Abstract
Intraocular pressure (IOP) is an important measurement that needs to be taken during ophthalmic examinations, especially in ocular hypertension subjects, glaucoma patients and in patients with risk factors for developing glaucoma. The gold standard technique in measuring IOP is still Goldmann applanation tonometry (GAT); however, this procedure requires local anesthetics, can be difficult in patients with scarce compliance, surgical patients and children, and is influenced by several corneal parameters. Numerous tonometers have been proposed in the past to address the problems related to GAT. The authors review the various devices currently in use for the measurement of intraocular pressure (IOP), highlighting the main advantages and limits of the various tools. The continuous monitoring of IOP, which is still under evaluation, will be an important step for a more complete and reliable management of patients affected by glaucoma.
Keywords: intraocular pressure (IOP), tonometry, Goldmann applanation tonometer (GAT), central corneal thickness (CCT), ocular hypertension, glaucoma
1. Introduction
Intraocular pressure (IOP) is an important measurement, which should be taken in every patient over the age of 40 that undergoes a complete ophthalmic examination and in all patients with ocular hypertension (OHT) or with risk factors for developing primary open-angle glaucoma (POAG) (i.e., family history, myopia, increased cup-to-disc ratio, etc.). IOP measurement is obviously a fundamental tool in subjects with diagnosed ocular hypertension or glaucoma. Even if the IOP measurement in vivo is only an estimate of the true IOP (which is only possible with invasive manometry), this value, rightly or wrongly, is often taken as an indicator of the efficacy of any treatment for glaucoma and to assess glaucoma severity and progression in patient management. It is thus of great importance to acquire accurate and precise IOP measurements in clinical practice.
Numerous instruments, called tonometers, have been proposed since the 19th century to obtain IOP measurements [1,2,3]. Based on the operating principle, these instruments can be differentiated into two main groups: (1) indentation tonometers; (2) applanation tonometers.
2. Indentation Tonometry
The prototype of the indentation tonometers is the Schiøtz tonometer that was introduced many years ago [4] and is no longer currently used (Figure 1).
Figure 1.
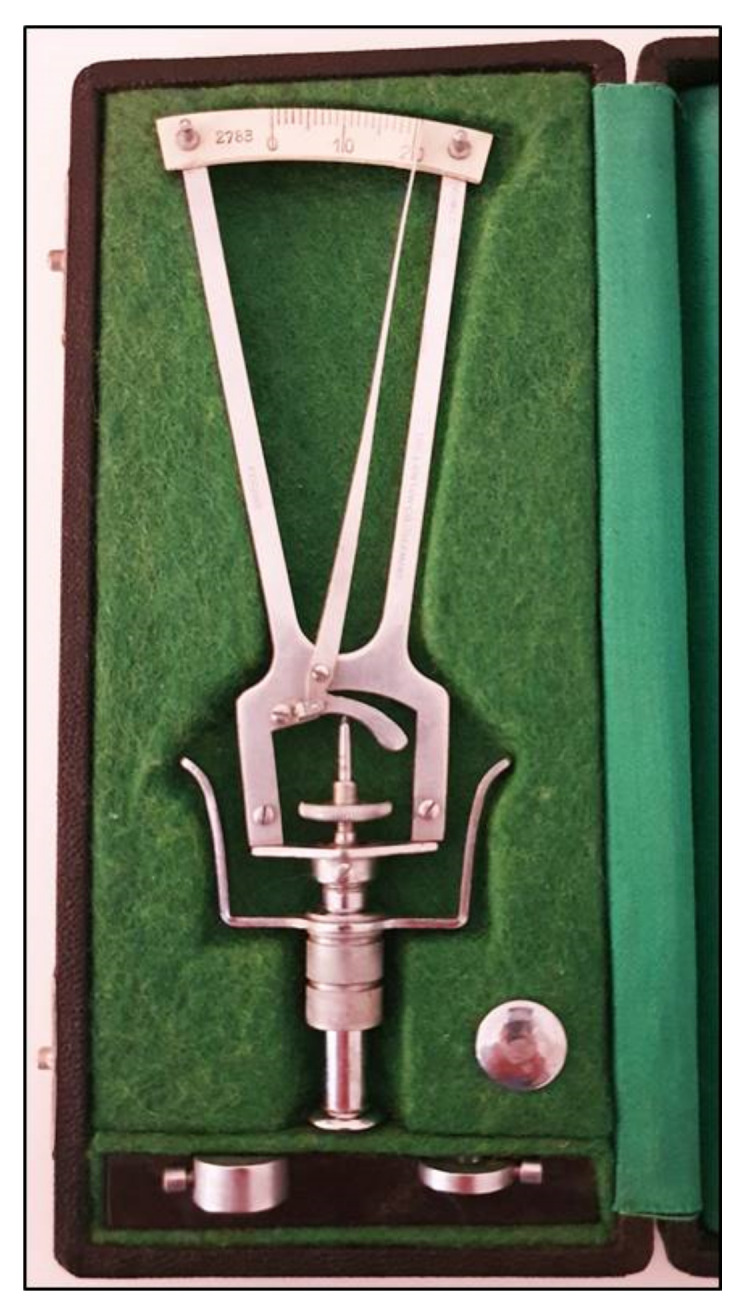
Schiøtz tonometer with different weights.
Using this instrument, the cornea is indented by a plunger loaded with different weights. The IOP is based on the depth of indentation. The values are shown on a scale ranging from 0 to 20 units, in which the protrusion of the plunger of 0.05 mm represents each unit of measurement. The value indicated on the handle needs to be converted in mmHg using a conversion scale. The coefficient of ocular rigidity, which can differ amongst eyes, should be taken into consideration to obtain corrected measurements of IOP. The Schiøtz tonometer is a simple and relatively inexpensive instrument. It is still sometimes used in developing countries [5,6] and in children under general anesthesia [7]. This tonometer, however, is subject to several sources of error, which include improper positioning on the eye, defective or dirty instruments, high variability in comparison with other devices and measurements influenced by individual ocular rigidity [8]. Moreover, patients must be in a supine position when taking measurements with this tonometer.
3. Applanation Tonometry
Applanation tonometers are currently considered the most reliable instruments for an accurate IOP measurement. Such tonometers use the Imbert–Fick law: P = F/S, in which P is pressure, S represents the surface of the flattened area, and F is the force needed to flatten a fixed corneal area. Apart from the tonometer by Maklakoff and several other instruments that are no longer currently in use, in which the force is provided by the weight of the tonometer itself, applanation tonometry is based on the area of flattened cornea that is calculated and converted in mmHg [2]. In almost all instruments of this type, the F value is varied to get the proper corneal applanation for a predetermined area. The Goldmann applanation tonometer (GAT) was first invented in 1948 by Hans Goldmann [9] and is still considered the gold standard to date. The tonometer needs to be positioned on a slit lamp.
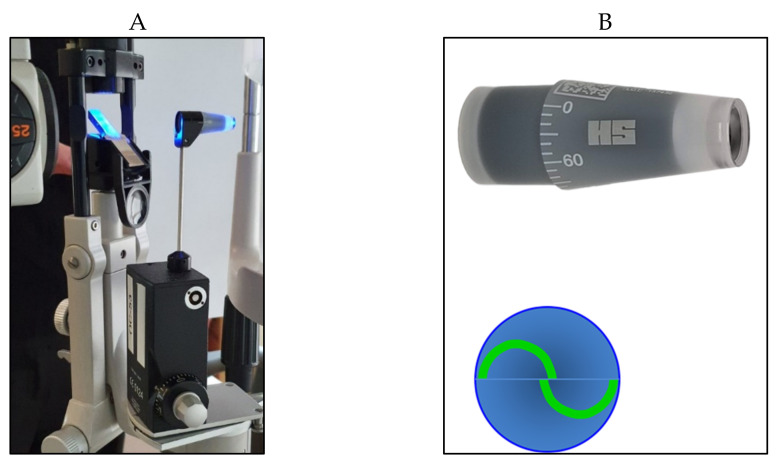
A truncated cone, with a 7.35 mm2 surface area and a dimeter of 3.06 mm, illuminated by a blue light, is pushed on the center of the anaesthetized cornea. A doubling prism embedded in the cone divides the circular meniscus on the surface of the flattened cornea e into two arcs, which need to be aligned in order to obtain a precise and standardized applanation (Figure 2).
Figure 2.
Goldmann applanation tonometer positioned on the slit lamp (A) with its cone prism (B) (on the top right); the two arcs appear correctly aligned (B) (on the bottom right).
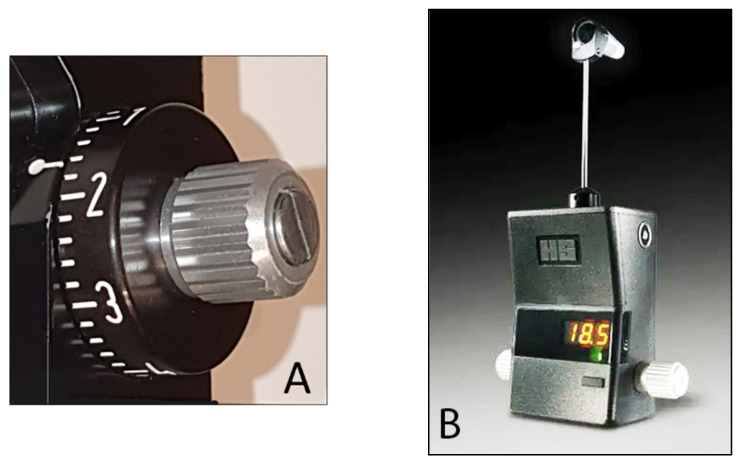
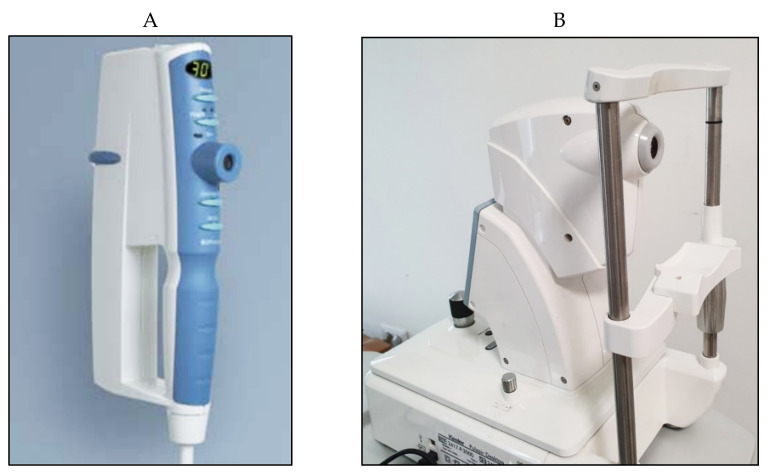
The force used needed to flatten the corresponding surface of the cornea is directly proportional to the IOP, expressed in mmHg that can be directly read in the scale of the measuring drum or in the posterior window for the digital version (Figure 3A,B).
Figure 3.
(A) Scale with IOP values in the Goldmann tonometer; (B) digital Goldmann tonometer (posterior view).
Contrary to what Hans Goldmann believed, corneal thickness may show a significant effect on IOP measurements. Thin corneas can give rise to an underestimation of the IOP and vice versa. Several authors have tried to address this problem by proposing a number of correction formulae [10,11,12,13,14]; however, none have been shown to be of widespread use. Several corneal biomechanical properties, which are not all completely known, may be involved, thus rendering the proposed correction factors misleading and limiting their clinical use [15,16,17]. Studies have reported that a thin cornea can be a factor of risk for developing glaucoma [18], in addition to the underestimated IOP with GAT.
GAT it is still the tonometer most commonly used in clinics, thanks to the ease of use, accuracy, reproducibility and affordability. There are, however, several drawbacks that should be kept in mind, as recently reported by Gazzard et al. [19]. GAT is affected by parameters of the cornea, which include central corneal thickness when this is far from the average (540 microns) [14,15], in addition to corneal curvature, axial length, hysteresis, etc. Moreover, GAT measurements are subjective and can depend on the physician experience. Studies have reported that even for the same physician, clinically significant differences can be found with a 95% repeatability coefficient of ±2 mmHg [20]. Other possible errors and drawbacks are due to the tear film with too little or too much fluorescein or an irregular or scarred cornea. GAT needs to be positioned on a slit lamp, and the subject must be in an upright position [21]. It is also important to remember that topic anesthesia is needed and that GAT should be periodically calibrated to provide good precision [22].
Portable handheld versions, such as the Perkins and Draeger tonometers (Figure 4), allow for measurements of IOP to also be taken supine and can be particularly useful in bedridden subjects and patients under general anesthesia.
Figure 4.
Handheld Perkins tonometer.
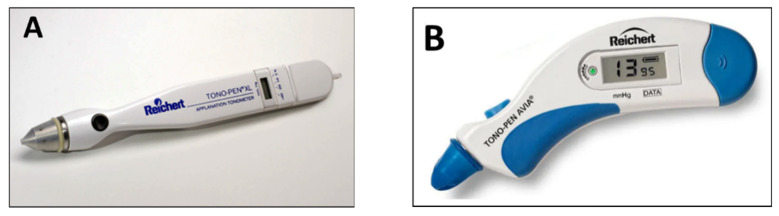
Other applanation instruments that use the same principle have been introduced several years ago. The Tono-Pen and the more recent Tono-Pen Avia (Reichert Ophthalmic Instruments, Depew, NY, USA) are portable lightweight battery-powered devices, which use the principles of applanation and indentation (Figure 5A,B). The reliability of each measurement is reported on a small display based on the standard deviation of the average of 10 readings. A disposable latex cap is used for each patient, which helps to reduces the risk of infection between patients.
Figure 5.
(A) Tono-Pen; (B) Tono-Pen Avia.
Numerous studies have reported the usefulness of these devices in clinics in comparison with GAT [23,24,25,26], but the repeatability coefficients for intra-session repeated measurement have been shown to be quite high (±4.3 mmHg) [27]. Clinical studies have shown that Tono-Pen can be significantly affected by CCT [28]. This tonometry, however, seems to provide better accuracy in edematous corneas in comparison with GAT and dynamic contour tonometry [29]. It is important to note that different tonometers cannot be used interchangeably [30,31].
The advantages of Tono-Pen include portability and instrumentation that does not require a slit lamp or electricity. IOP readings can be measured in both supine and upright positions. Topo-Pen can be especially useful in patients with eye scarring or irregular corneas, and in children and bedridden subjects.
Studies have shown the clinical limits of this instrument. Tono-pen was found to consistently underestimate IOP, with a significant error for IOP values >30 mmHg [32]. Several concerns still remain regarding the reproducibility of measurements when used in a routine clinical setting, considering that significant variations from Goldmann readings may occur in some patients.
4. Non-Contact Tonometry (Air-Puff Tonometry)
Non-contact tonometry (NCT) was first designed by Zeiss and developed by Grolman in 1972 [33]. Several models have been proposed in the past few decades that use a pulse of air to flatten the cornea without the need for touching the eye (Figure 6); such models, therefore, do not require anesthesia or fluorescein drops. In the Pulsair tonometer, a light beam is used in combination with a sensor that stops the production of air and measures the force used at the moment of corneal flattening.
Figure 6.
Pulsair EasyEye handheld (A) and Pulsair desktop (B) non-contact tonometers.
Numerous studies have examined the differences in IOP measured with various types of NCT instruments and other non-conventional tonometers compared to GAT [34,35]. Demirci et al. showed that IOP measurements with NCT were significantly higher than those obtained with both GAT and rebound tonometry, with significant differences (p < 0.001) in all age groups [36]. A recent study confirmed that NCT tends to overestimate IOP GAT measurements in patients with IOP > 16 mmHg, which was more evident when IOP > 20 mmHg [37], showing a decrease in accuracy at higher values.
Early studies in 1989 based on the comparisons with GAT, showed that up to 70% of NCT measurements fell within ± 3 mmHg of readings taken with GAT. When using a screening criteria of IOP > 21 mmHg, NCT showed a sensitivity of 85% and a specificity of 95% [38].
Similar to GAT and other instruments, NCT is influenced by corneal parameters. Kyei et al. showed a significant association between CCT and NCT, which is greater than that reported with GAT [39]. These finding were confirmed in a recent study, which concluded that GAT measurements are not equivalent and cannot be interchanged with those obtained by NCT [40].
The pros of NCT are mostly based on the ease of use, non-contact nature and portability of several devices. Measurements can be taken by non-medical staff and patient compliance is relatively good in most casesNCT does not require slit-lamp positioning; thus, it is easily used in cases with elderly individuals, children, disabled patients and patients with limited collaboration. NCT can be considered for patients that may not tolerate topical anesthetics, patients with limited collaboration or those at greater risk of infection.
The disadvantages of NCT include the fact that NCT is less accurate when IOP > 20 mmHg. Studies have shown that NCT results depend on the instrument brand, unit and model of the device used [41]. Comparison studies between three NTC devices showed that, when taking GAT as the gold standard and aiming to detect IOP > 21 mmHg, sensitivities greatly differed from 40%, 48% and 80%, which showed that NCT readings are device dependent and that devices require regular calibration [38]. Although NCT offers a non-contact mode of measuring that limits the risk of infection due to contaminated drops or Goldmann prisms, the risk of air-borne infection could be greater considering the air-puff nature, which should be considered in the midst of the recent COVID era [42,43,44,45].
NCT could be helpful in a day-to-day clinical setting that involves dealing mostly with normal patients undergoing routine checkups. This type of tonometry can be ideal as a screening tool, which can easily be performed by non-medical staff. Although studies have shown that NCT tends to overestimate GAT measurements, NCT can prove to be useful for post-operative patients with lid edema, limited collaboration, ocular pain, discomfort and increased tear film meniscus size, which are all factors that influence proper GAT measurements. NCT can be a useful screening tool, but should never replace or be interchanged with GAT, especially in the management of patients with risk factors, ocular hypertension, suspect patients and glaucoma.
Other types of non-contact tonometers, with new interesting features, have recently been introduced. In addition to the traditional tonometers, these devices show IOP values that take CCT and corneal biomechanics into account, claiming to provide more accurate IOP measurements [19,46].
The Ocular Response Analyzer (Reichert Technologies, Depew, NY, USA) or ORA, developed in 2005 by Luce et al. [46], is a non-contact air-puff tonometer that provides an optical electrical device to measure the deformation of the cornea caused by the impact of the air (Figure 7).
Figure 7.
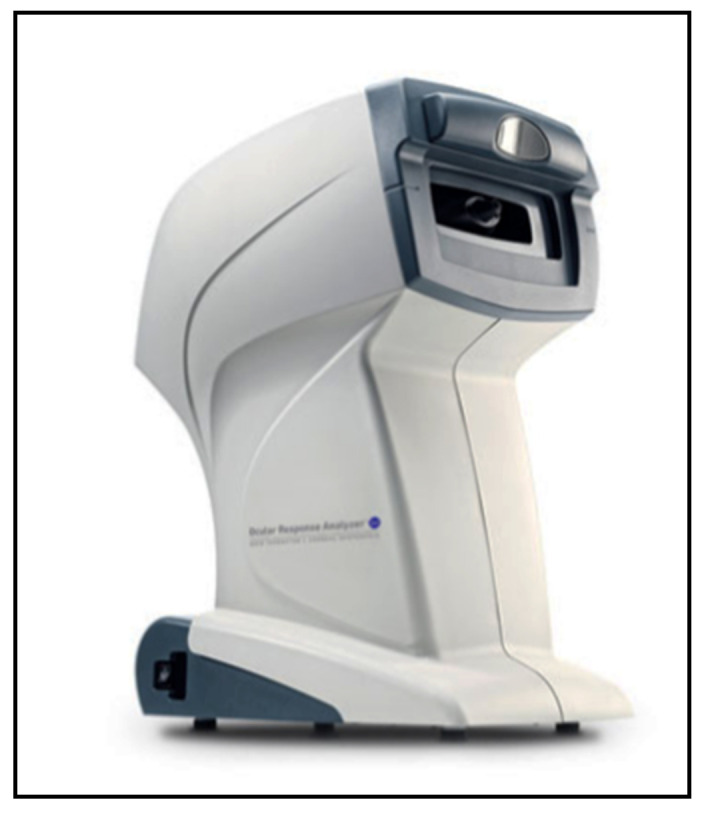
Ocular Response Analyzer (ORA) mod.G3.
The force of the air makes the cornea move in an inward fashion in a first applanation state, causing it to take on a slight concave shape, to then move outward in a further applanation state, and finally to take on a normal configuration state. The electro-optical applanation detection system registers the curvature of the cornea in a diameter of 3 mm in the center for 20 msec. The two inward and outward applanation events, which are delayed by the viscoelastic corneal damping, allow for the calculations of two different IOP values based on the applanation principle. The instruments provide an average of these two pressure measurements and supply the so-called Goldmann-correlated IOP value (IOPg); the corneal hysteresis (CH) parameter is based on the difference between these two measurements of pressure. The instrument also provides a corneal-compensated IOP (IOPcc), which is based on the biomechanical properties of the cornea (elasticity and viscosity), to compensate for the measured IOP values [46,47].
There are several pros and cons to this device. The ORA is a relatively expensive device, but it is easy to handle, and topical anesthesia and fluorescein are not needed. In comparison with GAT, ORA has been demonstrated to significantly overestimate the IOP values, especially at high IOP levels [47,48]. Several authors have demonstrated that the IOPcc is less affected by corneal properties [48,49,50,51] and may better reflect the true IOP after refractive surgery of the cornea when compared to GAT IOP values [52].
Recent studies have shown that the ORA IOPcc values were superior to the GAT IOP measurements in predicting rates of glaucoma progression [53,54]. The CH parameter has been associated with other parameters of damage due to glaucoma, such as high cup-to-disc ratio and defects in the visual field. Several studies have shown a low CH value to be an independent predictor of functional damage occurrence or progression in the visual field in patients affected by ocular hypertension or glaucoma [55,56,57,58]. Moreover, the CH value can help in detecting patients with pathologies of the cornea such as keratoconus [59]. It may also be a useful parameter for patients at risk of developing corneal ectasia after refractive LASIK surgery [60].
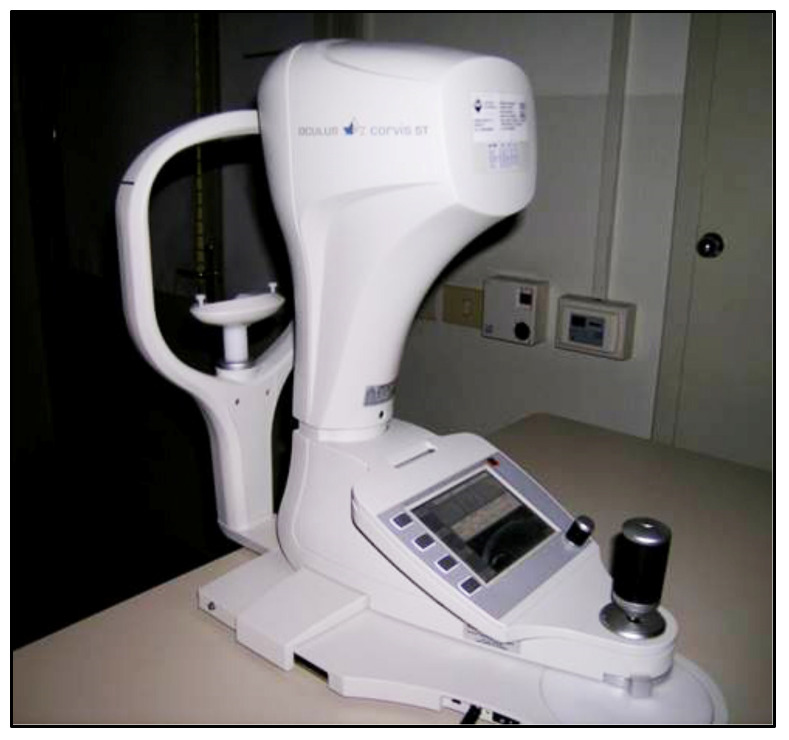
The Corvis ST (Oculus, Wezlar, Germany), a novel non-contact instrument, released in 2011 [61], is based on the system that causes indentation of the cornea by a jet of air. The tonometer has a built-in Scheimpflug ultra-high-speed device (Figure 8).
Figure 8.
Corvis tonometer.
This instrument provides IOP measurements based on the indentation principle, in addition to pachymetry taken by on optical device and other biomechanical parameters of the cornea obtained by registering the surface deformation due to an applied air pulse, similar to an ORA device. A Scheimpflug camera visualizes an 8.5 mm diameter of the center of the surface of the cornea and precisely records the corneal deformation induced by the air-jet and its return to its normal shape with a high resolution and more than 4300 frames per second. A biomechanically corrected IOP value (bIOP), which takes the individual corneal deformation parameters into account, is also provided by the device.
The Corvis ST precision for the CCT and IOP values has been shown to be excellent; however, it is moderate for the corneal deformation parameters [62,63,64]. Previous studies demonstrated that Corvis ST tends to underestimate IOP readings obtained with GAT [61,62,65,66]. The Corvis ST biomechanically corrected IOP values (bIOP) have been shown to be less influenced by the CCT and corneal biomechanics and to be more effective in measuring the IOP in subjects who underwent refractive surgery [67]. Moreover, the Corvis ST corneal deformation parameters have been shown to be effective in discriminating between normal and keratoconic eyes [68].
5. Pneumotonometry
Pneumotonometers are devices based on the applanation principle, which use a different technology [69,70]: the tonometer probe consists of a hollow central tube flanked by a side exhaust, and the sensor is air pressure, which is dependent on the resistance of the exhaust. During the cornea applanation, the pressure within the central tubes increases to match the force generated by the IOP. A pneumatic electronic transducer converts the air pressure to a tracer on a strip of paper (Figure 9).
Figure 9.
Pneumotonometer.
In several studies, pneumotonometry proved to be quite accurate and reliable in glaucoma screening and showed a greater reliability compared to GAT after PRK and LASIK [71,72,73]. Pneumotonometers such as the Pulsatile Ocular Blood Flow (OBF, Figure 10) have been used in the past to measure the pulse fluctuation and thereby give indirect information regarding the ocular blood pulse [74,75,76,77]. OBF measurements, however, appear to be more influenced by CCT and more variable than GAT readings, with a significant overestimation [78,79,80]. The clinical usefulness of this instrument in clinics still remains controversial.
Figure 10.
Langham Ocular Blood Flow pneumotonometer.
6. Rebound Tonometry
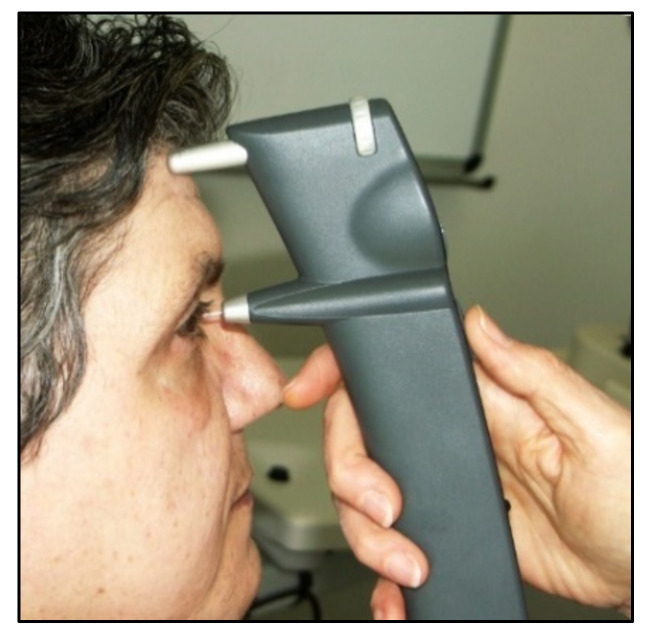
From a clinical point of view, the iCare rebound tonometer, introduced in 2000 by Kontiola [81], is currently one of the most interesting and widespread instruments used in practice (Figure 11).
Figure 11.
iCare rebound tonometer.
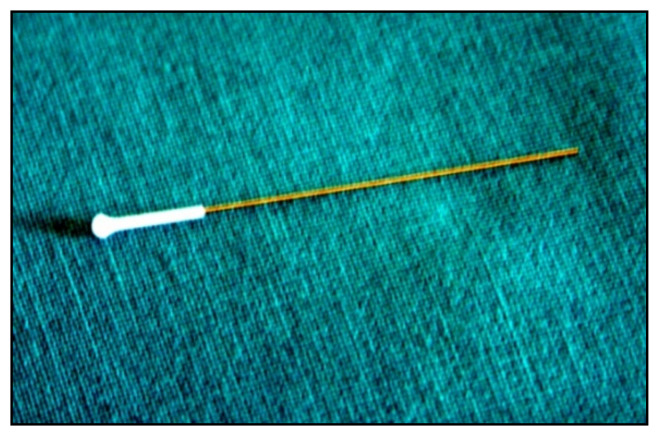
A subtle probe impacts onto the cornea and then rebounds from the eye with a different velocity, which varies according to the IOP (Figure 12).
Figure 12.
Disposable iCare probe.
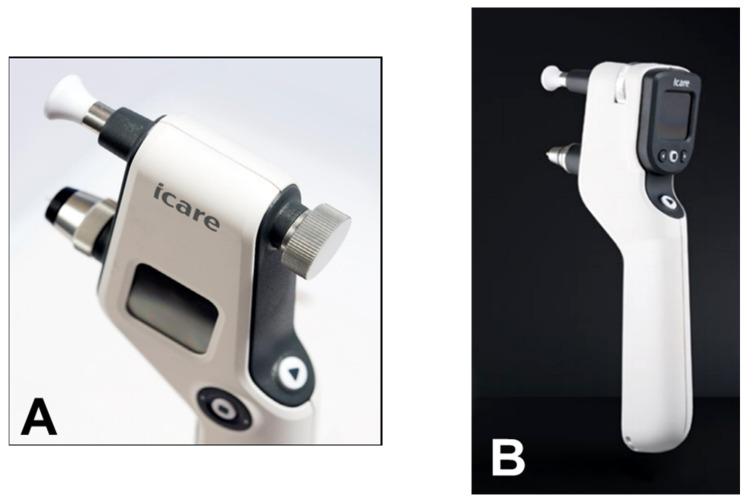
The movement of the probe causes a voltage in the internal solenoid that is then amplified and digitally changed by a microprocessor. The IOP value is averaged from six consecutive measurements. The reliability of the final value is also displayed. The iCare tonometer is a reliable and precise instrument. It is rapid and easy to use, which is particularly helpful in busy clinics and with children, considering that there is no need for topic anesthesia [82,83,84,85]. The small surface contact makes it suitable to measure IOP after keratoplasty and in damaged corneas [85,86]. The iCare PRO version released in 2011 uses a shorter probe, which can also be used to measure IOP in a supine position. The most recent versions of this instrument, which are updated versions of the iCare PRO with a long probe (iCare IC100 and IC200) (Figure 13A,B), provide new features, such as a red or green light to show if the position of the probe is correct, in addition to providing the possibility of measuring IOP in a supine position [87,88].
Figure 13.
(A) iCare 100; (B) iCare 200 version.
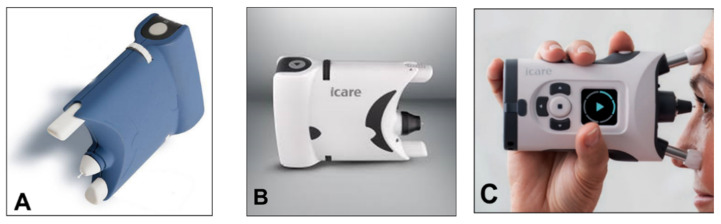
A simplified version (iCare One, replaced at first by the iCare Home and, recently, by the iCare Home2 (Figure 14A–C)), which can autonomously be used by patients, has recently been introduced for at-home auto-tonometry. It can be helpful for detecting IOP peaks, especially in suspect glaucoma and in normal tension glaucoma subjects, when IOP measurements appear to be normal during office hours [89,90,91,92].
Figure 14.
(A) iCare One; (B) iCare Home; (C) iCare Home2.
Numerous studies have compared the different versions of iCare with GAT and other non-conventional tonometers. When compared to gold standard GAT, clinical results report a good correlation of tonometry readings, with r values greater than 0.8 for low-to-moderate GAT readings [93]. A recent study showed agreement between GAT readings and iCare to be good, with a <2 mmHg mean difference for all ranges of IOP [87]. For IOP > 23 mmHg, rebound tonometry tends to underestimate IOP compared to GAT, showing readings that are significantly lower [93].
Considering that rebound tonometry may be less traumatic on the cornea compared to GAT, it could offer a better alternative in post-operative patients to provide information regarding IOP. It is important to note that GAT measurements tend to be lower than iCare for post-operative patients with corneal edema [84,94]. The agreement between iCare and GAT has been reported to be acceptable in lamellar keratoplasty subjects; yet, it has been reported to be poor for patients with penetrating keratoplasty [84]. Rebound tonometry surely cannot replace GAT. However, it may prove to be clinically useful in post-operative eyes with fragile anterior segments, or eyes with increased risk of infection, in which GAT is impractical or not indicated.
In comparing the reliability and reproducibility of IOP values with iCare tonometry, air tonometry and GAT, Valero et al. reported ICC > 0.85 and low differences with GAT [95]. Several studies have reported that iCare provides reproducible and reliability measurements when compared to other tonometers, although it appears to slightly underestimate GAT readings [88,95,96,97,98,99,100,101]. The repeatability of iCare has been shown to be excellent, with ICC > 0.9 [88,99]. Reliability results with iCare compared to GAT have been shown to be good (ICC > 0.87) for patients with IOP with low-to-moderate measurements; however, such results are moderate (ICC = 0.52) for IOP < 16 mmHg and >23 mmHg [88]. Studies have reported sensitivity and specificity rates greater than 0.90 for rebound tonometry [98,99].
IOP readings taken with iCare do not appear to be affected by axial length, refractive error, age and gender [87,100]. GAT and rebound tonometry, however, are influenced by corneal characteristics. IOP tends to be overestimated for central corneal thicknesses greater than 520 microns [82,93,96,99,100,101]. Rebound tonometry also appears to be affected by corneal curvature, corneal hysteresis and disease [96,100,101]. Tonometer measurements tend to be more accurate with iCare for middle levels of IOP, ranging 16–23 mmHg [100].
Rebound tonometry has several advantages, which include having a short learning curve, being user friendly in nature, being well tolerated, and being safe for staff and patients. The instruments are portable, self-calibrated, affordable and do not require a slit lamp, topical anesthesia or fluorescein dye. These iCare instruments can also be used by trained non-medical staff. Multiple readings can be taken if needed without the fear of corneal abrasion or other complications [88]. Unlike GAT that uses a prism in contact with the cornea, the minimal contact and duration with the disposable iCare tips limits the risk of iatrogenic damage and cross-infection, which is of great importance in the recent COVID era [101]. Moreover, iCare does not induce IOP reduction caused by ocular massaging that can be observed in GAT [102]. The iCare home version can be useful in self-twenty-four-hour measurements of IOP to monitor diurnal variations in IOP, which may assist in treatment decision making and surgical timing, especially in patients at greater risks of IOP spikes such as pigment dispersion glaucoma, angle-closure suspects and pseudoexfoliation glaucoma [100].
The rebound tonometers offer numerous advantages; however, several cons should be noted. A recent study comparing iCare with GAT in 1000 eyes showed larger mean differences between tonometers in eyes with IOP > 22 mmHg and in the group of glaucoma patients with medications [103]. The precision and accuracy of iCare can be influenced by peripheral measurements of the cornea as opposed to proper central positioning of the tip [104]. Based on the good agreement with GAT for IOP values <21 mmHg, iCare can be a time-saving and wise alternative in a routine busy clinical screening setting, in which the majority of healthy patients show low-to-moderate IOP. These instruments can offer additional helpful information in a community-based setting when used together with other pertinent screening tools. High IOP readings taken with iCare tonometers need to be checked and confirmed with gold standard GAT. Rebound tonometry applies minimal pressure on the cornea; thus, it can be used to provide indicative IOP readings in first-day post-operative patients, keeping in mind the good, yet limiting, agreement with GAT readings. The ease of use, portability, rapidity, and use in supine positions make it an excellent tool for examining children, disabled and/or bedridden patients and patients with limited collaboration, in which GAT cannot be performed or is impractical.
7. Dynamic Contour Tonometry
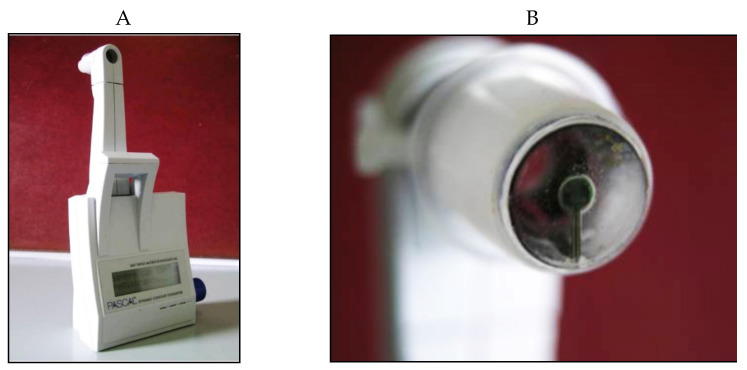
The Dynamic Contour Tonometer (PASCAL, DCT) (SMT Swiss Microtechnology AG, Port, Switzerland) is a relatively new device developed by Kaufmann et al. in 2003 [105] and implemented by Kanngiesser et al. in 2005 [106]. The DCT, which is not based on the applanation principle, calculates the IOP using the Pascal principle, according to which the pressure change is applied to all parts of a fluid in a contained enclosed space. The tonometer is positioned on the slit-lamp, requires the use of anesthetic drops (no fluorescein) and is automatically calibrated. It uses a concave contour tip that is equipped with a tiny sensor in the center of the contact surface (Figure 15).
Figure 15.
Dynamic Contour Pascal (A) with its sensor tip (B).
When the “contour matching” between the surface of the cornea and the tip of the instrument is reached, the tangential forces of the cornea are cancelled, and the embedded pressure sensor directly measures the IOP without any cornea deformation and bias related to corneal factors, at least theoretically [107,108,109,110]. The pressure sensor tip is protected with a thin silicone membrane covered by disposable sensor caps in order to avoid the risk of infection. The DCT requires about 8-10 s of corneal contact in order to provide IOP measurements, which are shown on an LCD screen. Quality scores and ocular pulse amplitude (OPA) values are also provided. The DCT IOP is based on the diastolic IOP, which should be considered when comparing DCT and GAT IOP measurements. The OPA provides indirect information regarding perfusion of the choroid, which has been demonstrated to be important in the onset and progression of glaucoma [111].
The DCT has been shown to be less influenced by the properties of the cornea and can be therefore helpful in taking IOP readings in subjects with previous photorefractive surgeries [112,113,114,115,116]. The DCT has shown high precision, with higher reproducibility than GAT [109,117]. In comparison with GAT, however, the DCT IOP measurements, although highly correlated, tend to be significantly higher [26].
The principal drawbacks of the DCT include: the need for a slit lamp, anesthetic and corneal contact; the need for trained staff and highly cooperative patients that can keep a good head and eye position for at least 8 s, meaning that this tonometer may prove to be difficult to use and not rapid in busy clinics [118]; and reduced accuracy in the presence of irregular corneas [119].
8. Applanation Resonance Tonometry
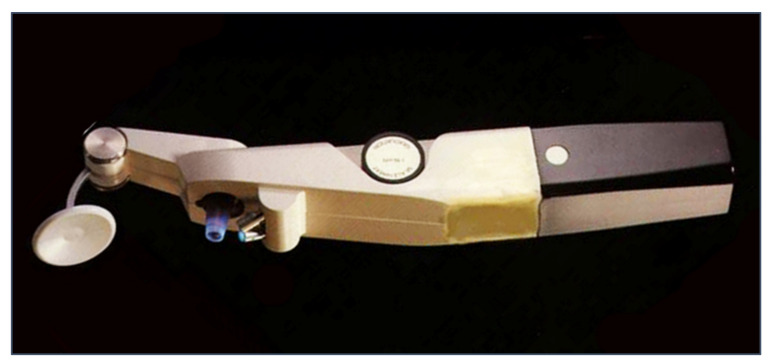
The Applanation Resonance Tonometer (ART), known in the current commercial version as BioResonator ART (BioResonator AB, Umea, Sweden) (Figure 16), was developed by Eklund et al. in 2003 [120]. It was released as both a manual and automatic version in 2012 [121]. This tonometer uses the applanation tonometry principle combined with the resonance technique. The device needs must be mounted on a slit lamp, requires the use of local anesthetic drops before IOP measurement and uses a concave surface sensor tip, which is positioned on the cornea. The sensor tip is manually pushed towards the cornea in the manual version of the instrument, whereas the automatic version provides a tiny motor for movement of the tip. A resonance piezoelectric device is found in the tip of the sensor that generates a shift in frequency which is proportional to the area of contact. The IOP is based on the contact area and force measurement parameters, which are taken continuously throughout the test [120,121].
Figure 16.

The BioResonator ART tonometer.
The ART probe must be carefully disinfected before each subject. This tonometer is self-calibrated and gives the repeated IOP measurement median and a quality index reflecting the standard deviation of the IOP values. The IOP measurement provided by the BioResonator ART is claimed to be more accurate than that of GAT considering that it represents the median of repeated measures; however, the precision of the instrument has been questioned [122,123,124].
Previous studies have reported that this tonometer can provide an overestimate in IOP values when compared to GAT [123,124,125]. Furthermore, it seems to be affected by CCT and corneal biomechanics [120,123,126]. Despite some advantages (repeatable and reliable measurements, no fluorescein is needed), the BioResonator ART has some drawbacks that may reduce its clinical usefulness, including: the need for a slit lamp, anesthetic and corneal contact, with sterilization issues; can be affected by various artifacts and measurement errors; moreover, its accuracy is influenced by the thickness and biomechanics of the cornea.
9. Continuous IOP Monitoring
All the aforementioned devices can be usefully employed for taking spot IOP measurements during office time. This can be acceptable in a screening setting, but, unfortunately, undetected elevated IOP spikes tend to occur during the night in many glaucomatous patients [127]. IOP readings during clinical office hours fail to detect these peaks in more than 50% of cases with a significant underestimation of IOP [128,129,130,131]. Hughes et al. reported that data obtained with continuous monitoring in IOP using a 24 h device had an influence in therapeutic decisions in 79.3% of enrolled subjects [132]. Keeping these data in mind, it can be inferred that our current standards in clinics with regard to taking IOP measurements may not suffice and thus need to be modified [133].
An important step towards a more precise management of patients affected by ocular hypertension and chronic glaucoma would be the possibility of continuously monitoring IOP values not only during the day but also in the night, as occurs with the 24 h blood pressure Holter. This information could be particularly useful in the so-called normal tension glaucoma patients, which show significant damage progression despite an apparently normalized IOP. In these cases, an elevated IOP can sometimes be found during the night, especially early in the morning, outside office hours [134,135]. A number of devices, most of them only experimental, have been proposed for this purpose over the past 20 years [136,137,138]. Some of them need to be surgically inserted into the eye, either during a cataract extraction procedure, usually embedded in an intraocular lens [139,140,141], or positioned in the anterior chamber [142,143], or in the suprachoroidal space [144]. A non-invasive continuous IOP measurement is also possible using special contact lenses with different types of miniaturized sensors and a wireless power transmission of data to a recorder.
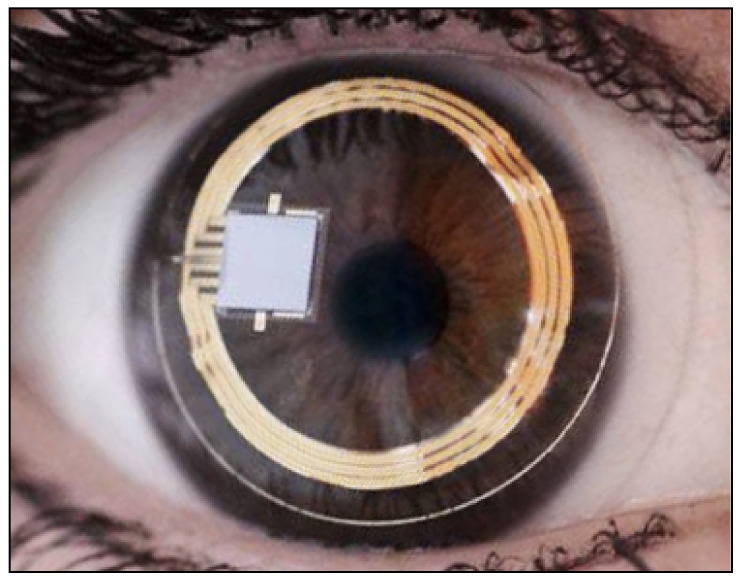
The contact lens sensor Sensimed Triggerfish (Triggerfish CLS, Sensimed AG, Lausanne, Switzerland) is a miniaturized electromechanical system with a microprocessor embedded in a disposable silicon contact lens, which transmits a signal to an external wireless antenna located in the periocular surface (Figure 17 and Figure 18). The data are then transferred to a portable recorder, for a total of 288 data sets in 24 h. This device can measure small modifications in the curvature of the cornea believed to be due to variations of IOP [145,146,147,148,149].
Figure 17.
Sensimed Triggerfish.
Figure 18.
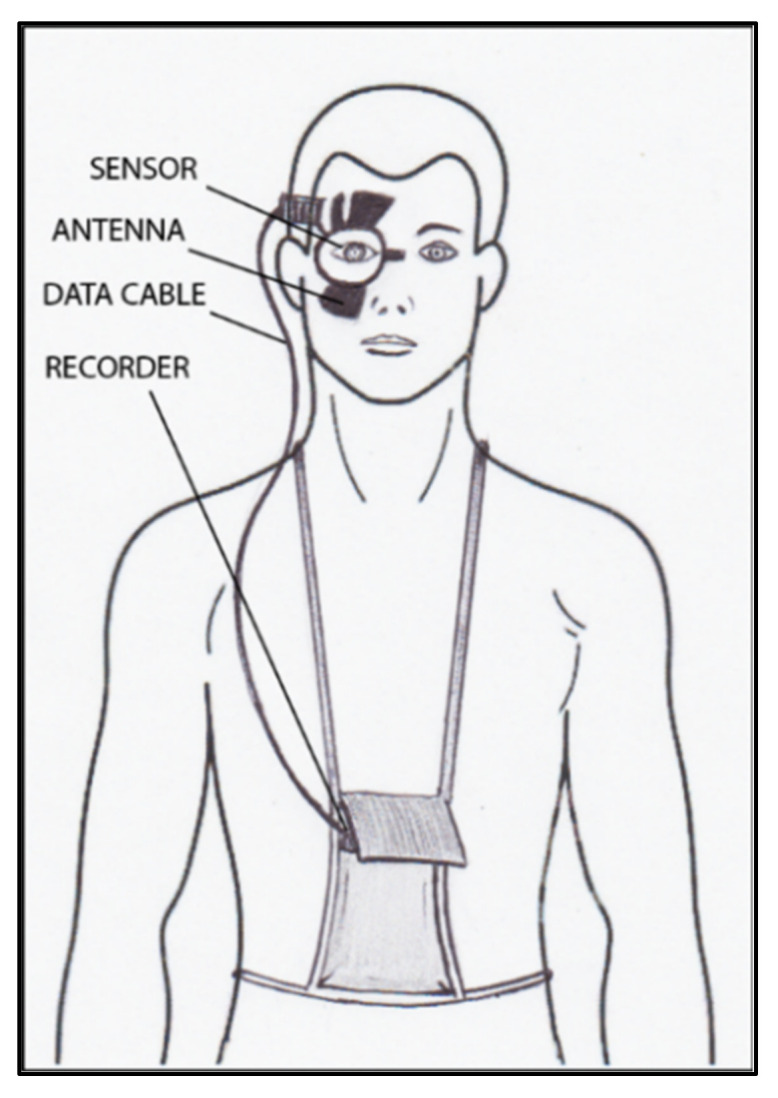
Schematic view of Triggerfish, wireless antenna and portable recorder.
Triggerfish CLS is usually well tolerated [150,151,152] and has also been shown to have high reproducibility [150,151,152,153,154,155]. The information obtained with these device parameters might be useful in assessing changes and IOP fluctuations in subjects with pseudoexfoliation syndrome, pigment dispersion, and in predicting the visual field loss progression rate [156,157]. Several studies have shown the usefulness of this contact lens sensor for assessment of the risk of glaucoma, which may prove to be important in subjects with NTG, in which IOP tends to be normal with diurnal readings [158,159,160,161].
The main problem with this device is that there is no direct correlation between corneal changes, expressed in millivolt equivalent (mVeq), and IOP values. Studies have shown that IOP measurements taken with GAT and Triggerfish values tend to have a high correlation at the beginning, after the insertion of CLS [148]; however, the correlation becomes poor after 24 h [153,154].
CLS is advantageous because it is not invasive, can be easily removed and dismantled [155], readily available [155], accepted and tolerated by patients [150,151,152], and provides good reproducibility [151,153,154]. The validity (i.e., considering the estimation accuracy of IOP readings) and relatively costly equipment of CLS are important drawbacks, which render the clinical usefulness of this instrument still debatable in literature [153,154].
Other types of devices able to measure IOP, either implantable or non-invasive [162,163,164,165,166,167,168,169,170,171,172,173], have been proposed, but almost all are still experimental and need further studies before being introduced into clinical practice.
10. Conclusions
As shown in Table 1, numerous tonometers have been proposed in the past. It is important to note that in managing and treating glaucoma patients, it is preferable and more reliable to measure IOP every time with the same type of equipment for each individual glaucoma patient. Several instruments have provided specific advantages compared to Goldmann tonometry. Alternative systems reported in literature, either non-invasive or implantable, remain experimental. Despite promising preliminary results, none have obtained widespread use and are adaptable in a routine clinal setting. New is not always better. The Goldmann tonometer, despite its limitations and a lack of innovative and novel advancements in the past 70 years, continues to be theoretically more precise and considered the gold standard tonometer to diagnose and manage patients with ocular hypertension and glaucoma.
Table 1.
Summary of the characteristics of the various available tonometers.
| Tonometer Type | Production Year | Working Principle | Contact/ Noncontact |
Advantages | Disadvantages | Clinical Suitability |
Cost * |
|---|---|---|---|---|---|---|---|
| Schioetz tonometer | 1905 | indentation | contact | Simple | High variability | still used only in developing countries |
feasible |
| Inexpensive | Can be used in a supine position only | ||||||
| No need of slit-lam, electricity or charging batteries | Affected by various sources of error | ||||||
| Topical anesthesia is needed | |||||||
| Goldmann applanation tonometer (GAT) |
1955 | applanation | contact | Quite simple to use | Affected by corneal thickness and other corneal parameters | good | feasible |
| Accurate and reproducible measurements | The accuracy depends on the clinician’s experience | ||||||
| It is currently considered as the gold standard in IOP measurement | Needs to be used with a slit-lamp | ||||||
| Can be used in the upright position only | |||||||
| Topical anesthesia and fluorescein are needed | |||||||
| Perkins tonometer | 1965 | applanation | contact | Can be used in a supine position too | Affected by corneal thickness and other corneal parameters | good | feasible |
| Topical anesthesia and fluorescein are needed | |||||||
| TonoPen | 1989 | indentation/ applanation |
contact | Lightweight and portable | Hight variability and quite poor repeatability | moderate | feasible |
| Quick and simple to use | Can underestimate IOP values | ||||||
| Can be used in any position | Topical anesthesia is needed | ||||||
| No need of slit-lamp or electricity | Influenced by corneal parameters | ||||||
| Self-calibration, provides quality index | |||||||
| Air-puff tonometers | 1973 | applanation | noncontact | Easy and fast to use | Need of regular calibration | ideal as a screening tool |
medium |
| No need to touch the cornea | Readings are device-dependent | ||||||
| No need of topical anesthesia and fluorescein | Possible germs aerosol | ||||||
| Can be used by paramedical staff | Influenced by corneal parameters | ||||||
| Less accurate when IOP > 20 mmHg | |||||||
| Ocular Response Analyzer |
2005 | applanation | noncontact | Simple to use, self-calibration, provides quality index | Possible germs aerosol | good | expensive |
| No need of topical anesthesia and fluorescein | |||||||
| Can be used by paramedical staff | |||||||
| Provides additional information (corneal central thickness and biomechanics) | |||||||
| IOP correction for corneal biomechanical parameters | |||||||
| Useful after corneal refractive surgery | |||||||
| Detection of corneal diseases | |||||||
| Corvis ST | 2011 | indentation/ applanation |
noncontact | Simple to use, self-calibration, provides quality index | Tends to underestimate the GAT IOP values |
good | expensive |
| No need of fluorescein and topical anesthesia | |||||||
| Can be used by paramedical staff | |||||||
| Provides additional information (corneal central thickness and biomechanics) | |||||||
| IOP correction for corneal biomechanical parameters | |||||||
| Useful after corneal refractive surgery | |||||||
| Detection of corneal diseases | |||||||
| Pneumotonometers | 1969 | applanation | contact | OBF provides information on the ocular blood pulse | Affected by corneal thickness | controversial | expensive |
| Overestimates IOP values | |||||||
| iCare tonometer | 1997 | ballistic probe (rebound) | contact | Ease of use with a short learning curve | Needs a proper central positioning of the tip | excellent | feasible |
| Portable and self-calibrated | Influenced by corneal thickness | ||||||
| No slit-lamp or topical anesthesia or fluorescein dye required | |||||||
| Can be used by trained non-medical staff | |||||||
| Can be used in supine positions (iCare PRO and iCare 200) | |||||||
| Minimal corneal trauma (useful in post-operative patients) | |||||||
| The home version can be useful in self-twenty-four-hour monitoring of IOP | |||||||
| Dynamic contour tonometer (DCT, PASCAL) |
2005 | contour matching | contact | No need of fluorescein, disposable probes | Need of slit lamp and topical anesthesia | poor | medium |
| Self-calibration, provides quality index | Difficult to use | ||||||
| Independent from corneal properties | Need of highly cooperative patients | ||||||
| Useful after corneal refractive surgery | |||||||
| High precision | |||||||
| Additional information (ocular pulse amplitude) | |||||||
| BioResonator ART | 2003 | applanation | contact | No need of fluorescein | Need of slitlamp and local anesthetic | moderate | medium |
| Self-calibration, provides quality index | Need of probe disinfection | ||||||
| High reliability (median of repeated IOP measurements) | Required training to use | ||||||
| Affected by corneal properties | |||||||
| Sensimed Triggerfish | 2004 | corneal curvature monitoring |
contact | Continuous measurements over a 24-hour period | It does not provide direct IOP values | moderate | feasible |
| Good tolerability | IOP estimation accuracy not known | ||||||
| High reproducibility |
* feasible: 0–5000 euros; medium range 5000–10,000 euros; expensive: >10,000 euros.
Author Contributions
All authors (P.B., M.L.S. and M.Z.) contributed equally to all aspects of the manuscript, including conceptualization, methodology, validation, formal analysis, investigation, resources, writing and preparation of all drafts, editing, visualization, supervision, and project administration. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kniestedt C., Punjabi O., Lin S., Stamper R.L. Tonometry through the Ages. Surv. Ophthalmol. 2008;53:568–591. doi: 10.1016/j.survophthal.2008.08.024. [DOI] [PubMed] [Google Scholar]
- 2.Stamper R.L. A History of Intraocular Pressure and Its Measurement. Optom. Vis. Sci. 2011;88:E16–E28. doi: 10.1097/OPX.0b013e318205a4e7. [DOI] [PubMed] [Google Scholar]
- 3.Brusini P. Intraocular Pressure and Its Measurement. In: Choplin N.T., Traverso C.E., editors. Atlas of Glaucoma. 3rd ed. CRC Press; Boca Raton, FL, USA: 2014. pp. 29–36. [Google Scholar]
- 4.Albert D.M., Keeler R. The Pressure: Before and after Schiøtz. Ophthalmol. Glaucoma. 2020;3:409–413. doi: 10.1016/j.ogla.2020.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kyari F., Nolan W., Gilbert C. Ophthalmologists’ practice patterns and challenges in achieving optimal management for glaucoma in Nigeria: Results from a nationwide survey. BMJ Open. 2016;6:e012230. doi: 10.1136/bmjopen-2016-012230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagarajan S., Velayutham V., Ezhumalai G. Comparative evaluation of applanation and indentation tonometers in a community ophthalmology setting in Southern India. Saudi. J. Ophthalmol. 2016;30:83–87. doi: 10.1016/j.sjopt.2015.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lasseck J., Jehle T., Feltgen N., Lagrèze W.A. Comparison of intraocular tonometry using three different non-invasive tonometers in children. Graefe’s Arch. Clin. Exp. Ophthalmol. 2008;246:1463–1466. doi: 10.1007/s00417-008-0863-y. [DOI] [PubMed] [Google Scholar]
- 8.Ohana O., Varssano D., Shemesh G. Comparison of intraocular pressure measurements using Goldmann tonometer, I-care pro, Tonopen XL, and Schiotz tonometer in patients after Descemet stripping endothelial keratoplasty. Indian J. Ophthalmol. 2017;65:579–583. doi: 10.4103/ijo.IJO_31_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goldmann H., Schmidt T. Über Applanationstonometrie. Acta. Ophthalmol. 1957;134:221–242. doi: 10.1159/000303213. [DOI] [PubMed] [Google Scholar]
- 10.Ehlers N., Bramsen T., Sperling S. Applanation tonometry and central corneal thickness. Acta. Ophthalmol. 2009;53:34–43. doi: 10.1111/j.1755-3768.1975.tb01135.x. [DOI] [PubMed] [Google Scholar]
- 11.Whitacre M.M., Stein R.A., Hassanein K. The Effect of Corneal Thickness on Applanation Tonometry. Am. J. Ophthalmol. 1993;115:592–596. doi: 10.1016/S0002-9394(14)71455-2. [DOI] [PubMed] [Google Scholar]
- 12.Herndon L.W., Choudhri S.A., Cox T., Damji K.F., Shields M.B., Allingham R.R. Central Corneal Thickness in Normal, Glaucomatous, and Ocular Hypertensive Eyes. Arch. Ophthalmol. 1997;115:1137–1141. doi: 10.1001/archopht.1997.01100160307007. [DOI] [PubMed] [Google Scholar]
- 13.Wolfs R.C., Klaver C., Vingerling J.R., Grobbee D.E., Hofman A., de Jong P.T. Distribution of Central Corneal Thickness and Its Association With Intraocular Pressure: The Rotterdam Study. Am. J. Ophthalmol. 1997;123:767–772. doi: 10.1016/S0002-9394(14)71125-0. [DOI] [PubMed] [Google Scholar]
- 14.Doughty M.J., Zaman M.L. Human Corneal Thickness and Its Impact on Intraocular Pressure Measures: A Review and Meta-analysis Approach. Surv. Ophthalmol. 2000;44:367–408. doi: 10.1016/S0039-6257(00)00110-7. [DOI] [PubMed] [Google Scholar]
- 15.Park S.J., Ang G.S., Nicholas S., Wells A.P. The Effect of Thin, Thick, and Normal Corneas on Goldmann Intraocular Pressure Measurements and Correction Formulae in Individual Eyes. Ophthalmology. 2012;119:443–449. doi: 10.1016/j.ophtha.2011.07.058. [DOI] [PubMed] [Google Scholar]
- 16.Brandt J.D., Gordon M.O., Gao F., Beiser J.A., Miller J.P., Kass M. Adjusting Intraocular Pressure for Central Corneal Thickness Does Not Improve Prediction Models for Primary Open-Angle Glaucoma. Ophthalmology. 2012;119:437–442. doi: 10.1016/j.ophtha.2011.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gunvant P., O’Leary D.J., Baskaran M., Broadway D.C., Watkins R.J., Vijaya L. Evaluation of Tonometric Correction Factors. J. Glaucoma. 2005;14:337–343. doi: 10.1097/01.ijg.0000176940.81799.33. [DOI] [PubMed] [Google Scholar]
- 18.Brandt J.D., Beiser J.A., Kass M., Gordon M.O. Central corneal thickness in the ocular hypertension treatment study (OHTS) Ophthalmology. 2001;108:1779–1788. doi: 10.1016/S0161-6420(01)00760-6. [DOI] [PubMed] [Google Scholar]
- 19.Gazzard G., Jayaram H., Roldan A.M., Friedman D.S. When gold standards change: Time to move on from Goldmann tonometry? Br. J. Ophthalmol. 2021;105:1–2. doi: 10.1136/bjophthalmol-2020-317112. [DOI] [PubMed] [Google Scholar]
- 20.Kotecha A., White E., Schlottmann P.G., Garway-Heath D. Intraocular Pressure Measurement Precision with the Goldmann Applanation, Dynamic Contour, and Ocular Response Analyzer Tonometers. Ophthalmology. 2010;117:730–737. doi: 10.1016/j.ophtha.2009.09.020. [DOI] [PubMed] [Google Scholar]
- 21.Whitacre M.M., Stein R. Sources of error with use of Goldmann-type tonometers. Surv. Ophthalmol. 1993;38:1–30. doi: 10.1016/0039-6257(93)90053-A. [DOI] [PubMed] [Google Scholar]
- 22.Choudhari N.S., Rao H.L., Ramavath S., Rekha G., Rao A., Senthil S., Garudadri C.S. How Often the Goldmann Applanation Tonometer Should be Checked for Calibration Error? J. Glaucoma. 2016;25:908–913. doi: 10.1097/IJG.0000000000000545. [DOI] [PubMed] [Google Scholar]
- 23.Frenkel R.E.P., Hong Y.J., Shin D.H. Comparison of the Tono-Pen to the Goldmann Applanation Tonometer. Arch. Ophthalmol. 1988;106:750–753. doi: 10.1001/archopht.1988.01060130820030. [DOI] [PubMed] [Google Scholar]
- 24.Iester M., Mermoud A., Achache F., Roy S. New TonoPen XL: Comparison with the Goldmann tonometer. Eye. 2001;15:52–58. doi: 10.1038/eye.2001.13. [DOI] [PubMed] [Google Scholar]
- 25.Blumberg M.J., Varikuti V.N.V., Weiner A. Real-world comparison between the Tonopen and Goldmann applanation tonometry in a university glaucoma clinic. Int. Ophthalmol. 2021;41:1815–1825. doi: 10.1007/s10792-021-01742-z. [DOI] [PubMed] [Google Scholar]
- 26.Salvetat M.L., Zeppieri M., Tosoni C., Brusini P. Comparisons between Pascal dynamic contour tonometry, the TonoPen, and Goldmann applanation tonometry in patients with glaucoma. Acta. Ophthalmol. Scand. 2006;85:272–279. doi: 10.1111/j.1600-0420.2006.00834.x. [DOI] [PubMed] [Google Scholar]
- 27.Tonnu P.-A., Ho T., Sharma K., White E., Bunce C., Garway-Heath D. A comparison of four methods of tonometry: Method agreement and interobserver variability. Br. J. Ophthalmol. 2005;89:847–850. doi: 10.1136/bjo.2004.056614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tonnu P.-A., Ho T., Newson T., El Sheikh A., Sharma K., White E., Bunce C., Garway-Heath D. The influence of central corneal thickness and age on intraocular pressure measured by pneumotonometry, non-contact tonometry, the Tono-Pen XL, and Goldmann applanation tonometry. Br. J. Ophthalmol. 2005;89:851–854. doi: 10.1136/bjo.2004.056622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kontadakis G.A., Pennos A., Pentari I., Kymionis G.D., Pallikaris I.G., Ginis H. Accuracy of dynamic contour tonometry, Goldmann applanation tonometry, and Tono-Pen XL in edematous corneas. Ther. Adv. Ophthalmol. 2020;12 doi: 10.1177/2515841420923190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cronemberger S., Veloso A.W. Comparison of Tono-Pen Avia and Handheld Applanation Tonometry in Primary Congenital Glaucoma. J. Glaucoma. 2021;30:e227–e230. doi: 10.1097/IJG.0000000000001820. [DOI] [PubMed] [Google Scholar]
- 31.Deuter C.M.E., Schlote T., Hahn G.A., Bende T., Derse M. Messung des Augeninnendrucks mit dem Tono-Pen im Vergleich zum Applanationstonometer nach Goldmann—eine klinische Studie an 100 Augen. Klin. Mon. Für Augenheilkd. 2002;219:138–142. doi: 10.1055/s-2002-26718. [DOI] [PubMed] [Google Scholar]
- 32.Gopesh T., Camp A., Unanian M., Friend J., Weinreb R.N. Rapid and Accurate Pressure Sensing Device for Direct Measurement of Intraocular Pressure. Transl. Vis. Sci. Technol. 2020;9:28. doi: 10.1167/tvst.9.3.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grolman B. A new tonometer system. Optom. Vis. Sci. 1972;49:646–660. doi: 10.1097/00006324-197208000-00005. [DOI] [PubMed] [Google Scholar]
- 34.Hansen M.K. Clinical comparison of the XPERT non-contact tonometer and the conventional Goldmann applanation tonometer. Acta. Ophtahlmol. Scand. 1995;73:176–180. doi: 10.1111/j.1600-0420.1995.tb00664.x. [DOI] [PubMed] [Google Scholar]
- 35.Vincent S., Vincent R.A., Shields D., Lee G.A. Comparison of intraocular pressure measurement between rebound, non-contact and Goldmann applanation tonometry in treated glaucoma patients. Clin. Exp. Ophthalmol. 2011;40:e163–e170. doi: 10.1111/j.1442-9071.2011.02670.x. [DOI] [PubMed] [Google Scholar]
- 36.Demirci G., Erdur S.K., Tanriverdi C., Gulkilik G., Ozsutçu M. Comparison of rebound tonometry and non-contact airpuff tonometry to Goldmann applanation tonometry. Ther. Adv. Ophthalmol. 2019;11 doi: 10.1177/2515841419835731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stock R.A., Ströher C., Sampaio R.R., Mergener R.A., Bonamigo E.L. A Comparative Study between the Goldmann Applanation Tonometer and the Non-Contact Air-Puff Tonometer (Huvitz HNT 7000) in Normal Eyes. Clin. Ophthalmol. 2021;15:445–451. doi: 10.2147/OPTH.S294710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moseley M.J., Evans N.M., Fielder A.R. Comparison of a new non-contact tonometer with Goldmann applanation. Eye. 1989;3:332–337. doi: 10.1038/eye.1989.48. [DOI] [PubMed] [Google Scholar]
- 39.Kyei S., Assiamah F., Kwarteng M.A., Gboglu C.P. The Association of Central Corneal Thickness and Intraocular Pressure Measures by Non-Contact Tonometry and Goldmann Applanation Tonometry among Glaucoma Patients. Ethiop. J. Health Sci. 2020;30:999–1004. doi: 10.4314/ejhs.v30i6.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rebours A.K., Kouassi F., Soumahoro M., Ellalie C.K.C., Siméon K.A.N., Agbohoun R. Comparaison de la tonomérie de Goldmann à la tonométrie à air pulsé à propos de 159 patients à Abidjan. J. Français D’Ophtalmol. 2021;44:41–47. doi: 10.1016/j.jfo.2020.03.023. [DOI] [PubMed] [Google Scholar]
- 41.Atkinson P.L., Wishart P.K., James J.N., Vernon S.A., Reid F. Deterioration in the accuracy of the Pulsair non-contact tonometer with use: Need for regular calibration. Eye. 1992;6:530–534. doi: 10.1038/eye.1992.112. [DOI] [PubMed] [Google Scholar]
- 42.Britt J.M. Microaerosol Formation in Noncontact ’Air-Puff’ Tonometry. Arch. Ophthalmol. 1991;109:225–228. doi: 10.1001/archopht.1991.01080020071046. [DOI] [PubMed] [Google Scholar]
- 43.Lai T.H.T., Tang E.W., Chau S.K.Y., Fung K.S.C., Li K.K.W. Stepping up infection control measures in ophthalmology during the novel coronavirus outbreak: An experience from Hong Kong. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020;258:1049–1055. doi: 10.1007/s00417-020-04641-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Almazyad E.M., Ameen S., Khan M.A., Malik R. Guidelines and recommendations for tonometry use during the COVID-19 era. Middle East Afr. J. Ophthalmol. 2020;27:73–78. doi: 10.4103/meajo.MEAJO_237_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mostafa I., Bianchi E., Brown L., Tatham A.J. What is the best way to measure intraocular pressure (IOP) in a virtual clinic? Eye. 2021;35:448–454. doi: 10.1038/s41433-020-0868-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luce D.A. Determining in vivo biomechanical properties of the cornea with an ocular response analyzer. J. Cataract. Refract. Surg. 2005;31:156–162. doi: 10.1016/j.jcrs.2004.10.044. [DOI] [PubMed] [Google Scholar]
- 47.Kynigopoulos M., Schlote T., Kotecha A., Tzamalis A., Pajic B., Haefliger I. Repeatability of Intraocular Pressure and Corneal Biomechanical Properties Measurements by the Ocular Response Analyser. Klin. Mon. Für Augenheilkd. 2008;225:357–360. doi: 10.1055/s-2008-1027256. [DOI] [PubMed] [Google Scholar]
- 48.Martinez-De-La-Casa J.M., García-Feijóo J., Fernández-Vidal A., Mendez-Hernandez C., Garcia-Sanchez J. Ocular Response Analyzer versus Goldmann Applanation Tonometry for Intraocular Pressure Measurements. Investig. Opthalmol. Vis. Sci. 2006;47:4410–4414. doi: 10.1167/iovs.06-0158. [DOI] [PubMed] [Google Scholar]
- 49.Medeiros F.A., Weinreb R.N. Evaluation of the Influence of Corneal Biomechanical Properties on Intraocular Pressure Measurements Using the Ocular Response Analyzer. J. Glaucoma. 2006;15:364–370. doi: 10.1097/01.ijg.0000212268.42606.97. [DOI] [PubMed] [Google Scholar]
- 50.Touboul D., Roberts C., Kérautret J., Garra C., Maurice-Tison S., Saubusse E., Colin J. Correlations between corneal hysteresis, intraocular pressure, and corneal central pachymetry. J. Cataract. Refract. Surg. 2008;34:616–622. doi: 10.1016/j.jcrs.2007.11.051. [DOI] [PubMed] [Google Scholar]
- 51.Bayoumi N.H.L., Bessa A.S., El Massry A.A.K. Ocular Response Analyzer and Goldmann Applanation Tonometry. J. Glaucoma. 2010;19:627–631. doi: 10.1097/IJG.0b013e3181ca7e01. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H., Sun Z., Li L., Sun R., Zhang H. Comparison of intraocular pressure measured by ocular response analyzer and Goldmann applanation tonometer after corneal refractive surgery: A systematic review and meta-analysis. BMC Ophthalmol. 2020;20:23–29. doi: 10.1186/s12886-019-1288-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lascaratos G., Garway-Heath D.F., Russell R.A., Crabb D.P., Zhu H., Hirn C., Kotecha A., Suzuki K., UKGTS Investigators Intraocular pressure (IOP) measured with the ocular response ana-lyser is a better predictor of glaucoma progression than Goldmann IOP in the United Kingdom Glaucoma Treatment study (UKGTS) Investig. Ophthalmol. Vis. Sci. 2014;55:128–138. [Google Scholar]
- 54.Susanna B.N., Ogata N.G., Daga F.B., Susanna C.N., Diniz-Filho A., Medeiros F.A. Association between Rates of Visual Field Progression and Intraocular Pressure Measurements Obtained by Different Tonometers. Ophthalmology. 2019;126:49–54. doi: 10.1016/j.ophtha.2018.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Congdon N.G., Broman A.T., Bandeen-Roche K., Grover D., Quigley H.A. Central Corneal Thickness and Corneal Hysteresis Associated With Glaucoma Damage. Am. J. Ophthalmol. 2006;141:868–875. doi: 10.1016/j.ajo.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Medeiros F.A., Meira-Freitas D., Lisboa R., Kuang T.-M., Zangwill L.M., Weinreb R.N. Corneal Hysteresis as a Risk Factor for Glaucoma Progression: A Prospective Longitudinal Study. Ophthalmology. 2013;120:1533–1540. doi: 10.1016/j.ophtha.2013.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Susanna B.N., Ogata N.G., Jammal A.A., Susanna C.N., Berchuck S.I., Medeiros F.A. Corneal Biomechanics and Visual Field Progression in Eyes with Seemingly Well-Controlled Intraocular Pressure. Ophthalmology. 2019;126:1640–1646. doi: 10.1016/j.ophtha.2019.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Fujino Y., Murata H., Matsuura M., Nakakura S., Shoji N., Nakao Y., Kiuchi Y., Asaoka R. The Relationship between Corneal Hysteresis and Progression of Glaucoma After Trabeculectomy. J. Glaucoma. 2020;29:912–917. doi: 10.1097/IJG.0000000000001581. [DOI] [PubMed] [Google Scholar]
- 59.Kirgiz A., Erdur S.K., Atalay K., Gurez C. The Role of Ocular Response Analyzer in Differentiation of Forme Fruste Keratoconus from Corneal Astigmatism. Eye Contact Lens. Sci. Clin. Pr. 2019;45:83–87. doi: 10.1097/ICL.0000000000000541. [DOI] [PubMed] [Google Scholar]
- 60.Randleman J.B. Post-laser in-situ keratomileusis ectasia: Current understanding and future directions. Curr. Opin. Ophthalmol. 2006;17:406–412. doi: 10.1097/01.icu.0000233963.26628.f0. [DOI] [PubMed] [Google Scholar]
- 61.Reznicek L., Muth D., Kampik A., Neubauer A.S., Hirneiss C. Evaluation of a novel Scheimpflug-based non-contact tonometer in healthy subjects and patients with ocular hypertension and glaucoma. Br. J. Ophthalmol. 2013;97:1410–1414. doi: 10.1136/bjophthalmol-2013-303400. [DOI] [PubMed] [Google Scholar]
- 62.Salvetat M.L., Zeppieri M., Tosoni C., Felletti M., Grasso L., Brusini P. Corneal Deformation Parameters Provided by the Corvis-ST Pachy-Tonometer in Healthy Subjects and Glaucoma Patients. J. Glaucoma. 2015;24:568–574. doi: 10.1097/IJG.0000000000000133. [DOI] [PubMed] [Google Scholar]
- 63.Lopes B.T., Roberts C.J., Elsheikh A., Vinciguerra R., Vinciguerra P., Reisdorf S., Berger S., Koprowski R., Ambrósio R. Repeatability and Reproducibility of Intraocular Pressure and Dynamic Corneal Response Parameters Assessed by the Corvis ST. J. Ophthalmol. 2017;2017:8515742. doi: 10.1155/2017/8515742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serbecic N., Beutelspacher S., Markovic L., Roy A.S., Shetty R. Repeatability and reproducibility of corneal biomechanical parameters derived from Corvis ST. Eur. J. Ophthalmol. 2020;30:1287–1294. doi: 10.1177/1120672119864554. [DOI] [PubMed] [Google Scholar]
- 65.Luebke J., Bryniok L., Neuburger M., Jordan J.F., Boehringer D., Reinhard T., Wecker T., Anton A. Intraocular pressure measurement with Corvis ST in comparison with applanation tonometry and Tomey non-contact tonometry. Int. Ophthalmol. 2019;39:2517–2521. doi: 10.1007/s10792-019-01098-5. [DOI] [PubMed] [Google Scholar]
- 66.Nakao Y., Kiuchi Y., Okumichi H. Evaluation of biomechanically corrected intraocular pressure using Corvis ST and comparison of the Corvis ST, noncontact tonometer, and Goldmann applanation tonometer in patients with glaucoma. PLoS ONE. 2020;15:e0238395. doi: 10.1371/journal.pone.0238395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bao F., Huang W., Zhu R., Lu N., Wang Y., Li H., Wu S., Lin H., Wang J., Zheng X., et al. Effectiveness of the Goldmann Applanation Tonometer, the Dynamic Contour Tonometer, the Ocular Response Analyzer and the Corvis ST in Measuring Intraocular Pressure following FS-LASIK. Curr. Eye Res. 2019;45:144–152. doi: 10.1080/02713683.2019.1660794. [DOI] [PubMed] [Google Scholar]
- 68.Yang K., Xu L., Fan Q., Gu Y., Song P., Zhang B., Zhao D., Pang C., Ren S. Evaluation of new Corvis ST parameters in normal, Post-LASIK, Post-LASIK keratectasia and keratoconus eyes. Sci. Rep. 2020;10:5676. doi: 10.1038/s41598-020-62825-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Durham D.G., Bigliano R.P., Masino J.A. Pneumatic applanation tonometer. Trans. Am. Acad. Ophthalmol. Otolaryngol. Am. Acad. Ophthalmol. Otolaryngol. 1965;69:1029–1047. [PubMed] [Google Scholar]
- 70.West C.E., Capella J.A., Kaufman H.E. Measurement of Intraocular Pressure with a Pneumatic Applanation Tonometer. Am. J. Ophthalmol. 1972;74:505–509. doi: 10.1016/0002-9394(72)90917-8. [DOI] [PubMed] [Google Scholar]
- 71.Guildford J., O’day D.M. Applanation Pneumotonometry in Screening for Glaucoma. South. Med. J. 1985;78:1081–1083. doi: 10.1097/00007611-198509000-00015. [DOI] [PubMed] [Google Scholar]
- 72.Abbasoglu Özlem E., Bowman R., Cavanagh H., McCulley J.P. Reliability of intraocular pressure measurements after myopic excimer photorefractive keratectomy. Ophthalmology. 1998;105:2193–2196. doi: 10.1016/S0161-6420(98)91215-5. [DOI] [PubMed] [Google Scholar]
- 73.Zadok D., Tran D.B., Twa M., Carpenter M., Schanzlin D.J. Pneumotonometry versus Goldmann tonometry after laser in situ keratomileusis for myopia. J. Cataract. Refract. Surg. 1999;25:1344–1348. doi: 10.1016/S0886-3350(99)00202-3. [DOI] [PubMed] [Google Scholar]
- 74.Langham M.E. Discussion on pneumatic applanation tonometer. Tr. Am. Acad. Ophth. Otolaryng. 1965;69:1042. [Google Scholar]
- 75.Langham M.E., McCarthy E. A Rapid Pneumatic Applanation Tonometer. Arch. Ophthalmol. 1968;79:389–399. doi: 10.1001/archopht.1968.03850040391006. [DOI] [PubMed] [Google Scholar]
- 76.Silver D.M., Farrell R.A. Validity of pulsatile ocular blood flow measurements. Surv. Ophthalmol. 1994;38:S72–S80. doi: 10.1016/0039-6257(94)90049-3. [DOI] [PubMed] [Google Scholar]
- 77.Esgin H., Alimgil M., Erda S. Clinical comparison of the ocular blood flow tonograph and the Goldmann applanation tonometer. Eur. J. Ophthalmol. 1998;8:162–166. doi: 10.1177/112067219800800308. [DOI] [PubMed] [Google Scholar]
- 78.Gunvant P., Baskaran M., Vijaya L., Joseph I.S., Watkins R.J., Nallapothula M., Broadway D.C., O’Leary D.J. Effect of corneal parameters on measurements using the pulsatile ocular blood flow tonograph and Goldmann applanation tonometer. Br. J. Ophthalmol. 2004;88:518–522. doi: 10.1136/bjo.2003.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bhan A., Bhargava J., Vernon S.A., Armstrong S., Bhan K., Tong L., Sung V. Repeatability of ocular blood flow pneumotonometry. Ophthalmology. 2003;110:1551–1554. doi: 10.1016/S0161-6420(03)00492-5. [DOI] [PubMed] [Google Scholar]
- 80.Spraul C.W., Lang G.E., Ronzani M., Högel J., Lang G.K. Reproducibility of measurements with a new slit lamp-mounted ocular blood flow tonograph. Graefe’s Arch. Clin. Exp. Ophthalmol. 1998;236:274–279. doi: 10.1007/s004170050077. [DOI] [PubMed] [Google Scholar]
- 81.Kontiola A.I. A new induction-based impact method for measuring intraocular pressure. Acta. Ophthalmol. Scand. 2000;78:142–145. doi: 10.1034/j.1600-0420.2000.078002142.x. [DOI] [PubMed] [Google Scholar]
- 82.Brusini P., Salvetat M.L., Zeppieri M., Tosoni C., Parisi L. Comparison of ICare Tonometer with Goldmann Applanation Tonometer in Glaucoma Patients. J. Glaucoma. 2006;15:213–217. doi: 10.1097/01.ijg.0000212208.87523.66. [DOI] [PubMed] [Google Scholar]
- 83.Kageyama M., Hirooka K., Baba T., Shiraga F. Comparison of ICare Rebound Tonometer with Noncontact Tonometer in Healthy Children. J. Glaucoma. 2011;20:63–66. doi: 10.1097/IJG.0b013e3181d12dc4. [DOI] [PubMed] [Google Scholar]
- 84.Salvetat M.L., Zeppieri M., Miani F., Tosoni C., Parisi L., Brusini P. Comparison of iCare tonometer and Goldmann applanation tonometry in normal corneas and in eyes with automated lamellar and penetrating keratoplasty. Eye. 2011;25:642–650. doi: 10.1038/eye.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Realini T., McMillan B., Gross R.L., Devience E., Balasubramani G.K. Assessing the Reliability of Intraocular Pressure Measurements Using Rebound Tonometry. J. Glaucoma. 2021;30:629–633. doi: 10.1097/IJG.0000000000001892. [DOI] [PubMed] [Google Scholar]
- 86.Rosentreter A., Athanasopoulos A., Schild A.M., Lappas A., Cursiefen C., Dietlein T.S. Rebound, Applanation, and Dynamic Contour Tonometry in Pathologic Corneas. Cornea. 2013;32:313–318. doi: 10.1097/ICO.0b013e318254a3fb. [DOI] [PubMed] [Google Scholar]
- 87.Badakere S.V., Chary R., Choudhari N.S., Rao H.L., Garudadri C., Senthil S. Agreement of Intraocular Pressure Measurement of Icare ic200 with Goldmann Applanation Tonometer in Adult Eyes with Normal Cornea. Ophthalmol. Glaucoma. 2021;4:89–94. doi: 10.1016/j.ogla.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 88.Ve R.S., Jose J., Pai H.V., Biswas S., Parimi V., Poojary P., Nagarajan T. Agreement and repeatability of Icare ic100 tonometer. Indian J. Ophthalmol. 2020;68:2122–2125. doi: 10.4103/ijo.IJO_546_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Halkiadakis I., Stratos A., Stergiopoulos G., Patsea E., Skouriotis S., Mitropoulos P., Papaconstantinou D., Georgopoulos G. Evaluation of the Icare-ONE rebound tonometer as a self-measuring intraocular pressure device in normal subjects. Graefe’s Arch. Clin. Exp. Ophthalmol. 2012;250:1207–1211. doi: 10.1007/s00417-011-1875-6. [DOI] [PubMed] [Google Scholar]
- 90.Rosenfeld E., Barequet D., Mimouni M., Fischer N., Kurtz S. Role of home monitoring with iCare ONE rebound tonometer in glaucoma patients management. Int. J. Ophthalmol. 2021;14:405–408. doi: 10.18240/ijo.2021.03.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Cvenkel B., Velkovska M.A., Jordanova V.D. Self-measurement with Icare HOME tonometer, patients’ feasibility and acceptability. Eur. J. Ophthalmol. 2019;30:258–263. doi: 10.1177/1120672118823124. [DOI] [PubMed] [Google Scholar]
- 92.Quérat L., Chen E. Clinical Use of iC are Home® tonometer. Acta. Ophthalmol. 2020;98:e131–e132. doi: 10.1111/aos.14169. [DOI] [PubMed] [Google Scholar]
- 93.Gao F., Liu X., Zhao Q., Pan Y. Comparison of the iCare rebound tonometer and the Goldmann applanation tonometer. Exp. Ther. Med. 2017;13:1912–1916. doi: 10.3892/etm.2017.4164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kiddee W., Tanjana A. Variations of Intraocular Pressure Measured by Goldmann Applanation Tonometer, Tono-Pen, iCare Rebound Tonometer, and Pascal Dynamic Contour Tonometer in Patients With Corneal Edema After Phacoemulsification. J. Glaucoma. 2021;30:317–324. doi: 10.1097/IJG.0000000000001725. [DOI] [PubMed] [Google Scholar]
- 95.Valero B., Fénolland J.-R., Rosenberg R., Sendon D., Mesnard C., Sigaux M., Giraud J.-M., Renard J.-P. Fiabilité et reproductibilité des mesures de la pression intraoculaire par le tonomètre Icare ® Home (modèle TA022) et comparaison avec les mesures au tonomètre à aplanation de Goldmann chez des patients glaucomateux. J. Français D’Ophtalmol. 2017;40:865–875. doi: 10.1016/j.jfo.2017.06.008. [DOI] [PubMed] [Google Scholar]
- 96.Nakakura S., Mori E., Fujio Y., Fujisawa Y., Matsuya K., Kobayashi Y., Tabuchi H., Asaoka R., Kiuchi Y. Comparison of the Intraocular Pressure Measured Using the New Rebound Tonometer Icare ic100 and Icare TA01i or Goldmann Applanation Tonometer. J. Glaucoma. 2019;28:172–177. doi: 10.1097/IJG.0000000000001138. [DOI] [PubMed] [Google Scholar]
- 97.Kato Y., Nakakura S., Matsuo N., Yoshitomi K., Handa M., Tabuchi H., Kiuchi Y. Agreement among Goldmann applanation tonometer, iCare, and Icare PRO rebound tonometers; non-contact tonometer; and Tonopen XL in healthy elderly subjects. Int. Ophthalmol. 2017;38:687–696. doi: 10.1007/s10792-017-0518-2. [DOI] [PubMed] [Google Scholar]
- 98.Scuderi G., Cascone N.C., Regine F., Perdicchi A., Cerulli A., Recupero S.M. Validity and Limits of the Rebound Tonometer (ICare®): Clinical Study. Eur. J. Ophthalmol. 2011;21:251–257. doi: 10.5301/EJO.2010.3712. [DOI] [PubMed] [Google Scholar]
- 99.Nakakura S., Asaoka R., Terao E., Nagata Y., Fukuma Y., Oogi S., Shiraishi M., Kiuchi Y. Evaluation of rebound tonometer iCare IC200 as compared with IcarePRO and Goldmann applanation tonometer in patients with glaucoma. Eye Vis. 2021;8:25. doi: 10.1186/s40662-021-00249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Liu J., De Francesco T., Schlenker M., Ahmed I.I. Icare Home Tonometer: A Review of Characteristics and Clinical Utility. Clin. Ophthalmol. 2020;14:4031–4045. doi: 10.2147/OPTH.S284844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chen M., Zhang L., Xu J., Chen X., Gu Y., Ren Y., Wang K. Comparability of three intraocular pressure measurement: iCare pro rebound, non-contact and Goldmann applanation tonometry in different IOP group. BMC Ophthalmol. 2019;19:225. doi: 10.1186/s12886-019-1236-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ting S.L., Lim L.T., Ooi C.Y., Rahman M. Comparison of Icare Rebound Tonometer and Perkins Applanation Tonometer in Community Eye Screening. Asia-Pac. J. Ophthalmol. 2019;8:229–232. doi: 10.22608/apo.2018433. [DOI] [PubMed] [Google Scholar]
- 103.Subramaniam A.G., Allen P., Toh T. Comparison of the Icare ic100 rebound tonometer and the Goldmann applanation tonometer in 1000 eyes. Ophthalmic Res. 2020;64:321–326. doi: 10.1159/000511455. [DOI] [PubMed] [Google Scholar]
- 104.Muttuvelu D.V., Baggesen K., Ehlers N. Precision and accuracy of the ICare tonometer—Peripheral and central IOP measurements by rebound tonometry. Acta. Ophthalmol. 2010;90:322–326. doi: 10.1111/j.1755-3768.2010.01987.x. [DOI] [PubMed] [Google Scholar]
- 105.Kaufmann C., Bachmann L.M., Thiel M.A. Intraocular Pressure Measurements Using Dynamic Contour Tonometry after Laser In Situ Keratomileusis. Investig. Opthalmol. Vis. Sci. 2003;44:3790–3794. doi: 10.1167/iovs.02-0946. [DOI] [PubMed] [Google Scholar]
- 106.Kanngiesser H.E., Kniestedt C., Robert Y.C.A. Dynamic Contour Tonometry. J. Glaucoma. 2005;14:344–350. doi: 10.1097/01.ijg.0000176936.16015.4e. [DOI] [PubMed] [Google Scholar]
- 107.Kamppeter B.A., Jonas J.B. Dynamic Contour Tonometry for Intraocular Pressure Measurement. Am. J. Ophthalmol. 2005;140:318–320. doi: 10.1016/j.ajo.2005.01.039. [DOI] [PubMed] [Google Scholar]
- 108.Ceruti P., Morbio R., Marraffa M., Marchini G. Comparison of Goldmann applanation tonometry and dynamic contour tonometry in healthy and glaucomatous eyes. Eye. 2008;23:262–269. doi: 10.1038/sj.eye.6703102. [DOI] [PubMed] [Google Scholar]
- 109.Fogagnolo P., Figus M., Frezzotti P., Iester M., Oddone F., Zeppieri M., Ferreras A., Brusini P., Rossetti L., Orzalesi N. Test-retest variability of intraocular pressure and ocular pulse amplitude for dynamic contour tonometry: A multicentre study. Br. J. Ophthalmol. 2009;94:419–423. doi: 10.1136/bjo.2009.165142. [DOI] [PubMed] [Google Scholar]
- 110.Katsimpris J.M., Theoulakis P.E., Vasilopoulos K., Skourtis G., Papadopoulos G.E., Petropoulos I.K. Correlation between Central Corneal Thickness and Intraocular Pressure Measured by Goldmann Applanation Tonometry or Pascal Dynamic Contour Tonometry. Klin. Mon. Für Augenheilkd. 2015;232:414–418. doi: 10.1055/s-0035-1545792. [DOI] [PubMed] [Google Scholar]
- 111.Schwenn O. Ocular pulse amplitude in patients with open angle glaucoma, normal tension glaucoma, and ocular hypertension. Br. J. Ophthalmol. 2002;86:981–984. doi: 10.1136/bjo.86.9.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kniestedt C., Kanngiesser H., Stamper R.L. Assessment of Pascal dynamic contour tonometer in monitoring IOP after LASIK. J. Cataract. Refract. Surg. 2005;31:458–459. doi: 10.1016/j.jcrs.2005.02.021. [DOI] [PubMed] [Google Scholar]
- 113.Siganos D.S., Papastergiou G.I., Moedas C. Assessment of the Pascal dynamic contour tonometer in monitoring intraocular pressure in unoperated eyes and eyes after LASIK. J. Cataract. Refract. Surg. 2004;30:746–751. doi: 10.1016/j.jcrs.2003.12.033. [DOI] [PubMed] [Google Scholar]
- 114.Lee S.Y., Kim E.W., Choi W., Park C.K., Kim S., Bae H.W., Seong G.J., Kim C.Y. Significance of dynamic contour tonometry in evaluation of progression of glaucoma in patients with a history of laser refractive surgery. Br. J. Ophthalmol. 2019;104:276–281. doi: 10.1136/bjophthalmol-2018-313771. [DOI] [PubMed] [Google Scholar]
- 115.Kandarakis A., Soumplis V., Pitsas C., Kandarakis S., Halikias J., Karagiannis D. Comparison of dynamic contour tonometry and Goldmann applanation tonometry following penetrating keratoplasty. Can. J. Ophthalmol. 2010;45:489–493. doi: 10.3129/i10-035. [DOI] [PubMed] [Google Scholar]
- 116.Sales-Sanz M., Arranz-Marquez E., Arruabarrena C., Teus M.A. Influence of LASEK on Schiøtz, Goldmann and dynamic contour Tonometry. Graefe’s Arch. Clin. Exp. Ophthalmol. 2017;256:173–179. doi: 10.1007/s00417-017-3825-4. [DOI] [PubMed] [Google Scholar]
- 117.Wang A.S., Alencar L., Weinreb R.N., Tafreshi A., Deokule S., Vizzeri G., Medeiros F.A. Repeatability and Reproducibility of Goldmann Applanation, Dynamic Contour, and Ocular Response Analyzer Tonometry. J. Glaucoma. 2013;22:127–132. doi: 10.1097/IJG.0b013e3182254ba3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bochmann F., Kaufmann C., Theil M.A. Dynamic contour tonometry versus Goldmann applanation tonometry: Challenging the gold standard. Expert. Rev. Ophthalmol. 2015;5:743–749. doi: 10.1586/eop.10.68. [DOI] [Google Scholar]
- 119.Okafor K.C., Brandt J.D. Measuring intraocular pressure. Curr. Opin. Ophthalmol. 2015;26:103–109. doi: 10.1097/ICU.0000000000000129. [DOI] [PubMed] [Google Scholar]
- 120.Eklund A., Hallberg P., Linden C., Lindahl O.A. An Applanation Resonator Sensor for Measuring Intraocular Pressure Using Combined Continuous Force and Area Measurement. Investig. Opthalmol. Vis. Sci. 2003;44:3017–3024. doi: 10.1167/iovs.02-1116. [DOI] [PubMed] [Google Scholar]
- 121.Jóhannesson G., Hallberg P., Eklund A., Lindén C. Introduction and clinical evaluation of servo-controlled applanation resonance tonometry. Acta. Ophthalmol. 2011;90:677–682. doi: 10.1111/j.1755-3768.2011.02111.x. [DOI] [PubMed] [Google Scholar]
- 122.Hallberg P., Eklund A., Bäcklund T., Lindén C. Clinical Evaluation of Applanation Resonance Tonometry. J. Glaucoma. 2007;16:88–93. doi: 10.1097/01.ijg.0000243468.28590.8e. [DOI] [PubMed] [Google Scholar]
- 123.Salvetat M.L., Zeppieri M., Tosoni C., Brusini P. Repeatability and accuracy of applanation resonance tonometry in healthy subjects and patients with glaucoma. Acta. Ophthalmol. 2013;92:e66–e73. doi: 10.1111/aos.12209. [DOI] [PubMed] [Google Scholar]
- 124.Ottobelli L., Fogagnolo P., Frezzotti P., De Cillà S., Vallenzasca E., Digiuni M., Paderni R., Motolese I., Bagaglia S.A., Motolese E., et al. Repeatability and reproducibility of applanation resonance tonometry: A cross-sectional study. BMC Ophthalmol. 2015;15:36. doi: 10.1186/s12886-015-0028-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mulak M., Czak W.A., Mimier M., Kaczmarek R. A comparison of intraocular pressure values obtained using a Goldmann applanation tonometer and a handheld version of applanation resonance tonometer: A preliminary report. Adv. Clin. Exp. Med. 2018;27:481–485. doi: 10.17219/acem/68559. [DOI] [PubMed] [Google Scholar]
- 126.Jóhannesson G., Hallberg P., Eklund A., Koskela T., Lindén C. Change in Intraocular Pressure Measurement After Myopic LASEK. J. Glaucoma. 2012;21:255–259. doi: 10.1097/IJG.0b013e31820719c8. [DOI] [PubMed] [Google Scholar]
- 127.Liu J.H., Kripke D.F., Hoffman R.E., Twa M., Loving R.T., Rex K.M., Gupta N., Weinreb R.N. Nocturnal elevation of intraocular pressure in young adults. Investig. Ophthalmol. Vis. Sci. 1998;39:2707–2712. [PubMed] [Google Scholar]
- 128.Konstas A.G.P., Mantziris D.A., Stewart W.C. Diurnal Intraocular Pressure in Untreated Exfoliation and Primary Open-angle Glaucoma. Arch. Ophthalmol. 1997;115:182–185. doi: 10.1001/archopht.1997.01100150184006. [DOI] [PubMed] [Google Scholar]
- 129.Asrani S., Zeimer R., Wilensky J., Gieser D., Vitale S., Lindenmuth K. Large Diurnal Fluctuations in Intraocular Pressure Are an Independent Risk Factor in Patients With Glaucoma. J. Glaucoma. 2000;9:134–142. doi: 10.1097/00061198-200004000-00002. [DOI] [PubMed] [Google Scholar]
- 130.Tan S., Baig N., Hansapinyo L., Jhanji V., Wei S., Tham C.Y.C. Comparison of self-measured diurnal intraocular pressure profiles using rebound tonometry between primary angle closure glaucoma and primary open angle glaucoma patients. PLoS ONE. 2017;12:e0173905. doi: 10.1371/journal.pone.0173905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Barkana Y., Anis S., Liebmann J., Tello C., Ritch R. Clinical Utility of Intraocular Pressure Monitoring Outside of Normal Office Hours in Patients With Glaucoma. Arch. Ophthalmol. 2006;124:793–797. doi: 10.1001/archopht.124.6.793. [DOI] [PubMed] [Google Scholar]
- 132.Hughes E., Spry P., Diamond J. 24-Hour Monitoring of Intraocular Pressure in Glaucoma Management: A Retrospective Review. J. Glaucoma. 2003;12:232–236. doi: 10.1097/00061198-200306000-00009. [DOI] [PubMed] [Google Scholar]
- 133.Konstas A.G.P., Quaranta L., Bozkurt B., Katsanos A., Feijoo J.G., Rossetti L., Shaarawy T., Pfeiffer N., Miglior S. 24-h Efficacy of Glaucoma Treatment Options. Adv. Ther. 2016;33:481–517. doi: 10.1007/s12325-016-0302-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ho C.H., Wong J.K.W. Role of 24-Hour Intraocular Pressure Monitoring in Glaucoma Management. J. Ophthalmol. 2019;2019:3632197–3632213. doi: 10.1155/2019/3632197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mcmonnies C.W. The importance of and potential for continuous monitoring of intraocular pressure. Clin. Exp. Optom. 2017;100:203–207. doi: 10.1111/cxo.12497. [DOI] [PubMed] [Google Scholar]
- 136.Ittoop S.M., SooHoo J.R., Seibold L.K., Mansouri K., Kahook M.Y. Systematic Review of Current Devices for 24-h Intraocular Pressure Monitoring. Adv. Ther. 2016;33:1679–1690. doi: 10.1007/s12325-016-0388-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Molaei A., Karamzadeh V., Safi S., Esfandiari H., Dargahi J., Khosravi M. Upcoming methods and specifications of continuous intraocular pressure monitoring systems for glaucoma. J. Ophthalmic Vis. Res. 2018;13:66–71. doi: 10.4103/jovr.jovr_208_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dick H.B., Schultz T., Gerste R.D. Miniaturization in Glaucoma Monitoring and Treatment: A Review of New Technologies That Require a Minimal Surgical Approach. Ophthalmol. Ther. 2019;8:19–30. doi: 10.1007/s40123-019-0161-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee J.O., Park H., Du J., Balakrishna A., Chen O., Sretavan D., Choo H. A microscale optical implant for continuous in vivo monitoring of intraocular pressure. Microsyst. Nanoeng. 2017;3:17057. doi: 10.1038/micronano.2017.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Koutsonas A., Walter P., Roessler G., Plange N. Implantation of a Novel Telemetric Intraocular Pressure Sensor in Patients with Glaucoma (ARGOS Study): 1-Year Results. Investig. Opthalmol. Vis. Sci. 2015;56:1063–1069. doi: 10.1167/iovs.14-14925. [DOI] [PubMed] [Google Scholar]
- 141.Choritz L., Mansouri K., Bosch J.V.D., Weigel M., Dick H.B., Wagner M., Thieme H., Rüfer F., Szurmann P., Wehner W., et al. Telemetric Measurement of Intraocular Pressure via an Implantable Pressure Sensor—12-Month Results from the ARGOS-02 Trial. Am. J. Ophthalmol. 2020;209:187–196. doi: 10.1016/j.ajo.2019.09.011. [DOI] [PubMed] [Google Scholar]
- 142.Chen P.-J., Rodger D., Humayun M.S., Tai Y.-C. Unpowered spiral-tube parylene pressure sensor for intraocular pressure sensing. Sens. Actuators A Phys. 2006;127:276–282. doi: 10.1016/j.sna.2005.08.027. [DOI] [Google Scholar]
- 143.Demeng L., Niansong M., Zhaofeng Z. An ultralow power wireless intraocular pressure monitoring system. J. Semicond. 2014;35:105014. [Google Scholar]
- 144.Mariacher S., Ebner M., Januschowski K., Hurst J., Schnichels S., Szurman P. Investigation of a novel implantable suprachoroidal pressure transducer for telemetric intraocular pressure monitoring. Exp. Eye Res. 2016;151:54–60. doi: 10.1016/j.exer.2016.07.016. [DOI] [PubMed] [Google Scholar]
- 145.Leonardi M., Leuenberger P., Bertrand D., Bertsch A., Renaud P. First Steps toward Noninvasive Intraocular Pressure Monitoring with a Sensing Contact Lens. Investig. Opthalmol. Vis. Sci. 2004;45:3113–3117. doi: 10.1167/iovs.04-0015. [DOI] [PubMed] [Google Scholar]
- 146.Hediger A., Kniestedt C., Zweifel S., Knecht P., Funk J., Kanngiesser H. Kontinuierliche Augeninnendruckmessung. Der. Ophthalmol. 2009;106:1111–1115. doi: 10.1007/s00347-009-1919-z. [DOI] [PubMed] [Google Scholar]
- 147.Twa M., Roberts C.J., Karol H.J., Mahmoud A.M., Weber P.A., Small R.H. Evaluation of a Contact Lens-Embedded Sensor for Intraocular Pressure Measurement. J. Glaucoma. 2010;19:382–390. doi: 10.1097/IJG.0b013e3181c4ac3d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Mansouri K., Shaarawy T. Continuous intraocular pressure monitoring with a wireless ocular telemetry sensor: Initial clinical experience in patients with open angle glaucoma. Br. J. Ophthalmol. 2011;95:627–629. doi: 10.1136/bjo.2010.192922. [DOI] [PubMed] [Google Scholar]
- 149.Mansouri K., Weinreb R.N., Liu J.H.K. Efficacy of a Contact Lens Sensor for Monitoring 24-H Intraocular Pressure Related Patterns. PLoS ONE. 2015;10:e0125530. doi: 10.1371/journal.pone.0125530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Mansouri K., Medeiros F.A., Tafreshi A., Weinreb R.N. Error in PubMed in: Global Burden of Visual Impairment and Blindness. Arch. Ophthalmol. 2012;130:1559. doi: 10.1001/jamaophthalmol.2013.1350. [DOI] [PubMed] [Google Scholar]
- 151.Lorenz K., Korb C., Herzog N., Vetter J.M., Elflein H., Keilani M.M., Pfeiffer N. Tolerability of 24-Hour Intraocular Pressure Monitoring of a Pressure-sensitive Contact Lens. J. Glaucoma. 2013;22:311–316. doi: 10.1097/IJG.0b013e318241b874. [DOI] [PubMed] [Google Scholar]
- 152.Dunbar G.E., Shen B.Y., Aref A.A. The Sensimed Triggerfish contact lens sensor: Efficacy, safety, and patient perspectives. Clin. Ophthalmol. 2017;11:875–882. doi: 10.2147/OPTH.S109708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mottet B., Aptel F., Romanet J.-P., Hubanova R., Pépin J.-L., Chiquet C. 24-Hour Intraocular Pressure Rhythm in Young Healthy Subjects Evaluated With Continuous Monitoring Using a Contact Lens Sensor. JAMA Ophthalmol. 2013;131:1507–1516. doi: 10.1001/jamaophthalmol.2013.5297. [DOI] [PubMed] [Google Scholar]
- 154.Holló G., Kóthy P., Vargha P. Evaluation of Continuous 24-Hour Intraocular Pressure Monitoring for Assessment of Prostaglandin-induced Pressure Reduction in Glaucoma. J. Glaucoma. 2014;23:e6–e12. doi: 10.1097/IJG.0b013e31829e5635. [DOI] [PubMed] [Google Scholar]
- 155.Mansouri K. The Road Ahead to Continuous 24-Hour Intraocular Pressure Monitoring in Glaucoma. J. Ophthalmic Vis. Res. 2014;9:260–268. [PMC free article] [PubMed] [Google Scholar]
- 156.Tojo N., Hayashi A., Otsuka M. Correlation between 24-h continuous intraocular pressure measurement with a contact lens sensor and visual field progression. Graefe’s Arch. Clin. Exp. Ophthalmol. 2020;258:175–182. doi: 10.1007/s00417-019-04487-9. [DOI] [PubMed] [Google Scholar]
- 157.Tojo N., Hayashi A., Otsuka M., Miyakoshi A. Fluctuations of the Intraocular Pressure in Pseudoexfoliation Syndrome and Normal Eyes Measured by a Contact Lens Sensor. J. Glaucoma. 2016;25:e463–e468. doi: 10.1097/IJG.0000000000000292. [DOI] [PubMed] [Google Scholar]
- 158.Pajic B., Pajic-Eggspuchler B., Haefliger I. Continuous IOP Fluctuation Recording in Normal Tension Glaucoma Patients. Curr. Eye Res. 2011;36:1129–1138. doi: 10.3109/02713683.2011.608240. [DOI] [PubMed] [Google Scholar]
- 159.Agnifili L., Mastropasqua R., Frezzotti P., Fasanella V., Motolese I., Pedrotti E., Di Iorio A., Mattei P.A., Motolese E., Mastropasqua L. Circadian intraocular pressure patterns in healthy subjects, primary open angle and normal tension glaucoma patients with a contact lens sensor. Acta. Ophthalmol. 2014;93:e14–e21. doi: 10.1111/aos.12408. [DOI] [PubMed] [Google Scholar]
- 160.Tojo N., Abe S., Ishida M., Yagou T., Hayashi A. The Fluctuation of Intraocular Pressure Measured by a Contact Lens Sensor in Normal-Tension Glaucoma Patients and Nonglaucoma Subjects. J. Glaucoma. 2017;26:195–200. doi: 10.1097/IJG.0000000000000517. [DOI] [PubMed] [Google Scholar]
- 161.Kim Y.W., Kim J.-S., Lee S.Y., Ha A., Lee J., Park Y.J., Kim Y.K., Jeoung J.W., Park K.H. Twenty-four–Hour Intraocular Pressure–Related Patterns from Contact Lens Sensors in Normal-Tension Glaucoma and Healthy Eyes. Ophthalmology. 2020;127:1487–1497. doi: 10.1016/j.ophtha.2020.05.010. [DOI] [PubMed] [Google Scholar]
- 162.Kim Y.W., Kim M.J., Park K.H., Jeoung J.W., Kim S.H., Jang C.I., Lee S.H., Kim J.H., Lee S., Kang J.Y. Preliminary study on implantable inductive-type sensor for continuous monitoring of intraocular pressure. Clin. Exp. Ophthalmol. 2015;43:830–837. doi: 10.1111/ceo.12573. [DOI] [PubMed] [Google Scholar]
- 163.Mansouri K., Rao H.L., Weinreb R.N. Short-Term and Long-Term Variability of Intraocular Pressure Measured with an Intraocular Telemetry Sensor in Patients with Glaucoma. Ophthalmology. 2021;128:227–233. doi: 10.1016/j.ophtha.2020.07.016. [DOI] [PubMed] [Google Scholar]
- 164.Xu S.C., Gauthier A.C., Liu J. The Application of a Contact Lens Sensor in Detecting 24-Hour Intraocular Pressure-Related Patterns. J. Ophthalmol. 2016;2016:4727423. doi: 10.1155/2016/4727423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Kouhani M.H.M., Wu J., Tavakoli A., Weber A.J., Li W. Wireless, passive strain sensor in a doughnut-shaped contact lens for continuous non-invasive self-monitoring of intraocular pressure. Lab. Chip. 2020;20:332–342. doi: 10.1039/C9LC00735K. [DOI] [PubMed] [Google Scholar]
- 166.Xu J., Cui T., Hirtz T., Qiao Y., Li X., Zhong F., Han X., Yang Y., Zhang S., Ren T.-L. Highly Transparent and Sensitive Graphene Sensors for Continuous and Non-invasive Intraocular Pressure Monitoring. ACS Appl. Mater. Interfaces. 2020;12:18375–18384. doi: 10.1021/acsami.0c02991. [DOI] [PubMed] [Google Scholar]
- 167.Maeng B., Chang H.-K., Park J. Photonic crystal-based smart contact lens for continuous intraocular pressure monitoring. Lab. Chip. 2020;20:1740–1750. doi: 10.1039/C9LC01268K. [DOI] [PubMed] [Google Scholar]
- 168.Wasilewicz R., Varidel T., Simon-Zoula S., Schlund M., Cerboni S., Mansouri K. First-in-human continuous 24-hour measurement of intraocular pressure and ocular pulsation using a novel contact lens sensor. Br. J. Ophthalmol. 2020;104:1519–1523. doi: 10.1136/bjophthalmol-2019-315276. [DOI] [PubMed] [Google Scholar]
- 169.Agaoglu S., Diep P., Martini M., Kt S., Baday M., Araci I.E. Ultra-sensitive microfluidic wearable strain sensor for intraocular pressure monitoring. Lab. Chip. 2018;18:3471–3483. doi: 10.1039/C8LC00758F. [DOI] [PubMed] [Google Scholar]
- 170.Campigotto A., Leahy S., Zhao G., Campbell R.J., Lai Y. Non-invasive Intraocular pressure monitoring with contact lens. Br. J. Ophthalmol. 2019;104:1324–1328. doi: 10.1136/bjophthalmol-2018-313714. [DOI] [PubMed] [Google Scholar]
- 171.Fan Y., Tu H., Zhao H., Wei F., Yang Y., Ren T. A wearable contact lens sensor for noninvasive in-situ monitoring of intraocular pressure. Nanotechnology. 2021;32:095106. doi: 10.1088/1361-6528/abca5f. [DOI] [PubMed] [Google Scholar]
- 172.Dou Z., Tang J., Liu Z., Sun Q., Wang Y., Li Y., Yuan M., Wu H., Wang Y., Pei W., et al. Wearable Contact Lens Sensor for Non-invasive Continuous Monitoring of Intraocular Pressure. Micromachines. 2021;12:108. doi: 10.3390/mi12020108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gillmann K., Wasilewicz R., Hoskens K., Simon-Zoula S., Mansouri K. Continuous 24-hour measurement of intraocular pressure in millimeters of mercury (mmHg) using a novel contact lens sensor: Comparison with pneumatonometry. PLoS ONE. 2021;16:e0248211. doi: 10.1371/journal.pone.0248211. [DOI] [PMC free article] [PubMed] [Google Scholar]