Abstract
Highly porous 3d transition metal oxide nanostructures are opening up the exciting area of oxygen evolution reaction (OER) catalysts in alkaline medium thanks to their good thermal and chemical stability, excellent physiochemical properties, high specific surface area and abundant nanopores. In this paper, highly porous Co-doped NiO nanorods were successfully synthesized by a simple hydrothermal method. The porous rod-like nanostructures were preserved with the added cobalt dopant ranging from 1 to 5 at% but were broken into aggregated nanoparticles at higher concentrations of additional cobalt. The catalytic activity of Co-doped NiO nanostructures for OER in an alkaline medium was assayed. The 5%Co-NiO sample showed a drastically enhanced activity. This result could originate from the combination of advantageous characteristics of highly porous NiO nanorods such as large surface area and high porosity as well as the important role of Co dopant that could provide more catalytic active sites, leading to an enhanced catalytic activity of the nanocatalyst.
Keywords: nickel oxide, Co dopant, highly porous nanorods, oxygen evolution reaction, synergistic effect
1. Introduction
Hydrogen has been considered as a sustainable energy carrier thanks to its high energy density and environmental benignity [1,2]. Water splitting represents a promising route for the large-scale production of H2. It consists of two half-reactions, namely the anodic oxygen evolution reaction (OER) and cathodic hydrogen evolution reaction [3,4]. The OER has slow kinetics, thus requires a high overpotential to sustain an appropriate reaction rate, because of its multiple electron, multiple proton nature being relevant to O–H bond breaking and O=O bond formation [5–7]. Thus, boosting the rate of OER is critical in order to improve the overall efficiency of water electrolysis for H2 generation [8]. To date, noble metal oxide IrO2 and RuO2 catalysts have been considered as the most efficient OER catalysts [9,10]. However, the extensive application of these materials has been impeded by their rareness and high cost. Thus, efforts are being focused to design and develop efficient oxygen evolution catalysts based on elements that are found abundantly in the Earth's crust [11].
3d transition metal oxide-based nanomaterials including iron oxide, nickel oxide, cobalt oxide and manganese oxide have attracted great interest as fascinating OER catalysts in alkaline medium thanks to their good thermal and chemical stability and excellent physiochemical properties [12–14]. MnO2 nanorod arrays showed good activity and stability for OER in alkaline medium because of the enhancement of the MnO2 activity relating to its nanorod array structures [15]. Ni-Fe2O3 nanoclews exhibited excellent electrocatalytic activity for OER with sustainability and low overpotential due to the atomic-scale synergistic effect arising from Fe and Ni contribution as well as their unique nanostructures promoting highly exposed active catalytic sites [16]. Ultrathin porous Co3O4 nanoplates with large specific surface area, small crystalline size and high porosity possess great catalytic activity for OER, which may be supported by their structural features that provide more surface-active sites and efficient chemical diffusion [17]. The direct growth of dual active site NiCo2O4 catalyst on three-dimensional (3D) porous N-doped graphene to form 3D hybrid nanocomposites with some remarkable characteristics such as 3D conductive network and in- and out-of-plane pores can promote charge transport in electrodes, leading to outstanding catalytic efficiency for OER [18]. The results demonstrate that the advantages of nanostructures such as large specific surface area, the increase of edges and defective sites on the surface and high porosity may enhance the intrinsic properties of electrocatalytic materials, subsequently increasing the catalytic OER activity. Thus, the development of a simple method to tailor the composition and control the morphology of metal oxide nanostructures is of considerable interest to explore novel electrocatalytic properties for OER.
Nickel oxide nanostructures, as a promising p-type semiconductor, have been widely used in catalysis [19,20]. In some circumstances, the single nickel oxide showed better catalytic OER performance in comparison with a cobalt, iron or manganese equivalent [21]. However, the nickel oxide-based OER electrocatalysts are less attractive because of their low efficiency and poor stability [22]. Some strategies have been proposed to improve the electrocatalytic activity of nickel oxide for the OER such as the introduction of N dopants into NiO nanosheets [23], embedding nickel/nickel oxide within a N-graphene matrix [24], designing of rich nickel vacancies within mesoporous nickel oxide [25] as well as employing different morphological nanostructures [26,27]. Despite tremendous efforts, the development of efficient and robust OER catalysts based on nickel oxide nanostructures is still a great challenge and needs further investigation. Herein, we introduce a simple post-synthesis approach to prepare highly porous Co-doped NiO nanorods using Co-doped NiC2O4 nanorods as a precursor. The remarkable features of highly porous NiO nanorods such as large surface area and high porosity may provide better charge transfer, more catalytic active sites on the surface and efficient chemical diffusion. Additionally, the synergic effect of cobalt in the nickel oxide matrix is an essential factor for enhancing catalytic activity for OER.
2. Experimental
2.1. Synthesis of highly porous undoped and Co-doped NiO nanorods
Nickel(II) chloride (NiCl2 · 6H2O) and oxalic acid (H2C2O4 · H2O) were used as the starting materials for the preparation of pure NiC2O4 nanorod precursor and highly porous NiO nanorods. Cobalt(II) nitrate (Co(NO3)2 · 6H2O) was used as a dopant precursor for the synthesis of Co-doped NiC2O4 nanorod precursors and highly porous Co-doped NiO nanorods. The solvent used for the synthesis of these samples was glycerol. All chemicals were purchased from Sigma-Aldrich and used as received without further purification.
Highly porous undoped and Co-doped NiO nanorods were prepared by a simple post-synthesis route. For the synthesis of undoped NiC2O4 nanorods, 2 mmol NiCl2 · 6H2O and 0.7 mmol oxalic acid were dissolved in 9 ml distilled water and 16 ml glycerol by magnetic stirring for 1 h to obtain a clear solution. The solution was transferred to a 50 ml Teflon-lined stainless-steel autoclave, and then aged at 150°C for 12 h. After cooling to room temperature, the solid products were collected by filtration and washed thoroughly by distilled water and ethanol. The precipitates were dried at 80°C for 24 h to obtain NiC2O4 nanorods. The NiC2O4 nanorod precursor was annealed at 500°C for 3 h with a heating rate of 2°C min−1 to form highly porous NiO nanorods. The Co-doped NiC2O4 nanorods were synthesized using a similar process as described for pure NiC2O4. Therein, the concentration of the Co(NO3)2 · 6H2O dopant was 1–7% in molarity as starting sources. The as-synthesized Co-doped NiC2O4 nanorods were transformed into highly porous Co-doped NiO nanorods by a similar annealing process. The resultant nanomaterials were named as X%Co-NiO wherein X represents the amount of Co dopant introduced.
2.2. Material characterizations
X-ray diffraction (XRD) measurements of the samples were conducted using a Bruker D8 Advance X-ray diffractometer. The morphology and chemical composition of the products were analysed by scanning electron microscopy (SEM) and energy-dispersive X-ray spectroscopy (EDS) with a JSM-5300LV instrument and high-resolution transmission electron microscopy (HRTEM), transmission electron microscopy (TEM) as well as selected area electron diffraction (SAED) with a JEOL JEM 1230. Thermogravimetric analysis (TG) and differential thermal analysis (DTA) were conducted using a Labsys TG/DTA-SETARAM simultaneous thermogravimetric analyser with a heating rate of 2°C min−1 in air environment. A Nicolet 6700 FTIR spectrometer was used to record the infrared spectra of the samples. The Brunauer–Emmett–Teller (BET) specific surface area and Barrett–Joyner–Halenda pore size distribution of the highly porous NiO nanorods were obtained from nitrogen adsorption/desorption isotherm measurement.
The extended X-ray absorption fine-structured (EXAFS) spectra of the Co K-edge (7709 eV) and Ni K-edge (8333 eV) were recorded in fluorescence mode using a Ge (220) double-crystal monochromator at the beam line BL8 at Synchrotron Light Research Institute (SLRI), Nakhon Ratchasima, Thailand (at the 1.2 GeV storage ring, with an average beam current of 80–120 mA) [28]. Measurements were performed at room temperature. All data analysis was performed using ATHENA software.
2.3 . Catalytic assay
Three milligrams of highly porous NiO nanorod-based catalyst (pure and Co-doped NiO nanorods) was dispersed in a solvent mixture constituted of 0.2 ml ethanol and 0.8 ml deionized water to prepare catalyst ink. It was then drop-cast onto a clean fluorine-doped tin oxide (FTO)-coated glass electrode with NiO nanorod-based catalyst loading of 100 µg cm−2. The resultant catalyst-loaded FTO electrode was then naturally dried in air prior to use. For example, a photograph of 3%Co-doped NiO nanorod catalyst-loaded FTO electrode is shown in the electronic supplementary material, figure S1.
The activity of the catalyst electrode was assayed in a pH 14 NaOH solution using a conventional three-electrode configuration with the catalyst working electrode, Ag/AgCl in 1 M KCl as reference electrode and a Pt mesh counter electrode. Linear sweep voltammograms were recorded with a potential scan rate of 2 mV s−1. All potentials were quoted against the reversible hydrogen electrode (RHE).
3. Results and discussion
3.1 . Synthesis and characterization of highly porous NiO nanorods with and without Co dopant
As revealed by SEM analysis, the pristine NiC2O4 · 2H2O precursor consists of nanorods with an average diameter of 50–100 nm and length of 0.5–1 µm (electronic supplementary material, figure S2(a)). The respective TEM images indicate that the surface of nanorods is smooth (electronic supplementary material, figure S3(a,b)). Introduction of Co dopant at 1–5 at% did not induce a significant change in the morphology of NiC2O4 · 2H2O nanorods (electronic supplementary material, figure S2(b–f)). However, at higher content of Co, the morphology of the sample was changed significantly. Actually, the 6%Co-NiC2O4 and 7%Co-NiC2O4 samples showed irregular shapes (electronic supplementary material, figure S2(g,h)), and the length of rods decreased in comparison with that of 0–5%Co samples. A large number of nanoparticles were observed clearly in the SEM images of 6%Co-NiC2O4 and 7%Co-NiC2O4 samples.
The calcination resulted in a drastic change of morphology. The calcined products with Co dopant content ranging from 0 to 5 at% consisted of porous rod-like nanostructures with rough surface (figure 1a–f). The TEM images of samples (figure 2a–f) revealed that the nanorods were constructed of nanosized particles as building blocks. The clear contrast between dark and light regions in an individual rod of pure NiO (electronic supplementary material, figure S3(c)) implies the formation of porous nanorods which could be originated from the oxidation reaction of the organic component as well as phase transformation of the precursors into NiO during annealing under air environment [29]. For 6%Co-NiC2O4 and 7%Co-NiC2O4 samples, the nanorods were broken and transformed into particle agglomerates, as observed in SEM (figure 1g,h) and TEM images (figure 2g,h). The shape inhomogeneity may be related to the strain induced in the lattice by the increase of Co concentration or the formation of highly reactive, weak bonding environments when a cobalt cation attaches to a surface site [30].
Figure 1.
SEM images of calcined porous NiO nanorods (a) and Co-doped porous NiO nanorod samples: (b) 1%Co-NiO, (c) 2%Co-NiO, (d) 3%Co-NiO, (e) 4%Co-NiO, (f) 5%Co-NiO, (g) 6%Co-NiO and (h) 7%Co-NiO.
Figure 2.
TEM images of calcined porous NiO nanorods (a) and Co-doped porous NiO nanorod samples: (b) 1%Co-NiO, (c) 2%Co-NiO, (d) 3%Co-NiO, (e) 4%Co-NiO, (f) 5%Co-NiO, (g) 6%Co-NiO and (h) 7%Co-NiO.
The TEM image of a single rod of 5%Co-doped NiO nanorod sample (figure 3a) shows a similar morphology to that of a pure NiO nanorod sample. The nanopores on the doped nanorods were observed significantly, formed by the aggregation of nanoparticles (figure 3b). The HRTEM analysis of 5%Co-doped NiO sample showed its lattice spacing values of 2.084 Å, assigning to the (200) plane face centred cubic NiO, and its SAED pattern corresponding to crystalline nature and phase purity (figure 3c,d).
Figure 3.
TEM image (a), HRTEM images (b,c) and SAED pattern (d) of 5%Co-doped NiO nanorods.
In order to substantiate the formation of porous NiO nanorods under the annealing process, the thermal characterization of the precursor NiC2O4 nanorods was analysed by TG-DTA. Two weight loss steps were observed clearly in TG-DTA curves (electronic supplementary material, figure S4(a)). The first step with 9.04% weight loss in the temperature range of 30–230°C can be attributed to the evaporation of intercalated water molecules to form anhydrous oxalate. On further increasing the temperature, the second step showing ca 44.49% of weight loss in the range of 230–500°C with a sharp exotherm at 324°C in DTA curves was observed. It is assignable to the decomposition reaction of anhydrous nickel oxalate into nickel oxide (equation (3.2)) [31–33]. No other peak was observed beyond 500°C, implying the complete decomposition of precursor NiC2O4 · 2H2O nanorods into porous NiO nanorods. The thermal decomposition process can be explained by the following equations [32]:
| 3.1 |
and
| 3.2 |
The XRD patterns of precursor samples are shown in the electronic supplementary material, figure S4(b). The XRD pattern of undoped sample shows diffraction peaks at 22.6°, 30.4°, 35.5°, 40.9°, 43.8°, 47.7°, 48.7° and 37.73° that are perfectly indexed to (002), (), (402), (021), (), (), () and (023) crystal planes of monoclinic nickel oxalate dihydrate (JCPDS card no. 25-0582). In the Co-doped samples, characteristic peaks of cobalt oxalate, cobalt oxide, cobalt hydroxide or other impurities are not visible. The XRD patterns of calcined samples are shown in figure 4. These patterns are well matched to the standard cubic nickel oxide (JCPDS card no. 04-0835), indicating that the NiC2O4 precursor was completely transformed into NiO phase at 500°C. No obvious peaks of impurities were present in the XRD patterns. Slight shifts in XRD peak position of (200) crystal planes of the cubic NiO phase of Co-doped NiO samples towards lower angle were observed (electronic supplementary material, figure S5), demonstrating the incorporation of Co dopant atoms in the host matrix [34]. The similar radius of Co (0.79 Å) and Ni (0.83 Å) atoms makes Co atoms substitute Ni atoms in lattices of NiO [35], leading to Co ions being highly dispersed in the matrix of NiO.
Figure 4.
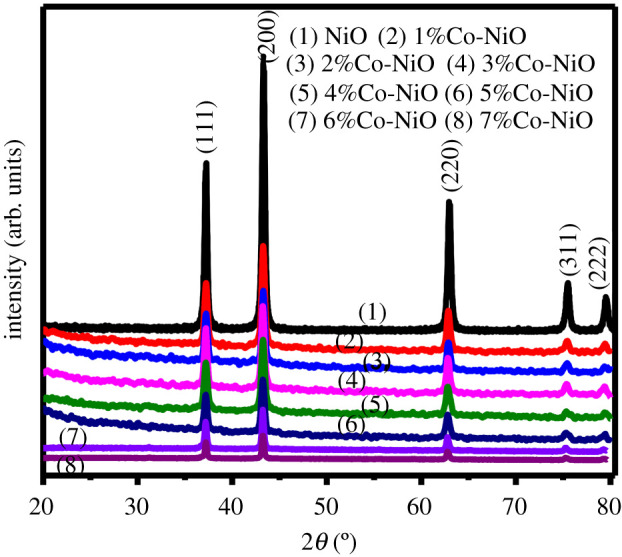
XRD patterns of calcined samples 0–7%Co-NiO.
In the FTIR analysis, the NiC2O4 and Co-doped NiC2O4 samples with cobalt dopant range of 1–7% show characteristic bending vibration bands of nickel oxalate dihydrate (electronic supplementary material, figure S6(a,b)). For 0–5%Co-NiC2O4 samples (electronic supplementary material, figure S5(a)), the absorption band at about 3400 cm−1 was assigned to the O–H stretching vibration of hydrate and absorbed water [36]. The peak at approximately 1627 cm−1 could be related to both antisymmetric C=O stretching vibration and bending vibration of hydration water [37]. The symmetric C=O stretching vibration band was found at 1357 cm−1. In addition, the absorption bands at 1317 cm−1 and 829 cm−1 were assigned to the C–O and C–C stretching vibrations of coordinated oxalic acid, respectively [38]. The Ni–O bending of oxalate moiety was assigned to the absorption band at about 491 cm−1 [39]. The FTIR spectra of 6%Co-NiC2O4 and 7%Co-NiC2O4 showed similar features to that of NiC2O4 (electronic supplementary material, figure S6(b)), excepting slight shifts were observed for the symmetric C=O, C–O, C–C and Ni–O bending vibration bands. After the calcination process, the feature absorption bands of NiC2O4-based samples disappeared in FTIR spectra of calcined samples (electronic supplementary material, figure S6(c,d)). For 0–5%Co-NiO samples (electronic supplementary material, figure S6(c)), the strong band at about 416 cm−1 was assignable to the stretching vibration of Ni–O [40]. The broad absorption band centred at 3400 cm−1 and the band at approximately 1627 cm−1 were indexed to O–H and H–O–H bending vibration modes [41]. When the Co-dopant concentration was increased, the Ni–O stretching vibration band was slightly shifted (electronic supplementary material, figure S6(d)), which may be related to the defect states of NiO structure [42]. The FTIR spectra further confirmed the successful conversion of undoped and Co-doped NiC2O4 · 2H2O nanorods to undoped and Co-doped porous NiO nanorods, respectively.
In order to demonstrate clearly the incorporation of Co within Co-NiC2O4 precursors and Co-NiO catalysts, the elemental composition of products was analysed by EDS as shown in the electronic supplementary material, figures S7 and S8. As compared to NiC2O4, the Co-doped NiC2O4 samples exhibited the presence of a significant amount of Co along with Ni, O and C as a result of cobalt ion incorporation during the synthesis. Cobalt/nickel atomic ratio is very close to the theoretically calculated value in all samples. After the calcining process, the pure NiO confirms the presence of Ni and O elements (electronic supplementary material, figure S9(a)) with a Ni/O atomic ratio of approximately 1 : 1 (table 1). The doped samples showed the existence of Ni, O and Co, indicating the successful doping (electronic supplementary material, figure S9(b–h)). The Co/Ni atomic ratio is basically consistent with the Co-doping concentration in starting experiment (table 1).
Table 1.
Composition of calcined samples measured from EDS.
| elements | samples |
|||||||
|---|---|---|---|---|---|---|---|---|
| NiO |
1%Co-NiO |
2%Co-NiO |
3%Co-NiO |
|||||
| wt% | at% | wt% | at% | wt% | at% | wt% | at% | |
| O K | 23.30 | 52.72 | 23.44 | 52.89 | 23.35 | 52.77 | 23.55 | 53.05 |
| Co K | — | — | 0.83 | 0.51 | 1.43 | 0.88 | 2.31 | 1.41 |
| Ni K | 76.70 | 47.28 | 75.73 | 46.6 | 75.22 | 46.35 | 74.14 | 45.54 |
| total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
| samples |
||||||||
| 4%Co-NiO |
5%Co-NiO |
6%Co-NiO |
7%Co-NiO |
|||||
| elements | wt% | at% | wt% | at% | wt% | at% | wt% | at% |
| O K | 22.49 | 51.56 | 22.3 | 51.29 | 22.04 | 50.92 | 22.13 | 51.04 |
| Co K | 3.05 | 1.90 | 3.82 | 2.39 | 4.45 | 2.79 | 5.02 | 3.15 |
| Ni K | 74.46 | 46.54 | 73.88 | 46.32 | 73.51 | 46.29 | 72.85 | 45.81 |
| total | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 | 100.00 |
The textural features of the 2%Co-NiO, 5%Co-Ni and 6%Co-NiO samples and that of the undoped NiO were explored by nitrogen adsorption/desorption isotherm analysis. Results are presented in figure 5. In all samples, the N2 adsorption/desorption curves, which possess the same shapes, are typical type IV isotherms with an H1 type loop hysteresis, confirming a mesoporous structure in the nanomaterials [43]. The BET surface area of NiO nanorods was 33 m2 g−1, which represented a significant increment in comparison with the NiC2O4 · 2H2O nanorods (SBET approx. 17 m2 g−1) (electronic supplementary material, figure S9). The BET specific surface areas of 2%Co-NiO and 5%Co-NiO rod-like nanostructures were 35 and 32 m2 g−1, respectively, which were slightly changed in comparison with that of pure NiO nanorods. While the specific surface area of 6%Co-NiO sample slightly decreased (28 m2 g−1), which might be because the highly porous nanorod was broken and agglomerated nanoparticles were formed after the annealing process. The EXAFS spectrum of the Ni-K edge recorded for the 5%Co-NiO nanorod sample (figure 6a, red trace) showed identical features to those of a NiO reference crystal (figure 6a, black trace). The Fourier transform spectral analysis in the k range between 2.5 and 11.5 Å−1 showed a similar Ni–Ni distance between the 5%Co-NiO and the NiO reference sample (figure 6b). However, a small alternation was observed in the shorter range, being assignable to Ni–O interaction. For the 5%Co-NiO sample, the Co-K edge spectrum showed similar features to that of CoO [44,45] (figure 6c,d, red trace). The signal at radial distance of 2.5 Å can be assigned to Co–Ni distance while Co–O bonds are found in shorter distances of 1–2 Å. From the available data, we conclude highly porous NiO nanorods and highly porous Co-doped nanorods were successfully prepared. We then investigated the contribution of the Co dopant to the electrochemical catalytic activity of the NiO nanorods for the OER in alkaline solution.
Figure 5.
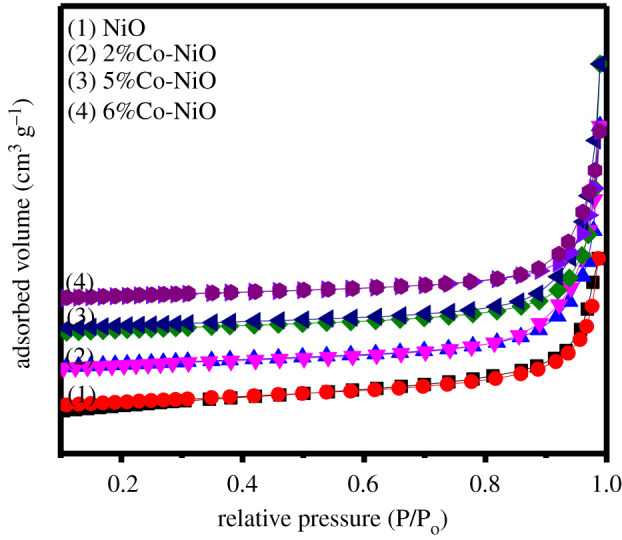
N2 adsorption/desorption isotherms of pure highly porous NiO nanorods, and of 2%Co-NiO, 5%Co-NiO and 6%Co-NiO samples.
Figure 6.
Ni-K edge EXAFS spectra of 5%Co-NiO as-prepared, 5%Co-NiO after catalysis and NiO reference (a) and Fourier transform of the experimental EXAFS spectra in K space (b); Co-K edge EXAFS spectra of 5%Co-NiO as-prepared, 5%Co-NiO after catalysis and CoO reference (c) and Fourier transform of the experimental EXAFS spectra in K space (d).
3.2. Catalytic activity of Co-doped highly porous NiO nanorods
The catalytic activity of NiO nanorod-loaded FTO electrode was assayed in an alkaline solution being saturated with oxygen. A NiO nanorod electrode without Co dopant showed a negligible catalytic current density (figure 7a, red trace). The introduction of Co dopant altered the electrochemical property and therefore the catalytic activity of NiO nanorods (figure 7a). The 5%Co-NiO sample shows an oxidation peak at 1.5 V and a shoulder at 1.4 V versus RHE prior to the catalytic event at onset potential of 1.55 V versus RHE, representing an onset overpotential of 320 mV. This onset overpotential is comparable to that determined for a FeNiOx catalyst [46]. The pre-catalytic oxidation peak can be assigned to CoIII/CoII redox couple. This oxidation event shifted as a function of the amount of Co dopant introduced, likely due to different incorporation within the crystal structure of NiO nanorods. A variation of CoIII/CoII oxidation peak as a function of Co dopant content has been reported for the case of CoFeOOH catalyst [47]. In the kinetic limiting regime, 5%Co-NiO showed the lowest value of Tafel slope of 89 mV decade−1 (figure 7b). To generate a catalytic current density of 10 mA cm−2, this catalyst required an overpotential of 450 mV. We then pick up the catalytic current density generated at 1.7 V versus RHE as a descriptor to track the contribution of Co dopant to the catalytic activity. It can be seen that 5% of Co dopant shows the optimal contribution (figure 7c). Given that the 5%Co-NiO sample has a slightly lower surface area than the pristine NiO and 2%Co-NiO samples, this result clearly evidences the role of Co dopant in promoting the catalytic activity of NiO nanorods.
Figure 7.
O2-evolving catalytic activity of NiO nanorods without and with different Co dopant content assayed in a NaOH electrolyte solution. (a) Linear sweep voltammograms recorded with a potential scan rate of 2 mV s−1. (b) Tafel plots. (c) Plot of catalytic current collected at 1.7 V versus RHE as a function of amount of Co dopant. (d) Chronoamperometry recorded at 1.7 V versus RHE.
We then investigated the robustness of the 5%Co-doped NiO nanorod catalyst during the catalytic operation. To do so, the catalyst electrode was held at 1.7 V versus RHE (figure 7d) in the electrolyte solution for 3 h. The catalytic current density showed an obvious degradation over time. This could be linked to the physical detachment of catalyst powder from the electrode surface (together with the O2 bubbles generated) or the degradation of the catalyst itself during the catalytic operation. To examine the latter possibility, the morphology and chemical nature of the 5%Co-NiO catalyst after catalysis were examined by SEM and EXAFS. No obvious change in the nanorod morphology was observed as revealed by SEM analysis (electronic supplementary material, figure S10). Furthermore, EDS of all Co-doped NiO nanostructures after catalysis (electronic supplementary material, figure S11) indicated that the cobalt was not washed away during the reaction, confirming that Co was tightly bound in the NiO crystal lattice. EXAFS analysis in the Ni-K and Co-K edges showed similar features for the as-prepared 5%Co-NiO nanorods and the same sample after being operated for 3 h (figure 6a,b, red and blue traces). This suggests intact chemical environments of the Ni and Co within the 5%Co-NiO nanorod catalyst after the catalysis. Only small deviations were observed in the short-range interactions, corresponding to Ni–O and Co–O bonds (figure 6c,d).
In general, the catalytic activity of metal oxide nanostructured electrocatalysts can be improved by controlling the morphologies and doping by metal because they possess an increased active surface area and synergistic interaction [48,49]. The role of Co doping within catalysts for enhanced OER has been reported for MoS2 [50], RuO2 [51], TiO2 [52] and SnS2 [53], attributed to some effects such as additional catalytic active sites and modifying the electronic structure of catalysts. In this report, the optimal cobalt content added into NiO nanorods was about 5 at% to modify their electronic structures without breaking their nanorod structures. Thus, we speculate that the 5%Co-doped NiO nanorods showed an enhanced activity for OER thanks to the combination of the porous nanoarchitecture and the effect of Co dopant that could provide more abundant catalytic active sites.
4. Conclusion
Highly porous Co-doped NiO nanorods with high specific surface area and abundant nanopores were synthesized by a facile post-synthesis route using Co-doped NiC2O4 nanorods as a precursor. With a reasonable content of Co dopant added into NiO crystal structure (5%Co-NiO sample), the highly porous NiO nanorods are still maintained. The obtained nanocomposite showed a significant increase of catalytic activity for OER, which may be attributed to a combination of highly porous NiO nanoarchitecture and the effect of Co dopant. The result demonstrates the important role of highly porous nanostructures and doping composition of electrocatalysts for enhancing catalytic activity and providing efficient and inexpensive catalysts for OER.
Supplementary Material
Acknowledgement
We thank the SLRI BL8 staff for their experimental support.
Contributor Information
Nguyen Duc Cuong, Email: nguyenduccuong@hueuni.edu.vn.
Phong D. Tran, Email: tran-dinh.phong@usth.edu.vn.
Data accessibility
Our data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g79cnp5p8.
Authors' contributions
N.D.C. provided the research plan, assisted in scientific discussion, performed the experiments, analysed the data and contributed to writing the manuscript. T.D.T performed the experiments, prepared the figures and contributed to writing the manuscript. Q.T.N. helped in performing experiments and assisted in manuscript writing. H.V.M.H and T.T.H. helped in performing the experiments, prepared figures and assisted in the manuscript preparation. D.T.Q and W.K. analysed the data and assisted in scientific discussion. P.D.T. provided the research plan, assisted in scientific discussion and wrote the manuscript. All authors reviewed the manuscript.
Competing interests
We declare we have no competing interests.
Funding
This work was supported by the Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant no. 103.02-2019.43.
References
- 1.Song J, Wei C, Huang Z, Liu C, Zeng L. 2020A review on fundamentals for designing oxygen evolution electrocatalysts. Chem. Soc. Rev. 49, 2196-2214. ( 10.1039/C9CS00607A) [DOI] [PubMed] [Google Scholar]
- 2.Schäfer H, et al. 2015Surface oxidation of stainless steel: oxygen evolution electrocatalysts with high catalytic activity. ACS Catal. 5, 2671-2680. ( 10.1021/acscatal.5b00221) [DOI] [Google Scholar]
- 3.Velikokhatnyi OI, Kadakia K, Datta MK, Kumta PN. 2013Fluorine-doped IrO2: a potential electrocatalyst for water electrolysis. J. Phys. Chem. C 117, 20 542-20 547. ( 10.1021/jp308947h) [DOI] [Google Scholar]
- 4.Cuong ND, Quang DT. 2020Progress through synergistic effects of heterojunction in nanocatalysts: review. Vietnam J. Chem. 58, 434-463. ( 10.1002/vjch.202000072) [DOI] [Google Scholar]
- 5.Tang F, et al. 2017Strong surface hydrophilicity in Co-based electrocatalysts for water oxidation. ACS Appl. Mater. Interfaces 9, 26 867-26 873. ( 10.1021/acsami.7b07088) [DOI] [PubMed] [Google Scholar]
- 6.Barforoush JM, Seuferling TE, Jantz DT, Song KR, Leonard KC. 2018Insights into the active electrocatalytic areas of layered double hydroxide and amorphous nickel–iron oxide oxygen evolution electrocatalysts. ACS Appl. Energy Mater. 1, 1415-1423. ( 10.1021/acsaem.8b00190) [DOI] [Google Scholar]
- 7.Indra A, Menezes PW, Zaharieva I, Dau H, Driess M. 2020Detecting structural transformation of cobalt phosphonate to active bifunctional catalysts for electrochemical water-splitting. J. Mater. Chem. A 8, 2637-2643. ( 10.1039/C9TA09775A) [DOI] [Google Scholar]
- 8.Sadiek IM, Mohammad AM, El-Shakre ME, El-Deab MS. 2012Electrocatalytic activity of nickel oxide nanoparticles-modified electrodes: optimization of the loading level and operating pH towards the oxygen evolution reaction. Int. J. Hydrogen Energy 37, 68-77. ( 10.1016/j.ijhydene.2011.09.097) [DOI] [Google Scholar]
- 9.Hu W, Wang Y, Hu X, Zhou Y, Chen S. 2012Three-dimensional ordered macroporous IrO2 as electrocatalyst for oxygen evolution reaction in acidic medium. J. Mater. Chem. 22, 6010-6016. ( 10.1039/c2jm16506f) [DOI] [Google Scholar]
- 10.Tsuji E, Imanishi A, Fukui KI, Nakato Y. 2011Electrocatalytic activity of amorphous RuO2 electrode for oxygen evolution in an aqueous solution. Electrochim. Acta 56, 2009-2016. ( 10.1016/j.electacta.2010.11.062) [DOI] [Google Scholar]
- 11.Du J, Liu G, Li F, Zhu Y, Sun L. 2019Iron–salen complex and Co2+ ion-derived cobalt–iron hydroxide/carbon nanohybrid as an efficient oxygen evolution electrocatalyst. Adv. Sci. 6, 1900117. ( 10.1002/advs.201900117) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song F, Bai L, Moysiadou A, Lee S, Hu C, Liardet L, Hu X. 2018Transition metal oxides as electrocatalysts for the oxygen evolution reaction in alkaline solutions: an application-inspired renaissance. J. Am. Chem. Soc. 140, 7748-7759. ( 10.1021/jacs.8b04546) [DOI] [PubMed] [Google Scholar]
- 13.Wu X, Scott K. 2011CuxCo3−xO4 (0 ≤ x < 1) nanoparticles for oxygen evolution in high performance alkaline exchange membrane water electrolysers. J. Mater. Chem. 21, 12 344-12 351. ( 10.1039/c1jm11312g) [DOI] [Google Scholar]
- 14.Yeo BS, Bell AT. 2011Enhanced activity of gold-supported cobalt oxide for the electrochemical evolution of oxygen. J. Am. Chem. Soc. 133, 5587-5593. ( 10.1021/ja200559j) [DOI] [PubMed] [Google Scholar]
- 15.Chen S, Zhai T, Lu XH, Zhang MZ, Li ZY, Xu CW, Tong Y. 2012Large-area manganese oxide nanorod arrays as efficient electrocatalyst for oxygen evolution reaction. Int. J. Hydrogen Energy 37, 13 350-13 354. ( 10.1016/j.ijhydene.2012.06.059) [DOI] [Google Scholar]
- 16.Samanta A, Das S, Jana S. 2019Doping of Ni in α-Fe2O3 nanoclews to boost oxygen evolution electrocatalysis. ACS Sustain. Chem. Eng. 7, 12 117-12 124. ( 10.1021/acssuschemeng.9b01208) [DOI] [Google Scholar]
- 17.Gomez IJ, Arnaiz B, Cacioppo M, Arcudi F, Prato M. 2018Nitrogen-doped carbon nanodots for bioimaging and delivery of paclitaxel. J. Mater. Chem. B 6, 5540-5548. ( 10.1039/C8TB01796D) [DOI] [PubMed] [Google Scholar]
- 18.Chen S, Qiao SZ. 2013Hierarchically porous nitrogen-doped graphene–NiCo2O4 hybrid paper as an advanced electrocatalytic water-splitting material. ACS Nano 7, 10 190-10 196. ( 10.1021/nn404444r) [DOI] [PubMed] [Google Scholar]
- 19.Dang D, Duc N, Long P, Thi N, Anh N. 2019Facile post-synthesis and gas sensing properties of highly porous NiO microspheres. Sens. Actuators A 296, 110-120. ( 10.1016/j.sna.2019.07.014) [DOI] [Google Scholar]
- 20.Zhao Y, Zhang X, Xu X, Zhao Y, Zhou H, Li J, Jin H. 2016Synthesis of NiO nanostructures and their catalytic activity in the thermal decomposition of ammonium perchlorate. CrystEngComm 18, 4836-4843. ( 10.1039/C6CE00627B) [DOI] [Google Scholar]
- 21.Zhuang L, Ge L, Yang Y, Li M, Jia Y, Yao X, Zhu Z. 2017Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 29, 1606793. ( 10.1002/adma.201606793) [DOI] [PubMed] [Google Scholar]
- 22.Zhou X, Xia Z, Zhang Z, Ma Y, Qu Y. 2014One-step synthesis of multi-walled carbon nanotubes/ultra-thin Ni(OH)2 nanoplate composite as efficient catalysts for water oxidation. J. Mater. Chem. A 2, 11 799-11 806. ( 10.1039/C4TA01952K) [DOI] [Google Scholar]
- 23.Xia B, Wang T, Jiang X, Li J, Zhang T, Xi P, Gao D, Xue D. 2019N+-ion irradiation engineering towards the efficient oxygen evolution reaction on NiO nanosheet arrays. J. Mater. Chem. A 7, 4729-4733. ( 10.1039/C9TA00023B) [DOI] [Google Scholar]
- 24.Faisal SN, et al. 2018A quadrafunctional electrocatalyst of nickel/nickel oxide embedded N-graphene for oxygen reduction, oxygen evolution, hydrogen evolution and hydrogen peroxide oxidation reactions. Sustain. Energy Fuels 2, 2081-2089. ( 10.1039/c8se00068a) [DOI] [Google Scholar]
- 25.Liu X, Zhai ZY, Chen Z, Zhang LZ, Zhao XF, Si FZ, Li JH. 2018Engineering mesoporous NiO with enriched electrophilic Ni3+ and O− toward efficient oxygen evolution. Catalysts 8, 310. ( 10.3390/catal8080310) [DOI] [Google Scholar]
- 26.Babar PT, Lokhande AC, Gang MG, Pawar BS, Pawar SM, Kim JH. 2018Thermally oxidized porous NiO as an efficient oxygen evolution reaction (OER) electrocatalyst for electrochemical water splitting application. J. Ind. Eng. Chem. 60, 493-497. ( 10.1016/j.jiec.2017.11.037) [DOI] [Google Scholar]
- 27.Silva VD, Simões TA, Grilo JPF, Medeiros ES, Macedo DA. 2020Impact of the NiO nanostructure morphology on the oxygen evolution reaction catalysis. J. Mater. Sci. 55, 6648-6659. ( 10.1007/s10853-020-04481-1) [DOI] [Google Scholar]
- 28.Klysubun W, Tarawarakarn P, Thamsanong N, Amonpattaratkit P, Cholsuk C, Lapboonrueng S, Chaichuay S, Wongtepa W. 2020Upgrade of SLRI BL8 beamline for XAFS spectroscopy in a photon energy range of 1–13 keV. Radiat. Phys. Chem. 175, 108145. ( 10.1016/j.radphyschem.2019.02.004) [DOI] [Google Scholar]
- 29.Son LL, Van Thi TT, Trung KQ, Van Hieu N, Trung DD, Cuong ND. 2019Facile and scalable fabrication of highly porous Co3O4 and α-Fe2O3 nanosheets and their catalytic properties. J. Electron. Mater. 48, 7897-7905. ( 10.1007/s11664-019-07616-6) [DOI] [Google Scholar]
- 30.Mehra S, Bergerud A, Milliron DJ, Chan EM, Salleo A. 2016Core/shell approach to dopant incorporation and shape control in colloidal zinc oxide nanorods. Chem. Mater. 28, 3454-3461. ( 10.1021/acs.chemmater.6b00981) [DOI] [Google Scholar]
- 31.Tian WG, Wang PP, Ren L, Sun GB, Sun LN, Yang K, Wei BQ, Hu CW. 2007Controllable synthesis of NiC2O4·2H2O nanorods precursor and applications in the synthesis of nickel-based nanostructures. J. Solid State Chem. 180, 3551-3559. ( 10.1016/j.jssc.2007.09.036) [DOI] [Google Scholar]
- 32.Said AEA, El-wahab MMA, Soliman SA, Goda MN. 2014Synthesis and characterization of nano CuO-NiO mixed oxides. Nanosci. Nanoeng. 2, 17-28. ( 10.13189/nn.2014.020103) [DOI] [Google Scholar]
- 33.Vaidya S, Rastogi P, Agarwal S, Gupta SK, Ahmad T, Antonelli AM, Ramanujachary KV, Lofland SE, Ganguli AK. 2008Nanospheres, nanocubes, and nanorods of nickel oxalate: control of shape and size by surfactant and solvent. J. Phys. Chem. C 112, 12 610-12 615. ( 10.1021/jp803575h) [DOI] [Google Scholar]
- 34.Mishra AK, Das D. 2011Effect of transition metal (Fe, Co) doping in NiO nanostructures prepared by a wet chemical route. AIP Conf. Proc. 1349, 1139-1140. ( 10.1063/1.3606265) [DOI] [Google Scholar]
- 35.Wu Z, Wang X, Huang J, Gao F. 2017A Co-doped Ni–Fe mixed oxide mesoporous nanosheet array with low overpotential and high stability towards overall water splitting. J. Mater. Chem. A 6, 167-178. ( 10.1039/C7TA07956G) [DOI] [Google Scholar]
- 36.Małecka B, Małecki A, Drozdz-Cieśla E, Tortet L, Llewellyn P, Rouquerol F. 2007Some aspects of thermal decomposition of NiC2O4·2H2O. Thermochim. Acta 466, 57-62. ( 10.1016/j.tca.2007.10.010) [DOI] [Google Scholar]
- 37.Lin Z, et al. 2016Morphology-controllable synthesis and thermal decomposition of Ag and Ni oxalate for Ag-Ni alloy electrical contact materials. Mater. Des. 108, 640-647. ( 10.1016/j.matdes.2016.06.123) [DOI] [Google Scholar]
- 38.Wang L, Zhang R, Jiang Y, Tian H, Tan Y, Zhu K, Yu Z, Li W. 2019Interfacial synthesis of micro-cuboid Ni0.55 Co0.45 C2O4 solid solution with enhanced electrochemical performance for hybrid supercapacitors. Nanoscale 11, 13 894-13 902. ( 10.1039/C9NR03790J) [DOI] [PubMed] [Google Scholar]
- 39.Behnoudnia F, Dehghani H. 2014Anion effect on the control of morphology for NiC2O4·2H2O nanostructures as precursors for synthesis of Ni(OH)2 and NiO nanostructures and their application for removing heavy metal ions of cadmium(II) and lead(II). Dalton Trans. 43, 3471-3478. ( 10.1039/c3dt52049h) [DOI] [PubMed] [Google Scholar]
- 40.Son LL, Cuong D, Van Thi T, Hieu T. 2019Konjac glucomannan-templated synthesis of three-dimensional NiO nanostructures assembled from porous NiO nanoplates for gas sensors. RSC Adv. 9, 9584-9593. ( 10.1039/C9RA00285E) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.López-Ortiz A, Collins-Martínez VH, Hernández-Escobar CA, Flores-Gallardo SG, Zaragoza-Contreras EA. 2008Protection of NiO nanoparticles against leaching in acid medium by grafting of polyacrylic acid. Mater. Chem. Phys. 109, 306-310. ( 10.1016/j.matchemphys.2007.11.031) [DOI] [Google Scholar]
- 42.Kumar V, Singh K, Sharma J, Kumar A, Vij A, Thakur A. 2017Zn-doped SnO2 nanostructures: structural, morphological and spectroscopic properties. J. Mater. Sci. Mater. Electron. 28, 18 849-18 856. ( 10.1007/s10854-017-7836-z) [DOI] [Google Scholar]
- 43.Online VA, Wang Q, Zhao X, Yuan B. 2016Cross-linked porous α-Fe2O3 nanorods as high performance anode materials for lithium ion batteries. RSC Adv. 6, 97 385-97 390. ( 10.1039/C6RA22034G) [DOI] [Google Scholar]
- 44.Ha DH, Moreau LM, Honrao S, Hennig RG, Robinson RD. 2013The oxidation of cobalt nanoparticles into Kirkendall-Hollowed CoO and Co3O4: the diffusion mechanisms and atomic structural transformations. J. Phys. Chem. C 117, 14 303-14 312. ( 10.1021/jp402939e) [DOI] [Google Scholar]
- 45.Gawai UP, Gaikwad DK, Bodke MR, Khawal HA, Pandey KK, Yadav AK, Jha SN, Bhattacharyya D, Dole BN. 2019Doping effect on the local structure of metamagnetic Co doped Ni/NiO:GO core–shell nanoparticles using X-ray absorption spectroscopy and the pair distribution function. Phys. Chem. Chem. Phys. 21, 1294-1307. ( 10.1039/C8CP05267K) [DOI] [PubMed] [Google Scholar]
- 46.Loos S, Zaharieva I, Chernev P, Lißner A, Dau H. 2019Electromodified NiFe alloys as electrocatalysts for water oxidation: mechanistic implications of time-resolved UV/Vis tracking of oxidation state changes. ChemSusChem 12, 1966-1976. ( 10.1002/cssc.201802737) [DOI] [PubMed] [Google Scholar]
- 47.Burke MS, Kast MG, Trotochaud L, Smith AM, Boettcher SW. 2015Cobalt–iron (oxy)hydroxide oxygen evolution electrocatalysts: the role of structure and composition on activity, stability, and mechanism. J. Am. Chem. Soc. 137, 3638-3648. ( 10.1021/jacs.5b00281) [DOI] [PubMed] [Google Scholar]
- 48.Jung S, McCrory CCL, Ferrer IM, Peters JC, Jaramillo TF. 2016Benchmarking nanoparticulate metal oxide electrocatalysts for the alkaline water oxidation reaction. J. Mater. Chem. A 4, 3068-3076. ( 10.1039/C5TA07586F) [DOI] [Google Scholar]
- 49.Sidhureddy B, Dondapati JS, Chen A. 2019Shape-controlled synthesis of Co3O4 for enhanced electrocatalysis of the oxygen evolution reaction. Chem. Commun. 55, 3626-3629. ( 10.1039/C8CC10194A) [DOI] [PubMed] [Google Scholar]
- 50.Xiong Q, Zhang X, Wang H, Liu G, Wang G, Zhang H, Zhao H. 2018One-step synthesis of cobalt-doped MoS2 nanosheets as bifunctional electrocatalysts for overall water splitting under both acidic and alkaline conditions. Chem. Commun. 54, 3859-3862. ( 10.1039/C8CC00766G) [DOI] [PubMed] [Google Scholar]
- 51.Tian Y, et al. 2020A Co-doped nanorod-like RuO2 electrocatalyst with abundant oxygen vacancies for acidic water oxidation. iScience 23, 100756. ( 10.1016/j.isci.2019.100756) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbas M, ul Haq T, Arshad SN, Zaheer M. 2020Fabrication of cobalt doped titania for enhanced oxygen evolution reaction. Mol. Catal. 488, 110894. ( 10.1016/j.mcat.2020.110894) [DOI] [Google Scholar]
- 53.Jiang M, Huang Y, Sun W, Zhang X. 2019Co-doped SnS2 nanosheet array for efficient oxygen evolution reaction electrocatalyst. J. Mater. Sci. 54, 13 715-13 723. ( 10.1007/s10853-019-03856-3) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Our data are available from the Dryad Digital Repository: https://doi.org/10.5061/dryad.g79cnp5p8.







