Abstract
Background
For different lymph node metastasis (LNM) and distant metastasis (DM), the diagnosis, treatment and prognosis of T1-2 non-small cell lung cancer (NSCLC) are different. It is essential to figure out the risk factors and establish prediction models related to LNM and DM.
Methods
Based on the surveillance, epidemiology, and end results (SEER) database from 1973 to 2015, a total of 43,156 eligible T1-2 NSCLC patients were enrolled in the retrospective study. Logistic regression analysis was used to determine the risk factors of LNM and DM. Risk factors were applied to construct the nomograms of LNM and DM. The predictive nomograms were discriminated against and evaluated by Concordance index (C-index) and calibration plots, respectively. Decision curve analysis (DCAs) was accepted to measure the clinical application of the nomogram. Cumulative incidence function (CIF) was performed further to detect the prognostic role of LNM and DM in NSCLC-specific death (NCSD).
Results
Eight factors (age at diagnosis, race, sex, histology, T-stage, marital status, tumor size, and grade) were significant in predicting LNM and nine factors (race, sex, histology, T-stage, N-stage, marital status, tumor size, grade, and laterality) were important in predicting DM(all, P< 0.05). The calibration curves displayed that the prediction nomograms were effective and discriminative, of which the C-index were 0.723 and 0.808. The DCAs and clinical impact curves exhibited that the prediction nomograms were clinically effective.
Conclusions
The newly constructed nomograms can objectively and accurately predict LNM and DM in patients suffering from T1-2 NSCLC, which may help clinicians make individual clinical decisions before clinical management.
Keywords: SEER database, T1-2 non-small cell lung cancer, lymph node metastasis, distant metastasis, nomogram
Introduction
Lung cancer is one of the most common malignant tumors. According to statistics, another 228,820 lung cancer cases were discovered in the United States in 2020, while 135,720 patients died of the disease. The incidence rate and mortality rate of lung cancer were the highest in all malignant tumors (1). Lung cancer can be divided into small cell lung cancer (SCLC) and non-small cell lung cancer (NSCLC) based on the pathological classification, in which NSCLC accounts for 85% of the newly diagnosed lung cancer (2). According to the American Joint Commission on Cancer (AJCC) TNM 7th edition staging system, T1-2 refers to that the maximum diameter of the primary tumor is ≤ 7cm; the chest lesions are limited; the chest wall, transverse septum, mediastinal pleura, pericardium, trachea, and esophagus were not involved; no satellite nodules were found in the lung, either. In terms of patients with newly diagnosed pulmonary space occupying lesions, if they are suspected of malignant tumor, percutaneous biopsy, bronchoscopy, sputum cytology, and other methods will be adopted to clarify the pathology before the operation. High-resolution CT (HRCT) can make the clinical T-stage. Most of T1-2 NSCLC patients had no LNM and DM at the initial diagnosis, but some T1-2 NSCLC patients had LNM and/or DM. For N and M staging, further examination is quite necessary. The standard examinations are as follows: PET-CT, lymph node biopsy, mediastinoscopy, surgery. Although there are clinical guidelines for further assessment and treatment, many doctors still cannot fully understand and remember the contents of the guidelines, or cannot keep up with the disciplines’ progress, so they often make plans on the basis of local experience and personal experience. For example, for the clinical suspected 2R/2L, 4R/4L, and 10R/10L regional lymph node metastasis, the guidelines recommend esophageal ultrasound-guided biopsy. Still in clinical practices, some thoracic surgeons did not carry out this evaluation before the operation, while performed lymph node biopsy or dissection according to experience. If LNM and DM can be predicted accurately from the outset, the examination and treatment can be more targeted, diagnosis time may be shorter, the unnecessary examination can be rolled out, and patients’ economic burden can be reduced as well. Besides, as for whether there are lymph nodes and distant metastasis or not, the results of the prognosis of T1-2 NSCLC are entirely different. If we can predict LNM and DM, we can also more accurately judge the prognosis.
Nomogram, a graphical and straightforward prediction tool, can be used to numerically calculate the risk probability of clinical events for individual patients (3, 4). For many malignant tumors, the better predictive ability of nomogram has been confirmed, compared with the widely used TNM staging system (5, 6). However, up to now, it is still impossible to obtain an accurate nomogram for the prediction of LNM and DM in T1-2 lung cancer patients. Therefore, we aimed to evaluate T1-2 NSCLC using the LNM and DM nomograms in the SEER database.
Methods
Patient Enrollment and Characteristics
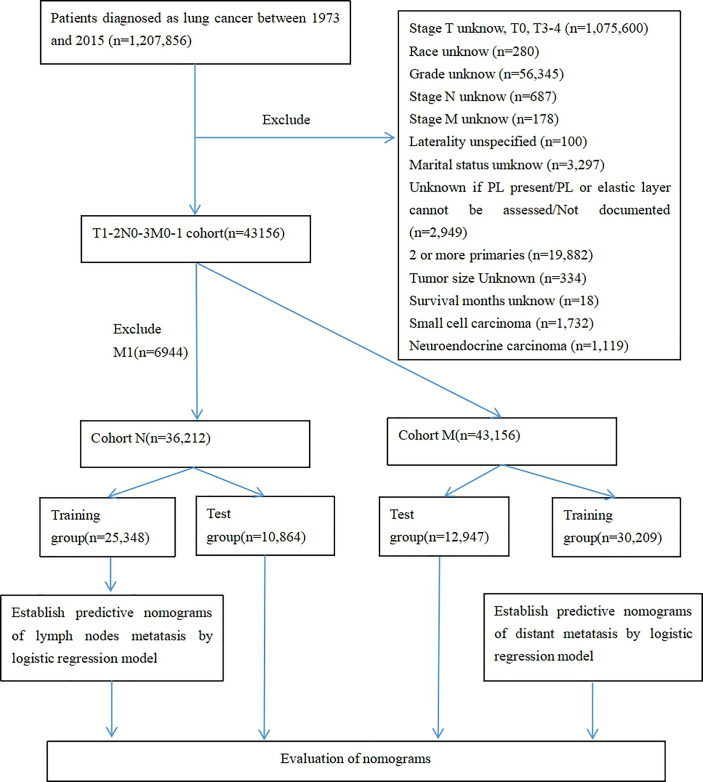
We used seerstat8.3.6 software to extract data from the SEER database(http://seer.cancer.gov/seerstat/), and the authoritative cancer statistics database of US cover 34.6% of US population up to now. Within the SEER database, we enrolled 43,156 patients who were diagnosed with primary T1-2 non-small cell lung between 1973 and 2015. Lung cancer cases were screened according to the following factors: year of diagnosis, sex, age of diagnosis, race recode, marital status at diagnosis, laterality, ICD-O-3 Hist/behav,malignant, grade, CS tumor size (2004+), Derived AJCC TNM(7th), RX Summ–Scope Reg LN Sur(2003+), SEER cause of death classification, Vital status recode, survival months, and other SEER cause of death classification. The flowchart of case selection is illustrated in Figure 1. The optimal cut-off values for age and tumor size were assessed by X-tile software (Yale University, New Haven, Connecticut, USA) ( Figure 2 ). Patients in cohort N and cohort M were divided into the training group and test group in the ratio of 7:3, randomly.
Figure 1.
Case screening flow chart.
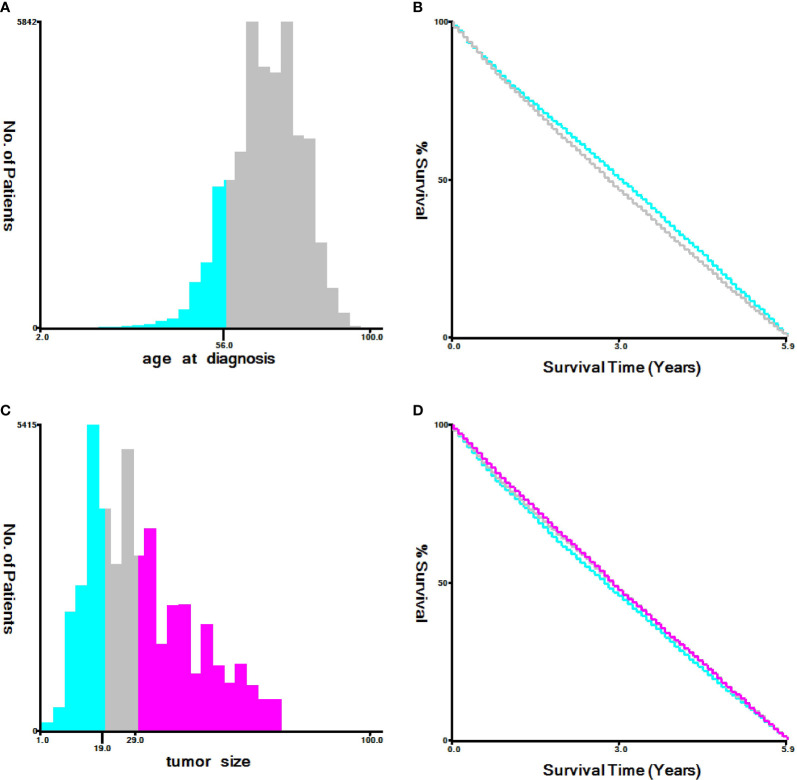
Figure 2.
Identification of optimal cut-off values of age (A, B) and tumor size (C, D) via X-tile software analysis. Optimal cut-off values of age were identified as 56 based on NCSD. Optimal cut-off values of tumor size were determined as 19 mm and 29 mm based on NCSD.
Variable Declaration
The histology variable was classified as “Adenocarcinomas,” “Squamous cell carcinoma,” “Large cell carcinoma” or “Other”. The cause-specific death was classified as “alive,” “dead due to cancer” or “dead due to other cause”. Meanwhile, “stage M1a” and “stage M1b” were classified as “stage M1”.
Nomograms Construction
Univariate and multivariate logistic regression analyses were performed to identify risk factors for LNM and DM cases. The factors screened out by multiple logistic regression models (P < 0.05) were applied to construct the nomograms. C-index and calibration plots conducted by a bootstrapping method with 1000 resamples were used to validate the nomograms in the discriminatory power. The DCAs were plotted to validate the nomograms in clinical application value. Based on the DCAs, clinical impact curves were chosen to show the significant value of the nomograms. In addition, the CIF was carried out further to determine the prognostic role of LNM and DM in NSCLC-specific death (NCSD). All models and images were conducted by R software (version 3.5.1) with various packages, including foreign, rms, nom1, rmda, tibble, survival, cmprsk, and stdca (https://rstudio.com/products/rpackages/).
Statistical Analysis
The optimal cut-off values for age and tumor size were assessed by the X-tile software Kaplan Meier curve. The baseline of patients between the training group and the test group was tested through Chi-square tests. The general situation of patients was summarized by Spss23.0. The difference was statistically significant if P < 0.05. Other data analyses were carried out through the corresponding functions of R software.
Results
Patients and Characteristics
After strict screening, 43,156 patients diagnosed with T1-2 NSCLC during 2010-2015 were finally included in this study from the SEER database. There were cohort N (T1-2N0-2M0 stage NSCLC, n = 36,212) and cohort M (T1-2 NSCLC, n = 43,156). The patients in cohort N were divided into training group (n = 25,348) and test group (n = 10,864). The patients in cohort M were divide into training group (n =30,209) and test group (n = 12,947). Totally, 9,439 of 36,212 patients (26.07%) occurred LNM in cohort N, and 6,944 of 43,156 patients (16.09%) occurred DM in cohort M. Patients’ characteristics were listed in Tables 1, 2.
Table 1.
Characteristics of patients in cohort N.
| Characteristic | Nt (n=36212) | Training group (n = 25348) | Test group (n = 10864) | p | ||
|---|---|---|---|---|---|---|
| Ne (%) | Nne (%) | Ne (%) | Nne (%) | |||
| N=6582 | N=18766 | N=2857 | N=8007 | |||
| Age | 0.405 | |||||
| ≤56 | 4535 (12.52) | 1012 (15.38) | 2187 (11.65) | 395 (13.83) | 941 (11.75) | |
| >56 | 31677 (87.48) | 5570 (84.62) | 16579 (88.35) | 2462 (86.17) | 7066 (88.25) | |
| Race | <0.001 | |||||
| White | 30107 (83.14) | 5384 (81.80) | 15750 (83.93) | 2309 (80.82) | 6664 (83.23) | |
| Black | 3433 (9.48) | 693 (10.53) | 1626 (8.66) | 345 (12.08) | 769 (9.60) | |
| American Indian /Alaska Native |
175 (0.48) | 40 (0.61) | 99 (0.53) | 8 (0.28) | 28 (0.35) | |
| Asian or Pacific Islander | 2497 (6.90) | 465 (7.06) | 1291 (6.88) | 195 (6.83) | 546 (6.82) | |
| Sex | 0.681 | |||||
| Male | 17054 (47.09) | 3458 (52.54) | 8498 (45.28) | 1484 (51.94) | 3614 (45.14) | |
| Female | 19158 (52.91) | 3124 (47.46) | 10268 (54.72) | 1373 (48.06) | 4393 (54.86) | |
| Histology | 0.453 | |||||
| Adenocarcinomas | 21240 (58.65) | 3775 (57.35) | 11069 (58.98) | 1634 (57.19) | 4762 (59.47) | |
| Squamous cell carcinoma | 11283 (31.16) | 2362 (35.89) | 5531 (29.47) | 1028 (35.98) | 2362 (29.50) | |
| Large cell carcinoma | 278 (0.77) | 75 (1.14) | 113 (0.60) | 28 (0.98) | 62 (0.77) | |
| Others | 3411 (9.42) | 370 (5.62) | 2053 (10.94) | 167 (5.85) | 821 (10.25) | |
| T-stage | 0.310 | |||||
| T1a | 10967 (30.29) | 937 (14.24) | 6767 (36.06) | 391 (13.69) | 2872 (35.87) | |
| T1b | 7401 (20.44) | 1041 (15.82) | 4074 (21.71) | 505 (17.68) | 1781 (22.24) | |
| T2a | 13930 (38.47) | 3212 (48.80) | 6561 (34.96) | 1387 (48.55) | 2770 (34.59) | |
| T2b | 3914 (10.81) | 1392 (21.15) | 1364 (7.27) | 574 (20.09) | 584 (7.29) | |
| Marital status | 0.896 | |||||
| Married | 20070 (55.42) | 3719 (56.50) | 10334 (55.07) | 1595 (55.83) | 4422 (55.23) | |
| Single | 5145 (14.21) | 937 (14.24) | 2661 (14.18) | 419 (14.67) | 1128 (14.09) | |
| Divorced | 4676 (12.91) | 881 (13.38) | 2410 (12.84) | 378 (13.23) | 1007 (12.58) | |
| Widowed | 6321 (17.46) | 1045 (15.88) | 3361 (17.91) | 465 (16.28) | 1450 (18.11) | |
| Tumor size | 0.437 | |||||
| 1-19 | 10642 (29.39) | 884 (13.43) | 6587 (35.10) | 384 (13.44) | 2787 (34.81) | |
| 20-29 | 10271 (28.36) | 1467 (22.29) | 5672 (30.22) | 668 (23.38) | 2464 (30.77) | |
| >29 | 15299 (42.25) | 4231 (64.28) | 6507 (34.67) | 1805 (63.18) | 2756 (34.42) | |
| Grade | 0.496 | |||||
| Well differentiated; Grade I | 6754 (18.65) | 479 (7.28) | 4299 (22.91) | 209 (7.32) | 1767 (22.07) | |
| Moderately differentiated; Grade II |
16178 (44.68) | 2754 (41.84) | 8560 (45.61) | 1163 (40.71) | 3701 (46.22) | |
| Poorly differentiated; Grade III |
12853 (35.49) | 3241 (49.24) | 5717 (30.46) | 1439 (50.37) | 2456 (30.67) | |
| Undifferentiated; Grade IV |
427 (1.18) | 108 (1.64) | 190 (1.01) | 46 (1.61) | 83 (1.04) | |
| Laterality | 0.567 | |||||
| Left | 14883 (41.10) | 2764 (41.99) | 7679 (40.92) | 1174 (41.09) | 3266 (40.79) | |
| Right | 21329 (58.90) | 3818 (58.01) | 11087 (59.08) | 1683 (58.91) | 4741 (59.21) | |
| Survival statue | 0.452 | |||||
| Dead | 11290 (31.18) | 3271 (49.70) | 4601 (24.52) | 1384 (48.44) | 2034 (25.40) | |
| Alive | 24922 (68.82) | 3311 (50.30) | 14165 (75.48) | 1473 (51.56) | 5973 (74.60) | |
P-value indicates the baseline comparison between training group and test group.
Table 2.
Characteristic of patients in cohort M.
| Characteristic | Mt (%)N=43156 | Training group N = 30209 | Test group N = 12947 | p | ||
|---|---|---|---|---|---|---|
| Me (%) | Mne (%) | Me (%) | Mne (%) | |||
| N=4837 | N=25372 | N=2107 | N=10840 | |||
| Age | 0.416 | |||||
| ≤56 | 5615 (13.01) | 765 (15.82) | 3192 (12.58) | 315 (14.95) | 1343 (12.39) | |
| >56 | 37541 (86.99) | 4072 (84.18) | 22180 (87.42) | 1792 (85.05) | 9497 (87.61) | |
| Race | 0.633 | |||||
| White | 35597 (82.48) | 3803 (78.62) | 21118 (82.23) | 1687 (80.07) | 8989 (82.92) | |
| Black | 4304 (9.97) | 618 (12.78) | 2405 (9.48) | 253 (12.01) | 1028 (9.48) | |
| American Indian/ Alaska Native |
198 (0.46) | 19 (0.39) | 126 (0.50) | 4 (0.19) | 49 (0.45) | |
| Asian or Pacific Islander | 30579 (7.08) | 397 (8.21) | 1723 (6.79) | 163 (7.74) | 774 (7.14) | |
| Sex | 0.822 | |||||
| Male | 20747 (48.07) | 2569 (53.11) | 11965 (47.16) | 1124 (53.35) | 5089 (46.95) | |
| Female | 22409 (51.93) | 2268 (46.89) | 13407 (52.84) | 983 (46.65) | 5751 (53.05) | |
| Histology | 0.136 | |||||
| Adenocarcinomas | 26065 (60.40) | 3397 (70.23) | 14918 (58.80) | 1428 (67.77) | 6322 (58.32) | |
| Squamous cell carcinoma | 13082 (30.31) | 1235 (25.53) | 7871 (31.02) | 564 (26.77) | 3412 (31.48) | |
| Large cell carcinoma | 395 (0.92) | 76 (1.57) | 183 (0.72) | 41 (1.95) | 95 (0.88) | |
| Others | 3614 (8.37) | 129 (2.67) | 2400 (9.46) | 74 (3.51) | 1011 (9.33) | |
| T-stage | 0.722 | |||||
| T1a | 11673 (27.05) | 486 (10.05) | 7709 (30.38) | 220 (10.44) | 3258 (30.06) | |
| T1b | 8466 (19.62) | 749 (15.48) | 5171 (20.38) | 316 (15.00) | 2230 (20.57) | |
| T2a | 17309 (40.11) | 2398 (49.58) | 9735 (38.37) | 981 (46.56) | 4195 (38.70) | |
| T2b | 5708 (13.23) | 1204 (24.89) | 2757 (10.87) | 590 (28.00) | 1157 (10.67) | |
| N-stage | 0.972 | |||||
| N0 | 28948 (67.08) | 1510 (31.22) | 18755 (73.92) | 665 (31.56) | 8018 (73.97) | |
| N1 | 4299 (9.96) | 501 (10.36) | 2499 (9.85) | 219 (10.39) | 1080 (9.96) | |
| N2 | 8014 (18.57) | 2076 (42.92) | 3546 (13.98) | 889 (42.19) | 1503 (13.87) | |
| N3 | 1895 (4.39) | 750 (15.51) | 572 (2.25) | 334 (15.85) | 239 (2.20) | |
| Marital status | 0.994 | |||||
| Married | 23913 (55.41) | 2657 (54.93) | 14094 (55.55) | 1186 (56.29) | 5976 (55.13) | |
| Single | 6314 (14.63) | 839 (17.35) | 3580 (14.11) | 330 (15.66) | 1565 (14.44) | |
| Divorced | 5503 (12.75) | 586 (12.11) | 3260 (12.85) | 241 (11.44) | 1416 (13.06) | |
| Widowed | 7426 (17.21) | 755 (15.61) | 4438 (17.49) | 350 (16.61) | 1883 (17.37) | |
| Tumor size | 0.579 | |||||
| 1-19 | 11352 (26.30) | 488 (10.09) | 7499 (29.56) | 222 (10.54) | 3143 (28.99) | |
| 20-29 | 11606 (26.89) | 943 (19.50) | 7151 (28.18) | 392 (18.60) | 3120 (28.78) | |
| >29 | 20198 (46.80) | 3406 (70.42) | 10722 (42.26) | 1493 (70.86) | 4577 (42.22) | |
| Grade | 0.390 | |||||
| Well differentiated; Grade I | 7158 (16.59) | 281 (5.81) | 4727 (18.63) | 123 (5.84) | 2027 (18.70) | |
| Moderately differentiated; Grade II |
18538 (42.96) | 1689 (34.92) | 11363 (44.79) | 671 (31.85) | 4815 (44.42) | |
| Poorly differentiated; Grade III |
16863 (39.07) | 2750 (56.85) | 8987 (35.42) | 1260 (59.80) | 3866 (35.66) | |
| Undifferentiated; Grade IV | 597 (1.38) | 117 (2.42) | 295 (1.16) | 53 (2.52) | 132 (1.22) | |
| Laterality | 0.812 | |||||
| Left | 17882 (41.44) | 2075 (42.90) | 10454 (41.20) | 924 (43.85) | 4429 (40.86) | |
| Right | 25274 (58.56) | 2762 (57.10) | 14918 (58.80) | 1183 (56.15) | 6411 (59.14) | |
| Survival statue | 0.833 | |||||
| Dead | 16714 (38.73) | 3794 (78.44) | 7916 (31.20) | 1630 (77.36) | 3374 (31.13) | |
| Alive | 26442 (61.27) | 1043 (21.56) | 17456 (68.80) | 477 (22.64) | 7466 (68.87) | |
P-value indicates the baseline comparison between training group and test group.
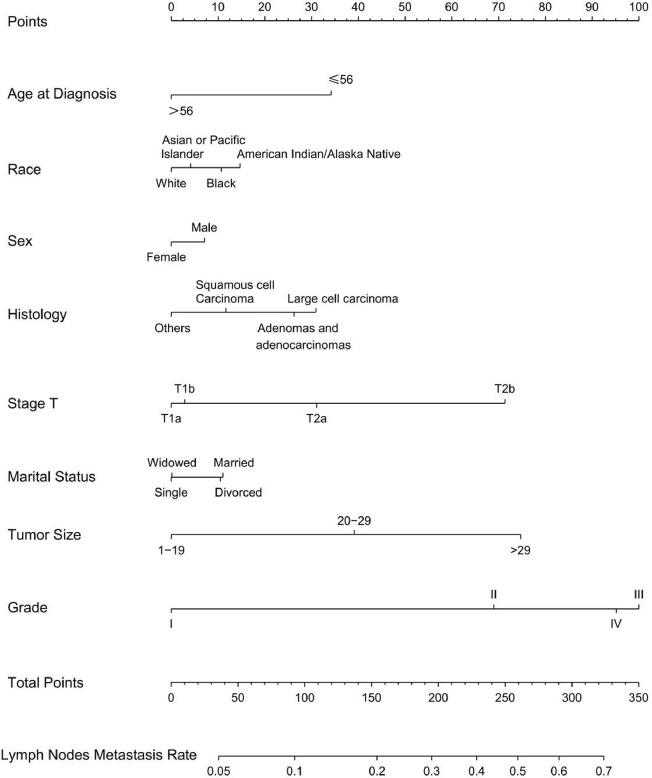
Independent Risk Factor and Model Construction for Lymph Node Metastasis
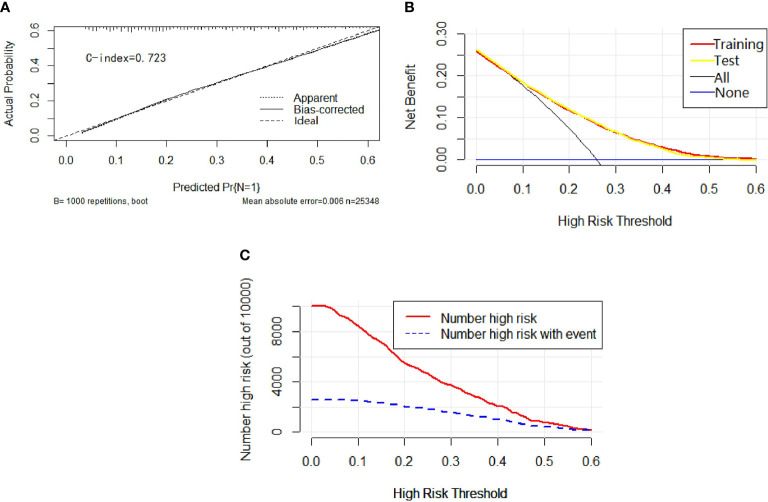
Univariable and multivariable binary logistic regression analyses were conducted to screen the independent risk factors for lymph node metastasis. Eight factors, including age, race, sex, histology, T-stage, marital status, tumor size, and grade, were confirmed to work in the prediction of LNM (Table 3). Scores assignments and predictive probability for each risk factor in the nomogram (Figure 3) were calculated in Table 5. The score of each independent predictor is the corresponding upper scale. The total points of each subject are the sum of the scores of each independent predictor. The value of the total points corresponding to the risk axis is the risk of LNM. The higher the total point is, the higher the risk of LNM is. In the training set, the nomogram has good discrimination and calibration in predicting the risk of LNM, and the C index is 0.723 (Figure 4A). Decision curve analysis (DCA) of nomogram evaluates the net benefit of patients. The larger the net benefit rate is, the better the predictive performance of the prognostic risk model is (Figure 4B). In addition, the clinical impact curve (CIC) detects the predictive value of nomogram in LNM prognosis (Figure 4C).
Table 3.
Logistic regression analysis of the risk factors for lymph node metastasis in cohort N.
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age | ||||||
| ≤56 | Reference | Reference | ||||
| >56 | 0.73 | 0.67-0.79 | <0.001 | 0.64 | 0.58-0.70 | <0.001 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.25 | 1.13-1.37 | <0.001 | 1.15 | 1.04-1.28 | 0.007 |
| American Indian/ Alaska Native |
1.18 | 0.81-1.69 | 0.374 | 1.21 | 0.81-1.79 | 0.337 |
| Asian or Pacific Islander | 1.05 | 0.94-1.18 | 0.354 | 1.06 | 0.94-1.19 | 0.373 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.75 | 0.71-0.79 | <0.001 | 0.91 | 0.86-0.97 | 0.004 |
| Histology | ||||||
| Adenocarcinomas | Reference | Reference | ||||
| Squamous cell carcinoma | 1.25 | 1.18-1.33 | <0.001 | 0.82 | 0.77-0.88 | <0.001 |
| Large cell carcinoma | 1.95 | 1.45-2.61 | <0.001 | 1.06 | 0.76-1.49 | 0.718 |
| Others | 0.53 | 0.47-0.59 | <0.001 | 0.71 | 0.62-0.80 | <0.001 |
| T-stage | ||||||
| T1a | Reference | Reference | ||||
| T1b | 1.85 | 1.68-2.03 | <0.001 | 1.04 | 0.91-1.19 | 0.578 |
| T2a | 3.54 | 3.26-3.83 | <0.001 | 1.50 | 1.33-1.70 | <0.001 |
| T2b | 7.37 | 6.66-8.16 | <0.001 | 2.56 | 2.20-2.97 | <0.001 |
| Marital status | ||||||
| Married | Reference | Reference | ||||
| Single | 0.98 | 0.90-1.06 | 0.608 | 0.86 | 0.79-0.95 | 0.002 |
| Divorced | 1.02 | 0.93-1.11 | 0.720 | 0.99 | 0.90-1.09 | 0.882 |
| Widowed | 0.86 | 0.80-0.93 | <0.001 | 0.87 | 0.79-0.94 | 0.001 |
| Tumor size | ||||||
| 1-19 | Reference | Reference | ||||
| 20-29 | 1.93 | 1.76-2.11 | <0.001 | 1.67 | 1.48-1.89 | <0.001 |
| >29 | 4.85 | 4.47-5.25 | <0.001 | 2.67 | 2.35-3.04 | <0.001 |
| Grade | ||||||
| Well differentiated; Grade I | Reference | Reference | ||||
| Moderately differentiated; Grade II |
2.89 | 2.61-3.21 | <0.001 | 2.48 | 2.22-2.77 | <0.001 |
| Poorly differentiated; Grade III |
5.09 | 4.59-5.65 | <0.001 | 3.73 | 3.34-4.17 | <0.001 |
| Undifferentiated; anaplastic; Grade IV |
5.10 | 3.95-6.57 | <0.001 | 3.49 | 2.62-4.64 | <0.001 |
| Laterality | ||||||
| Left | Reference | Reference | ||||
| Right | 0.96 | 0.90-1.01 | 0.128 | 0.96 | 0.91-1.02 | 0.214 |
Figure 3.
Nomogram for predicting LNM in T1-2N0-3M0 NSCLC. Eight factors were calculated into the LNM prediction nomogram.
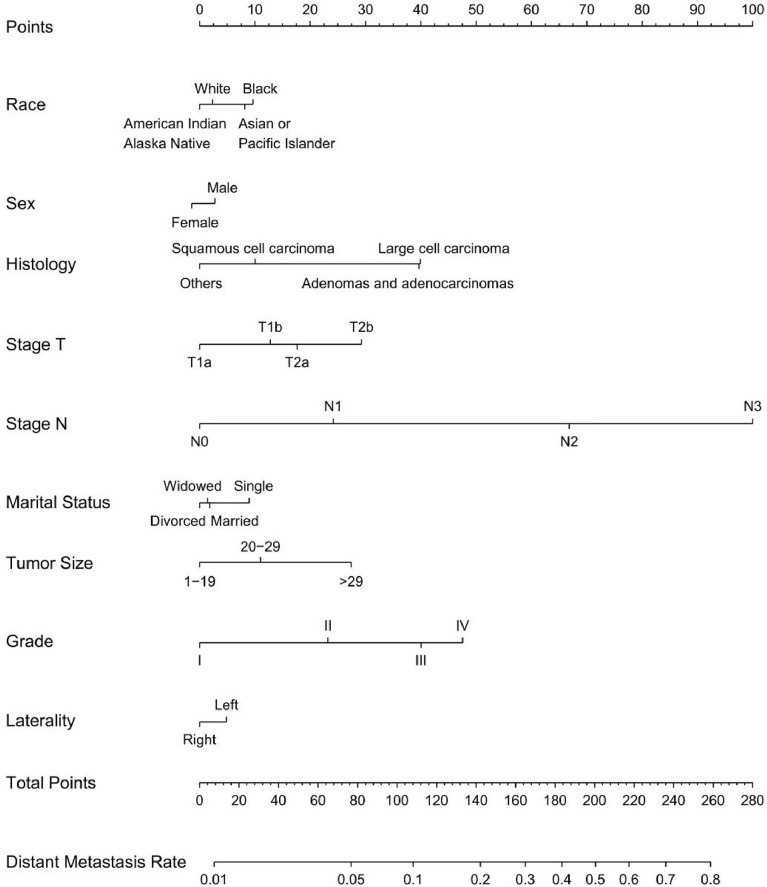
Table 5.
Nomogram score of risk factors for LNM and DM in T1-2 NSCLC.
| Risk factors | Nomogram score | |
|---|---|---|
| Lymph nodes metastasis | Distant metastasis | |
| Age | ||
| ≤56 | 34 | / |
| >56 | 0 | / |
| Race | ||
| White | 0 | 2 |
| Black | 11 | 10 |
| American Indian/ Alaska Native |
15 | 0 |
| Asian or Pacific Islander |
4 | 8 |
| Sex | ||
| Male | 7 | 4 |
| Female | 0 | 0 |
| Histology | ||
| Adenocarcinomas | 26 | 40 |
| Squamous cell carcinoma |
12 | 10 |
| Large cell carcinoma |
31 | 40 |
| Others | 0 | 0 |
| T-stage | ||
| T1a | 0 | 0 |
| T1b | 3 | 13 |
| T2a | 31 | 18 |
| T2b | 71 | 29 |
| N-stage | ||
| N0 | / | 0 |
| N1 | / | 24 |
| N2 | / | 67 |
| N3 | / | 100 |
| Marital status | ||
| Married | 11 | 2 |
| Single | 0 | 9 |
| Divorced | 11 | 0 |
| Widowed | 0 | 1 |
| Tumor size (mm) | ||
| 1-19 | 0 | 0 |
| 20-29 | 39 | 11 |
| >29 | 75 | 27 |
| Grade | ||
| Well differentiated; Grade I |
0 | 0 |
| Moderately differentiated; Grade II |
69 | 23 |
| Poorly differentiated; Grade III |
100 | 40 |
| Undifferentiated; anaplastic; Grade IV |
95 | 48 |
| Laterality | ||
| Left | / | 5 |
| Right | / | 0 |
Figure 4.
In calibration curve (A), the x-axis represents the predicted probability of LNM, the y-axis refers to the actual probability of LNM, and the ideal line marks the diagonal of the graph, indicating that the predicted probability is utterly equal to the true likelihood, which is the ideal condition of the prediction model. The apparent line represents the theoretical prediction ability, and the bias-corrected stands for the prediction ability of the corrected model. The figure shows that the above three lines are very close, and c-index = 0.723, suggesting that the prediction model is very accurate. In the decision curve (B), the x-axis represents the threshold probability, the y-axis marks the net income, the horizontal blue line is an extreme case that no patients suffer from LNM, and the black line represents another extreme case that all patients have LNM. The red line and the yellow line are predicted by the training group and the test group, respectively. The two lines basically coincide, indicating that the prediction ability of the model is stable and reliable. In the clinical impact curve (C), the x-axis represents the different thresholds of LNM, and the y-axis stands for the number of people. The red line displays the number of high-risk patients under different thresholds, and the blue line illustrates the actual number of LNM in high-risk patients under different thresholds.
Independent Risk Factor and Model Construction for Distant Metastasis
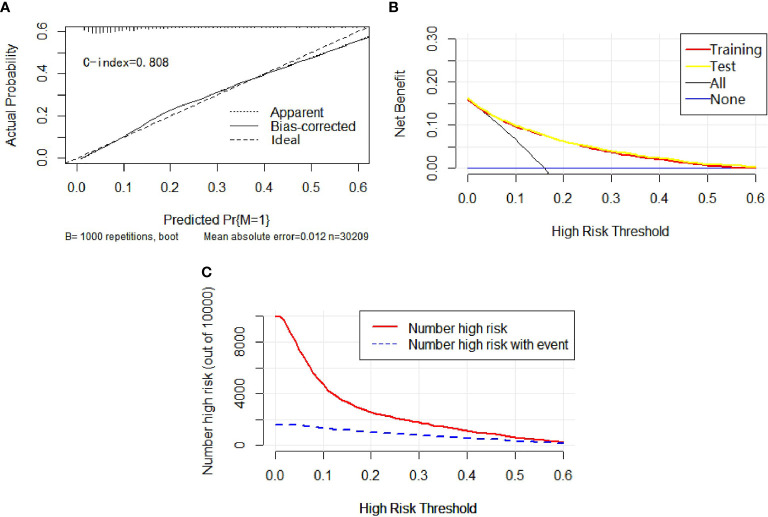
Univariable and multivariable binary logistic regression analyses were conducted to screen the independent risk factors for distant metastasis. Nine factors, including race, sex, histology, T-stage, N-stage, marital status, tumor size, grade, and laterality, were confirmed to work in the prediction of DM (Table 4). Scores assignments and predictive probability for each risk factor in the nomogram (Figure 5) are exhibited in Table 5. The score of each independent predictor is the corresponding upper scale. The total point of each subject is the sum of the scores of each independent predictor. The value of the total points corresponding to the risk axis is the risk of DM. The higher the total point is, the higher the risk of LNM is. In the training set, the nomogram has good discrimination and calibration in predicting the risk of DM, and the C index is 0.808 (Figure 6A). Decision curve analysis (DCA) of nomogram evaluates the net benefit of patients. The larger the net benefit rate is, the better the predictive performance of the prognostic risk model is (Figure 6B). Furthermore, the clinical impact curve (CIC) detects the predictive value of nomogram in LNM prognosis (Figure 6C).
Table 4.
Logistic regression analysis of the risk factors for distant metastasis in cohort M.
| Factors | Univariate analysis | Multivariate analysis | ||||
|---|---|---|---|---|---|---|
| OR | 95%CI | P | OR | 95%CI | P | |
| Age | ||||||
| ≤56 | Reference | Reference | ||||
| >56 | 0.77 | 0.70-0.84 | <0.001 | 0.92 | 0.83-1.02 | 0.108 |
| Race | ||||||
| White | Reference | Reference | ||||
| Black | 1.43 | 1.30-1.57 | <0.001 | 1.18 | 1.06-1.32 | 0.002 |
| American Indian/ Alaska Native |
0.84 | 0.50-1.32 | 0.472 | 0.95 | 0.54-1.57 | 0.846 |
| Asian or Pacific Islander | 1.28 | 1.14-1.43 | <0.001 | 1.15 | 1.00-1.30 | 0.039 |
| Sex | ||||||
| Male | Reference | Reference | ||||
| Female | 0.79 | 0.74-0.84 | <0.001 | 0.90 | 0.84-0.97 | 0.006 |
| Histology | ||||||
| Adenocarcinomas | Reference | Reference | ||||
| Squamous cell carcinoma | 0.69 | 0.64-0.74 | <0.001 | 0.50 | 0.46-0.54 | <0.001 |
| Large cell carcinoma | 1.82 | 1.38-2.38 | <0.001 | 1.01 | 0.72-1.39 | 0.965 |
| Others | 0.24 | 0.20-0.28 | <0.001 | 0.39 | 0.32-0.47 | <0.001 |
| T-stage | ||||||
| T1a | Reference | Reference | ||||
| T1b | 2.30 | 2.04-2.59 | <0.001 | 1.35 | 1.14-1.61 | <0.001 |
| T2a | 3.91 | 3.53-4.33 | <0.001 | 1.52 | 1.29-1.79 | <0.001 |
| T2b | 6.93 | 6.19-7.77 | <0.001 | 2.00 | 1.67-2.40 | <0.001 |
| N-stage | ||||||
| N0 | Reference | Reference | ||||
| N1 | 2.49 | 2.23-2.78 | <0.001 | 1.77 | 1.58-1.98 | <0.001 |
| N2 | 7.27 | 6.74-7.84 | <0.001 | 4.89 | 4.51-5.30 | <0.001 |
| N3 | 16.29 | 14.44-18.38 | <0.001 | 10.76 | 9.49-12.22 | <0.001 |
| Marital status | ||||||
| Married | Reference | Reference | ||||
| Single | 1.24 | 1.14-1.35 | <0.001 | 1.17 | 1.06-1.30 | 0.002 |
| Divorced | 0.95 | 0.86-1.05 | 0.3371 | 0.96 | 0.86-1.07 | 0.413 |
| Widowed | 0.90 | 0.83-0.98 | 0.0216 | 1.00 | 0.90-1.11 | 0.998 |
| Tumor size(mm) | ||||||
| 1-19 | Reference | Reference | ||||
| 20-29 | 2.03 | 1.81-2.27 | <0.001 | 1.30 | 1.10-1.53 | 0.002 |
| >29 | 4.88 | 4.42-5.40 | <0.001 | 1.93 | 1.64-2.27 | <0.001 |
| Grade | ||||||
| Well differentiated; Grade I | Reference | Reference | ||||
| Moderately differentiated; Grade II |
2.50 | 2.20-2.85 | <0.001 | 1.74 | 1.51-2.00 | <0.001 |
| Poorly differentiated; Grade III |
5.15 | 4.54-5.86 | <0.001 | 2.59 | 2.26-2.98 | <0.001 |
| Undifferentiated; anaplastic; Grade IV |
6.67 | 5.21-8.51 | <0.001 | 3.10 | 2.31-4.13 | <0.001 |
| Laterality | ||||||
| Left | Reference | Reference | ||||
| Right | 0.93 | 0.88-0.99 | 0.028 | 0.89 | 0.83-0.95 | 0.001 |
Figure 5.
Nomogram for predicting DM in T1-2N0-3M0-1 NSCLC. Nine factors were calculated into DM prediction nomogram.
Figure 6.
In calibration curve (A), the x-axis represents the predicted probability of DM, the y-axis stands for the actual probability of DM, and the ideal line is the diagonal of the graph, indicating that the predicted probability is utterly equal to the true likelihood, which is the ideal condition of the prediction model. The apparent line represents the theoretical prediction ability, and the bias-corrected marks the prediction ability of the corrected model. The figure reveals that the above three lines are very close, and c-index = 0.808, suggesting that the prediction model is very accurate. In the decision curve (B), the x-axis represents the threshold probability, the y-axis refers to the net income, the horizontal blue line is an extreme case that no patients suffer from DM, and the black line represents another extreme case that all patients have DM. The red line and the yellow line are predicted by the training group and the test group, respectively. The two lines basically coincide, indicating that the prediction ability of the model is stable and reliable. In the clinical impact curve (C), the x-axis represents the different thresholds of DM, and the y-axis marks the number of people. The red line indicates the number of high-risk patients under different thresholds, and the blue line indicates the actual number of DM in high-risk patients under different thresholds.
Survival Analyses
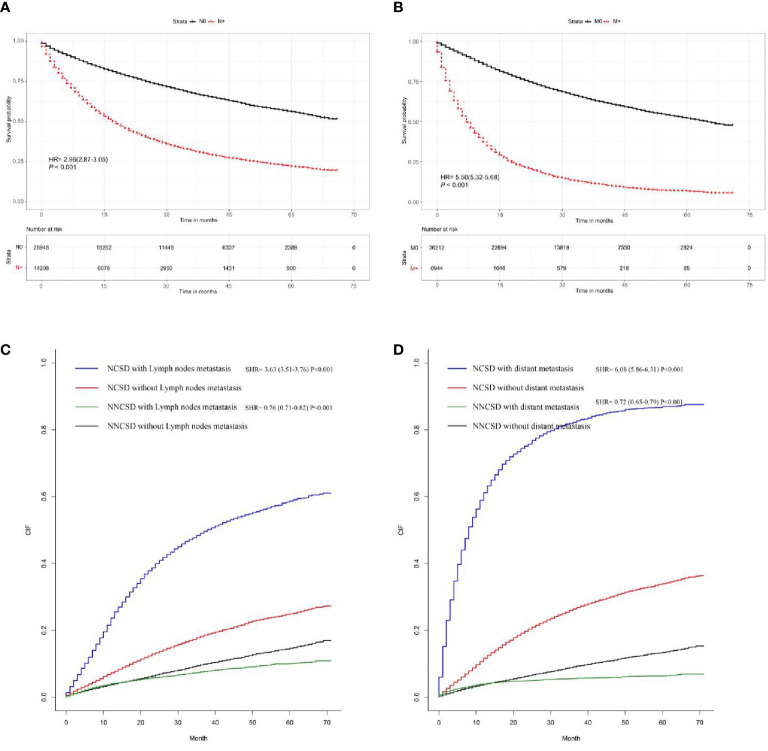
Based on the Kaplan-Meier and Gray method, we analyzed LNM and DM related deaths. The results proved that positive lymph node involvement (hazard ratio (HR) = 2.96, 95%CI = (2.87-3.05), P < 0.001) and distant metastasis (HR = 5.50, 95%CI = (5.32-5.68), P < 0.001) are significantly correlated with overall survival using Kaplan-Meier curves (Figures 7A, B). At the same time, the Gray method displayed that LNM (subdistribution hazard ratio (SHR) = 3.63, 95%CI=(3.51-3.76), P < 0.001) and DM (SHR = 6.08, 95%CI = (5.86-6.13), P < 0.001) are significantly correlated with cancer-specific (Figures 7C, D).
Figure 7.
The survival rate of T1-2 NSCLC patients was evaluated by the presence or absence of LNM and DM; the results showed that LNM and DM are closely related to survival rate (A, B). The cancer-specific survival rate of T1-2 NSCLC patients was evaluated by the presence or absence of LNM and DM; the results demonstrated that LNM and DM are closely correlated with cancer-specific survival rates (C, D).
T1-2 stage NSCLC patients have the following characteristics: the maximum diameter of the primary tumor is ≤7cm, and other tissues in the chest (except visceral pleura) are not involved. Due to different lymph nodes and distant metastasis situations, patients need different diagnosis and treatment methods, and there will be different prognoses. This study showed that about 74% of newly diagnosed T1-2 NSCLC patients are in stage I-IIA without lymph node and distant metastasis. According to NCCN Guidelines(Small Cell Lung Cancer, Version 2020.V6), surgical resection is feasible, and there is no need for radiotherapy or chemotherapy after surgical resection. About 26% of newly diagnosed NSCLC in the T1-2 stage has lymph node metastasis but has no distant metastasis. Some patients (T2bN1M0, T1-2N2-3M0) need postoperative chemoradiotherapy (7). About 16% of newly diagnosed NSCLC in the T1-2 stage has distant metastasis, so there is no indication of radical operation, and other treatments such as chemotherapy, targeted drugs, and immunity are recommended (8). LNM and DM are important factors for making diagnosis and treatment plans and predicting prognosis. At present, the pathological biopsy is still the gold standard for diagnosing LNM and DM in NSCLC. Although there are simple examination methods, PET-CT, LNM and DM can be evaluated preliminarily. Still, the price is higher, and there are false negative and false positive (9). Therefore, non-invasive and effective methods to evaluate the presence of LNM and DM in NSCLC patients are urgently needed. According to the prediction results of the models, further selections of examination and treatment can be more reasonably chose.
In recent years, more and more researches participated in this field, but there are still many shortcomings and limitations. First, previous studies (10, 11) established Cox regression analysis models based on logistic regression analysis. On the contrary, these models cannot be used in clinical practices for their low predictive ability. As a new display form, nomogram can directly display the predicted LNM and DM. This method forms a nomogram to predict the correlation probability, thus providing a reference for further examination and clinical decision-making.
There are a lot of nomograms to predict the diagnosis and prognosis of cancer, but some problems still exist. Some studies include a small sample size (12, 13); some studies contain few factors (14, 15); some studies do not set cut-off value (16, 17); some studies are not divided into training and test groups (18, 19). This is the first and the only study that developed a nomogram to predict the probability of LNM and DM in T1-2 NSCLC patients as far as we know. We divided the included population into the N cohort (T1-2N0-3M0 NSCLC for LNM) and M cohort (T1-2N0-3M0-1 NSCLC for DM). Besides, we divided the LNM cohort and DM cohort into the training group and the test group on the basis of the ratio of 7:3, respectively. The incidence of LNM and DM of T1-2 NSCLC were analyzed, and the corresponding nomograms were constructed through the training group, and then verified by the test group. Two nomograms were established and validated for predicting LNM and DM in patients with T1-2 NSCLC. LNM nomogram includes eight factors, namely age, race, sex, histology, T-stage, marital status, tumor size, and grade, whereas DM nomogram incorporates nine factors, namely race, sex, histology, T-stage, N-stage, marital status, tumor size, grade, and laterality. Both of the nomograms indicated good agreement between predictions and observations. C-index of the LNM nomogram, and DM nomogram were calculated with the values of 0.723 and 0.808, respectively. These nomograms reveal good clinical utility in the proper threshold probability range.
In this population-based study, from the score table of nomogram (Table 5), it is obvious that grade, tumor size, and T-stage account for the most significant score. It has been reported that grade is closely related to LNM in NSCLC (20). Our data also showed that the LNM risk of moderately differentiated, poorly differentiated and undifferentiated cancer increased to 2.48, 3.73 and 3.49 compared with well-differentiated carcinoma (both P < 0.001). In this study, patients with squamous cell carcinoma have lower risk in comparison with adenocarcinomas patients. Previous studies (21) have revealed that young age at diagnosis is associated with the increased risk of LNM in patients with NSCLC. As in these studies, we noticed that the risk of LNM was higher in the younger T1-2 NSCLC group (age ≤ 56 years) than that in the older T1-2 NSCLC group (age > 56 years). The reason may be that young patients with lower tumor differentiation grade are more likely to escape from the immune surveillance of the body. But for this conjecture, we still have not come to any conclusive data, which needs further study. Adenocarcinomas are more likely to have lymph node metastasis than squamous cell carcinoma (P < 0.001). No significant differences in LNM between left and right lung cancer were found.
For the DM nomogram, the largest proportion in risk scores factors are T-stage, Grade, and Histology. Of note, patients with the worse N-stage and worse grade are more prone to occur distant metastasis. As are consistent with previous studies (22), adenocarcinomas are more likely to have distant metastasis than squamous cell carcinoma, and female are more prone to have distant metastasis than male. Different from the predicted LNM, there were no significant differences between the older group (> 56) and the younger group (≤56). Laterality has been found to be predictive of distant metastasis in T1-2 NSCLC. Compared with left lung cancer, right lung cancer has a lower risk of distant metastasis(HR=0.89, 95%CI=0.83-0.95, P<0.001). The reason may be related to the anatomical structure of the lung. For instance, the left lung is divided into 2 pages and 8 lung segments, and the right lung is divided into 3 pages and 10 lung segments. The right pulmonary artery is longer, while the left pulmonary artery is shorter.
Furthermore, we found that LNM and DM in T1-2 NSCLC were associated with cancer-specific death and non-cancer-specific death. In this database-based study, we screened 43,156 eligible patients with a median follow-up of 70 months from real-world data. We analyzed the data by adopting appropriate statistical methods and found these convincing conclusions.
However, there are some limitations in this study. Firstly, this is a population-based retrospective analysis that lacks prospective data for verification. Although the 8th edition of TNM is currently used, we can only continue to refer to the 7th edition (Table 6) because the study is a retrospective study and the seventh edition was used for case entry at that time. Secondly, this database’ information is insufficient in terms of smoking, tumor markers, imaging examination, and important molecular factors (EGFR, rose1 and ALK gene status), thus leading to that our nomogram failed to include these important factors. Finally, our data are only from one institution. Although the data are divided into training and test groups, it inevitably causes internal bias. We can further collect multi center data and combine other factors for the improvement of the model.
Table 6.
7th Edition AJCC Cancer Staging Manual for NSCLC.
| T component | |
|---|---|
| 0 cm (pure lepidic adenocarcinoma ≤3 cm in total size) | T1a if ≤ 2 cm; T1b f>2-3cm |
| ≤0.5 cm invasive size (lepidic predominant adenocarcinoma ≤ 3 cm total size) | T1a if ≤ 2cm; T1b if>2-3cm |
| ≤1cm | T1a |
| >1-2 cm | T1a |
| >2-3 cm | T1b |
| >3-4 cm | T2a |
| >4-5 cm | T2a |
| >5-7 cm | T2b |
| >7 cm | T3 |
| Bronchus <2 cm from carin | T3 |
| Total atelectasis/pneumonitis | T3 |
| Invasion of diaphragm | T3 |
| Invasion of mediastinal pleura | T3 |
| N component | |
| Regional lymph nodes cannot be assessed | NX |
| No regional lymph node metastasis | N0 |
| Metastasis in ipsilateral peribronchial and/or ipsilateral hilar lymph nodes andintrapulmonary nodes, including involvement by direct extension | N1 |
| Metastasis in ipsilateral mediastinal and/or subcarinal lymph node(s) | N2 |
| Metastasis in contralateral mediastinal, contralateral hilar, ipsilateral or contralateral scalene, or supraclavicular lymph node(s)contralateral scalene, or supraclavicular lymph node(s) | N3 |
| M component | |
| Metastasis within the thoracic cavity | M1a |
| Single extrathoracic metastasis | M1b |
| Multiple extrathoracic metastasis | M1b |
In conclusion, based on the independent risk factors screened from the large database, we constructed two nomograms that can accurately predict LNM and DM in different stages of T1-2 NSCLC patients. The listed factors can be easily obtained from clinical and pathological data. Through the verification of discrimination and correction, our nomogram has high accuracy and reliability, so it can be applied to clinical practices. Combined with other clinical data, it can help doctors make better diagnosis and investigation, individualized treatment, and follow-up management decisions for t1-2 NSCLC patients.
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author Contributions
YQ, SW, and JL designed the study. WY, LZ, and YS extracted and analyzed the data. BZ and LT wrote and edited the manuscript. Authors were ranked according to their contributions. YQ and SW contributed equally to this work and should be considered as co-first authors. All authors contributed to the article and approved the submitted version.
Funding
National Natural Science Foundation of China (81673809); Science and technology project of traditional Chinese medicine in Zhejiang Province (2020ZB049); Project of Zhejiang lung cancer prevention and treatment center of traditional Chinese medicine (2A11801); Zhejiang Provincial natural science foundation (LQ19H030004); Key research department project of Oncology Department of Tongde Hospital of Zhejiang Province.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.683282/full#supplementary-material
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2020. CA Cancer J Clin (2020) 70:7–30. 10.3322/caac.21590 [DOI] [PubMed] [Google Scholar]
- 2.Duma N, Santana-Davila R, Molina JR. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin Proc (2019) 94:1623–40. 10.1016/j.mayocp.2019.01.013 [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Du L, Yang X, Li J, Li P, Zhao Y, et al. A Nomogram Combining Long non-Coding RNA Expression Profiles and Clinical Factors Predicts Survival in Patients With Bladder Cancer. Aging (Albany NY) (2020) 12:2857–79. 10.18632/aging.102782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao L, Gong J, Xi Y, Xu M, Li C, Kang X, et al. MRI-Based Radiomics Nomogram may Predict the Response to Induction Chemotherapy and Survival in Locally Advanced Nasopharyngeal Carcinoma. Eur Radiol (2020) 30:537–46. 10.1007/s00330-019-06211-x [DOI] [PubMed] [Google Scholar]
- 5.Dong D, Fang MJ, Tang L, Shan XH, Gao JB, Giganti F, et al. Deep Learning Radiomic Nomogram can Predict the Number of Lymph Node Metastasis in Locally Advanced Gastric Cancer: An International Multicenter Study. Ann Oncol (2020) 31:912–20. 10.1016/j.annonc.2020.04.003 [DOI] [PubMed] [Google Scholar]
- 6.Zheng XQ, Huang JF, Lin JL, Chen L, Zhou TT, Chen D, et al. Incidence, Prognostic Factors, and a Nomogram of Lung Cancer With Bone Metastasis at Initial Diagnosis: A Population-Based Study. Transl Lung Cancer Res (2019) 8:367–79. 10.21037/tlcr.2019.08.16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kris MG, Gaspar LE, Chaft JE, Kennedy EB, Azzoli CG, Ellis PM, et al. Adjuvant Systemic Therapy and Adjuvant Radiation Therapy for Stage I to IIIA Completely Resected Non-Small-Cell Lung Cancers: American Society of Clinical Oncology/Cancer Care Ontario Clinical Practice Guideline Update. J Clin Oncol (2017) 35:2960–74. 10.1200/JCO.2017.72.4401 [DOI] [PubMed] [Google Scholar]
- 8.Arbour KC, Riely GJ. Systemic Therapy for Locally Advanced and Metastatic Non-Small Cell Lung Cancer: A Review. JAMA (2019) 322:764–74. 10.1001/jama.2019.11058 [DOI] [PubMed] [Google Scholar]
- 9.Finessi M, Bisi G, Deandreis D. Hyperglycemia and 18F-FDG PET/CT, Issues and Problem Solving: A Literature Review. Acta Diabetol (2020) 57:253–62. 10.1007/s00592-019-01385-8 [DOI] [PubMed] [Google Scholar]
- 10.Shen L, Yun T, Guo J, Liu Y, Liang C. Clinicopathological Characteristics and Risk Factors of Station 4L Lymph Node Metastasis of Left Non-Small Cell Lung Cancer. Nan Fang Yi Ke Da Xue Xue Bao (2020) 40:1793–8. 10.12122/j.issn.1673-4254.2020.12.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ding N, Mao Y, Gao S, Xue Q, Wang D, Zhao J, et al. Predictors of Lymph Node Metastasis and Possible Selective Lymph Node Dissection in Clinical Stage IA non-Small Cell Lung Cancer. J Thorac Dis (2018) 10:4061–8. 10.21037/jtd.2018.06.129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Zheng YZ, Liao HY, Guo X, Li Y, Wang Z, et al. A Clinical Nomogram and Heat Map for Assessing Survival in Patients With Stage I Non-Small Cell Lung Cancer After Complete Resection. Ther Adv Med Oncol (2020) 12:1758835920970063. 10.1177/1758835920970063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Qiu B, Li HQ, Chang QG, Yin DT. Nomograms Predict Survival in Patients With Anaplastic Thyroid Carcinoma. Med Sci Monit (2019) 25:8447–56. 10.12659/MSM.918245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang Y, Liu W, Yu Y, Liu JJ, Xue HD, Qi YF, et al. CT Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Gastric Cancer. Eur Radiol (2020) 30:976–86. 10.1007/s00330-019-06398-z [DOI] [PubMed] [Google Scholar]
- 15.Jeong SH, Kim RB, Park SY, Park J, Jung EJ, Ju YT, et al. Nomogram for Predicting Gastric Cancer Recurrence Using Biomarker Gene Expression. Eur J Surg Oncol (2020) 46:195–201. 10.1016/j.ejso.2019.09.143 [DOI] [PubMed] [Google Scholar]
- 16.Kim SM, Min BH, Ahn JH, Jung SH, An JY, Choi MG, et al. Nomogram to Predict Lymph Node Metastasis in Patients With Early Gastric Cancer: A Useful Clinical Tool to Reduce Gastrectomy After Endoscopic Resection. Endoscopy (2020) 52:435–43. 10.1055/a-1117-3059 [DOI] [PubMed] [Google Scholar]
- 17.Zhang Y, Hong YK, Zhuang DW, He XJ, Lin ME. Bladder Cancer Survival Nomogram: Development and Validation of a Prediction Tool, Using the SEER and TCGA Databases. Med (Baltimore) (2019) 98:e17725. 10.1097/MD.0000000000017725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guo K, Feng Y, Yuan L, Wasan HS, Sun L, Shen M, et al. Risk Factors and Predictors of Lymph Node Metastasis and Distant Metastasis in Newly Diagnosed T1 Colorectal Cancer. Cancer Med (2020) 9:5095–113. 10.1002/cam4.3114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hou G, Zheng Y, Wei D, Li X, Wang F, Tian J, et al. Development and Validation of a SEER-Based Prognostic Nomogram for Patients With Bone Metastatic Prostate Cancer. Med (Baltimore) (2019) 98:e17197. 10.1097/MD.0000000000017197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhai X, Guo Y, Qian X. Combination of Fluorine-18 Fluorodeoxyglucose Positron-Emission Tomography/Computed Tomography (¹⁸F-FDG PET/CT) and Tumor Markers to Diagnose Lymph Node Metastasis in Non-Small Cell Lung Cancer (NSCLC): A Retrospective and Prospective Study. Med Sci Monit (2020) 26:e922675. 10.12659/MSM.922675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen B, Wang X, Yu X, Xia WJ, Zhao H, Li XF, et al. Lymph Node Metastasis in Chinese Patients With Clinical T1 non-Small Cell Lung Cancer: A Multicenter Real-World Observational Study. Thorac Cancer (2019) 10:533–42. 10.1111/1759-7714.12970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolwin Y, Gillis R, Peled N. Gender and Lung Cancer-SEER-Based Analysis. Ann Epidemiol (2020) 46:14–9. 10.1016/j.annepidem.2020.04.003 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.