Abstract
In age-matched controlled studies performed in Japan, enterotoxigenic Bacteroides fragilis was isolated from 14.9% of 114 children aged 1 to 14 years with antibiotic-unassociated diarrhea (AUD) and 6.5% of 108 children aged 1 to 6 years with antibiotic-associated diarrhea (AAD). The difference in comparison with control children, was significant for AUD children but not AAD children.
Bacteroides fragilis, an anaerobic gram-negative bacillus, may cause various types of endogenous extraintestinal infections such as intra-abdominal infection and sepsis. In 1984, enterotoxigenic B. fragilis (ETBF) strains were found in newborn lambs with diarrheal disease (6), and they have been recognized as diarrheal pathogens in young domestic animals (1, 7–9). Early investigations producing evidence that ETBF may have a role in human diarrhea focused on children in developing areas (10, 13) or countries (12) who had close contact with animals. Two studies carried out in developed countries gave contrasting results: a significant association of ETBF with childhood diarrheal disease was found in the United States (14) but not in Italy (18). Thus, additional investigations are required to uncover the role of ETBF in human diarrheal disease in developed countries. In this study, we investigated the prevalence of ETBF in children who lived in an urban area of Nagoya, Japan.
A total of 416 children aged between 1 month and 14 years who visited the Department of Pediatrics, Meitetsu Hospital, Nagoya, Japan, between February and August 1996 and were negative for common enteropathogenic bacteria were enrolled in this study. Subjects consisted of 137 children with antibiotic-unassociated diarrhea (AUD), 166 with antibiotic-associated diarrhea (AAD), and 113 without diarrhea (controls). To obtain age-matched results, AUD children were compared with control children aged ≤14 years and AAD children were compared with controls aged ≤6 years.
Stool specimens were frozen in Kenki-porter vials (Clinical Supply, Hashima, Japan), used as anaerobic transporters, at −80°C until they were cultured anaerobically on Bacteroides bile esculin (BBE) agar (Kyokuto Seiyaku, Tokyo, Japan) for isolation of B. fragilis. Stool cultures were carried out within a month after sampling. Five colonies per sample, if available, were selected randomly from BBE agar and subjected to PCR as described below. Rotavirus was detected directly from stool specimens by using the Rotavirus TestPack kit (Abbott Laboratories, Abbott Park, Ill.), and adenovirus was detected by using the ADENOCLONE E kit (Meridian Diagnostics, Inc., Cincinnati, Ohio) according to the manufacturers’ protocols.
Bacterial DNA was extracted by heating for 10 min at 95°C. The primer set used for identification of B. fragilis was GBI-11 (5′-GCCGGTCAGAATGGAGTAGGAGACC-3′) and GBI-12 (5′-CCCGACCCGGACCTTGCAACAGA-3′), which amplified a 262-bp segment of the neuraminidase gene (3). The bft gene, encoding B. fragilis enterotoxin (BFT), was identified by using a primer set consisting of GBF-201, 5′-GAACCTAAAACGGTATATGT-3′ (corresponding to bases 729 to 748), and GBF-210, 5′-GTTGTAGACATCCCACTGGC-3′ (bases 1077 to 1096) (2). The expected DNA product was 368 bp. Amplification was performed for 35 cycles as follows: 95°C for 20 s and 62°C for 2 min, followed by a final 5-min extension at 74°C. PCR products were separated by electrophoresis on a 5% polyacrylamide gel and visualized under UV light following ethidium bromide staining.
A cell culture assay using HT29/C1 cells to detect BFT was performed as described previously (4, 5), with the exception that cell morphological changes were read after 2- and 24-h incubation periods.
Age distribution of each group was analyzed by the Wilcoxon rank sum test. Fisher’s exact test was used for contingency tables.
The specificity of PCR primers GBF-201 and GBF-210 was tested by using 133 B. fragilis strains comprising 82 strains that were BFT positive and 51 that were BFT negative by cell culture assay. The results of the PCR assay were consistent with those of the cell culture assay, except for one strain from a control child which was PCR positive but cell culture negative. Repeated assays gave the same results. Although it is unknown whether the strain produces BFT at too low a level to be detected by cell culture assay or whether its bft gene is silent or incomplete, the strain was counted as ETBF in this study.
Subjects were divided into two groups according to age, <1 year and ≥1 year. Mean ages of AUD children aged 1 to 14 years and their counterpart controls were 4.8 ± 3.7 and 4.9 ± 3.9 years, respectively. Mean ages of AAD children aged 1 to 6 years and their counterpart controls were 2.2 ± 1.4 and 2.7 ± 1.7 years, respectively. The differences between the two groups in each case were not statistically significant.
Rates of recovery of B. fragilis, ETBF, rotavirus, and adenovirus from AUD children and controls are shown in Table 1. Although the rate of isolation of B. fragilis from AUD children aged 1 to 14 years was comparable to that for controls, the rate of carriage of ETBF was significantly higher in AUD children (14.9%) than in controls (4.9%). AUD children harbored rotavirus and adenovirus more frequently than did their control counterparts (P < 0.01).
TABLE 1.
Recovery of B. fragilis, ETBF, rotavirus, and adenovirus from children with AUD and control children
| Organism | No. (%) of children from whom indicated
organism was recovered in group:
|
|||
|---|---|---|---|---|
| AUD (n =
137)
|
Controls (n = 113)
|
|||
| <1 yr (n = 23) | 1–14 yr (n = 114) | <1 yr (n = 32) | 1–14 yr (n = 81) | |
| B. fragilis | 6 (26.1) | 61 (53.5) | 5 (15.6) | 42 (51.9) |
| ETBF | 2 (8.7) | 17 (14.9)a | 0 (0) | 4 (4.9) |
| Rotavirus | 9 (39.1)b | 23 (20.2) | 3 (9.4) | 11 (13.6) |
| Adenovirus | 0 (0) | 10 (8.8)b | 0 (0) | 0 (0) |
P < 0.05 compared with age-matched controls.
P < 0.01.
Isolation rates for all organisms but B. fragilis were rather similar between AAD children and controls (Table 2). B. fragilis was less frequently isolated from AAD children aged 1 to 6 years than from their control counterparts (P < 0.01).
TABLE 2.
Recovery of B. fragilis, ETBF, rotavirus, and adenovirus from children with AAD and control children
| Organism | No. (%) of children from whom indicated
organism was recovered in group:
|
|||
|---|---|---|---|---|
| AAD (n =
166)
|
Controls (n = 88)
|
|||
| <1 yr (n = 58) | 1–6 yr (n = 108) | <1 yr (n = 32) | 1–6 yr (n = 56) | |
| B. fragilis | 10 (17.2) | 25 (23.1)a | 5 (15.6) | 27 (48.2) |
| ETBF | 3 (5.2) | 7 (6.5) | 0 (0) | 3 (5.4) |
| Rotavirus | 10 (17.2) | 17 (15.7) | 3 (9.4) | 9 (16.1) |
| Adenovirus | 0 (0) | 2 (1.9) | 0 (0) | 0 (0) |
P < 0.01 compared with age-matched controls.
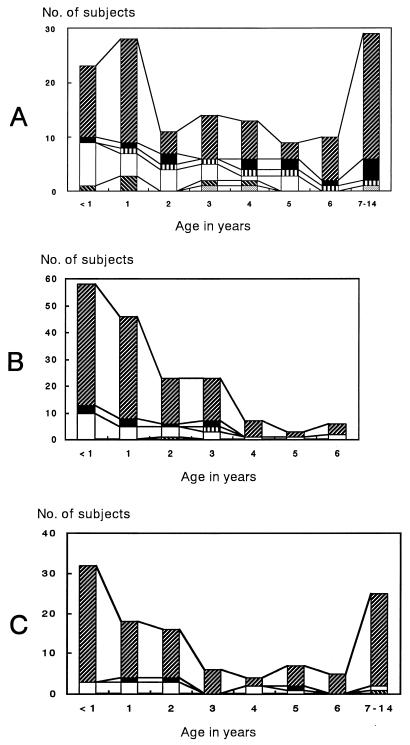
The incidence of concomitant recoveries of ETBF, rotavirus, and adenovirus by age is illustrated in Fig. 1. Concomitant detection of ETBF and rotavirus was observed with six AUD children aged <1 to 4 years, but no children studied had ETBF and adenovirus concurrently. ETBF alone was carried in 13 of the 19 ETBF-positive AUD children, 1 of the 10 ETBF-positive AAD children, and 1 of the 3 ETBF-positive controls. Mixed recoveries were very rare for AAD children and controls.
FIG. 1.
Incidence of the concomitant recoveries of ETBF, rotavirus, and adenovirus by age. Panels A, B, and C show distribution profiles for children with AUD, children with AAD, and control children, respectively. ▨, rotavirus negative, adenovirus negative, ETBF negative; ■, rotavirus negative, adenovirus negative, ETBF positive; ▥, rotavirus negative, adenovirus positive, ETBF negative; □, rotavirus positive, adenovirus negative, ETBF negative; ▧, rotavirus positive, adenovirus negative, ETBF positive; ░⃞, rotavirus positive, adenovirus positive, ETBF negative.
After omission of subjects positive for rotavirus, adenovirus, or both, rates of isolation of B. fragilis and ETBF were reevaluated. Of the 84 AUD children aged 1 to 14 years, 52.4 and 14.3% had B. fragilis and ETBF, respectively, while 54.3 and 4.3% of the 70 control counterparts carried B. fragilis and ETBF, respectively; the difference between rates of ETBF isolation was significant (P < 0.05). Of 89 AAD children aged 1 to 6 years, 20.2 and 6.7% were positive for B. fragilis and ETBF, respectively, while 51.1 and 6.4% of control counterparts were positive for B. fragilis and ETBF, respectively; the difference for B. fragilis was significant (P < 0.001).
Five colonies per sample, if available, were subjected to PCR. B. fragilis strains isolated from each child were always ETBF or nontoxigenic strains.
In previous studies, rates of isolation of ETBF from children aged ≥1 year with diarrhea varied: 4.8% in an urban setting in the United States (14), 9% in Bangladesh (12), 12% in a native-American setting (13), and 17% in Italy (11). B. fragilis was recovered from 32.1% of children with diarrhea in an urban setting in the United States (14) and 43% of children in Italy (11). The possible reason for higher rates of isolation of B. fragilis from AUD children observed in this study may be that we used BBE agar for primary culture and examined five colonies per subject.
In conclusion, in comparison with controls, ETBF was significantly associated with AUD but not AAD in Japanese children.
Acknowledgments
We thank George E. Killgore, Centers for Disease Control and Prevention, for editing the manuscript.
REFERENCES
- 1.Border M, Firehammer B D, Shoop D S, Myers L L. Isolation of Bacteroides fragilisfrom the feces of diarrheic calves and lambs. J Clin Microbiol. 1985;21:472–473. doi: 10.1128/jcm.21.3.472-473.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco A A, Mundy L M, Trucksis M, Wu S, Kaper J B, Sears C L. Cloning and characterization of the Bacteroides fragilismetalloprotease toxin gene. Infect Immun. 1997;65:1007–1013. doi: 10.1128/iai.65.3.1007-1013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jotwani R, Kato N, Kato H, Watanabe K, Ueno K. Detection of Bacteroides fragilisin clinical specimens by polymerase chain reaction amplification of the neuraminidase gene. Curr Microbiol. 1995;31:215–219. doi: 10.1007/BF00298376. [DOI] [PubMed] [Google Scholar]
- 4.Kato N, Karuniawati A, Jotwani R, Kato H, Watanabe K, Ueno K. Isolation of enterotoxigenic Bacteroides fragilisfrom extraintestinal sites by cell culture assay. Clin Infect Dis. 1995;20(Suppl. 2):S141. doi: 10.1093/clinids/20.supplement_2.s141. [DOI] [PubMed] [Google Scholar]
- 5.Kato N, Kato H, Watanabe K, Ueno K. Association of enterotoxigenic Bacteroides fragiliswith bacteremia. Clin Infect Dis. 1996;23(Suppl. 1):S83–S86. doi: 10.1093/clinids/23.supplement_1.s83. [DOI] [PubMed] [Google Scholar]
- 6.Myers L L, Firehammer B D, Shoop D S, Border M M. Bacteroides fragilis: a possible cause of acute diarrheal disease in newborn lambs. Infect Immun. 1984;44:241–244. doi: 10.1128/iai.44.2.241-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Myers L L, Shoop D S. Association of enterotoxigenic Bacteroides fragiliswith diarrheal disease in young pigs. Am J Vet Res. 1987;48:774–775. [PubMed] [Google Scholar]
- 8.Myers L L, Shoop D S, Byars T D. Diarrhea associated with enterotoxigenic Bacteroides fragilisin foals. Am J Vet Res. 1987;48:1565–1567. [PubMed] [Google Scholar]
- 9.Myers L L, Shoop D S, Firehammer B D, Border M M. Association of enterotoxigenic Bacteroides fragiliswith diarrheal disease in calves. J Infect Dis. 1985;152:1344–1347. doi: 10.1093/infdis/152.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Myers L L, Shoop D S, Stackhouse L L, Newman F S, Flaherty R J, Letson G W, Sack R B. Isolation of enterotoxigenic Bacteroides fragilisfrom humans with diarrhea. J Clin Microbiol. 1987;25:2330–2333. doi: 10.1128/jcm.25.12.2330-2333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pantosti A, Menozzi M G, Frate A, Sanfilippo L, D’Ambrosio F, Malpeli M. Detection of enterotoxigenic Bacteroides fragilisand its toxin in stool samples from adults and children in Italy. Clin Infect Dis. 1997;24:12–16. doi: 10.1093/clinids/24.1.12. [DOI] [PubMed] [Google Scholar]
- 12.Sack R B, Albert M J, Alam K, Neogi P K, Akbar M S. Isolation of enterotoxigenic Bacteroides fragilisfrom Bangladeshi children with diarrhea: a controlled study. J Clin Microbiol. 1994;32:960–963. doi: 10.1128/jcm.32.4.960-963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sack R B, Myers L L, Almeido-Hill J, Shoop D S, Bradbury W C, Reid R, Santosham M. Enterotoxigenic Bacteroides fragilis: epidemiologic studies of its role as a human diarrhoeal pathogen. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 14.San Joaquin V H, Griffis J C, Lee C, Sears C L. Association of Bacteroides fragiliswith childhood diarrhea. Scand J Infect Dis. 1995;27:211–215. doi: 10.3109/00365549509019011. [DOI] [PubMed] [Google Scholar]