Supplemental Digital Content is available in the text.
Keywords: acute coronary syndrome, anti-arrhythmia agents, atrial fibrillation, atrial fibrillation ablation, controlled clinical trial, death, heart failure, stroke
Background:
Even on optimal therapy, many patients with heart failure and atrial fibrillation experience cardiovascular complications. Additional treatments are needed to reduce these events, especially in patients with heart failure and preserved left ventricular ejection fraction.
Methods:
This prespecified subanalysis of the randomized EAST-AFNET4 trial (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) assessed the effect of systematic, early rhythm control therapy (ERC; using antiarrhythmic drugs or catheter ablation) compared with usual care (allowing rhythm control therapy to improve symptoms) on the 2 primary outcomes of the trial and on selected secondary outcomes in patients with heart failure, defined as heart failure symptoms New York Heart Association II to III or left ventricular ejection fraction [LVEF] <50%.
Results:
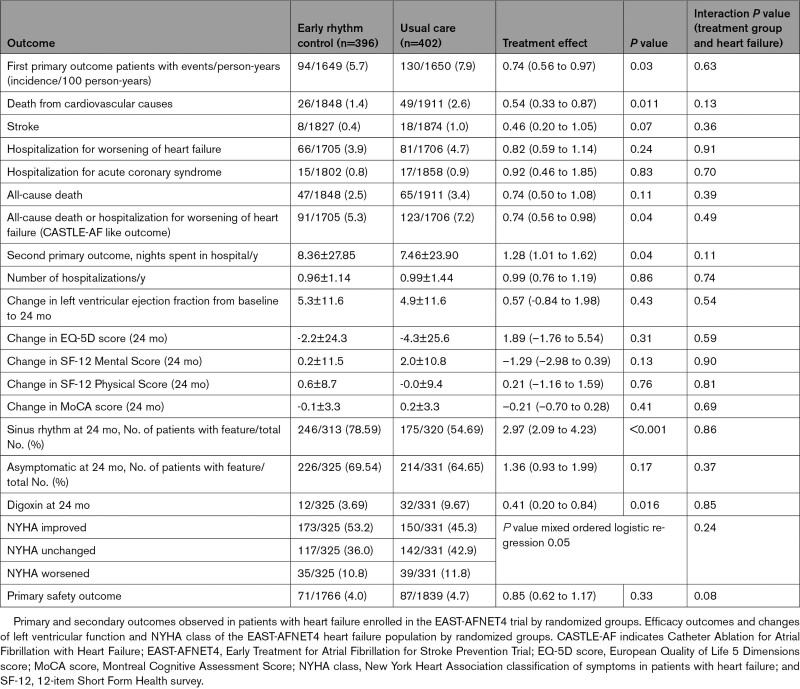
This analysis included 798 patients (300 [37.6%] female, median age 71.0 [64.0, 76.0] years, 785 with known LVEF). The majority of patients (n=442) had heart failure and preserved LVEF (LVEF≥50%; mean LVEF 61±6.3%), the others had heart failure with midrange ejection fraction (n=211; LVEF 40%–49%; mean LVEF 44 ± 2.9%) or heart failure with reduced ejection fraction (n=132; LVEF<40%; mean LVEF 31±5.5%). Over the 5.1-year median follow-up, the composite primary outcome of cardiovascular death, stroke, or hospitalization for worsening of heart failure or for acute coronary syndrome occurred less often in patients randomly assigned to ERC (94/396; 5.7 per 100 patient-years) compared with patients randomly assigned to usual care (130/402; 7.9 per 100 patient-years; hazard ratio, 0.74 [0.56–0.97]; P=0.03), not altered by heart failure status (interaction P value=0.63). The primary safety outcome (death, stroke, or serious adverse events related to rhythm control therapy) occurred in 71 of 396 (17.9%) patients with heart failure randomly assigned to ERC and in 87 of 402 (21.6%) patients with heart failure randomly assigned to usual care (hazard ratio, 0.85 [0.62–1.17]; P=0.33). LVEF improved in both groups (LVEF change at 2 years: ERC 5.3±11.6%, usual care 4.9±11.6%, P=0.43). ERC also improved the composite outcome of death or hospitalization for worsening of heart failure.
Conclusions:
Rhythm control therapy conveys clinical benefit when initiated within 1 year of diagnosing atrial fibrillation in patients with signs or symptoms of heart failure.
Registration:
URL: https://www.clinicaltrials.gov; Unique identifier: NCT01288352. URL: http://www.controlled-trials.com; Unique identifier: ISRCTN04708680. URL: https://www.clinicaltrialsregister.eu; Unique identifier: 2010-021258-20.
Clinical Perspective.
What Is New?
This prespecified subanalysis of the randomized EAST-AFNET4 trial (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) demonstrates that systematic, early rhythm control therapy using antiarrhythmic drugs and atrial fibrillation ablation is safe and reduces cardiovascular outcomes in patients with atrial fibrillation and heart failure compared with the current strategy of delayed, symptom-directed rhythm control.
The clinical benefit of early rhythm control therapy was observed in patients with preserved, midrange, and reduced left ventricular ejection fraction.
Early rhythm control therapy was delivered using a combination of antiarrhythmic drugs and atrial fibrillation ablation within guideline recommendations.
Left ventricular function, symptoms, and quality of life improved equally in both treatment strategies.
What Are the Clinical Implications?
Our study supports a treatment strategy of systematic rhythm control therapy (with antiarrhythmic drugs or atrial fibrillation ablation) within a year of diagnosing atrial fibrillation in patients with signs or symptoms of heart failure to reduce cardiovascular outcomes.
Atrial fibrillation and heart failure are 2 associated, common cardiovascular diseases.1 Approximately 30% of patients with atrial fibrillation also have heart failure.2–4 The sequence of presentation varies, but patients with both conditions are at particular risk of cardiovascular complications,5,6 including all-cause and cardiovascular death,5,7 stroke, and worsening of heart failure8 across the spectrum of left ventricular functions.7,8 Several smaller studies evaluated whether rhythm control therapy using atrial fibrillation ablation can improve outcomes in patients with atrial fibrillation and heart failure with severely reduced ejection fraction, providing homogeneous data demonstrating improved left ventricular function9,10 and a signal for better outcomes.11 These findings led to an increased use of rhythm control therapy, often atrial fibrillation ablation, in patients with heart failure and reduced ejection fraction.12,13 Whereas the majority of these trials used catheter ablation to deliver rhythm control therapy, the EAST-AFNET4 trial (Early Treatment for Atrial Fibrillation for Stroke Prevention Trial) recently demonstrated a clinical benefit of early rhythm control (ERC) therapy by using a combination of antiarrhythmic drugs and atrial fibrillation ablation.14 It is less clear whether rhythm control therapy conveys clinical benefit in patients with moderately reduced or preserved left ventricular ejection fraction (LVEF).15 Whether the clinical benefit of the EAST-AFNET4 trial can be transferred to patients with stable heart failure, especially patients with heart failure (HF) with preserved ejection fraction (HFpEF), and whether the beneficial effects found using atrial fibrillation ablation in patients with reduced ejection fraction can be replicated by ERC using either antiarrhythmic drugs or atrial fibrillation ablation, is not known.
Methods
EAST-AFNET4 was conducted as an international, investigator-initiated, parallel-group, randomized, open, blinded outcome-assessment trial.16 Access to the data will be made available on request. Please contact info@kompetenznetz-vorhofflimmern.de. The EAST-AFNET4 trial protocol was approved by ethical review boards and competent authorities for all institutions including approval for the analyses outlined in its statistical analysis plan. All participants gave informed consent.
Trial Population and Trial Intervention
The EAST-AFNET4 trial enrolled adults with early atrial fibrillation, defined as atrial fibrillation diagnosed ≤12 months before enrollment. For inclusion, patients were required to be either older than 75 years of age, to have had a previous transient ischemic attack or stroke, or to meet 2 of the following criteria: age >65 years, female sex, HF, hypertension, diabetes mellitus, severe coronary artery disease, chronic kidney disease (Modification of Diet in Renal Disease stage 3 or 4 [glomerular filtration rate 15–59 mL/1.73 m2 of body surface area]16), and left ventricular hypertrophy (diastolic septal wall width >15 mm). Overall, 2789 patients were randomly assigned in a 1:1 fashion to be treated by ERC (n=1395) or usual care (n=1394).14 In the ERC group, antiarrhythmic drug therapy, atrial fibrillation ablation, or cardioversion were required to be initiated early after randomization.
In patients randomly assigned to usual care, the initial treatment consisted of rate control therapy without rhythm control therapy. Rhythm control was used only in the context of symptom-restricted rhythm control therapy, that is, to treat uncontrolled atrial fibrillation–related symptoms despite adequate rate control.14
HF Subgroup Analysis and Outcomes
For this prespecified subgroup analysis, all patients with signs or symptoms of HF at enrollment into the EAST-AFNET4 trial14,16 were analyzed. HF and asymptomatic left ventricular dysfunction were defined as symptoms according to New York Heart Association (NYHA) class II to III or LVEF <50%. Patients were stratified according to baseline LVEF into patients with reduced ejection fraction (HFrEF, LVEF <40%), moderately reduced ejection fraction (HFmrEF, LVEF 40%–49%), and preserved ejection fraction (HFpEF, LVEF ≥50%). The effects of ERC and usual care between randomized groups (intention-to-treat analysis) were compared in patients with HF as a whole and categorized by left ventricular function. Effects on the first primary outcome (composite of death from cardiovascular causes, stroke, or hospitalization with worsening of HF or acute coronary syndrome), the second primary outcome (number of nights spent in the hospital per year), and key secondary outcomes (heart rhythm, LVEF, quality of life, atrial fibrillation–related symptoms, and cognitive function) of the EAST-AFNET4 trial14 were analyzed. Furthermore, the primary safety outcome, a composite of death from any cause, stroke, or prespecified serious adverse events was evaluated.
In addition, a CASTLE-AF–like outcome of death or hospitalization for worsening of HF according to the primary outcome of the CASTLE-AF trial (Catheter Ablation for Atrial Fibrillation with Heart Failure)11 and a CABANA-like composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest according to the outcome of the CABANA trial (Catheter Ablation Versus Antiarrhythmic Drug Therapy for Atrial Fibrillation),17 were analyzed. Both were calculated by using the correlating defined outcomes of the EAST-AFNET4 trial as assessed by the EAST-AFNET4 end point review committee.14
Statistical Analyses
Baseline characteristics of patients are summarized with descriptive statistical methods. Continuous variables are described by mean and standard deviation or median, 1st and 3rd quantile. Categorical data are summarized as absolute and relative frequencies.
The first primary and second primary outcomes of the overall EAST-AFNET4 trial were prespecified for this analysis. For the analysis of the first primary outcome, a Cox proportional hazards model with a frailty, that is, gamma-distributed random effect, for the cluster center was applied. This model was also used for the analysis of further time-to-event outcomes, that is, time to cardiovascular death, time to first stroke, time to first hospitalization for worsening HF, time to first hospitalization for acute coronary syndrome, time to all-cause death, time to the primary safety outcome, a composite of all-cause death and hospitalization for worsening HF, and a composite of all-cause death, major bleeding, or ischemic stroke with a Rankin score ≥2. The Aalen-Johansen estimator for estimating cumulative incidences was used to account for the competing event all-cause death within the primary outcome analysis. Kaplan-Meier–based cumulative incidences were used if all-cause death was a component of the outcome.
The second primary outcome was calculated as the observed sum of nights in the hospital divided by the individual follow-up time (in days; in the case of a follow-up time of 0 days, 0.01 days of follow-up was assumed) and was analyzed by using a negative binomial mixed model. This model was also used for the analyses of number of hospitalizations.
Baseline-adjusted mixed linear models were used for continuous secondary outcomes, that is, LVEF change (baseline to 24 months), change in European Quality of Life 5 Dimensions score, change in 12-item Short Form Survey (Mental and Physical Score), and change in Montreal Cognitive Assessment score. A random intercept for center was assumed and restricted maximum likelihood method was used.
Sinus rhythm and symptoms at 24 months were analyzed by using logistic mixed models. Ordered logistic mixed models were used to analyze improvement in European Heart Rhythm Association score and NYHA class from baseline to 24 months.
To analyze whether catheter ablation had an impact on time-to-event outcomes, a time-varying covariate was used for catheter ablation, that is, the group changed if catheter ablation was observed before the first event within the primary outcome. The same holds for other outcomes, where only the ablation is counted that was observed before the outcome.
Safety outcomes were analyzed through the χ2 test. Multivariable regression was applied to gain adjusted effects. An interaction term between treatment group and HF was considered in the models.
Subgroup analysis was conducted in the same manner as for the first primary outcome but also included a corresponding interaction term between treatment group and subgroup of interest. Analyses for multivariable models and secondary outcomes within linear or (ordered) logistic models are based on multiple imputed baseline data with 60 imputations to replace missing values for continuous outcomes and covariates defined for adjustment (see more details in the main article14 and its data supplement). All effects, that is, mean differences or ratios, are given with corresponding 95% confidence intervals. Because of the explorative design of the study, no adjustment for multiple testing was conducted, that is, P values are descriptive. Statistic software R Version 4.0.3. was used.
Results
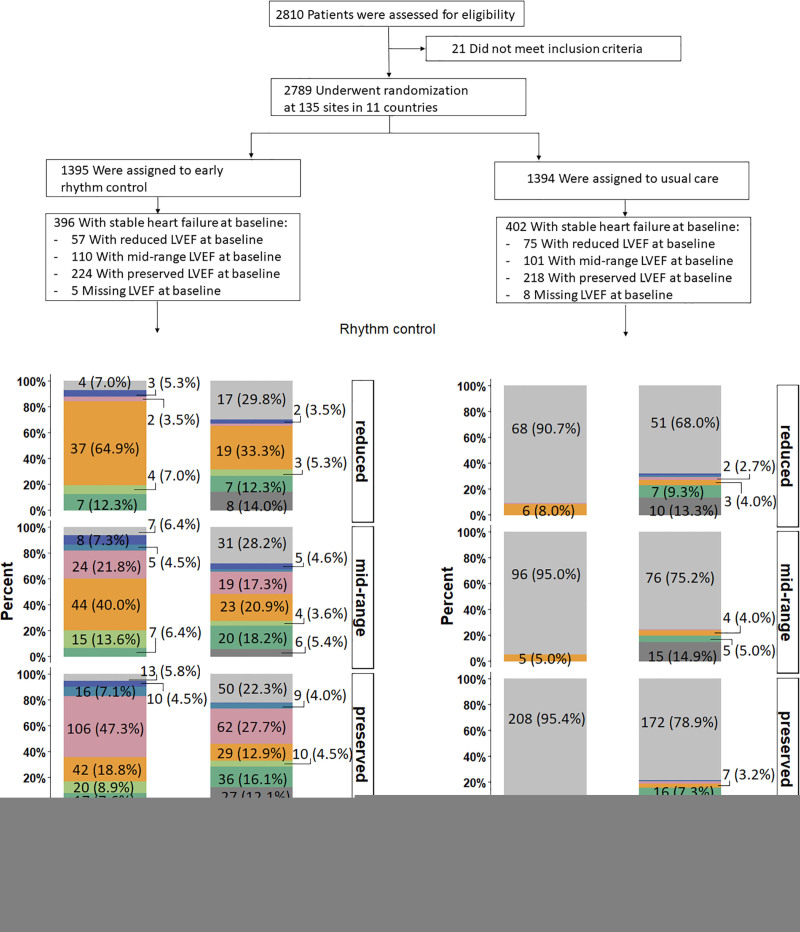
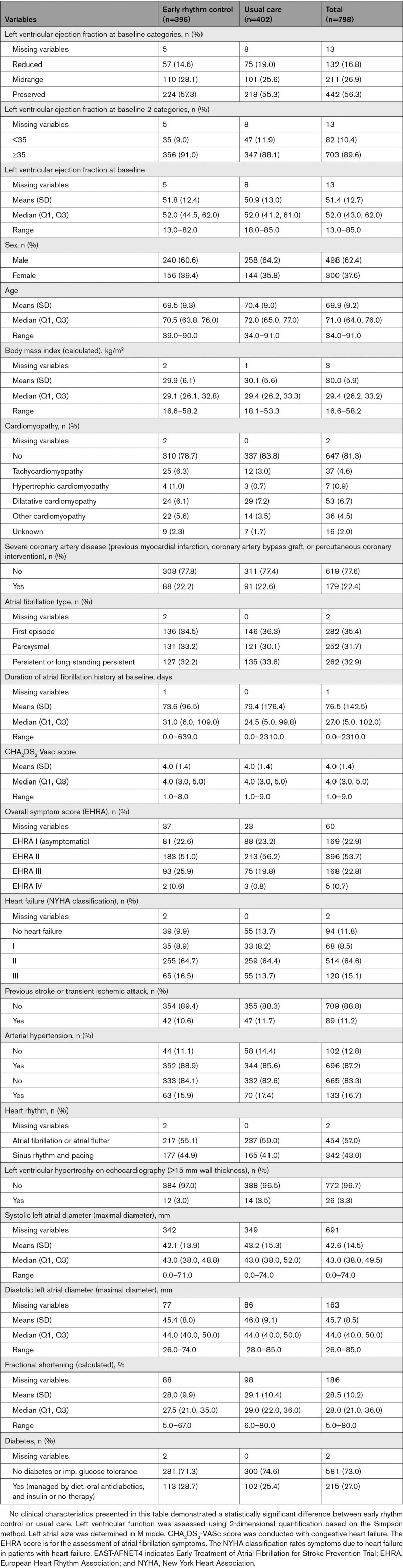
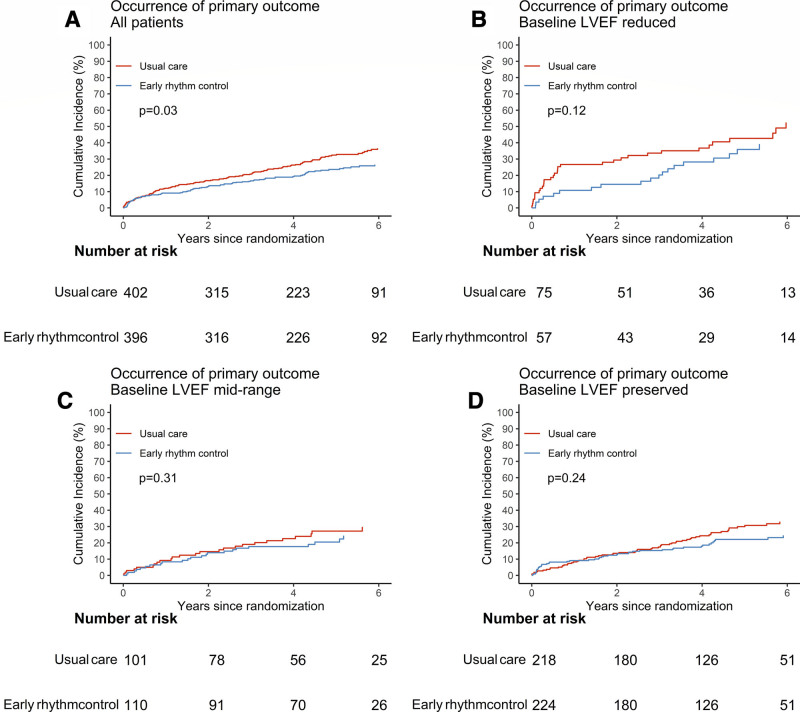
EAST-AFNET4 randomly assigned 798 patients with stable HF, including 442 (56.3%) patients with HFpEF, 211 (26.9%) patients with HFmrEF, and 132 (16.8%) patients with HFrEF. Baseline LVEF was missing in 13 patients and imputed for the analysis (Figure 1). Patient characteristics were not different between randomized groups (Table 1). Patient characteristics as per LVEF subgroup are listed in Table 1 and Table I in the Data Supplement. Follow-up was available in all patients. The primary outcome occurred in 94 of 396 patients randomly assigned to ERC and in 130 of 402 patients randomly assigned to usual care (univariable hazard ratio [HR], 0.74 [95% CI, 0.56–0.97]; P=0.03; Table 2 and Figure 2): The effect was not different from the treatment effect in patients with normal left ventricular function and without signs of HF (HR, 0.81 [0.66–1.01]; P=0.06; interaction P (between treatment and HF)=0.63). Patients with and without ischemic cardiomyopathy had a similar risk for the first primary and second primary outcomes; also, changes in left ventricular function occurred with comparable incidence for both groups (Table II in the Data Supplement). Patients with preserved LVEF had a lower risk for the first primary outcome than patients with reduced LVEF (reduced versus preserved HR, 1.76 [1.19–2.59]). Patients with preserved and midrange LVEF had a similar risk for the first primary outcome (midrange versus preserved HR, 1.01 [0.68–1.50]). Total nights spent in the hospital were higher in patients randomly assigned to ERC compared with usual care (8.36±27.85 versus 7.46±23.9, univariable treatment effect, 1.28 [1.01–1.62]; P=0.04; Table 2; Figure I in the Data Supplement; for non-HF: 1.00 [0.86–1.17]; P=0.96; interaction P=0.11). Secondary outcomes were observed as depicted in Table 2. Subgroup analysis is shown in Figure II in the Data Supplement.
Figure 1.
Consort flow chart of the EAST-AFNET4 heart failure subanalysis. A total of 798 patients with heart failure were included in this analysis; 396 were randomly assigned to early rhythm control, and 402 were randomly assigned to usual care. During follow-up, in the early rhythm control group 201 of 2049 total follow-up years were lost (147 follow-up years lost because 31 patients withdrew; 54 follow-up years lost because 36 patients were lost to follow-up) and 159 of 2070 total follow-up years were lost in the usual care group (108 follow-up years lost because 26 patients withdrew; 51 follow-up years lost because 33 patients were lost to follow-up). Screening and randomization are replicated from the main article.14 AF indicates atrial fibrillation; EAST-AFNET4, Early Treatment of Atrial Fibrillation for Stroke Prevention Trial; fu, follow-up; and LVEF, left ventricular ejection fraction.
Table 1.
Clinical Characteristics of the EAST-AFNET4 Patients With Heart Failure at Baseline by Randomized Groups
Table 2.
Outcomes of Early Rhythm Control and Usual Care in Patients With Heart Failure
Figure 2.
Primary outcome in EAST-AFNET4 patients with heart failure by randomized groups. Aalen-Johansen cumulative-incidence curves for the effects of early rhythm control on the primary outcome. Primary outcome is defined as a composite of death from cardiovascular causes, stroke, or hospitalization with worsening of heart failure or acute coronary syndrome. A, All patients with heart failure. B, Heart failure with reduced ejection fraction. C, Heart failure with midrange ejection fraction. D, Heart failure with preserved ejection fraction. EAST-AFNET4 indicates Early Treatment of Atrial Fibrillation for Stroke Prevention Trial; and LVEF, left ventricular ejection fraction.
Outcomes Based on the CASTLE-AF and CABANA Trials
ERC also improved a combined outcome of death or hospitalization for worsening of HF. Ninety-one of 396 patients randomly assigned to ERC experienced a combined outcome of death or hospitalization for worsening of HF compared with 123 of 402 patients with events in those randomly assigned to usual care (P=0.04; Figure III in the Data Supplement; treatment group-HF interaction P=0.49). The composite outcome of death, disabling stroke, serious bleeding, or cardiac arrest was numerically lower in patients randomly assigned to ERC (51/396 patients with event) than in those randomly assigned to usual care (71/402 patients with event), without significant intergroup difference (P=0.10; Table III and Figure IV in the Data Supplement; treatment group-HF interaction P=0.32).
Rhythm Control Therapy
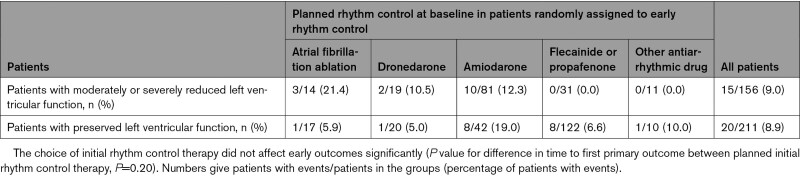
Rhythm control therapy was initiated in most patients (367/391, 93.9%; Figure 1) randomly assigned to ERC and was prescribed only in a minority of patients in the usual care arm (23/394, 5.8%; Figure 1) at randomization. The difference remained substantial after 2 years (ERC 252/351, 71.8%; usual care 69/352, 19.6%; Figure 1). Most patients randomly assigned to ERC received flecainide, dronedarone, or amiodarone (Figure 1 and Table IV in the Data Supplement). Sinus rhythm at baseline was recorded more often in patients with ERC than in patients with usual care (Table 1) and was not associated with better outcome for both primary outcomes within multivariable analysis. Based on resting ECG evaluation at 12 and 24 months, sinus rhythm was observed more often in the ERC group (Figure V in the Data Supplement). Catheter ablation was performed in 140 patients with HF including 88 patients randomly assigned to ERC and 52 patients randomly assigned to usual care. Characteristics and distribution of patients treated with or without catheter ablation are shown in Table V and Figure VI in the Data Supplement. The effect of ERC did not differ between patients treated with atrial fibrillation ablation and patients treated with antiarrhythmic drugs. Visual inspection identified a slight (nonsignificant) early excess of first primary outcomes in the subgroup of patients with HFpEF. The tabulated outcomes suggest numerically more early HF events in patients treated with amiodarone (Table 3).
Table 3.
Exploratory Analysis of Primary Outcomes Within 12 Months After Randomization in patients Randomly Assigned to Early Rhythm Control, Split by Planned Initial Rhythm Therapy
Safety Outcome
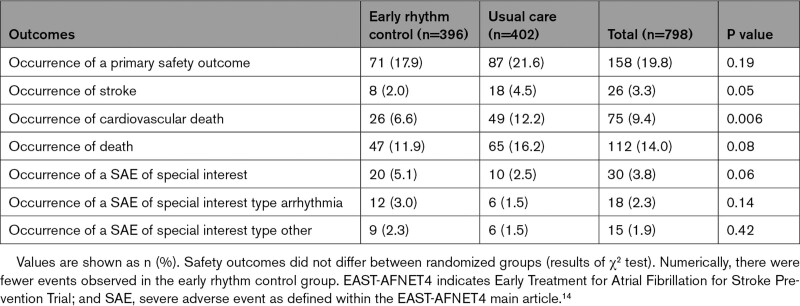
There were no significant differences between ERC and usual care for the primary safety outcome (Table 4 and Table VI in the Data Supplement).
Table 4.
Safety Outcomes in the EAST-AFNET4 Heart Failure Study Population by Randomized Groups
Improvement of LVEF
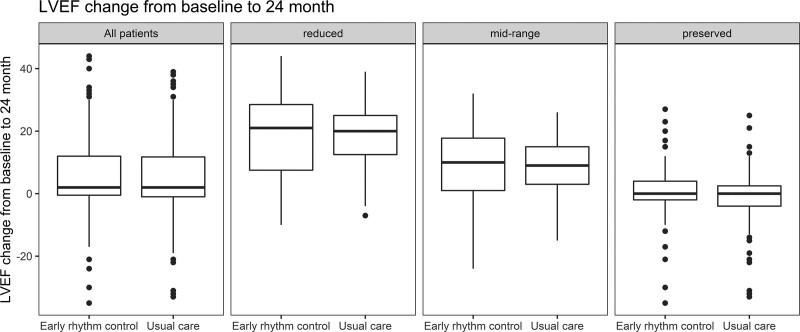
LVEF improved in both groups, resulting in similar improvement of LVEF in patients randomly assigned to ERC or to usual care (mean improvement in LVEF 5.3±11.6% versus 4.9±11.6%, respectively; univariable P=0.43; Table 2; interaction P value (between treatment group and HF)=0.38). LVEF improved mainly in patients with reduced or midrange LVEF (Figure 3 and Figure VII in the Data Supplement) without differences between randomized groups. A complete recovery of initially reduced LVEF was observed in 24 patients randomly assigned to ERC and in 26 patients randomly assigned to usual care, whereas an increase of LVEF above the recommended threshold for implantable cardioverter defibrillator implantation (35%) occurred in 24 patients of the ERC group and in 29 patients treated with usual care (Table VII in the Data Supplement). Sensitivity analysis using only complete cases did not show significant differences compared with imputed data (mean improvement in LVEF ERC 5.6±11.7%; usual care 4.4±11.5%; univariable P=0.24; Table VIII in the Data Supplement)
Figure 3.
Left ventricular function and changes in left ventricular function of EAST-AFNET4 patients with heart failure by randomized groups. Changes in LVEF between baseline and 2 years are given in the overall heart failure population (all patients, Left) and split by LVEF groups (reduced, midrange, and preserved). The numeric changes in LVEF, split by randomized group, were early rhythm control, reduced LVEF 17.28±13.45; usual care, reduced LVEF 18.10±10.73, mean difference –0.83 (–4.44 to 2.79; P=0.66); early rhythm control, midrange LVEF 9.25±10.44; usual care, midrange LVEF 8.68±8.97 (mean difference 0.66 [–1.99 to 3.31]; P=0.63); early rhythm control, preserved LVEF 0.33±8.33; usual care, preserved LVEF –0.93±8.34 (mean difference 0.98 [(–0.83 to 2.79]; P=0.29). EAST-AFNET4 indicates Early Treatment of Atrial Fibrillation for Stroke Prevention Trial; and LVEF, left ventricular ejection fraction.
Symptoms and Quality of Life
At the end of the follow-up, a similar number of patients without atrial fibrillation–related symptoms were seen in the ERC and the usual care arm (ERC 226 [69.54%], usual care 214 [64.65%]) and similar outcomes regarding quality of life (European Quality of Life 5 Dimensions score ERC –2.2±24.3 versus usual care –4.3±25.6) were observed (Table 2). Atrial fibrillation symptoms improved at 24 months in both randomized groups (ERC 56.4%; usual care 54.2%; Tables IX and X in the Data Supplement) without intergroup differences.
Anticoagulation and HF Therapy
The vast majority of patients (≈90%) received guideline-recommended oral anticoagulation throughout the follow-up without differences between both groups. Vitamin K antagonists and the novel oral anticoagulants were evenly distributed (Table XI in the Data Supplement). Therapy of concomitant cardiovascular conditions appeared well balanced, and a normal average blood pressure throughout follow-up was seen in both groups (Figure VIII in the Data Supplement). HF medication did not show differences between randomized groups at discharge, including the high use of β-blockers (79.1%; ERC 78.4%, usual care 79.9%), angiotensin-converting enzyme inhibitors or angiotensin II receptor blockers (62.5%; ERC 60.1%, usual care 64.9%), or diuretics (50.4%; ERC 49.7%, usual care 51.0%; Tables XII and XIII in the Data Supplement). Mineral corticoid receptor antagonist use was not as high as recommended, but similar between randomized groups (overall 12.4%; ERC 13.7%; usual care 11.2%). Digitalis glycoside use at discharge was higher in the usual care group (usual care 9.7%; ERC 5.8%) but was not associated with worse outcomes (Tables XIV and XV in the Data Supplement).
Rate Control Therapy
Rate control therapy as the mainstay of usual care was initiated in 366 patients, resulting in a well-controlled median heart rate of 65.5 beats/min in the usual care group. It is important to know that rate control therapy was given in addition to rhythm control in 85.1% (337) of patients randomly assigned to ERC, mainly using β-blockers (78.4%; Figure 1; Tables XIII and XVI in the Data Supplement).
Outcomes According to NYHA Class
Within multivariable analysis, NYHA class at baseline showed some association with primary and secondary outcomes, that is, NYHA II or III compared with asymptomatic HF. NYHA classes II and III were not related to LVEF changes (Figure IX in the Data Supplement). HF symptoms estimated by NYHA class improved after 24 months in both groups (ERC 53.2%, usual care 45.3%) with a slightly higher improvement in patients randomly assigned to ERC (P=0.05; Table 2). The highest improvement in NYHA class occurred in patients with preserved ejection fraction (Table VII in the Data Supplement).
Multivariable Analysis
Adjusted effects associated with the first primary outcome were observed for sex (female versus male HR 0.65 [0.47–0.89]), ejection fraction (reduced LVEF versus preserved LVEF HR 1.76 [1.48–2.10]), NYHA class II (NYHA class II versus no HF HR 2.31 [1.31–4.07]), and NYHA class III (NYHA class III versus no HF HR 3.93 [2.09–7.39]).
Complete Case Analysis
A complete case analysis of EAST-AFNET4 patients with HF for secondary outcomes where imputation was necessary is provided in Table XVII in the Data Supplement.
Discussion
Main Findings
This analysis demonstrates that ERC therapy reduces a composite of cardiovascular death, stroke, or hospitalization for worsening of HF or for acute coronary syndrome compared with usual care (including rhythm control use to improve atrial fibrillation–related symptoms) in patients with signs or symptoms of HF. A similar clinical benefit of ERC was found when a CASTLE-AF–like outcome was calculated, extending the clinical benefit found in that study to an unselected cohort of patients with HF with reduced and preserved ejection fraction receiving rhythm control therapy by using either antiarrhythmic drugs or atrial fibrillation ablation. Unlike CASTLE-AF, the clinical benefit of ERC was achieved using antiarrhythmic drugs or atrial fibrillation ablation, chosen by the site investigators within guideline recommendations. The majority of patients in this analysis presented with HFpEF, similar to the recently published subanalysis of the CABANA trial comparing catheter ablation and antiarrhythmic drug therapy in patients with atrial fibrillation and HF.18 The clinical benefit of ERC was not associated with improved LVEF at 2 years compared with usual care. Strengths of the analysis are the long median follow-up duration of 5.1 years and the enrollment of a broad spectrum of patients with HF and recently diagnosed atrial fibrillation.
Type of Rhythm Control Therapy
Most patients were treated with antiarrhythmic drugs, with amiodarone (in HFrEF19), and flecainide, dronedarone, or amiodarone (in HFpEF, Figure 1) as the main agents. Antiarrhythmic drugs were prescribed according to the current guidelines and at the recommended dose.12,13 Approximately 17% of patients randomly assigned to ERC (25% of those still in follow-up at that time point) were treated with atrial fibrillation ablation in the first 2 years after randomization. This suggests that the clinical benefit found in this subanalysis can be achieved by using antiarrhythmic drugs as initial therapy. It is worthwhile to note that flecainide was used in a relatively high number of patients without safety concerns. All treatments were given following the guidance of international atrial fibrillation guidelines, potentially enabling the safe use of antiarrhythmic drugs in this population.
As expected, patients treated by ERC were more likely to present in sinus rhythm at the 24-month follow-up than patients treated by usual care. Also, the proportion of patients with atrial fibrillation at 2 years was higher in this analysis than in the overall cohort of the EAST-AFNET4 trial. This is in line with previously published data,14,20 because HF is believed to contribute to recurrent atrial fibrillation and to atrial cardiomyopathy21 in patients with atrial fibrillation.22 It seems plausible that early initiation of therapy was one of the factors that rendered antiarrhythmic drug therapy relatively effective in this analysis. Catheter ablation of atrial fibrillation improves quality of life and reduces arrhythmia recurrence to a higher extent than antiarrhythmic drug therapy, with signals that there may be clinical benefit, especially in patients with reduced left ventricular function.20,23–25 In view of the clinical benefit of catheter ablation compared with antiarrhythmic drug therapy seen in the CABANA HF subanalysis,18 it is tempting to speculate that ERC using catheter ablation could convey an even larger clinical benefit than the treatment pattern chosen by the investigators of the EAST-AFNET4 trial. Alternatively, antiarrhythmic drugs may be sufficient to achieve ERC therapy because of the lower risk of recurrent atrial fibrillation. The value of catheter ablation for ERC awaits testing in a controlled clinical trial.
Timing of Rhythm Control Therapy
Patients with HF who have atrial fibrillation are at high risk of cardiovascular events including cardiovascular death,5,7 stroke, and worsening of HF.8 Recent-onset atrial fibrillation is associated with worse outcomes than established atrial fibrillation.2,26 The early timing of rhythm control therapy in this study could have amplified the clinical benefit of ERC compared with usual care. It is also possible that the early initiation of rhythm control therapy led to an improved efficacy of antiarrhythmic drug therapy in comparison with other trials testing antiarrhythmic drugs for rhythm control therapy in patients with HF.18,19
Role of Left Ventricular Function
Patients with preserved LVEF had a lower risk for the first primary outcome compared with patients with reduced LVEF. This is in line with the findings of several previous studies on patients with reduced ejection fraction and points to the fact that left ventricular function retains prognostic importance in patients with HF and atrial fibrillation.20,23,25 Reduction of cardiovascular events by HF therapies such as inhibitors of the renin-angiotensin-aldosterone system or cardiac resynchronization therapy is accompanied by improvements of cardiac function in patients with HF and reduced ejection fraction. Early studies found that catheter ablation can improve left ventricular function in patients with atrial fibrillation and tachycardiomyopathy.27 The findings of the CASTLE-AF trial corroborate this theory, because catheter ablation in patients with HF who have atrial fibrillation resulted not only in better outcomes of the death from any cause or hospitalization for worsening HF, but also in a clinically relevant improvement of left ventricular function.20 The present analysis showed a similar clinical benefit of ERC therapy, but improvement of LVEF was not different between ERC and usual care. It is possible that rhythm control therapy given to symptomatic patients with HF and atrial fibrillation led to improved left ventricular function in patients randomly assigned to usual care. Exploratory analyses suggest that treatment with amiodarone, but not treatment with flecainide, propafenone, or dronedarone, was potentially associated with early HF hospitalizations in patients with HF and preserved left ventricular function. This is unexpected because amiodarone is considered a safe antiarrhythmic drug in patients with HF13,28–30 and calls for further clinical research to determine the optimal antiarrhythmic drug therapy in patients with HFpEF.
In summary, this analysis suggests that ERC can prevent clinical outcomes in patients with HF and that left ventricular function remains a predictor of outcomes in patients with HF and atrial fibrillation. In addition, sinus rhythm was not associated with better primary outcomes. This might at least in part be explained by the fact that rhythm control therapy was allowed when symptoms or signs of tachycardiomyopathy occurred in the usual care group.
HF Therapy and Anticoagulation Therapy
Patients with HF in the EAST-AFNET4 trial were medically well treated in both study arms, without differences between randomized groups. Treatment included a high use of recommended HF therapies with β-blockers, angiotensin-converting enzyme inhibitors, or angiotensin-II receptor antagonists. Mineralocorticoid antagonists were prescribed less often, but with no difference between randomized groups. In accordance with the recommendations for HF treatment valid at the time of recruitment and earlier follow-up period, only a few patients received angiotensin receptor-neprilysin inhibitor, and there was no use of sodium-glucose cotransporter-2 inhibitors. These novel drugs for HF have shown additional benefits including reduced outcomes and improved LVEF,31,32 yet they are unlikely to interact with the intervention of the EAST-AFNET4 trial.
Over 90% of patients with atrial fibrillation and HF received oral anticoagulation without differences between randomized groups. Most patients were treated with novel oral anticoagulants. This implies that oral anticoagulation as a confounder on relevant clinical outcomes such as stroke or cardiovascular mortality is unlikely.
Rate Control Therapy
Rate control therapy was delivered as recommended by current guidelines.12,13 Most patients in both randomized groups received rate control therapy. The proportion of patients treated with selective rate-controlling medication was higher in the patients randomly assigned to usual care. When the rate-controlling effects of antiarrhythmic drugs (amiodarone, dronedarone, propafenone) are considered, this difference is smaller. Although we cannot exclude a theoretical effect on outcomes associated with a more intensive rate control therapy, this is unlikely, in view of the neutral outcome of the RACE II trial (Rate Control Efficacy in Permanent Atrial Fibrillation).33 Besides the rate-controlling effects of antiarrhythmic drugs, the high use of β-blocker therapy as a standard of care in patients with HF explains the high rate of prescription of rate-controlling therapy in the ERC group.
Safety Aspects
Both antiarrhythmic drug therapy and catheter ablation in patients with HF and atrial fibrillation were evenly safe in this analysis, supporting the main findings of the EAST-AFNET4 trial.
Limitations and Strengths
This analysis was prespecified in the statistical analysis plan of the EAST-AFNET4 trial, but the trial was not powered specifically for this subanalysis. EAST-AFNET4 is a strategy trial, the intervention was not blinded, and there are no data on left ventricular function or quality of life beyond 2 years of follow-up. Despite these limitations, this analysis reports the first contemporary comparison of systematic ERC therapy compared with restricted and delayed rhythm control in patients with atrial fibrillation and HF. The size of the population is larger than most randomized trials published so far and comparable to the HF subanalysis of CABANA. A strength of the analysis is the control group receiving treatment according to contemporary atrial fibrillation guidelines.
Conclusions
This subanalysis of the EAST-AFNET4 trial demonstrates that ERC therapy using antiarrhythmic drugs or atrial fibrillation ablation is safe and reduces cardiovascular events in patients with HF. The clinical benefit of ERC is not associated with greater improvement in LVEF compared with that observed with usual care. Clinical benefit is observed across the spectrum of HF subtypes, suggesting that restoring and maintaining sinus rhythm via rhythm control therapy conveys the clinical benefit. In the view of the authors, all patients with signs or symptoms of HF should be considered for rhythm control therapy within a year of being diagnosed with atrial fibrillation.
Sources of Funding
EAST-AFNET4 (Early Treatment of Atrial Fibrillation for Stroke Prevention Trial) was funded in part by BMBF (German Ministry of Education and Research, Berlin, Germany, Grant 01 GI 0204), DZHK (German Center for Cardiovascular Research, Berlin, Germany), AFNET (Atrial Fibrillation Network), European Heart Rhythm Association, St Jude Medical/Abbott, Sanofi, and the German Heart Foundation. Further support came from the European Union (grant agreement No. 633196 [CATCH ME] to Dr Kirchhof and AFNET; grant agreement EU IMI 116074 [BigData@Heart] to Dr Kirchhof), the British Heart Foundation (FS/13/43/30324; PG/17/30/32961; PG/20/22/35093; AA/18/2/34218, all to Dr Kirchhof), and Leducq Foundation to Dr Kirchhof.
Disclosures
Dr Brandes receives grants or contracts from Region of Southern Denmark/Zealand Region (Research grant paid to institution); royalties or licenses from Gyldendals forlag; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing, or educational events from Bayer, Boehringer Ingelheim, BMS; support for attending meetings and travel from Biotronik and reports leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Specialist council in Cardiology of the Region of Southern Denmark. Dr Goette receives all support for the present manuscript from AFNET (Atrial Fibrillation Network), Sanofi-Aventis, St. Jude Medical; grants or contracts from EU Horizon 2020; Grant No. 965286; consulting fees from Berlin Chemie, Boston Scientific, Medtronic, Omeicos, Daiichi Sankyo, and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing or educational events: Bayer, BMS, Pfizer, Boehringer Ingelheim, Berlin Chemie, Boston Scientific, Medtronic, Menarini. Dr Camm receives support for the present manuscript from Sanofi, St. Jude (now Abbott); grants or contracts from Boehringer Ingelheim, Bayer, Pfizer/BMS, Daiichi Sankyo; royalties or licenses from ESC Textbook of Cardiovascular Medicine; consulting fees from Boehringer Ingelheim, Medtronic, Boston Scientific, Bayer, Pfizer/BMS, Daiichi Sankyo; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Bayer, Menarini; reports participation on a Data Safety Monitoring Board or Advisory Board for Biotronik, Anthos, Glaxo Smith Kline, Abbott, Johnson and Johnson and leadership or fiduciary role in other board, society, committee or advocacy group, paid or unpaid for Atrial fibrillation Association, Arrhythmia Alliance, European Heart Rhythm Association, World Society of Arrhythmia. Dr Ozga receives all support for the present manuscript from AFNET. Dr Rillig receives grants from Philips (KODEX-EPD), Medtronik, Biosense webster, Cardiofocus; consulting fees from Philips (KODEX -EPD), Medtronik, Ablamap, Cardiofocus, Biosense webster; payment or honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Philips (KODEX -EPD), Medtronik, Ablamap, Cardiofocus, Biosense, Bayer and Novartis; payment for expert testimony from Philips (KODEX-EPD) and support for attending meetings and/or travel from Philips (KODEX -EPD), Medtronik, Ablamap, Cardiofocus, Biosense webster. Dr Suling receives all support for the present manuscript from AFNET. Dr Magnussen receives research support from the German Center for Cardiovascular Research (DZHK; Promotion of women scientists’ program) and from the Deutsche Stiftung für Herzforschung unrelated to the current work. She has received speaker fees from Astra Zeneca, Novartis, and Loewenstein medical in the last 3 years outside this work. Dr Ng reports grants from Boston Scientific, grants and personal fees from Abbott, personal fees from Biosense Webster, Catheter Precision, and Daiichi Sankyo, outside the submitted work. Dr Breithardt reports grants to AFNET for the EAST Trial from Sanofi-Aventis, grants from Abbott Vascular, BMBF (German Ministry of Education and Research, Grant Number: 01 GI 0204), DZHK (German Center for Cardiovascular Research), EHRA (European Heart Rhythm Association, a branch of the European Society of Cardiology), and Deutsche Herzstiftung (German Heart Foundation), during the conduct of the study. Outside the submitted work: personal fees from Boehringer Ingelheim, BMS, Bayer Health Care, Johnson & Johnson, Sanofi-Aventis, Portola, Biosense, Biotronik, Daiichi Sankyo, and grants to AFNET from BMS and Biosense. Dr Heidbuchel receives grants or contracts from Abbott, Medtronic, Biotronik, Boston Scientific, Bayer, Boehringer-Ingelheim, Daiichi Sankyo, Pfizer, BMS; and payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Bayer, Pfizer-BMS, Daiichi Sankyo, Springer Healthcare Ltd. Dr van Gelder receives grants or contracts from Dutch heart Foundation, Medtronic; consulting fees from Daiichi Sankyo, BMS, Pfizer; payment or honoraria for lectures, presentations, speakers’ bureaus, manuscript writing, or educational events from Daiichi Sankyo, BMS, Pfizer and reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid in EHRA program committee Chair. Dr Crijns reports the Netherlands Cardiovascular Research Initiative: an initiative with support of the Dutch Heart Foundation, CVON 2014-9: Reappraisal of Atrial Fibrillation: Interaction Between Hypercoagulability, Electrical Remodeling, and Vascular Destabilisation in the Progression of Atrial Fibrillation (RACE V). Dr Kuck receives grants or contracts from Medtronic, Biosense-Webste, and consulting fees from CardioValve. Dr Wegscheider reports grants from AFNET, during the conduct of the study; grants from Biotronik, personal fees from Biotronik, personal fees from Boston Scientific, from Resmed, from Novartis, outside the submitted work. Dr Haegeli receives institutional grants outside of the present manuscript from Abbott, Abiomed, Amgen, AstraZeneca, Bayer, Biosense Webster, Biotronik, Boston Scientific, Bracco, B. Braun, Daiichi-Sankyo, Edwards Lifesciences, Medtronic, MicroPort, Novartis, Vascular Medical, Zoll. Dr Szumowski reports leadership or fiduciary role in other board, society, committee, or advocacy group, paid or unpaid for Advisory Board for Cardiology for Polish Ministry of Health. Dr Kirchhof receives research support for basic, translational, and clinical research projects from European Union, British Heart Foundation, Leducq Foundation, Medical Research Council (UK), and German Center for Cardiovascular Research, from several drug and device companies active in atrial fibrillation and has received honoraria from several such companies in the past, but not in the last three years. He is listed as inventor on two patents held by University of Birmingham (Atrial Fibrillation Therapy WO 2015140571, Markers for Atrial Fibrillation WO 2016012783). The other authors report no conflicts.
Supplemental Materials
Data Supplement Tables I–XVII
Data Supplement Figures I–IX
Supplementary Material
Nonstandard Abbreviations and Acronyms
- EAST-AFNET4
- Early Treatment of Atrial Fibrillation for Stroke Prevention Trial
- ERC
- early rhythm control
- HFmEF
- heart failure with midrange ejection fraction
- HFpEF
- heart failure with preserved ejection fraction
- HFrEF
- heart failure with reduced ejection fraction
- LVEF
- left ventricular ejection fraction
- NYHA
- New York Heart Association
Continuing medical education (CME) credit is available for this article. Go to http://cme.ahajournals.org to take the quiz.
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/CIRCULATIONAHA.121.056323.
For Sources of Funding and Disclosures, see page 857.
This work was presented as an abstract at the Heart Rhythm 2021 meeting, Boston, MA, July 28 to July 31, 2021.
Contributor Information
Andreas Rillig, Email: a.rillig@uke.de.
Christina Magnussen, Email: c.magnussen@uke.de.
Ann-Kathrin Ozga, Email: a.ozga@uke.de.
Anna Suling, Email: asuling@uke.de.
Axel Brandes, Email: axel.brandes@rsyd.dk.
Günter Breithardt, Email: g.breithardt@uni-muenster.de.
A. John Camm, Email: jcamm@sgul.ac.uk.
Harry J.G.M. Crijns, Email: hjgm.crijns@mumc.nl.
Lars Eckardt, Email: Lars.Eckardt@ukmuenster.de.
Arif Elvan, Email: a.elvan@isala.nl.
Andreas Goette, Email: andreas.goette@af-net.eu.
Michele Gulizia, Email: michele.gulizia60@gmail.com.
Laurent Haegeli, Email: Laurent.Haegeli@usz.ch.
Hein Heidbuchel, Email: heinheid@gmail.com.
Karl-Heinz Kuck, Email: kuckkh@aol.com.
Andre Ng, Email: gan1@le.ac.uk.
Lukasz Szumowski, Email: lszumowski@ikard.pl.
Isabelle van Gelder, Email: i.c.van.gelder@umcg.nl.
Karl Wegscheider, Email: k.wegscheider@uke.de.
References
- 1.Braunwald E. Shattuck lecture–cardiovascular medicine at the turn of the millennium: triumphs, concerns, and opportunities. N Engl J Med. 1997;337:1360–1369. doi: 10.1056/NEJM199711063371906 [DOI] [PubMed] [Google Scholar]
- 2.Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, Ellinor PT, Cheng S, Vasan RS, Lee DS, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133:484–492. doi: 10.1161/CIRCULATIONAHA.115.018614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fabritz L, Crijns HJGM, Guasch E, Goette A, Häusler KG, Kotecha D, Lewalter T, Meyer C, Potpara TS, et al. Dynamic risk assessment to improve quality of care in patients with atrial fibrillation: the 7th AFNET/EHRA Consensus Conference. Europace. 2021;23:329–344. doi: 10.1093/europace/euaa279 [DOI] [PubMed] [Google Scholar]
- 4.Chua W, Purmah Y, Cardoso VR, Gkoutos GV, Tull SP, Neculau G, Thomas MR, Kotecha D, Lip GYH, Kirchhof P, et al. Data-driven discovery and validation of circulating blood-based biomarkers associated with prevalent atrial fibrillation. Eur Heart J. 2019;40:1268–1276. doi: 10.1093/eurheartj/ehy815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 6.Schrage B, Geelhoed B, Niiranen TJ, Gianfagna F, Vishram-Nielsen JKK, Costanzo S, Söderberg S, Ojeda FM, Vartiainen E, Donati MB, et al. Comparison of cardiovascular risk factors in European population cohorts for predicting atrial fibrillation and heart failure, their subsequent onset, and death. J Am Heart Assoc. 2020;9:e015218. doi: 10.1161/JAHA.119.015218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, et al. ; Beta-Blockers in Heart Failure Collaborative Group. Efficacy of β blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis. Lancet. 2014;384:2235–2243. doi: 10.1016/S0140-6736(14)61373-8 [DOI] [PubMed] [Google Scholar]
- 8.Zafrir B, Lund LH, Laroche C, Ruschitzka F, Crespo-Leiro MG, Coats AJS, Anker SD, Filippatos G, Seferovic PM, Maggioni AP, et al. ; ESC-HFA HF Long-Term Registry Investigators. Prognostic implications of atrial fibrillation in heart failure with reduced, mid-range, and preserved ejection fraction: a report from 14 964 patients in the European Society of Cardiology Heart Failure Long-Term Registry. Eur Heart J. 2018;39:4277–4284. doi: 10.1093/eurheartj/ehy626 [DOI] [PubMed] [Google Scholar]
- 9.Willems S, Meyer C, de Bono J, Brandes A, Eckardt L, Elvan A, van Gelder I, Goette A, Gulizia M, Haegeli L, et al. Cabins, castles, and constant hearts: rhythm control therapy in patients with atrial fibrillation. Eur Heart J. 2019;40:3793–3799c. doi: 10.1093/eurheartj/ehz782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rillig A, Makimoto H, Wegner J, Lin T, Heeger C, Lemes C, Fink T, Metzner A, Wissner E, Mathew S, et al. Six-year clinical outcomes after catheter ablation of atrial fibrillation in patients with impaired left ventricular function. J Cardiovasc Electrophysiol. 2015;26:1169–1179. doi: 10.1111/jce.12765 [DOI] [PubMed] [Google Scholar]
- 11.Marrouche NF, Brachmann J, Andresen D, Siebels J, Boersma L, Jordaens L, Merkely B, Pokushalov E, Sanders P, Proff J, et al. ; CASTLE-AF Investigators. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;378:417–427. doi: 10.1056/NEJMoa1707855 [DOI] [PubMed] [Google Scholar]
- 12.Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, et al. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Europace. 2016;18:1609–1678. doi: 10.1093/europace/euw295 [DOI] [PubMed] [Google Scholar]
- 13.Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomström-Lundqvist C, Boriani G, Castella M, Dan GA, Dilaveris PE, et al. ; ESC Scientific Document Group. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42:373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 14.Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, et al. ; EAST-AFNET 4 Trial Investigators. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med. 2020;383:1305–1316. doi: 10.1056/NEJMoa2019422 [DOI] [PubMed] [Google Scholar]
- 15.Gopinathannair R, Chen LY, Chung MK, Cornwell WK, Furie KL, Lakkireddy DR, Marrouche NF, Natale A, Olshansky B, Joglar JAAmerican Heart Association Electrocardiography and Arrhythmias Committee and Heart Failure and Transplantation Committee of the Council on Clinical Cardiology; Council on Arteriosclerosis, Thrombosis and Vascular Biology; Council on Hypertension; Council on Lifestyle and Cardiometabolic Health; and the Stroke Council. Managing atrial fibrillation in patients with heart failure and reduced ejection fraction: a scientific statement from the American Heart Association. Circ Arrhythm Electrophysiol. 2021;14:HAE0000000000000078. doi: 10.1161/HAE.0000000000000078 [DOI] [PubMed] [Google Scholar]
- 16.Kirchhof P, Breithardt G, Camm AJ, Crijns HJ, Kuck KH, Vardas P, Wegscheider K. Improving outcomes in patients with atrial fibrillation: rationale and design of the Early treatment of Atrial fibrillation for Stroke prevention Trial. Am Heart J. 2013;166:442–448. doi: 10.1016/j.ahj.2013.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Packer DL, Mark DB, Robb RA, Monahan KH, Bahnson TD, Poole JE, Noseworthy PA, Rosenberg YD, Jeffries N, Mitchell LB, et al. ; CABANA Investigators. Effect of catheter ablation vs antiarrhythmic drug therapy on mortality, stroke, bleeding, and cardiac arrest among patients with atrial fibrillation: the CABANA randomized clinical trial. JAMA. 2019;321:1261–1274. doi: 10.1001/jama.2019.0693 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Packer DL, Piccini JP, Monahan KH, Al-Khalidi HR, Silverstein AP, Noseworthy PA, Poole JE, Bahnson TD, Lee KL, Mark DBCABANA Investigators. Ablation versus drug therapy for atrial fibrillation in heart failure: results from the CABANA trial. Circulation. 2021;143:1377–1390. doi: 10.1161/CIRCULATIONAHA.120.050991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, et al. ; Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789 [DOI] [PubMed] [Google Scholar]
- 20.Marrouche NF, Kheirkhahan M, Brachmann J. Catheter ablation for atrial fibrillation with heart failure. N Engl J Med. 2018;379:492. doi: 10.1056/NEJMc1806519 [DOI] [PubMed] [Google Scholar]
- 21.Goette A, Kalman JM, Aguinaga L, Akar J, Cabrera JA, Chen SA, Chugh SS, Corradi D, D’Avila A, Dobrev D, et al. ; Document Reviewers:. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18:1455–1490. doi: 10.1093/europace/euw161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marrouche NF, Wilber D, Hindricks G, Jais P, Akoum N, Marchlinski F, Kholmovski E, Burgon N, Hu N, Mont L, et al. Association of atrial tissue fibrosis identified by delayed enhancement MRI and atrial fibrillation catheter ablation: the DECAAF study. JAMA. 2014;311:498–506. doi: 10.1001/jama.2014.3 [DOI] [PubMed] [Google Scholar]
- 23.Di Biase L, Mohanty P, Mohanty S, Santangeli P, Trivedi C, Lakkireddy D, Reddy M, Jais P, Themistoclakis S, Dello Russo A, et al. Ablation versus amiodarone for treatment of persistent atrial fibrillation in patients with congestive heart failure and an implanted device: results from the AATAC multicenter randomized trial. Circulation. 2016;133:1637–1644. doi: 10.1161/CIRCULATIONAHA.115.019406 [DOI] [PubMed] [Google Scholar]
- 24.Kuck KH, Merkely B, Zahn R, Arentz T, Seidl K, Schlüter M, Tilz RR, Piorkowski C, Gellér L, Kleemann T, et al. Catheter ablation versus best medical therapy in patients with persistent atrial fibrillation and congestive heart failure: the randomized AMICA trial. Circ Arrhythm Electrophysiol. 2019;12:e007731. doi: 10.1161/CIRCEP.119.007731 [DOI] [PubMed] [Google Scholar]
- 25.Prabhu S, Taylor AJ, Costello BT, Kaye DM, McLellan AJA, Voskoboinik A, Sugumar H, Lockwood SM, Stokes MB, Pathik B, et al. Catheter ablation versus medical rate control in atrial fibrillation and systolic dysfunction: the CAMERA-MRI study. J Am Coll Cardiol. 2017;70:1949–1961. doi: 10.1016/j.jacc.2017.08.041 [DOI] [PubMed] [Google Scholar]
- 26.Wang TJ, Larson MG, Levy D, Vasan RS, Leip EP, Wolf PA, D’Agostino RB, Murabito JM, Kannel WB, Benjamin EJ. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation. 2003;107:2920–2925. doi: 10.1161/01.CIR.0000072767.89944.6E [DOI] [PubMed] [Google Scholar]
- 27.Khan MN, Jaïs P, Cummings J, Di Biase L, Sanders P, Martin DO, Kautzner J, Hao S, Themistoclakis S, Fanelli R, et al. ; PABA-CHF Investigators. Pulmonary-vein isolation for atrial fibrillation in patients with heart failure. N Engl J Med. 2008;359:1778–1785. doi: 10.1056/NEJMoa0708234 [DOI] [PubMed] [Google Scholar]
- 28.Roy D, Talajic M, Nattel S, Wyse DG, Dorian P, Lee KL, Bourassa MG, Arnold JM, Buxton AE, Camm AJ, et al. ; Atrial Fibrillation and Congestive Heart Failure Investigators. Rhythm control versus rate control for atrial fibrillation and heart failure. N Engl J Med. 2008;358:2667–2677. doi: 10.1056/NEJMoa0708789 [DOI] [PubMed] [Google Scholar]
- 29.Al-Jazairi MIH, Nguyen BO, De With RR, Smit MD, Weijs B, Hobbelt AH, Alings M, Tijssen JGP, Geelhoed B, Hillege HL, et al. Antiarrhythmic drugs in patients with early persistent atrial fibrillation and heart failure: results of the RACE 3 study. Europace. Published online April 26, 2021. doi 10.1093/europace/euab062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC, Jr, Ellinor PT, Ezekowitz MD, Field ME, Furie KL, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS Guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Rhythm Society in Collaboration With the Society of Thoracic Surgeons. Circulation. 2019;140:e125–e151. doi: 10.1161/CIR.0000000000000665 [DOI] [PubMed] [Google Scholar]
- 31.Zannad F, Ferreira JP, Pocock SJ, Anker SD, Butler J, Filippatos G, Brueckmann M, Ofstad AP, Pfarr E, Jamal W, et al. SGLT2 inhibitors in patients with heart failure with reduced ejection fraction: a meta-analysis of the EMPEROR-Reduced and DAPA-HF trials. Lancet. 2020;396:819–829. doi: 10.1016/S0140-6736(20)31824-9 [DOI] [PubMed] [Google Scholar]
- 32.Docherty KF, Jhund PS, Inzucchi SE, Køber L, Kosiborod MN, Martinez FA, Ponikowski P, DeMets DL, Sabatine MS, Bengtsson O, et al. Effects of dapagliflozin in DAPA-HF according to background heart failure therapy. Eur Heart J. 2020;41:2379–2392. doi: 10.1093/eurheartj/ehaa183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Van Gelder IC, Groenveld HF, Crijns HJ, Tuininga YS, Tijssen JG, Alings AM, Hillege HL, Bergsma-Kadijk JA, Cornel JH, Kamp O, et al. ; RACE II Investigators. Lenient versus strict rate control in patients with atrial fibrillation. N Engl J Med. 2010;362:1363–1373. doi: 10.1056/NEJMoa1001337 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.