Abstract
Background
Amniotomy (deliberate rupture of the membranes) is a simple procedure which can be used alone for induction of labour if the membranes are accessible, thus avoiding the need for pharmacological intervention. However, the time interval from amniotomy to established labour may not be acceptable to clinicians and women, and in a number of cases labour may not ensue. This is one of a series of reviews of methods of cervical ripening and labour induction using standardised methodology.
Objectives
To determine the effects of amniotomy alone for third trimester labour induction in women with a live fetus.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register and bibliographies of relevant papers (January 2007). We updated this search on 23 May 2012 and added the results to the awaiting classification section of the review.
Selection criteria
Clinical trials comparing amniotomy alone for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction. We assessed trial quality and contacted study authors for additional information.
Main results
Two trials, comprising 50 and 260 women, respectively were eligible for inclusion in this review. No conclusions could be drawn from comparisons of amniotomy alone versus no intervention, and amniotomy alone versus oxytocin alone (small trial, only one pre‐specified outcome reported). No trials compared amniotomy alone with intracervical prostaglandins. One trial compared amniotomy alone with a single dose of vaginal prostaglandins for women with a favourable cervix, and found a significant increase in the need for oxytocin augmentation in the amniotomy alone group (44% versus 15%; relative risk 2.85, 95% confidence interval 1.82 to 4.46). This should be viewed with caution as this was the result of a single‐centre trial. Furthermore, secondary intervention occurred four hours after amniotomy, and this time interval may not have been appropriate.
Authors' conclusions
Data are lacking about the value of amniotomy alone for induction of labour. While there are now other modern methods available for induction of labour (pharmacological agents), there remain clinical scenarios where amniotomy alone may be desirable and appropriate, and this method is worthy of further research. This research should include evaluation of the appropriate time interval from amniotomy to secondary intervention, women and caregivers' satisfaction and economic analysis.
[Note: the two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Amnion; Amnion/surgery; Cesarean Section; Labor, Induced; Labor, Induced/methods; Pregnancy Trimester, Third; Randomized Controlled Trials as Topic
Plain language summary
Amniotomy alone for induction of labour
There is not enough evidence about the effects of amniotomy alone (deliberate rupture of the membranes) to induce labour.
Sometimes it is advisable to get labour started (induction) because of concerns about either the pregnant woman or her unborn baby. Amniotomy has been used as either the only method of inducing labour if the membranes can be reached, or used with drugs such as oxytocin or prostaglandin. Amniotomy may be preferred by women wanting a drug‐free labour and it is cheap. However, it can be uncomfortable and, if after amniotomy there is a long time interval before the baby is born, there is a risk of infection. There is also the risk of the cord coming out before the baby. This review of trials found that there is not enough evidence about the effects of amniotomy alone for the induction of labour.
Background
This review is one of a series of reviews of methods of labour induction using a standardised protocol (Hofmeyr 2000).
Induction of labour is a common obstetric intervention which is usually undertaken for a clinical indication (for example concerns about fetal wellbeing, post term pregnancy). It may also, rightly or wrongly, be undertaken for other reasons, such as a woman's request or clinician's convenience.
Amniotomy, i.e. deliberate artificial rupture of the membranes, was first described over 200 years ago by Thomas Denman of the Middlesex Hospital in the United Kingdom. This procedure is performed vaginally, whereby the clinician identifies the cervix and membranes digitally, introduces the instrument to be used and pierces the membranes. The instrument most commonly used is a specially designed plastic hook, but sometimes steel surgical forceps are used. The procedure itself is not painful as there are no nerve endings on the membranes, and anaesthesia is not required, however, the vaginal examination required to gain access to the cervix and membranes may be uncomfortable, and in some cases painful, particularly if the membranes are difficult to reach.
Amniotomy may be performed during labour for various clinical reasons, such as failure to progress in the first stage of labour and need to assess the status of the liquor in the presence of fetal heart rate abnormalities. In the past it has been advocated in combination with oxytocin for the active management of labour (O'Driscoll 1973). However, a systematic review of 9 trials entitled 'amniotomy for shortening spontaneous labour' (Fraser 2000), concluded that it is associated with both benefit and risk and that it should be reserved for women with abnormal labour progress. In particular although it may reduce the length of spontaneous labour and the need for oxytocin augmentation, there is a trend toward an increase in the caesarean section rate.
It may also be employed prelabour as the sole method of induction of labour or in combination with the use of pharmacological agents for induction of labour, such as oxytocin or prostaglandins. Theoretically, amniotomy releases endogenous prostaglandins which in turn may result in cervical changes and spontaneous labour. A rise in prostaglandin metabolites following amniotomy has been demonstrated and the concentration does seem to be related to the induction‐delivery interval (Husslein 1983).
This procedure is only possible if the membranes are physically accessible. Even if the cervix is unfavourable (low Bishop's score), the membranes may be accessible. Potential hazards associated with amniotomy include cord prolapse if the presenting part is high at the time of the procedure and when the membranes have been breeched, the fetal environment is vulnerable to ascending infection. Indeed, infective agents can be introduced at the time of amniotomy and during subsequent vaginal examinations. Amniotomy for induction of labour may be contraindicated in the presence of known HIV positivity as duration of membrane rupture has been identified as an independent risk factor for mother‐to‐child transmission of HIV infection (Landesman 1996).
Booth found approximately 88% of women with a favourable Bishop's score will labour after amniotomy alone (Booth 1970). However the amniotomy to labour interval may be long, for example, Saleh 1975 reported in a prospective observational study of amniotomy versus amniotomy and early intravenous oxytocin infusion, significantly more women undelivered after 24 hours in the amniotomy alone group. The long amniotomy to labour interval may not be acceptable to women and/or clinicians, particularly in view of the risk of ascending infection, and if there is a clinical indication for induction favouring labour and delivery without unnecessary delay. Of course, labour may not ensue, even after a prolonged interval, in which case pharmacological agents would be indicated.
In modern day obstetric practice amniotomy is more commonly used in combination with pharmacological agents for induction of labour. However, knowledge of its efficacy as an induction method remains valuable for a number of reasons. In countries where resources are limited, it may be worthwhile as a first line induction method, to avoid using expensive pharmacological agents. In some situations where the woman prefers to avoid pharmacological intervention if at all possible, it could be employed initially, and pharmacological agents introduced, if after a specified time interval, labour does not ensue. In women with a previous caesarean section it may be advantageous to avoid pharmacological uterine stimulation.
Objectives
To determine, from the best available evidence, the effectiveness and safety of amniotomy alone for third trimester cervical ripening and induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing amniotomy alone for cervical ripening or labour induction, with placebo/no treatment or other methods listed above it on a predefined list of methods of labour induction (see 'Methods of the review'); the trials included some form of random allocation to either group; and they reported one or more of the prestated outcomes. Due to anticipated paucity of trials, pseudo‐randomised trials were considered.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a live fetus.
Predefined subgroup analyses will be (see list below): previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data will appear in the analysis tables.
Types of interventions
Amniotomy alone compared with placebo/no treatment or any other method above it on a predefined list of methods of labour induction. It was intended that comparisons with three methods, which are placed above amniotomy alone on a predefined list, would be included in this review as follows: (i) vaginal prostaglandins; (ii) intracervical prostaglandins; (iii) oxytocin alone.
For details of the predefined list of induction methods please refer to 'Methods of the review'.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). Differences were settled by discussion amongst registered Cochrane Pregnancy and Childbirth reviewers with an interest in labour induction.
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. subgroup analyses will be limited to the primary outcomes: (1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this is unlikely. All these events will be rare, and a modest change in their incidence will be easier to detect if composite outcomes are presented. It was intended that the incidence of individual components be explored as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction:
Measures of effectiveness:
(6) cervix unfavourable/unchanged after 12‐24 hours; (7) oxytocin augmentation.
Complications:
(8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia (11) instrumental vaginal delivery; (12) meconium stained liquor; (13) Apgar score <7 at 5 minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side effects (all) (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction:
(26) woman not satisfied; (27) caregiver not satisfied.
While all the above outcomes were sought, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we will use the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (>5 contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short term variability).
One non‐prespecified outcome is included in the analysis tables, namely, antibiotics given to baby.
Outcomes were included in the analysis: if reasonable measures were taken to minimise observer bias; and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register by contacting the Trials Search Co‐ordinator (January 2007). We updated this search on 23 May 2012 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
The original search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2000).
Searching other resources
We searched the reference lists of trial reports. We did not apply any language restrictions.
Data collection and analysis
A strategy has been developed to deal with the large volume and complexity of trial data relating to labour induction. This strategy was developed as the result of a collaboration between the Cochrane Pregnancy and Childbirth Group and the Clinical Effectiveness Support Unit of the Royal College of Obstetricians and Gynaecologists, UK. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We have therefore developed a two‐stage method of data extraction. The initial data extraction was done in a series of primary reviews (including this review) arranged by methods of induction of labour, following a standardised methodology. The data will then be extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each primary review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, for example, this review (5) was to include only comparisons with intravenous oxytocin (4) intracervical prostaglandins (3), vaginal prostaglandins (2) or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
placebo/no treatment;
vaginal prostaglandins (Kelly 2003);
intracervical prostaglandins (Boulvain 2008);
intravenous oxytocin (Kelly 2001a);
amniotomy;
amniotomy plus intravenous oxytocin (Howarth 2001);
vaginal misoprostol (Hofmeyr 2003);
oral misoprostol (Alfirevic 2006);
mechanical methods including extra‐amniotic Foley catheter (Boulvain 2001);
membrane sweeping (Boulvain 2005);
extra‐amniotic prostaglandins (Hutton 2001);
intravenous prostaglandins (Luckas 2000);
oral prostaglandins (French 2001);
mifepristone (Neilson 2000);
oestrogens alone of with amniotomy (Thomas 2001);
corticosteroids (Kavanagh 2006a);
relaxin (Kelly 2001c);
hyaluronidase (Kavanagh 2006b);
castor oil, bath and/or enema (Kelly 2001b);
acupuncture (Smith 2004);
breast stimulation (Kavanagh 2005);
sexual intercourse (Kavanagh 2001);
homeopathic methods (Smith 2003);
nitric oxide donors (Kelly 2011);
buccal or sublingual misoprostol (Muzonzini 2004);
hypnosis;
other methods for induction of labour.
The primary reviews were to be analysed by the following subgroups:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
The secondary reviews will include all methods of labour induction for each of the categories of women for which subgroup analysis has been done in the primary reviews, and will include only five primary outcome measures. There will thus be six secondary reviews, of methods of labour induction in the following groups of women:
nulliparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
nulliparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined);
multiparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
multiparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined);
previous caesarean section, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
previous caesarean section, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined).
Each time a primary review is updated with new data, those secondary reviews which include data which have changed, will also be updated.
The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of the Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials were initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous reviewers in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Appendix 1 for the purpose of the reviews.
Performance bias was examined with regards to whom was blinded in the trials i.e. patient, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. Details of the feasibility and appropriateness of blinding at all levels were sought.
Individual outcome data were included in the analysis if they met the prestated criteria in 'Types of outcome measures'. Included trial data were processed as described in the Cochrane Collaboration Handbook (Clarke 1999). Data extracted from the trials were analysed on an intention to treat basis (when this was not done in the original report, re‐analysis was performed if possible). If data were missing, clarification would have been sought from the original authors. If the attrition was such that it might significantly affect the results, these data would have been excluded from the analysis. This decision rested with the reviewers of primary reviews and is clearly documented. If there were missing data which later became available, they would have been included in the analyses.
Data would have been extracted from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting is poor, methodological issues would have been reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors was therefore assessed on a random sample of trials.
Once the data had been extracted, they were distributed to individual reviewers for entry onto the Review Manager computer software (RevMan 1999), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, relative risks and 95% confidence intervals were calculated, and in the absence of heterogeneity, results were pooled using a fixed effects model.
The predefined criteria for sensitivity analysis include all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis was limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these were to be analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Results
Description of studies
Seven potential trials were identified.
Five excluded trials:
Three studies were excluded because they did not report any of the pre‐specified primary or secondary outcomes. Sivasuriya 1978 evaluated the association between three methods of induction of labour and neonatal jaundice. Thornton 1989 determined oxytocin concentration in women undergoing amniotomy for induction of labour. Ward 1991 is a conference abstract which presents a randomised trial of three methods of induction, but does not contain any data for extraction relating to the outcome measures sought.
Two studies (Secher 1981; Westergaard 1983) are methodologically good randomised trials of prostaglandins versus oxytocin for induction of labour, in two subgroups of women, namely those with favourable cervix where amniotomy alone was performed prior to randomisation and those with unfavourable cervix where amniotomy was not performed prior to randomisation. Therefore amniotomy was not randomised, but pragmatically allocated, thus rendering these trials not eligible for inclusion in this review. They will, however be considered for other relevant primary induction of labour reviews.
Two included trials:
Jagani 1982 is a small pseudo‐randomised trial comprising 50 women which aimed to evaluate whether alteration of the cervix affects the ability to induce labour if the cervix was unfavourable (Bishop's score is 4 or less). There were five subgroups, each of 10 women, namely (1) control (no intervention); (2) laminaria; (3) foley catheter; (4) amniotomy; and (5) oxytocin with intact membranes, to which women were allocated by chart number. After 12 hours of the allocated intervention, amniotomy and oxytocin were employed to induce labour. The analysis aimed to evaluate whether any of the primary interventions improved the Bishop's score and the ability to induce labour, as well as reduce the induction to delivery interval.
Mahmood 1995 is a randomised trial comparing a single dose of vaginal prostaglandin E2 gel (primiparae 2 mg, multiparae 1 mg) with forewater amniotomy for induction of labour at term in 260 women with a favourable cervix (Bishop's score 6 or greater). In the prostaglandin group amniotomy was performed four hours after insertion of the gel or sooner if analgesia requested. In the amniotomy group review was undertaken four hours after amniotomy and oxytocin commenced if there was no uterine activity and/or no change in cervical status. In both groups at six hours oxytocin was commenced if there was evidence of unsatisfactory uterine activity.
(Two reports from an updated search on 23 May 2012 have been added to Studies awaiting classification.)
Risk of bias in included studies
Jagani 1982 used chart numbers and hence pseudo‐randomised (inadequate concealment allocation). Blinding of the patient and the therapist was not feasible in this trial. It is unclear whether the outcome assessor or analyst were blinded to the initial intervention. Analysis was performed on an intention to treat basis and there were no losses to follow up, thus reducing attrition bias. This trial was small with only 10 women in each of the subgroups and therefore unlikely to produce meaningful results. Furthermore, only three of the prespecified outcomes were reported, two of which were not reported in absolute figures (but as mean and standard deviations) and thus the data were not usable for this review.
The trial by Mahmood 1995 was very well designed. Following a retrospective audit for pilot data, a power calculation was undertaken to determine the required sample size. The method of randomisation was consecutive numbered sealed opaque envelopes which contained allocation from a table of random numbers (adequate concealment allocation). Blinding to reduce performance and detection bias was unfeasible. Analysis was performed on an intention to treat basis, there were no reported protocol violations and no losses to follow up, thus reducing attrition bias. In the amniotomy alone group, further intervention occurred at four hours (i.e. oxytocin if labour not established), and this arbitrary time interval is important, as it is possible that the longer a women is left with ruptured membranes, the more likely labour will ensue without the need for intervention. However, the 'ideal' time interval is not known.
Effects of interventions
Amniotomy alone versus no intervention
This comparison included only one small trial with 10 women in each group (Jagani 1982). The only primary outcome reported where data could be extracted was caesarean section, and although this was not significantly increased in the amniotomy alone group (40% versus 0%; relative risk (RR) 9.00, 95% confidence interval (CI) 0.55 to 147.95), in view of the wide confidence intervals due to limited numbers this should be viewed with caution. None of the prespecified secondary outcomes were reported.
Amniotomy alone versus oxytocin
This comparison included only one small trial with 10 women in each group (Jagani 1982). The only outcome reported where data could be extracted was caesarean section, and there was no significant difference between the two interventions (40% versus 30%; RR 1.33, 95% CI 0.40 to 4.49), but this should be viewed with caution due to limited numbers. None of the prespecified secondary outcomes were reported.
Amniotomy alone versus vaginal prostaglandin
This comparison included only one trial comprising 260 women, 130 in each group (Mahmood 1995). Data were reported separately for primiparae (110 women) and multiparae (150 women).
Primary outcomes
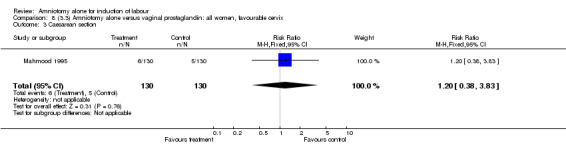
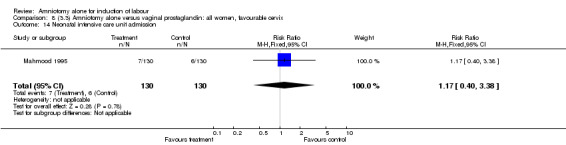
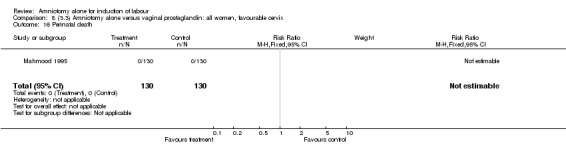
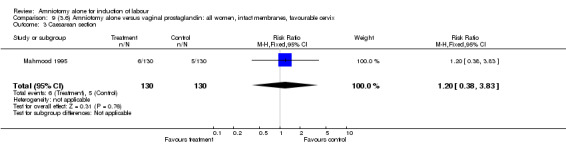
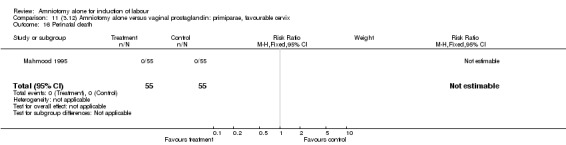
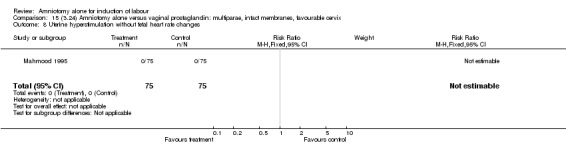
There were no cases of uterine hyperstimulation with fetal heart rate changes. The caesarean section rates were similar for both groups (46% versus 39%). There were no perinatal deaths. Other primary outcomes were not reported (vaginal delivery not achieved within 24 hours, serious neonatal morbidity and serious maternal morbidity or death).
Secondary outcomes
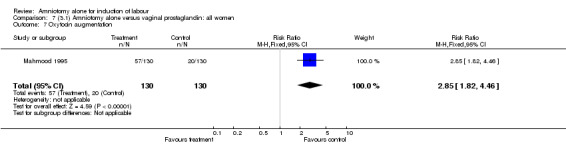
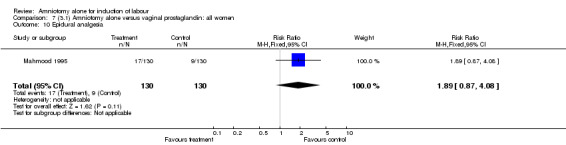
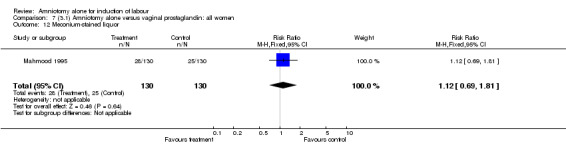
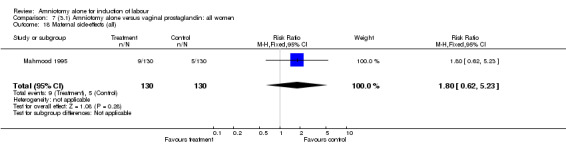
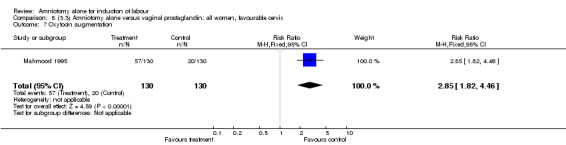
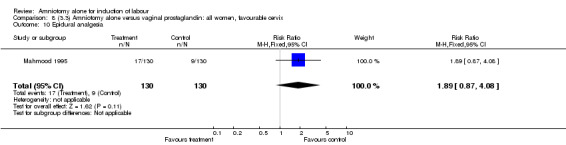
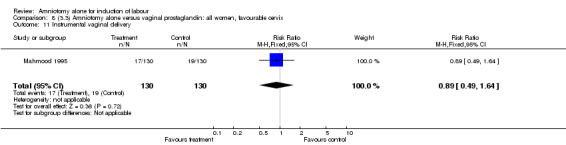
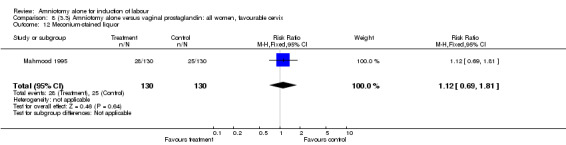
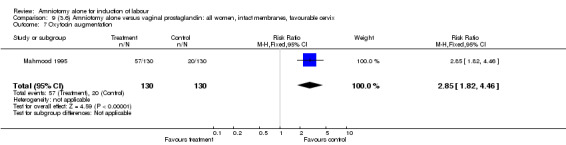
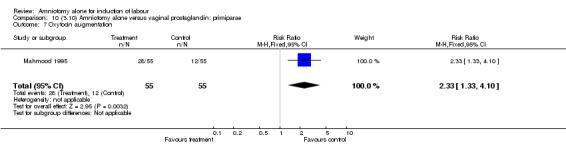
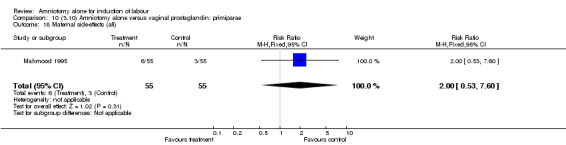
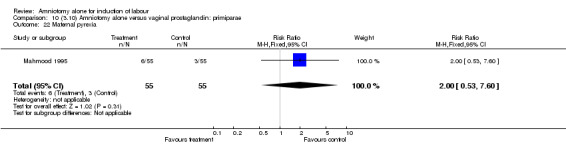
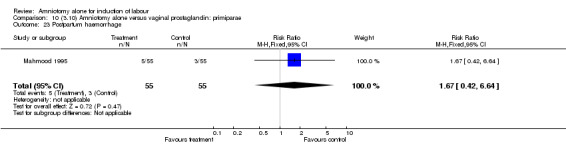
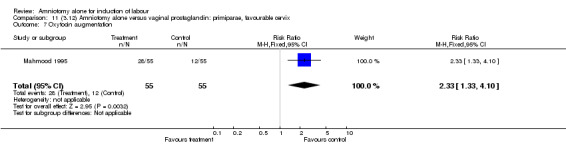
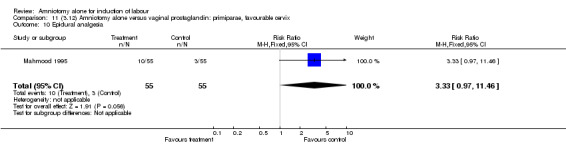
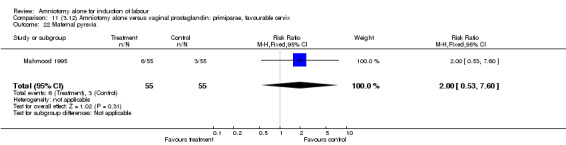
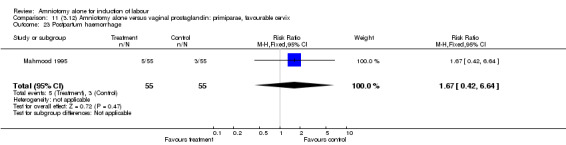
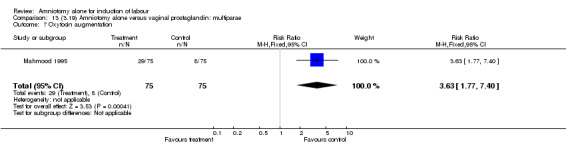
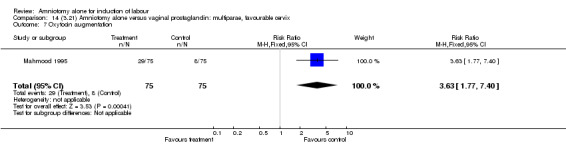
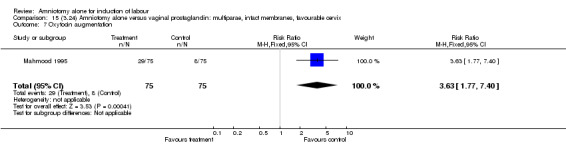
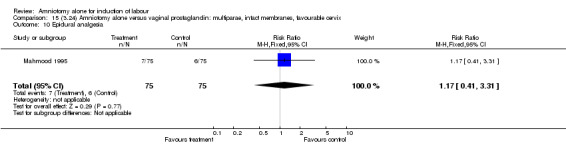
There was a significant increase in oxytocin augmentation in the amniotomy alone group compared with the vaginal prostaglandin group (44% versus 15%; RR 2.85, 95% CI 1.82 to 4.46). This was apparent for both primiparae and multiparae, but more so for multiparae (51% versus 22%; RR 2.33, 95% CI 1.33 to 4.10 and 39% versus 11%; RR 3.63, 95% CI 1.77 to 7.40 respectively). There was a non‐significant increase in epidural analgesia in the amniotomy alone group (13% versus 7%; RR 1.89, 95% CI 0.87 to 4.08), particularly amongst primiparae (18% versus 6%; RR 3.33, 95% CI 0.97 to 11.46). There was a non‐significant increase in intrapartum maternal pyrexia in the amniotomy alone group (7% versus 4%; RR 1.80, 95% CI 0.62 to 5.23). In the amniotomy group, amongst primiparae there was a non‐significant increase in meconium‐stained liquor (22% versus 13%; RR 1.71, 95% CI 0.73 to 4.03). In the amniotomy group, amongst primiparae there was a non‐significant increase in postpartum haemorrhage (9% versus 6%, RR 1.67, 95% CI 0.42 to 6.64) and by contrast this was the opposite in multiparae (9% versus 12%; RR 0.78, 95% CI 0.31 to 1.98).
Amniotomy alone versus intracervical prostaglandin
There were no trials which addressed this comparison.
Discussion
Presumably, amniotomy alone is widely used for induction of labour, particularly in countries where resources are scarce, yet there is surprisingly little research in this area. Due to the paucity of trials, firm conclusions cannot be drawn about amniotomy alone for induction of labour. Furthermore one of the two included trials (Jagani 1982) reported only one of the prespecified outcomes (caesarean section), thus compounding this lack of data.
The only trial which reported a number of the pre‐specified outcome measures (Mahmood 1995) was well designed. The only significant finding was the increased need for oxytocin augmentation following amniotomy alone versus a single dose of vaginal prostaglandin in women with a favourable cervix. However, this finding would ideally need to be validated in other clinical settings and different populations. Furthermore, as secondary intervention occurred four hours after amniotomy, it could be argued that if a longer time interval was allowed this difference may not have arisen, as the longer allowed from amniotomy to intervention, the more likely labour would ensue. The nonsignificant differences in the amniotomy alone group towards an increase in epidural analgesia (particularly amongst primiparae), intrapartum maternal pyrexia, meconium‐stained liquor (amongst primiparae) are interesting but not conclusive. The contrast in incidence of postpartum haemorrhage amongst primiparae (nonsignificant increase) versus multiparae (nonsignificant decrease) is difficult to interpret and may be meaningless, and a chance effect.
With such small numbers it is impossible to comment on implications for perinatal and maternal morbidity and mortality, as trials or meta‐analysis of large power are required (thousands of participants).
Authors' conclusions
Implications for practice.
Data on the effectiveness and safety of amniotomy alone for induction of labour are lacking. No recommendations for clinical practice can be made on the basis of this review.
Implications for research.
Although there are numerous pharmacological methods for induction of labour, the clinical effectiveness and safety of which will be reported in other primary reviews, there are scenarios where amniotomy alone may be favoured. For example, in clinical settings where resources are limited and cost savings on pharmacological agents may be welcomed, and if an individual woman is not keen on pharmacological intervention. In women with previous caesarean section, avoiding pharmacological uterine stimulation may be advantageous. Therefore, we feel it is reasonable to recommend that further research into the method of amniotomy alone for induction of labour is needed, and would urge researchers to evaluate this method in the context of different time intervals between the primary (amniotomy alone) and secondary intervention (addition of a pharmacological agent). This research should include assessment of women and caregiver satisfaction and economic analysis.
[Note: the two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 23 May 2012 | Amended | Search updated. Two reports added to Studies awaiting classification (Macones 2011; Rijnders 2007). |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 28 August 2008 | Amended | Converted to new review format. |
| 31 January 2007 | New search has been performed | Search updated. One new trial excluded (Chanrachakul 2003). |
Acknowledgements
The authors would like to thank Tony Kelly and Jo Kavanagh ‐ Research fellows of the Clinical Effectiveness Support Unit, RCOG, UK and the team involved in co‐ordinating the induction of labour primary reviews. We would also like to thank Zarko Alfirevic for expert and much needed advice in compiling the review.
Appendices
Appendix 1. Methodological quality of trials
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
Data and analyses
Comparison 1. (1.1) Amniotomy alone versus no intervention: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
1.3. Analysis.
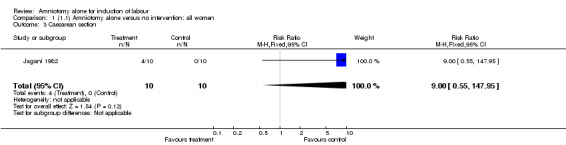
Comparison 1 (1.1) Amniotomy alone versus no intervention: all women, Outcome 3 Caesarean section.
Comparison 2. (1.2) Amniotomy alone versus no intervention: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
2.3. Analysis.
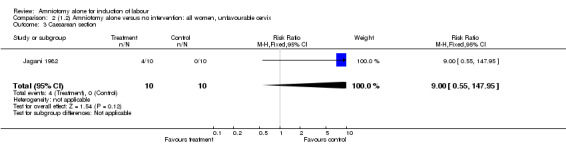
Comparison 2 (1.2) Amniotomy alone versus no intervention: all women, unfavourable cervix, Outcome 3 Caesarean section.
Comparison 3. (1.5) Amniotomy alone versus no intervention: all women, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 9.0 [0.55, 147.95] |
3.3. Analysis.
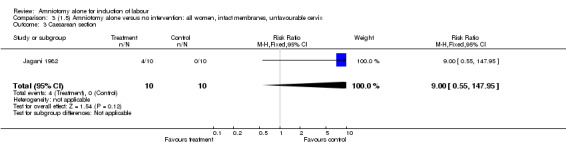
Comparison 3 (1.5) Amniotomy alone versus no intervention: all women, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
Comparison 4. (2.1) Amniotomy alone versus oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.40, 4.49] |
4.3. Analysis.
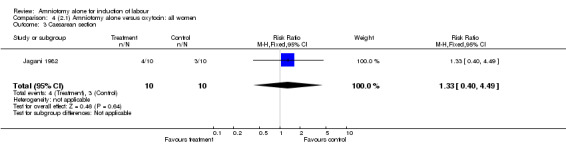
Comparison 4 (2.1) Amniotomy alone versus oxytocin: all women, Outcome 3 Caesarean section.
Comparison 5. (2.2) Amniotomy alone versus oxytocin: all women, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.40, 4.49] |
5.3. Analysis.
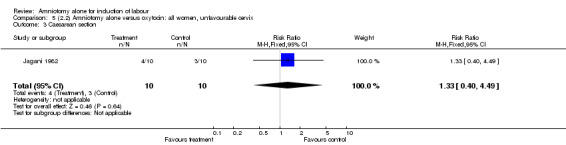
Comparison 5 (2.2) Amniotomy alone versus oxytocin: all women, unfavourable cervix, Outcome 3 Caesarean section.
Comparison 6. (2.5) Amniotomy alone versus oxytocin: all women, intact membranes, unfavourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.40, 4.49] |
6.3. Analysis.
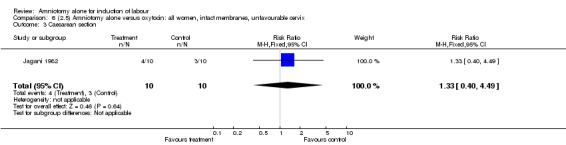
Comparison 6 (2.5) Amniotomy alone versus oxytocin: all women, intact membranes, unfavourable cervix, Outcome 3 Caesarean section.
Comparison 7. (3.1) Amniotomy alone versus vaginal prostaglandin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
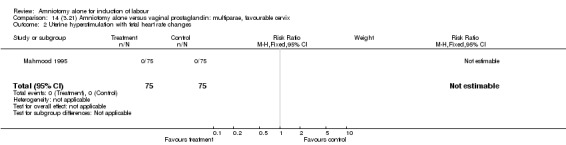
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
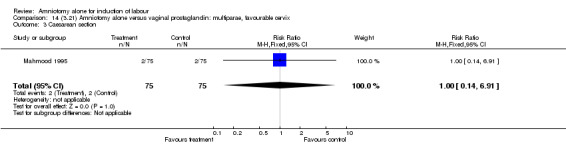
| 3 Caesarean section | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.83] |
| 7 Oxytocin augmentation | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.82, 4.46] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.87, 4.08] |
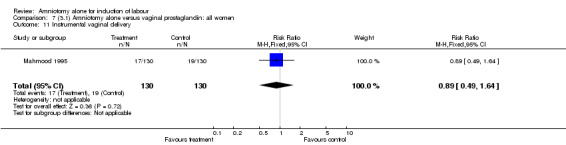
| 11 Instrumental vaginal delivery | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.64] |
| 12 Meconium‐stained liquor | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.69, 1.81] |
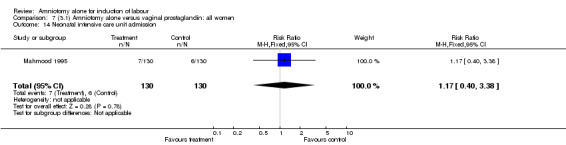
| 14 Neonatal intensive care unit admission | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
| 16 Perinatal death | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 22 Maternal pyrexia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 23 Postpartum haemorrhage | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
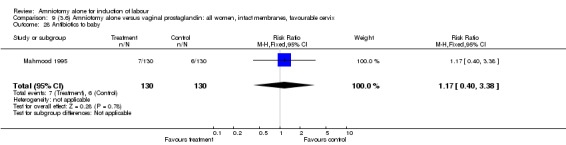
| 28 Antibiotics to baby | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
7.2. Analysis.
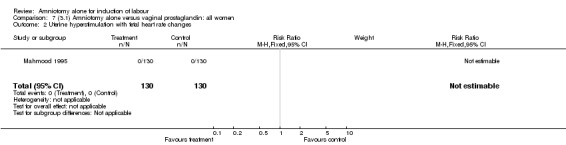
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
7.3. Analysis.
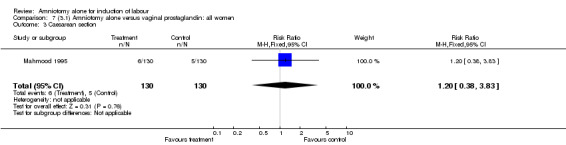
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 3 Caesarean section.
7.7. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 7 Oxytocin augmentation.
7.8. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
7.10. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 10 Epidural analgesia.
7.11. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 11 Instrumental vaginal delivery.
7.12. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 12 Meconium‐stained liquor.
7.14. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 14 Neonatal intensive care unit admission.
7.16. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 16 Perinatal death.
7.18. Analysis.
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 18 Maternal side‐effects (all).
7.22. Analysis.
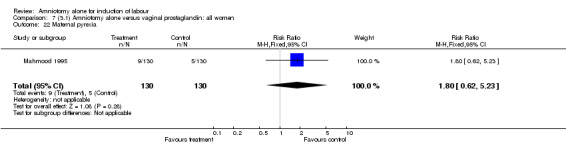
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 22 Maternal pyrexia.
7.23. Analysis.
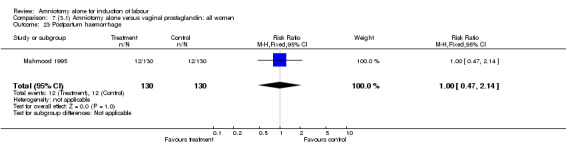
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 23 Postpartum haemorrhage.
7.28. Analysis.
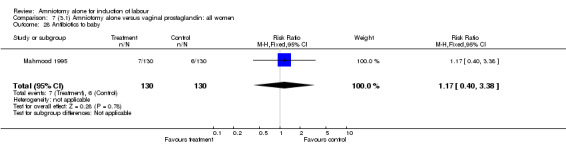
Comparison 7 (3.1) Amniotomy alone versus vaginal prostaglandin: all women, Outcome 28 Antibiotics to baby.
Comparison 8. (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.83] |
| 7 Oxytocin augmentation | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.82, 4.46] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.87, 4.08] |
| 11 Instrumental vaginal delivery | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.64] |
| 12 Meconium‐stained liquor | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.69, 1.81] |
| 14 Neonatal intensive care unit admission | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
| 16 Perinatal death | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 22 Maternal pyrexia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 23 Postpartum haemorrhage | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 28 Antibiotics to baby | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
8.2. Analysis.
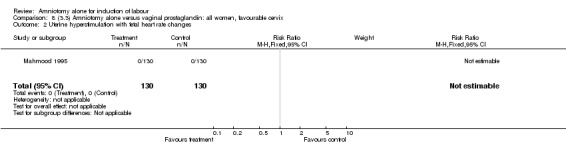
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
8.3. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 3 Caesarean section.
8.7. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 7 Oxytocin augmentation.
8.8. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
8.10. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 10 Epidural analgesia.
8.11. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 11 Instrumental vaginal delivery.
8.12. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 12 Meconium‐stained liquor.
8.14. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
8.16. Analysis.
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 16 Perinatal death.
8.18. Analysis.
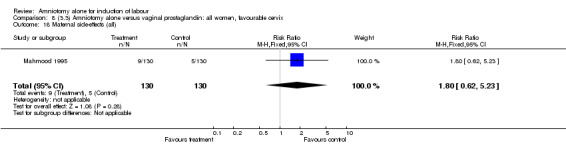
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 18 Maternal side‐effects (all).
8.22. Analysis.
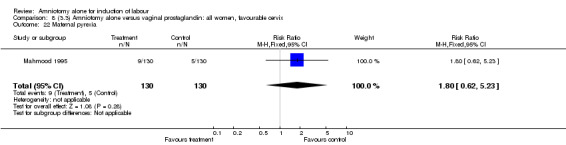
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 22 Maternal pyrexia.
8.23. Analysis.
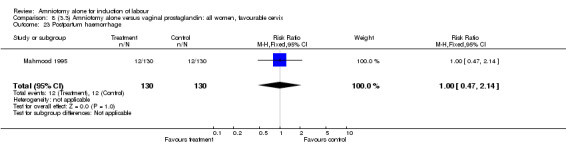
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 23 Postpartum haemorrhage.
8.28. Analysis.
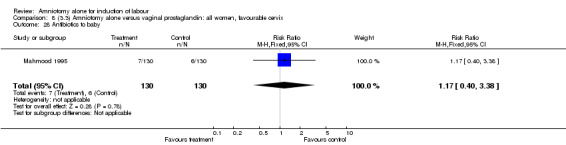
Comparison 8 (3.3) Amniotomy alone versus vaginal prostaglandin: all women, favourable cervix, Outcome 28 Antibiotics to baby.
Comparison 9. (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.83] |
| 7 Oxytocin augmentation | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.85 [1.82, 4.46] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.89 [0.87, 4.08] |
| 11 Instrumental vaginal delivery | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.64] |
| 12 Meconium‐stained liquor | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.12 [0.69, 1.81] |
| 14 Neonatal intensive care unit admission | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
| 16 Perinatal death | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 22 Maternal pyrexia | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.8 [0.62, 5.23] |
| 23 Postpartum haemorrhage | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.47, 2.14] |
| 28 Antibiotics to baby | 1 | 260 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.40, 3.38] |
9.2. Analysis.
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
9.3. Analysis.
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 3 Caesarean section.
9.7. Analysis.
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 7 Oxytocin augmentation.
9.8. Analysis.
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
9.10. Analysis.
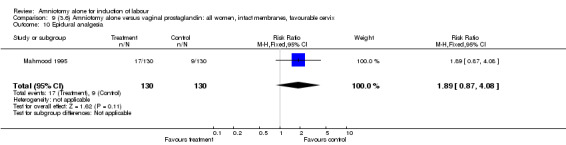
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 10 Epidural analgesia.
9.11. Analysis.
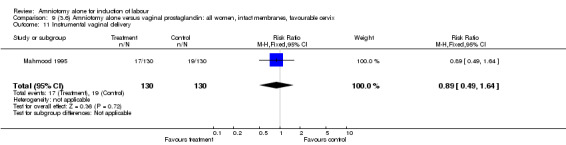
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
9.12. Analysis.
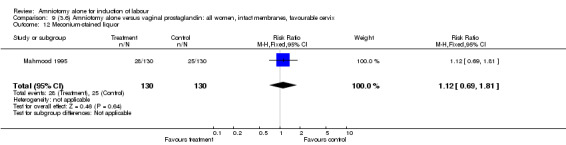
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 12 Meconium‐stained liquor.
9.14. Analysis.
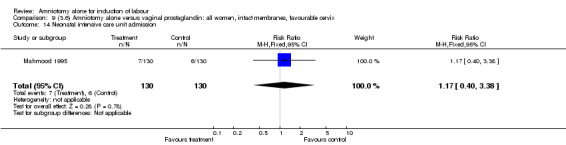
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
9.16. Analysis.
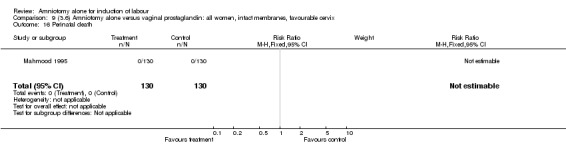
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 16 Perinatal death.
9.18. Analysis.
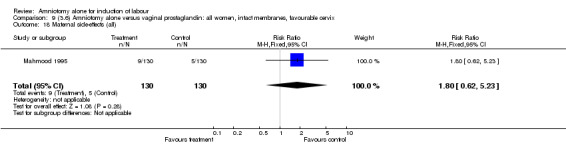
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 18 Maternal side‐effects (all).
9.22. Analysis.
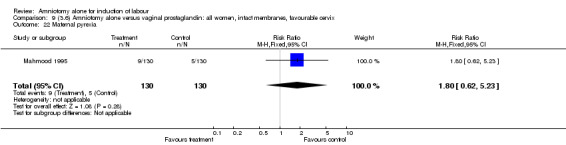
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 22 Maternal pyrexia.
9.23. Analysis.
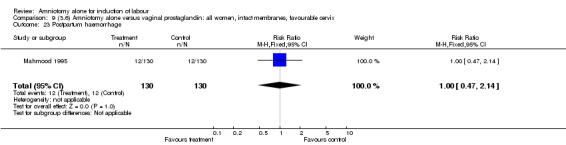
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 23 Postpartum haemorrhage.
9.28. Analysis.
Comparison 9 (3.6) Amniotomy alone versus vaginal prostaglandin: all women, intact membranes, favourable cervix, Outcome 28 Antibiotics to baby.
Comparison 10. (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
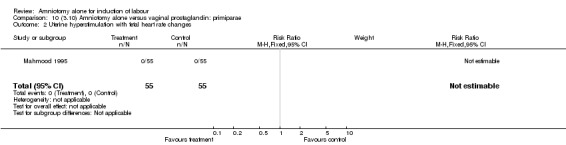
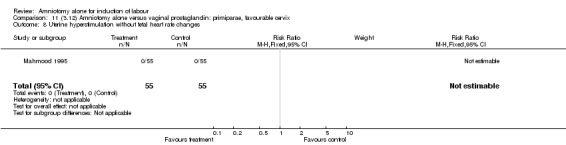
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
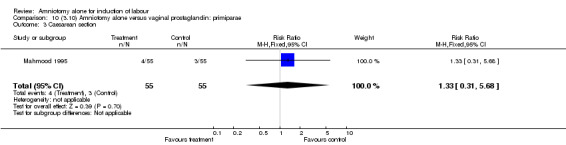
| 3 Caesarean section | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.68] |
| 7 Oxytocin augmentation | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.33, 4.10] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.97, 11.46] |
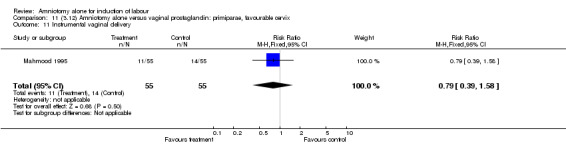
| 11 Instrumental vaginal delivery | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.58] |
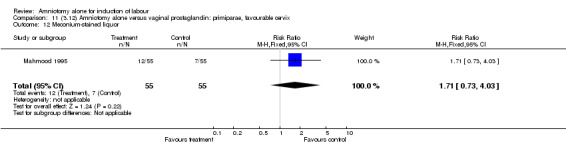
| 12 Meconium‐stained liquor | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.73, 4.03] |
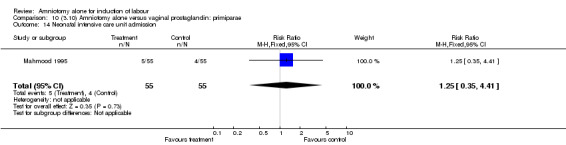
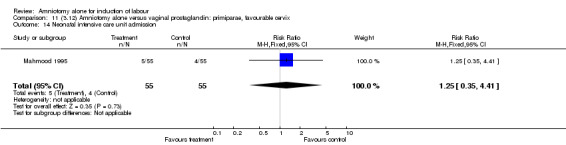
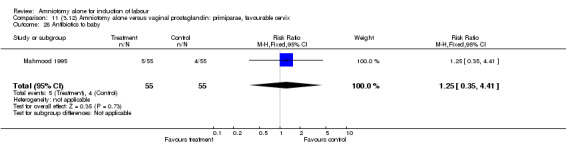
| 14 Neonatal intensive care unit admission | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
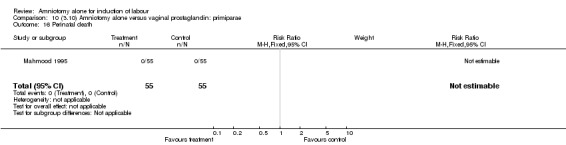
| 16 Perinatal death | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
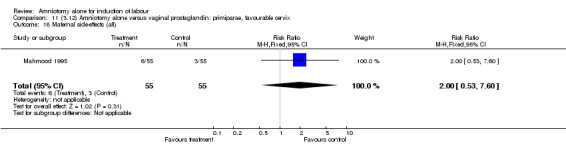
| 18 Maternal side‐effects (all) | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 22 Maternal pyrexia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 23 Postpartum haemorrhage | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.64] |
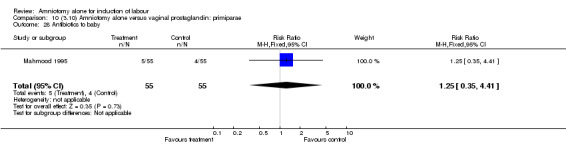
| 28 Antibiotics to baby | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
10.2. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
10.3. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 3 Caesarean section.
10.7. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 7 Oxytocin augmentation.
10.8. Analysis.
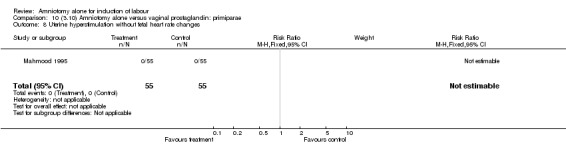
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
10.10. Analysis.
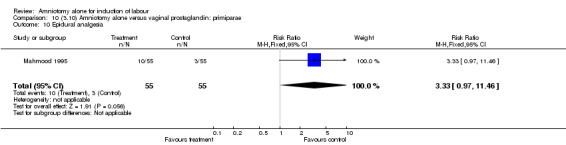
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 10 Epidural analgesia.
10.11. Analysis.
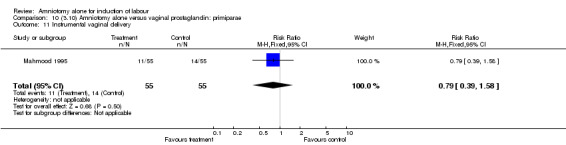
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 11 Instrumental vaginal delivery.
10.12. Analysis.
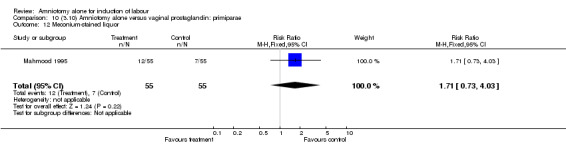
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 12 Meconium‐stained liquor.
10.14. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 14 Neonatal intensive care unit admission.
10.16. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 16 Perinatal death.
10.18. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 18 Maternal side‐effects (all).
10.22. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 22 Maternal pyrexia.
10.23. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 23 Postpartum haemorrhage.
10.28. Analysis.
Comparison 10 (3.10) Amniotomy alone versus vaginal prostaglandin: primiparae, Outcome 28 Antibiotics to baby.
Comparison 11. (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
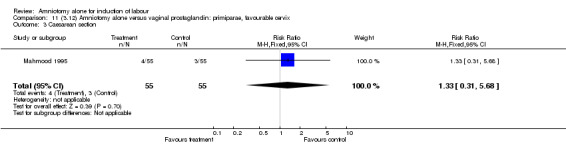
| 3 Caesarean section | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.68] |
| 7 Oxytocin augmentation | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.33, 4.10] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.97, 11.46] |
| 11 Instrumental vaginal delivery | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.58] |
| 12 Meconium‐stained liquor | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.73, 4.03] |
| 14 Neonatal intensive care unit admission | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
| 16 Perinatal death | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 22 Maternal pyrexia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 23 Postpartum haemorrhage | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.64] |
| 28 Antibiotics to baby | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
11.2. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
11.3. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 3 Caesarean section.
11.7. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 7 Oxytocin augmentation.
11.8. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
11.10. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 10 Epidural analgesia.
11.11. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 11 Instrumental vaginal delivery.
11.12. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 12 Meconium‐stained liquor.
11.14. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
11.16. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 16 Perinatal death.
11.18. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 18 Maternal side‐effects (all).
11.22. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 22 Maternal pyrexia.
11.23. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 23 Postpartum haemorrhage.
11.28. Analysis.
Comparison 11 (3.12) Amniotomy alone versus vaginal prostaglandin: primiparae, favourable cervix, Outcome 28 Antibiotics to baby.
Comparison 12. (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.33 [0.31, 5.68] |
| 7 Oxytocin augmentation | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.33 [1.33, 4.10] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.33 [0.97, 11.46] |
| 11 Instrumental vaginal delivery | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.39, 1.58] |
| 12 Meconium‐stained liquor | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.71 [0.73, 4.03] |
| 14 Neonatal intensive care unit admission | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
| 16 Perinatal death | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 22 Maternal pyrexia | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.53, 7.60] |
| 23 Postpartum haemorrhage | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.67 [0.42, 6.64] |
| 28 Antibiotics to baby | 1 | 110 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.25 [0.35, 4.41] |
12.2. Analysis.
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
12.3. Analysis.
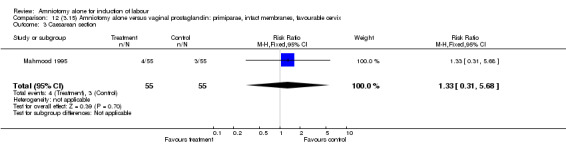
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 3 Caesarean section.
12.7. Analysis.
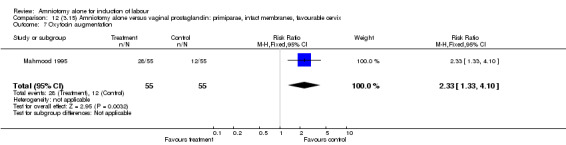
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 7 Oxytocin augmentation.
12.8. Analysis.
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
12.10. Analysis.
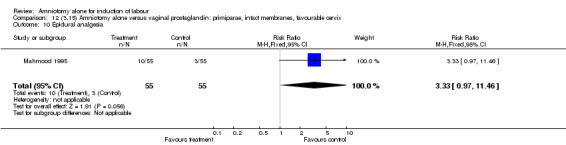
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 10 Epidural analgesia.
12.11. Analysis.
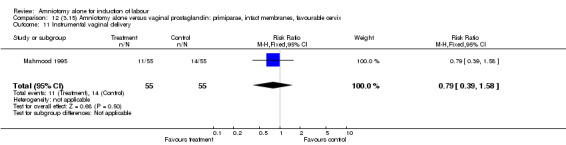
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
12.12. Analysis.
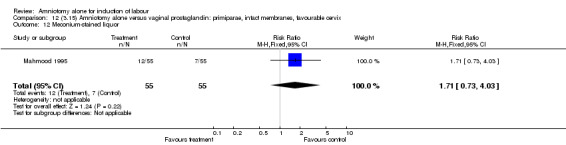
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 12 Meconium‐stained liquor.
12.14. Analysis.
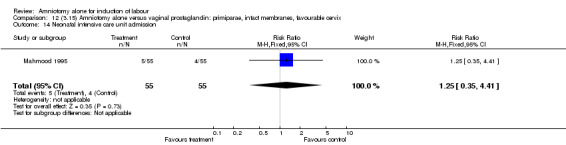
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
12.16. Analysis.
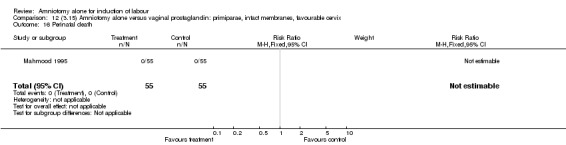
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 16 Perinatal death.
12.18. Analysis.
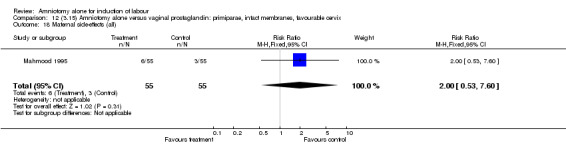
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 18 Maternal side‐effects (all).
12.22. Analysis.
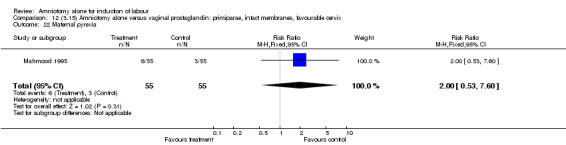
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 22 Maternal pyrexia.
12.23. Analysis.
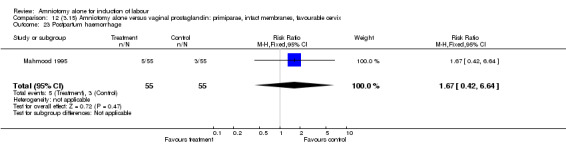
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 23 Postpartum haemorrhage.
12.28. Analysis.
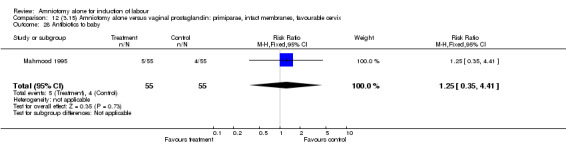
Comparison 12 (3.15) Amniotomy alone versus vaginal prostaglandin: primiparae, intact membranes, favourable cervix, Outcome 28 Antibiotics to baby.
Comparison 13. (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
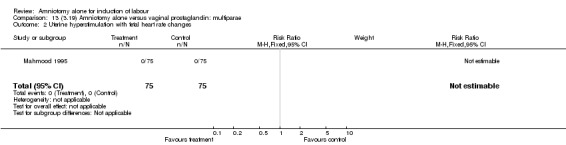
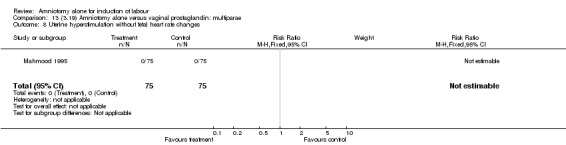
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
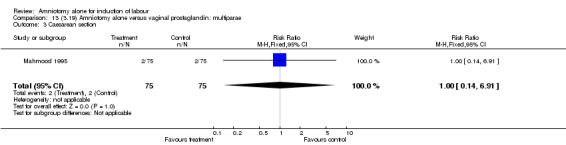
| 3 Caesarean section | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 7 Oxytocin augmentation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.77, 7.40] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
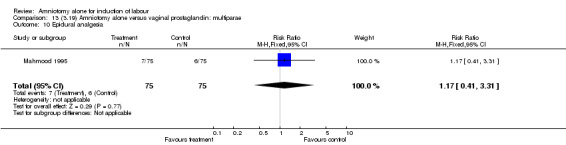
| 10 Epidural analgesia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.31] |
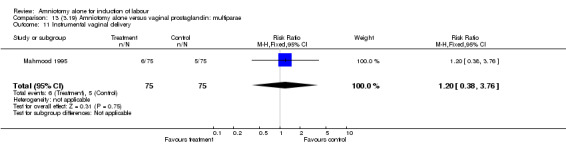
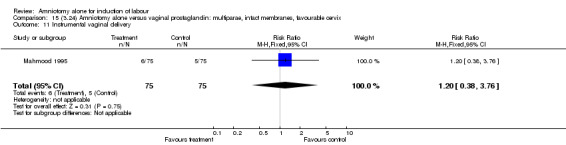
| 11 Instrumental vaginal delivery | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.76] |
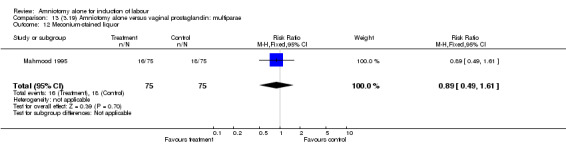
| 12 Meconium‐stained liquor | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.61] |
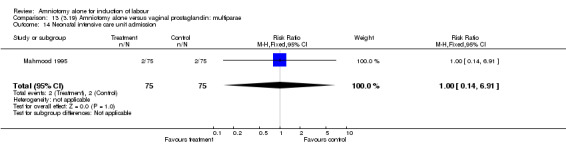
| 14 Neonatal intensive care unit admission | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 16 Perinatal death | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 22 Maternal pyrexia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 23 Postpartum haemorrhage | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.98] |
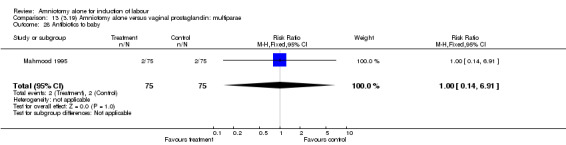
| 28 Antibiotics to baby | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
13.2. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
13.3. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 3 Caesarean section.
13.7. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 7 Oxytocin augmentation.
13.8. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
13.10. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 10 Epidural analgesia.
13.11. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 11 Instrumental vaginal delivery.
13.12. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 12 Meconium‐stained liquor.
13.14. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 14 Neonatal intensive care unit admission.
13.16. Analysis.
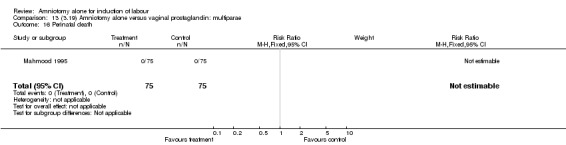
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 16 Perinatal death.
13.18. Analysis.
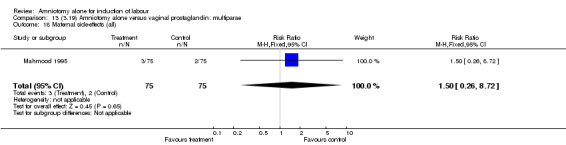
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 18 Maternal side‐effects (all).
13.22. Analysis.
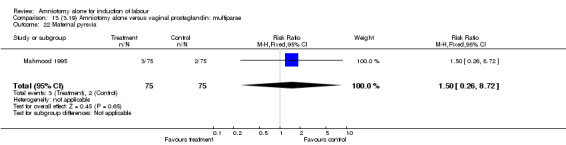
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 22 Maternal pyrexia.
13.23. Analysis.
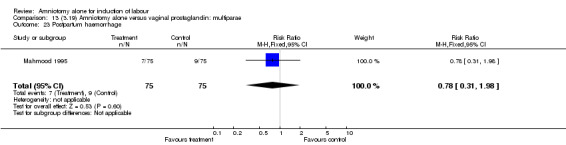
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 23 Postpartum haemorrhage.
13.28. Analysis.
Comparison 13 (3.19) Amniotomy alone versus vaginal prostaglandin: multiparae, Outcome 28 Antibiotics to baby.
Comparison 14. (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 7 Oxytocin augmentation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.77, 7.40] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.31] |
| 11 Instrumental vaginal delivery | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.76] |
| 12 Meconium‐stained liquor | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.61] |
| 14 Neonatal intensive care unit admission | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 16 Perinatal death | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 22 Maternal pyrexia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 23 Postpartum haemorrhage | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.98] |
| 28 Antibiotics to baby | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
14.2. Analysis.
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
14.3. Analysis.
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 3 Caesarean section.
14.7. Analysis.
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 7 Oxytocin augmentation.
14.8. Analysis.
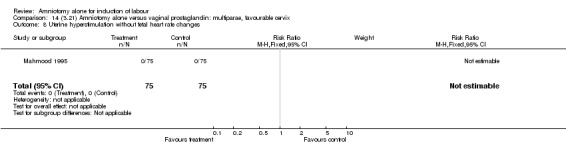
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
14.10. Analysis.
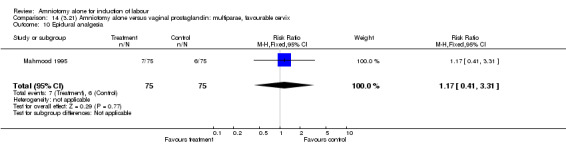
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 10 Epidural analgesia.
14.11. Analysis.
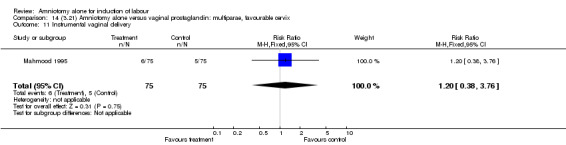
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 11 Instrumental vaginal delivery.
14.12. Analysis.
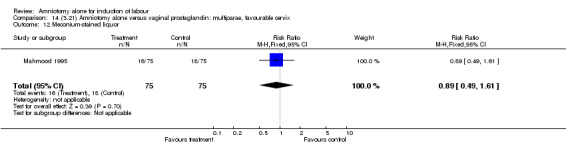
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 12 Meconium‐stained liquor.
14.14. Analysis.
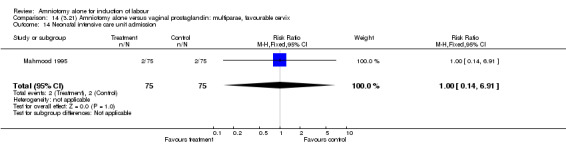
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
14.16. Analysis.
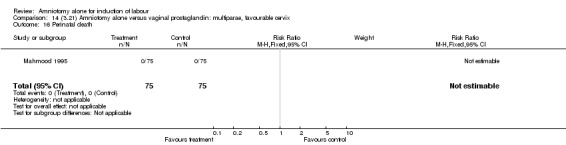
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 16 Perinatal death.
14.18. Analysis.
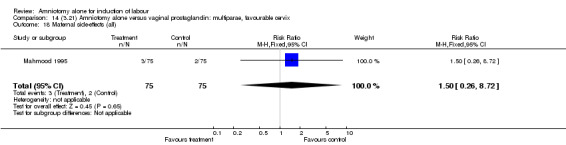
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 18 Maternal side‐effects (all).
14.22. Analysis.
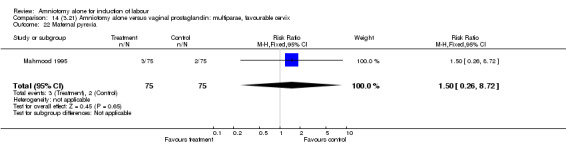
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 22 Maternal pyrexia.
14.23. Analysis.
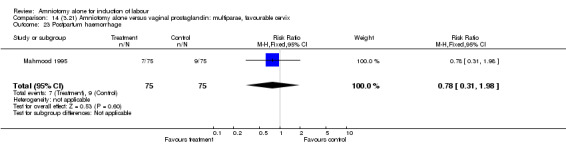
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 23 Postpartum haemorrhage.
14.28. Analysis.
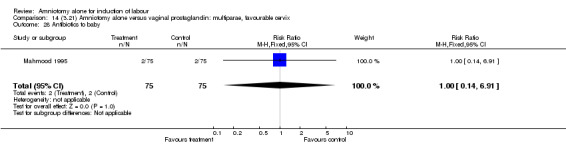
Comparison 14 (3.21) Amniotomy alone versus vaginal prostaglandin: multiparae, favourable cervix, Outcome 28 Antibiotics to baby.
Comparison 15. (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 7 Oxytocin augmentation | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.63 [1.77, 7.40] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 10 Epidural analgesia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.17 [0.41, 3.31] |
| 11 Instrumental vaginal delivery | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.2 [0.38, 3.76] |
| 12 Meconium‐stained liquor | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.49, 1.61] |
| 14 Neonatal intensive care unit admission | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
| 16 Perinatal death | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 18 Maternal side‐effects (all) | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 22 Maternal pyrexia | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.5 [0.26, 8.72] |
| 23 Postpartum haemorrhage | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.78 [0.31, 1.98] |
| 28 Antibiotics to baby | 1 | 150 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.14, 6.91] |
15.2. Analysis.
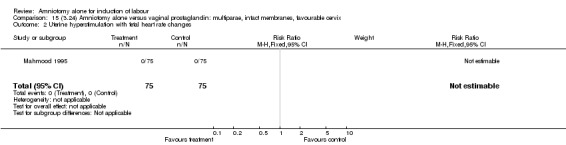
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
15.3. Analysis.
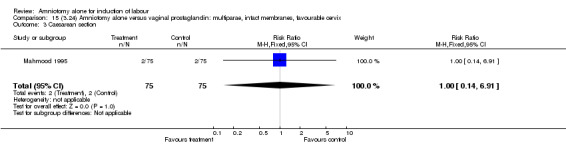
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 3 Caesarean section.
15.7. Analysis.
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 7 Oxytocin augmentation.
15.8. Analysis.
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
15.10. Analysis.
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 10 Epidural analgesia.
15.11. Analysis.
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 11 Instrumental vaginal delivery.
15.12. Analysis.
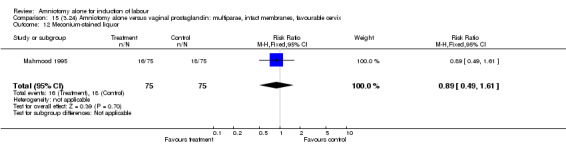
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 12 Meconium‐stained liquor.
15.14. Analysis.
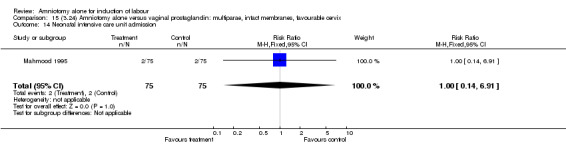
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 14 Neonatal intensive care unit admission.
15.16. Analysis.
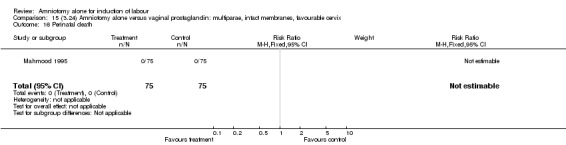
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 16 Perinatal death.
15.18. Analysis.
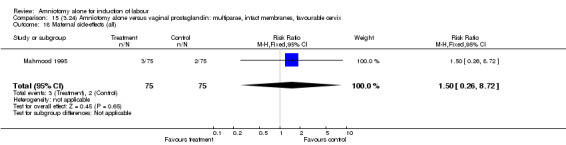
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 18 Maternal side‐effects (all).
15.22. Analysis.
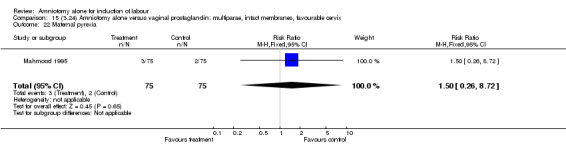
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 22 Maternal pyrexia.
15.23. Analysis.
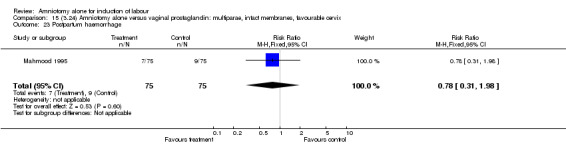
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 23 Postpartum haemorrhage.
15.28. Analysis.
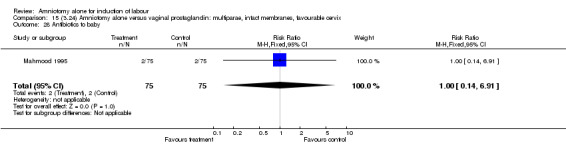
Comparison 15 (3.24) Amniotomy alone versus vaginal prostaglandin: multiparae, intact membranes, favourable cervix, Outcome 28 Antibiotics to baby.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Jagani 1982.
| Methods | Pseudo‐randomisation according to chart number. | |
| Participants | 50 women with live fetus at term and Bishop's score of 4 or less. Indication for induction ‐ 25 post term; 17 hypertensive disorder; 4 diabetes; 1 pyelonephritis; 2 IUGR; 1 congenital abnormality of fetus. | |
| Interventions | Aim was to evaluate whether 'alteration of the cervix affects the ability to induce labour if the Bishop's score is 4 or less'. 5 groups: control; laminaria; foley catheter; amniotomy; oxytocin with intact membranes. After 12 hours amniotomy and oxytocin employed. | |
| Outcomes | Primary: caesarean section. Secondary: change in Bishop's score; induction‐to‐delivery interval. | |
| Notes | Unable to extract data for the first two outcomes ‐ no absolute numbers given ‐ reported as mean and SD. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | C ‐ Inadequate |
Mahmood 1995.
| Methods | Numbered, sealed opaque envelopes with allocation from table of random numbers. | |
| Participants | 260 women with singleton uncomplicated pregnancy, cephalic presentation, favourable cervix (Bishop's score 6 or more) | |
| Interventions | 2 groups: Amniotomy alone ‐ reviewed in 4 hours and oxytocin infusion commenced if not contracting or no cervical change. Vaginal prostaglandin E2 gel (primips 2 mg, multips 1 mg) ‐ reviewed at 4 hours for amniotomy or sooner if requested analgesia or if spontaneous ROM. Oxytocin infusion 2 hours later if uterine activity not established. | |
| Outcomes | Primary: uterine hyperstimulation with FHR changes; caesarean section. Secondary: oxytocin augmentation; uterine hyperstimulation without FHR changes; epidural analgesia; instrumental vaginal delivery; meconium stained liquor; neonatal intensive care admission; perinatal death; maternal side effects (intrapartum pyrexia), postpartum haemorrhage. Not prespecified: antibiotics to baby. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
IUGR: intrauterine growth retardation ROM: rupture of membranes FHR: fetal heart rate SD: standard deviation
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chanrachakul 2003 | Trial comparing expectant management with immediate induction of labour. The women in the induction group were sent to labour ward for induction by artificial rupture of membranes or oxytocin, or both. |
| Secher 1981 | Randomised trial of oral prostaglandin E2 vs intravenous oxytocin. Prior to randomisation, amniotomy was performed if the cervix was favourable (subjective decision by attending clinician). The group who did not have primary amniotomy were immediately randomised. The group who had primary amniotomy were left for four hours following which, if labour had not ensued, they were randomised to prostaglandin or oxytocin. Therefore, they were not randomised in terms of amniotomy alone (pragmatic allocation) and hence excluded from this review. This study will be considered in the amniotomy and oxytocin, and the oral prostaglandin reviews. |
| Sivasuriya 1978 | This study was undertaken to evaluate the association between neonatal jaundice and three methods of induction of labour (amniotomy alone; amniotomy and oxytocin infusion; and amniotomy and oral prostaglandins) compared with spontaneous labour. None of the pre‐specified primary outcomes for this review were reported. |
| Thornton 1989 | This study was undertaken to determine oxytocin concentration in women undergoing amniotomy for induction of labour at term. None of the pre‐specified primary outcomes for this review were reported. |
| Ward 1991 | Conference abstract. In this study, women were randomly allocated to one of three groups: prostaglandin gel; amniotomy alone, and amniotomy with oxytocin infusion. No primary outcomes relevant to this review reported. The authors comment on the potential advantages of prostaglandin in reducing the need for intravenous oxytocin and thus allowing women to mobilise more readily in early labour. These potential advantages in terms of consumer satisfaction were not evaluated formally, but merely speculated upon. |
| Westergaard 1983 | Randomised trial of oral prostaglandin E2 vs buccal oxytocin. Prior to randomisation, primary amniotomy was performed if the cervix was favourable (subjective decision by attending clinician). The group who did not have primary amniotomy was immediately randomised. The group who had primary amniotomy was left for four hours following which, if labour had not ensued. the participants were randomised to prostaglandin or oxytocin. Therefore, they were not randomised in terms of amniotomy alone (pragmatic allocation) and hence excluded from this review. This study will be considered in the amniotomy and oxytocin, and the oral prostaglandin reviews. |
vs: versus
Contributions of authors
Both authors contributed equally to the development of the review.
Sources of support
Internal sources
University of Liverpool, UK.
External sources
No sources of support supplied
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Jagani 1982 {published data only}
- Jagani N, Schulman H, Fleischer A, Mitchell J, Randolph G. Role of the cervix in the induction of labor. Obstetrics & Gynecology 1982;59:21‐6. [PubMed] [Google Scholar]
Mahmood 1995 {published data only}
- Mahmood TA, Rayner A, Smith NC, Beat I. A randomised prospective trial comparing single dose prostaglandin E2 vaginal gel with forewater amniotomy for induction of labour. European Journal of Obstetrics & Gynecology and Reproductive Biology 1995;58:111‐7. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Chanrachakul 2003 {published data only}
- Chanrachakul B, Herabutya Y. Postterm with favorable cervix: is induction necessary?. European Journal of Obstetrics & Gynecology and Reproductive Biology 2003;106:154‐7. [DOI] [PubMed] [Google Scholar]
Secher 1981 {published data only}
- Secher NJ, Lange AP, Hassing Nielsen F, Thomson Pedersen G, Westergaard JG. Induction of labor with and without primary amniotomy. A randomized study of prostaglandin E2 tablets and intravenous oxytocin. Acta Obstetricia et Gynecologica Scandinavica 1981;60:237‐41. [DOI] [PubMed] [Google Scholar]
Sivasuriya 1978 {published data only}
- Sivasuriya M, Tan KL, Salmon YM, Karim SMM. Neonatal serum bilirubin levels in spontaneous and induced labour. British Journal of Obstetrics and Gynaecology 1978;85:619‐23. [DOI] [PubMed] [Google Scholar]
Thornton 1989 {published data only}
- Thornton S, Davison JM, Baylis PH. Amniotomy‐induced labour is not mediated by endogenous oxytocin. British Journal of Obstetrics and Gynaecology 1989;96:945‐8. [DOI] [PubMed] [Google Scholar]
Ward 1991 {published data only}
- Ward SJ. Induction of labour using prostaglandin gel in patients with a favourable cervix. Proceedings of 2nd European Congress on Prostaglandins in Reproduction; 1991 April 30‐May 3; The Hague, Netherlands. 1991:143.
Westergaard 1983 {published data only}
- Westergaard JG, Lange AP, Pedersen GT, Secher NJ. Oral oxytocics for induction of labor. Acta Obstetricia et Gynecologica Scandinavica 1983;62:103‐10. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Macones 2011 {published data only}
- Macones G, Stamilio D, Rampersad R, Cahill AG, Odibo AO. The efficacy of early amniotomy in nulliparous labor induction: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2011;204(1 Suppl):S4. [DOI] [PubMed] [Google Scholar]
Rijnders 2007 {published data only}
- Rijnders MEB. Costs and effects of amniotomy at home for induction of post term pregnancy (ongoing trial). Current Controlled Trials (www.controlled‐trials.com) (accessed 15 February 2007).
Additional references
Alfirevic 2006
- Alfirevic Z, Weeks A. Oral misoprostol for induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD001338.pub2] [DOI] [PubMed] [Google Scholar]
Booth 1970
- Booth JH, Kurdizak VB. Elective induction of labour: a controlled study. Canadian Medical Association Journal 1970;103:245‐8. [PMC free article] [PubMed] [Google Scholar]
Boulvain 2001
- Boulvain M, Kelly A, Lohse C, Stan C, Irion O. Mechanical methods for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD001233] [DOI] [PubMed] [Google Scholar]
Boulvain 2005
- Boulvain M, Stan C, Irion O. Membrane sweeping for induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 1. [DOI: 10.1002/14651858.CD000451.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Boulvain 2008
- Boulvain M, Kelly AJ, Irion O. Intracervical prostaglandins for induction of labour. Cochrane Database of Systematic Reviews 2008, Issue 1. [DOI: 10.1002/14651858.CD006971] [DOI] [PubMed] [Google Scholar]
Clarke 1999
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.0 [updated July 1999]. In: Review Manager (RevMan) [Computer program]. Version 4.0 Oxford, England: The Cochrane Collaboration, 1999.
Curtis 1987
- Curtis P, Evans S, Resnick J. Uterine hyperstimulation. The need for standard terminology. Journal of Reproductive Medicine 1987;32:91‐5. [PubMed] [Google Scholar]
Fraser 2000
- Fraser WD, Turcot L, Krauss I, Brisson‐Carrol G. Amniotomy for shortening spontaneous labour. Cochrane Database of Systematic Reviews 2006, Issue 3. [DOI: 10.1002/14651858.CD000015.pub2] [DOI] [Google Scholar]
French 2001
- French L. Oral prostaglandin E2 for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003098] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hofmeyr 2000
- Hofmeyr GJ, Alfirevic Z, Kelly T, Kavanagh J, Thomas J, Brocklehurst P, Neilson JP. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002074] [DOI] [PubMed] [Google Scholar]
Hofmeyr 2003
- Hofmeyr GJ, Gulmezoglu AM. Vaginal misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD000941] [DOI] [PubMed] [Google Scholar]
Howarth 2001
- Howarth GR, Botha DJ. Amniotomy plus intravenous oxytocin for induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003250] [DOI] [PMC free article] [PubMed] [Google Scholar]
Husslein 1983
- Husslein P, Kofler E, Rasmussen A, Sumulong L, Fuchs AR, Fuchs F. Oxytocin and initiation of human parturition: IV. Plasma concentrations of oxytocin and 13, 14‐dihydro‐15‐keto‐prostaglandin F2alpha during induction of labor by artificial rupture of membranes. American Journal of Obstetrics and Gynecology 1983;147:503‐7. [PubMed] [Google Scholar]
Hutton 2001
- Hutton E, Mozurkewich E. Extra‐amniotic prostaglandin for induction of labour.. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003092] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2001
- Kavanagh J, Kelly AJ, Thomas J. Sexual intercourse for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003093] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2005
- Kavanagh J, Kelly AJ, Thomas J. Breast stimulation for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2005, Issue 3. [DOI: 10.1002/14651858.CD003392.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006a
- Kavanagh J, Kelly AJ, Thomas J. Corticosteroids for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003100.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kavanagh 2006b
- Kavanagh J, Kelly AJ, Thomas J. Hyaluronidase for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2006, Issue 2. [DOI: 10.1002/14651858.CD003097.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2001a
- Kelly AJ, Tan B. Intravenous oxytocin alone for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 3. [DOI: 10.1002/14651858.CD003246] [DOI] [PubMed] [Google Scholar]
Kelly 2001b
- Kelly A, Kavanagh J, Thomas J. Castor oil, bath and/or enema for cervical priming and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003099] [DOI] [PubMed] [Google Scholar]
Kelly 2001c
- Kelly AJ, Kavanagh J, Thomas J. Relaxin for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 2. [DOI: 10.1002/14651858.CD003103] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kelly 2003
- Kelly AJ, Kavanagh J, Thomas J. Vaginal prostaglandin (PGE2 and PGF2a) for induction of labour at term. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003101] [DOI] [PubMed] [Google Scholar]
Kelly 2011
- Kelly AJ, Munson C, Minden L. Nitric oxide donors for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2011, Issue 6. [DOI: 10.1002/14651858.CD006901.pub2] [DOI] [PubMed] [Google Scholar]
Landesman 1996
- Landesman SH, Kalish LA, Burns DN, Minkoff H, Fox HE, Zorilla C, et al. Obstetrical factors and the transmission of human immunodeficiency virus type 1from mother to child. New England Journal of Medicine 1996;334:1617‐23. [DOI] [PubMed] [Google Scholar]
Luckas 2000
- Luckas M, Bricker L. Intravenous prostaglandin for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002864] [DOI] [PMC free article] [PubMed] [Google Scholar]
Muzonzini 2004
- Muzonzini G, Hofmeyr GJ. Buccal or sublingual misoprostol for cervical ripening and induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD004221.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Neilson 2000
- Neilson JP. Mifepristone for induction of labour. Cochrane Database of Systematic Reviews 2000, Issue 4. [DOI: 10.1002/14651858.CD002865] [DOI] [PubMed] [Google Scholar]
O'Driscoll 1973
- O'Driscoll K, Stronge JM, Minogue M. Active management of labour. BMJ 1973;iii:135‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
RevMan 1999 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.0 for Windows. Oxford, England: The Cochrane Collaboration, 1999.
Saleh 1975
- Saleh YZ. Surgical induction of labour with and without oxytocin infusion: A prospective study. Australian and New Zealand Journal of Obstetrics and Gynaecology 1975;15:80‐3. [Google Scholar]
Smith 2003
- Smith CA. Homoeopathy for induction of labour. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003399] [DOI] [PubMed] [Google Scholar]
Smith 2004
- Smith CA, Crowther CA. Acupuncture for induction of labour. Cochrane Database of Systematic Reviews 2004, Issue 1. [DOI: 10.1002/14651858.CD002962.pub2] [DOI] [PubMed] [Google Scholar]
Thomas 2001
- Thomas J, Kelly AJ, Kavanagh J. Oestrogens alone or with amniotomy for cervical ripening or induction of labour. Cochrane Database of Systematic Reviews 2001, Issue 4. [DOI: 10.1002/14651858.CD003393] [DOI] [PMC free article] [PubMed] [Google Scholar]


