Abstract
Acquired dermal macular hyperpigmentation (ADMH) is an umbrella term that includes disorders clinically characterized by small and large pigmented macules/patches and histopathologically showing an evidence of current or resolved interface dermatitis with pigment incontinence, without clinically significant prior inflammatory phase. The term intends to include diseases previously described in the literature as lichen planus pigmentosus, Riehl's melanosis/pigmented cosmetic dermatitis and ashy dermatosis/erythema dyschromicum perstans. The nomenclature and origin of these disorders have always been a matter of discussion. These disorders share many clinicopathological similarities, are difficult to treat and adversely affect the quality of life. Recent consensus points towards the need for a unifying term to facilitate research and therapeutic trials. This article aims to provide a comprehensive review of the recent advances in ADMH.
Keywords: Acquired dermal macular hyperpigmentation, ashy dermatosis, erythema dyschromia perstans, lichen planus pigmentosus, pigmented cosmetic dermatitis, Reihl's melanosis
Introduction
Acquired dermal macular hyperpigmentation (ADMH) is an umbrella term that includes disorders, which are clinically characterized by small and large pigmented macules/patches and histopathologically show evidence of current or resolved interface dermatitis with pigment incontinence, without any clinically evident prior inflammatory skin lesions.[1,2] The term intends to include diseases previously described in the literature as Riehl's melanosis/pigmented cosmetic dermatitis (PCD),[3] lichen planus pigmentosus (LPP)[4] and ashy dermatosis.[5] Although a global consensus attempted to define these dermatoses recently, uncertainty still exists in patient categorization due to the existence of overlapping entities.[6]
Recently, there has been considerable debate on the nomenclature of these disorders.[7,8,9] Progress has been made in epidemiology and pathogenesis, and new morphological variants have been described.[4,10,11,12,13,14] Recent studies have defined the dermoscopic features of ADMH, and now, a validated scale is available to assess the disease severity.[1,15,16]
Need for a Uniform Nomenclature
There is a significant degree of clinico-pathological and dermoscopic overlap amongst disorders group under ADMH.[1,7,17] The group overall represents a lichenoid tissue reaction pattern in response to chronic subclinical injury; and represents a distinctive pattern of diseases, which are expressive of the same pathologic process with a similar histopathologic pattern, but with different, as yet unknown, etiologies.[1,7,17]
Further, lack of specific diagnostic/clinical indicators[6] and uncertainties in classifying patients into distinct subgroups hampers defining patient population in clinical trials. To ease the communication amongst researchers, the need for introduction of an umbrella term was long felt. In 2016, Chandran and Kumarasinghe had proposed the term 'acquired macular (hyper) pigmentation of uncertain etiology'.[2] Gupta and Sharma reiterated the same terminology as 'macular hyperpigmentation of uncertain etiology'.[8] Our group has preferred the name 'acquired dermal macular hyperpigmentation of varied etiology'.[7]
Some of the salient similarities and differences between entities grouped under ADMH namely LPP, PCD and ashy dermatosis are summarized in Table 1. Various other disorders listed in Table 2 have clinical and histopathological similarity to ADMH, but differ by manifesting a prior inflammatory phase clinically or by the lack of interface dermatitis and melanin incontinence histologically.
Table 1.
Clinical similarities and differences between various causes of acquired dermal macular pigmentation
| Characteristic | LPP | Ashy dermatosis | Riehl’s melanosis |
|---|---|---|---|
| Epidemiology | Reported primarily from India | Reported primarily from Mexico and rest of the Latin world | Reported primarily from Asia, especially Japan |
| Age | Any age | Any age | Middle age |
| Sex | Female > Male | Female > Male | Female>Male |
| Etiology | Unknown? Mustard oil,? cosmetics,? amla oil,? hair dyes | Unknown,? ammonium nitrite,? radiographic contrast media,? intestinal whipworm infestation,? cobalt allergy | Pigmented contact dermatitis to antigens present in cosmetics and textiles. Commonly para-phenylenediamine |
| Morphology | Discrete dark brown macules coalescing to form patches with usually well-defined margins over face, trunk, extensor aspect of arms Perioral involvement is typical |
Ash-colored, polycyclic macules primarily distributed over trunk and proximal arms. Sometimes, the lesions are surrounded by a rim of erythema with an elevated border. | Diffuse or patchy brown pigmentation with ill-defined margins on the face, bilaterally symmetrical. It is more intense on the forehead and the temples, and severe cases may look black, purple, or blue-black. |
| Associations | Lichen planus Alopecia areata Autoimmune thyroiditis Frontal fibrosing alopecia Vitiligo Atopy |
None | None |
LPP - Lichen planus pigmentosus
Table 2.
Clinical differentials of acquired dermal macular hyperpigmentation
| Diseases with a prior inflammatory phase |
| Resolved benign lichenoid keratosis |
| Fixed drug eruption/resolved drug eruption |
| Drug-induced hyperpigmentation |
| Resolved viral exanthema |
| Dermatomyositis |
| Burnt out graft vs host disease |
| Resolved lesions of prurigo pigmentosa |
| Actinic lichen planus |
| Hyperpigmented phase of incontinentia pigmenti |
| Post-inflammatory hyperpigmentation |
| Pigmentation following psoriasis/pityriasis rosea |
| Post-radiotherapy hyperpigmentation |
| Malignancies |
| Metastatic melanoma |
| Patch stage of cutaneous T-cell lymphoma |
| Nevi and related benign dermatoses |
| Nevi and associated conditions |
| Aberrant persistent Mongolian spots |
| Phakomatosis pigmentovascularis |
| Bilateral nevus of Ota-like macules |
| Systemic disorders |
| Addison’s disease |
| Hyperthyroidism |
| Vitamin B12 deficiency |
| Mastocytosis |
| Non-melanin pigmentation |
| Argyria |
| Drug-induced pigmentation (e.g., amiodarone, minocycline) |
| Ochronosis |
| Miscellaneous |
| Melasma |
| Macular amyloidosis |
| Flat pigmented seborrheic keratosis |
| Freckles and lentigenes |
| Confluent and reticulate papillomatosis |
| Partially treated pityriasis versicolor |
| Phytophotodermatitis |
| Erythema-ab-igne |
| Multiple café-au-lait macules |
| Idiopathic eruptive macular pigmentation (IEMP) |
Lichen Planus Pigmentosus (LPP)
As a clinical entity, LPP was first described by Bhutani et al.[18] Its occurrence is fairly frequent in the Indian population although the precise figures of its incidence and prevalence have not been established.[11,19]
Etiology and pathogenesis
The exact etiology of LPP is not known till date. The suspected culprits include use of cosmetics along with fragrances, henna, hair dyes, mustard oil and amla oil. Mustard oil contains allyl thiocyanate, a potential photosensitizer, which could play a role in the pathogenesis of LPP.[4] A significant proportion of patients report worsening of their pigmentation after sun exposure. Considering this and the fact that exposed sites are frequently the first to be involved, sunlight has been postulated to play a role in inciting LPP [Figure 1a-c].[19]
Figure 1.
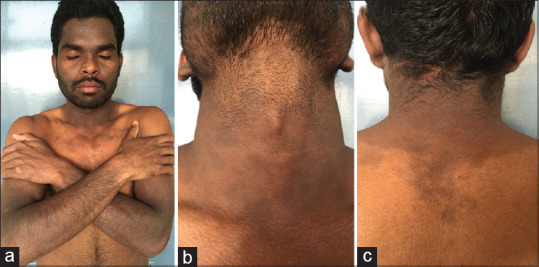
(a-c) A case of lichen planus pigmentosus typically showing photo-distributed lesions with involvement of the V area of the neck and upper back followed by an abrupt cut-off. Photo-protected areas, including the upper eyelids, infra-orbital area and chin, are relatively spared
However, apart from sun-exposed areas of the face and neck, unprotected flexural skin, such as the axillae and inguinal areas, are also commonly involved in LPP [Figure 2a].[20] An increased incidence of hepatitis C infection among LPP patients has been reported from Kuwait.[21] However, such an association was not noticed in our practice (unpublished observation). The possible role of hormonal factors might explain its slightly increased incidence in perimenopausal females.[4] Role of contact sensitization to nickel and sensitivity to nickel containing foods might be implicated as evidenced by patch tests.[19,22]
Figure 2.
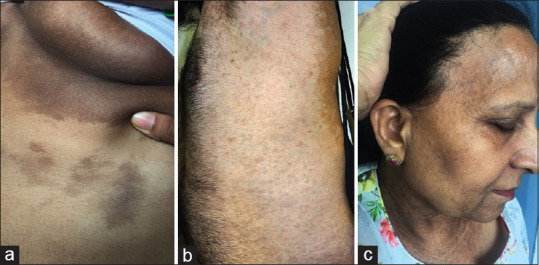
(a) Flexural lichen planus pigmentosus involving the infra-mammary area (b) co-existing vitiligo with lichen planus pigmentosus (c) lichen planus pigmentosus associated with frontal fibrosing alopecia
LPP can be associated with other autoimmune diseases like lichen planus, lichen planopilaris, vitiligo [Figure 2b], frontal fibrosing alopecia [Figure 2c] and hypothyroidism suggesting a role of autoimmunity in disease pathogenesis.[23] Interestingly, LPP has been known to occur in Blaschkoidal pattern [Figure 3a and b]. These findings suggest that there is a strong genetic predisposition for occurrence of LPP.
Figure 3.
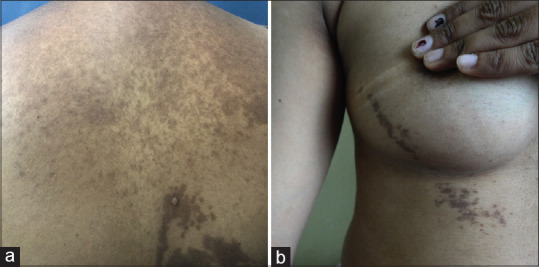
Cases of Blaschkoidal lichen planus pigmentosus. (a) A middle-aged lady with classical lichen planus pigmentosus of upper back with Blaschkoidal lesion over lower back, which were extending to the right upper arm. She also had involvement of face and infra-mammary areas. (b) Blaschkoidal lichen planus pigmentosus over the chest
Many investigators consider LPP to be an abortive form of lichen planus. In a recent study, the cytokine profile in patients of LPP was compared with lichen planus and post-inflammatory hyperpigmentation due to lichen planus.[12] Unlike lichen planus, patients of LPP had minimal expression of Interferon gamma, Foxp3 and cytokines IL-17A, IL-22, IL 23 representing Th17 pathway suggesting a baseline difference in the etiopathogenesis of LPP and LP.
Epidemiology
Although LPP as a clinical entity was initially described in Indians, this disorder has subsequently been seen in other racial and ethnic groups also. In a study of 344 patients of LPP, there was an overall female preponderance (2:1).[11] The mean age of onset was 37 years, with females having a significantly higher age at onset. There was a positive correlation between disease duration and its extent; and a longer disease duration was associated with more widespread disease.[11]
Clinical features
LPP is insidious in onset and has a chronic course. The patient may continue to develop new lesions while old ones enlarge with gradual extension of size and deepening of color. Although lesions are generally asymptomatic, mild pruritus and burning sensations are present in about one-third of the patients.[11] There is usually no preceding or associated erythema around the lesions.
Lesions of LPP initially appear as small, ill-defined oval to round macules, which later become confluent to form large areas of pigmentation. Pigmentation in different patients varies from slate-grey to brownish-black, although in a single patient, it is generally uniform. The distribution of the lesions of LPP is variable, but face and neck are the most frequent initial sites of involvement.[11] With time, the upper extremities and upper part of the back and trunk may also be involved. Infrequently, the flexures, i.e., the axillae, groin, infra-mammary areas and bald scalp may be involved. The preauricular region, including the temples and forehead, is predominantly the initial site of onset and is involved in almost all the patients. Lesions are generally bilaterally symmetrical, predominantly, in the exposed areas. Rarely, involvement can be generalized. Although rare, there can be palmoplantar [Figure 4a and b] and mucosal involvement.[13]
Figure 4.
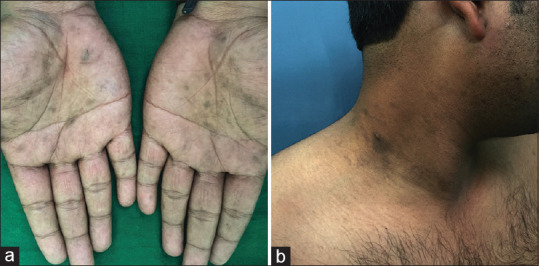
(a) Palmo-plantar involvement in lichen planus pigmentosus. (b) The patient also had typical lesions of lichen planus pigmentosus over face and axilla
Compared to PCD, patients of LPP are more likely to be younger, have perioral onset of the disease with well-defined margins and more likely to have concomitant lichen planus.[17] The most common morphological pattern of LPP is diffuse.[11] Other patterns seen include reticular, blotchy and perifollicular. Rare variants include inverse, mucosal, linear and zosteriform patterns.[10,24]
Associated diseases
LPP can be associated with other cutaneous and systemic inflammatory and autoimmune diseases. A retrospective study found LPP to be associated with atopic diathesis and autoimmune diseases in 14% and 13% of the patients, respectively.[11] Lichen planus was the most common association; cutaneous lichen planus in 11% patients and oral lichen planus in 5%, other associations being hypothyroidism (11.3%), vitiligo and alopecia areata.[11] In Hispanic/Latin ethnicity, frontal fibrosing alopecia is commonly seen as a herald sign for LPP. This association was more strongly seen in premenopausal women. The application of hair care products seems to be a common link between the development of frontal fibrosing alopecia and LPP.[23]
Riehl's Melanosis
Synonym: Pigmented cosmetic dermatitis; pigmented contact dermatitis
Riehl first described it during the First World War in 1917 when he saw several patients with a striking dark pigmentation of the face in Vienna. They were of both sexes, and the children were also affected. Riehl could not explain the cause of the condition, but discussed the possibility that wartime food substitutes might be of importance.[25]
Etiology
Since the first description of this condition by Riehl, many authors have studied and observed this condition and there is a consensus that Riehl's melanosis is probably a pigmented contact dermatitis to antigens present in cosmetics and textiles with anecdotal reports of air-borne contact dermatitis to musk ambrette and other plants.[26] Cosmetic allergens, red and yellow pigments in textile dyes, chromium hydroxide, aniline and azo dyes, bactericidal agents (carbanilides, ricinoleic acids), hair dyes, red kumkum, and fragrances have also been implicated. In Indian patients, contact sensitization to para-phenylenediamine in hair dyes is an important etiological factor.[17] Textile allergens including optical whiteners, dyes, textile finishes, mercury compounds, formaldehyde and rubber components are also thought to induce pigmented contact dermatitis. Sometimes, occupational allergens like coal tar, pitch, asphalt, mineral oil and chromates have been incriminated.[27]
Pathogenesis
Repeated contact with low levels of allergens (present in cosmetics and textiles) produces a type IV cytolytic reaction characterized by vacuolar basal cell degeneration and pigment incontinence rather than a frank eczematous reaction. Ultraviolet light exposure may contribute in some, since pigmentation is often photo-localized and some of the chemicals implicated not only stimulate melanogenesis but are also known photosensitizers. Photo patch testing can produce identical pigmentation at patch test sites, supporting the role of ultraviolet rays in disease pathogenesis.[17]
Epidemiology
Incidence and prevalence of Riehl's melanosis is not known and though most reports are from Japan, cases have also been reported from Europe, South America, India and South Africa.[28] In general, it is most pronounced in ethnic races and in people with dark complexion. Women appear to have a greater predilection; with majority of patients being young-middle-aged women, although, it has also been reported in men and children. In a recent study, patients suspected of having PCD (patch-test positive group) were found to be significantly older and had a longer duration of hair color usage.[17]
Clinical Features
Riehl originally described the entity as 'the dark brown pigmentation engaging the entire face, but most pronounced on the forehead and in the zygomatic and temporal regions and in some cases had a greyish nuance. The pigmentation was generally more pronounced laterally on the face, rather than in the central area. It extended to the ears, neck and nape of the neck, and onto the scalp for a varying distance. The skin surface looked as though it were covered with flour or was slightly scaly, and on the forehead, cheeks and ears horny plugs were seen in widened follicular orifices. The diseased skin was thickened and slightly rough. There was no atrophy, no exudation and almost no hyperemia. The areas of pigmentation were not demarcated sharply. The uniform discoloration of the head and neck gradually diminished towards the thorax, breaking up into small-pigmented macules or discrete papules, usually follicular. New, isolated efflorescence was to start with reddish-brown, only later becoming dark brown. The hands, forearms, axillae and sub-mammary and umbilical skin were less commonly involved, and here the pigmentation was also less pronounced. The patients had no signs of general disease.'[25]
Compared to LPP, patients of PCD are more likely to have symptomatic hyperpigmentation and mild superficial scaling may be apparent. Lesions due to cosmetics and hair dye usually starts over hair margins, are ill-defined hyperpigmented patches with preferential involvement of outer surface, helix and lobule of ear, temples, preauricular area (sites affected by application of hair dyes) and upper back [Figure 5a and b]. Sites of involvement also depends on the allergen responsible—those due to textiles more often involve anterior aspect of thighs and axillae (sparing the vault). However, these clinical features are not absolute and overlapping entities are common in clinical practice. Patients may show positive patch test and photo patch test to cosmetics or their ingredients [Figure 6a and b].[17]
Figure 5.
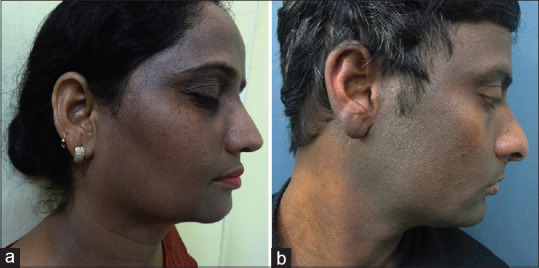
(a) Case of lichen planus pigmentosus showing sparing of the ears (b) pigmented contact dermatitis to hair dye showing predominant involvement of helix of the ear and ear lobule
Figure 6.
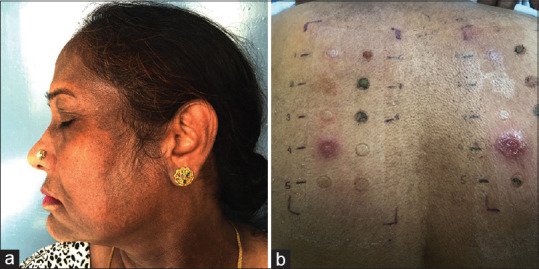
A case of pigmented contact dermatitis to mehendi. (a) Diffuse brownish-black discoloration with characteristic involvement of the lobule of the ear. Note can also be made of colored hair (b) Same patient showing patch-test positivity (2+) to para-phenylenediamine. Photo-patch test (right side) showing photo-aggravated contact dermatitis (3+)
Ashy Dermatosis
Synonym: Erythema dyschromicum perstans
Ramirez in 1957 described a condition called “dermatosis cenicienta” (ashy dermatosis),[29] which presented with small greyish macules, and later in 1961, Convit et al.[30] described a dermatosis from Venezuela consisting of five cases characterized by large greyish macules with a papular, slowly extending erythematous border. Convit et al. termed this condition as “erythema chronicum figuratum melanodermicum”, but it was subsequently renamed as “erythema dyschromicum perstans” (EDP) by Sulzberger. Later, in 1966 and 1967, Ramirez reviewed these conditions and unified EDP and ashy dermatosis concluding that EDP was an early form of ashy dermatosis.[29] The origin and nomenclature of EDP and ashy dermatosis is still controversial, and a matter of debate.
Epidemiology
Though ashy dermatosis has been reported from many countries including India, it is most common in Latin America and Asia.[29] It occurs in both sexes, but causes greater concern in women. Though it can affect any age group, characteristically lesions affect people less than 30 years of age.
Etiology
The etiology of ashy dermatosis is unknown, but anecdotal reports have incriminated exposure to ammonium nitrite, radiographic contrast media and chlorothalonil, intestinal whipworm infestation, cobalt allergy and HIV infection. The relation of ashy dermatosis to lichen planus is uncertain; both have several clinical, histological, and immunohistochemical similarities and can co-exist.[31] The pigmentation in ashy dermatosis is due to the presence of melanin in the melanosome complexes in dermis (frequent) and in epidermis (sometimes).
Clinical features
The ashy dermatosis lesions are asymptomatic and rarely pruritic. The pigmented macules appear spontaneously; they typically have an ashy color but show various hues from grey-brown or blue-grey to black-grey. They measure 0.5-2 cm and have a sharply-limited, flat border; they may rarely be preceded by an erythematous macule or temporarily surrounded by an erythematous, flat or slightly papular but non-extensive border [Figure 7a and b]. Fully developed lesions consist of a constellation of oval-shaped, occasionally confluent macules. The lesions extend by multiplication of macules. They are disseminated and symmetrical, located on the trunk and proximal limbs, and may extend to the neck and the face. Some cases have been described having segmental and linear distribution. Palms, soles, scalp and mucous membranes are usually spared. Once developed, the dermatosis is stable but may disappear spontaneously or subside within 23 years.[29] Distinctly, different clinical forms have been described on the basis of varying hues or particular location of skin lesion.
Figure 7.
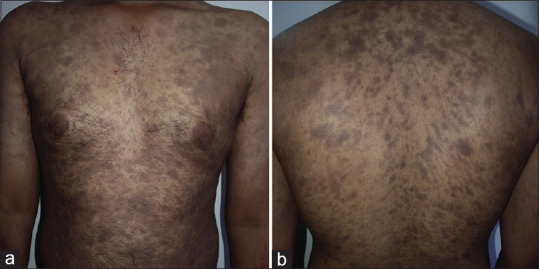
(a and b) A case of erythema dyschromicum perstans showing discrete round to oval pigmented macules over trunk and proximal extremities. Subtle erythema surrounding the pigmented macules can be appreciated
Dermoscopic Features of ADMH
ADMH as a group share lichenoid tissue damage and melanin incontinence as a common feature. Though few authors have reported subjective differences in the dermoscopy of LPP compared to PCD and EDP, we are of the opinion that no dermoscopic feature is specific enough to differentiate among these entities. Consequently, the predominant dermoscopic features of ADMH are pigment dots, globules and blotches.[1,22] This is unlike that of melasma, which shows exacerbated pseudo reticular network and nevus of Ota, which shows slate-grey structureless areas. These pigment structures are arranged in various configurations as described in Figure 8a-d.[1] There is a correlation between density of melanin incontinence on histopathology with dermoscopic grades of disease severity.[1]
Figure 8.
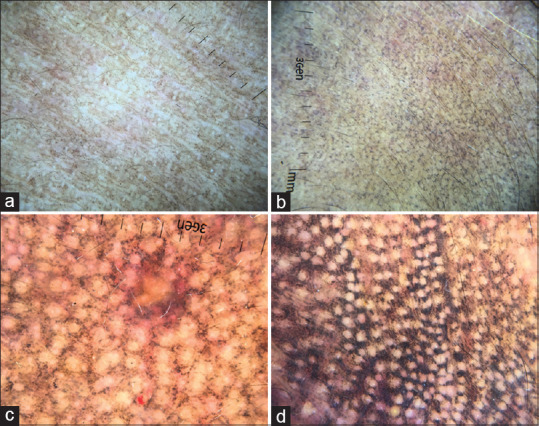
Dermoscopic grades of acquired dermal macular hyperpigmentation. (a) Grade 1: Predominantly, pigment dots situated discreetly without any pattern arrangement (b) Grade 2: Pigment dots and globules coalescing with each other in a broken netlike/Chinese letter pattern (c) Grade 3: Pigment dots and globules showing well-formed netlike pattern (d) Grade 4: Diffuse involvement with pigment dots, globules and blotches, sparing just the eccrine and sebaceous gland openings
Other dermoscopic features include owl-eye-like structures consisting of a central brown dot surrounded by a hyperpigmented halo, which histopathologically correlates with follicular plugging; exaggerated pseudoreticular pattern, which corresponds to increased melanin content in basal keratinocytes; and hem like pattern.[1,22]
Histological Aspects of ADMH
The unifying histological features of all the conditions categorized under ADMH include interface dermatitis and basal cell vacuolization with pigment incontinence. There are subtle differences in the pathology of individual conditions.
Similar to LP, LPP on histological examination shows lichenoid infiltrate, basal cell vacuolization and melanin incontinence. Apoptotic keratinocytes may be found rarely. Although it is considered a variety of LP, a dense lichenoid infiltrate is rarely observed in LPP[32] and recent studies have demonstrated significant differences in the cytokine and cellular milieu of LPP in comparison to LP.[12] In a previous histological study, a band-like lichenoid infiltrate was observed in 18% cases of LPP.[19] Subsequent studies have also found lichenoid infiltrate in a small percentage of LPP cases. In fact, most of the LPP cases show only mild lymphoid infiltrate and focal basal cell degeneration.[32] The most prominent histological finding in LPP biopsy is the presence of melanophages in the upper dermis with focal basal cell vacuolization. The epidermis is usually unremarkable or show atrophy with loss of rete ridges. Mild perivascular and periadnexal inflammation is also noted. Sasidharanpillai et al.[33] described three histological patterns of LPP: pattern 1 showing prominent interface dermatitis with melanin incontinence, pattern 2 showing sparse inflammation without basal cell degeneration and prominent pigment incontinence and pattern 3 without significant inflammation, basal cell degeneration or pigment incontinence. These changes do not correlate with the duration of clinical symptoms.
Riehl's melanosis (PCD), also shows similar histological findings. Contrary to its name, pigment contact dermatitis on histology does not show spongiotic changes, rather shows interface dermatitis with basal cell vacuolization and melanin incontinence. The dermis shows variable perivascular inflammation. Patients with PCD are more likely to show epidermal melanization extending into the upper layers of the epidermis compared to LPP.[17,34] However, histology of LPP, AD and PCD is largely overlapping and the final diagnosis is reached based on clinical correlation.[32]
AD, on histological examination, shows upper dermal lichenoid infiltrate, basal cell vacuolization and pigment incontinence. Few studies have examined the histological difference between LPP and AD. While most authors have concluded that the histological features of these two conditions are indistinguishable, some authors have reported contradictory histological difference between LPP and AD. In our opinion, these histological criteria can't differentiate between AD and LPP in a given biopsy.
Quantitative Assessment of Disease Severity in ADMH
For long, a quantitative tool to assess disease severity in ADMH was lacking. This hampered an objective assessment of treatment response and patient counseling on disease severity. Acquired dermal macular hyperpigmentation area and severity index (DPASI), a validated tool is now available to quantitatively assess disease severity in ADMH.[15,16] In this system, the face and neck is divided into six different segments. The dermoscopic disease severity of each segment is graded and multiplied with the area of involvement and a multiplication factor, the maximum possible score being 40.
Psychological Burden in ADMH
Disorders of hyperpigmentation usually cause psychological and emotional distress to the patient and can pose a significant negative impact on a person's health-related quality of life. However, formal assessment of the psychological burden of ADMH was long overdue. A recent study found associated anxiety and depression in 18.7% and 24.1% of patients with ADMH.[35] A significant proportion (14.3%) also experienced somatoform disorders. This was comparable to psychosocial disturbance seen in vitiligo.[35] Till date, there is no health-related quality-of-life (HR-QOL) measures, which specifically address the impact of ADMH in any population. Future studies are required to derive ADMH-specific HR-QOL measures.
Treatment of Acquired Macular Pigmentation Disorders
The natural course of the disorders included in ADMH is not clear with some cases showing spontaneous resolution and others having persistence of pigmentation for years. Because of these issues, there is not much evidence on the efficacy of the various treatment options. Currently, none of the available treatment options show consistent response or a clear superiority to the other modalities—the evidence on efficacy being restricted to a few case series. Given the paucity of the ineffectiveness of various treatment modalities, patients often have to rely on cosmetic camouflage to even out the skin tone. Physical sunscreens which come in tinted blends are also useful. A literature review of all the published studies was done in PubMed and Embase using the keywords LPP + treatment; Riehl's melanosis + treatment; PCD + treatment; pigmented contact dermatitis + treatment; ashy dermatosis + treatment; erythema dyschromia perstans + treatment as on 31-05-2020. Relevant articles with more than five treated patients are tabulated in Table 3.
Table 3.
Summary of treatment options for ADMH
| Author | Patients | Treatment schedule | Follow-up | Treatment response | Complications | Comment |
|---|---|---|---|---|---|---|
| Lichen planus pigmentosus | ||||||
| Al Mutairi and El Khalawany [21] | 33 (Middle eastern, Fitzpatrick skin type III-V) | Topical tacrolimus 0.03% ointment twice daily for 6-12 weeks | Once in 4 weeks for 16 weeks | 53% of patients improved of which 57% had >75% improvement and 42% had >50% improvement | None | Open label, non-randomized study |
| Bhari et al. [36]. | 9 (Indian, Fitzpatrick skin type IV-V) | Six sessions of Q-switched Nd- Yag laser, post toning protocol at 2 weekly intervals | Fortnightly for 14 weeks | 25% mean clinical improvement. No significant reduction in erythema and melanin index | Post-inflammatory hypopigmentation in one patient | Only modest histological and clinical improvement with this modality |
| Sonthalia et al.[39] | 17 (Indian, Fitzpatrick skin type IV-V) | Six sessions of Croton oil-free phenol combination every 3 weeks | 9-12 months | 29% experienced >75% reduction in pigmentation. Majority (76%) had at least 25% or more reduction in pigmentation |
Burning (3), nasopharyngeal irritation (11). Erythema, scaling, and crusting Prolonged peel reaction and temporary PIH were noted in 2 patients |
Safe and well-tolerated method, with effect being maintained for 1 year |
| Muthu SK et al.[40] | 32 (Indian, Fitzpatrick skin type IV-V) | 20 mg OD oral isotretinoin for 6 months with topical sunscreen | 6 months | 22% >50% improvement, 55% had 25-50% improvement. | One patient developed menorrhagia. Many experienced mild symptoms like cheilitis, xerosis and transient transaminitis | Patients with shorter duration of disease and limited body surface area involvement had better outcome |
| Sindhura et al.[14] | 6 (Indian, Fitzpatrick skin type IV-V) | 2.5 mg dexamethasone oral minipulse twice weekly, with cream mometasone furoate 0.1% topically and 0.1% tacrolimus ointment | 16 weeks | One case had satisfactory improvement | None | Retrospective study |
| Vinay et al.[11] | 344 (Indian, Fitzpatrick skin type IV-VI) | Group 1: Topical steroids. Group 2: Topical tacrolimus Group 3: Oral mini pulse (OMP) Group 4: Oral drugs other than OMP. |
3-67 months (mean of 11 months) | 42.3% of patients experienced overall improvement. Group 3: 63% had a satisfactory response. Group 4: Improvement was only 42.3% |
The adverse effects to treatment were seen in 12% of cases, mostly in patients receiving systemic therapies | Retrospective study |
| Shah S D[37] | 13 | Q-switched Nd-Yag laser, 4-8 weekly × 5 sessions | 24 weeks | 38% had >90% improvement. Another 38% showed >75% improvement. 23% experienced >50% response. |
One patient had confetti-like leukoderma. Another patient experienced scarring and hypopigmentation | Lack of control group and small sample size |
| Riehl’s Melanosis/Pigmented Contact Dermatitis | ||||||
| Cho MY et al.[41] | 21 (Koreans, Fitzpatrick skin type III to IV) | 6 sessions of mid fluence (laser intensity of 3.5-5 J/cm2, 5-mm spot size, and 10 Hz frequency). Q-switched Nd-Yag 1064 nm laser with 40 days in between each session | 34 weeks | 2/21 patients showed >75% improvement. 8/21 had 50-75% improvement. 6/21 had 25-50% improvement. 2/21 had <25% improvement. 3/21 had no improvement | One patient developed itching One patient experienced prolonged erythema | Retrospective study |
| Xu Z et al.[43] | 10 (Chinese, Fitzpatrick skin type III to V) | Oral tranexamic acid 250 mg BD for 6 months with 150 mg oral glycyrrhizin compound for the first 3 months | 24 weeks | 7/10 experienced 50-75% improvement. 2/10 had 25-50% improvement. 1/10 patient had <25% improvement | None | Absence of a control group Low sample size |
| Kwon H et al.[44] | 8 (Koreans, Fitzpatrick skin type III to V) | Low-fluence Q-switched 1064 Nd-Yag laser at 3-week intervals. Hydroquinone 4% cream was applied every night, along with 250 mg/day of oral tranexamic acid | 54 weeks | 3/8 patients had >75% improvement. 5/8 had 50-75% improvement. | None | Absence of a control group Low sample size |
| Chung et al.[45] | 6 (Koreans, Fitzpatrick III and IV) | Dual-pulse mode Q-switched Nd-Yag laser with fluence of 2-4 J/cm2, frequency 10 Hz and spot size of 7 mm every 2 weeks for 4 months | 20 weeks | 4/6 patients had 50-75% improvement. 2/6 patients had 25-50% improvement. |
None | Small sample size |
| Li et al.[46] | 6 (Chinese, Fitzpatrick skin type IV) | Split face study, wherein one-half of the face was treated with IPL (fluence 17 J/cm2) in triple-pulsed mode with pulse width of 3 ms and delay time of 40 ms for 8-10 sessions | 24 weeks | 1 patient experienced excellent improvement and 5 experienced good improvement as per assessment of clinical photograph. Histopathology also showed greater reduction of melanin on the treatment side | Self-resolving post-inflammatory hyperpigmentation was noted in one patient | Novel safe and effective method |
| Ashy Dermatosis/Erythema Dyschromicum Perstans | ||||||
| Piquero MJ et al.[47] | 8 Latin Americans | Clofazamine 100 mg for 3 months, followed by gradual dose reduction | 3-8 months | 3 patients had excellent cure. 4 of them had good improvement. In 1 patient, the lesions persisted | All experienced reddish hue of the skin, epigastric pain. 2 of them had dry skin | Suggests immune down regulation as a component of the disease |
Lichen planus pigmentosus
Management of LPP includes avoidance of exacerbating factors; photoprotection in the actinic variant, and measures to reduce friction in the inversus variant such as weight reduction and avoidance of tight clothes. Topical steroids have been commonly used but are of doubtful efficacy. Topical tacrolimus, specific suppressor of T-cell-mediated inflammation, is considered a reasonable treatment option. An open-label study of topical tacrolimus (0.03%) used for 4 months showed improvement in 7/13 patients, with effects starting at 8 weeks.[21] In the authors' practice, topical tacrolimus is a commonly prescribed agent for treating LPP.
Low-fluence Q-switched Nd-YAG laser has been increasingly used to treat LPP. Bhari et al.[36] showed a mean clinical improvement of 25% after six sessions of Q-switched Nd-YAG laser at 2 weekly intervals. A similar study by Shah et al.[37] showed a maximal improvement of 38% in LPP patients. The combination of topical tacrolimus 0.1%, hydroxychloroquine 200 mg twice a day and Vitamin C iontophoresis for 6 months resulted in the stabilization of the hyperpigmentation. This was then combined with laser to reduce the residual hyperpigmentation.[38] Among chemical peels, croton oil-free phenol combination peel every 3 weeks was found to reduce the pigmentation by up to 75%.[39] In our experience, patients are mostly dissatisfied with minimal if any response obtained after lasers and peels.
Systemic treatment modalities that have been tried include systemic corticosteroids, dapsone, colchicine and isotretinoin.[11,19,40] Combining it with topical tacrolimus may be a reasonable option, especially in cases of predominant facial pigmentation. In a recent prospective study, we have assessed the use of mycophenolate mofetil in patients of ADMH. A significant reduction in DPASI score was seen at 24 weeks of follow-up (Bishnoi et al., under preparation).
Treatment in LPP patients aims to stabilize the disease and to reverse the hyperpigmentation. In our practice, for rapidly progressive and widespread disease, oral mini pulse (OMP) (dexamethasone 2.5 mg-5 mg twice weekly) or mycophenolate mofetil (2 g/day) is generally prescribed for disease stabilization. Topical treatments in the form of topical corticosteroids or tacrolimus and antioxidants are advised at a later date to reverse the pigmentation in localized and stable disease.[11]
Riehl's melanosis
The treatment of Riehl's melanosis remains inconclusive. Avoidance of the suspected causal agents is essential in preventing disease progression. In an Indian scenario, avoidance of hair dye/henna is crucial in the management of Riehl's melanosis.[17] Topical bleaching agents containing hydroquinone, tretinoin or glycolic acid have also been used to treat this disease with unsatisfactory clinical outcomes. Recent studies have demonstrated good efficacy on treatment of Riehl's melanosis with low-pulse energy 1064-nm Q-switched Nd-YAG laser; combination of low-fluence Q-switched 1064 nm Nd-YAG laser, hydroquinone cream and oral tranexamic acid; and Q-switched 1064 nm Nd-YAG laser-operated as a dualpulse at half-fluence and 140-μs intervals in Asian skin [Table 3].[41,42,43,44,45,46] However, in our experience, the improvement offered by Q-switched ND-YAG laser is modest and short lasting.
Ashy dermatosis
For ashy dermatosis, no consistently efficacious treatment exists. The successful outcomes obtained with griseofulvin and dithiazamine iodide, an antihelminthic drug, seem anecdotal. In two studies, clofazimine was reported to provide promising results.[47,48]
Conclusion
With an ever-increasing patient awareness, it is imperative for dermatologists to be aware of the various entities reported under the umbrella term of ADMH. The histopathology is usually similar, and treatment responses are dismal. New treatment options need to be deciphered and protocols for lasers need to be standardized. Also, future therapeutic trials should use validated severity assessment tools to define the degree of improvement to maintain uniformity in reporting outcomes across various study groups.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form the patient(s) has/have given his/her/their consent for his/her/their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Vinay K, Bishnoi A, Parsad D, Saikia UN, Sendhil Kumaran M. Dermoscopic evaluation and histopathological correlation of acquired dermal macular hyperpigmentation. Int J Dermatol. 2017;56:1395–9. doi: 10.1111/ijd.13782. [DOI] [PubMed] [Google Scholar]
- 2.Chandran V, Kumarasinghe SP. Macular pigmentation of uncertain aetiology revisited: Two case reports and a proposed algorithm for clinical classification. Australas J Dermatol. 2017;58:45–9. doi: 10.1111/ajd.12428. [DOI] [PubMed] [Google Scholar]
- 3.Ebihara T, Nakayama H. Pigmented contact dermatitis. Clin Dermatol. 1997;15:593–9. doi: 10.1016/s0738-081x(97)00072-2. [DOI] [PubMed] [Google Scholar]
- 4.Robles-Méndez JC, Rizo-Frías P, Herz-Ruelas ME, Pandya AG, Ocampo Candiani J. Lichen planus pigmentosus and its variants: Review and update. Int J Dermatol. 2018;57:505–14. doi: 10.1111/ijd.13806. [DOI] [PubMed] [Google Scholar]
- 5.Zaynoun S, Rubeiz N, Kibbi A-G. Ashy dermatoses – A critical review of the literature and a proposed simplified clinical classification. Int J Dermatol. 2008;47:542–4. doi: 10.1111/j.1365-4632.2008.03625.x. [DOI] [PubMed] [Google Scholar]
- 6.Kumarasinghe SPW, Pandya A, Chandran V, Rodrigues M, Dlova NC, Kang HY, et al. A global consensus statement on ashy dermatosis, erythema dyschromicum perstans, lichen planus pigmentosus, idiopathic eruptive macular pigmentation, and Riehl's melanosis. Int J Dermatol. 2019;58:263–72. doi: 10.1111/ijd.14189. [DOI] [PubMed] [Google Scholar]
- 7.Bishnoi A, Vinay K, Kumaran SM, Parsad D. Everything is in the name: Macular hyperpigmentation of uncertain etiology or acquired dermal macular hyperpigmentation of varied etiologies? Indian J Dermatol Venereol Leprol. 2018;85:85–7. doi: 10.4103/ijdvl.IJDVL_373_18. [DOI] [PubMed] [Google Scholar]
- 8.Gupta V, Sharma VK. Ashy dermatosis, lichen planus pigmentosus and pigmented cosmetic dermatitis: Are we splitting the hair? Indian J Dermatol Venereol Leprol. 2018;84:470–4. doi: 10.4103/ijdvl.IJDVL_549_17. [DOI] [PubMed] [Google Scholar]
- 9.Kumaran MS, Dabas G, Parsad D, Vinay K. Lichen planus pigmentosus-An appraisal. Int J Dermatol. 2018;57:748–50. doi: 10.1111/ijd.13982. [DOI] [PubMed] [Google Scholar]
- 10.Daroach M, Guliani A, Keshavmurthy V, Vishwajeet V, Saikia UN, Kumaran MS. Follicular lichen planus pigmentosus in blaschkoid pattern: Superimposed segmental mosaicism. Indian J Dermatol Venereol Leprol. 2020;86:305–7. doi: 10.4103/ijdvl.IJDVL_680_18. [DOI] [PubMed] [Google Scholar]
- 11.Vinay K, Kumar S, Bishnoi A, Aggarwal D, Radotra BD, Parsad D, et al. A clinico-demographic study of 344 patients with lichen planus pigmentosus seen in a tertiary care center in India over an 8-year period. Int J Dermatol. 2020;59:245–52. doi: 10.1111/ijd.14540. [DOI] [PubMed] [Google Scholar]
- 12.Kumaran MS, Bishnoi A, Srivastava N, Tekumalla S, Vinay K, Bhatia A, et al. Significant reduction in the expression of interleukins-17A, 22 and 23A, forkhead box p3 and interferon gamma delineates lichen planus pigmentosus from lichen planus. Arch Dermatol Res. 2019;311:519–27. doi: 10.1007/s00403-019-01926-9. [DOI] [PubMed] [Google Scholar]
- 13.Dabas G, Vinay K, Parsad D, Chatterjee D, Kumaran MS. A retrospective study of lichen planus pigmentosus with focus on palmoplantar involvement. Clin Exp Dermatol. 2019;44:190–3. doi: 10.1111/ced.13696. [DOI] [PubMed] [Google Scholar]
- 14.Sindhura KB, Vinay K, Kumaran MS, Saikia UN, Parsad D. Lichen planus pigmentosus: A retrospective clinico-epidemiologic study with emphasis on the rare follicular variant. J Eur Acad Dermatology Venereol. 2016;30:e142–4. doi: 10.1111/jdv.13454. [DOI] [PubMed] [Google Scholar]
- 15.Kumaran MS, Dabas G, Vinay K, Parsad D. Reliability assessment and validation of the dermal pigmentation area and severity index: A new scoring method for acquired dermal macular hyperpigmentation. J Eur Acad Dermatology Venereol. 2019;33:1386–92. doi: 10.1111/jdv.15516. [DOI] [PubMed] [Google Scholar]
- 16.Vinay K, Dabas G, Parsad D, Kumaran MS. A novel scale for measurement of acquired dermal macular hyperpigmentation severity. J Eur Acad Dermatology Venereol. 2018;32:e251–3. doi: 10.1111/jdv.14772. [DOI] [PubMed] [Google Scholar]
- 17.Bishnoi A, Vinay K, Arshdeep, Parsad D, Handa S, Saikia UN, et al. Contact sensitization to hair colours in acquired dermal macular hyperpigmentation: Results from a patch and photo-patch test study of 108 patients. J Eur Acad Dermatology Venereol. 2019;33:1349–57. doi: 10.1111/jdv.15576. [DOI] [PubMed] [Google Scholar]
- 18.Bhutani LK, Bedi TR, Pandhi RK, Nayak NC. Lichen planus pigmentosus. Dermatologica. 1974;149:43–50. doi: 10.1159/000251470. [DOI] [PubMed] [Google Scholar]
- 19.Kanwar AJ, Dogra S, Handa S, Parsad D, Radotra BD. A study of 124 Indian patients with lichen planus pigmentosus. Clin Exp Dermatol. 2003;28:481–5. doi: 10.1046/j.1365-2230.2003.01367.x. [DOI] [PubMed] [Google Scholar]
- 20.Pock L, Jelínková L, Drlík L, Abrhámová S, Vojtechovská S, Sezemská D, et al. Lichen planus pigmentosus-inversus. J Eur Acad Dermatology Venereol. 2001;15:452–4. doi: 10.1046/j.1468-3083.2001.00347.x. [DOI] [PubMed] [Google Scholar]
- 21.Al Mutairi N, El-Khalawany M. Clinicopathological characteristics of lichen planus pigmentosus and its response to tacrolimus ointment: An open label, non-randomized, prospective study. J Eur Acad Dermatol Venereol. 2010;24:535–40. doi: 10.1111/j.1468-3083.2009.03460.x. [DOI] [PubMed] [Google Scholar]
- 22.Sharma VK, Gupta V, Pahadiya P, Vedi KK, Arava S, Ramam M. Dermoscopy and patch testing in patients with lichen planus pigmentosus on face: A cross-sectional observational study in fifty Indian patients. Indian J Dermatology, Venereol Leprol. 2017;83:656–62. doi: 10.4103/ijdvl.IJDVL_469_16. [DOI] [PubMed] [Google Scholar]
- 23.Kumaran MS, Razmi TM, Vinay K, Parsad D. Clinical, dermoscopic, and trichoscopic analysis of frontal fibrosing alopecia associated with acquired dermal macular hyperpigmentation: A cross sectional observational case-control study. J Am Acad Dermatol. 2018;79:588–91. doi: 10.1016/j.jaad.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Jin H. Image Gallery: Lichen planus pigmentosus of the tongue. Br J Dermatol. 2020;182:e3. doi: 10.1111/bjd.18418. [DOI] [PubMed] [Google Scholar]
- 25.Rorsman H. Riehl's Melanosis. Int J Dermatol. 1982;21:75–8. doi: 10.1111/j.1365-4362.1982.tb00500.x. [DOI] [PubMed] [Google Scholar]
- 26.Pires MC, Manoel Silva dos Reis V, Mitelmann R, Moreira F. Pigmented contact dermatitis due to Plathymenia foliosa dust. Contact Dermatitis. 1999;40:339. doi: 10.1111/j.1600-0536.1999.tb06099.x. [DOI] [PubMed] [Google Scholar]
- 27.Khanna N, Rasool S. Facial melanoses: Indian perspective. Indian J Dermatology, Venereol Leprol. 2011;77:552–63. doi: 10.4103/0378-6323.84046. [DOI] [PubMed] [Google Scholar]
- 28.Sugai T, Takahasmi Y, Takagi T. Pigmented cosmetic dermatitis and coal tar dyes *. Contact Dermatitis. 1977;3:249–56. doi: 10.1111/j.1600-0536.1977.tb03670.x. [DOI] [PubMed] [Google Scholar]
- 29.Combemale P, Faisant M, Guennoc B, Dupin M, Heyraud JD. Erythema dyschromicum perstans: Report of a new case and critical review of the literature. J Dermatol. 1998;25:747–53. doi: 10.1111/j.1346-8138.1998.tb02495.x. [DOI] [PubMed] [Google Scholar]
- 30.Convit J, Kerdel-Vegas FRG. Erythema dyschromicum perstans. J Invest Dermatol. 1961;36:457–62. [Google Scholar]
- 31.Berger RS, Hayes TJ, Dixon SL. Erythema dyschromicum perstans and lichen planus: Are they related? J Am Acad Dermatol. 1989;21:438–42. doi: 10.1016/s0190-9622(89)80055-6. [DOI] [PubMed] [Google Scholar]
- 32.Kubba A, Patel A, Kubba R. Clinicopathological correlation of acquired hyperpigmentary disorders. Indian J Dermatol, Venereol Leprol. 2013;79:367–75. doi: 10.4103/0378-6323.110798. [DOI] [PubMed] [Google Scholar]
- 33.Sasidharanpillai S, Govindan A, Ajithkumar Ky, Mahadevan ST, Bindu V, Khader A, et al. Histological evaluation of acquired dermal macular hyperpigmentation. Indian Dermatol Online J. 2019;10:542–46. doi: 10.4103/idoj.IDOJ_426_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bishnoi A, Vinay K, Parsad D, Kumaran MS. Exaggerated epidermal pigmentation in acquired dermal macular hyperpigmentation: An important finding. Clin Exp Dermatol. 2019;44:e211. doi: 10.1111/ced.13978. [DOI] [PubMed] [Google Scholar]
- 35.Dabas G, Vinay K, Parsad D, Kumar A, Kumaran MS. Psychological disturbances in patients with pigmentary disorders: A cross-sectional study. J Eur Acad Dermatology Venereol. 2020;34:392–9. doi: 10.1111/jdv.15987. [DOI] [PubMed] [Google Scholar]
- 36.Bhari N, Sharma VK, Singh S, Parihar A, Arava S. Effect of Q-switched Nd-YAG laser on the clinical, pigmentary, and immunological markers in patients with lichen planus pigmentosus: A pilot study. Dermatol Ther. 2020;33:e13208. doi: 10.1111/dth.13208. [DOI] [PubMed] [Google Scholar]
- 37.Shah DSD, Aurangabadkar DS, Nikam DB. An open-label non-randomized prospective pilot study of the efficacy of Q-switched Nd-YAG laser in management of facial lichen planus pigmentosus. J Cosmet Laser Ther. 2019;21:108–15. doi: 10.1080/14764172.2018.1469770. [DOI] [PubMed] [Google Scholar]
- 38.Wu CY, Lin FL. A successful combination therapy of tacrolimus, hydroxychloroquine and picosecond laser for lichen planus pigmentosus. Australas J Dermatol. 2019;60:e336–7. doi: 10.1111/ajd.13060. [DOI] [PubMed] [Google Scholar]
- 39.Sonthalia S, Vedamurthy M, Thomas M, Goldust M, Jha AK, Srivastava S, Aggarwal I. Modified phenol peels for treatment-refractory hyperpigmentation of lichen planus pigmentosus: A retrospective clinico-dermoscopic analysis. J Cosmet Dermatol. 2019;18:1479–86. doi: 10.1111/jocd.12862. [DOI] [PubMed] [Google Scholar]
- 40.Muthu SK, Narang T, Saikia UN, Kanwar AJ, Parsad D, Dogra S. Low-dose oral isotretinoin therapy in lichen planus pigmentosus: An open-label non-randomized prospective pilot study. Int J Dermatol. 2016;55:1048–54. doi: 10.1111/ijd.13293. [DOI] [PubMed] [Google Scholar]
- 41.Cho MY, Roh MR. Successful treatment of Riehl's melanosis with mid-fluence Q-switched Nd: YAG 1064-nm laser. Lasers Surg Med. 2020;52:753–60. doi: 10.1002/lsm.23214. [DOI] [PubMed] [Google Scholar]
- 42.Wang L, Wen X, Hao D, Li Y, Du D, Jiang X. Combination therapy with salicylic acid chemical peels, glycyrrhizin compound, and vitamin C for Riehl's melanosis. J Cosmet Dermatol. 2020;19:1377–80. doi: 10.1111/jocd.13153. [DOI] [PubMed] [Google Scholar]
- 43.Xu Z, Xing X, Zhang C, Chen L, Flora Xiang L. A pilot study of oral tranexamic acid and Glycyrrhizin compound in the treatment of recalcitrant Riehl's melanosis. J Cosmet Dermatol. 2019;18:286–92. doi: 10.1111/jocd.12797. [DOI] [PubMed] [Google Scholar]
- 44.Kwon HH, Ohn J, Suh DH, Park HY, Choi SC, Jung JY, et al. A pilot study for triple combination therapy with a low-fluence 1064 nm Q-switched Nd: YAG laser, hydroquinone cream and oral tranexamic acid for recalcitrant Riehl's Melanosis. J Dermatolog Treat. 2017;28:155–9. doi: 10.1080/09546634.2016.1187706. [DOI] [PubMed] [Google Scholar]
- 45.Chung BY, Kim JE, Ko JY, Chang SE. A pilot study of a novel dual-Pulsed 1064 nm Q-switched Nd: YAG laser to treat Riehl's melanosis. J Cosmet Laser Ther. 2014;16:290–2. doi: 10.3109/14764172.2014.946054. [DOI] [PubMed] [Google Scholar]
- 46.Li YH, Liu J, Chen JZ, Wu Y, Xu TH, Zhu X, et al. A pilot study of intense pulsed light in the treatment of Riehl's melanosis. Dermatologic Surg. 2011;37:119–22. doi: 10.1111/j.1524-4725.2010.01831.x. [DOI] [PubMed] [Google Scholar]
- 47.Piquero-Martín J, Pérez-Alfonzo R, Abrusci V, Briceño L, Gross A, Mosca W, et al. Clinical trial with clofazimine for treating erythema dyschromicum perstans. Evaluation of cell-mediated immunity. Int J Dermatol. 1989;28:198–200. doi: 10.1111/j.1365-4362.1989.tb02466.x. [DOI] [PubMed] [Google Scholar]
- 48.Baranda L, Torres-Alvarez B, Cortes-Franco R, Moncada B, Portales-Perez DP, Gonzalez-Amaro R. Involvement of cell adhesion and activation molecules in the pathogenesis of erythema dyschromicum perstans (ashy dermatitis). The effect of clofazimine therapy. Arch Dermatol. 1997;133:325–9. [PubMed] [Google Scholar]


