Abstract
Introduction:
Besides autoimmunity, recent studies have reported a role of the coagulation cascade in the pathogenesis of urticaria. However, the real-world data regarding the utility of measuring D-dimer levels in patients chronic spontaneous urticaria (CSU) is missing. This study was done to evaluate the D-dimer levels of CSU patients and study the relationship between raised D-dimer levels and disease severity and treatment response.
Methods:
A retrospective chart review of all adult (>18 years) CSU patients was done. Complete clinicodemographic data regarding the disease duration and treatment response were noted. Urticaria activity score over 7 days (UAS7) was used to assess disease severity.
Results:
Of total 141 patients, D-dimer levels were raised in 46 CSU patients (32.6%) with mean D-dimer levels at baseline in these patients being 329.53 ± 546.94 ng/ml. The proportion of patients with raised plasma D-dimer levels was higher in patients with severe CSU (12.9%, 27.2%, 54.5% in mild, moderate, and severe disease, respectively P < 0.001). No significant differences were found between those with raised D-dimers and those having normal D-dimer levels with respect to age, gender, presence of angioedema, history of atopy, presence of thyroid abnormality, ASST/APST positivity, and serum IgE.
Conclusion:
D-dimer levels parallels the disease severity and can help predict the need for higher dose of antihistamines and second-line therapy in CSU patients.
Keywords: Chronic spontaneous urticaria, coagulation, D-dimer, fibrinolysis, tranexamic acid, urticaria
Introduction
Although the role of coagulation cascade was proposed in urticaria pathogenesis as early as 2007, its exact portrayal has been elusive.[1] The coagulation pathway and inflammation are interlinked closely, and they actuate and promulgate each other. Thrombin-antithrombin and other products of coagulation/fibrinolysis cascade play an important part in inflammation and coagulation pathways by mast cell activation and increasing vascular permeability.[2,3] There is scarcity of data pertaining to relationship between D-dimer levels in chronic spontaneous urticaria (CSU) from Indian subcontinent, despite a significant large number of affected patients.
Methods
We performed a retrospective chart review of all adult (>18 years) CSU patients enrolled in our urticaria clinic between January 2017 to June 2018. The primary objective was to correlate the relationship between D-dimer levels and CSU disease severity, duration, atopy, thyroid abnormalities, total serum IgE, urticaria activity score (UAS), and treatment response to first and second-line drugs. Complete clinicodemographic data regarding the disease duration and treatment response were noted, and patients with insufficient data were excluded from the study. Urticaria activity score over 7 days (UAS7) was used to assess disease severity.[4] UAS7 was graded as urticaria-free = 0; well-controlled urticaria = 1–6; mild = 7–15; moderate = 16–27; and severe urticaria = 28–42.
All patients in our urticaria clinic are routinely started on standard dosages of second-generation antihistamines (AH1) once daily. Further dose titration is performed fortnightly according to patient response and EACCI guidelines.[5] Antihistamine non-responsiveness was defined as UAS7 >6 or <75% decrease from baseline UAS7 after 4 weeks of 4-times dose of antihistamines. Second-line drugs (cyclosporine 3 mg/kg/day, azathioprine 1-2 mg/kg/day, or omalizumab) are given to patients not controlled with four-fold increased dosage of antihistamines given for at least 4-weeks and require repeated courses of oral corticosteroids for symptomatic control. D-dimer, prothrombin time (PT), activated partial thromboplastin time (APTT) levels are usually performed in patients having severe or antihistamine non-responsive disease; and are usually repeated at 0, 8, and 16 weeks. Plasma D-dimer levels are measured using latex immunoassay with automated coagulation analyzer (ACL TOP 500) and level >250 ng/mL are indicated as raised (manufacturer's instruction). The reference ranges of PT and APTT are 9-12 seconds and 28-38 seconds, respectively.
Statistical analyses were performed using the Fisher's exact test (for categorical data) or Kruskal-Wallis H test (for continuous data) as appropriate using Statistical Package for the Social Sciences (SPSS) V23 (IBM, Armonk, NY) with P < 0.05 as significant. Binary logistic regression was applied to find an independent association of raised D-dimer in CSU patients after adjusting confounding variables which were found to be significantly associated in univariate analysis (p < 0.05).
Results
Of total urticaria patients screened during January 2017 to June 2018; 141 patients had sufficient information available regarding D-dimer levels and other clinicodemographic variables. Median level of D-dimer in these 141 CSU patients was 165 ng/ml (IQR, 310-112). Of 141, D-dimer levels were raised in 46 CSU patients (32.6%) with mean D-dimer levels at baseline in these patients being 329.53 ± 546.94 ng/ml.
The proportion of patients with raised plasma D-dimer levels was higher in patients with severe CSU (12.9%, 27.2%, 54.5% in mild, moderate, and severe disease, respectively P < 0.001). Mean D-dimer levels were high in patients with a higher UAS7 at baseline (p < 0.001). Patients with raised D-dimer levels required upgrading doses of AH1 (4 times normal dosing) for disease control (p < 0.001) and an increased need to be initiated on second-line drugs (immunosuppressants) significantly more frequently than those with normal D-dimer levels; P < 0.001, Table 1. No significant differences were found between those with raised D-dimers and those having normal D-dimer levels with respect to age, gender, presence of angioedema, history of atopy, presence of thyroid abnormality, ASST/APST positivity, and serum IgE, Table 1.
Table 1.
Factors associated with increased D-dimer (>250 ng/mL) in chronic spontaneous urticaria patients
| Parameter | CSU patients with normal D-dimer (=<250 ng/mL) (Total n=95) | CSU patients with raised D-dimer (>250 ng/mL) (Total n=46) | P |
|---|---|---|---|
| Age (years; mean±SD) | 34.45±11.87 | 36.17±11.17 | 0.41 |
| Sex (F/M) | 59/36 | 29/17 | 0.91 |
| Duration (months) | 45.6±23.8 | 44.9±22.5 | 0.85 |
| Presence of angioedema | 40 (42.1%) | 25 (54.3%) | 0.17 |
| History of Atopy | 31 (32.6%) | 13 (28.3%) | 0.59 |
| Presence of thyroid abnormality | 10 (10.5%) | 5 (12.2%) | 1.00 |
| Family history of urticaria | 12 (12.6%) | 5 (12.2%) | 0.76 |
| Positive ASST | 37 (38.9%) | 18 (39.1%) | 0.98 |
| Positive APST | 24 (25.3%) | 13 (28.3%) | 0.70 |
| Mean Serum Ig E (kU/L) (Mean±SD) | 440.6±569.12 | 756.5±1724.41 | 0.10 |
| Serum Ig E raised (>100 kU/L) | 70 (73.7%) | 35 (76.1%) | 0.75 |
| Mean UAS 0 weeks (Mean±SD) | 12.46±4.87 | 16.45±5.12 | <0.001* |
| Number of patients having mild, moderate and severe grading of baseline UAS7 | |||
| Mild | 27 | 4 | <0.001 |
| Moderate | 48 | 18 | * |
| Severe | 20 | 24 | |
| PT (in seconds) (Mean±SD) | 11.06±1.34 | 11.88±1.55 | 0.002* |
| PT raised | 7 (7.4%) | 17 (36.9%) | <0.001* |
| APTT (in seconds) (Mean±SD) | 33.33±3.26 | 36.48±4.32 | <0.001* |
| APTT raised | 4 (4.2%) | 18 (39.1%) | <0.001* |
| Dose of antihistamine required | |||
| Single | 19 | 2 | <0.001 |
| Two-fold | 21 | 1 | * |
| Three-fold | 20 | 5 | |
| Four-fold | 35 | 38 | |
| Second-line drugs used | 22 (23.1%) | 34 (73.9%) | <0.001 |
| Azathioprine | 12 (12.6%) | 19 (41.3%) | * |
| Cyclosporine | 10 (10.5%) | 15 (32.6%) |
*Significant P values; +- SD- Standard Deviation
Binary logistic regression was applied to detect if an independent association existed between raised D-dimer levels in CSU patients and raised PT, raised APTT, UAS7 at 0 weeks, dose of antihistamine and second-line agents. Patients having raised D-dimer had 15.75 (adjusted OR = 15.75; 95% CI: 3.56 to 69.65, P = 0.001), 77.35 (adjusted OR = 77.35; 95% CI: 10.91 to 548.14, P = 0.001), and 7.89 odds of having raised PT, raised APTT and severe UAS7 at baseline compared to the patients with normal levels, Table 2.
Table 2.
Multivariate binary logistic regression to find out independent association among confounding variables and raised D-dimer
| Variables | Beta- coefficient | S.E. | P | Adjusted Odds Ratio (OR) | 95% CI for Adjusted OR | |
|---|---|---|---|---|---|---|
|
| ||||||
| Lower | Upper | |||||
| PT raised | 2.757 | 0.758 | 0.001 | 15.75 | 3.56 | 69.65 |
| APTT raised | 4.348 | 0.999 | 0.001 | 77.35 | 10.91 | 548.14 |
| Mild UAS at 0 week | 0.129 | |||||
| Reference | ||||||
| Moderate UAS at 0 week | 1.445 | 0.987 | 0.143 | 4.24 | 0.61 | 29.347 |
| Severe UAS at 0 week | 2.066 | 1.025 | 0.044 | 7.89 | 1.059 | 58.788 |
| Dose of antihistamine | 0.716 | 0.433 | 0.098 | 2.05 | 00.877 | 4.78 |
| Adjuvant requirement | 0.592 | 1.922 | 0.758 | 1.81 | 0.042 | 78.16 |
| Constant | -6.455 | 1.699 | 0.001 | 0.002 | ||
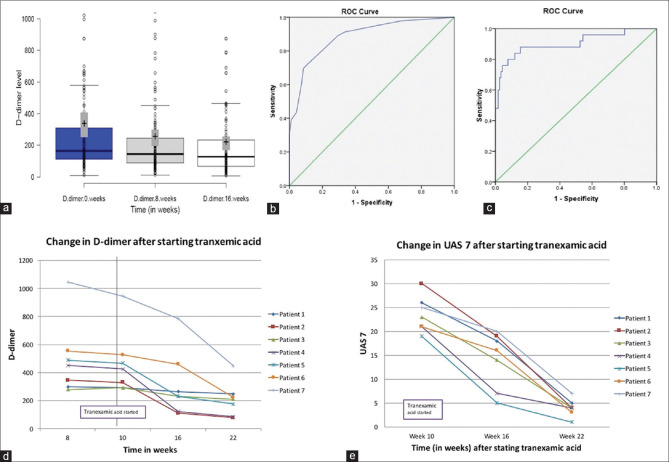
Mean D-dimer levels in those having raised levels decreased on treatment with AH1 and paralleled disease severity. Mean D-dimer levels at 0, 8 and 16 weeks after initiating treatment were 329.53 ± 546.94 ng/mL, 247.75 ± 373.05 ng/mL and 212.49 ± 301.25 ng/mL, respectively. Second-line drugs was started in 34/46 patients, the mean D-dimer level reduced to <250 ng/ml in 22 patients with elevated baseline D-dimer [Figure 1a].
Figure 1.
(a) Change in D-dimer levels after starting treatment with only antihistamines (8 weeks) and second line agents (16 weeks). (b) Receiver operating characteristics curves analysis with D-dimer level (b) 250 ng/ml and (c) 500 ng/ml, change in (d) D-dimer, and (e) UAS7 after starting tranexamic acid
Receiver operating characteristics curves analysis was performed with D-dimer level 250 ng/ml and 500 ng/ml; and area under the curve was found to be 0.88 and 0.90, respectively [Figure 1b and c] indicating higher cut-off level may be more beneficial in detecting patients with severe urticaria. However, for this study we took 250 ng/ml as cut-off owing to manufacturer's instructions.
It was also noted that in 7 patients with persistently raised D-dimer levels, 1 gm oral tranexamic acid was prescribed for 3 months along with AH1. Two patients responded with a decrease in UAS7 by 6 weeks while another 3 improved by 12 weeks, 2 patients failed to respond to TXA treatment. Mean UAS7 in 7 patients before starting TXA, and after 6 and 12 weeks of initiation of TXA were 23.57 ± 3.74, 14.14 ± 5.93, and 4.0 ± 1.83, respectively (p < 0.000) [Figure 1d and e].
Discussion
D-dimer is a sensitive marker for cross-linked fibrin degradation and coagulation activation and fibrinolysis. Earlier reports demonstrated a rise in D-dimer levels in 2/21 (10%),[1] 14/68 (20%)[6] and 58/120 (48.3%)[7] patients, respectively. In our study the D-dimer levels were elevated in 32.6% CSU patients. Higher D-dimer levels in CSU patients suggest role of coagulation cascade in the pathogenesis of the disease. Activation of coagulation cascade and generation of thrombin may result in mast cell degranulation leading to urticaria.[8] D-dimer has been found to correlate with the disease severity in CSU.[3,7] Our study had high UAS7 in patients with higher D-dimer levels as compared to controls. Moreover, levels of D-dimer were found higher in patients who did not respond to AH1 which is in accordance to previous studies.[9] In a study by Asero et al., 39% patients with increased d-dimer levels responded well to cyclosporine and d-dimer levels decreased after the clinical response to cyclosporine.[10] In another study evaluating the efficacy of monthly omalizumab 300 mg in anti-histamine refractory CSU showed complete response in 79% patients with elevated D-dimer levels.[11]
Tranexamic acid (TXA) inhibits plasmin formation, displaces plasminogen from fibrin surface and partially inhibits fibrinogenolysis at higher concentrations.[12] TXA exerts an anti-inflammatory effect by inhibiting plasmin-mediated activation of complement, monocytes, and neutrophils, there are only 2 previous studies and 3 isolated case reports of TXA use in CSU patients.[13] Laurberg et al.[14] reported no clinical benefit was observed with TXA in CSU patients with depressed C-1 esterase inhibitor value and Asero et al.[15] reported improvement in CSU patients on treatment with heparin and tranexamic acid both. In our study 5/7 patients with persistently raised D-dimer levels had responded well to TXA, thus TXA can be a helpful adjuvant in these specific cohort of patients just short of initiation of immunosuppressive therapy.
A major limitation of our study is its retrospective nature. To conclude, our study suggests that D-dimer levels parallels the disease severity in CSU and can help predict the need for higher dose requirement of antihistamines and a frequent need for second-line immunosuppressant therapy for better symptomatic relief. TXA can be useful adjuvant in this group of patients just short of immunosuppressive treatment. Large-scale, prospective, long-term follow up studies are required to exemplify our observations.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Asero R, Tedeschi A, Coppola R, Griffini S, Paparella P, Riboldi P, et al. Activation of the tissue factor pathway of blood coagulation in patients with chronic urticaria. J Allergy Clin Immunol. 2007;119:705–10. doi: 10.1016/j.jaci.2006.08.043. [DOI] [PubMed] [Google Scholar]
- 2.Wang F, Tang H, Xu JH, Kang KF. Activation of the blood coagulation cascade is involved in patients with chronic urticaria. J Allergy Clin Immunol. 2009;123:972–3. doi: 10.1016/j.jaci.2009.01.039. [DOI] [PubMed] [Google Scholar]
- 3.Asero R, Tedeschi A, Riboldi P, Griffini S, Bonanni E, Cugno M. Severe chronic urticaria is associated with elevated plasma levels of D-dimer. Allergy. 2008;63:176–80. doi: 10.1111/j.1398-9995.2007.01514.x. [DOI] [PubMed] [Google Scholar]
- 4.Erbagci Z. The leukotriene receptor antagonist montelukast in the treatment of chronic idiopathic urticaria: A single-blind, placebo-controlled, crossover clinical study. J Allergy Clin Immunol. 2002;110:484–8. doi: 10.1067/mai.2002.126676. [DOI] [PubMed] [Google Scholar]
- 5.Zuberbier T, Aberer W, Asero R, Abdul Latiff AH, Baker D, Ballmer-Weber B, et al. The EAACI/GA (2) LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy. 2018;73:1393–414. doi: 10.1111/all.13397. [DOI] [PubMed] [Google Scholar]
- 6.Asero R, Tedeschi A, Riboldi P, Cugno M. Plasma of patients with chronic urticaria shows signs of thrombin generation, and its intradermal injection causes wheal-and-flare reactions much more frequently than autologous serum. J Allergy Clin Immunol. 2006;117:1113–7. doi: 10.1016/j.jaci.2005.12.1343. [DOI] [PubMed] [Google Scholar]
- 7.Triwongwaranat D, Kulthanan K, Chularojanamontri L, Pinkaew S. Correlation between plasma D-dimer levels and the severity of patients with chronic urticaria. Asia Pac Allergy. 2013;3:100–5. doi: 10.5415/apallergy.2013.3.2.100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Obtułowicz A, Migacz-Gruszka K, Pirowska M, Basta-Klonowska K, Wojas-Pelc A. Participation of the coagulation system and fibrinolysis as well as selected biomarkers in pathogenesis of chronic urticaria with various activity degree. Postepy Dermatol Alergol. 2020;37:608–12. doi: 10.5114/ada.2020.98270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Asero R. D-dimer: A biomarker for antihistamine-resistant chronic urticaria. J Allergy Clin Immunol. 2013;132:983–6. doi: 10.1016/j.jaci.2013.04.037. [DOI] [PubMed] [Google Scholar]
- 10.Asero R. Plasma D-dimer levels and clinical response to ciclosporin in severe chronic spontaneous urticaria. J Allergy Clin Immunol. 2015;135:1401–3. doi: 10.1016/j.jaci.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 11.Asero R, Marzano AV, Ferrucci S, Cugno M. D-dimer plasma levels parallel the clinical response to omalizumab in patients with severe chronic spontaneous urticaria. Int Arch Allergy Immunol. 2017;172:40–4. doi: 10.1159/000453453. [DOI] [PubMed] [Google Scholar]
- 12.Shedden C, Highet AS. Delayed pressure urticaria controlled by tranexamic acid. Clin Exp Dermatol. 2006;31:295–6. doi: 10.1111/j.1365-2230.2005.02014.x. [DOI] [PubMed] [Google Scholar]
- 13.Holm JG, Ivyanskiy I, Thomsen SF. Use of nonbiologic treatments in antihistamine-refractory chronic urticaria: A review of published evidence. J Dermatolog Treat. 2018;29:80–97. doi: 10.1080/09546634.2017.1329505. [DOI] [PubMed] [Google Scholar]
- 14.Laurberg G. Tranexamic acid (Cyklokapron) in chronic urticaria: A double-blind study. Acta Derm Venereol. 1977;57:369–70. [PubMed] [Google Scholar]
- 15.Asero R, Tedeschi A, Cugno M. Heparin and tranexamic Acid therapy may be effective in treatment-resistant chronic urticaria with elevated D-dimer: A pilot study. Int Arch Allergy Immunol. 2010;152:384–9. doi: 10.1159/000292947. [DOI] [PubMed] [Google Scholar]