Abstract
Identification, visualization, and quantitation of cardiolipin (CL) in biological membranes is of great interest because of the important structural and physiological roles of this lipid. Selective fluorescent detection of CL using noncovalently bound fluorophore 1,1,2,2-tetrakis[4-(2-trimethylammonioethoxy)-phenylethene (TTAPE-Me) has been recently proposed. However, this dye was only tested on wild-type mitochondria or liposomes containing negligible amounts of other anionic lipids, such as phosphatidylglycerol (PG) and phosphatidylserine (PS). No clear preference of TTAPE-Me for binding to CL compared to PG and PS was found in our experiments on artificial liposomes, Escherichia coli inside-out vesicles, or Saccharomyces cerevisiae mitochondria in vitro or in situ, respectively. The shapes of the emission spectra for these anionic phospholipids were also found to be indistinguishable. Thus, TTAPE-Me is not suitable for detection, visualization, and localization of CL in the presence of other anionic lipids present in substantial physiological amounts. Our experiments and complementary molecular dynamics simulations suggest that fluorescence intensity of TTAPE-Me is regulated by dynamic equilibrium between emitting dye aggregates, stabilized by unspecific but thermodynamically favorable electrostatic interactions with anionic lipids, and nonemitting dye monomers. These results should be taken into consideration when interpreting past and future results of CL detection and localization studies with this probe in vitro and in vivo. Provided methodology emphasizes minimal experimental requirements, which should be considered as a guideline during the development of novel lipid-specific probes.
Significance
1,1,2,2-tetrakis[4-(2-trimethylammonioethoxy)-phenylethene (TTAPE-Me) was recently invented as turn-on cardiolipin (CL)-specific fluorescent probe. However, we found that its fluorescent responses for different anionic lipids were essentially the same and did not allow discrimination between CL, PG, and PS by either emission intensities or the shape of corresponding spectra. Therefore, TTAPE-Me is not suitable for detection, visualization, and localization of CL in the presence of other anionic lipids neither in vitro nor in vivo. Our data suggest that fluorescence intensity of TTAPE-Me is regulated by the shift in dynamic equilibrium between nonemitting monomers and emitting dye aggregates stabilized by nonspecific electrostatic interactions with anionic lipids. Our results provide a guideline for the successful development of novel lipid-specific fluorescent probes.
Introduction
Cardiolipin (CL) imparts cell membranes with a unique set of physical and chemical characteristics because of its unique chemical and structural properties (1). The functions of CL are diverse. Besides a crucial role in bioenergetics (2, 3, 4, 5, 6), CL is associated with many cellular abnormalities and human diseases (7,8); modifies ordering and rigidity of the lipid bilayer (9) and forms lipid microdomains (1,10,11); serves as a targets for antimicrobials (12,13); participates in bacterial cell division (12, 13, 14) and adaptation to environmental stress (15,16); connects size and growth rate of bacterial cells (17,18); drives the generation of membrane-derived vesicles by pathogenic bacteria (19); participates in signaling in preapoptotic cells (20,21) and mitophagy (22,23); and guides membrane protein translocation (5,24).
The distribution of CL between the membrane monolayers in either bacterial or eukaryotic cells remains unknown. Current methods of quantifying and visualizing CL are hampered by the lack of specificity to this particular lipid. 10-N-nonyl acridine orange (NAO) is widely used for fluorescent detection of CL in bacteria and mitochondria. NAO exhibits a characteristic red shift of emission spectra, which was previously considered to be unique for the interaction between this probe and CL. However, the lack of specificity of NAO toward CL was recently reported in mitochondria (25), gram-negative (11) and gram-positive bacteria (26), and archaea (27). Thus, NAO cannot be utilized for the mapping of CL sidedness because of its uncertain anionic phospholipid specificity, full membrane permeability, and concentration dependency of red spectral shift. So, selective nonpermeant leaflet-specific probes are of great demand for mapping a transmembrane distribution of CL.
Recently, a water-soluble 1,1,2,2-tetrakis[4-(2-trimethylammonioethoxy)-phenylethene (TTAPE-Me) dye, with positively charged cationic functional groups such as a quaternary amines, was proposed as a promising CL-specific fluorescent probe suitable for CL detection and sidedness studies (28,29). TTAPE-Me belongs to the fluorophores with so-called aggregation-induced emission characteristics. TTAPE-Me is nonfluorescent in aqueous solutions, but its fluorescence is turned on in CL-containing liposomes in a concentration-dependent manner, whereas very weak fluorescence is observed in vesicles containing phosphatidylcholine (PC) and phosphatidylethanolamine (PE) lipids (28).
Although TTAPE-Me is claimed to be selective to CL and is marketed in this way, this probe has never been tested properly for interactions with other anionic lipids. Indeed, in the seminal work of Leung et al. (28), only the wild-type mitochondria and liposomes representing a wild-type mitochondrial lipid composition were used. CL is indeed a predominant anionic lipid in wild-type mitochondria at the standard conditions in which other anionic lipids (PS, phosphatidylinositol (PI), and PG) are found in very small amounts. However, in mitochondrial membranes, not only CL deficiency but also excess PG or unbalanced ratios of anionic phospholipids are frequent events (30). Thus, mitochondrial membranes and their lipid mimetics alone are not suitable for determining CL specificity of the dye. Obviously, these results are not relevant for the membranes of Gram-negative and Gram-positive bacteria, which are rich in PG, and mammalian plasma membranes in which PS is abundant.
Surprisingly, TTAPE-Me was never tested for equimolar concentrations of CL and other anionic lipids, particularly PG and PS. It was reported recently that TTAPE-Me responds similarly and binds to 100 nm large unilamellar vesicles made of 100% PG and CL with comparable affinity (29), but these experiments were performed in the presence of drastic excess of the probe.
To fill this knowledge gap, we performed a thorough investigation of TTAPE-Me combining fluorescence measurements with all-atom molecular dynamics (MD) simulations. We first assessed dye fluorescence in organic solvents with different degrees of polarity and simulated its behavior in water. Then, we measured dye fluorescence in liposomes constituted from major anionic lipids CL, PG, and PS, pristine or mixed with PE in different proportions. We also simulated the interaction of TTAPE-Me with lipid bilayers of corresponding compositions. This complementary approach allowed us 1) to elucidate further the molecular mechanism of TTAPE-Me turn-on fluorescence in lipid membranes, 2) to show that TTAPE-Me fluorescence differs drastically in different solvents, and 3) to demonstrate that this probe is not CL specific and binds promiscuously to main anionic phospholipids.
Materials and methods
MD simulations
MD simulations were performed in Gromacs (31) version 2019.2. Topologies were generated by CHARMM GUI (32,33). Analysis was performed with Pteros molecular modeling library (34). The details of system preparation, simulations, and analysis are provided in Supporting materials and methods.
Reagents
The following reagents were used: TTAPE-Me (BioVision, Milpitas, CA); anhydrotetracycline (aTC) was from Spectrum Chemical (Gardena, CA); phosphatidylethanolamine total extract from Escherichia coli (Avanti Polar Lipids, Alabaster, AL); CL total extract from E. coli (Avanti Polar Lipids); phosphatidylglycerol total extract from E. coli (Avanti Polar Lipids); and phosphatidylserine extract from porcine brain (Avanti Polar Lipids).
Growth of bacterial and yeast strains
E. coli strain W3110 is wild-type with respect to glycerophospholipid biosynthesis and composition. BKT12 (ΔclsA, ΔclsB, ΔclsC1::KanR) lacks all three CL synthases and is completely devoid of CL (35). Although YmdB (C2) is required for full activity of the ClsC1-YmdB complex, lack of ClsC1 prevents the utilization of PG and PE in a novel mode of CL biosynthesis. Both strains were grown on Luria-Bertani (LB) medium until reaching the logarithmic phase of growth (optical density at 600 nm (OD600) of 0.5–0.6). Liquid LB media was made with 10 g of tryptone, 5 g of yeast extract, and 10 g of NaCl per liter (high-salt LB Miller medium). Strain AT2033 (PLtetO-1-pssA + pss93::kanR lacY::Tn9 recA srl::Tn10), in which the PE content of the cell can be regulated from near zero to wild-type level (75%) being proportional to the amount of gene inducer aTC in the growth medium (36,37), was used. Strain AT2033 was grown first overnight on LB medium supplemented with 50 mM MgCl2 and then outgrown in the same medium until an OD600 of 0.5–0.6. Cultures were supplemented with 20 ng/mL of aTC and harvested at the logarithmic phase of growth (OD600 of 0.5–0.6) when the desired amount of PE and anionic phospholipids was reached (38).
Wild-type yeast strain DL1 and its CRD1 null derivatives (Δcrd1) mutant YZD2 (ade2–101, his3Δ200, leu2Δ1, lys2–801, trp1Δ63, ura3–52, crd1Δ::HIS3, MATα) with entire disruption of crd1 (encodes CL synthase) open reading frame synthesizes 6–10% of PG instead CL (39). Both strains were grown at 30°C in nonfermentable YPEG growth medium consisting of 1% Bacto yeast extract, 2% Bacto peptone, and 1% ethanol (v/v) and 3% glycerol as carbon sources. The cells were harvested (OD600 of 2.0) by centrifugation and washed with cold Tris-buffered saline buffer (50 mM Tris-HCl (pH 7.4), containing 150 mM NaCl).
Small unilamellar vesicles preparation
The small unilamellar vesicles (SUVs) were prepared by sonication method as it was described elsewhere (40,41). The details are provided in Supporting materials and methods.
Preparation of uniformly oriented inside-out vesicles from different E. coli lipid mutant cells
Inside-out vesicles (ISOv) were prepared from E. coli wild-type W3110 and AT2033 outgrown in the presence of 20 ng/mL and therefore containing different amounts of CL and PG and BKT12 lacking CL by rupture of cells using a French press at 560 kg/cm2 (8000 psi) with subsequent differential centrifugation as described previously (18).
Isolation of mitochondria from Saccharomyces cerevisiae
Mitochondria were isolated from spheroplasts of yeast cells as previously described (6) except that 3 mg of Zymolase-20T per gram of cells was used, and incubation time was 90 min. Isolated mitochondria were further purified by centrifugation at 30,000 rpm (SW41 Ti) at 4°C for 90 min through a density gradient containing 60, 32, 23, and 15% layers of sucrose in 15 mM Tris-HCl buffer (pH 7.4) and 20 mM KCl. Pellets were washed with 20 mL of 10 mM 3-(N-Morpholino)propane sulfonic acid (MOPS) buffer containing 250 mM sucrose and 1 mM EDTA (pH 7.2) and centrifuged at 1500 × g for 5 min. Supernatant from the last centrifugation was centrifuged at 12,000 × g for 10 min at +4°C. Mitochondria (pellets) were suspended in sucrose/EDTA (SE) buffer and frozen in 0.1-mL aliquots at −80°C.
Before labeling with TTAPE-Me, the mitochondria were incubated with 1 mM EDTA for 20 min, according to the protocol described in (28).
Labeling procedures
Initially, the stock powder of TTAPE-Me probe was diluted in DMSO. For monitoring of the TTAPE-Me response to different solvents, the probe in DMSO was directly added to the respective solvent to receive 20 μM final concentration of the probe and <0.25% of DMSO (except the samples with pure DMSO).
To label the SUVs, TTAPE-Me was diluted in 600 μL 10 mM HEPES/Na buffer (pH 7.4) and added to 150 μL of 1 mM mixture of the lipids. As the result, 200 μM solution of the lipids (<0.25% of DMSO) was received. The mixture was incubated during 10 min in the dark at room temperature before the measurements.
To label ISOv or mitochondria, the appropriate amount of each sample was diluted in 250 μL of 100 mM HEPES/Na buffer (pH 7.4) and mixed with 250 μL of buffer with premixed TTAPE-Me probe. As a result, 100 μg/mL (by BCA assay) solution of ISOv or mitochondria with 10 μM of TTAPE-Me (<0.25% of DMSO) was received. The mixture was incubated during 10 min in the dark at room temperature before the measurements.
Spectroscopy
Spectra were recorded in half-microcuvette with PTI Quantamaster 40 spectrofluorometer at 25°C. 3- or 5-nm slits on excitation and emission were applied to receive a reasonably high signal. 5-nm slits were applied to the samples described on Fig. 1, C and D and Fig. 3, G and H (along with Fig. S2, C and D).
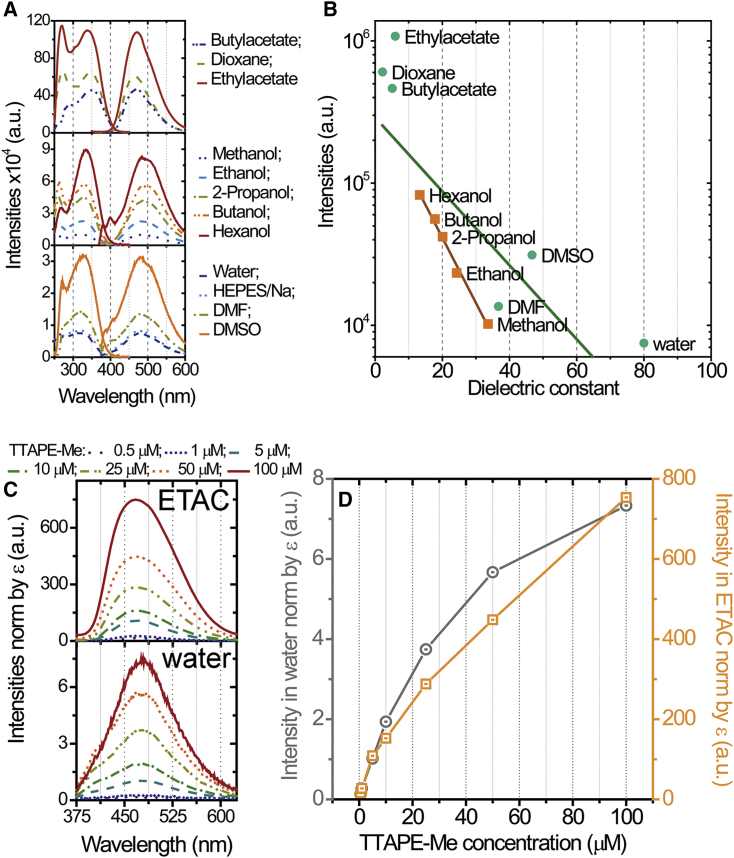
Figure 1.
Fluorescent response of 20 μM TTAPE-Me probe to different solvents. (A) Excitation (left curves) and emission (right curves) spectra in various solvents. The excitation and emission spectra were recorded in respect to appropriate maximal values for each sample. The spectra are represented as the averaged records from three independent experiments. Excitation and emission were provided by respective maximum for each solvent. (B) The maximal fluorescent intensities (in log scale) as a function of the dielectric constant of the solvent. The longer (green) line shows linear fit of all point. The shorter (brown) line shows linear fit for monatomic alcohols (showed as squares) only. (C) The spectra of TTAPE-Me in different concentrations in ethyl acetate (ETAC) (top panel) and water (bottom panel) normalized by the molar extinction coefficient (ϵ) at 325 nm wavelength for each concentration. Excitation was provided by 325-nm wavelength for both water and ETAC. (D) Maximal fluorescence intensities of TTAPE-Me in ETAC (squares) and water (circles) as a function of dye concentration computed from the normalized spectra in (C). To see this figure in color, go online.
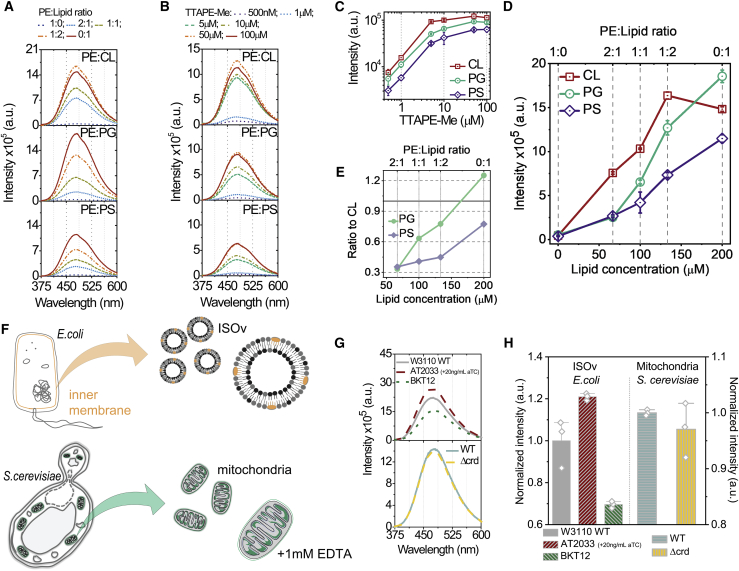
Figure 3.
Fluorescent spectra of TTAPE-Me in 200 μM SUVs composed of PE mixed with CL, PG, or PS in different proportions. (A) Response of SUVs composed of PE/lipid = 1:1 mixtures (in which “lipid” refers to CL, PG, or PS) to increasing concentration of TTAPE-Me. (B) Response of SUVs with 10 μM of TTAPE-Me to an increasing proportion of CL, PG, and PS. (C) Fluorescence intensity as a function of TTAPE-Me concentration corresponding to (A). (D) Fluorescence intensity as a function of concentrations of CL, PG, and PS in model SUVs corresponding to (B). Absolute concentration is shown on the bottom axis, whereas the relative abundance of the lipid in relation to PE is shown on the top axis. (E) The ratios of fluorescent intensity in PG and PS in comparison to CL based on (D). (F) The scheme of the sample’s origin and nature for bacterial vesicles and isolated mitochondria. (G) Spectra of ISOv from W3110 wild-type (∼60% PE, ∼30% PG, and ∼10% CL), BKT12 (∼62% PE, ∼32% PG, and no CL), and AT2033 + 20 ng/mL aTC (∼10% PE, ∼50% PG, and ∼40% CL) strains E. coli (top panel) and purified mitochondria from wild-type and Δcrd (CL substituted by PG) strains of S. cerevisiae. (H) Normalized (by wild-type of each organism) fluorescent maxima intensities for samples in (G). Data on (C and D) are represented as the mean ± SD. The spectra for each sample are represented as averaged ones from several respective experiments. Excitation at 325-nm wavelength was used for all shown spectra (see Fig. S2C). To see this figure in color, go online. For (F) the combined schemes from Servier Medical Art by Servier (licensed under a creative commons attribution 3.0 unported license) and KP were used.
To minimize the influence of autofluorescence and scattering on the probe response, the spectra of the TTAPE-Me in SUVs, mitochondria from S. cerevisiae, ISOv from E. coli, or solution were normalized on the curve provided by the respective vesicles or solvent without the probe (for details, see Fig. S2).
Results
TTAPE-Me behavior in different solvents
Both the excitation and emission of TTAPE-Me were examined in different aqueous and organic solvents (Fig. 1 A) because an original report (28) considered excitation in water only.
The probe was essentially nonemissive in water; however, the fluorescence increases drastically with a decrease of dielectric constant of the solvent (Fig. 1 B). Although there are unsystematic deviations from this trend caused by the chemical nature of the solvent, there is a clear indication that TTAPE-Me is more fluorescent in a less polar environment. In the case of compounds of the same chemical nature (a series of monatomic alcohols), there is a perfect linear dependence of the logarithm of emission intensity on dielectric constant (Fig. 1 B).
We also investigated concentration dependence of the emission spectra in the solvents with minimal and maximal fluorescent responses: water and ethyl acetate, respectively (Fig. 1, C and D). The spectra were normalized by the molar extinction coefficient computed according to Beer-Lambert law (42) , where A is an absorbance at 325 nm, c is a concentration of TTAPE-Me, and l is an optical path length. This eliminated the influence of significant absorbance and scattering of light by the dye aggregates, which becomes evident at high dye concentrations (Fig. S2 D). The spectra were also corrected for the scattering and autofluorescence of the samples (see Supporting materials and methods and Fig. S2 for details).
The maximum of emission spectra does not change in a broad range of dye concentrations varied from 0.5 to 100 μM. A clearly visible shoulder appears at ∼400 nm in a range of concentrations 10–50 μM in water but not in ethyl acetate. The fluorescence intensity in ethyl acetate is significantly more linear than in water at high dye concentrations, which suggests nontrivial dependence of the fluorescent response on the nature of the solvent.
To understand this puzzling behavior of the dye, we performed MD simulations of TTAPE-Me in water and ethyl acetate. It appears that TTAPE-Me molecules are prone to fast spontaneous aggregation in both media, but the structures of aggregates differ drastically depending on the solvent (Fig. 2).
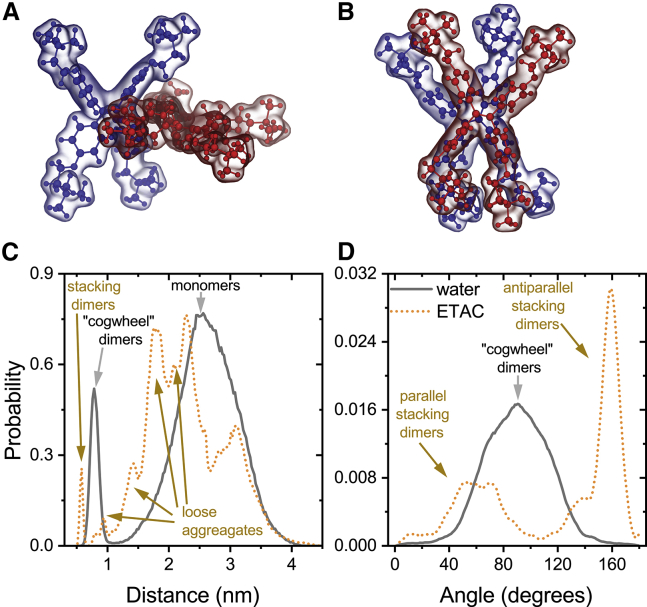
Figure 2.
Characterization of TTAPE-Me aggregates by means of atomistic MD simulations. (A) Typical “cogwheel” dimer in water. (B) Typical stacking dimer in (ETAC. (C) Distribution of distances between the centers of masses of TTAPE-Me molecules in MD simulations. (D) Distribution of angles between chromophore normals in MD simulations. To see this figure in color, go online.
In water, transient dimers and trimers with a “cogwheel” topology are formed (Fig. 2 A). The distance between centers of masses in such dimers is ∼0.8 nm (Fig. 2 C, gray line), and the normals of both chromophores are mostly perpendicular to each other (Fig. 2 D, gray line), resulting in “face-to-edge” orientation. This arrangement decreases energetically unfavorable hydrophobic mismatch of the water-exposed chromophores by partially screening them from water.
In contrast, in ethyl acetate, the stacking dimers with parallel (“face-to-tail”) or antiparallel (“face-to-face”) orientation of the normals are formed (Fig. 2 B). The distance between the centers of masses in the stacks is ∼0.6 nm, and the angle between the normals of chromophores is ∼60° in the parallel and ∼160° in the antiparallel arrangements (Fig. 2, C and D, yellow lines). Moreover, there is a surprising variety of other aggregates of various sizes and topologies, which are visible as multiple peaks on the distance distribution (Fig. 2 C).
Visual inspection of MD trajectories show that TTAPE-Me molecules in ethyl acetate form a loosely packed disordered network in which the monomers contact by either their hydrophobic chromophores or hydrophilic “arms” (Fig. S1 B). In contrast, in water, only monomers and distinct cogwheel-like dimers and trimers, held together by hydrophobic interactions, are observed (Fig. S1 A).
Interaction of TTAPE-Me with lipid membranes
To study interaction of TTAPE-Me with different anionic phospholipids, we prepared SUVs consisting of CL, PG, or PS alone or in binary mixture with PE at different mole ratios and compared TTAPE-Me emission upon its binding. The optimal concentration of TTAPE-Me was determined by titration of different probe/lipid ratios (Fig. 3 C). The saturation of the signal starts at the ratio 20:1 for all lipids. Thus, this ratio, corresponding to dye concentration of 10 μM, was used for all further experiments.
TTAPE-Me fluorescence intensity was measured then as a function of different probe/lipid, e.g., [TTAPE-Me]/[Lipid], ratios in which the amount of probe (Fig. 3, A and C) or amount of individual target lipid (Fig. 3, B, E, and D) were varied, respectively.
Fig. 3, A and B show that shapes of emission spectra for pure CL, PG, and PS as well as for their binary mixtures with PE are indistinguishable (also see Fig. S2, D and E). It is necessary to note that several published experiments (29) were performed in the presence of drastic excess of the probe, and the background signal was not subtracted properly from the emission spectra (28,29). In such cases, spurious differences in the shapes of spectra (as shown in Fig. S2 G versus Fig. S2 H) could be interpreted incorrectly as apparent selectivity of TTAPE-Me to particular lipids.
There is no significant fluorescence signal in pure PE, which allows for using mixtures with this aminolipid for measuring concentration dependencies for CL, PG, and PS. The fluorescent signal increases in a very similar manner with the increase of probe concentration in 1:1 mixtures of PE with CL, PG, and PS, respectively (Fig. 3 C), demonstrating the ability of TTAPE-Me to interact with all three anionic lipids by means of the same unspecific physical mechanism. The absolute fluorescent intensities depend both on the nature of the anionic lipid and its content (Fig. 3 D). CL produces a stronger response than PG and PS for probe concentrations up to 150 μM, but the ratio of intensities never exceeds the factor of 3 (Fig. 3 E). For the concentration of 200 μM, the fluorescence of PG becomes even stronger than CL (Fig. 3, D and E). These data support the recent finding that demonstrates that TTAPE-Me is capable of detecting the presence of anionic lipid but cannot distinguish between mono- and dianionic lipid headgroups, at least for CL and PG molecules populating the surface of 100-nm large unilamellar vesicles with the same net charge (29).
To address an effectiveness of CL detection by TTAPE-Me in natural membranes, we performed a series of experiments on the ISOv from the inner membrane of E. coli and on the purified mitochondria from S. cerevisiae (Fig. 3 F) with well-defined, controlled, and established lipid composition. Namely, three strains of E. coli with different compositions of inner membrane were studied:
-
1)
W3110 wild-type strain with ∼60% of PE, ∼30% of PG, and ∼10% of CL in the inner membrane (18);
-
2)
BKT12 strain with ∼62% of PE, ∼32% of PG, and no CL (18); and
-
3)
AT2033 strain outgrown with 20 ng/mL of aTC, resulting in ∼10% of PE, ∼50% of PG, and ∼40% of CL from total cell amount (43,44).
In yeast, CL and PG are the major and minor mitochondrial phospholipids under standard conditions, respectively. However, full disruption of crd1 gene encoding CL synthase in S. cerevisiae results in a complete loss of CL and the compensatory increase of PG content (39,45).
The mitochondria and ISOv from different E. coli strains differ by their PG + CL content; thus, it was expectable to obtain different fluorescent intensities. The consistent decrease of intensity was observed as a total percentage of anionic lipids progressively decreased from AT2033 + 20 ng/mL aTC (∼90% of PG + CL) to W3110 (∼40% PG + CL) and to BKT12 (∼32% of PG only) (Fig. 3, G and H). The signal remained nearly the same in the mitochondria from both strains of S. cerevisiae with statistically insignificant lower intensity in Δcrd, which lacks CL completely (Fig. 3, G and H). This demonstrates that CL and PG present in mitochondria at nearly the same percentage lead to indistinguishable fluorescent signals of TTAPE-Me probe.
These complementary results demonstrated confidently that TTAPE-Me probe exhibits essentially the same concentration dependence on the amount of individual target lipid or their sum, either upon binding to liposomal (Fig. 3, A–D), bacterial, or mitochondrial CL or PG (Fig. 3, F–H) and therefore is not specific for CL.
To rationalize the observed results, we performed MD simulations of TTAPE-Me with lipid bilayers made of CL, PG, PS, and PC/PE. We performed independent sets of simulations with either TTAPE-Me monomers or the “cogwheel” dimers with each of the bilayers.
Analysis of trajectories shows that neither monomers nor the dimers interact with any of the studied lipids in a specific manner. Only unspecific transient complexes of the probe with the lipids are formed.
The visual inspection (Fig. 4, A–D) and the distance distributions (Fig. 4 E) show that the probe is mostly adsorbed on the membrane surface in CL- and PG-containing membranes, whereas it is less frequently bound to the PS membrane and only occasionally contacts the PC/PE membrane in the course of random diffusion. There is no significant difference in adsorption propensity between monomers and dimers.
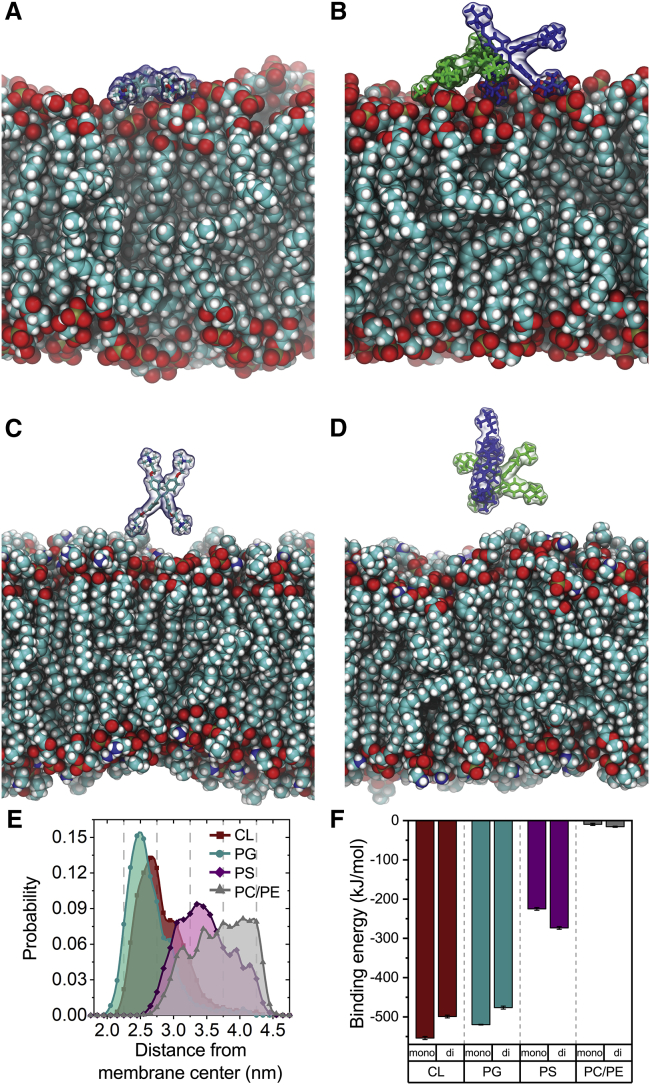
Figure 4.
Snapshots of equilibrated MD trajectories for CL with probe monomer (A), CL with probe dimer (B), PC/PE with probe monomer (C), and PC/PE with probe dimer (D). The lipids are shown in space-fill representation. The TTAPE-Me molecules are shown as balls and sticks with a semitransparent molecular surface. In the case of dimers, monomers are shown in a different color. (E) Distributions of the distances from TTAPE-Me monomer to the center of lipid bilayer in MD simulations. (F) Interaction energies of TTAPE-Me (per monomer) with different lipid bilayers computed over equilibrated parts of MD trajectories. Error bars correspond to the standard error. To see this figure in color, go online.
The probe molecule adsorbs on top of the lipid headgroups. Multiple sorption/desorption events are observed in the course of simulations with one or more choline groups of TTAPE-Me in contact with lipid headgroups (Fig. 4, A–D). The probe does not penetrate into the bilayer and does not intercalate between the lipid headgroups. This is consistent with the previous finding demonstrating that the mode of TTAPE-Me interaction with anionic lipids is primarily electrostatic by nature (28,29) because of interaction energy between the probe, and the membrane is dominated by electrostatic interactions and correlates strongly with lipid net charge and the charge of the headgroup (Fig. 4 E; Table 1). The probe is attracted to the membrane by the lipid net charge, whereas the strength of binding is likely to be further tuned by the part of the headgroup located above the phosphate moiety, which is in direct contact with the probe. Indeed, the probe binds strongly to CL and PG, which lack any charged moieties above the phosphate. The binding to PS, which possesses a serine group, is weaker because of unfavorable interactions with the exposed primary amine. Finally, there is no interaction at all with PC and PE because these lipids hold a net neutral charge and own an exposed amine (choline and ethanolamine, respectively), which repel strongly cationic TTAPE-Me molecule.
Table 1.
Binding of TTAPE-Me to the lipid bilayer as a function of the lipid total charge and the charge of headgroup above the phosphate moiety
| Lipid type | Monomer binding energy (kJ/mol) | Dimer binding energy (per monomer) (kJ/mol) | Relative binding strength (monomer) | Relative binding strength (dimer) | Lipid total charge | Headgroup charge, excluding phosphate |
|---|---|---|---|---|---|---|
| CL | −553.7 ± 3.8 | −499.1 ± 3.5 | 1 | 1 | −2 | 0 |
| PG | −519.6 ± 0.9 | −476.2 ± 3.7 | 0.94 | 0.95 | −1 | 0 |
| PS | −224.9 ± 3.4 | −273.2 ± 3.5 | 0.41 | 0.55 | −1 | 0 (−1/+1) |
| PC/PE | −9.5 ± 3.4 | −15.7 ± 1.4 | 0.02 | 0.03 | 0 | +1 |
The mean binding energies and the standard errors are the same as in Fig. 4F. Relative binding strength is computed in comparison to CL.
The binding energies computed in MD simulations of monomers and dimers (Fig. 4 F) are fully consistent with observed fluorescence intensities (Fig. 3, A–D) and follow the same fluorescence and interaction efficiencies order: CL ≥ PG > PS ≫ PE.
Stability of TTAPE-Me dimers
To rationalize the role of probe monomers and aggregates in observed fluorescent response, we computed the potentials of mean force (PMFs) of TTAPE-Me dimer formation in three different environments: in ethyl acetate, in bulk water, and adsorbed on top of the CL bilayer by the means of umbrella sampling MD simulations. The results are shown in Fig. 5.
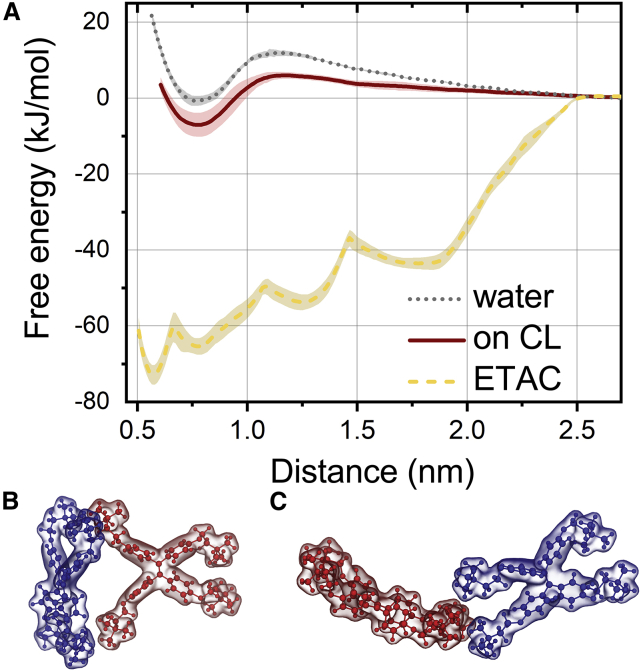
Figure 5.
Stability of TTAPE-Me dimers. (A) The PMFs of TTAPE-Me dimer formation in bulk water, ETAC, and adsorbed on top of CL lipid bilayer. (B and C) Representative structures of the “loose” TTAPE-Me dimers in ETAC with the center of masses distances of 1.2 and 1.8 nm, respectively. To see this figure in color, go online.
In bulk water, the free energies of dimers and monomers are comparable, and the barrier between them is only ∼12 kJ/mol. This suggests that the dynamic equilibrium between monomers and dimers in water could be shifted easily by the change of probe concentration. The optimal distance in the dimers is ∼0.8 nm, which is in perfect agreement with the results of free simulations in water (Fig. 5 A).
For the probe molecules adsorbed on top of the CL bilayer, the PMF is similar to one in bulk water, but the dimers are more energetically favorable (by ∼7 kJ/mol) in this case. This suggests that the membrane-bound fraction of the probe should be enriched in dimers in comparison to bulk solvent. The distance in the dimer is the same as in bulk water, which confirms our observation that the cogwheel dimers are adsorbed as is on the membranes (Fig. 5 A).
Finally, in ethyl acetate, much more complex behavior is observed. There is an extremely stable stacking dimer with the distance of ∼0.6 nm (Fig. 5 A, shown in Fig. 2 B). The energy barrier of its complete dissociation is very high (∼72 kJ/mol), which means that in experiments, almost all molecules stack in bulk ethyl acetate. Moreover, changes of probe concentration would have very little effect on equilibrium between monomers and dimers. In addition to the stable stacking dimer, there are three metastable aggregates: a cogwheel-like dimer (∼0.8 nm, similar to one observed in water, Fig. 2 A) and two looser dimers (∼1.2 and ∼1.8 nm). In the latter two, the monomers are in contact only by their “arms” (Fig. 5, B and C). All metastable aggregates have much higher energies in comparison to the stable stacking dimer and should not be populated significantly in experimental conditions.
Discussion
Our results show that the behavior of TTAPE-Me probe is much more complex than it was thought before, as follows:
-
1)
The fluorescence is much stronger in apolar solvents than in aqueous solution.
-
2)
The probe forms dimers, trimers, and higher-order aggregates eagerly. The topology of these aggregates and their relative abundance depend on the solvent polarity, probe concentration, and the presence of anionic lipids.
-
3)
TTAPE-Me is not selective to CL or to any other anionic lipid. The probe adsorbs on top of the membrane surface by means of unspecific electrostatic attraction modified slightly by the nature of exposed lipid headgroup moieties. Probe monomers and dimers exhibit very similar adsorption propensities.
The mechanism which makes TTAPE-Me fluorescent in the presence of anionic lipids and nonfluorescent in the bulk water is still not understood completely. Leung et al. (28) hypothesized that the probe forms fluorescent aggregates in the presence of anionic lipid but remains monomeric and nonfluorescent in water. However, no proofs of this hypothesis were reported to date.
Our data confirm higher fluorescence of TTAPE-Me dimers and aggregates. Indeed, the strongest fluorescence is observed in ethyl acetate solvent, in which all probe molecules exist in the form of extremely stable stacking aggregates, according to MD simulations (Fig. 2 B; Fig. 5 A). Very large Stokes shift of TTAPE-Me emission (Fig. 1 A) also strongly suggests a multimeric structure of emitting moieties.
However, we have shown that the structures of dimers and higher-order aggregates of TTAPE-Me in water and in ethyl acetate are different, which raises the question of whether their fluorescent properties are directly comparable.
The emission wavelength of TTAPE-Me is not affected by the probe concentration and is only slightly affected by the solvent polarity (Fig. 1 C). This precludes the formation of emitting J-aggregates, which are characterized by pronounced spectral shifts (46,47) or the presence of solvatochromic effects, based on the dipole-dipole interaction between the probe and the solvent molecules (47). Thus, the most plausible explanation of the dramatic decrease of the fluorescent intensity of TTAPE-Me in water and other highly polar solvents is strong quenching because of the hydrogen bonds formation between the probe and solvent molecules (48). Four ether groups of TTAPE-Me are likely to serve as effective proton acceptors in water and polar solvents, which facilitate such quenching. The details of these phenomena require investigation with dedicated quantum chemical computations, which are beyond the scope of this article.
Using the PMFs in Fig. 5 A, it is easy to compute the equilibrium ratio between dye monomers and dimers. According to Boltzmann distribution, the ratio of probabilities of monomeric and dimeric states is P = Pmonomer/Pdimer = exp(Edimer − Emonomer/kBT), where E is the free energy corresponding to dimer or monomer, respectively, kB is the Boltzmann constant, and T = 300 K as the absolute temperature. Because Pmonomer + Pdimer = 1, the equilibrium ratio of monomers and dimers in the system is r = 1/(1 + P).
In bulk water, r = 0.57 (there are 57% of dimers), whereas on top of CL bilayer, r = 0.94 (there are 94% of dimers). If we assume that the monomers are not fluorescent at all and that all probe molecules are adsorbed on the membrane, then the ideal fluorescence ratio between bulk water and CL bilayer is ∼0.94/0.57 = 1.6 times. The difference in our experiments is much higher (up to ∼10 times) at TTAPE-Me concentration of 10 μM.
This discrepancy is easily explainable by the absence of trimers and higher-order aggregates in our simplified calculations, inaccuracy of PMFs because of force field limitations, and the fact that all simulations were done in the ground state of the probe, whereas excitation may alter the energy of dimer formation significantly. Nevertheless, our simulations confirm qualitatively the hypothesis of Leung et al. (28) about the nature of TTAPE-Me nonfluorescence in bulk water.
However, our results completely disprove the conclusion of Leung et al. (28) concerning selectivity of TTAPE-Me to CL. In our experiments the fluorescent responses of TTAPE-Me for different anionic phospholipids have comparable intensities and indistinguishable spectral shapes. We have shown that TTAPE-Me is unable to discriminate between CL, PG, and PS, neither in small artificial liposomes nor in bacterial ISOv and isolated yeast mitochondria with different anionic lipid compositions. The unbalanced ratios of PG and CL are frequent events in eukaryotic mitochondrial membranes (30). In the plasma membranes of eukaryotic cells, coincidental surface exposure of CL and PS is also not unprecedented. CL could be missorted from mitochondria to the cell surface on the early stages of apoptosis, which acts as an in vivo trigger for the production of anti-CL antibodies (20,21).
TTAPE-Me could only be used for detecting CL in the wild-type mitochondrial membranes, which lack significant amounts of any other anionic lipids at standard conditions. This probe is not applicable for this purpose in eukaryotic cells, Gram-positive and Gram-negative bacteria, and the cell-derived vesicles neither in vitro nor in vivo.
Our data allow for formulating the following hypothesis of TTAPE-Me action in the presence of anionic lipid membranes. The fluorescence comes from emitting probe aggregates, which are more energetically favorable in contact with the headgroups of anionic lipids than in bulk water (Fig. 5). The higher is the net negative charge of the membrane, the more emitting probe aggregates would adsorb on its surface unspecifically, and the stronger is the fluorescence signal. This scheme describes all our experimental and simulation data in a simple and elegant way and explains observed fluorescent intensities in a row CL ≥ PG > PS ≫ PC/PE.
We want to emphasize that TTAPE-Me is an interesting molecule with surprising reach and nontrivial behavior, which deserves further study. However, we demonstrated that the TTAPE-Me probe exhibits enhanced fluorescence of similar magnitude upon binding to CL or PG and with somewhat smaller magnitude to PS and therefore is not specific for CL and detected PG with the same efficiency. Therefore, it is not suitable as a selective fluorescent probe for CL and, in our opinion, should not be used, advertised, or marketed in this manner.
Conclusions
Our data show that TTAPE-Me is not selective to CL or to any other anionic lipid. This probe is sensitive to the net change of the membrane surface and the charge of exposed moieties of the lipid headgroups in a generic manner. The most plausible mechanism of this sensitivity is higher stability of emitting probe aggregates adsorbed on the headgroups of anionic lipids by the means of unspecific but thermodynamically favorable electrostatic interactions. The fluorescent responses of TTAPE-Me for different anionic lipids are comparable and do not allow for discriminating between CL, PG, and PS lipids by either shape of the spectra or emission intensities.
TTAPE-Me could still be relevant for detecting CL in wild-type mitochondria, which lack significant amounts of any other anionic lipids. However, this probe is expected to bind promiscuously to all anionic phospholipids in Gram-positive and Gram-negative bacteria, mitochondria with unbalanced ratios of anionic phospholipids, all nonmitochondrial membranes of eukaryotic cells, cell-derived vesicles, exosomes, etc. Therefore, marketing of TTAPE-Me as “cardiolipin probe” is highly misleading because this probe is not suitable for selective labeling of CL in the membranes in which other anionic lipids are present in non-negligible physiological amounts.
These results should be taken into consideration when interpreting past and future results of CL detection and localization studies with TTAPE-Me probe in vivo and in vitro.
Author contributions
M.B. initiated the research. M.B., S.Y., and K.P. designed the experiments. E. coli strains were cultivated by M.B., and the ISOv were isolated with the assistance of K.P. S.Y. performed in silico experiments. K.P. performed in vitro experiments. All authors interpreted the data and wrote the manuscript.
Acknowledgments
We are grateful to Venkata K. P. S. Mallampalli and Dr. Eugenia Mileykovskaya for providing isolated mitochondria and to Prof. A. P. Demchenko for discussion and useful suggestions.
This work was supported by North Atlantic Treaty Organization Science for Peace and Security Programme SPS 985291 (to M.B.), the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreements 690853 (to M.B. and S.Y.), Kazan Federal University Strategic Academic Leadership Program, Russia (to M.B.), Russian Science Foundation, Russia grant RCF 20-14-00166 (to M.B. as co-investigator), National Institutes of General Medical Sciences, USA grants GM115969 and GM121493, and the John S. Dunn Foundation, USA (to M.B. as co-investigator). K.P. and S.Y. were supported by a stipend from North Atlantic Treaty Organization Science for Peace and Security Programme SPS 985291. S.Y. was supported by the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No. 796245.
Editor: John Conboy.
Footnotes
Kyrylo Pyrshev, Semen Yesylevskyy, and Mikhail Bogdanov contributed equally to this work.
A preliminary version of this work, [doi.org/10.1101/2020.09.10.292433], was deposited in BioRxiv on September 11, 2020.
Supporting material can be found online at https://doi.org/10.1016/j.bpj.2021.06.039.
Contributor Information
Kyrylo Pyrshev, Email: kyrylo.a.pyrshev@uth.tmc.edu.
Mikhail Bogdanov, Email: mikhail.v.bogdanov@uth.tmc.edu.
Supporting citations
References (49, 50, 51) appear in the Supporting material.
Supporting material
References
- 1.Lewis R.N., McElhaney R.N. The physicochemical properties of cardiolipin bilayers and cardiolipin-containing lipid membranes. Biochim. Biophys. Acta. 2009;1788:2069–2079. doi: 10.1016/j.bbamem.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 2.Paradies G., Paradies V., Petrosillo G. Role of cardiolipin in mitochondrial function and dynamics in health and disease: molecular and pharmacological aspects. Cells. 2019;8:728. doi: 10.3390/cells8070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Planas-Iglesias J., Dwarakanath H., Klein-Seetharaman J. Cardiolipin interactions with proteins. Biophys. J. 2015;109:1282–1294. doi: 10.1016/j.bpj.2015.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gupta K., Donlan J.A.C., Robinson C.V. The role of interfacial lipids in stabilizing membrane protein oligomers. Nature. 2017;541:421–424. doi: 10.1038/nature20820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryabichko S., Ferreira V.M., Bogdanov M. Cardiolipin is required in vivo for the stability of bacterial translocon and optimal membrane protein translocation and insertion. Sci. Rep. 2020;10:6296. doi: 10.1038/s41598-020-63280-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang M., Mileykovskaya E., Dowhan W. Cardiolipin is essential for organization of complexes III and IV into a supercomplex in intact yeast mitochondria. J. Biol. Chem. 2005;280:29403–29408. doi: 10.1074/jbc.M504955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schlame M., Greenberg M.L. Biosynthesis, remodeling and turnover of mitochondrial cardiolipin. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:3–7. doi: 10.1016/j.bbalip.2016.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghio S., Camilleri A., Vassallo N. Cardiolipin promotes pore-forming activity of alpha-synuclein oligomers in mitochondrial membranes. ACS Chem. Neurosci. 2019;10:3815–3829. doi: 10.1021/acschemneuro.9b00320. [DOI] [PubMed] [Google Scholar]
- 9.Etienne F., Roche Y., Bernard S. Cardiolipin packing ability studied by grazing incidence X-ray diffraction. Chem. Phys. Lipids. 2008;152:13–23. doi: 10.1016/j.chemphyslip.2007.12.002. [DOI] [PubMed] [Google Scholar]
- 10.Mileykovskaya E., Dowhan W. Cardiolipin membrane domains in prokaryotes and eukaryotes. Biochim. Biophys. Acta. 2009;1788:2084–2091. doi: 10.1016/j.bbamem.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oliver P.M., Crooks J.A., Weibel D.B. Localization of anionic phospholipids in Escherichia coli cells. J. Bacteriol. 2014;196:3386–3398. doi: 10.1128/JB.01877-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tran T.T., Panesso D., Arias C.A. Daptomycin-resistant Enterococcus faecalis diverts the antibiotic molecule from the division septum and remodels cell membrane phospholipids. mBio. 2013;4 doi: 10.1128/mBio.00281-13. e00281-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.El Khoury M., Swain J., Mingeot-Leclercq M.-P. Targeting bacterial cardiolipin enriched microdomains: an antimicrobial strategy used by amphiphilic aminoglycoside antibiotics. Sci. Rep. 2017;7:10697. doi: 10.1038/s41598-017-10543-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Renner L.D., Weibel D.B. MinD and MinE interact with anionic phospholipids and regulate division plane formation in Escherichia coli. J. Biol. Chem. 2012;287:38835–38844. doi: 10.1074/jbc.M112.407817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Luévano-Martínez L.A., Kowaltowski A.J. Phosphatidylglycerol-derived phospholipids have a universal, domain-crossing role in stress responses. Arch. Biochem. Biophys. 2015;585:90–97. doi: 10.1016/j.abb.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 16.Dalebroux Z.D., Edrozo M.B., Miller S.I. Delivery of cardiolipins to the Salmonella outer membrane is necessary for survival within host tissues and virulence. Cell Host Microbe. 2015;17:441–451. doi: 10.1016/j.chom.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Z., Zhang Q., Shi H. Quantitative connection between cell size and growth rate by phospholipid metabolism. Cells. 2020;9:391. doi: 10.3390/cells9020391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bogdanov M., Pyrshev K., Dowhan W. Phospholipid distribution in the cytoplasmic membrane of Gram-negative bacteria is highly asymmetric, dynamic, and cell shape-dependent. Sci. Adv. 2020;6:eaaz6333. doi: 10.1126/sciadv.aaz6333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kudryakova I.V., Suzina N.E., Vasilyeva N.V. Studying factors involved in biogenesis of Lysobacter sp. XL1 outer membrane vesicles. Biochemistry (Mosc.) 2017;82:501–509. doi: 10.1134/S0006297917040125. [DOI] [PubMed] [Google Scholar]
- 20.Sorice M., Circella A., Valesini G. Cardiolipin on the surface of apoptotic cells as a possible trigger for antiphospholipids antibodies. Clin. Exp. Immunol. 2000;122:277–284. doi: 10.1046/j.1365-2249.2000.01353.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sorice M., Circella A., Esposti M.D. Cardiolipin and its metabolites move from mitochondria to other cellular membranes during death receptor-mediated apoptosis. Cell Death Differ. 2004;11:1133–1145. doi: 10.1038/sj.cdd.4401457. [DOI] [PubMed] [Google Scholar]
- 22.Kagan V.E., Bayır H., Balasubramanian K. Elimination of the unnecessary: intra- and extracellular signaling by anionic phospholipids. Biochem. Biophys. Res. Commun. 2017;482:482–490. doi: 10.1016/j.bbrc.2016.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maguire J.J., Tyurina Y.Y., Kagan V.E. Known unknowns of cardiolipin signaling: the best is yet to come. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862:8–24. doi: 10.1016/j.bbalip.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Corey R.A., Pyle E., Collinson I. Specific cardiolipin-SecY interactions are required for proton-motive force stimulation of protein secretion. Proc. Natl. Acad. Sci. USA. 2018;115:7967–7972. doi: 10.1073/pnas.1721536115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gohil V.M., Gvozdenovic-Jeremic J., Greenberg M.L. Binding of 10-N-nonyl acridine orange to cardiolipin-deficient yeast cells: implications for assay of cardiolipin. Anal. Biochem. 2005;343:350–352. doi: 10.1016/j.ab.2005.04.039. [DOI] [PubMed] [Google Scholar]
- 26.Pogmore A.-R., Seistrup K.H., Strahl H. The Gram-positive model organism Bacillus subtilis does not form microscopically detectable cardiolipin-specific lipid domains. Microbiology (Reading) 2018;164:475–482. doi: 10.1099/mic.0.000639. [DOI] [PubMed] [Google Scholar]
- 27.Lobasso S., Saponetti M.S., Corcelli A. Archaebacterial lipid membranes as models to study the interaction of 10-N-nonyl acridine orange with phospholipids. Chem. Phys. Lipids. 2009;157:12–20. doi: 10.1016/j.chemphyslip.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 28.Leung C.W., Hong Y., Tang B.Z. Superior fluorescent probe for detection of cardiolipin. Anal. Chem. 2014;86:1263–1268. doi: 10.1021/ac403616c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elmer-Dixon M.M., Hoody J., Bowler B.E. Cardiolipin preferentially partitions to the inner leaflet of mixed lipid large unilamellar vesicles. J. Phys. Chem. B. 2019;123:9111–9122. doi: 10.1021/acs.jpcb.9b07690. [DOI] [PubMed] [Google Scholar]
- 30.Matsumura A., Higuchi J., Oka T. Inactivation of cardiolipin synthase triggers changes in mitochondrial morphology. FEBS Lett. 2018;592:209–218. doi: 10.1002/1873-3468.12948. [DOI] [PubMed] [Google Scholar]
- 31.Hess B., Kutzner C., Lindahl E. GROMACS 4: algorithms for highly efficient, load-balanced, and scalable molecular simulation. J. Chem. Theory Comput. 2008;4:435–447. doi: 10.1021/ct700301q. [DOI] [PubMed] [Google Scholar]
- 32.Jo S., Kim T., Im W. CHARMM-GUI: a web-based graphical user interface for CHARMM. J. Comput. Chem. 2008;29:1859–1865. doi: 10.1002/jcc.20945. [DOI] [PubMed] [Google Scholar]
- 33.Lee J., Cheng X., Im W. CHARMM-GUI input generator for NAMD, GROMACS, AMBER, OpenMM, and CHARMM/OpenMM simulations using the CHARMM36 additive force field. J. Chem. Theory Comput. 2016;12:405–413. doi: 10.1021/acs.jctc.5b00935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yesylevskyy S.O. Pteros 2.0: evolution of the fast parallel molecular analysis library for C++ and python. J. Comput. Chem. 2015;36:1480–1488. doi: 10.1002/jcc.23943. [DOI] [PubMed] [Google Scholar]
- 35.Tan B.K., Bogdanov M., Guan Z. Discovery of a cardiolipin synthase utilizing phosphatidylethanolamine and phosphatidylglycerol as substrates. Proc. Natl. Acad. Sci. USA. 2012;109:16504–16509. doi: 10.1073/pnas.1212797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bogdanov M., Dowhan W. Lipid-dependent generation of dual topology for a membrane protein. J. Biol. Chem. 2012;287:37939–37948. doi: 10.1074/jbc.M112.404103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bogdanov M., Xie J., Dowhan W. To flip or not to flip: lipid-protein charge interactions are a determinant of final membrane protein topology. J. Cell Biol. 2008;182:925–935. doi: 10.1083/jcb.200803097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bogdanov M., Heacock P.N., Dowhan W. Study of polytopic membrane protein topological organization as a function of membrane lipid composition. Methods Mol. Biol. 2010;619:79–101. doi: 10.1007/978-1-60327-412-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang M., Mileykovskaya E., Dowhan W. Gluing the respiratory chain together. Cardiolipin is required for supercomplex formation in the inner mitochondrial membrane. J. Biol. Chem. 2002;277:43553–43556. doi: 10.1074/jbc.C200551200. [DOI] [PubMed] [Google Scholar]
- 40.Feitosa E., Barreleiro P.C., Olofsson G. Phase transition in dioctadecyldimethylammonium bromide and chloride vesicles prepared by different methods. Chem. Phys. Lipids. 2000;105:201–213. doi: 10.1016/s0009-3084(00)00127-4. [DOI] [PubMed] [Google Scholar]
- 41.Pyrshev K.A., Klymchenko A.S., Demchenko A.P. Apoptosis and eryptosis: striking differences on biomembrane level. Biochim. Biophys. Acta Biomembr. 2018;1860:1362–1371. doi: 10.1016/j.bbamem.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 42.Demchenko A.P. Third Edition. Springer International Publishing; Basel, Switzerland: 2020. Introduction to Fluorescence Sensing: Volume 1: Materials and Devices. [Google Scholar]
- 43.Rowlett V.W., Mallampalli V.K.P.S., Vitrac H. Impact of membrane phospholipid alterations in Escherichia coli on cellular function and bacterial stress adaptation. J. Bacteriol. 2017;199 doi: 10.1128/JB.00849-16. e00849-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vitrac H., Dowhan W., Bogdanov M. Effects of mixed proximal and distal topogenic signals on the topological sensitivity of a membrane protein to the lipid environment. Biochim. Biophys. Acta Biomembr. 2017;1859:1291–1300. doi: 10.1016/j.bbamem.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Jiang F., Ryan M.T., Greenberg M.L. Absence of cardiolipin in the crd1 null mutant results in decreased mitochondrial membrane potential and reduced mitochondrial function. J. Biol. Chem. 2000;275:22387–22394. doi: 10.1074/jbc.M909868199. [DOI] [PubMed] [Google Scholar]
- 46.Hestand N.J., Spano F.C. Expanded theory of H- and J-molecular aggregates: the effects of vibronic coupling and intermolecular charge transfer. Chem. Rev. 2018;118:7069–7163. doi: 10.1021/acs.chemrev.7b00581. [DOI] [PubMed] [Google Scholar]
- 47.Demchenko A.P. Second Edition. Springer International Publishing; Basel, Switzerland: 2015. Introduction to Fluorescence Sensing. [Google Scholar]
- 48.Pivovarenko V.G., Klueva A.V., Demchenko A.P. Bands separation in fluorescence spectra of ketocyanine dyes: evidence for their complex formation with monohydric alcohols. Chem. Phys. Lett. 2000;325:389–398. [Google Scholar]
- 49.Huang J., MacKerell A.D., Jr. CHARMM36 all-atom additive protein force field: validation based on comparison to NMR data. J. Comput. Chem. 2013;34:2135–2145. doi: 10.1002/jcc.23354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Humphrey W., Dalke A., Schulten K. VMD: visual molecular dynamics. J. Mol. Graph. 1996;14:33–38, 27–28.. doi: 10.1016/0263-7855(96)00018-5. [DOI] [PubMed] [Google Scholar]
- 51.Bozelli J.C., Jr., Hou Y.H., Epand R.M. Lipid asymmetry of a model mitochondrial outer membrane affects Bax-dependent permeabilization. Biochim. Biophys. Acta Biomembr. 2020;1862:183241. doi: 10.1016/j.bbamem.2020.183241. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.