curation, Investigation, Writing - review & editing. Laura Mählmann: Data curation, Investigation, Writing - review & editing. Cedric Kettelhack: Data curation, Investigation, Writing - review & editing. Etna E. Engeli: Data curation, Investigation, Writing - review & editing. Jessica P.K. Doll: Data curation, Writing - review & editing. Stefan Borgwardt: Funding acquisition, Project administration, Resources, Writing - review & editing. Undine E. Lang: Funding acquisition, Project administration, Resources, Writing - review & editing. Stefan Kaiser: Funding acquisition, Project administration, Resources, Writing - review & editing. Marc Walter: Funding acquisition, Project administration, Resources, Writing - review & editing. Marcus Herdener: Funding acquisition, Project administration, Resources, Writing - review & editing. Johannes Wrege: Funding acquisition, Project administration, Resources, Writing - review & editing. André Schmidt: Conceptualization, Formal analysis, Funding acquisition, Methodology, Project administration, Resources, Supervision, Validation, Writing - original draft, Writing - review & editing.
Acknowledgments
Acknowledgments
This work was supported by the Stiftung zur Förderung der gastroenterologischen und allgemeinen klinischen Forschung sowie der medizinischen Bildauswertung (AS).
Disclosures
The authors report no biomedical financial interests or potential conflicts of interest.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.nicl.2021.102825.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- Admon R., Pizzagalli D.A. Dysfunctional reward processing in depression. Curr. Opin. Psychol. 2015;4:114–118. doi: 10.1016/j.copsyc.2014.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D., DeLong M.R. Basal ganglia-thalamocortical circuits: parallel substrates for motor, oculomotor, “prefrontal” and “limbic” functions. Prog. Brain Res. 1990;85:119–146. [PubMed] [Google Scholar]
- Alexander G.E., Crutcher M.D. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13(7):266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association, 2000. Diagnostic and Statistical Manual of Mental Disorders (4th edition). American Psychiatric Association, Washington, DC.
- Andreasen N.C. Negative symptoms in schizophrenia. Definition and reliability. Arch. Gen. Psychiatry. 1982;39:784–788. doi: 10.1001/archpsyc.1982.04290070020005. [DOI] [PubMed] [Google Scholar]
- Auerbach R.P., Pisoni A., Bondy E., Kumar P., Stewart J.G., Yendiki A., Pizzagalli D.A. Neuroanatomical prediction of anhedonia in adolescents. Neuropsychopharmacology. 2017;42:2087–2095. doi: 10.1038/npp.2017.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck A.T., Ward C.H., Mendelson M., Mock J., Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Berridge K.C. Food reward: brain substrates of wanting and liking. Neurosci. Biobehav. Rev. 1996;20:1–25. doi: 10.1016/0149-7634(95)00033-b. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Res. Brain Res. Rev. 1998;28:309–369. doi: 10.1016/s0165-0173(98)00019-8. [DOI] [PubMed] [Google Scholar]
- Berridge K.C., Robinson T.E. Liking, wanting, and the incentive-sensitization theory of addiction. Am. Psychol. 2016;71:670–679. doi: 10.1037/amp0000059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogoian H.R., King T.Z., Turner J.A., Semmel E.S., Dotson V.M. Linking depressive symptom dimensions to cerebellar subregion volumes in later life. Transl. Psychiatry. 2020;10:201. doi: 10.1038/s41398-020-00883-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bostan A.C., Strick P.L. The basal ganglia and the cerebellum: nodes in an integrated network. Nat. Rev. Neurosci. 2018;19:338–350. doi: 10.1038/s41583-018-0002-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady R.O., Gonsalvez I., Lee I., Öngür D., Seidman L.J., Schmahmann J.D., Eack S.M., Keshavan M.S., Pascual-Leone A., Halko M.A. Cerebellar-prefrontal network connectivity and negative symptoms in schizophrenia. Am. J. Psychiatry. 2019;176:512–520. doi: 10.1176/appi.ajp.2018.18040429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carta I., Chen C.H., Schott A.L., Dorizan S., Khodakhah K. Cerebellar modulation of the reward circuitry and social behavior. Science. 2019;363 doi: 10.1126/science.aav0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox J., Witten I.B. Striatal circuits for reward learning and decision-making. Nat. Rev. Neurosci. 2019;20:482–494. doi: 10.1038/s41583-019-0189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuthbert B.N., Insel T.R. Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med. 2013;11:126. doi: 10.1186/1741-7015-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Der-Avakian A., Markou A. The neurobiology of anhedonia and other reward-related deficits. Trends Neurosci. 2012;35:68–77. doi: 10.1016/j.tins.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douaud G., Smith S., Jenkinson M., Behrens T., Johansen-Berg H., Vickers J., James S., Voets N., Watkins K., Matthews P.M., James A. Anatomically related grey and white matter abnormalities in adolescent-onset schizophrenia. Brain. 2007;130:2375–2386. doi: 10.1093/brain/awm184. [DOI] [PubMed] [Google Scholar]
- Dukart J., Smieskova R., Harrisberger F., Lenz C., Schmidt A., Walter A., Huber C., Riecher-Rössler A., Simon A., Lang U.E., Fusar-Poli P., Borgwardt S. Age-related brain structural alterations as an intermediate phenotype of psychosis. J. Psychiatry Neurosci. 2017;42:307–319. doi: 10.1503/jpn.160179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engeli E.J.E., Zoelch N., Hock A., Nordt C., Hulka L.M., Kirschner M., Scheidegger M., Esposito F., Baumgartner M.R., Henning A., Seifritz E., Quednow B.B., Herdener M. Impaired glutamate homeostasis in the nucleus accumbens in human cocaine addiction. Mol. Psychiatry. 2020 doi: 10.1038/s41380-020-0828-z. [DOI] [PubMed] [Google Scholar]
- Enneking V., Krüssel P., Zaremba D., Dohm K., Grotegerd D., Förster K., Meinert S., Bürger C., Dzvonyar F., Leehr E.J., Böhnlein J., Repple J., Opel N., Winter N.R., Hahn T., Redlich R., Dannlowski U. Social anhedonia in major depressive disorder: a symptom-specific neuroimaging approach. Neuropsychopharmacology. 2019;44:883–889. doi: 10.1038/s41386-018-0283-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt B.J., Robbins T.W. From the ventral to the dorsal striatum: devolving views of their roles in drug addiction. Neurosci. Biobehav. Rev. 2013;37:1946–1954. doi: 10.1016/j.neubiorev.2013.02.010. [DOI] [PubMed] [Google Scholar]
- Ferenczi, E.A., Zalocusky, K.A., Liston, C., Grosenick, L., Warden, M.R., Amatya, D., Katovich, K., Mehta, H., Patenaude, B., Ramakrishnan, C., Kalanithi, P., Etkin, A., Knutson, B., Glover, G.H., Deisseroth, K., 2016. Prefrontal cortical regulation of brainwide circuit dynamics and reward-related behavior. Science 351, aac9698. [DOI] [PMC free article] [PubMed]
- Friedel R.O. Dopamine dysfunction in borderline personality disorder: a hypothesis. Neuropsychopharmacology. 2004;29:1029–1039. doi: 10.1038/sj.npp.1300424. [DOI] [PubMed] [Google Scholar]
- Gard D.E., Gard M.G., Kring A.M., John O.P. Anticipatory and consummatory components of the experience of pleasure: a scale development study. J. Res. Pers. 2006;40:1086–1102. [Google Scholar]
- Gard D.E., Kring A.M., Gard M.G., Horan W.P., Green M.F. Anhedonia in schizophrenia: distinctions between anticipatory and consummatory pleasure. Schizophr. Res. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfield J.B.B., Lubman D.I., Yücel M. Anhedonia in substance use disorders: a systematic review of its nature, course and clinical correlates. Aust. N. Z. J. Psychiatry. 2014;48:36–51. doi: 10.1177/0004867413508455. [DOI] [PubMed] [Google Scholar]
- Garrison J., Erdeniz B., Done J. Prediction error in reinforcement learning: a meta-analysis of neuroimaging studies. Neurosci. Biobehav. Rev. 2013;37:1297–1310. doi: 10.1016/j.neubiorev.2013.03.023. [DOI] [PubMed] [Google Scholar]
- Gerardin E., Lehéricy S., Pochon J.B., Tézenas du Montcel S., Mangin J.F., Poupon F., Agid Y., Le Bihan D., Marsault C. Foot, hand, face and eye representation in the human striatum. Cereb. Cortex. 2003;13:162–169. doi: 10.1093/cercor/13.2.162. [DOI] [PubMed] [Google Scholar]
- Goghari V.M., Sponheim S.R., MacDonald A.W. The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neurosci. Biobehav. Rev. 2010;34:468–486. doi: 10.1016/j.neubiorev.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Good C.D., Johnsrude I.S., Ashburner J., Henson R.N.A., Friston K.J., Frackowiak R.S.J. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Good C.D., Scahill R.I., Fox N.C., Ashburner J., Friston K.J., Chan D., Crum W.R., Rossor M.N., Frackowiak R.S.J. Automatic differentiation of anatomical patterns in the human brain: validation with studies of degenerative dementias. Neuroimage. 2002;17:29–46. doi: 10.1006/nimg.2002.1202. [DOI] [PubMed] [Google Scholar]
- Grace A.A. Dysregulation of the dopamine system in the pathophysiology of schizophrenia and depression. Nat. Rev. Neurosci. 2016;17:524–532. doi: 10.1038/nrn.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haber S.N. The primate basal ganglia: parallel and integrative networks. J. Chem. Neuroanat. 2003;26:317–330. doi: 10.1016/j.jchemneu.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Haber S.N. Corticostriatal circuitry. Dialogues Clin. Neurosci. 2016;18:7–21. doi: 10.31887/DCNS.2016.18.1/shaber. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruno M., Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J. Neurophysiol. 2006;95:948–959. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- Harvey P.-O., Pruessner J., Czechowska Y., Lepage M. Individual differences in trait anhedonia: a structural and functional magnetic resonance imaging study in non-clinical subjects. Mol. Psychiatry. 2007;12:767–775. doi: 10.1038/sj.mp.4002021. [DOI] [PubMed] [Google Scholar]
- Harvey P.-O., Armony J., Malla A., Lepage M. Functional neural substrates of self-reported physical anhedonia in non-clinical individuals and in patients with schizophrenia. J. Psychiatr. Res. 2010;44:707–716. doi: 10.1016/j.jpsychires.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Heinz A., Schmidt L., Reischies F. Anhedonia in schizophrenic, depressed, or alcohol-dependent patients–neurobiological correlates. Pharmacopsychiatry. 1994;27(Suppl 1):7–10. doi: 10.1055/s-2007-1014317. [DOI] [PubMed] [Google Scholar]
- Ho B.-C., Andreasen N.C., Ziebell S., Pierson R., Magnotta V. Long-term antipsychotic treatment and brain volumes: a longitudinal study of first-episode schizophrenia. Arch. Gen. Psychiatry. 2011;68:128–137. doi: 10.1001/archgenpsychiatry.2010.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horan W.P., Kring A.M., Blanchard J.J. Anhedonia in schizophrenia: a review of assessment strategies. Schizophr. Bull. 2006;32:259–273. doi: 10.1093/schbul/sbj009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Husain M., Roiser J.P. Neuroscience of apathy and anhedonia: a transdiagnostic approach. Nat. Rev. Neurosci. 2018;19:470–484. doi: 10.1038/s41583-018-0029-9. [DOI] [PubMed] [Google Scholar]
- Insel T., Cuthbert B., Garvey M., Heinssen R., Pine D.S., Quinn K., Sanislow C., Wang P. Research domain criteria (RDoC): toward a new classification framework for research on mental disorders. Am. J. Psychiatry. 2010;167:748–751. doi: 10.1176/appi.ajp.2010.09091379. [DOI] [PubMed] [Google Scholar]
- Keedwell P.A., Andrew C., Williams S.C.R., Brammer M.J., Phillips M.L. The neural correlates of anhedonia in major depressive disorder. Biol. Psychiatry. 2005;58:843–853. doi: 10.1016/j.biopsych.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Kelley A.E., Bakshi V.P., Haber S.N., Steininger T.L., Will M.J., Zhang M. Opioid modulation of taste hedonics within the ventral striatum. Physiol. Behav. 2002;76:365–377. doi: 10.1016/s0031-9384(02)00751-5. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Sladky R., Haugg A., Stämpfli P., Jehli E., Hodel M., Engeli E., Hösli S., Baumgartner M.R., Sulzer J., Huys Q.J.M., Seifritz E., Quednow B.B., Scharnowski F., Herdener M. Self-regulation of the dopaminergic reward circuit in cocaine users with mental imagery and neurofeedback. EBioMedicine. 2018;37:489–498. doi: 10.1016/j.ebiom.2018.10.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar P., Berghorst L.H., Nickerson L.D., Dutra S.J., Goer F.K., Greve D.N., Pizzagalli D.A. Differential effects of acute stress on anticipatory and consummatory phases of reward processing. Neuroscience. 2014;266:1–12. doi: 10.1016/j.neuroscience.2014.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lally, N., Nugent, A.C., Luckenbaugh, D.A., Ameli, R., Roiser, J.P., Zarate, C.A., 2014. Anti-anhedonic effect of ketamine and its neural correlates in treatment-resistant bipolar depression. Transl Psychiatry 4, e469. [DOI] [PMC free article] [PubMed]
- Lally N., Nugent A.C., Luckenbaugh D.A., Niciu M.J., Roiser J.P., Zarate C.A. Neural correlates of change in major depressive disorder anhedonia following open-label ketamine. J. Psychopharmacol. 2015;29:596–607. doi: 10.1177/0269881114568041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecrubier, Y., Sheehan, D., Weiller, E., Amorim, P., Bonora, I., Sheehan, K., Janavs, J., amp, Dunbar, G., 1997. The Mini International Neuropsychiatric Interview. (MINI), a short diagnostic interview: Reliability and validity according to the CIDI.
- Liberg B., Klauser P., Harding I.H., Adler M., Rahm C., Lundberg J., Masterman T., Wachtler C., Jonsson T., Kristoffersen-Wiberg M., Pantelis C., Wahlund B. Functional and structural alterations in the cingulate motor area relate to decreased fronto-striatal coupling in major depressive disorder with psychomotor disturbances. Front. Psychiatry. 2014;5:176. doi: 10.3389/fpsyt.2014.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Gadian D.G., Johnsrude I.S., Good C.D., Ashburner J., Frackowiak R.S.J., Frith C.D. Navigation-related structural change in the hippocampi of taxi drivers. Proc. Natl. Acad. Sci. U.S.A. 2000;97:4398–4403. doi: 10.1073/pnas.070039597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marissen Marlies A.E., Arnold Nienke, Franken Ingmar H.A. Anhedonia in borderline personality disorder and its relation to symptoms of impulsivity. Psychopathology. 2012;45:179–184. doi: 10.1159/000330893. [DOI] [PubMed] [Google Scholar]
- Marquand A.F., Haak K.V., Beckmann C.F. Functional corticostriatal connection topographies predict goal directed behaviour in humans. Nat. Hum. Behav. 2017;1:0146. doi: 10.1038/s41562-017-0146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez Diana, Slifstein Mark, Broft Allegra, Mawlawi Osama, Hwang Dah-Ren, Huang Yiyun, Cooper Thomas, Kegeles Lawrence, Zarahn Eric, Abi-Dargham Anissa, Haber Suzanne N., Laruelle Marc. Imaging human mesolimbic dopamine transmission with positron emission tomography. Part II: amphetamine-induced dopamine release in the functional subdivisions of the striatum. J. Cereb. Blood Flow Metab. 2003;23:285–300. doi: 10.1097/01.WCB.0000048520.34839.1A. [DOI] [PubMed] [Google Scholar]
- Mawlawi Osama, Martinez Diana, Slifstein Mark, Broft Allegra, Chatterjee Rano, Hwang Dah-Ren, Huang Yiyun, Simpson Norman, Ngo Kim, Van Heertum Ronald, Laruelle Marc. Imaging human mesolimbic dopamine transmission with positron emission tomography: I. Accuracy and precision of D(2) receptor parameter measurements in ventral striatum. J. Cereb. Blood Flow Metab. 2001;21:1034–1057. doi: 10.1097/00004647-200109000-00002. [DOI] [PubMed] [Google Scholar]
- Moberget Torgeir, Ivry Richard B. Prediction, psychosis, and the cerebellum. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2019;4:820–831. doi: 10.1016/j.bpsc.2019.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nestler Eric J., Carlezon William A. The mesolimbic dopamine reward circuit in depression. Biol. Psychiatry. 2006;59:1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- Nichols Thomas E., Holmes Andrew P. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum. Brain Mapp. 2002;15:1–25. doi: 10.1002/hbm.1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nusslock Robin, Alloy Lauren B. Reward processing and mood-related symptoms: an RDoC and translational neuroscience perspective. J. Affect. Disord. 2017;216:3–16. doi: 10.1016/j.jad.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Doherty J., Dayan P., Schultz J., Deichmann R., Friston K., Dolan R.J. Dissociable roles of ventral and dorsal striatum in instrumental conditioning. Science. 2004;304:452–454. doi: 10.1126/science.1094285. [DOI] [PubMed] [Google Scholar]
- Peciña S., Berridge K.C. Hedonic hot spot in nucleus accumbens shell: where do mu-opioids cause increased hedonic impact of sweetness? J. Neurosci. 2005;25:11777–11786. doi: 10.1523/JNEUROSCI.2329-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli Diego A., Jahn Allison L., O’Shea James P. Toward an objective characterization of an anhedonic phenotype: a signal-detection approach. Biol. Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli Diego A., Iosifescu Dan, Hallett Lindsay A., Ratner Kyle G., Fava Maurizio. Reduced hedonic capacity in major depressive disorder: evidence from a probabilistic reward task. J. Psychiatr. Res. 2008;43:76–87. doi: 10.1016/j.jpsychires.2008.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli Diego A., Holmes Avram J., Dillon Daniel G., Goetz Elena L., Birk Jeffrey L., Bogdan Ryan, Dougherty Darin D., Iosifescu Dan V., Rauch Scott L., Fava Maurizio. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry. 2009;166:702–710. doi: 10.1176/appi.ajp.2008.08081201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs-Ericsson Natalie J., Hajcak Greg, Sheffler Julia L., Stanley Ian H., Selby Edward A., Potter Guy G., Steffens David C. Putamen volume differences among older adults: depression status, melancholia, and age. J. Geriatr. Psychiatry Neurol. 2018;31:39–49. doi: 10.1177/0891988717747049. [DOI] [PubMed] [Google Scholar]
- Schmidt K., Nolte-Zenker B., Patzer J., Bauer M., Schmidt L.G., Heinz A. Psychopathological correlates of reduced dopamine receptor sensitivity in depression, schizophrenia, and opiate and alcohol dependence. Pharmacopsychiatry. 2001;34:66–72. doi: 10.1055/s-2001-15184. [DOI] [PubMed] [Google Scholar]
- Schmidt André, Vogel Marc, Baumgartner Sophie, Wiesbeck Gerhard A., Lang Undine, Borgwardt Stefan, Walter Marc. Brain volume changes after long-term injectable opioid treatment: a longitudinal voxel-based morphometry study. Addict. Biol. 2020;26 doi: 10.1111/adb.12970. e12970. [DOI] [PubMed] [Google Scholar]
- Schultz W., Tremblay L., Hollerman J.R. Reward processing in primate orbitofrontal cortex and basal ganglia. Cereb. Cortex. 2000;10:272–284. doi: 10.1093/cercor/10.3.272. [DOI] [PubMed] [Google Scholar]
- Sheehan D., Lecrubier Y., Sheehan K., Amorim P., Janavs J., Weiller E., Hergueta T., Baker R., Dunbar G. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Sherdell L., Waugh C.E., Gotlib I.H. Anticipatory pleasure predicts motivation for reward in major depression. J. Abnorm. Psychol. 2012;121:51–60. doi: 10.1037/a0024945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M., Jenkinson M., Woolrich M.W., Beckmann C.F., Behrens T.E., Johansen-Berg H., Bannister P.R., De Luca M., Drobnjak I., Flitney D.E., Niazy R.K., Saunders J., Vickers J., Zhang Y., De Stefano N., Brady J.M., Matthews P.M. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Smith S.M., Nichols T.E. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. Neuroimage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Stepien M., Manoliu A., Kubli R., Schneider K., Tobler P.N., Seifritz E., Herdener M., Kaiser S., Kirschner M. Investigating the association of ventral and dorsal striatal dysfunction during reward anticipation with negative symptoms in patients with schizophrenia and healthy individuals. PLoS ONE. 2018;13 doi: 10.1371/journal.pone.0198215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss G.P., Wilbur R.C., Warren K.R., August S.M., Gold J.M. Anticipatory vs. consummatory pleasure: what is the nature of hedonic deficits in schizophrenia? Psychiatry Res. 2011;187:36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway M.T., Zald D.H. Reconsidering anhedonia in depression: lessons from translational neuroscience. Neurosci. Biobehav. Rev. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tziortzi A.C., Haber S.N., Searle G.E., Tsoumpas C., Long C.J., Shotbolt P., Douaud G., Jbabdi S., Behrens T.E., Rabiner E.A., Jenkinson M., Gunn R.N. Connectivity-based functional analysis of dopamine release in the striatum using diffusion-weighted MRI and positron emission tomography. Cereb. Cortex. 2014;24:1165–1177. doi: 10.1093/cercor/bhs397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzvi E., Stoldt A., Witt K., Krämer U.M. Striatal-cerebellar networks mediate consolidation in a motor sequence learning task: an fMRI study using dynamic causal modelling. Neuroimage. 2015;122:52–64. doi: 10.1016/j.neuroimage.2015.07.077. [DOI] [PubMed] [Google Scholar]
- Voets N.L., Hough M.G., Douaud G., Matthews P.M., James A., Winmill L., Webster P., Smith S. Evidence for abnormalities of cortical development in adolescent-onset schizophrenia. Neuroimage. 2008;43:665–675. doi: 10.1016/j.neuroimage.2008.08.013. [DOI] [PubMed] [Google Scholar]
- Volkow N.D., Wise R.A., Baler R. The dopamine motive system: implications for drug and food addiction. Nat. Rev. Neurosci. 2017;18:741–752. doi: 10.1038/nrn.2017.130. [DOI] [PubMed] [Google Scholar]
- Vrieze E., Pizzagalli D.A., Demyttenaere K., Hompes T., Sienaert P., de Boer P., Schmidt M., Claes S. Reduced reward learning predicts outcome in major depressive disorder. Biol. Psychiatry. 2013;73:639–645. doi: 10.1016/j.biopsych.2012.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton A.E., Treadway M.T., Pizzagalli D.A. Reward processing dysfunction in major depression, bipolar disorder and schizophrenia. Curr. Opin. Psychiatry. 2015;28:7–12. doi: 10.1097/YCO.0000000000000122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise R.A., Spindler J., deWit H., Gerberg G.J. Neuroleptic-induced “anhedonia” in rats: pimozide blocks reward quality of food. Science. 1978;201:262–264. doi: 10.1126/science.566469. [DOI] [PubMed] [Google Scholar]
- Wittchen, H.-U., Zaudig, M., Fydrich, T., 1997. SKID. Strukturiertes Klinisches Interview für DSM-IV. Achse I und II. Handanweisung. Hogrefe, Göttingen.
- Worbe Y., Baup N., Grabli D., Chaigneau M., Mounayar S., McCairn K., Féger J., Tremblay L. Behavioral and movement disorders induced by local inhibitory dysfunction in primate striatum. Cereb. Cortex. 2009;19:1844–1856. doi: 10.1093/cercor/bhn214. [DOI] [PubMed] [Google Scholar]
- World Health Organisation, 1992. ICD-10: the ICD-10 classification of mental and behavioural disorders: clinical descriptions and diagnostic guidelines. World Health Organisation, Geneva.
- Wrege J.S., Ruocco A.C., Euler S., Preller K.H., Busmann M., Meya L., Schmidt A., Lang U.E., Borgwardt S., Walter M. Negative affect moderates the effect of social rejection on frontal and anterior cingulate cortex activation in borderline personality disorder. Cogn. Affect Behav. Neurosci. 2019;19:1273–1285. doi: 10.3758/s13415-019-00716-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X.H., Huang J., Zhu C.Y., Wang Y.F., Cheung E.F., Chan R.C., Xie G.R. Motivational deficits in effort-based decision making in individuals with subsyndromal depression, first-episode and remitted depression patients. Psychiatry Res. 2014;220:874–882. doi: 10.1016/j.psychres.2014.08.056. [DOI] [PubMed] [Google Scholar]
- Zhang B., Lin P., Shi H., Öngür D., Auerbach R.P., Wang X., Yao S. Mapping anhedonia-specific dysfunction in a transdiagnostic approach: an ALE meta-analysis. Brain Imaging Behav. 2016;10:920–939. doi: 10.1007/s11682-015-9457-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.