Abstract
Three hundred sixty-two Streptococcus pneumoniae strains were isolated from children under 5 years of age at Dhaka Shishu (Children) Hospital from 1993 to 1997. The strains were isolated from blood (n = 105), CSF (n = 164), ear swab (n = 61), eye swab (n = 20), and pus (n = 12). Of the 362 isolates, 42 (11.6%) showed intermediate resistance (MIC, <0.1 μg/ml) and only 4 (1.1%) showed complete resistance (MIC, >2.0 μg/ml) to penicillin. Penicillin resistance exhibited a strong relationship with serotype 14; 47.8% of the penicillin-resistant strains belonged to this type. A remarkably high (64.1%) resistance to co-trimoxazole was observed, along with a significant increase during the time period studied; there was no relationship to capsular type. By way of contrast, penicillin resistance did not show any significant change during the study period. Resistance to chloramphenicol (2.2%) and erythromycin (1.1%) was rare. The high resistance to co-trimoxazole and its increasing trend demand elucidation of the clinical impact of pneumonia treatment by this antimicrobial and reconsideration of the World Health Organization recommendation for co-trimoxazole administration to children with community-acquired pneumonia at the health care worker level in Bangladesh.
Streptococcus pneumoniae, the leading cause of childhood pneumonia, is responsible for 20 to 40% of the global total of 4 million deaths of children below 5 years of age from pneumonia per year. This organism is also a common cause of pneumonia and the second leading cause of meningitis in Bangladesh (10, 12). The acute respiratory tract infection control program designed for developing countries by the World Health Organization includes patient management protocols that recommend the use of an antimicrobial drug (penicillin, ampicillin, or co-trimoxazole) for children with pneumonia. In Bangladesh, as in other developing countries, empirical therapy is the rule rather than the exception. The health care workers of Bangladesh routinely give co-trimoxazole tablets, popularly known as pneumonia bori, to patients with community-acquired pneumonia. However, information about the antimicrobial susceptibility of pneumococci from most of the developing world, which could potentially benefit the design of acute respiratory tract infection control programs, is severely deficient. The only preliminary report form Bangladesh showed that 10% of the S. pneumoniae strains tested were resistant to penicillin (14).
We present here an analysis of drug resistance patterns of 362 pneumococcal isolates from cerebrospinal fluid (CSF), blood, ear swabs, pus, and eye swabs prospectively collected in 1993 to 1997.
The strains were collected from out- and inpatients of Dhaka Shishu (Children) Hospital (DSH), which is the only national pediatric hospital in Bangladesh for primary and tertiary care. All children 0 to 5 years of age were included in the study. The strains studied were isolated from blood (n = 105), CSF (n = 164), ear swabs (n = 61), eye swabs (n = 20), and pus (n = 12).
MICs of penicillin, ampicillin, cephalosporin, co-trimoxazole, chloramphenicol, and erythromycin for the strains were determined by E test. The test was performed on Mueller-Hinton agar (Unipath) supplemented with 5% defibrinated sheep blood. Inocula were prepared in Mueller-Hinton broth by direct suspension of pneumococcal colonies grown overnight on sheep blood agar and matched to a 0.5 McFarland opacity standard tube as described by Jorgensen et al. (6). Disc diffusion tests for the antimicrobials mentioned above were done by following the recommendation of the National Committee for Clinical Laboratory Standards (NCCLS) (9). Serotyping of the strains was done by using antisera from Statens Serum Institut (Copenhagen, Denmark) as described earlier (5, 13). In accordance with the NCCLS recommendation, only the penicillin-resistant strains were tested for sensitivity to ampicillin and cephalosporins (9).
Resistance patterns of S. pneumoniae strains were determined by putting the disc diffusion zone and MIC on MS Excel 1997, which was customized for the recommended cutoff value (9). Epi-Info 6.02 was used to analyze the results. The diameters of the zones of inhibition for oxacillin (for penicillin), co-trimoxazole, chloramphenicol, and erythromycin discs were compared with the MICs of the respective antibiotics for the same strains. The sensitivity and specificity of the disc diffusion results for detection of resistant strains were determined by the method described by Barker and Hall (2).
The serogroup distribution (Table 1) of the isolates was similar to that of the previous report (13). Of 362 strains, 70.2% (254) were resistant to at least one antibiotic; 69.6% (252) and 64.1% (232) of the strains were found to be resistant to co-trimoxazole by disc diffusion and E test, respectively, with 100% sensitivity and 86.1% specificity of disc diffusion. A remarkable increase (P < 0.001) in co-trimoxazole resistance was observed among the strains when they were grouped and analyzed by year (Table 1). Capsular types did not bear any relationship to co-trimoxazole resistance.
TABLE 1.
Serotype distribution of S. pneumoniae isolates from pediatric patients in Bangladesh from 1993 to 1997
| Serotype | No. of isolates | Percentage | Rank |
|---|---|---|---|
| 1 | 20 | 5.5 | 7 |
| 2 | 10 | 2.8 | 11 |
| 5 | 14 | 3.9 | 10 |
| 6 | 20 | 5.5 | 8 |
| 7F | 46 | 12.7 | 1 |
| 9V | 8 | 2.2 | 13 |
| 12 | 24 | 6.6 | 5 |
| 14 | 32 | 8.8 | 3 |
| 15 | 34 | 9.4 | 2 |
| 18 | 30 | 8.3 | 4 |
| 19 | 22 | 6.1 | 6 |
| 20 | 8 | 2.2 | 14 |
| 33 | 10 | 2.8 | 12 |
| 45 | 18 | 5.0 | 9 |
| Others | 44 | 12.1 | |
| Nontypeable | 22 | 6.1 | |
| Total | 362 | 100 |
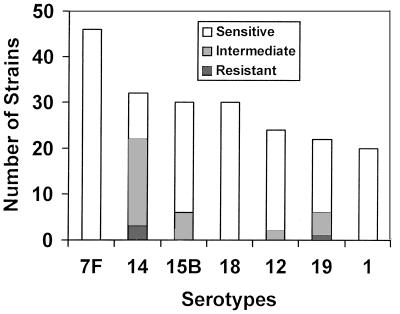
Penicillin resistance was observed in 46 (12.7%) of the 362 strains; however, only 4 (1.1%) showed complete resistance (MIC, ≥2.0). The frequency of penicillin resistance varied by capsular type, and resistance was found in nine different serotypes, although 34 (74%) of the 46 resistant strains were confined to three different serogroups or serotypes: 14 (47.8%; n = 22), 15B (13.0%; n = 6), and 19 (13.0%; n = 6). Figure 1 shows penicillin resistance among the seven predominant serotypes (13), which comprised 36 (78.2%) of the 46 penicillin-resistant strains. Remarkably, 22 (68.7%) of the 32 serotype 14 strains were penicillin resistant but none of serotype 7F, the most predominant serotype, showed resistance to penicillin. Penicillin resistance remained stable through out the study period (P = 0.87) (Table 2).
FIG. 1.
Occurrence of penicillin resistance in seven predominant pneumococcal serotypes of Bangladesh.
TABLE 2.
Pneumococcal isolates resistant to penicillin and co-trimoxazole by year from 1993 to 1997
| Yr (no. of strains) | No. (%) of strains resistant to:
|
|||
|---|---|---|---|---|
| Penicillin
|
Co-trimoxazole
|
|||
| Intermediate resistancea | Total resistanceb | Intermediate resistancec | Total resistanced | |
| 1993 (53) | 6 (11.3) | 0 (0) | 12 (22.6) | 14 (26.4) |
| 1994 (56) | 7 (12.5) | 0 (0) | 17 (30.4) | 14 (25.0) |
| 1995 (95) | 13 (13.7) | 2 (2.7) | 21 (22.1) | 30 (31.6) |
| 1996 (53) | 5 (9.4) | 2 (3.7) | 15 (28.3) | 28 (52.8) |
| 1997 (105) | 11 (10.5) | 0 (0) | 16 (15.2) | 65 (61.9) |
MIC, 0.1 to 1.0 μg/ml.
MIC, ≥2.0 μg/ml.
MIC, 1/19 to 2/38 μg/ml.
MIC, ≥4/76 μg/ml.
When the strains were screened for penicillin resistance by using oxacillin discs, none of the resistant strains were missed (sensitivity, 100%) and 28% of them (18 of 64) were classified as sensitive when tested by E strips (specificity, 94.5%). Among the oxacillin-resistant strains, 16 did not show any zone of inhibition; however, 12 (75%) of them were only relatively resistant (MIC, 0.1 to 1.0 μg/ml) and 4 (25%) showed complete resistance (MIC, ≥2.0 μg/ml). Chloramphenicol and erythromycin resistance was observed in 10 (2.8%) and 4 (1.1%) of the 362 strains, respectively.
These results show that Bangladesh is marginally in the group of countries with high (>10%) penicillin resistance (1, 7). However, unlike other countries, such as Spain, South Africa, France, Hungary, etc. (1), resistance to penicillin in Bangladesh did not show any change during the study period, and further, it is similar to that in the previous report (14). Most (91%; 42 of 46) of the penicillin-resistant strains in this study showed only relative resistance, and for 97.2% (352 of 362) of the strains the MICs were less than 0.50 μg/ml. Further, as pneumococcus strains isolated from blood and CSF are more likely to be resistant to penicillin than are strains isolated from carriers in the community (11), penicillin is probably the best empirical choice for treatment of pneumonia cases, as a high concentration in serum of >100 μg/ml can easily be reached (16).
In this study, resistance to penicillin was mostly (47.8%; 22 of 46) observed in serotype 14 strains. A similar correlation was also observed among the strains from Slovakia (11) and the United States (15). In contrast to the high resistance rate among serotype 14 strains (73.3%; 22 to 30), none of the strains of serotype 7F, the most predominant type in Bangladesh (13), was resistant to penicillin. Comparison of zone sizes with MICs revealed that the 20-mm cutoff for penicillin resistance recommended by the NCCLS (9) is 100% sensitive and 94.5% specific. Any zone size cutoff of less than 20 mm compromises the sensitivity. This finding is in contrast to that of Evans et al. (4), who proposed 9 mm as the cutoff point. Similarly, when the NCCLS cutoff point is strictly followed, disc diffusion was found to be 100% sensitive for detection of co-trimoxazole and chloramphenicol resistance. Further, we found the E test to be a very simple and reproducible method for MIC determination in routine diagnostic laboratories with limited facilities and highly cost effective if it is selectively used only for strains which are found to be resistant by disc diffusion following the stringent criteria of the NCCLS.
None of the strains were resistant to ampicillin. Chloramphenicol resistance was observed in only 2.8% of the strains, without any relationship to penicillin resistance. Therefore, the combination of ampicillin and chloramphenicol, the most common regimen used in the hospitals of our country, is still a good empirical choice for the treatment of pneumococcal meningitis. The most remarkable findings of this study are the high co-trimoxazole resistance and its increasing rate (P < 0.001). This can possibly be correlated with the wide use this antibiotic in the community because of its dose convenience, cost effectiveness, and easy availability over the counter. In the last several years, its use has increased as health care workers have been using it for community-acquired pneumonia cases, as recommended by the World Health Organization. Recommendation of co-trimoxazole is based on previous Gambian data showing that all S. pneumoniae isolates from community-acquired pneumonia cases were sensitive to co-trimoxazole (3). In recent years, however, resistance to co-trimoxazole has been emerging among isolates of S. pneumoniae, and it is now recommended that the use of co-trimoxazole should immediately be discontinued in areas with a high prevalence of resistant strains (7). At this point, it is very important to elucidate the clinical impact of co-trimoxazole treatment of pneumonia cases with proven resistant or sensitive pneumococcus strains to decide whether (i) the use of co-trimoxazole should immediately be discouraged in a population with a high percentage of co-trimoxazole resistance or (ii) the resistance of S. pneumoniae to this antimicrobial, in terms the cutoff point, has to be redefined.
We are continuing to monitor the drug resistance of our isolates at DSH to see its trend and are in the process of collecting a large number of nasopharyngeal S. pneumoniae strains from the community of Dhaka and other districts to get representative strains from the whole country (8) to see if there are differences in the resistance patterns.
Acknowledgments
This research was supported by the Bangladesh Medical Research Council; the Programme Coordination Committee of the International Centre for Diarrhoeal Disease and Research, Bangladesh; and DSH. The Ministry of Health and Welfare, Government of Japan, also donated generously in completing the work.
REFERENCES
- 1.Appelbaum P C. Antimicrobial resistance in Streptococcus pneumoniae: an overview. Clin Infect Dis. 1992;15:77–83. doi: 10.1093/clinids/15.1.77. [DOI] [PubMed] [Google Scholar]
- 2.Barker D J P, Hall A J. Practical epidemiology. 4th ed. Edinburgh, Scotland: Churchill Livingstone; 1991. [Google Scholar]
- 3.Campbell H, Byass P, Forgie I M, et al. Trial of cotrimoxazole versus procaine penicillin with ampicillin in treatment of community-acquired pneumonia in young Gambian children. Lancet. 1988;ii:1182–1184. doi: 10.1016/s0140-6736(88)90244-9. [DOI] [PubMed] [Google Scholar]
- 4.Evans T G, Kamara A, Minnic K, Blevins D, Sosnowski K. Pneumococcal resistance in Southwest Virginia. Antimicrob Agents Chemother. 1995;39:985–986. doi: 10.1128/aac.39.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Facklam R R, Washington J A., II . Streptococcus and related catalase-negative gram-positive cocci. In: Balows A, Hausler W J Jr, Herrmann K, Isenberg H D, Shadomy H J, editors. Manual of clinical microbiology. 5th ed. Washington, D.C: American Society for Microbiology; 1991. pp. 238–257. [Google Scholar]
- 6.Jorgensen J H, Howell A W, Maher L A. Quantitative antimicrobial susceptibility testing of Haemophilus influenzae and Streptococcus pneumoniae by using the E-test. J Clin Microbiol. 1991;29:109–114. doi: 10.1128/jcm.29.1.109-114.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Klugman K P. Pneumococcal resistance to antibiotics. Clin Microbiol Rev. 1990;3:171–196. doi: 10.1128/cmr.3.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lehmann D, Gratten M, Montgomery J. Susceptibility of pneumococcal carriage isolates to penicillin provides a conservative estimate of susceptibility of invasive pneumococci. Pediatr Infect Dis J. 1997;16:297–305. doi: 10.1097/00006454-199703000-00009. [DOI] [PubMed] [Google Scholar]
- 9.National Committee for Clinical Laboratory Standards. Performance of standards for antimicrobial susceptibility testing: fifth international supplement. NCCLS document M100-S5. 14, no. 16. Villanova, Pa: National Committee for Clinical Laboratory Standards; 1994. [Google Scholar]
- 10.Rahaman M, Huq F, Sack D A. Acute lower respiratory infection in hospitalised patients with diarrhea in Dhaka, Bangladesh. Rev Infect Dis. 1990;12:S889–S906. doi: 10.1093/clinids/12.supplement_8.s899. [DOI] [PubMed] [Google Scholar]
- 11.Reichler M R, Rakovsky J, Sobotova A, et al. Multiple antimicrobial resistance of pneumococci in children with otitis media, bacteremia, and meningitis in Slovakia. J Infect Dis. 1995;171:1491–1496. doi: 10.1093/infdis/171.6.1491. [DOI] [PubMed] [Google Scholar]
- 12.Saha S K, Rikitomi N, Ruhulamin M, et al. The increasing burden of disease in Bangladeshi children due to Haemophilus influenzae type meningitis. Ann Trop Paediatr. 1997;17:5–8. doi: 10.1080/02724936.1997.11747855. [DOI] [PubMed] [Google Scholar]
- 13.Saha S K, Rikitomi N, Biswas D, Watanabe K, Ruhulamin M, Ahmed K, Hanif M, Matsumoto K, Sack R B, Nagatake T. Serotypes of Streptococcus pneumoniae causing invasive childhood infections in Bangladesh, 1992–1995. J Clin Microbiol. 1997;35:785–787. doi: 10.1128/jcm.35.3.785-787.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saha S K, Khan W A, Hoq M S, Selim A F M, Akbar M S. Penicillin resistant pneumococci in Bangladeshi children. Lancet. 1992;337:734–735. doi: 10.1016/0140-6736(91)90320-o. [DOI] [PubMed] [Google Scholar]
- 15.Spika J S, Facklam R R, Plikaytis B D, Oxtboy M J, et al. Antimicrobial resistance of Streptococcus pneumoniae in the United States, 1979–1987. J Infect Dis. 1991;163:1273–1278. doi: 10.1093/infdis/163.6.1273. [DOI] [PubMed] [Google Scholar]
- 16.Ward J. Antibiotic-resistant Streptococcus pneumoniae clinical and epidemiologic aspects. Rev Infect Dis. 1981;3:254–265. doi: 10.1093/clinids/3.2.254. [DOI] [PubMed] [Google Scholar]