Abstract
Background:
Dermatophytosis has recently emerged as a major public health problem in the Indian subcontinent, most cases becoming chronic and recurrent.
Aims:
Assessing the clinico-epidemiologic and mycologic profile of treatment naïve, chronic, recurrent and steroid-modified dermatophytosis.
Materials and Methods:
We conducted across-sectional study involving 111 cases of dermatophytosis. Detailed epidemiology, clinical parameters, treatment history and other host factors were assessed along with scraping for potassium hydroxide (KOH) and fungal culture.
Results:
Among 111 patients,(F: M 1.7:1; mean age 44.4 ± 18.2 years), 51.4% were treatment naïve, while 34.2% and 14.4% presented with chronic and recurrent tinea respectively. Family history and sharing of fomites among infected family members was commoner in the latter groups (P = 0.001). Topical steroid application was reported in 49.5%, however only 7.2% presented with steroid modified tinea. Tinea corporis et cruris (41.4%) was the predominant clinical type followed by tinea corporis (34.2%) and tinea cruris (27.9%). KOH mount and culture were positive in 62.2% and 39.6% cases respectively; commonest isolates being Trichophyton rubrum, and Trichophyton mentagrophytes complex in 15.3% cases each. Trichophyton rubrum was the commonest etiology for treatment naïve and recurrent cases, while Trichophyton mentagrophytes was the commonest isolate from chronic and steroid-modified cases (P = 0.0003). Interestingly, T.mentagrophytes complex and T. rubrum were the commonest causes of tinea corporis and tinea cruris respectively (P = 0.07).
Conclusion:
Trichophyton rubrum was the commonest organism in treatment naïve and recurrent cases, while Trichophyton mentagrophytes complex accounted for most cases of chronic and steroid modified tinea. The difference in predominant species seems to be a major contributory factor for chronicity and recurrence. However, several host factors like topical steroid use and sharing of fomites also play additional roles.
Keywords: Chronic, clinico-mycology, dermatophytosis, recurrent, steroid-modified tinea
Introduction
Dermatophytosis includes infection of skin and its appendages by dermatophyte group of fungi encompassing Trichophyton sp., Microsporum sp. And Epidermophyton sp.[1] These infections are a common reason for dermatologic consultation, affecting 20-25% of the global population.[1] Tropical countries like India demonstrate higher prevalence due to conducive hot and humid climate. However, the predominant species varies across regions, being responsible for heterogeneous severity and treatment responsiveness.Other contributory factors include overcrowding, poor hygiene and poverty. Despite lack of mortality, there is significant morbidity impairing the quality of life.
Recently, a changing trend has been observed in the Indian dermatophytosis patients with maximum cases not responding to conventional treatment,th us resulting inan explosion of cases reaching epidemic proportions. Additionally, most are presenting as chronic/recurrent dermatophytosis. Although a standard definition is lacking, chronic dermatophytosis has been arbitrarily defined as disease continuing for more than 6 months to 1 year, with or without recurrence despite adequate treatment.[2,3] Similarly, Panda et al.,[3] have defined recurrent dermatophytosis as “cutaneous dermatophytosis in which the infection reoccurred within 6 weeks of stopping the adequate antifungal treatment with at least 2 such episodes in last 6 months.” Theoretically, some of these cases may be relapse, without adequatefungal clearance due to missed sources of infection such as an unnoticed nail or vellus hair involvement; while re-infection from untreated family members or inadequate hygiene (infected clothing and fomites) is another possibility.[3] Some authors have proposed antifungal drug resistance[3] or modifiable host factors viz. CARD9 mutation resulting in defective antifungal response[4] or biofilm formation (for both T. rubrum and T. mentagrophytes)[5] as the probable causes of treatment failure, however further conclusive studies are necessary.
Rather, a more common cause of reduced therapeutic benefit might be the indiscriminate use of topical steroid-antifungal cocktails, which are available as over-the-counter (OTC) preparations in India. These preparations contain low concentrations of steroids (absorption is further reduced during penetration through the skin layers) which stimulate fungal metabolism; however higher steroid concentrations may inhibit fungal metabolism by their cytostatic properties.[3] All those cases having received treatment withDermatophytosis altered by topical corticosteroid preparations are called “steroid-modified tinea” or “tinea incognito”, a working definition being “patients with application of topical steroid for a minimum duration of six-weeks (alone or in combination with other drugs)”.[6]
We aimed to explore the clinico-epidemiologic distribution and identify the causative organisms of chronic, recurrent and steroid modified dermatophytosis and compare them with treatment-naïve dermatophytosis. Dearth of similar literature in the present set-up has prompted this study.
Materials and Methods
We conducted a cross-sectional study involving 111 consecutive patients with clinically diagnosed dermatophytosisat the departments of Dermatology and Microbiology of a tertiary care centre, after obtainingwritten informed consent. Pregnancy, lactation, underlying systemic conditions like diabetes, cardiac or hepato-renal dysfunction, and intake of systemic immunosuppressants within past 14 days, and tinea capitis served as ourexclusion criteria. Initially we screened 123 patients, but 12 denied culture and were excluded. Requisite approval was obtained from the institutional Ethics committee and study was conducted in accordance with the Declaration of Helsinki (Brazil, 2013) and the ICH-GCP (1996) guidelines.
All study participants (n = 111) were subjected to detailed clinical history concerning socio-demographic details, duration of disease, family history, use and sharing of fomites like towels, soaps, clothing and treatment received including use of over-the-counter (OTC) products. Based on clinical history, all patients were categorised into 4 groups- treatment-naïve (those who have not taken any treatment (topical/oral) including self-medication), chronic,[2] recurrent[3] and steroid modified tinea/tinea incognito.[6]
Subsequently, general survey, systemic examination and thorough dermatological examination was performed by a single dermatologist to assess the clinical type of tinea along with number of lesions, distribution and to rule out any atypical morphology e.g., pseudoimbricata [Figure 1]. Routine biochemistryand retrovirus screening was undertaken in all patients. A lesional skin sample was collected from each patient and sent to the Microbiology department for further evaluation.
Figure 1.

Tinea Psudoimbricata along with Peripheral Pustules Suggestive of Steroid Modified Tinea
Sample collection
Samples were collected after cleansing the affected skin surface with 70% alcohol and allowing it to evaporate. They were collected from the advancing lesional edge using the blunt end of a sterile surgical blade (No. 15), held at 90 degrees. Nail samples were collected by scrapping infected nail area/undersurface, or clipping infected nails. If multiple sites were involved, scrapings were collected from the site of maximum activity. All samples were preserved in small, sterile black paper envelopes for easy visualization and absorption of moisture to reduce/eliminate bacterial load. Each specimen was divided into 2 parts- one for KOH mount and another for fungal culture.
Examination of direct KOH mount
One part of each specimen was immersed in 10% KOH (overnight for nail clippings), mounted on a clean, grease-free glass slide and observed directly under low-power magnification (10x and 40x) of microscope to detect fungal hyphae, spores or yeast cells.
Isolation of dermatophytes on culture
The other part of each sample was inoculated in Sabouraud Dextrose Agar (SDA) containing Chloramphenicol (0.05%) with and without Cycloheximide (0.5%) and Dermatophyte Test Medium (DTM) supplemented with Gentamicin and Chlortetracycline to grow dermatophytes. These wereincubated in biological oxygen demand incubator at room temperature (25°C-37°C), after 1 week of adequate drying in the envelopes to get rid of moisture. All cultures were examined twice weeklyfor growth and incubated for 4 weeks before declaring them negative.
The growths were noted for colony characteristics like texture, surface, colour on surface and reverse, and any diffusible pigment [Figures 2 and 3]. The causative organisms were identified based on typical macroscopic colony morphology e.g. velvety, red pigment on reverse (T. rubrum) and white to tan, cottony or powdery, variable pigment (T. mentagrophytes complex).[7] Wet mount and slide cultures were undertaken for microscopic morphology and species identification e.g., tear-drop microconidia with few, long pencil-shaped macroconidia (T. rubrum) and grape-like clusters of microconidia, cigar-shaped macroconidia with terminal rat-tail filaments (T. mentagrophytes complex).[7] Germ tube tests were performed on all growths identified as yeasts. Cycloheximide in agar was used to isolate the dermatophyte by inhibiting several fungi, including Aspergillus and the mucoraceous moulds Rhizopus, Absidia, and Mucor.
Figure 2.

Test Tube Containing Dermatophyte Test Medium Showing Velvety White Colony on the Obverse and Red Pigment on the Reverse Suggestive of T. Rubrum
Figure 3.

Test Tube Containing Dermatophyte Test Medium Showing White to Tan, Cottony Colony on the Obverse along with Variable Pigmentation Suggestive of T.Mentagrophytes
Repeat cultures were performed in cases where primary culture was negative for dermatophytes but positive for non-dermatophyte moulds (NDM) or yeasts to rule out the possibility of contamination. Confirmed diagnosis of NDM (non-dermatophyte moulds) was performed according to the following criteria:
(i) abnormality consistent with superficial mycoses,
(ii) positive KOH preparation, the presence of filamentous fungi in biological fluid material,
(iii) failure to isolate dermatophyte culture, and
(iv) the growth of non-dermatophyte moulds in three successive occasions at least, with a minimum of 2-week interval.
All cultures were evaluated both macroscopically and microscopically under lactophenol cotton blue (LPCB) mount using tease-mount preparation and slide culture techniques to detect the formation of macroconidia and microconidia or other typical fungal morphologies.Due to non-availability of differentiation test media for yeasts and dermatophytes, no further bio- chemical tests were performed and the cultures reported based on macroscopic and microscopic examination and germ tube tests only.
Statistical analysis
All data were tested for normality using Kolmogorov-Smirnov test. Numerical data (presented as mean ± SD) were analyzed using paired t-test. Qualitative data (presented as frequencies and proportions) was compared using chi square test or Fischer's exact test. The statistical software SPSS v 10.0 and Medcalc® v 9.6.4.0 was used for analysis. A p value < 0.05 has been considered statistically significant.
Results
In our study (n = 111), females outnumbered males (M: F 1:1.7), mean age being 44.4 ± 18.2 years. Twenty-three patients (20.7%, n = 111) belonged to 21-30 year followed by 19.8% (22/111) and 18.9% (21/111) in 61-70 year and 41-50 year age groups respectively. Overall, more than half of our patients (51.4%, 57/111) presented after 6 months of disease onset. [Table 1] Family history was positive in 82 (73.9%) patients; significantly more in chronic/recurrent or steroid-modified cases (P = 0.02). The different demographic details have been depicted in Table 1.
Table 1.
Demographic Details of Study Patients (n=111)
| Parameters | Total Number of Patients (n=111) | T/T Naive (n=51) | Chronic/Recurrent/Steroid Modified (n=60) | P |
|---|---|---|---|---|
| Age in years (mean±SD) | 44.4±18.2 | 42.1±18.1 | 46.3±18.2 | 0.2* |
| Age group, n (%) | ||||
| 11-20 years | 11 (9.9) | 7 (13.7) | 4 (6.7) | 0.2¶ |
| 21-30 years | 23 (20.7) | 11 (21.6) | 12 (20) | |
| 31-40 years | 16 (14.4) | 9 (17.6) | 7 (11.7) | |
| 41-50 years | 21 (18.9) | 6 (11.8) | 15 (25) | |
| 51-60 years | 18 (16.2) | 8 (15.7) | 10 (16.7) | |
| 61-70 years | 22 (19.8) | 10 (19.6) | 12 (20) | |
| Sex (M: F) | 41:70 | 16:35 | 25:35 | 0.3¶ |
| Residence (Rural: urban) | 13:98 | 5:46 | 8:52 | 0.6¶ |
| Duration of disease, n (%) | ||||
| <6 months | 54 (48.6) | 28 (54.9) | 26 (43.3) | 0.3# |
| >6 months | 57 (51.4) | 23 (45.1) | 34 (56.7) | |
| Family history, n (%) | ||||
| Positive | 82 (73.9) | 32 (62.7%) | 50 (83.3%) | 0.02# |
| Negative | 29 (26.1) | 19 (37.3%) | 10 (16.7%) |
Tests used to obtain statistical significance: *t-test; ¶Chi-square test; #Fisher’s exact test
Almost half of the patients [51 (45.9%)] were treatment-naive, while 38 (34.2%)] and 14 (12.6%) subjects presented with chronic and recurrent cases respectively. History of topical corticosteroid application was obtained in 55 (49.5%; n = 111) subjects, however only 8 patients (7.2%, n = 111) presented with steroid modified tinea having corroborating clinical features like erythematous follicular and non - follicular papules [62.5%, 5/8], peripheral pustules at margin [25%, 2/5] and pseudoimbricata in 1 (12.5%; n = 8) case [Figure 1]. Topical steroid applications were mostly in the form of steroid-antifungal combinations, purchased directly over-the counter (OTC). Family history and sharing of fomites (e.g., towels) among infected family members was maximum in chronic cases (94.7%, 36/38) followed by 87.5% (12/14) and 62.7% (32/51) in recurrent and treatment naïve cases respectively; this association being statistically significant (P = 0.001, Chi-square test). All patients with tinea cruris and corporis reported exacerbation of symptoms with tightly fitting clothing.
Majority of our patients (82.9%, 92/111) presented with involvement of multiple sites. Tinea corporis et cruris was the commonest clinical presentation (41.4%, 46/111) followed by tinea corporis (34.2%, 38/111), tinea cruris (27.9%, 31/111) [Figure 4] and tinea unguium (9%, 10/111) cases. Other rare presentations included tinea faciei[Figure 5], tinea manuum, tinea pedis, tinea barbae and scrotal tinea.
Figure 4.

Extensive Tinea Cruris. Several Striae can be Observed Suggestive of Topical Steroid Application
Figure 5.
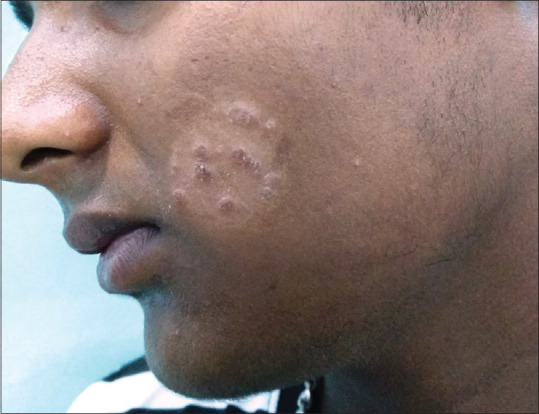
Tinea Faciei
KOH mount was positive for fungal elements in 69 (62.2%, n = 111) patients, most common finding being thin hyaline branched septate hyphae. Among the positive samples, 6 were from nails (all showing dermatophytes) In these patients, chronic dermatophytosis predominated (37.7%, 26/69), followed by treatment naïve (31.9%, 22/69), recurrent (20.3%, 14/69) and steroid modified cases (10.1%, 7/69).
In our study, 44 patients (39.6%; n = 111) showed a positive culture for causative organisms. The commonest organisms isolated were Trichophyton rubrum and Trichophyton mentagrophytes complex in 17 cases each (15.3%; n = 111) [Figures 2 and 3] followed by non-dermatophyte moulds (NDM) [6.3%, 7/111] and single isolates of Epidermophyton sp., Microsporum sp. and Candida albicans. [Table 2] Amongst the NDMs, Aspergillus sp. (5 cases) was commoner than Rhizopus sp. (2 cases). Interestingly, Trichophyton rubrum was the commonest causative agent in treatment naïve cases (13.7%, 7/51) and recurrent cases (35.7%, 5/14) while Trichophyton mentagrophytes complexwas the commonest isolate in chronic dermatophytosis (15.8%, 6/38) and steroid-modified cases (62.5%, 5/8) [P = 0.0003, Chi-square]. Trichophyton rubrum was the commonest isolated organism from those with a positive family history (n = 82) followed by Trichophyton mentagrophytes (19.5% [16/82] vs. 15.9% [13/82], P = 0.7, Chi-square).Trichophyton rubrum and Trichophyton mentagrophytes complexwere the commonest isolated organisms from tinea cruris [29%, 9/31] and tinea corporis [31.6%, 12/38] respectively, being comparable statistically (P = 0.07, Chi-square). Table 2 highlights the correlation of isolated organisms with clinical presentation.
Table 2.
Correlation of Isolated Organism with Site and Clinical Presentation of Dermatophytoses (n=44)
| Name of Isolated Organism | Site of sample | Treatment-Naïve (n=12) | Chronic (n=13) | Recurrent (n=12) | Steroid Modified Tinea (n=7) | ||
|---|---|---|---|---|---|---|---|
|
| |||||||
| Glabrous Skin | Nail | ||||||
|
| |||||||
| Tinea Corporis | Tinea Cruris | ||||||
| Trichophyton rubrum (n=17) | 5 | 9 | 3 | 7 | 4 | 5 | 1 |
| Trichopyton mentagrophytes complex (n=17) | 12 | 1 | 4 | 2 | 6 | 4 | 5 |
| Non-dermatophyte moulds (n=7) | 2 | 5 | 0 | 2 | 2 | 2 | 1 |
| Candida albicans (n=1) | 0 | 1 | 0 | 0 | 1 | 0 | 0 |
| Epidermophyton sp. (n=1) | 1 | 0 | 0 | 1 | 0 | 0 | 0 |
| Microsporum sp.(n=1) | 1 | 0 | 0 | 0 | 0 | 1 | 0 |
This table includes only culture positive patients (n=44). All the values mentioned represent actual number of patients
Discussion
In our study, 21-30 year agegroup was affected most commonly, consistent with most authors[8,9,10] Sharing of accommodation and fomites, for work/study possibly make this group most vulnerable. We observed chronic and recurrent cases to be common in the 5th decade, similar to Vineetha et al.,[11] probably attributable to waning immunity with advancing age.
In our study, females outnumbered males (M:F 1:1.7); contradicting most authors [Table 3][8,10,12] In our part of country, most females are housewives staying indoors, so they have higher rates of reporting to the doctors compared to their male counterparts who are mostly engaged in outdoor work. Additionally, increased exposure to heat while cooking in our hot and humid weather may be an additional contributory factor.
Table 3.
Salient Features of Recent Indian Studies Concerning Dermatophyte Infections
| Authors and year, Place of study | Most Common Age Group Affected (Years) | Gender | Most Common Sites Affected | Most Common Clinical Presentation | Family History Positive (%) | KOH Positive (%) | Culture Positive (%) | Predominant Species Isolated (%) |
|---|---|---|---|---|---|---|---|---|
| Kalita et al., 2019; Rajasthan[1] | 21-30 | M>F (248:115) | Tinea corporis (75%)> tinea cruris (18.75%) | - | - | 58.4 | 40.22 |
T. mentagrophytes (55) T. tonsurans (22.5) |
| Janardhan et al., 2017; Telangana[8] | 31-40 | M>F (1.86:1) | Tinea corporis (45%)> Tinea cruris (28%) | - | 4.5%; Friendsà 16% | 90 | 72 |
T. rubrum (52) T. mentagrophytes (14) |
| Krishan et al., 2018; Mumbai[10] | 16-30 | M>F (2.29:1) | Tinea cruris (53%)> Tinea corporis (23.5%) | - | - | 53 | 67.8 |
T. rubrum (54.5) T. mentagrophytes (45.5) |
| Vineetha et al., 2018; Kerala[11] | 10-20 | F>M (1.1:1) | Tinea corporis (28.7) > Tinea cruris (17.2) | 68%- Chronic; 32%- 1st episode | 28%- chronic, 21%- 1st episode | 79%- 1st episode, 34%- chronic | 34 |
T. rubrum (21) T. mentagrophytes (10) |
| Singh et al., 2019; Orissa[12] | 20-30 | M>F (1.22:1) | Tinea corporis et cruris (39.5%) > tinea corporis (27%)> tinea cruris (15.1%) | Most patients (42.76%) presented within 1 month of disease. | 48.8 | 97.7 | 73.6 |
T. mentagrophytes (79.9) T. rubrum (13.5) |
| Mahajan et al., 2017; Uttar Pradesh[15] | 20-40 | M>F (3:1) | Tinea corporis et cruris (27.2%)> Tinea corporis (20.8%)> Tinea cruris (18.9%) | 40.4% - Intermittent or continuous infection for 1 to 6 months. 35.8% - Longer duration (>6 months upto 2 years) |
30.9 | 79.6 | 52.4 |
T. mentagrophytes (75.9) T. rubrum (21.9) |
| Present study, 2020; West Bengal | 21-30 | F>M (1.7:1) | Tinea corporis et cruris (41.4%)> Tinea corporis (34.2%)> Tinea cruris (27.9%) | 45.9% -1st episode/treatment naïve 34.2% - Chronic 12.6% à Recurrent 7.2% - Steroid modified tinea |
73.9 | 62.2 | 39.6 |
T. mentagrophytes complex (15.3) T. rubrum (15.3) |
In our study, most patients (51.4%) presented after 6 months of disease, consistent with most authors[9,13]; while Singh et al.,[12] recorded a lower disease duration.[Table 3] This difference may be attributed to heterogeneous levels of awareness and socio-cultural norms, as most of our patients tried indigenous remedies and OTC products before approaching a dermatologist.
Family history was positive in 73.9% of patients, higher than that observed in other studies.[Table 3][11,12,14] Sharing of fomites among infected family members viz. towels was significantly more in chronic (94.7%) and recurrent (87.5%) cases, compared to treatment naïve cases (56.1%); in agreement withVineetha et al.[11] Increased prevalence of overcrowding and shared accommodation in our set-up may be responsible for higher incidence of positive family history.
In the present study, almost half of the patients (45.9%) were treatment naïve followed by chronic (34.2%), recurrent (12.6%) and steroid-modified (7.2%) cases. However, Vineetha et al.,[11] reported most cases to be chronic (68%) followed by 1st episode (32%). 49.5% of our patients used unsupervised OTC topical medications containing steroids, lower than that reported by most authors (63%-77.9%),[11,15,16] but more than double reported by Singh et al. (21.7%).[12] Thus, most studies including ours highlight the improper and rampant use of steroid containing preparations (easy availability, low cost, early relief from inflammatory symptoms like itching) to be a major contributory factor for resurgence of dermatophytosis in recent times. These medications suppress the host cell mediated immunity (CMI) resulting in persistence and chronicity of the disease despite treatment.[11]
In cases of steroid modified tinea/tinea incognito, erythema along with follicular and non-follicular papules was the commonest presentation followed by peripheral pustules and pseudoimbricata. Similar atypical presentations have been reported by Vineetha et al.,[11] and Verma et al.,[17] along with eczematous lesions and genital involvement. Dermatologists should be aware of these presentations for early suspicion of steroid abuse to initiate appropriate counselling and treatment.
Almost 83% of our patients presented with multi-site involvement,(tinea corporis et cruris> tinea corporis> tinea cruris> tinea unguium), consistent with most authors.[Table 3][1,8,12,14,15] However some authors have reported tinea cruris to be the commonest clinical type[10,17] while Grover et al.,[18] reported tinea pedis to the commonest type in north-east India, possibly due to topographical variation. Two of our male patients presented with solitary genital involvement. Multiple-site and solitary genital involvement are suggestive of topical steroid abuse, as reported by Verma et al.[17]
KOH positivity was observed in 62.2% of our cases, consistent with most authors.[10,19,20] In contrast, some authors have reported higher KOH positivity.[8,12,15] [Table 3]. Interestingly, most of our KOH positive cases presented with chronic dermatophytosis, followed by treatment-naïve cases.
Isolation of dermatophyte species by culture was possible in 39.6% of our patients, while literature shows a variable culture positivity rate ranging from 34%-74%.[8,10,11,12,13,21] [Table 3]Our low yield on culture may be attributed to current or previous use of antifungals or topical steroids leading to deeper cutaneous fungal penetration, similar to Vineetha et al.[11] The most common isolated organisms were Trichophyton rubrum and Trichophyton mentagrophytes complex in 15.3% cases each, followed by non-dermatophyte moulds (NDM) in 6.3% cases (Aspergillus sp.>Rhizopus sp.]. While some studies have depicted T. rubrum as the commonest organism, mostly from South India,[8,21,22] several recent ones have depicted T. mentagrophytes as the commonest etiology[12,15] [Table 3]; in contrast, our study detected both these organisms in equal proportions.
We have also highlighted the rare role of other NDMsin dermatophytosis.Interestingly, we detected T. mentagrophytes to be the commonest causative organism for chronic and steroid modified cases, while treatment naïve and recurrent cases predominantly demonstrated T. rubrum (P = 0.0003). This finding is consistent with recent reports which have detected T. mentagrophytes as the commonest organism in recalcitrant dermatophytosis, suggesting its role in impartingtreatment unresponsiveness.[23,24] Recently Nenoff et al.,[25] have identified a new genotype of T. mentagrophytes (T.mentagrophytes ITS type VIII) to be responsible for the current epidemic of dermatophytosis. However, equal prevalence of T. rubrum in our study suggests that causative organism is not the only cause for persistent disease, other contributory factors like family history, overcrowding and sharing of fomites are also important. Table 3 compares the salient features of recent Indian studies.
Limitations
Small sample size and inability to perform anti-fungal sensitivity and resistance were our major limitations. We also included patients who have taken or are currently taking antifungals or applied topical steroids thereby reducing the chance of growth on fungal culture.
Conclusion
Chronicity, recurrence and injudicious use of OTC topical steroid preparations are major causes of persistent disease, resulting in the recentepidemic of dermatophytosis.Infection of multiple family members, delayed seeking of medical advice, use of tight-fitting clothing and sharing of fomites are other contributory factors. Both T.rubrum and T.mentagrophyes complex affected our patients in equal proportions, although the latter was significantly predominant in chronic and steroid modified cases. This probably indicates the role of etiological species in treatment response. Thus, appropriate counselling measures to avoid exacerbating factors like sharing infected fomites and tight garments, along with adequate treatment is essential to curb this menace. Another important factor for the persistent disease might be drug related such as poor compliance, poor-quality of drugs and drug resistance. Thus, we need large scale studies in the future to address these concerns and analyse their role in the current epidemic of persistent dermatophytosis.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Kalita JM, Sharma A, Bhardwaj A, Nag VL. Dermatophytoses and spectrum of dermatophytes in patients attending a teaching hospital in Western Rajasthan.India. J Family Med Prim Care. 2019;8:14–8. doi: 10.4103/jfmpc.jfmpc_159_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dogra S, Uprety S. The menace of chronic and recurrent dermatophytosis in India: Is the problem deeper than we perceive? Indian Dermatol Online J. 2016;7:73–6. doi: 10.4103/2229-5178.178100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Panda S, Verma S. The menace of dermatophytosis in India: The evidence that we need. Indian J Dermatol VenereolLeprol. 2017;83:281–4. doi: 10.4103/ijdvl.IJDVL_224_17. [DOI] [PubMed] [Google Scholar]
- 4.Lanternier F, Pathan S, Vincent QB, Liu L, Cypowyj S, Prando C, et al. Deep dermatophytosis and inherited CARD9 deficiency. N Engl J Med. 2013;369:1704–14. doi: 10.1056/NEJMoa1208487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Costa-Orlandi CB, Sardi JC, Santos CT, Fusco-Almeida AM, Mendes-Giannini MJ. In vitro characterization of Trichophyton rubrum and T.mentagrophytes biofilms. Biofouling. 2014;30:719–27. doi: 10.1080/08927014.2014.919282. [DOI] [PubMed] [Google Scholar]
- 6.Dutta B, Rasul ES, Boro B. Clinico-epidemiological study of tinea incognito with microbiological correlation. Indian J Dermatol VenereolLeprol. 2017;83:326–31. doi: 10.4103/ijdvl.IJDVL_297_16. [DOI] [PubMed] [Google Scholar]
- 7.Chander J. Dermatophytosis. In: Chander J, editor. Textbook of Medical Mycology. 4th ed. India: Jaypee Publishers; 2018. pp. 162–202. [Google Scholar]
- 8.Janardhan B, Vani G. Clinico-mycological study of dermatophytosis. Int J Res Med Sci. 2017;5:31–9. [Google Scholar]
- 9.Agarwal U, Saran J, Agarwal P. Clinico-mycological study of dermatophytes in a tertiary care centre in northwest India. Indian J Dermatol VenereolLeprol. 2014;80:194. doi: 10.4103/0378-6323.129434. [DOI] [PubMed] [Google Scholar]
- 10.Krishan SA, Ray R, Chatterjee M, Khandare M. A cross sectional descriptive study on clinical type and etiological agent of superficial dermatophytosis. Indian JClin Dermatol. 2018;1:71–4. [Google Scholar]
- 11.Vineetha M, Sheeja S, Celine MI, Sadeep MS, Palackal S, Shanimole PE, et al. Profile of dermatophytosis in a tertiary care center. Indian Journal Dermatol. 2018;63:490–5. doi: 10.4103/ijd.IJD_177_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Singh BS, Tripathy T, Kar BR, Ray A. Clinicomycological study of dermatophytosis in a tertiary care hospital in eastern India: A cross-sectional study. Indian Dermatol Online J. 2020;11:46–50. doi: 10.4103/idoj.IDOJ_62_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar S, Mallya PS, Kumari P. Clinico-mycological study of dermatophytosis in a tertiary care hospital. Int J Scientific Study. 2014;1:27–32. [Google Scholar]
- 14.Bindu V, Pavithran K. Clinico-mycological study of dermatophytosis in Calicut. Indian J Dermatol VenereolLeprol. 2002;68:259–61. [PubMed] [Google Scholar]
- 15.Mahajan S, Tilak R, Kaushal SK, Mishra RN, Pandey SS. Clinico-mycological study of dermatophytic infections and their sensitivity to antifungal drugs in a tertiary care center. Indian J Dermatol VenereolLeprol. 2017;83:436–40. doi: 10.4103/ijdvl.IJDVL_519_16. [DOI] [PubMed] [Google Scholar]
- 16.Dabas R, Janney MS, Subramaniyan R, Arora S, Lal VS, Donaparthi N. Use of over-the-counter topical medications in dermatophytosis: A cross-sectional, single-center, pilot study from a tertiary care hospital. Indian J Drugs Dermatol. 2018;4:13–7. [Google Scholar]
- 17.Verma S, Madhu R. The great Indian epidemic of superficial dermatophytosis: An appraisal. Indian J Dermatol. 2017;62:227–36. doi: 10.4103/ijd.IJD_206_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grover S, Roy P. Clinico-mycological profile of superficial mycosis in a hospital in North-East India. Med J Armed Forces India. 2003;59:114–6. doi: 10.1016/S0377-1237(03)80053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharma R, Adhikari L, Sharma RL. Recurrent dermatophytosis: A rising problem in Sikkim, a Himalayan state of India. Indian J PatholMicrobiol. 2017;60:541–5. doi: 10.4103/IJPM.IJPM_831_16. [DOI] [PubMed] [Google Scholar]
- 20.Poluri LV, Indugula JP, Kondapaneni SL. Clinicomycological study of dermatophytosis in South India. J Lab Physicians. 2015;7:84–9. doi: 10.4103/0974-2727.163135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Surendran KA, Bhat RM, Boloor R, Nandakishore B, Sukumar D. A clinical and mycological study of dermatophytic infections. Indian J Dermatol. 2014;59:262–7. doi: 10.4103/0019-5154.131391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Manjunath M, Koppad M, Dadapeer S. Clinicomycological study of Dermatomycosis in a tertiary care hospital. Indian J Microbiol Res. 2016;3:190–3. [Google Scholar]
- 23.Sardana K, Kaur R, Arora P, Goyal R, Ghunawat S. Is antifungal resistance a cause for treatment failure in dermatophytosis: A study focused on tinea corporis and cruris from a tertiary centre? Indian Dermatol Online J. 2018;9:90–5. doi: 10.4103/idoj.IDOJ_137_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pathania S, Rudramurthy SM, Narang T, Saikia UN, Dogra S. A prospective study of the epidemiological and clinical patterns of recurrent dermatophytosis at a tertiary care hospital in India. Indian J Dermatol VenereolLeprol. 2018;84:678–84. doi: 10.4103/ijdvl.IJDVL_645_17. [DOI] [PubMed] [Google Scholar]
- 25.Nenoff P, Verma SB, Vasani R, Burmester A, Hipler UC, Wittig F, et al. The current Indian epidemic of superficial dermatophytosis due to Trichophyton mentagrophytes—A molecular study. Mycoses. 2019;62:336–56. doi: 10.1111/myc.12878. [DOI] [PubMed] [Google Scholar]


