Abstract
This study was conducted to investigate the effects of lycopene (LYC) on mitochondrial oxidative injury and dysfunction in the liver of aflatoxin B1 (AFB1)-exposed broilers. A total of 192 healthy 1-day-old male broilers were randomly divided into 3 groups with 8 replicates of 8 birds each. Birds in the 3 groups were fed basal diet (control), basal diet with 100 µg/kg AFB1, and basal diet with 100 µg/kg AFB1 and 200 mg/kg LYC, respectively. The experiment lasted 42 d. The results showed that AFB1 decreased average daily body weight gain (ADG), average daily feed intake, and gain to feed ratio (G :F) compared to the control group, the LYC supplementation increased ADG and G/F compared to AFB1 group (P < 0.05). Broilers in the AFB1 group had lower mitochondrial glutathione (mGSH) concentration and glutathione peroxidase (GSH-Px), manganese superoxide dismutase (MnSOD), and thioredoxin reductase activities, and higher hydrogen peroxide (H2O2) and reactive oxygen species (ROS) concentrations than the control group (P < 0.05). The LYC increased mGSH concentration and GSH-Px and MnSOD activities, and decreased H2O2 and ROS concentrations compared to AFB1 group (P < 0.05). Broilers fed the AFB1 diet showed increased mitochondrial swelling and decreased adenosine triphosphate concentration than the control group, and LYC had opposite effects (P < 0.05). The AFB1 decreased the activities of mitochondrial electron transfer chain (ETC) complexes I, II, III, and V, downregulated the mRNA expression levels of hepatic MnSOD, thioredoxin 2, thioredoxin reductase, peroxiredoxin-3, peroxisome proliferator-activated receptor γ coactivator 1α, nuclear respiratory factor 1, and mitochondrial transcription factor A compared with the control group (P < 0.05), and LYC increased activities of mitochondrial ETC complexes III and V, and upregulated mRNA expression levels of these genes in comparison to AFB1 group (P < 0.05). In conclusion, the LYC protected broilers from AFB1-induced liver mitochondrial oxidative injury and dysfunction by stimulating mitochondrial antioxidant capacity and maintaining mitochondrial biogenesis.
Key words: lycopene, mitochondrion, aflatoxin B1, broiler
INTRODUCTION
Mycotoxins are feed-derived risk factors that are detrimental to human and animal health. Among these, aflatoxin B1 (AFB1) has been identified by the World Health Organization as a group I carcinogen. Oxidative stress is one of the toxic effects of AFB1. The AFB1 metabolism is accompanied by the overproduction of reactive oxygen species (ROS), leading to oxidative stress (Marin and Taranu, 2012). Mitochondria are the major sites of intracellular ROS production. Overproduction of ROS disturbs ROS homeostasis and causes oxidative stress, and the mitochondria are the first to be threatened (Vakifahmetoglu-Norberg et al., 2017). Furthermore, the AFB1-induced mitochondrial injury is the potential mechanism underlying its toxic effects (Yilmaz et al., 2017). Thus, the toxicity of AFB1 can be alleviated by improving mitochondrial function.
Lycopene (LYC) is a natural food-derived pigment belonging to carotenoids used in food processing, and is mainly enriched in fruits and vegetables with a red color, such as tomatoes, watermelon, and papaya (Liang et al., 2019). The LYC has been identified as a class A nutrient by the World Health Organization. The LYC can be used as a dietary botanical bioactive substance with various bioactivities, including antioxidant capacity, and has therapeutic potential against diseases (Grabowska et al., 2019; Liang et al., 2019). The LYC or LYC-rich materials have been reported to exhibit mitochondrial protective effects against toxicity damage caused by hydrogen peroxide (H2O2) (Reshmitha et al., 2017), D-galactosamine/lipopolysaccharide (D-GalN/LPS) (Sheriff et al., 2017), and 1-methyl-4-phenylpyridinium ion (Yi et al., 2013). However, the effects of LYC on mitochondrial redox status and function in AFB1-exposed broilers have not been elucidated.
We speculated that LYC could alleviate hepatic mitochondrial oxidative injury and dysfunction in AFB1-exposed broilers. Therefore, the present study was conducted to evaluate the effects of LYC on mitochondrial antioxidant capacity, mitochondrial swelling, activities of mitochondrial electron transfer chain (ETC) complexes, hepatic adenosine triphosphate (ATP) concentration, and expression levels of related genes in the liver of AFB1-exposed broilers.
MATERIALS AND METHODS
Experimental Design and Management
The experiment was approved by the Ethics Committee and performed in accordance with the Institutional Animal Care and Use Committee of Yangzhou University (Permit number: SYXK (Su) 2016–0020), Yangzhou, China. A total of 192 healthy 1-day-old male Arbor Acres broiler chicks were obtained from a commercial hatchery (Nantong, Jiangsu Province, P. R. China) for the 42-d experiment. All birds were randomly divided into 3 groups with 8 replicates, and each replicate contained 8 birds. The birds in the 3 groups were fed basal diet (control, AFB1 <5 µg/kg), basal diet with 100 µg/kg AFB1 (AFB1), and basal diet with 100 µg/kg AFB1 and 200 mg/kg LYC (AFB1 + LYC), respectively. The basal diets were formulated to meet the nutrient requirements of Arbor Acres broilers (Table 1). LYC (purity ≥80%) and AFB1 (purity ≥98%) were purchased from Shanghai Yuanye Bio-Technology Co., Ltd. (Shanghai, China). All birds were kept in an environmentally controlled room at 32 to 34°C for the first 3 d, with the temperature gradually decreased by 2 to 3°C per week until it reached 22 ± 1°C with a 23 h light/1 h dark cycle. All birds were provided with feed and water ad libitum.
Table 1.
Composition and nutrient level of basal diet (as fed basis).
| Items | 1–21 d | 22–42 d |
|---|---|---|
| Ingredients (%) | ||
| Corn | 57.10 | 61.00 |
| Soybean meal | 31.00 | 28.00 |
| Corn gluten meal | 4.00 | 2.40 |
| Soybean oil | 3.00 | 4.00 |
| Dicalcium phosphate | 2.00 | 1.60 |
| Limestone | 1.20 | 1.30 |
| L-lysine | 0.20 | 0.25 |
| DL-methionine | 0.20 | 0.15 |
| Premix1 | 1.00 | 1.00 |
| Sodium chloride | 0.30 | 0.30 |
| Total | 100.00 | 100.00 |
| Calculated nutrient levels (%) | ||
| Apparent metabolizable energy (Kcal/kg) | 3,011.40 | 3,095.30 |
| Crude protein | 21.36 | 19.44 |
| Calcium | 1.00 | 0.93 |
| Available phosphorus | 0.46 | 0.39 |
| Lysine | 1.09 | 1.05 |
| Methionine | 0.56 | 0.47 |
| Arginine | 1.27 | 1.16 |
| Methionine + cystine | 0.91 | 0.80 |
The premix provided per kilogram of diet: vitamin A (retinyl acetate), 12,000 IU; vitamin D3 (cholecalciferol), 2,500 IU; vitamin E (DL-α-tocopheryl acetate), 20 IU; menadione, 1.3 mg; thiamin, 2.2 mg; riboflavin, 8.0 mg; nicotinamide, 40 mg; choline chloride, 400 mg; calcium pantothenate, 10 mg; pyridoxine HCl, 4 mg; biotin, 0.04 mg; folic acid, 1 mg; vitamin B12 (cobalamin), 0.013 mg; Fe (from ferrous sulfate), 80 mg; Cu (from copper sulphate), 8.0 mg; Mn (from manganese sulphate), 110 mg; Zn (from zinc sulfate), 60 mg; I (from calcium iodate), 1.1 mg; Se (from sodium selenite), 0.3 mg.
Growth Performance
Body weight and feed intake were recorded for each replicate, and average daily body weight gain (ADG), average daily feed intake (ADFI), and gain to feed ratio (G : F) were calculated.
Sample Collection and Preparation
At the end of the experiment, after 12 h of starvation, one broiler with an average body weight from each replicate was slaughtered for sample collection. Liver samples of each broiler were collected and divided into two parts, one of which was snap-frozen in liquid nitrogen and stored at −70°C, and the other was used to isolate mitochondria using a Tissue Mitochondria Isolation Kit (Beyotime Biotechnology, Shanghai, China). The bicinchoninic acid method was used to quantify the protein concentration using a BCA Protein Assay Kit (Beyotime Biotechnology).
Hepatic ROS Concentration
The hepatic mitochondrial ROS concentration was determined with a 2,7-dichlorofluorescein-diacetate fluorescence probe using a commercial kit purchased from Beyotime Biotechnology. The result of the control group was set at 100%, and the results of the other groups were expressed as a percentage of the control group.
Mitochondrial Redox Status
The concentrations of mitochondrial glutathione (mGSH) and H2O2, the activities of manganese superoxide dismutase (MnSOD), glutathione peroxidase (GSH-Px), thioredoxin reductase (TrxR), and thioredoxin peroxidase (TPX) were determined using commercial kits purchased from Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The results were expressed as nanogram per gram of protein (ng/g protein) for mGSH; milligram per gram of protein (mg/g protein) for H2O2; units per gram of protein (U/g protein) for GSH-Px, TrxR, and TPX, and units per milligram of protein (U/mg protein) for MnSOD.
Determination of Mitochondrial Swelling
The opening of the mitochondrial permeability transition pore causes mitochondrial swelling, which can be evaluated using the percentage of absorbance decrease at 540 nm. Mitochondrial swelling was determined according to the mitochondrial swelling assay method by Shi et al. (2015), and the absorbance value of the mitochondrial suspension at 540 nm was immediately and continuously recorded at 5 min and 10 min to determine the swelling. The results were expressed as the percentage of the absorbance decrease at different times compared to the initial absorbance value.
Activities of Mitochondrial ETC Complexes
The activities of the mitochondrial ETC complexes I, II, III, IV, and V were measured using the colorimetric method with commercial kits from Suzhou Comin Biotechnology Co. Ltd (Suzhou, China), and operated according to the manufacturer's instructions. The results were expressed as nanomole per minute per milligram of protein (nmol/min/mgprot).
Hepatic ATP Concentration
Hepatic ATP concentration was determined using the reverse-phase HPLC method as previously described by Wan et al. (2018). The results were expressed as micromole per gram of wet weight (µmol/g).
Real-Time PCR Analysis of Gene Expression
Total hepatic RNA isolation was performed using the Trizol Reagent method. Agarose gel electrophoresis detection and a microspectrophotometer (NaroDrop 2000c, Thermo Scientific, Waltham, MA) were used to measure the quality and quantity of RNA. Then, a Prime Script RT Master Mix kit (TaKaRa Biotechnology Co. Ltd., Dalian, China) was used to reverse-transcribe total RNA into cDNA following the manufacturer's instructions. Real-time PCR analysis was performed using a 7500 Real-Time PCR System (Applied Biosystems, Foster City, CA) with a TB Green Premix Ex Taq II kit (TaKaRa Biotechnology) following the manufacturer's instructions. The reaction conditions were: 95°C for 30 s, followed by 40 cycles of 95°C for 5 s and 60°C for 30 s, then 95°C for 15 s, 60°C for 1 min, 95°C for 15 s. The primer sequences (listed in Table 2) were designed by Primer Premier 5.0, and synthesized by Sangon Biotech Co., Ltd. (Shanghai, China). The relative mRNA expression levels of target genes were calculated using the 2−ΔΔCt method after normalization to the reference gene β-actin.
Table 2.
Primer sequences of the target genes.
| Genes | Gene numbers | Primer sequences (5′-3′) | Product lengths (bp) |
|---|---|---|---|
| β-actin | NM_205518 | F: TGATATTGCTGCGCTCGTTG | 183 |
| R: ATACCTCTTTTGCTCTGGGCTT | |||
| MnSOD | NM_204211.1 | F: CACTCTTCCTGACCTGCCTTAC | 169 |
| R: CACCTGAGCTGTAACATCACCTT | |||
| Trx2 | NM_001031410.1 | F: GCCCGTGGTGGTGGATT | 165 |
| R: GGCACTGCTGACACCTCGTA | |||
| TrxR2 | NM_001122691.2 | F: CCTGCTGGTCATTGGTGG | 219 |
| R: CCGTAGTGCTGGGCATCTT | |||
| Prx3 | XM_426543.5 | F: GATGTGAACTGCGAGGTGGT | 140 |
| R: CGGGAGATTTGTTTCGTGAG | |||
| PGC-1α | NM_001006457.1 | F: TCCTTCCCGCTGACCAAA | 212 |
| R: TCCTGCACTGCCTCCACA | |||
| NRF1 | NM_001030646.1 | F: CCATCCATCCGTAAGAGGC | 135 |
| R: TTTGAAGACAGGGTTGGGTTT | |||
| TFAM | NM_204100.1 | F: GCTTCCTGAGGGACAACCA | 171 |
| R: CAGCCAACTGCTCTTCGTATT |
Abbreviations: MnSOD, manganese superoxide dismutase; NRF1, nuclear respiratory factor 1; Prx3, peroxiredoxin-3; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; TFAM, mitochondrial transcription factor A; Trx2, thioredoxin 2; TrxR2, thioredoxin reductase 2.
Statistical Analysis
Data analysis was performed using SPSS 22.0 statistical software (SPSS Inc, Chicago, IL). Data were analyzed with one-way analysis of variance followed by the least significant difference test as the post-hoc test, and P < 0.05 was considered statistically significant. The results are presented as means ± SEM.
RESULTS
Growth Performance
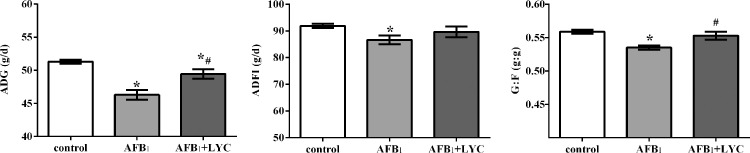
The effects of dietary LYC supplementation on the growth performance of AFB1-exposed broilers are presented in Figure 1. Broilers in the AFB1 and AFB1 + LYC group had lower ADG than the control group, and Broilers in the AFB1 group had lower ADFI and G : F than the control group. Whereas the dietary LYC supplementation group had higher ADG and G : F than the AFB1 group (P < 0.05).
Figure 1.
Effects of dietary lycopene (LYC) supplementation on growth performance of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight replicates. Abbreviations: ADFI, average daily feed intake; ADG, average daily body weight gain; G : F, gain to feed ratio. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
Hepatic ROS Concentration
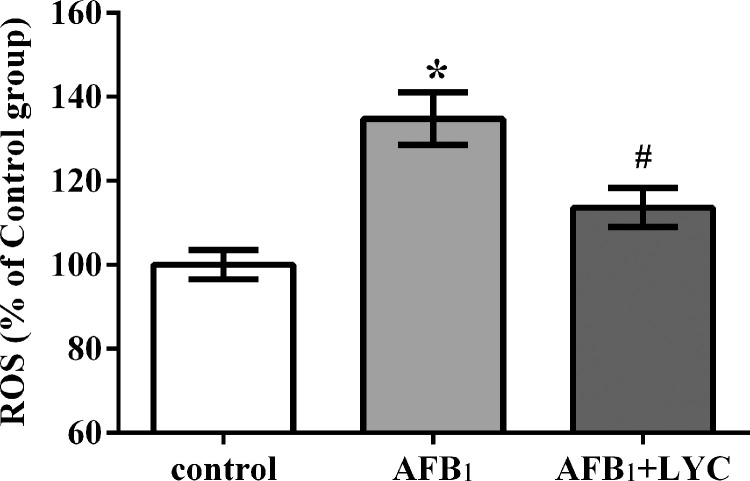
As shown in Figure 2, compared to the control group, AFB1 exposure increased hepatic ROS concentration, and LYC treatment decreased hepatic ROS concentration in AFB1-exposed broilers (P < 0.05).
Figure 2.
Effects of dietary lycopene (LYC) supplementation on hepatic reactive oxygen species (ROS) concentration of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight broilers. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1+LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
Mitochondrial Redox Status
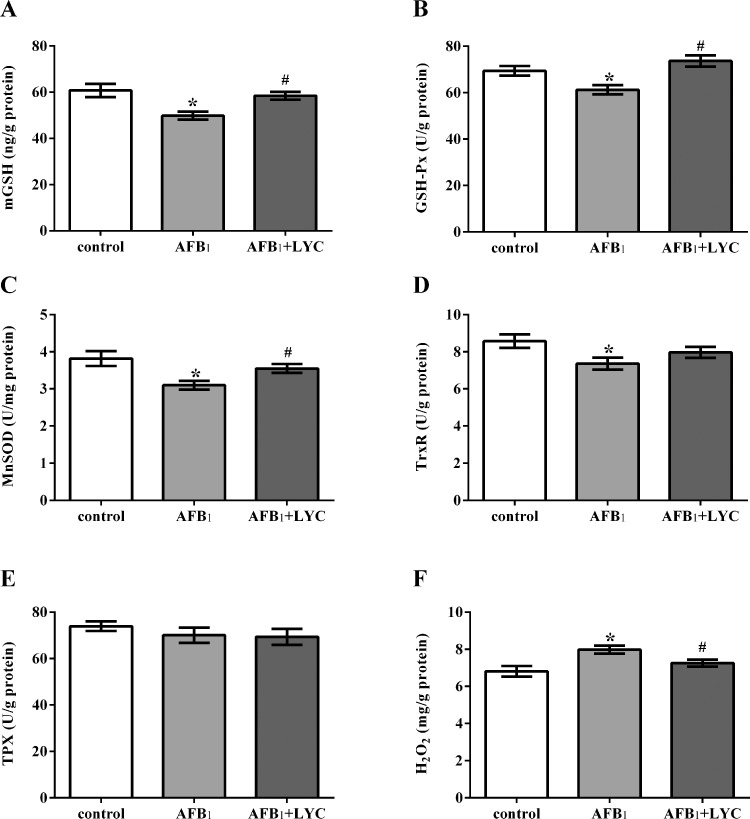
Broilers in the AFB1 group had lower mGSH concentration and GSH-Px, MnSOD, and TrxR activities, and higher H2O2 concentration than the control group (P < 0.05, Figure 3). The AFB1-exposed broilers receiving LYC showed higher mGSH concentrations and GSH-Px and MnSOD activities, and lower H2O2 concentrations than the broilers in the AFB1 group (P < 0.05).
Figure 3.
Effects of dietary lycopene (LYC) supplementation on mitochondrial glutathione (mGSH, A) concentration, the activities of glutathione peroxidase (GSH-Px, B), manganese superoxide dismutase (MnSOD, C), thioredoxin reductase (TrxR, D), thioredoxin peroxidase (TPX, E), and hydrogen peroxide concentration (H2O2, B) in hepatic mitochondria of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight broilers. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
Mitochondrial Swelling
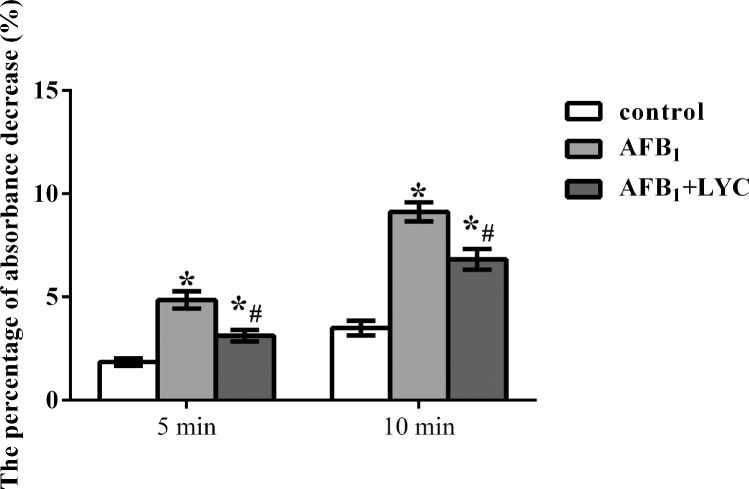
In the present study, broilers fed diets supplemented with AFB1 or AFB1 and LYC showed increased mitochondrial swelling, evidenced by the higher percentage of absorbance decrease at 5 min and 10 min than the control group (P < 0.05, Figure 4). However, broilers in the AFB1 + LYC group had a lower percentage of absorbance decrease at 5 min and 10 min than the AFB1 group (P < 0.05).
Figure 4.
Effects of dietary lycopene (LYC) supplementation on hepatic mitochondrial swelling (expressed as percentage of absorbance decrease) of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight broilers. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
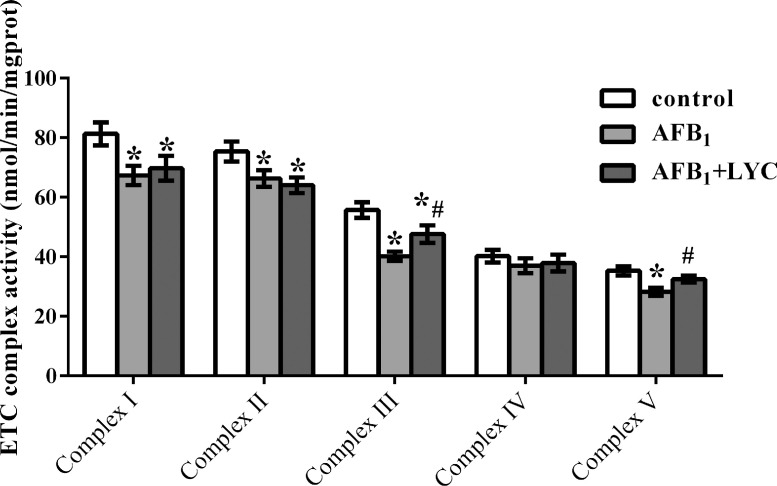
Activities of Mitochondrial ETC Complexes
The mitochondrial activities of ETC complexes I, II, III, and V in AFB1 group and the activity of ETC complex III in AFB1 + LYC group were decreased compared with the control group (P < 0.05, Figure 5). The LYC treatment increased activities of mitochondrial ETC complexes III and V in comparison with the nontreated AFB1-exposed broilers (P < 0.05).
Figure 5.
Effects of dietary lycopene (LYC) supplementation on activities of hepatic mitochondrial electron transfer chain (ETC) complexes of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight broilers. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
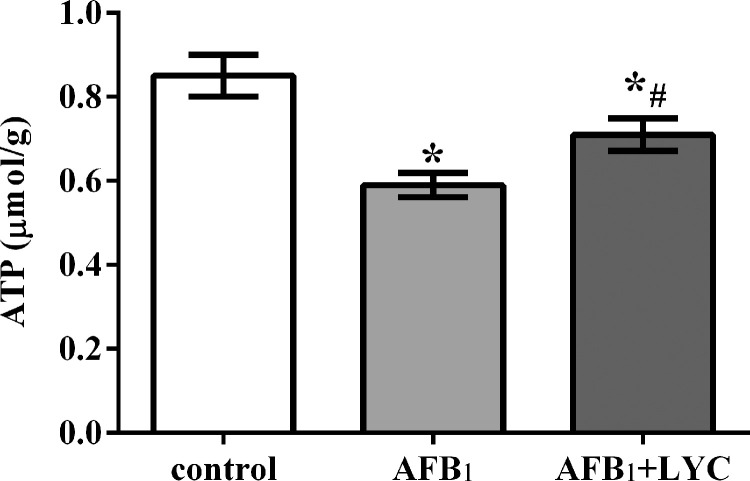
Hepatic ATP Concentration
As shown in Figure 6, broilers fed the AFB1 diet and AFB1 + LYC diet had lower hepatic ATP concentrations than those fed the basal diet (P < 0.05). In contrast, broilers in the AFB1 + LYC group had higher hepatic ATP concentrations than the AFB1 group (P < 0.05).
Figure 6.
Effects of dietary lycopene (LYC) supplementation on hepatic adenosine triphosphate (ATP) concentration of aflatoxin B1 (AFB1)-exposed broilers. Data are expressed as mean ± SEM from eight broilers. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group.
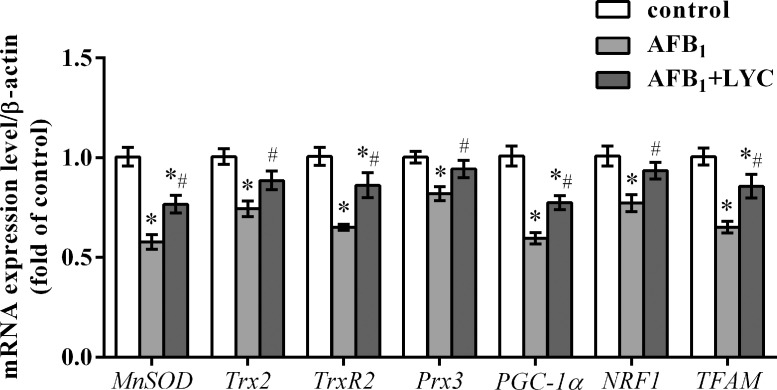
Gene Expression
The effects of LYC treatment and AFB1 exposure on the mRNA expression of hepatic genes relating to mitochondrial antioxidant capacity and biogenesis are shown in Figure 7. The mRNA expression levels of hepatic MnSOD, thioredoxin 2 (Trx2), thioredoxin reductase (TrxR2), peroxiredoxin-3 (Prx3), peroxisome proliferator-activated receptor γ coactivator 1α (PGC-1α), nuclear respiratory factor 1 (NRF1), and mitochondrial transcription factor A (TFAM) were downregulated in the AFB1-exposed broilers compared to the control group (P < 0.05). The mRNA expression levels of hepatic MnSOD, TrxR2, PGC-1α, and TFAM in AFB1 + LYC group were lower than the control group (P < 0.05). Compared to the AFB1 group, broilers in the AFB1+LYC group showed upregulated mRNA expression levels of the above-mentioned genes (P < 0.05).
Figure 7.
Effects of dietary lycopene (LYC) supplementation on relative mRNA expression levels of genes in liver of aflatoxin B1 (AFB1) exposed broilers. Data are expressed as mean ± SEM from eight broilers. Significant difference is indicated by * (P < 0.05) when compared with control group, and # (P < 0.05) when compared with AFB1 group. Abbreviations: MnSOD, manganese superoxide dismutase; NRF1, nuclear respiratory factor 1; Prx3, peroxiredoxin-3; PGC-1α, peroxisome proliferator-activated receptor γ coactivator 1α; TFAM, mitochondrial transcription factor A; Trx2, thioredoxin 2; TrxR2, thioredoxin reductase 2. Control, broilers were fed basal diet; AFB1, broilers were fed basal diet with 100 μg/kg AFB1; AFB1 + LYC, broilers were fed basal diet with 100 μg/kg AFB1 and 200 mg/kg LYC.
DISCUSSION
The detrimental effects of AFB1 on the growth performance of poultry have been recognized previously. Growth depression in broilers exposed to AFB1 was found in the present study. However, dietary LYC supplementation alleviated the adverse effects of AFB1 on the growth of broilers. Previous studies have shown that dietary 5% LYC-enriched dried tomato pomace increased the body weight and G/F of broilers from 1 to 28 d of age (Hosseini-Vashan et al., 2016), broilers fed a diet supplemented with 100 mg/kg LYC increased the final live weight at 35 d of age (Ševčíková et al., 2008), and LYC supplementation improved the growth performance of broilers under heat stress (Sahin et al., 2016). These observations indicate that LYC or LYC-enriched materials might have beneficial effects on poultry growth.
Mitochondria are identified as the key organelles in cellular redox signaling. The mGSH plays a pivotal role in the antioxidant defense system, and the depletion of mGSH makes cells sensitive to stimuli and induces oxidative stress (Marí et al., 2013). As a member of the GSH system, GSH-Px plays an important role in eliminating peroxides from the mitochondria (Rhee et al., 2005).The AFB1 exposure has been reported to deplete GSH and decrease the enzyme activity of GSH-Px in the kidney and heart tissues of rats (Yilmaz et al., 2018). Mitochondrial damage increases the production of superoxide anion free radicals, which are metabolized in the mitochondria by MnSOD and produce H2O2 (Marí et al., 2013). In the present study, the increased ROS and H2O2 level, decreased mGSH concentration and reduced activities of GSH-Px and MnSOD in the hepatic mitochondria of AFB1-exposed broilers indicated that the mitochondrial redox balance was disrupted and oxidative stress occurred. In addition, the mGSH and Trx2/Prx3 systems protect mitochondria from oxidative stress (Zhang et al., 2007). Trx2 plays a crucial role in protecting mitochondria from oxidative stress, and decreased mitochondrial Trx2 levels in mice livers showed increased oxidative damage (Pérez et al., 2008). Mitochondrial TrxR2 can decrease oxidized Trx2, and Prx3 is a mitochondrial peroxiredoxin (Prx) isoform that can reduce H2O2 and lipid hydroperoxides using thioredoxin (Trx) as a hydrogen donor (Patenaude et al., 2004). The decreased hepatic mRNA expression levels of Trx2, TrxR2, and Prx3 in AFB1-exposed broilers observed in the present study indicated that the mitochondrial Trx2/Prx3 antioxidant system was destroyed. Similarly, overproduction of mitochondrial ROS and decreased mRNA expression levels of antioxidant genes were observed in AFB1-exposed primary broiler hepatocytes (Liu and Wang, 2016). Thus, the AFB1 induced mitochondrial oxidative injury by affecting mitochondrial ROS homeostasis and damaging the antioxidant defense system.
Mitochondria are organelles that generate oxidative phosphorylation, transfer electrons, and synthesize ATP to provide energy. Mitochondria are sensitive to oxidative stress, leading to damaged membrane structures (Zorov et al., 2014). Pessayre et al. (2012) reported that mitochondrial membrane is disrupted and mitochondrial oxidative phosphorylation process is uncoupled in drug-induced liver damage. The AFB1 is a hepatotoxic substance, and hepatic mitochondria might be inevitably damaged. In ducklings that received AFB1, hepatic mitochondrial swelling and the increase of mitochondrial permeability transition pores were observed (Shi et al., 2015). Furthermore, excessive ROS production decreased the activities of mitochondrial ETC complexes (Simmons et al., 2005), and reduced Trx2 level led to decreased activities of mitochondrial ETC complexes and ATP production (Pérez et al., 2008). The PGC-1α is the most critical regulator of mitochondrial biogenesis, and the inhibition of PGC-1α expression can directly cause mitochondrial dysfunction (Cui et al., 2006). In contrast, the overexpression of PGC-1α can facilitate mitochondrial biogenesis and improve mitochondrial ETC activities, and activate energy metabolic pathways to increase ATP production (Finck and Kelly, 2006; Srivastava et al., 2009). The NRF1 is a downstream target gene of PGC-1α and responsible for regulating the expression of mitochondrial ETC genes (Hock and Kralli, 2009). NRF1 can regulate TFAM, which is mainly involved in mitochondrial DNA replication and transcription (Hock and Kralli, 2009). Huang et al. (2020) reported that AFB1 decreased ATP levels, reduced the activities of mitochondrial ETC I-IV, and downregulated mRNA expression of mitochondrial biogenesis genes, including PGC-1α, NRF1, and TFAM in mice testes. Similarly, increased mitochondrial swelling, reduced ATP concentration, and decreased activities of ETC mitochondrial complexes I, II, III, and V were observed in the present study. Furthermore, dietary AFB1 downregulated the mRNA expression levels of PGC-1α, NRF1, and TFAM in the liver of broilers, indicating that hepatic mitochondrial dysfunction was involved in AFB1-induced liver damage.
A previous study showed that the dietary inclusion of resveratrol enhanced antioxidant status and protected against AFB1-induced liver toxicity in broilers (Sridhar et al., 2015). Dietary selenium supplementation enhanced the hepatic antioxidant capacity and improved mitochondrial function by increasing the activities of mitochondrial antioxidant enzymes and ETC complexes I–IV, and improving mitochondrial structure in AFB1-exposed ducklings (Shi et al. 2012, 2015). Curcumin could protect ducks from ochratoxin A-induced impairment of mitochondrial integrity (Ruan et al., 2019). These studies demonstrate that the dietary modulation of antioxidant capacity and mitochondrial function were proposed as potential solutions to overcome mycotoxicosis.
In the present study, we evaluated the effects of LYC on hepatic mitochondrial antioxidant capacity and function in broilers exposed to AFB1. The LYC has high antioxidant property, which protects cells against oxidative damage under various oxidative stress conditions by scavenging free radicals (Grabowska et al., 2019). The LYC effectively alleviated H2O2 induced L6 myoblasts oxidative damage, and decreased mitochondrial membrane potential and DNA damage (Reshmitha et al., 2017). Toxic substances such as 3-nitropropionic acid, trimethyltin, and 1-methyl-4-phenylpyridine ions could induce neurotoxicity, including cell apoptosis, ROS accumulation, ATP level decrease, mitochondrial membrane permeability increase, mitochondrial membrane potential decrease, and mitochondrial DNA copy number and RNA transcription level decrease, whereas LYC could play a role in decreasing these toxic effects (Sandhir et al., 2010; Qu et al., 2011; Yi et al., 2013). In the fulminant liver failure model of rats induced by D-GalN/LPS, hepatic mitochondrial oxidative stress occurred, tricarboxylic acid cycle function was damaged, the ETC enzyme activity and ATP levels were decreased, whereas LYC pretreatment significantly inhibited the toxic effects of D-GalN/LPS on hepatic mitochondria (Sheriff et al., 2017). Qu et al. (2016) found that LYC decreased intracellular ROS and mitochondria-derived superoxide generation, ameliorated mitochondrial morphological alteration, increased activities of ETC complexes I–IV and ATP levels, prevented mitochondrial DNA damage, and improved the protein level of TFAM in β-amyloid treated neurons. The study of Reddy et al. (2006) revealed that LYC inhibited the mitochondrial activity decrease in AFB1-challenged human hepatocytes. Therefore, the protective effect of LYC is related to the alleviation of mitochondrial oxidative stress, and improving mitochondrial function and biogenesis, which is an important pathway for decreasing the adverse effects of toxic substances, including AFB1. In the present study, dietary LYC decreased ROS and H2O2 concentrations, increased mGSH concentration, enhanced the activities of GSH-Px and MnSOD, and increased the mRNA expression levels of MnSOD, Trx2, TrxR2, and Prx3 in AFB1-exposed broilers. These findings indicated that LYC improved the mitochondrial redox balance, relying on its ability of scavenging free radicals and stimulating the mitochondrial antioxidant components, including the GSH system, Trx2/Prx3 system, and antioxidant enzymes such as MnSOD. Furthermore, dietary LYC alleviated mitochondrial swelling, increased ATP levels and the activities of mitochondrial ETC complexes, and upregulated the mRNA expression levels of PGC-1α, NRF1, and TFAM in AFB1 challenged broilers. Thus, the LYC might alleviate mitochondrial dysfunction by improving mitochondrial biogenesis.
In summary, dietary LYC protected broilers from AFB1-induced liver mitochondrial oxidative injury and dysfunction, which might be related to its ability to scavenge free radicals, stimulate mitochondrial antioxidant capacity, and maintain mitochondrial biogenesis. The results of the present study expand our understanding that LYC or LYC-enriched materials could be used as promising dietary modulators for AFB1-induced injury in poultry.
Acknowledgments
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (grant number 31802095); the Poultry Genetics and Breeding Key Laboratory Open Project of Jiangsu Province (grant number JQLAB-KF-201801); and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD)
Disclosures
The authors have no conflicts of interest to report.
REFERENCES
- Cui L., Jeong H., Borovecki F., Parkhurst C.N., Tanese N., Krainc D. Transcriptional repression of PGC-1α by mutant huntingtin leads to mitochondrial dysfunction and neurodegeneration. Cell. 2006;127:59–69. doi: 10.1016/j.cell.2006.09.015. [DOI] [PubMed] [Google Scholar]
- Finck B.N., Kelly D.P. PGC-1 coactivators: Inducible regulators of energy metabolism in health and disease. J. Clin. Invest. 2006;116:615–622. doi: 10.1172/JCI27794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grabowska M., Wawrzyniak D., Rolle K., Chomczyński P., Oziewicz S., Jurga S., Barciszewski J. Let food be your medicine: nutraceutical properties of lycopene. Food Funct. 2019;10:3090–3102. doi: 10.1039/c9fo00580c. [DOI] [PubMed] [Google Scholar]
- Hock M.B., Kralli A. Transcriptional control of mitochondrial biogenesis and function. Annu. Rev. Physiol. 2009;71:177–203. doi: 10.1146/annurev.physiol.010908.163119. [DOI] [PubMed] [Google Scholar]
- Hosseini-Vashan S.J., Golian A., Yaghobfar A. Growth, immune, antioxidant, and bone responses of heat stress-exposed broilers fed diets supplemented with tomato pomace. Int. J. Biometeorol. 2016;60:1183–1192. doi: 10.1007/s00484-015-1112-9. [DOI] [PubMed] [Google Scholar]
- Huang W., Cao Z., Yao Q., Ji Q., Zhang J., Li Y. Mitochondrial damage are involved in Aflatoxin B1-induced testicular damage and spermatogenesis disorder in mice. Sci. Total Environ. 2020;701 doi: 10.1016/j.scitotenv.2019.135077. [DOI] [PubMed] [Google Scholar]
- Liang X., Ma C., Yan X., Liu X., Liu F. Advances in research on bioactivity, stability, metabolism and delivery systems of lycopene. Trends Food Sci. Tech. 2019;93:185–196. [Google Scholar]
- Liu Y., Wang W. Aflatoxin B1 impairs mitochondrial functions, activates ROS generation, induces apoptosis and involves Nrf2 signal pathway in primary broiler hepatocytes. Anim. Sci. J. 2016;87:1490–1500. doi: 10.1111/asj.12550. [DOI] [PubMed] [Google Scholar]
- Marí M., Morales A., Colell A., García-Ruiz C., Kaplowitz N., Fernández-Checa J.C. Mitochondrial glutathione: Features, regulation and role in disease. Biochim. Biophys. Acta. 2013;1830:3317–3328. doi: 10.1016/j.bbagen.2012.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marin D.E., Taranu I. Overview on aflatoxins and oxidative stress. Toxin Rev. 2012;31:32–43. [Google Scholar]
- Patenaude A., Murthy M.R.V., Mirault M.E. Mitochondrial thioredoxin system: effects of TrxR2 overexpression on redox balance, cell growth, and apoptosis. J. Biol. Chem. 2004;279:27302–27314. doi: 10.1074/jbc.M402496200. [DOI] [PubMed] [Google Scholar]
- Pérez V.I., Lew C.M., Cortez L.A., Webb C.R., Rodriguez M., Liu Y., Qi W., Li Y., Chaudhuri A., Remmen H.V., Richardson A., Ikeno Y. Thioredoxin 2 haploinsufficiency in mice results in impaired mitochondrial function and increased oxidative stress. Free Radical Bio. Med. 2008;44:882–892. doi: 10.1016/j.freeradbiomed.2007.11.018. [DOI] [PubMed] [Google Scholar]
- Pessayre D., Fromenty B., Berson A., Robin M.A., Lettéron P., Moreau R., Mansouri A. Central role of mitochondria in drug-induced liver injury. Drug Metab. Rev. 2012;44:34–87. doi: 10.3109/03602532.2011.604086. [DOI] [PubMed] [Google Scholar]
- Qu M., Jiang Z., Liao Y., Song Z., Nan X. Lycopene prevents amyloid [beta]-induced mitochondrial oxidative stress and dysfunctions in cultured rat cortical neurons. Neurochem. Res. 2016;41:1354–1364. doi: 10.1007/s11064-016-1837-9. [DOI] [PubMed] [Google Scholar]
- Qu M., Zhou Z., Chen C., Li M., Pei L., Chu F., Yang J., Wang Y., Li L., Liu C., Zhang L., Zhang G., Yu Z., Wang D. Lycopene protects against trimethyltin-induced neurotoxicity in primary cultured rat hippocampal neurons by inhibiting the mitochondrial apoptotic pathway. Neurochem. Int. 2011;59:1095–1103. doi: 10.1016/j.neuint.2011.10.005. [DOI] [PubMed] [Google Scholar]
- Reddy L., Odhav B., Bhoola K. Aflatoxin B1-induced toxicity in HepG2 cells inhibited by carotenoids: morphology, apoptosis and DNA damage. Biol. Chem. 2006;387:87–93. doi: 10.1515/BC.2006.012. [DOI] [PubMed] [Google Scholar]
- Reshmitha T.R., Thomas S., Geethanjali S., Arun K.B., Nisha P. DNA and mitochondrial protective effect of lycopene rich tomato (Solanum lycopersicum L.) peel extract prepared by enzyme assisted extraction against H2O2, induced oxidative damage in L6 myoblasts. J. Funct. Foods. 2017;28:147–156. [Google Scholar]
- Rhee S.G., Yang K.S., Kang S.W., Woo H.A., Chang T.S. Controlled elimination of intracellular H2O2: regulation of peroxiredoxin, catalase, and glutathione peroxidase via post-translational modification. Antioxid. Redox Sign. 2005;7:619–626. doi: 10.1089/ars.2005.7.619. [DOI] [PubMed] [Google Scholar]
- Ruan D., Wang W.C., Lin C.X., Fouad A.M., Chen W., Xia W.G., Wang S., Luo X., Zhang W.H., Yan S.J., Zheng C.T., Yang L. Effects of curcumin on performance, antioxidation, intestinal barrier and mitochondrial function in ducks fed corn contaminated with ochratoxin A. Animal. 2019;13:42–52. doi: 10.1017/S1751731118000678. [DOI] [PubMed] [Google Scholar]
- Sahin K., Orhan C., Tuzcu M., Sahin N., Hayirli A., Bilgili S., Kucuk O. Lycopene activates antioxidant enzymes and nuclear transcription factor systems in heat-stressed broilers. Poult. Sci. 2016;95:1088–1095. doi: 10.3382/ps/pew012. [DOI] [PubMed] [Google Scholar]
- Sandhir R., Mehrotra A., Kamboj S.S. Lycopene prevents 3-nitropropionic acid-induced mitochondrial oxidative stress and dysfunctions in nervous system. Neurochem. Int. 2010;57:579–587. doi: 10.1016/j.neuint.2010.07.005. [DOI] [PubMed] [Google Scholar]
- Ševčíková S., Skřivan M., Dlouhá G. The effect of lycopene supplementation on lipid profile and meat quality of broiler chickens. Czech J. Anim. Sci. 2008;53:431–440. [Google Scholar]
- Sheriff S.A., Ibrahim S.S., Devaki T., Chakraborty S., Agarwal S., Pérez-Sánchez H. Lycopene prevents mitochondrial dysfunction during d-galactosamine/lipopolysaccharide induced fulminant hepatic failure in albino rats. J. Proteome Res. 2017;16:3190–3199. doi: 10.1021/acs.jproteome.7b00176. [DOI] [PubMed] [Google Scholar]
- Shi D., Guo S., Liao S., Su R., Guo M., Liu N., Li P., Tang Z. Protection of selenium on hepatic mitochondrial respiratory control ratio and respiratory chain complex activities in ducklings intoxicated with aflatoxin B1. Biol. Trace Elem. Res. 2012;145:312–317. doi: 10.1007/s12011-011-9195-6. [DOI] [PubMed] [Google Scholar]
- Shi D., Liao S., Guo S., Li H., Yang M., Tang Z. Protective Effects of selenium on aflatoxin B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver. Biol. Trace Elem. Res. 2015;163:162–168. doi: 10.1007/s12011-014-0189-z. [DOI] [PubMed] [Google Scholar]
- Simmons R.A., Suponitsky-Kroyter I., Selak M.A. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to β-cell failure. J. Biol. Chem. 2005;280:28785–28791. doi: 10.1074/jbc.M505695200. [DOI] [PubMed] [Google Scholar]
- Sridhar M., Suganthi R.U., Thammiaha V. Effect of dietary resveratrol in ameliorating aflatoxin B1-induced changes in broiler birds. J. Anim. Physiol. An. N. 2015;99:1094–1104. doi: 10.1111/jpn.12260. [DOI] [PubMed] [Google Scholar]
- Srivastava S., Diaz F., Iommarini L., Aure K., Lombes A., Moraes C.T. PGC-1alpha/beta induced expression partially compensates for respiratory chain defects in cells from patients with mitochondrial disorders. Hum. Mol. Genet. 2009;18:1805–1812. doi: 10.1093/hmg/ddp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vakifahmetoglu-Norberg H., Ouchida A.T., Norberg E. The role of mitochondria in metabolism and cell death. Biochem. Bioph. Res. Co. 2017;482:426–431. doi: 10.1016/j.bbrc.2016.11.088. [DOI] [PubMed] [Google Scholar]
- Wan X., Ahmad H., Zhang L., Wang Z., Wang T. Dietary enzymatically treated Artemisia annua L. improves meat quality, antioxidant capacity and energy status of breast muscle in heat-stressed broilers. J. Sci. Food Agr. 2018;98:3715–3721. doi: 10.1002/jsfa.8879. [DOI] [PubMed] [Google Scholar]
- Yi F., He X., Wang D. Lycopene protects against MPP+- induced cytotoxicity by maintaining mitochondrial function in SH-SY5Y cells. Neurochem. Res. 2013;38:1747–1757. doi: 10.1007/s11064-013-1079-z. [DOI] [PubMed] [Google Scholar]
- Yilmaz S., Kaya E., Karaca A., Karatas O. Aflatoxin B1 induced renal and cardiac damage in rats: protective effect of lycopene. Res. Vet. Sci. 2018;119:268–275. doi: 10.1016/j.rvsc.2018.07.007. [DOI] [PubMed] [Google Scholar]
- Yilmaz S., Kaya E., Kisacam M.A. In: Pages 67-90 in Aflatoxin-Control, Analysis, Detection and Health Risks. Abdulra'Uf L.B., editor. InTech, London, UK; 2017. The effect on oxidative stress of aflatoxin and protective effect of lycopene on aflatoxin damage. [Google Scholar]
- Zhang H., Go Y.M., Jones D.P. Mitochondrial thioredoxin-2/peroxiredoxin-3 system functions in parallel with mitochondrial GSH system in protection against oxidative stress. Arch. Biochem. Biophys. 2007;465:119–126. doi: 10.1016/j.abb.2007.05.001. [DOI] [PubMed] [Google Scholar]
- Zorov D.B., Juhaszova M., Sollott S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014;94:909–950. doi: 10.1152/physrev.00026.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]