Abstract
Humans are highly adept at differentiating, regulating, and responding to their emotions. At the core of all these functions is emotional awareness: the conscious feeling states that are central to human mental life. Disrupted emotional awareness–a subclinical construct commonly referred to as ‘alexithymia’–is present in a range of psychiatric and neurological disorders and can have a deleterious impact on functional outcomes and treatment response. This chapter is a selective review of the current state of the science on alexithymia. We focus on two separate but related issues: i) the functional deficits associated with alexithymia and what they reveal about the importance of emotional awareness for shaping normative human functioning, and ii) the neural correlates of alexithymia and what they can inform us about the biological bases of emotional awareness. Lastly, we outline challenges and opportunities for alexithymia research, focusing on measurement issues and the potential utility of formal computational models of emotional awareness for advancing the fields of clinical and affective science.
Keywords: Alexithymia, Emotions, Emotional Awareness, Emotional Communication, Neurological Disorders, Interoception
Introduction
Emotions are salient internal states that can promote adaptive behavioral outputs (Darwin, 1872). Feeling anxious may inspire a tactical escape from an uncomfortable social situation, feeling unhappy might drive one to make a change in their career path, and feeling pleasure inspires another bite of chocolate ice cream. Recent decades have seen a rise in the use of modern neuroscientific tools to study emotional processes in humans and other species, leading to several–often conflicting–perspectives on the neurobiology of emotion and its emoted behavioral sequelae (Damasio, 1999; LeDoux, 2000; Dolan, 2002; Critchley, 2003; Lindquist et al., 2012; Seth and Friston, 2016; Adolphs, 2017; Barrett, 2017; Berridge, 2019). Across perspectives, there is a general consensus that emotion can be subdivided into a crude implicit valence and arousal signals that are generated primarily via subcortical and autonomic circuits, and a more complex explicit dimension (i.e., emotional awareness, or ‘feelings’) primarily computed via cortical circuits. For a thoughtful opinion on this two-system framework in the context of fear versus anxiety, see LeDoux and Pine (2016). The majority of experimental paradigms in human affective science have focused on subcortical and autonomic reactivity to emotional stimuli and fewer studies have been conducted that attempt to better understand the biology and function of conscious feelings. As a result, the neural circuits necessary for generating emotional awareness remain unclear and there is a lack of consensus on the fundamental question of whether emotional awareness represents an epiphenomenon or a functional dimension that mobilizes adaptive cognitive-behavioral responses to felt emotions (Adolphs et al., 2019).
The current chapter focuses on alexithymia, a construct that provides a unique opportunity to better understand the neural mechanisms and functions of emotional awareness and expression. Alexithymia is characterized by an impaired ability to be aware of, explicitly identify, and describe one’s feelings (Nemiah et al., 1976). Whereas implicit (e.g., physiological arousal) and explicit (e.g., self-reported emotion intensity) indicators of emotional reactivity tend to be highly associated in healthy volunteers, in alexithymia there is a decoupling of implicit and explicit emotional responses (Papciak et al., 1985; Stone and Nielson, 2001; Nielson and Meltzer, 2009; Gaigg et al., 2018). Multiple theoretical models of the alexithymia construct exist (Preece et al., 2017) but here we focus on the dominant Toronto model wherein alexithymia can be subdivided into an externally-oriented cognitive style (i.e., a tendency to focus on superficial information and avoid internal, affect-related thought), difficulty identifying feelings (i.e., diminished emotional awareness), and difficulty describing feelings (i.e., impaired expression of emotions through words; Bagby et al., 1994a). In the absence of neurological or psychiatric diseases or their treatment, alexithymia changes little over the lifespan in non-clinical populations, suggesting it is a relatively stable psychological trait (Luminet et al., 2001; Picardi et al., 2005; Rufer et al., 2006). Alexithymia can improve over the course of intervention for depressive symptoms and changes in alexithymia often correlate with the efficacy of pharmacological or psychotherapeutic treatment for depression (Özsahin et al., 2003; Spek et al., 2008; Cravello et al., 2009; Bressi et al., 2017). Much like trait anxiety or depression, alexithymia can be viewed as a dimension ranging from low-to-high in the general population and extending into the clinically pathological range (Parker et al., 2008). We will focus this chapter primarily on alexithymia in psychiatric and neurological illness. First, we review the transdiagnostic1 presence of alexithymia across affective, developmental, and neurological disorders, and – by extension – argue for the functional importance of emotional awareness for shaping adaptive functioning. Next, we will summarize extant evidence on the neural substrates of alexithymia and related constructs, summarizing how this work can provide insight into the neurobiology of emotional awareness. Lastly, we outline some challenges faced by alexithymia researchers and set the agenda for future research in this field, which should help to advance current understanding of alexithymia and its pathophysiology.
Alexithymia as a Transdiagnostic Clinical Symptom
Emotional Disorders & Alexithymia
Alexithymia was first described in patients seeking treatment for psychosomatic symptoms. A significant portion of patients experiencing somatic symptoms such as pain or fatigue also described being in a state of personal distress but had a striking inability to clearly articulate their feelings to clinicians (Sifneos, 1973; Nemiah et al., 1976). One might expect this blunted emotional awareness to protect individuals with alexithymia from negative feelings and perhaps to reduce anxiety and depression. However, the limited differentiation of emotional states in alexithymia seems to actually cause patients great difficulty with regulating and resolving negative affect. As a result, the prevalence of affective disorders is increased in this population (Lumley, 2000; Honkalampi et al., 2018).
The alexithymia construct has been criticized for strongly overlapping with depression, particularly given the high correlation between self-reported alexithymia and depression in healthy control participants (e.g. r=0.60; (Bagby et al., 1986)). Despite this association, subsequent factor analyses have indicated that the related constructs of alexithymia and depression are dissociable in both healthy and clinical populations (Parker et al., 1991; Marchesi et al., 2000). A recent meta-analysis of 19 studies (sampling N=3,572 participants) did suggest a moderate relationship between alexithymia and depressive symptoms, driven by associations between depression and the two affective dimensions of alexithymia (difficulty identifying feelings, and difficulty describing feelings; Li et al., 2015). Categorical studies of patients with major depressive disorder (MDD) suggest a high prevalence of comorbid clinically-significant alexithymia relative to control participants (Leweke et al., 2012). When compared to patients with non-alexithymic MDD, this comorbidity appears to be associated with a particularly harmful MDD symptom profile, characterized by more severe depression and increased rates of psychoticism and phobia (Honkalampi et al., 2007; Kim et al., 2008; Onur et al., 2013). Patients with generalized anxiety disorder, panic disorder, and posttraumatic stress disorder also demonstrate higher rates of clinically-significant alexithymia relative to the general population (Shipko et al., 1983; Zeitlin and McNally, 1993; Frewen et al., 2008a; Onur et al., 2013). Finally, there is some evidence for an association between alexithymia and obsessive-compulsive disorder (OCD). In contrast to other anxiety phenotypes, which are associated with disrupted emotional awareness per se (i.e., difficulty identifying and describing feelings), OCD appears to be more strongly associated with an externally-oriented cognitive style (Bankier et al., 2001).
Emotion dysregulation
The development of adaptive regulation strategies for modulating the trajectory of ongoing emotions is central to many treatments for emotional disorders (Campbell-Sills and Barlow, 2007). A recent re-conceptualization of alexithymia described it as a primary deficit in the key emotion regulation processes of attending to (i.e., a deficit in internally-directed cognition) and appraising (i.e., difficulty identifying and describing feelings) affective states (Preece et al., 2017). Several studies have found impaired emotion regulation abilities in individuals with elevated alexithymia (Barrett et al., 2001; Swart et al., 2009). Correspondingly, alexithymia has been found to have a negative impact on outcomes of therapy for anxiety and mood disorders (Kosten et al., 1992; Leweke et al., 2009; Ogrodniczuk et al., 2011). Furthermore, difficulties with emotion regulation that are associated with alexithymia appear to be associated with increased rates of addictive behaviors, e.g. pathological gambling and excessive mobile phone and internet use (Elmas et al., 2016; Schimmenti et al., 2017; Gao et al., 2018) and substance addiction (Stasiewicz et al., 2012; Betka et al., 2018).
Development of Alexithymia & Autism Spectrum Disorder (ASD)
In general, there have been relatively few studies on the epidemiology of alexithymia, making it difficult to make definitive claims about the milieu of cultural and environmental factors that shape risk for the development of alexithymia. One study suggests that there are cross-cultural differences in alexithymia between European American and Asian (both Asian American and Malaysian) college students (Berenbaum and Raghavan, 2003), while another suggests that alexithymia levels are elevated in rural relative to urban populations (Joukamaa et al., 2007). Whereas some studies have found small effects of male sex and reduced years of education on alexithymia (Joukamaa et al., 2007; Levant et al., 2009), others have failed to observe such differences (Parker et al., 1989). Given alexithymia is common in patients with anxiety and depression, factors that contribute to their development might be expected to influence risk for the development of alexithymia. Anxiety and depression have complex etiologies involving environmental and genetic factors (Kendler, 1992). Socioeconomic status is one environmental variable that has been shown to shape risk for the development of affective disorders in youth (Deng et al., 2006) but, in contrast, does not appear to shape one’s risk for developing alexithymia (Parker et al., 1989). In contrast, several studies have provided evidence that adverse childhood experiences and associated emotional trauma–which increase risk for anxiety and mood disorders (Deng et al., 2006; Sareen et al., 2013)–are associated with increased levels of alexithymia that continue into adulthood (Kench and Irwin, 2000; Kooiman et al., 2004; Bermond et al., 2008; Aust et al., 2013). Therefore, the development of alexithymia can be influenced by early childhood adversity.
Alexithymia is highly prevalent in several heritable neurodevelopmental disorders, suggesting that it may have, at least in part, a genetic component. In particular, individuals with autism spectrum disorder (ASD) have increased rates of clinically-significant alexithymia. A recent meta-analysis suggested that approximately 50% of individuals with ASD meet criteria for comorbid clinically-significant alexithymia, roughly 10 times the rate in the general population (Kinnaird et al., 2019). ASD has long been argued to comprise a fundamental defect in emotion. Impaired recognition of emotional faces (Uljarevic and Hamilton, 2013), decreased empathy (Baron-Cohen and Wheelwright, 2004), and blunted self-described experience of emotions (Losh and Capps, 2006) have been reported. These phenomena could simply reflect, at least in part, attenuated autonomic responses to affectively salient stimuli. However, while studies of autonomic responses of individuals with ASD in response to stimuli from the International Affective Picture System do demonstrate some complex patterns of differences from control subjects, they do not support the concept that those with ASD have substantially defective autonomic responses (Bölte et al., 2008; Mathersul et al., 2013). Unfortunately, the vast majority of studies documenting aberrant social and internal processing of emotional content in ASD have not measured comorbid levels of alexithymia. When alexithymia is assessed in such studies, it tends to explain a significant portion of variance in neural and behavioral assays of emotional reactivity across both ASD and typical development (e.g. Silani et al., 2008; Bird et al., 2010). In fact, a recent body of work from Bird and colleagues suggests that most classically-reported emotion deficits in ASD are in fact driven by heightened comorbid levels of alexithymia in ASD relative to typical development (for a review, see Bird and Cook, 2013). These studies suggest that deficits in emotional face recognition (e.g., Cook et al., 2013), empathy (e.g., Santiesteban et al., 2020), and autonomic reactivity (e.g., Gaigg et al., 2018) in ASD are driven by heightened levels of alexithymia in this population, and are independent of core ASD symptoms (i.e., difficulties with communication and social interaction, and the presentation of restricted and repetitive patterns of behavior, interests, and activities). In fact, alexithymia levels are likely to drive increased rates of clinically-significant anxiety in ASD (Maisel et al., 2016), which is among the most common and impairing psychiatric comorbidities in this population (comorbidity estimates range from 40–80% (Simonoff et al., 2008; van Steensel et al., 2011). Given that inherited genetic variants are thought to be the dominant mechanism driving the development of ASD (Wang et al., 2009; Constantino et al., 2013), it is likely that clinically-significant alexithymia–a common and impairing feature of the ASD phenotype–may also have a genetic basis. There are few well-powered genetic investigations of alexithymia, but a recent genome-wide association study sampling common variants in 585 healthy individuals revealed several potential candidate genes that may influence the risk of developing alexithymia over the course of neurodevelopment (Mezzavilla et al., 2015). Therefore, it seems likely that, like other affective disorders, a complex mixture of environmental and genetic factors contributes to developmental alexithymia. Regardless of its genetic or environmental origin, developmental alexithymia is a robust transdiagnostic indicator of the emergence of psychopathology in adolescence (Weissman et al., 2020).
Acquired Alexithymia in Neurological Disorders
Whereas developmental alexithymia has been the focus of much alexithymia research, research on ‘acquired alexithymia’ caused by brain damage or dysfunction in neurological disorders has been increasing (Ricciardi et al., 2015). Acquired alexithymia refers to the presence of reduced emotional awareness in patients following the onset of some acquired disease or trauma than induces a brain disorder. At the outset, it must be noted that the field is lacking a prospective study demonstrating that any neurological disorder involves a true pre- to postmorbid change in alexithymia (Ricciardi et al., 2015). Although this is a fundamental limitation in the literature on neurological disorders and alexithymia, it seems more likely that acquired alexithymia is driven by a neurological mechanism, rather than reflecting incremental amplification of premorbid alexithymia.
Traumatic Brain Injury (TBI)
Closed-head traumatic brain injury (cTBI)–e.g. via a motor vehicle accident or contact sports injury–is the most common form of brain injury and the most well-studied with respect to acquired alexithymia. cTBI involves widespread damage from the impact site to the deep cerebral white matter. These diffuse injuries cause complex neuropsychiatric sequelae (Sayer, 2012) and clinically-significant alexithymia is often prevalent in cTBI survivors. Alexithymia prevalence estimates in cTBI range from 30 to 60%, compared to 10 to 12% in non-brain-injured control participants (Koponen et al., 2005; Henry et al., 2006; Wood and Williams, 2007). Acquired alexithymia in cTBI appears to be independent of injury severity, presenting in mild, moderate, and severe cTBI patients (Koponen et al., 2005; Wood and Williams, 2007). Given the diffuse nature of the brain injury of cTBI, these studies have not enabled the identification of candidate brain regions or circuits that–if damaged–might cause acquired alexithymia symptoms. Such work is critical as it has the potential to elucidate the necessary neural substrates of emotional awareness. In a later section, Neurobiology of Emotional Awareness, we will consider cases of acquired alexithymia following focal, penetrating TBI that do enable such inferences.
Parkinson’s Disease (PD)
PD–a neurodegenerative disorder associated with profound loss of dopaminergic neurons in the substantia nigra (Dickson, 2012)–was originally characterized by a cluster of motor symptoms, but more recently there has been a surge in research directed at understanding the range of social and emotional impairments that progress over the course of PD. In particular, many studies have documented elevated rates of clinically-significant alexithymia in Parkinson’s disease. A recent review suggested that the prevalence of clinical alexithymia in PD is twice that in neurologically-healthy matched controls (Assogna et al., 2016). Patients with PD also present with a loss of motivation to obtain rewards (Ang et al., 2018) and an inability to learn from punishment when on dopaminergic medication (Frank et al., 2007), making it difficult to determine whether PD is associated with an aberrant implicit affective valence signal, or impaired emotional awareness per se. An examination of the specific functional role of the nigrostriatal pathway in affective processing will help to clarify whether alexithymia is likely to be a primary or secondary symptom in PD, a topic we will return to in the Neurobiology of Emotional Awareness section.
Neurovascular Diseases and Other Neurodegenerative Disorders.
In addition to TBI and PD, several neurovascular and neurodegenerative disorders have been thought to potentially cause acquired alexithymia. For example, patients with right brain stroke have higher rates of alexithymia than patients with left brain stroke (Spalletta et al., 2001; Bossu et al., 2009). Elevated levels of alexithymia have also been shown in patients with multiple sclerosis, semantic and frontotemporal dementia, Alzheimer’s disease, corticobasal syndrome, and Huntington’s disease (Bodini et al., 2008; Sturm and Levenson, 2011; Trinkler et al., 2013). However, whereas studies of alexithymia in TBI and PD have included a contrast between a well-characterized patient group and a neurologically healthy control group, most studies on alexithymia in stroke and other neurodegenerative disorders have not included controls. These studies have also typically collected small samples and very few of them have adequately controlled for potential confounding variance associated with comorbid depression (Ricciardi et al., 2015). Therefore, beyond TBI and PD, further research establishing the prevalence of acquired alexithymia in other neurological disorders is warranted.
Functional Consequences of Alexithymia in Neurological Disorders
Alexithymia has a disruptive effect on a variety of important outcomes in patients with neurologic disorders. In both PD and cTBI, clinically-significant alexithymia is associated with reduced quality of life and increased caregiver burden with a disruptive impact on interpersonal relationships (Williams and Wood, 2013; Klietz et al., 2020). The negative impact of alexithymia on functional outcomes has been particularly well-demonstrated in a series of cTBI studies by Williams, Wood, and colleagues, suggesting that alexithymia in cTBI is associated with decreased emotional empathy (Williams and Wood, 2010), increased somatic complaints and personal distress (Wood et al., 2009; Wood and Doughty, 2013), as well as increased suicidal ideation (Wood et al., 2010). In each of these studies, alexithymia–and most frequently the difficulty identifying feelings subscale–was found to account for variance in these outcomes above that accounted for by anxiety and depression. The disruptive impact of alexithymia on emotional empathy in cTBI has been replicated by an independent group (Neumann et al., 2014). An association between alexithymia and degraded interpersonal relationships has also been demonstrated in patients with neurodegenerative disorders (Sturm and Levenson, 2011). Finally, pathological reward-guided decision making has been linked to increased alexithymia in patients with neurological disorders. For example, in penetrating TBI alexithymia has been associated with impaired value-based decision making (Hogeveen et al., 2019) and alexithymia in PD has been associated with impulsivity-compulsivity disorders (Goerlich‐Dobre et al., 2014). These studies indicate the importance of intact emotional awareness for making adaptive value-based decisions. Collectively, studies of patients with psychiatric and neurological disorders provide evidence that feelings are not a mere epiphenomenon of human emotion. Instead, emotional awareness plays a functional role in shaping adaptive behaviors and, when disrupted by alexithymia, can drive a host of negative affective and interpersonal outcomes.
Neurobiology of Emotional Awareness
Several neural circuits process different forms of information that are critical for the generation of normal human emotional awareness. Here, we selectively review some of the subcortical, cingulo-insular, and prefrontal networks that seem to be the most important in mediating emotional awareness (Figure 1). For each of these networks, we will highlight evidence from human lesion-mapping studies bearing on their role in encoding emotional awareness.
Figure 1.
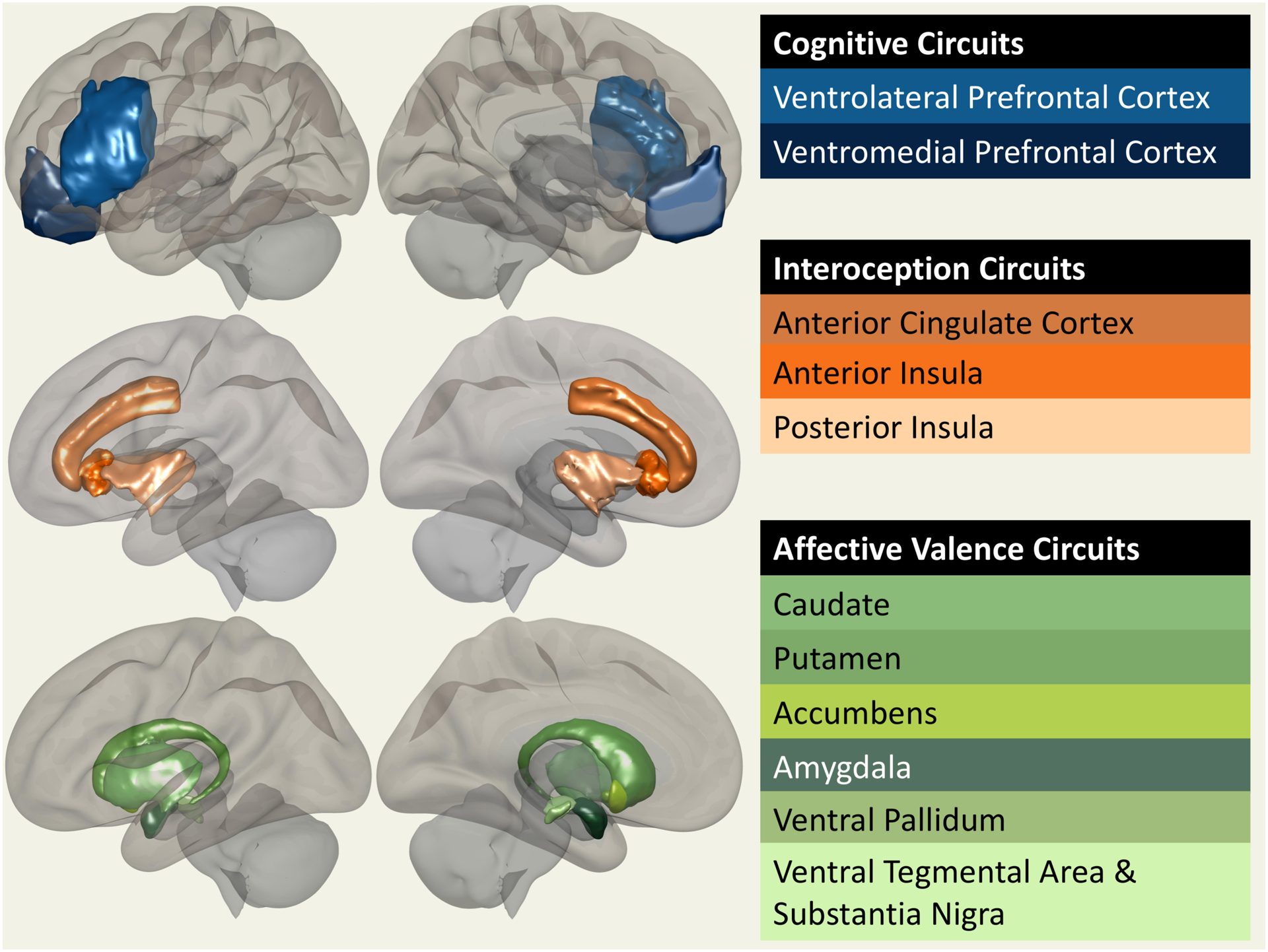
Surface display of the various structures that play a role in either coloring (e.g. valence circuits) or encoding (interoceptive and cognitive circuits) emotional awareness states.
Subcortical Systems
The explicit feeling states that are disrupted in alexithymia are shaped by affective valence signals. The targets of mesocorticolimbic dopaminergic projections play an important role in the modulation of emotional feelings and alexithymia is often associated with impairments in these systems. Dopaminergic input from the ventral tegmental area (VTA) to the nucleus accumbens (NAc) and activity-dependent modulation of amygdala (AMY)-NAc circuits are essential for driving motivated behaviors and likely play a role in encoding the reward value of outcomes (Cador et al., 1989; Berridge and Robinson, 1998; Stuber et al., 2011). Further, NAc and AMY have been implicated in negative valence encoding, with different subregions within NAc and AMY constituting modules for positive or negative valence encoding (Tye, 2018; Berridge, 2019). Further complicating the picture, it has also been suggested that these modules themselves can change between positive/negative affective modes depending on their inputs (Berridge, 2019). Dopamine projections from the substantia nigra to the dorsal striatum have been linked to reward learning and motivated behavior (Kravitz et al., 2012) and have been shown to play a causal role in shaping negative valence processing (Bouchet et al., 2018). Despite extensive work linking these subcortical dopaminergic circuits to affective valence and motivated behavior, the evidence linking any of these pathways to the subjective experience of emotion is limited. Some work suggests that hedonic impact–inferred from the presence of emotional facial expressions–is encoded in modules within NAc in rodents (Berridge and Kringelbach, 2015). However, it is unlikely that these facial expressions are indicative of emotional awareness per se, which may be unique to humans (Steklis and Lane, 2013; Smith et al., 2020).
The emotional sequelae of midbrain, striatal, and amygdala lesions or degeneration in humans can help to determine whether these subcortical substrates are necessary for emotional awareness. Lesions to the dorsal striatum in humans produce significant impairments in cognitive flexibility and reversal learning tasks (Cools et al., 2002; Bellebaum et al., 2008), likely reflecting connectivity with dorsolateral prefrontal cortex, but the degree to which these lesions impact emotional awareness and alexithymia has not been investigated. Given the high prevalence of alexithymia in PD, it is tempting to infer that the substantia nigra is necessary for emotional awareness in humans. However, there is ample evidence that PD is also associated with downstream effects at cortical sites including prefrontal cortex and insula (Kikuchi et al., 2001; Cools et al., 2002). Therefore, it is likely that alexithymia in PD represents a secondary consequence of the loss of dopaminergic input to fronto-opercular structures, rather than a primary consequence of disrupted emotional awareness encoding in nigrostriatal circuits.
Recent meta-analytic evidence has linked dysfunction of the amygdala to alexithymia scores in humans. Individuals with high alexithymia demonstrate hypoactivation of the amygdala in response to negatively valenced stimuli (van der Velde et al., 2013). Similarly, recognition of fearful facial expressions, or the experience of fear in response to negatively valenced visual stimuli, are both disrupted in Urbach-Wiethe Disease (UWD) patients with bilateral amygdala lesions (Adolphs, 2008; Feinstein et al., 2011). However, another classic lesion study suggested that day-to-day emotional experience was relatively intact in a patient with bilateral amygdala lesions (Anderson and Phelps, 2000). Pharmacologic manipulations (e.g., isoproterenol) and CO2 inhalation induce similar interoceptively-mediated experiences of anxiety and panic in UWD patients with bilateral amygdala lesions (Feinstein et al., 2013; Khalsa et al., 2016). Therefore, alexithymia and changes in emotional experience in neurological disorders associated with mesostriatal or amygdala dysfunction (e.g. PD and UWD) are likely to be associated with a basic impairment in the pathways that generate affective valence, rather than a disruption of emotional awareness per se. Thus, these subcortical circuits do not appear to play a central role in emotional awareness.
Prefrontal Cortex
Prominent theories of human consciousness argue that its contents are encoded in prefrontal cortex (PFC; Knight and Grabowecky, 1995; Lau and Rosenthal, 2011), and several regions within PFC likely contribute to processes that shape emotional awareness. Here, we focus on two regions that play a necessary role in emotional awareness: ventromedial prefrontal cortex (vmPFC2) and ventrolateral prefrontal cortex (vlPFC3). Tract tracing studies in nonhuman primates suggest that medial orbitofrontal regions homologous to human vmPFC are part of a monosynaptically connected network including both NAc and AMY (Amaral et al., 1992; Petrides and Pandya, 2007) and appear to encode value signals for shaping motivating behavior (Costa and Averbeck, 2020). In humans, meta-analytic functional neuroimaging evidence suggests that stimulus value is encoded in vmPFC during decision making (Bartra et al., 2013; Clithero and Rangel, 2014) and evidence from patients with focal lesions suggests that damage to this circuit causes patients to make pathological value-based decisions (Bechara et al., 2000; Hogeveen et al., 2017; Reber et al., 2017). These results suggest a role for vmPFC in value-based decision making. Additionally, neuroimaging studies have implicated vmPFC-AMY circuits in fear extinction and other forms of emotion regulation (Phelps et al., 2004; Braunstein et al., 2017), as well as emotion recognition and affective theory-of-mind (Shamay-Tsoory et al., 2005; Wolf et al., 2014). It has been speculated that vmPFC may contain several functionally-distinct modules for value-based decision making, emotion regulation, emotion recognition, and affective theory-of-mind (Hiser and Koenigs, 2018), though this hypothesis has not been tested within a single neuroimaging or lesion-symptom mapping study.
Clearly, vmPFC is critical to a variety of emotional processes. However, it is not clear whether or not the vmPFC is necessary for generating emotional awareness. There is human neuroimaging evidence suggesting that vmPFC regional cerebral blood-flow at rest is correlated with individual differences in the experience of negative affect (Zald et al., 2002) and damage to vmPFC can lead to decreased experience of negative affect (Hornak et al., 2003). The relatively small number of human neuroimaging studies that have assayed alexithymia suggest blunted vmPFC recruitment in individuals with high alexithymia who make less altruistic decisions during social decision making (FeldmanHall et al., 2013). In addition, there is increased vmPFC recruitment during trauma imagery in patients with post-traumatic stress disorder (PTSD), who have heightened alexithymia (Frewen et al., 2008b). Additionally, a recent voxel-based lesion-symptom mapping (VLSM) study of patients with penetrating TBI found that damage to several regions of vmPFC is associated with increased levels of acquired alexithymia–specifically driven by increased difficulty identifying feelings (Hogeveen et al., 2019). Therefore, it seems likely that some of the rich affective information encoded in vmPFC does enter into consciousness, and that damage to vmPFC is one contributor to low emotional awareness. vlPFC has also been linked to alexithymia. Damage to bilateral inferior frontal gyri (IFG), as defined by VLSM, has been associated with increased difficulty identifying feelings. Overlapping lesions involving left IFG caused deficits in performance on a language production task within the same cohort of patients (Boston Naming test; Hobson et al., 2018). It has been argued that language is a key ingredient to the construction and differentiation of emotional experiences (Lindquist, 2017) and therefore, disrupted language abilities may be one contributor to acquired alexithymia in patients with neurological disorders (Hobson et al., 2019). Many patients with Broca’s aphasia following left inferior frontal gyrus damage experience affective sequelae (Gainotti, 1997), which is unsurprising given the high degree of comorbidity between alexithymia and internalizing problems such as depression and anxiety.
Interoception Circuits
Theories of human emotion have long argued for a central role of physiological reactivity in shaping our feelings (James, 1884, 1894; Bard, 1934). Accordingly, neural circuits involved in ‘interoception’–i.e., perception of the current state of the viscera (e.g. heart rate, perspiration, etc.)– have been suggested to play a role in emotional awareness (Craig, 2002, 2009; Medford and Critchley, 2010; Damasio et al., 2013). In particular, the insula and pregenual anterior cingulate cortex (pACC) have both been implicated in interoceptive and emotional awareness, and both neuroimaging studies in healthy volunteers and lesion-mapping studies in patients have linked these structures to alexithymia.
Cytoarchitectonic and connectivity analyses suggest the primate insula is organized along a functional posterior-to-anterior axis. Afferent interoceptive inputs (i.e., visceral, gustatory, nociceptive, and vestibular) arrive at postero-dorsal sectors of the insula (pINS) and this information is integrated in the antero-ventral insula (aINS), which connects bidirectionally with cognitive and affective circuitry in the vlPFC, pACC, AMY, and NAc (Taylor et al., 2009; Benarroch, 2019; Palomero-Gallagher et al., 2019). aINS is therefore well-situated to contribute to emotional awareness by receiving ascending interoceptive inputs and communicating with structures encoding higher-order representations of goals, attention, and motor plans (namely, vlPFC and pACC; (Medford and Critchley, 2010; Gu et al., 2013). Human aINS is disproportionately large relative to aINS in other primate species (Bauernfeind et al., 2013); increased computational complexity enabled by cortical expansion of aINS and connected structures may provide essential support for the seemingly unique human capacity for emotional awareness (Smith et al., 2020). In line with this view, individuals with high alexithymia demonstrated blunted aINS recruitment when processing emotionally-salient images (Kano et al., 2003; Silani et al., 2008; Bird et al., 2010; Reker et al., 2010). aINS volumes are also reduced and aINS structural connectivity is aberrant in individuals with high alexithymia (Borsci et al., 2009; Ihme et al., 2013; Bernhardt et al., 2014). Perhaps most compellingly, penetrating TBI patients with aINS lesions demonstrate a significant increase in total alexithymia scores relative to patients with minimal aINS damage, patients with no aINS damage, or non-brain-injured controls. This effect is primarily driven by scores on the increased difficulty identifying feelings dimension of alexithymia (Hogeveen et al., 2016). Acquired alexithymia resulting from insular lesions can have significant and deleterious impacts on reward valuation abilities (Hogeveen et al., 2019) and the endorsement of altruistic beliefs (Chau et al., 2018), providing further evidence for the functional importance of emotional awareness for decision making and social cognition. Therefore, there is good reason to suggest that aINS is necessary for generating emotional awareness.
aINS and pACC are among the most commonly co-activated brain regions in the functional neuroimaging literature. It has been argued that they work together to form a salience network for shifting between interoceptive and exteroceptive information processing modes (Seeley et al., 2007; Menon, 2011; Uddin, 2014). However, the specific functional involvement of pACC in emotional awareness and how aINS and ACC interact to shape emotional awareness remain unclear. Functional neuroimaging studies have implicated increased pACC recruitment in a levels of emotional awareness scale that assays the complexity of the words one uses to describe feelings (Lane et al., 1998; McRae et al., 2008). A recent meta-analysis of functional neuroimaging findings related to alexithymia supported this finding, suggesting that a large region in ACC spanning subgenual, pregenual, and supragenual sectors is recruited more strongly in response to affective stimuli in individuals with high alexithymia relative to individuals with low alexithymia (van der Velde et al., 2013). Structural imaging findings have been less consistent for ACC, with some studies finding increased (Gündel et al., 2004; Goerlich-Dobre et al., 2015) and others finding decreased (van der Velde et al., 2014) pregenual and supragenual ACC volumes in patients with high alexithymia. A recent meta-analysis did not reliably implicate pACC or other ACC subregion volumetric differences in alexithymia (Xu et al., 2018). Perhaps the most compelling evidence for ACC involvement in emotional awareness comes from the change in emotional traits that accompany lesions to this region. Patients with cingulotomy show reduced tension and anger as a result of perilesional damage to cingulate cortex immediately adjacent to the cingulum bundle (Cohen et al., 2001), and a case study of a patient with a right anterior cingulate infarct found clinically-significant alexithymia symptoms, including a profound impairment in identifying and naming affective pictures along with difficulty connecting bodily sensations with their associated feeling states (Schäfer et al., 2007). Collectively, aINS and ACC (in particular, pACC) appear to play an essential role in emotional awareness and demonstrate aberrant functional recruitment in alexithymic individuals. Damage to these structures can lead to acquired alexithymic symptoms.
One possible mechanism by which aINS and pACC aberrations drive alexithymia is via impaired ‘interoceptive awareness.’ Functional neuroimaging tasks designed to probe interoceptive awareness (e.g. heartbeat tracking) have reliably evoked activation in the aINS. The degree of interoceptive awareness on these tasks and aINS recruitment are associated with conscious emotional experience (Critchley et al., 2004; Zaki et al., 2012). Further, patients with pACC damage demonstrate diminished scaling of cardiovascular activity as a function of different levels of cognitive effort, suggesting pACC plays a role in facilitating autonomic responses (Critchley et al., 2003). This line of work has led to the suggestion that aINS has a necessary role in integrating ascending visceral sensory data to generate interoceptive awareness, while pACC is critical for coordinating appropriate motor and non-motor responses to interoceptive events and plays a role in the formation and regulation of interoceptive experience (Medford and Critchley, 2010). Alexithymia has been associated with a multidomain deficit in interoceptive awareness involving cardiac, respiratory, muscular, and gustatory systems (Murphy et al., 2018). Therefore, further research on aINS and pACC recruitment and functional connectivity during different types of interoceptive processing will be essential to further clarifying their respective contributions to interoceptive and emotional awareness.
Interhemispheric Connectivity
Patients who underwent cerebral commissurotomy for treatment of intractable epilepsy often presented with “split-brain syndrome,” whereby tasks that required interhemispheric information transfer were impaired. For example, when presenting a visual stimulus selectively to the left hemifield, split-brain patients verbally reported that they did not see the stimulus, despite an intact ability to perform other types of manual or nonverbal responses that demonstrated that the stimulus had in fact been seen (Sperry et al., 1969). Given that this deficit was principally characterized by an impaired ability to verbally report information presented to the right hemisphere, Bogen and colleagues theorized that split-brain patients may also be impaired at generating words to express their feelings (i.e., may present with higher levels of alexithymia). In support of this view, commissurotomized patients demonstrated more factual and superficial responses in a semi-structured interview about their emotions, as well as when describing emotionally-salient stimuli (Hoppe and Bogen, 1977; TenHouten et al., 1986). Reduced alpha-band phase synchrony between hemispheres in these patients was associated with a more externally-oriented verbal description of their feelings (TenHouten et al., 1987). Acquired alexithymia in commissurotomized patients suggests that interhemispheric connectivity is necessary for normative emotional awareness. Further research is required on lateralization of emotional functioning but it is likely that interhemispheric connectivity facilitates emotional awareness by enabling individuals to simultaneously attend to and verbalize their feelings (cf., Hobson et al., 2019).
Challenges and Opportunities
Given the fact that emotional awareness is uniquely accessible to the individual, self-report has been the standard approach in alexithymia research “(e.g. Toronto Alexithymia Scale; Bagby et al., 1994). Self-report measures of alexithymia have yielded tremendous insight into the basics of the alexithymia construct, leading to several viable models of its underlying dimensions and estimates of its prevalence in the population and of its association with various psychiatric and neurological illnesses (Luminet et al., 2018). However, it has often been suggested that self-report measures reflect confounding traits that are independent of the actual constructs under investigation (Buchanan, 2016), and there may be a fundamental limitation on the degree to which self-report can reflect true underlying behavioral dispositions (Wilson and Dunn, 2004; Averbeck et al., 2013). As a result, total reliance on self-report measures limits our ability to gain insight into the neurocomputational mechanisms underlying emotional awareness and its disruption in alexithymia.
Promising approaches to moving beyond self-report in the study of emotional awareness and alexithymia include adopting objective performance-based measures, elucidating the association between objective and subjective assays, and the development of formalized computational models of emotional awareness. The Levels of Emotional Awareness Scale (LEAS) has been developed to provide a performance-based assay of trait emotional awareness abilities. It relies on participants’ abilities to describe their anticipated feelings and those of another person in response to a series of vignettes (Lane et al., 1990). Each response is coded with respect to the degree to which it reflects five different levels of emotional awareness of increasing complexity (1 = awareness of physiological cues; 2 = undifferentiated emotion; 3 = single differentiated emotion label; 4 = blends of emotions; and 5 = ability to distinguish self- and other-referential emotions). The LEAS appears to demonstrate convergent validity with self-report measures: individuals with high self-reported alexithymia score lower on the LEAS than individuals with low self-reported alexithymia (Lane et al., 1996). However, the LEAS provides a performance-based assay that might be more objective than self-report measures. Some LEAS variance likely reflects individual differences in general cognitive functioning rather than emotional awareness per se. This measure would not be effective in populations of people who may have emotionally rich mental lives but have neurological or psychiatric disorders characterized by limited verbal abilities (e.g. individuals with intellectual disability). Another approach for moving beyond pure reliance on self-report in the field is to quantify the magnitude of the correlation between subjective and objective assays. For example, individuals with a strong correlation between self-reported emotional intensity and evoked heart rate or skin conductance responses during emotionally salient events are likely to have clear insight into their own affective state, whereas a weak correlation may reflect diminished insight into one’s emotions. Adopting this approach, a recent study found that individuals with increased alexithymia demonstrated a reduced association between psychophysiological reactivity and self-reported emotion intensity (Gaigg et al., 2018). Another study found that trial-wise changes in subjective confidence on an emotional recognition memory test were related to objective moment-to-moment heart rate variability dynamics (Legrand et al., 2020). Finally, aberrant aINS resting-state functional connectivity with the default-mode network in adolescents with ASD was associated with a greater discrepancy between self- and parent-reported4 internalizing, suggesting that this neural correlate is associated with decreased insight into one’s own emotions in adolescents with ASD (Hogeveen et al., 2018). Therefore, probing the magnitude of the association between subjective and objective measures of one’s emotions could provide a more objective assay of trait emotional awareness than traditional self-report measures.
Lastly, the construct of emotional awareness, despite its intuitive appeal to clinical and affective scientists, remains poorly specified at the neurocomputational level. This lack of a clear mechanistic specification is a limitation for clinical work on alexithymia as it makes it difficult to move towards a precision medicine approach for facilitating emotional awareness in psychiatric and neurological populations (cf., Insel, 2014). In recent decades, there has been tremendous growth in the field of computational neuroscience, wherein mathematically formal, neurologically-plausible theories are developed and tested with the ultimate goal of establishing data-driven diagnoses and treatments for mental health disorders (Ferrante et al., 2019). A recent computational model of emotional awareness, incorporating a formal Markov Decision Process, has yielded clear and testable hypotheses about seven distinct neurocomputational mechanisms that might drive disrupted emotional awareness in clinical populations (Smith et al., 2019). Some of the mechanisms that map particularly clearly onto clinical presentations of alexithymia include somatically-biased prior expectations (e.g. individuals with psychosomatic disorders and high alexithymia might misread a panic attack for a heart attack), coarse emotion concept acquisition (e.g. individuals who experience parental neglect in childhood may have diminished social learning of emotion categories), and reduced emotional metacognition (e.g., individuals with an externally-oriented cognitive style may conceptually understand emotions, but may ignore the presence of their own emotional states and focus more on external events; Smith et al., 2019). Adoption of this mathematically formalized conceptualization of emotional awareness could facilitate the development of testable hypotheses that help to elucidate the functional neurobiology of emotional awareness. Additionally, this approach could facilitate clinical assessment and the adoption of personalized mental health interventions to strengthen emotional awareness.
Conclusion
We began this chapter by pointing out that conscious feeling states are central to determining how we perceive social behavior and determine our agency. While we have identified crucial neural structures mediating these states and some of the disorders that lead to abnormalities of awareness of feelings, we also note that the modeling of conscious feeling states is likely to further advance the science of conscious emotional states and their management by cognitive processes.
Footnotes
Here and elsewhere, transdiagnostic refers to clinical phenotypes that present across traditional psychiatric and neurologic diagnostic categories.
vmPFC: Comprising subgenual anterior cingulate, medial orbitofrontal cortex, and sectors of medial frontal cortex ventral to the anterior commissure
vlPFC: Comprising bilateral inferior frontal gyri and lateral orbitofrontal cortex
Note: In this study, parent-reports were in better agreement with gold standard structured clinical interviews than self-report, suggesting the former represented a more objective symptom measure.
References
- Adolphs R (2008) Fear, faces, and the human amygdala. Current Opinion in Neurobiology 18:166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R (2017) How should neuroscience study emotions? by distinguishing emotion states, concepts, and experiences. Soc Cogn Affect Neurosci 12:24–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adolphs R, Mlodinow L, Barrett LF (2019) What is an emotion? Current Biology 29:R1060–R1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkanen A, Carmichael ST (1992) Anatomical organization of the primate amygdaloid complex. In: The Amygdala: Neurobiological Aspects of Emotion, Memory, and Mental Dysfunction, pp 1–66. New York: Wiley-Liss. [Google Scholar]
- Anderson AK, Phelps EA (2000) Expression without recognition: Contributions of the human amygdala to emotional communication. Psychological Science 11:106–111. [DOI] [PubMed] [Google Scholar]
- Ang Y-S, Lockwood PL, Kienast A, Plant O, Drew D, Slavkova E, Tamm M, Husain M (2018) Differential impact of behavioral, social, and emotional apathy on Parkinson’s disease. Ann Clin Transl Neurol 5:1286–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assogna F, Cravello L, Orfei MD, Cellupica N, Caltagirone C, Spalletta G (2016) Alexithymia in Parkinson’s disease: A systematic review of the literature. Parkinsonism & Related Disorders 28:1–11. [DOI] [PubMed] [Google Scholar]
- Aust S, Härtwig EA, Heuser I, Bajbouj M (2013) The role of early emotional neglect in alexithymia. Psychological Trauma: Theory, Research, Practice, and Policy 5:225–232. [Google Scholar]
- Averbeck BB, Djamshidian A, O’Sullivan SS, Housden CR, Roiser JP, Lees AJ (2013) Uncertainty about mapping future actions into rewards may underlie performance on multiple measures of impulsivity in behavioral addiction: Evidence from Parkinson’s disease. Behavioral Neuroscience 127:245–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bagby M, Taylor GJ, Ryan D (1986) Toronto Alexithymia Scale: Relationship with Personality and Psychopathology Measures. Psychother Psychosom 45:207–215. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Parker JD, Taylor GJ (1994a) The twenty-item Toronto alexithymia scale--I. Item selection and cross-validation of the factor structure. Journal of Psychosomatic Research 38:23–32. [DOI] [PubMed] [Google Scholar]
- Bagby RM, Taylor GJ, Parker JDA (1994b) The twenty-item Toronto Alexithymia scale—II. Convergent, discriminant, and concurrent validity. Journal of Psychosomatic Research 38:33–40. [DOI] [PubMed] [Google Scholar]
- Bankier B, Aigner M, Bach M (2001) Alexithymia in DSM-IV Disorder: Comparative Evaluation of Somatoform Disorder, Panic Disorder, Obsessive-Compulsive Disorder, and Depression. Psychosomatics 42:235–240. [DOI] [PubMed] [Google Scholar]
- Bard P (1934) Emotion: I. The Neuro-humoral Basis of Emotional Reactions. In: A handbook of general experimental psychology, pp 264–311 International university series in psychology. Worcester, MA, US: Clark University Press. [Google Scholar]
- Baron-Cohen S, Wheelwright S (2004) The Empathy Quotient: An Investigation of Adults with Asperger Syndrome or High Functioning Autism, and Normal Sex Differences. J Autism Dev Disord 34:163–175. [DOI] [PubMed] [Google Scholar]
- Barrett LF (2017) The theory of constructed emotion: an active inference account of interoception and categorization. Soc Cogn Affect Neurosci 12:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett LF, Gross J, Christensen TC, Benvenuto M (2001) Knowing what you’re feeling and knowing what to do about it: Mapping the relation between emotion differentiation and emotion regulation. Cognition & Emotion 15:713–724. [Google Scholar]
- Bartra O, McGuire JT, Kable JW (2013) The valuation system: A coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. NeuroImage 76:412–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauernfeind AL, de Sousa AA, Avasthi T, Dobson SD, Raghanti MA, Lewandowski AH, Zilles K, Semendeferi K, Allman JM, Craig AD (Bud), Hof PR, Sherwood CC (2013) A volumetric comparison of the insular cortex and its subregions in primates. Journal of Human Evolution 64:263–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechara A, Tranel D, Damasio H (2000) Characterization of the decision-making deficit of patients with ventromedial prefrontal cortex lesions. Brain 123:2189–2202. [DOI] [PubMed] [Google Scholar]
- Bellebaum C, Koch B, Schwarz M, Daum I (2008) Focal basal ganglia lesions are associated with impairments in reward-based reversal learning. Brain 131:829–841. [DOI] [PubMed] [Google Scholar]
- Benarroch EE (2019) Insular cortex: Functional complexity and clinical correlations. Neurology 93:932–938. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Raghavan C (2003) Culture and Alexithymia : Mean Levels, Correlates, and the Role of Parental Socialization of Emotions. Emotion (Washington, DC) 2:341–360. [DOI] [PubMed] [Google Scholar]
- Bermond B, Moormann PP, Albach F, van Dijke A (2008) Impact of Severe Childhood Sexual Abuse on the Development of Alexithymia in Adulthood. Psychother Psychosom 77:260–262. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Valk SL, Silani G, Bird G, Frith U, Singer T (2014) Selective Disruption of Sociocognitive Structural Brain Networks in Autism and Alexithymia. Cerebral Cortex 24:3258–3267. [DOI] [PubMed] [Google Scholar]
- Berridge KC (2019) Affective valence in the brain: modules or modes? Nat Rev Neurosci 20:225–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Kringelbach ML (2015) Pleasure Systems in the Brain. Neuron 86:646–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC, Robinson TE (1998) What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? Brain Research Reviews 28:309–369. [DOI] [PubMed] [Google Scholar]
- Betka S, Pfeifer G, Garfinkel S, Prins H, Bond R, Sequeira H, Duka T, Critchley H (2018) How Do Self-Assessment of Alexithymia and Sensitivity to Bodily Sensations Relate to Alcohol Consumption? Alcohol Clin Exp Res 42:81–88. [DOI] [PubMed] [Google Scholar]
- Bird G, Cook R (2013) Mixed emotions: The contribution of alexithymia to the emotional symptoms of autism. Translational Psychiatry 3:e285–e285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bird G, Silani G, Brindley R, White S, Frith U, Singer T (2010) Empathic brain responses in insula are modulated by levels of alexithymia but not autism. Brain 133:1515–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodini B, Mandarelli G, Tomassini V, Tarsitani L, Pestalozza I, Gasperini C, Lenzi GL, Pancheri P, Pozzilli C (2008) Alexithymia in multiple sclerosis: relationship with fatigue and depression. Acta Neurol Scand 118:18–23. [DOI] [PubMed] [Google Scholar]
- Bölte S, Feineis-Matthews S, Poustka F (2008) Brief Report: Emotional Processing in High-Functioning Autism—Physiological Reactivity and Affective Report. J Autism Dev Disord 38:776–781. [DOI] [PubMed] [Google Scholar]
- Borsci G, Boccardi M, Rossi R, Rossi G, Perez J, Bonetti M, Frisoni GB (2009) Alexithymia in healthy women: A brain morphology study. Journal of Affective Disorders 114:208–215. [DOI] [PubMed] [Google Scholar]
- Bossu P, Salani F, Cacciari C, Picchetto L, Cao M, Bizzoni F, Rasura M, Caltagirone C, Robinson R, Orzi F, Spalletta G (2009) Disease Outcome, Alexithymia and Depression are Differently Associated with Serum IL-18 Levels in Acute Stroke. CNR 6:163–170. [DOI] [PubMed] [Google Scholar]
- Bouchet CA, Miner MA, Loetz EC, Rosberg AJ, Hake HS, Farmer CE, Ostrovskyy M, Gray N, Greenwood BN (2018) Activation of Nigrostriatal Dopamine Neurons during Fear Extinction Prevents the Renewal of Fear. Neuropsychopharmacology 43:665–672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braunstein LM, Gross JJ, Ochsner KN (2017) Explicit and implicit emotion regulation: a multi-level framework. Social Cognitive and Affective Neuroscience 12:1545–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bressi C, Fronza S, Minacapelli E, Nocito EP, Dipasquale E, Magri L, Lionetti F, Barone L (2017) Short-Term Psychodynamic Psychotherapy with Mentalization-Based Techniques in Major Depressive Disorder patients: Relationship among alexithymia, reflective functioning, and outcome variables – A Pilot study. Psychology and Psychotherapy: Theory, Research and Practice 90:299–313. [DOI] [PubMed] [Google Scholar]
- Buchanan T (2016) Self-report measures of executive function problems correlate with personality, not performance-based executive function measures, in nonclinical samples. Psychological Assessment 28:372–385. [DOI] [PubMed] [Google Scholar]
- Cador M, Robbins TW, Everitt BJ (1989) Involvement of the amygdala in stimulus-reward associations: Interaction with the ventral striatum. Neuroscience 30:77–86. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH (2007) Incorporating emotion regulation into conceptualizations and treatments of anxiety and mood disorders. In: Handbook of Emotion Regulation, pp 542–559. [Google Scholar]
- Chau A, Zhong W, Gordon B, Krueger F, Grafman J (2018) Anterior insula lesions and alexithymia reduce the endorsements of everyday altruistic attitudes. Neuropsychologia 117:428–439. [DOI] [PubMed] [Google Scholar]
- Clithero JA, Rangel A (2014) Informatic parcellation of the network involved in the computation of subjective value. Social Cognitive and Affective Neuroscience 9:1289–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RA, Paul R, Zawacki TM, Moser DJ, Sweet L, Wilkinson H (2001) Emotional and personality changes following cingulotomy. Emotion 1:38–50. [DOI] [PubMed] [Google Scholar]
- Constantino JN, Todorov A, Hilton C, Law P, Zhang Y, Molloy E, Fitzgerald R, Geschwind D (2013) Autism recurrence in half siblings: strong support for genetic mechanisms of transmission in ASD. Mol Psychiatry 18:137–138. [DOI] [PubMed] [Google Scholar]
- Cook R, Brewer R, Shah P, Bird G (2013) Alexithymia, not autism, predicts poor recognition of emotional facial expressions. Psychological Science 24:723–732. [DOI] [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM (2002) Dopaminergic modulation of high‐level cognition in Parkinson’s disease: the role of the prefrontal cortex revealed by PET. Brain 125:584–594. [DOI] [PubMed] [Google Scholar]
- Costa VD, Averbeck BB (2020) Primate orbitofrontal cortex codes information relevant for managing explore-exploit tradeoffs. J Neurosci:2355–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craig AD (2002) How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews Neuroscience 3:655–666. [DOI] [PubMed] [Google Scholar]
- Craig AD (2009) How do you feel--now? The anterior insula and human awareness. Nature Reviews Neuroscience 10:59–70. [DOI] [PubMed] [Google Scholar]
- Cravello L, Caltagirone C, Spalletta G (2009) The SNRI venlafaxine improves emotional unawareness in patients with post-stroke depression. Human Psychopharmacology: Clinical and Experimental 24:331–336. [DOI] [PubMed] [Google Scholar]
- Critchley H (2003) Emotion and its disorders. British Medical Bulletin 65:35–47. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Mathias CJ, Josephs O, O’Doherty J, Zanini S, Dewar BK, Cipolotti L, Shallice T, Dolan RJ (2003) Human cingulate cortex and autonomic control: Converging neuroimaging and clinical evidence. Brain 126:2139–2152. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ (2004) Neural systems supporting interoceptive awareness. Nature Neuroscience 7:189–195. [DOI] [PubMed] [Google Scholar]
- Damasio A, Damasio H, Tranel D (2013) Persistence of feelings and sentience after bilateral damage of the insula. Cerebral Cortex 23:833–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damasio AR (1999) THe feeling of what happens: Body and emotion in the making of consciousness. Orlando, FL: Harcourt. [Google Scholar]
- Darwin C (1872) The Expression of the Emotions in Man and Animals. London: Fontana Press. [Google Scholar]
- Deng S, Lopez V, Roosa MW, Ryu E, Burrell GL, Tein J-Y, Crowder S (2006) Family Processes Mediating the Relationship of Neighborhood Disadvantage to Early Adolescent Internalizing Problems. The Journal of Early Adolescence 26:206–231. [Google Scholar]
- Dickson DW (2012) Parkinson’s Disease and Parkinsonism: Neuropathology. Cold Spring Harbor Perspectives in Medicine 2:a009258–a009258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan RJ (2002) Emotion, Cognition, and Behavior. Science 298:1191–1194. [DOI] [PubMed] [Google Scholar]
- Elmas HG, Cesur G, Oral ET (2016) Alexithymia and Pathological Gambling: The Mediating Role of Difficulties in Emotion Regulation. Turkish Journal of Psychiatry Available at: http://www.turkpsikiyatri.com/default.aspx?modul=doi&doi=u13779 [Accessed July 15, 2020]. [PubMed] [Google Scholar]
- Feinstein JS, Adolphs R, Damasio A, Tranel D (2011) The Human Amygdala and the Induction and Experience of Fear. Current Biology 21:34–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinstein JS, Buzza C, Hurlemann R, Follmer RL, Dahdaleh NS, Coryell WH, Welsh MJ, Tranel D, Wemmie JA (2013) Fear and panic in humans with bilateral amygdala damage. Nature Neuroscience 16:270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FeldmanHall O, Dalgleish T, Mobbs D (2013) Alexithymia decreases altruism in real social decisions. Cortex 49:899–904. [DOI] [PubMed] [Google Scholar]
- Ferrante M, Redish AD, Oquendo MA, Averbeck BB, Kinnane ME, Gordon JA (2019) Computational psychiatry: a report from the 2017 NIMH workshop on opportunities and challenges. Mol Psychiatry 24:479–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank MJ, Samanta J, Moustafa AA, Sherman SJ (2007) Hold Your Horses: Impulsivity, Deep Brain Stimulation, and Medication in Parkinsonism. Science 318:1309–1312. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Dozois DJA, Neufeld RWJ, Lanius RA (2008a) Meta-analysis of alexithymia in posttraumatic stress disorder. J Traum Stress 21:243–246. [DOI] [PubMed] [Google Scholar]
- Frewen PA, Lanius RA, Dozois DJA, Neufeld RWJ, Pain C, Hopper JW, Densmore M, Stevens TK (2008b) Clinical and neural correlates of alexithymia in posttraumatic stress disorder. Journal of Abnormal Psychology 117:171–181. [DOI] [PubMed] [Google Scholar]
- Gaigg SB, Cornell AS, Bird G (2018) The psychophysiological mechanisms of alexithymia in autism spectrum disorder. Autism 22:227–231. [DOI] [PubMed] [Google Scholar]
- Gainotti G (1997) Emotional, psychological and psychosocial problems of aphasic patients: An introduction. Aphasiology 11:635–650. [Google Scholar]
- Gao T, Li J, Zhang H, Gao J, Kong Y, Hu Y, Mei S (2018) The influence of alexithymia on mobile phone addiction: The role of depression, anxiety and stress. Journal of Affective Disorders 225:761–766. [DOI] [PubMed] [Google Scholar]
- Goerlich‐Dobre KS, Probst C, Winter L, Witt K, Deuschl G, Möller B, van Eimeren T (2014) Alexithymia—an independent risk factor for impulsive-compulsive disorders in Parkinson’s disease. Movement Disorders 29:214–220. [DOI] [PubMed] [Google Scholar]
- Goerlich-Dobre KS, Votinov M, Habel U, Pripfl J, Lamm C (2015) Neuroanatomical profiles of alexithymia dimensions and subtypes: Structural Correlates of Alexithymia Subtypes. Hum Brain Mapp 36:3805–3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu X, Hof PR, Friston KJ, Fan J (2013) Anterior insular cortex and emotional awareness. Journal of Comparative Neurology 521:3371–3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gündel H, López-Sala A, Ceballos-Baumann AO, Deus J, Cardoner N, Marten-Mittag B, Soriano-Mas C, Pujol J (2004) Alexithymia Correlates With the Size of the Right Anterior Cingulate: Psychosomatic Medicine 66:132–140. [DOI] [PubMed] [Google Scholar]
- Henry JD, Phillips LH, Crawford JR, Theodorou G, Summers F (2006) Cognitive and psychosocial correlates of alexithymia following traumatic brain injury. Neuropsychologia 44:62–72. [DOI] [PubMed] [Google Scholar]
- Hiser J, Koenigs M (2018) The Multifaceted Role of the Ventromedial Prefrontal Cortex in Emotion, Decision Making, Social Cognition, and Psychopathology. Biological Psychiatry 83:638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobson H, Brewer R, Catmur C, Bird G (2019) The Role of Language in Alexithymia: Moving Towards a Multiroute Model of Alexithymia. Emotion Review 11:247–261. [Google Scholar]
- Hobson H, Hogeveen J, Brewer R, Catmur C, Gordon B, Krueger F, Chau A, Bird G, Grafman J (2018) Language and alexithymia: Evidence for the role of the inferior frontal gyrus in acquired alexithymia. Neuropsychologia 111:229–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J, Bird G, Chau A, Krueger F, Grafman J (2016) Acquired alexithymia following damage to the anterior insula. Neuropsychologia 82:142–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J, Hauner KK, Chau A, Krueger F, Grafman J (2017) Impaired valuation leads to increased apathy following ventromedial prefrontal cortex damage. Cerebral Cortex 27:1401–1408. [DOI] [PubMed] [Google Scholar]
- Hogeveen J, Krueger F, Grafman J (2019) Association Between Alexithymia and Impaired Reward Valuation in Patients With Fronto-insular Damage. Emotion. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogeveen J, Krug MK, Elliott MV, Solomon M (2018) Insula-Retrosplenial Cortex Overconnectivity Increases Internalizing via Reduced Insight in Autism. Biological Psychiatry Available at: http://linkinghub.elsevier.com/retrieve/pii/S0006322318300635 [Accessed June 14, 2018]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honkalampi K, De Berardis D, Vellante F, Viinamäki H (2018) Relations between alexithymia and depressive and anxiety disorders and personality. In: Alexithymia: Advances in research, theory, and clinical practice, pp 142–157. [Google Scholar]
- Honkalampi K, Hintikka J, Koivumaa-Honkanen H, Antikainen R, Haatainen K, Viinamäki H (2007) Long-Term Alexithymic Features Indicate Poor Recovery from Depression and Psychopathology. Psychother Psychosom 76:312–314. [DOI] [PubMed] [Google Scholar]
- Hoppe KD, Bogen JE (1977) Alexithymia in Twelve Commissurotomized Patients. Psychother Psychosom 28:148–155. [DOI] [PubMed] [Google Scholar]
- Hornak J, Bramham J, Rolls ET, Morris RG, O’Doherty J, Bullock PR, Polkey CE (2003) Changes in emotion after circumscribed surgical lesions of the orbitofrontal and cingulate cortices. Brain 126:1691–1712. [DOI] [PubMed] [Google Scholar]
- Ihme K, Dannlowski U, Lichev V, Stuhrmann A, Grotegerd D, Rosenberg N, Kugel H, Heindel W, Arolt V, Kersting A, Suslow T (2013) Alexithymia is related to differences in gray matter volume: A voxel-based morphometry study. Brain Research 1491:60–67. [DOI] [PubMed] [Google Scholar]
- Insel TR (2014) The NIMH Research Domain Criteria (RDoC) Project: precision medicine for psychiatry. The American Journal of Psychiatry 171:395–397. [DOI] [PubMed] [Google Scholar]
- James W (1884) What is an emotion? Mind 9:188–205. [Google Scholar]
- James W (1894) The physical basis of emotion. Psychological Review 1:516–529. [DOI] [PubMed] [Google Scholar]
- Joukamaa M, Taanila A, Miettunen J, Karvonen JT, Koskinen M, Veijola J (2007) Epidemiology of alexithymia among adolescents. Journal of Psychosomatic Research 63:373–376. [DOI] [PubMed] [Google Scholar]
- Kano M, Fukudo S, Gyoba J, Kamachi M, Tagawa M, Mochizuki H, Itoh M, Hongo M, Yanai K (2003) Specific brain processing of facial expressions in people with alexithymia: An H215O-PET study. Brain 126:1474–1484. [DOI] [PubMed] [Google Scholar]
- Kench S, Irwin HJ (2000) Alexithymia and childhood family environment. Journal of Clinical Psychology:9. [DOI] [PubMed] [Google Scholar]
- Kendler KS (1992) Major Depression and Generalized Anxiety Disorder: Same Genes, (Partly) Different Environments? Arch Gen Psychiatry 49:716. [DOI] [PubMed] [Google Scholar]
- Khalsa SS, Feinstein JS, Li W, Feusner JD, Adolphs R, Hurlemann R (2016) Panic Anxiety in Humans with Bilateral Amygdala Lesions: Pharmacological Induction via Cardiorespiratory Interoceptive Pathways. Journal of Neuroscience 36:3559–3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi A, Takeda A, Kimpara T, Nakagawa M, Kawashima R, Sugiura M, Kinomura S, Fukuda H, Chida K, Okita N, Takase S, Itoyama Y (2001) Hypoperfusion in the supplementary motor area, dorsolateral prefrontal cortex and insular cortex in Parkinson’s disease. Journal of the Neurological Sciences 193:29–36. [DOI] [PubMed] [Google Scholar]
- Kim JH, Lee SJ, Rim HD, Kim HW, Bae GY, Chang SM (2008) The Relationship between Alexithymia and General Symptoms of Patients with Depressive Disorders. Psychiatry Investig 5:179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnaird E, Stewart C, Tchanturia K (2019) Investigating alexithymia in autism: A systematic review and meta-analysis. Eur psychiatr 55:80–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klietz M, Schnur T, Drexel SC, Lange F, Paracka L, Huber MK, Dressler D, Höglinger GU, Wegner F (2020) Alexithymia Is Associated with Reduced Quality of Life and Increased Caregiver Burden in Parkinson’s Disease. Brain Sciences 10:401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight RT, Grabowecky M (1995) Escape from linear time: Prefrontal cortex and conscious experience. In: The cognitive neurosciences, pp 1357–1371. Cambridge, MA, US: The MIT Press. [Google Scholar]
- Kooiman CG, van Rees Vellinga S, Spinhoven P, Draijer N, Trijsburg RW, Rooijmans HGM (2004) Childhood Adversities as Risk Factors for Alexithymia and Other Aspects of Affect Dysregulation in Adulthood. Psychother Psychosom 73:107–116. [DOI] [PubMed] [Google Scholar]
- Koponen S, Taiminen T, Honkalampi K, Joukamaa M, Viinamäki H, Kurki T, Portin R, Himanen L, Isoniemi H, Hinkka S, Tenovuo O (2005) Alexithymia After Traumatic Brain Injury: Its Relation to Magnetic Resonance Imaging Findings and Psychiatric Disorders: Psychosomatic Medicine 67:807–812. [DOI] [PubMed] [Google Scholar]
- Kosten TR, Krystal JH, Giller EL, Frank J, Dan E (1992) Alexithymia as a predictor of treatment response in post-traumatic stress disorder. Journal of Traumatic Stress 5:563–573. [Google Scholar]
- Kravitz AV, Tye LD, Kreitzer AC (2012) Distinct roles for direct and indirect pathway striatal neurons in reinforcement. Nature Neuroscience 15:816–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RD, Quinlan DM, Schwartz GE, Walker PA, Zeitlin SB (1990) The Levels of Emotional Awareness Scale: a cognitive-developmental measure of emotion. Journal of Personality Assessment 55:124–134. [DOI] [PubMed] [Google Scholar]
- Lane RD, Reiman EM, Axelrod B, Yun LS, Holmes A, Schwartz GE (1998) Neural correlates of levels of emotional awareness: Evidence of an interaction between emotion and attention in the anterior cingulate cortex. Journal of Cognitive Neuroscience 10:525–535. [DOI] [PubMed] [Google Scholar]
- Lane RD, Sechrest L, Reidel R, Weldon V, Kaszniak A, Schwartz GE (1996) Impaired verbal and nonverbal emotion recognition in alexithymia. Psychosomatic Medicine 58:203–210. [DOI] [PubMed] [Google Scholar]
- Lau H, Rosenthal D (2011) Empirical support for higher-order theories of conscious awareness. Trends in Cognitive Sciences 15:365–373. [DOI] [PubMed] [Google Scholar]
- LeDoux JE (2000) Emotion Circuits in the Brain. 23:155–184. [DOI] [PubMed] [Google Scholar]
- LeDoux JE, Pine DS (2016) Using neuroscience to help understand fear and anxiety: A two-system framework. American Journal of Psychiatry 173:1083–1093. [DOI] [PubMed] [Google Scholar]
- Legrand N, Engen SS, Correa C, Mathiasen NK, Nikolova N, Fardo F, Allen M (2020) Emotional Metacognition: Stimulus Valence Modulates Cardiac Arousal and Metamemory. Neuroscience. Available at: http://biorxiv.org/lookup/doi/10.1101/2020.06.10.144428 [Accessed July 27, 2020]. [DOI] [PubMed] [Google Scholar]
- Levant R, Hall R, Williams C, Hasan N (2009) Gender Differences in Alexithymia. Psychology of Men & Masculinity - PSYCHOL MEN MASCULINITY 10:190–203. [Google Scholar]
- Leweke F, Bausch S, Leichsenring F, Walter B, Stingl M (2009) Alexithymia as a predictor of outcome of psychodynamically oriented inpatient treatment. Psychotherapy Research 19:323–331. [DOI] [PubMed] [Google Scholar]
- Leweke F, Leichsenring F, Kruse J, Hermes S (2012) Is Alexithymia Associated with Specific Mental Disorders? Psychopathology 45:22–28. [DOI] [PubMed] [Google Scholar]
- Li S, Zhang B, Guo Y, Zhang J (2015) The association between alexithymia as assessed by the 20-item Toronto Alexithymia Scale and depression: A meta-analysis. Psychiatry Research 227:1–9. [DOI] [PubMed] [Google Scholar]
- Lindquist KA (2017) The role of language in emotion: existing evidence and future directions. Current Opinion in Psychology 17:135–139. [DOI] [PubMed] [Google Scholar]
- Lindquist KA, Wager TD, Kober H, Bliss-Moreau E, Barrett LF (2012) The brain basis of emotion: A meta-analytic review. Behavior Brain Science 35:121–143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losh M, Capps L (2006) Understanding of emotional experience in autism: Insights from the personal accounts of high-functioning children with autism. Developmental Psychology 42:809–818. [DOI] [PubMed] [Google Scholar]
- Luminet O, Bagby RM, Taylor GJ (2001) An Evaluation of the Absolute and Relative Stability of Alexithymia in Patients with Major Depression. Psychother Psychosom 70:254–260. [DOI] [PubMed] [Google Scholar]
- Luminet OR, Bagby RM, Taylor GJ (2018) Alexithymia: Advances in research, theory, and clinical practice. Cambridge, UK: Cambridge University Press. [Google Scholar]
- Lumley MA (2000) Alexithymia and negative emotional conditions. Journal of Psychosomatic Research 49:51–54. [DOI] [PubMed] [Google Scholar]
- Maisel ME, Stephenson KG, South M, Rodgers J, Freeston MH, Gaigg SB (2016) Modeling the cognitive mechanisms linking autism symptoms and anxiety in adults. Journal of Abnormal Psychology 125:692–703. [DOI] [PubMed] [Google Scholar]
- Marchesi C, Brusamonti E, Maggini C (2000) Are alexithymia, depression, and anxiety distinct constructs in affective disorders? Journal of Psychosomatic Research 49:43–49. [DOI] [PubMed] [Google Scholar]
- Mathersul D, McDonald S, Rushby JA (2013) Automatic facial responses to affective stimuli in high-functioning adults with autism spectrum disorder. Physiology & Behavior 109:14–22. [DOI] [PubMed] [Google Scholar]
- McRae K, Reiman EM, Fort CL, Chen K, Lane RD (2008) Association between trait emotional awareness and dorsal anterior cingulate activity during emotion is arousal-dependent. NeuroImage 41:648–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medford N, Critchley HD (2010) Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function:1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon V (2011) Large-scale brain networks and psychopathology: A unifying triple network model. Trends in Cognitive Sciences 15:483–506. [DOI] [PubMed] [Google Scholar]
- Mezzavilla M, Ulivi S, Bianca ML, Carlino D, Gasparini P, Robino A (2015) Analysis of functional variants reveals new candidate genes associated with alexithymia. Psychiatry Research 227:363–365. [DOI] [PubMed] [Google Scholar]
- Murphy J, Catmur C, Bird G (2018) Alexithymia is associated with a multidomain, multidimensional failure of interoception: Evidence from novel tests. Journal of Experimental Psychology: General 147:398–408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemiah JC, Freyberger H, Sifneos PE (1976) Alexithymia: A view of the psychosomatic process. Modern Trends in Psychosomatic Medicine 3:430–439. [Google Scholar]
- Neumann D, Zupan B, Malec JF, Hammond F (2014) Relationships Between Alexithymia, Affect Recognition, and Empathy After Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation 29:E18. [DOI] [PubMed] [Google Scholar]
- Nielson KA, Meltzer MA (2009) Modulation of long-term memory by arousal in alexithymia: The role of interpretation. Consciousness and Cognition 18:786–793. [DOI] [PubMed] [Google Scholar]
- Ogrodniczuk JS, Piper WE, Joyce AS (2011) Effect of alexithymia on the process and outcome of psychotherapy: A programmatic review. Psychiatry Research 190:43–48. [DOI] [PubMed] [Google Scholar]
- Onur E, Alkın T, Sheridan MJ, Wise TN (2013) Alexithymia and Emotional Intelligence in Patients with Panic Disorder, Generalized Anxiety Disorder and Major Depressive Disorder. Psychiatr Q 84:303–311. [DOI] [PubMed] [Google Scholar]
- Özsahin A, Uzun Ö, Cansever A, Gulcat Z (2003) The effect of alexithymic features on response to antidepressant medication in patients with major depression. Depression and Anxiety 18:62–66. [DOI] [PubMed] [Google Scholar]
- Palomero-Gallagher N, Hoffstaedter F, Mohlberg H, Eickhoff SB, Amunts K, Zilles K (2019) Human Pregenual Anterior Cingulate Cortex: Structural, Functional, and Connectional Heterogeneity. Cerebral Cortex 29:2552–2574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papciak AS, Feuerstein M, Spiegel JA (1985) Stress Reactivity in Alexithymia: Decoupling of Physiological and Cognitive Responses. Journal of Human Stress 11:135–142. [DOI] [PubMed] [Google Scholar]
- Parker JDA, Bagby RM, Taylor GJ (1991) Alexithymia and depression: Distinct or overlapping constructs? Comprehensive Psychiatry 32:387–394. [DOI] [PubMed] [Google Scholar]
- Parker JDA, Keefer KV, Taylor GJ, Bagby RM (2008) Latent structure of the alexithymia construct: A taxometric investigation. Psychological Assessment 20:385–396. [DOI] [PubMed] [Google Scholar]
- Parker JDA, Taylor GJ, Bagby RM (1989) The alexithymia construct : relationship with sociodemographic variables and intelligence. Comprehensive Psychiatry:434–441. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN (2007) Efferent Association Pathways from the Rostral Prefrontal Cortex in the Macaque Monkey. Journal of Neuroscience 27:11573–11586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE (2004) Extinction Learning in Humans: Role of the Amygdala and vmPFC. Neuron 43:897–905. [DOI] [PubMed] [Google Scholar]
- Picardi A, Toni A, Caroppo E (2005) Stability of Alexithymia and Its Relationships with the ‘Big Five’ Factors, Temperament, Character, and Attachment Style. Psychother Psychosom 74:371–378. [DOI] [PubMed] [Google Scholar]
- Preece D, Becerra R, Allan A, Robinson K, Dandy J (2017) Establishing the theoretical components of alexithymia via factor analysis: Introduction and validation of the attention-appraisal model of alexithymia. Personality and Individual Differences 119:341–352. [Google Scholar]
- Reber J, Feinstein JS, O’Doherty JP, Liljeholm M, Adolphs R, Tranel D (2017) Selective impairment of goal-directed decision-making following lesions to the human ventromedial prefrontal cortex. Brain 140:1743–1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reker M, Ohrmann P, Rauch AV, Kugel H, Bauer J, Dannlowski U, Arolt V, Heindel W, Suslow T (2010) Individual differences in alexithymia and brain response to masked emotion faces. Cortex 46:658–667. [DOI] [PubMed] [Google Scholar]
- Ricciardi L, Demartini B, Fotopoulou A, Edwards MJ (2015) Alexithymia in Neurological Disease: A Review. JNP 27:179–187. [DOI] [PubMed] [Google Scholar]
- Rufer M, Ziegler A, Alsleben H, Fricke S, Ortmann J, Brückner E, Hand I, Peter H (2006) A prospective long-term follow-up study of alexithymia in obsessive-compulsive disorder. Comprehensive Psychiatry 47:394–398. [DOI] [PubMed] [Google Scholar]
- Santiesteban I, Gibbard C, Drucks H, Clayton N, Banissy MJ, Bird G (2020) Individuals with Autism Share Others’ Emotions: Evidence from the Continuous Affective Rating and Empathic Responses (CARER) Task. J Autism Dev Disord Available at: http://link.springer.com/10.1007/s10803-020-04535-y [Accessed August 24, 2020]. [DOI] [PubMed] [Google Scholar]
- Sareen J, Henriksen CA, Bolton S-L, Afifi TO, Stein MB, Asmundson GJG (2013) Adverse childhood experiences in relation to mood and anxiety disorders in a population-based sample of active military personnel. Psychol Med 43:73–84. [DOI] [PubMed] [Google Scholar]
- Sayer NA (2012) Traumatic Brain Injury and Its Neuropsychiatric Sequelae in War Veterans. Annu Rev Med 63:405–419. [DOI] [PubMed] [Google Scholar]
- Schäfer R, Popp K, Jörgens S, Lindenberg R, Franz M, Seitz RJ (2007) Alexithymia-like Disorder in Right Anterior Cingulate Infarction. Neurocase 13:201–208. [DOI] [PubMed] [Google Scholar]
- Schimmenti A, Passanisi A, Caretti V, La Marca L, Granieri A, Iacolino C, Gervasi AM, Maganuco NR, Billieux J (2017) Traumatic experiences, alexithymia, and Internet addiction symptoms among late adolescents: A moderated mediation analysis. Addictive Behaviors 64:314–320. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience 27:2349–2356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seth AK, Friston KJ (2016) Active interoceptive inference and the emotional brain. Philos Trans R Soc Lond B Biol Sci 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamay-Tsoory SG, Tomer R, Berger BD, Goldsher D, Aharon-Peretz J (2005) Impaired “affective theory of mind” is associated with right ventromedial prefrontal damage. Cognitive and Behavioral Neurology 18:55–67. [DOI] [PubMed] [Google Scholar]
- Shipko S, Alvarez WA, Noviello N (1983) Towards a Teleological Model of Alexithymia: Alexithymia and Post-Traumatic Stress Disorder. Psychother Psychosom 39:122–126. [DOI] [PubMed] [Google Scholar]
- Sifneos PE (1973) The prevalence of “alexithymic” characteristics in psychosomatic patients. Psychotherapy and Psychosomatics 22:255–262. [DOI] [PubMed] [Google Scholar]
- Silani G, Bird G, Brindley R, Singer T, Frith C, Frith U (2008) Levels of emotional awareness and autism: An fMRI study. Social Neuroscience 3:97–112. [DOI] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, Baird G (2008) Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. Journal of the American Academy of Child and Adolescent Psychiatry 47:921–929. [DOI] [PubMed] [Google Scholar]
- Smith R, Lane RD, Parr T, Friston KJ (2019) Neurocomputational mechanisms underlying emotional awareness: Insights afforded by deep active inference and their potential clinical relevance. Neuroscience & Biobehavioral Reviews 107:473–491. [DOI] [PubMed] [Google Scholar]
- Smith R, Steklis HD, Steklis NG, Weihs KL, Lane RD (2020) The evolution and development of the uniquely human capacity for emotional awareness: A synthesis of comparative anatomical, cognitive, neurocomputational, and evolutionary psychological perspectives. Biological Psychology 154:107925. [DOI] [PubMed] [Google Scholar]
- Spalletta G, Pasini A, Costa A, De Angelis D, Ramundo N, Paolucci S, Caltagirone C (2001) Alexithymic Features in Stroke: Effects of Laterality and Gender: Psychosomatic Medicine 63:944–950. [DOI] [PubMed] [Google Scholar]
- Spek V, Nyklíček I, Cuijpers P, Pop V (2008) Alexithymia and cognitive behaviour therapy outcome for subthreshold depression. Acta Psychiatrica Scandinavica 118:164–167. [DOI] [PubMed] [Google Scholar]
- Sperry RW, Gazzaniga MS, Bogen JE (1969) Interhemispheric relationships: The neocortical commissures; syndromes of hemisphere disconnection. Handbook of Clinical Neurology 4:273. [Google Scholar]
- Stasiewicz PR, Bradizza CM, Gudleski GD, Coffey SF, Schlauch RC, Bailey ST, Bole CW, Gulliver SB (2012) The relationship of alexithymia to emotional dysregulation within an alcohol dependent treatment sample. Addictive Behaviors 37:469–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steklis HD, Lane RD (2013) The Unique Human Capacity for Emotional Awareness: Psychological, Neuroanatomical, Comparative and Evolutionary Perspectives. In: Emotions of Animals and Humans: Comparative Perspectives (Watanabe S, Kuczaj S, eds), pp 165–205 The Science of the Mind. Tokyo: Springer Japan. Available at: 10.1007/978-4-431-54123-3_8 [Accessed July 22, 2020]. [DOI] [Google Scholar]
- Stone LA, Nielson KA (2001) Intact physiological response to arousal with impaired emotional recognition in alexithymia. Psychotherapy and Psychosomatics 70:92–102. [DOI] [PubMed] [Google Scholar]
- Stuber GD, Sparta DR, Stamatakis AM, van Leeuwen WA, Hardjoprajitno JE, Cho S, Tye KM, Kempadoo KA, Zhang F, Deisseroth K, Bonci A (2011) Excitatory transmission from the amygdala to nucleus accumbens facilitates reward seeking. Nature 475:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturm VE, Levenson RW (2011) Alexithymia in neurodegenerative disease. Neurocase 17:242–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swart M, Kortekaas R, Aleman A (2009) Dealing with feelings: Characterization of trait Alexithymia on emotion regulation strategies and cognitive-emotional processing. PLoS ONE 4:e5751–e5751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD (2009) Two systems of resting state connectivity between the insula and cingulate cortex. Hum Brain Mapp 30:2731–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TenHouten WD, Hoppe KD, Bogen JE, Walter DO (1986) Alexithymia: an experimental study of cerebral commissurotomy patients and normal control subjects. AJP 143:312–316. [DOI] [PubMed] [Google Scholar]
- TenHouten WD, Walter DO, Hoppe KD, Bogen JE (1987) Alexithymia and the Split Brain. Psychotherapy and Psychosomatics 47:1–10. [DOI] [PubMed] [Google Scholar]
- Trinkler I, Cleret de Langavant L, Bachoud-Lévi A-C (2013) Joint recognition–expression impairment of facial emotions in Huntington’s disease despite intact understanding of feelings. Cortex 49:549–558. [DOI] [PubMed] [Google Scholar]
- Tye KM (2018) Neural Circuit Motifs in Valence Processing. Neuron 100:436–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin LQ (2014) Salience processing and insular cortical function and dysfunction. Nature Reviews Neuroscience 16:55–61. [DOI] [PubMed] [Google Scholar]
- Uljarevic M, Hamilton A (2013) Recognition of Emotions in Autism: A Formal Meta-Analysis. J Autism Dev Disord 43:1517–1526. [DOI] [PubMed] [Google Scholar]
- van der Velde J, Servaas MN, Goerlich KS, Bruggeman R, Horton P, Costafreda SG, Aleman A (2013) Neural correlates of alexithymia: A meta-analysis of emotion processing studies. Neuroscience & Biobehavioral Reviews 37:1774–1785. [DOI] [PubMed] [Google Scholar]
- van der Velde J, van Tol M-J, Goerlich-Dobre KS, Gromann PM, Swart M, de Haan L, Wiersma D, Bruggeman R, Krabbendam L, Aleman A (2014) Dissociable morphometric profiles of the affective and cognitive dimensions of alexithymia. Cortex 54:190–199. [DOI] [PubMed] [Google Scholar]
- van Steensel FJA, Bögels SM, Perrin S (2011) Anxiety disorders in children and adolescents with autism spectrum disorders: A meta-analysis. Clinical Child and Family Psychology Review 14:302–317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K et al. (2009) Common genetic variants on 5p14.1 associate with autism spectrum disorders. Nature 459:528–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissman DG, Nook EC, Dews AA, Miller AB, Lambert HK, Sasse SF, Somerville LH, McLaughlin KA (2020) Low Emotional Awareness as a Transdiagnostic Mechanism Underlying Psychopathology in Adolescence. Clinical Psychological Science:2167702620923649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams C, Wood RL (2010) Alexithymia and emotional empathy following traumatic brain injury. Journal of Clinical and Experimental Neuropsychology 32:259–267. [DOI] [PubMed] [Google Scholar]
- Williams C, Wood RL (2013) The impact of alexithymia on relationship quality and satisfaction following traumatic brain injury. Journal of Head Trauma Rehabilitation 28:16–19. [DOI] [PubMed] [Google Scholar]
- Wilson TD, Dunn EW (2004) Self-Knowledge: Its Limits, Value, and Potential for Improvement. Annu Rev Psychol 55:493–518. [DOI] [PubMed] [Google Scholar]
- Wolf RC, Philippi CL, Motzkin JC, Baskaya MK, Koenigs M (2014) Ventromedial prefrontal cortex mediates visual attention during facial emotion recognition. Brain 137:1772–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood RL, Doughty C (2013) Alexithymia and Avoidance Coping Following Traumatic Brain Injury. The Journal of Head Trauma Rehabilitation 28:98–105. [DOI] [PubMed] [Google Scholar]
- Wood RLl, Williams C (2007) Neuropsychological correlates of organic alexithymia. J Inter Neuropsych Soc 13 Available at: http://www.journals.cambridge.org/abstract_S1355617707070518 [Accessed July 15, 2020]. [DOI] [PubMed] [Google Scholar]
- Wood RLL, Williams C, Kalyani T (2009) The impact of alexithymia on somatization after traumatic brain injury. Brain Injury 23:649–654. [DOI] [PubMed] [Google Scholar]
- Wood RLL, Williams C, Lewis R (2010) Role of alexithymia in suicide ideation after traumatic brain injury. Journal of the International Neuropsychological Society 16:1108–1114. [DOI] [PubMed] [Google Scholar]
- Xu P, Opmeer EM, van Tol M-J, Goerlich KS, Aleman A (2018) Structure of the alexithymic brain: A parametric coordinate-based meta-analysis. Neuroscience & Biobehavioral Reviews 87:50–55. [DOI] [PubMed] [Google Scholar]
- Zaki J, Davis JI, Ochsner KN (2012) Overlapping activity in anterior insula during interoception and emotional experience. NeuroImage 62:493–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zald DH, Mattson DL, Pardo JV (2002) Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences 99:2450–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeitlin SB, McNally RJ (1993) Alexithymia and anxiety sensitivity in panic disorder and obsessive-compulsive disorder. American Journal of Psychiatry 150:658–660. [DOI] [PubMed] [Google Scholar]


