Abstract
Background and Objectives
To evaluate the association between midlife plasma amyloid-β (Aβ1-42, Aβ1-40, Aβ42:Aβ40) and risk of mild cognitive impairment (MCI) and dementia.
Methods
Plasma Aβ42 and Aβ40 were retrospectively measured with a fluorometric bead-based immunoassay in a subsample of the Atherosclerosis Risk in Communities cohort study. We investigated the relationship of plasma Aβ42, Aβ40, and Aβ42:Aβ40 ratio measured in midlife and late life and the change from midlife to late life to risk of MCI, dementia, and combined MCI/dementia outcomes in late life (from 2011–2019). We used multinomial logistic regressions estimating relative risk ratios (RRRs) of these cognitive outcomes vs cognitively normal adjusted for age, sex, education, site-race, APOE, hypertension, diabetes, and body mass index.
Results
A total of 2,284 participants were included (midlife mean age 59.2 ± 5.2, 57% female, 22% Black). Each doubling of midlife Aβ42:Aβ40 was associated with 37% lower risk of MCI/dementia (RRR 0.63, 95% confidence interval [CI] 0.46–0.87), but only up to approximately the median (spline model threshold 0.20). Every 1-SD increase in plasma Aβ42 (10 pg/mL) was associated with 13% lower risk of MCI/dementia (RRR 0.87, 95% CI 0.77–0.98), whereas every 1-SD increase in plasma Aβ40 (67 pg/mL) was associated with 15% higher risk of MCI/dementia (RRR 1.15, 95% CI 1.01–1.29). Associations were comparable but slightly weaker statistically when models were repeated using late-life plasma Aβ predictors. Aβ42 and Aβ40 increased from midlife to late life, but changes were not associated with cognitive outcomes.
Discussion
Midlife measurement of plasma Aβ may have utility as a blood-based biomarker indicative of risk for future cognitive impairment.
The National Institute on Aging–Alzheimer's Association research framework for defining Alzheimer disease (AD) emphasizes elevated levels of aggregated amyloid-β (Aβ) as a biomarker of AD.1 The currently validated approaches to measure brain Aβ burden under this framework include using PET with an Aβ tracer or measuring Aβ peptides in CSF via lumbar puncture. These methods are restricted in application by expense or invasiveness, and a need remains to investigate more accessible alternatives to identify abnormal Aβ accumulation in asymptomatic individuals who could be at risk for dementia. To this end, plasma Aβ has shown promise as a blood-based biomarker.
Similar to associations seen with CSF Aβ, lower plasma Aβ42 or lower plasma Aβ42:Aβ40 ratio has been associated cerebral amyloidosis2-6 and increased risk of dementia and concurrent cognitive impairment.7-11 However, most investigations of plasma Aβ have focused on late-life plasma measurements and cognitive outcomes. Pathologic changes relating to AD begin decades before clinical symptoms appear,12,13 supporting a need to investigate the relationship of early measurements of plasma Aβ with clinical phenotypes in late life. Using data from the multisite community-based Atherosclerosis Risk in Communities (ARIC) study, we aimed to investigate the relationship between midlife plasma Aβ measurements and risk of cognitive impairment over 25 years of follow-up. In addition, we examined the association of late-life plasma Aβ and changes in plasma Aβ from midlife to late life with risk of cognitive impairment. Although race-differential associations were not hypothesized, White-Black stratified models were tested, acknowledging the influence of societal inequities to have downstream biological consequences potentially manifesting in known cerebrovascular disease and AD disparities14,15 and possibly to influence observed associations between plasma Aβ and cognitive impairment given the complex interaction of these pathologies in the development and presentation of dementia.16,17
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The ARIC study was approved by each site's institutional review board. Written informed consent was obtained from all participants.
Participants
The ARIC study began in 1987 with an initial cohort of 15,792 adults (age 45–64 years) recruited from 4 US communities (Washington County, Maryland; Forsyth County, North Carolina; Minneapolis, MN; and Jackson, MS), and has conducted 7 in-person examinations. At visit 5 (2011–2013; in-person study visit n = 6,538), plasma Aβ was measured in a subsample of 2,585 participants. This subsample was enriched for prevalent cognitive impairment at visit 5 (50% of subsample), with the remainder of the sample comprising cognitively unimpaired participants randomly selected across 2 age strata (<80 and ≥80 years). Evidence of prevalent cognitive impairment included low Mini-Mental State Examination (MMSE) score18 (<21 for White participants, <19 for Black participants), low scores on any cognitive domain from the visit 5 neuropsychological battery (<−1.5 z score), or significant decline in performance on any previously assessed cognitive test in ARIC (Digit Symbol Substitution Test, Delayed Word Recall, or Word Fluency). Further details regarding cognitive testing cutoffs and standardized factor scores have been reported previously.19,20 The specific MMSE score cutoffs were used to reduce the potential of misclassification of cognitive impairment, given the inherent limitation of psychometric instruments to adequately account for societal disparities related to education access and quality.19 All participants in this subsample additionally had plasma Aβ assayed at midlife from frozen blood samples from visit 3 (1993–1995) and were followed up prospectively beyond visit 5.
Participant sex, education, and race (White, Black, American Indian, or Asian) were self-reported at visit 1 (1987–1989). The analysis sample included only participants who self-reported race as White or Black. Covariates measured at visits 3 and 5 included hypertension (systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medications), diabetes (fasting glucose ≥126 mg/dL, nonfasting glucose ≥200 mg/dL [hemoglobin A1c ≥6.5% at visit 5 only], self-reported physician diagnosis, or use of oral diabetes medications or insulin), and body mass index (kilograms per meter squared). APOE ε4 carrier status was determined with the Taqman Assay (Applied Biosystems, Foster City, CA).
Plasma Aβ Measurements
Blood sampling procedures in ARIC have been described in detail.21 Briefly, 12-hour fasting EDTA whole-blood samples were collected and placed in an ice bath until plasma was separated out by centrifugation (10 minutes at 3,000g at 4°C). Plasma was divided into aliquots in 1.5-mL tubes, frozen, and stored in −80°C freezers. The plasma Aβ assay was performed in 2014 by the Department of Molecular Pharmacology and Experimental Therapeutics at the Mayo Clinic, Jacksonville, FL, with the commercially available INNO-BIA assay (Innogenetics, Ghent, Belgium). The INNO-BIA assay is a fluorometric bead-based (xMAP microspheres) immunoassay designed to simultaneously measure Aβ42 and Aβ40 in plasma, specifically peptides Aβ1-42 and Aβ1-40. Bound Aβ42 and Aβ40 emitted fluorescence that was detected by a Luminex 200 IS Total System instrument (Luminex Corp, Austin, TX). For each participant, the same plate was used to quantify plasma Aβ from visit 3 and 5 samples simultaneously. A logistic regression model predicted the concentrations of Aβ42 and Aβ40 (picograms per milliliter) by relating the observed fluorescence intensities to a standard curve. Intensities that were below the range of this curve could not be inferred. For these samples (visit 3: Aβ42 n = 163, Aβ40 n = 27; visit 5: Aβ42 n = 29, Aβ40 n = 3), the lower limits of detection threshold (12 pg/mL for Aβ42; 15 pg/mL for Aβ40) were assigned as values.
Dementia and Mild Cognitive Impairment Diagnosis
Full details of the mild cognitive impairment (MCI)/dementia diagnosis protocol in ARIC have been described elsewhere.22 Beginning at visit 5, all participants were identified as cognitively normal or possible MCI/dementia cases with the use of an algorithm that considered MMSE scores,18 Clinical Dementia Rating sum of boxes,23 concurrent performance on the neuropsychological test battery, and change in cognitive function from previous assessments. All cases that were identified as possible MCI/dementia by this algorithm and a sample of cognitively normal participants were reviewed by 2 experts (a physician and a neuropsychologist), who classified participants' cognitive status as normal, MCI, or dementia, with discordant diagnoses adjudicated by a third reviewer. The same procedure was used at follow-up during visits 6 (2016–2017) and 7 (2018–2019). Between ARIC visits, additional dementia, but not MCI, cases were identified over the phone by use of the Six-Item Screener24 and AD8,25 hospitalization records, and death certificates.
Statistical Analyses
Among the 2,585 participants sampled for plasma Aβ measurement, we excluded participants with missing/inconclusive cognition status (n = 7) or missing covariates (n = 294), resulting in an analytic sample of 2,284 Black and White adults. A comparison of the plasma Aβ sample to the full ARIC cohort at visit 3 is presented in eTable 1 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). For the current study, the primary outcome was defined as the most advanced stage of cognitive impairment a participant reached from visit 5 through visit 7 (normal, MCI, or dementia). For example, a participant classified as having MCI at visit 5 but dementia at visit 6 was considered to have dementia for the analysis. Similarly, a participant classified as having dementia at visit 5 but who died or did not return to visit 6 was considered to have dementia. Due to the sampling design, mortality (death) outcomes comprised only participants who were normal at visit 5 and died before any evidence of cognitive impairment. All participants were cognitively adjudicated as normal, MCI, or dementia at least once at visit 5, with follow-up adjudicated statuses at visits 6 and 7 if they had not died and returned for the clinic examination. We used multinomial logistic regression analyses for the 4-category outcome of normal status, MCI, dementia, and death to estimate relative risk ratios (RRR) as a function of plasma Aβ predictors adjusted for midlife age, sex, education, site-race, APOE ε4 carrier status, hypertension, diabetes, and body mass index. When estimates appeared similar for MCI and dementia, an additional analysis modeled RRR of a 3-category outcome of normal status, combined MCI/dementia, and death. All models were cross-temporal multinomial regressions and did not account for time to event because of the sampling design.
We used the midlife and late-life plasma Aβ predictors in 2 ways in our models. First, as is commonly done, we used a ratio of Aβ42 to Aβ40. Due to a considerably skewed distribution, the ratio term was base (2) log-transformed to better approximate a normal distribution. Second, we modeled Aβ42 and Aβ40 as separate terms in the same regression model. We examined nonlinear relationships between midlife plasma Aβ biomarkers and late-life cognitive outcome status using lowess smoothers. A single knot at 0.20 for plasma Aβ42:Aβ40 was statistically supported. For consistency, we used the same spline term when modeling late-life plasma Aβ42:Aβ40.
To investigate relations of change in Aβ42:Aβ40, Aβ42, and Aβ40 from midlife to late life to MCI and dementia risk, we tested additional multinomial logistic regression models including change score predictors (visit 5 − visit 3), adjusting for the same covariates and for baseline (visit 3) Aβ values. In these Aβ-change models, we additionally tested interaction terms of Aβ-change scores by Aβ baseline values to examine whether associations of Aβ-changes with cognitive outcomes might vary by initial (midlife) Aβ levels. Change models were also tested without adjustment for baseline values.
We conducted 3 sensitivity analyses. First, primary analyses were done excluding the participants (n = 190 at visit 3 and n = 32 at visit 5) with assay levels below the lower limit of detection, rather than assigning the lower limit values. Second, primary analyses were done without adjustment for APOE ε4 due to the strong association of this factor with Aβ clearance. Third, primary analyses were repeated in race-stratified samples (Black and White).
Data Availability
The ARIC study data used here are available to qualified investigators on request. Further details regarding data availability and study protocols are available elsewhere.26
Results
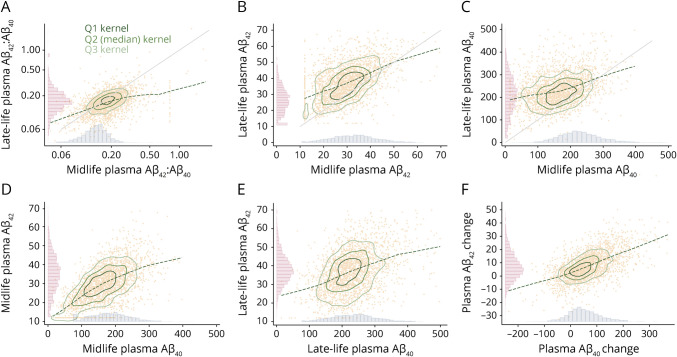
Descriptive statistics by cognitive outcome status are displayed in Table 1 (midlife) and eTable 2 (late life; data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). At the midlife plasma Aβ measurement, the average age of the sample was 59.2 ± 5.2 years (57% female, 22% Black), with an average Aβ42:Aβ40 ratio of 0.21 ± 0.12. At the time of late-life plasma Aβ measurements, the average age of the sample was 76.97 ± 5.30 years, with an average Aβ42:Aβ40 ratio of 0.17 ± 0.08. Figure 1 displays the distributions and scatterplots of Aβ42, Aβ40, and Aβ42:Aβ40 at both midlife and late-life measurements, as well as the change between measurements. Plasma Aβ42 and Aβ40 were positively related to each other concurrently and cross-temporally. Change in plasma Aβ42 and change in Aβ40 were similarly related. In addition, each visit 3 plasma Aβ measurement was positively related to the repeated visit 5 plasma Aβ measurement. Over 25 years of follow-up from the initial plasma Aβ assessment, 859 participants (38%) remained cognitively normal, 502 participants (22%) were classified as having dementia, 832 participants (36%) were classified as having MCI but not dementia, and 91 participants (4%) died without any classification of cognitive impairment. A comparison of midlife plasma Aβ measures across age, sex, and APOE ε4 carrier status is presented in eTable 3 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Older participants had higher levels of both plasma Aβ42 and Aβ40, but a slightly lower Aβ42:Aβ40. APOE ε4 carriers had lower plasma Aβ42 and Aβ42:Aβ40 compared to noncarriers and saw smaller increases in plasma Aβ42 from the midlife to late-life measurement. Compared to female participants, male participants had higher plasma Aβ42 and Aβ40, slightly lower Aβ42:Aβ40, and smaller increases in both Aβ42 and Aβ40 from the midlife to late-life measurement. All model estimates correspond to increases in the predictors (doubling of Aβ42:Aβ40; per 1-SD-higher Aβ42 or Aβ40), resulting in a lower estimated risk for an increase in Aβ42:Aβ40 or Aβ42 but an increased estimated risk for an increase in Aβ40.
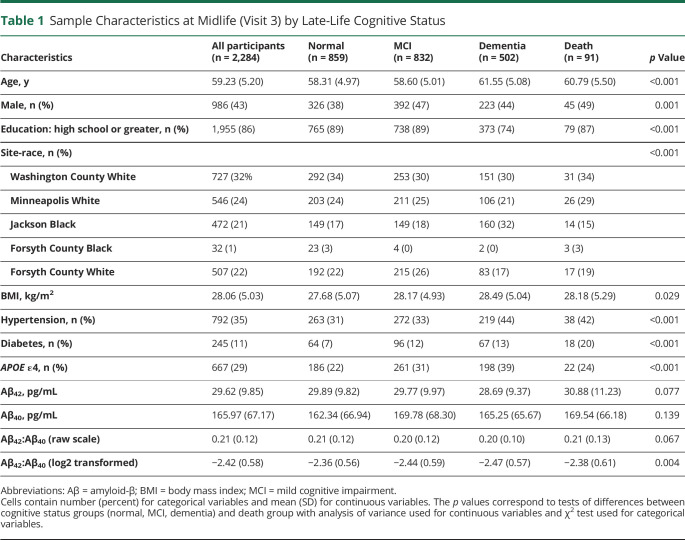
Table 1.
Sample Characteristics at Midlife (Visit 3) by Late-Life Cognitive Status
Figure 1. Midlife and Late-Life Plasma Aβ Distributions and Scatterplots Concurrently and Cross-Temporally.
Graphs display histograms of plasma amyloid-β (Aβ) distributions (picograms per milliliter or ratio) at midlife and late life. Change scores (midlife − late life; picograms per milliliter) reflect increases in Aβ42 and Aβ40 from midlife to late life. Individual observations are plotted with a lowess smoother and kernel density plots representing quartiles 1, 2 (median), and 3 of the data. Forty-six observations with Aβ42 or Aβ40 values exceeding 70 or 500 pg/mL, respectively, were removed for enhanced visualization. Ratio distributions in panel A have been log2 transformed to approximate normality. (A) Late-life and midlife plasma Aβ42:Aβ40; correlation 0.487. (B) Late-life and midlife plasma Aβ42 (picograms per milliliter); correlation 0.486. (C) Late-life and midlife plasma Aβ40 (picograms per milliliter); correlation 0.313. (D) Midlife plasma Aβ42 (picograms per milliliter) and Aβ40 (picograms per milliliter); correlation 0.612. (E) Late-life plasma Aβ42 (picograms per milliliter) and Aβ40 (picograms per milliliter); correlation 0.470. (F) Change in plasma Aβ42 (picograms per milliliter) and plasma Aβ40 (picograms per milliliter); correlation 0.612.
Midlife Plasma Aβ (Visit 3)
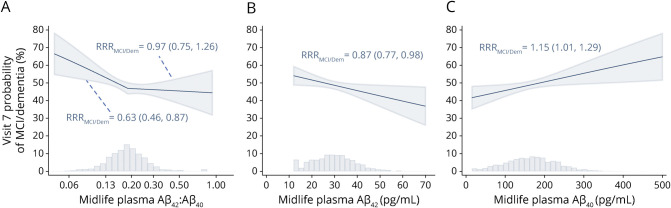
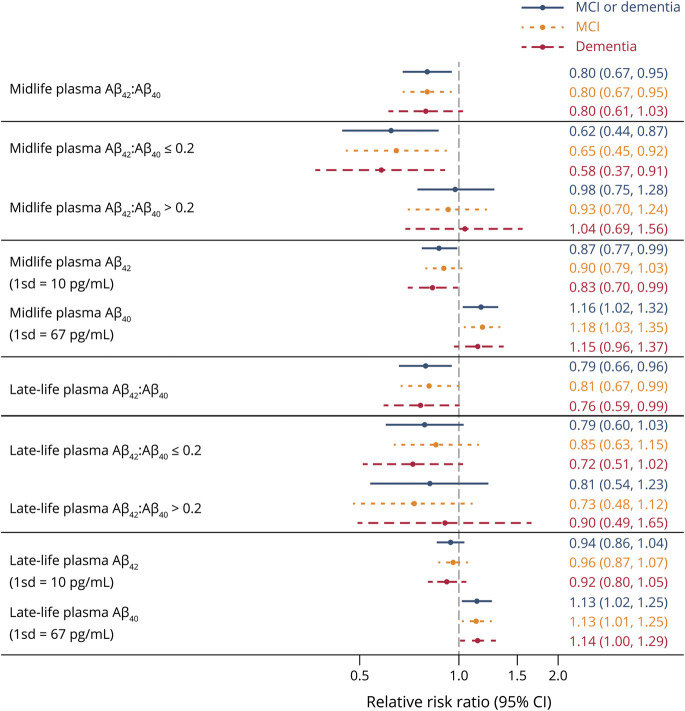
Combined MCI/dementia model-derived probabilities relative to cognitively normal as a function of increasing midlife plasma Aβ42:Aβ40, Aβ42, and Aβ40 are displayed in Figure 2. Each doubling of midlife Aβ42:Aβ40 was associated with a 37% lower risk of MCI/dementia compared to cognitively normal (RRR 0.63, 95% confidence interval [CI] 0.46–0.87), but only up to a threshold of 0.20, after which there was no relationship with risk (RRR 0.97, 95% CI 0.75–1.26). Each 1-SD-higher midlife Aβ42 (10 pg/mL) was associated with a 13% lower risk of MCI/dementia compared to cognitively normal (RRR 0.87, 95% CI 0.77–0.98), and each 1-SD-higher midlife Aβ40 (67 pg/mL) was associated with a 15% higher risk of MCI/dementia compared to cognitively normal (RRR 1.15, 95% CI 1.01–1.29). Similar estimates were seen when MCI and dementia were examined as separate outcomes (Figure 3). Additional model-derived probabilities of normal, MCI, and dementia by midlife plasma Aβ predictors are presented in eFigure 1 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Figure 3 presents RRR for all plasma Aβ predictors at midlife and late life comparing MCI, dementia, and combined MCI/dementia outcomes to cognitively normal. Model results for additional pairwise comparisons, including RRR for dementia vs MCI and death vs normal, are presented in eTable 4 for midlife plasma Aβ (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Full model results, including all covariate estimates for midlife plasma Aβ associations with MCI/dementia, are displayed in eTable 5 (Data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33).
Figure 2. Midlife (Visit 3) Plasma Aβ Associations With Visit 7 MCI/Dementia Probabilities.
Relative risk ratio (RRRs; normal referent) given with 95% confidence limits. Histograms display the midlife distributions of amyloid-β42 (Aβ42):Aβ40, Aβ42, and Aβ40. Two observations with Aβ42 or Aβ40 values exceeding 70 or 500 pg/mL, respectively, were removed for enhanced visualization. (A) Each doubling of Aβ42:Aβ40 when Aβ42:Aβ40 is ≤0.20 was associated with an RRR of mild cognitive impairment (MCI)/dementia vs normal of 0.63 (p = 0.005). Each doubling of Aβ42:Aβ40 when Aβ42:Aβ40 is >0.2 was associated with an RRR of MCI/dementia vs normal of 0.97 (p = 0.813). (B) Each 1-SD-higher Aβ42 (10 pg/mL) across the entire spectrum of Aβ42 was associated with an RRR of MCI/dementia vs normal of 0.87 (p = 0.026). (C) Each 1-SD-higher Aβ40 (67 pg/mL) across the entire spectrum of Aβ40 was associated with an RRR of MCI/dementia vs normal of 1.15 (p = 0.030).
Figure 3. Midlife (Visit 3) and Late-Life (Visit 5) Plasma Aβ Associations With Visit 7 Cognitive Status.
Estimates represent relative risk ratios (RRRs; and 95% confidence intervals [CIs]) from multinomial models adjusted for age, sex, race-site, education, hypertension, diabetes, body mass index, and APOE ε4. Referent group was cognitively normal. All estimates are from separate models for linear amyloid-β (Aβ) ratio, Aβ ratio with splines, and continuous Aβ42 and Aβ40 at each visit.
Late-Life Plasma Aβ (Visit 5)
Figure 3 further presents risk for MCI, dementia, and combined MCI/dementia outcomes compared to cognitively normal by late-life (visit 5) plasma Aβ predictors. Each doubling of midlife Aβ42:Aβ40 was associated with a 23% lower risk of MCI/dementia compared to cognitively normal (RRR 0.77, 95% CI 0.59–1.00), but only up to the threshold of 0.20 established from midlife plasma Aβ models, after which there was no relationship with risk (RRR 0.83, 95% CI 0.55–1.25). Contrasting midlife associations, higher late-life Aβ42 was not associated with MCI or dementia. However, each 1-SD-higher late-life Aβ40 (67 pg/mL) was associated with a 14% higher risk of MCI/dementia compared to cognitively normal (RRR 1.14, 95% CI 1.02–1.26). Model results for additional pairwise comparisons, including risk for death vs normal and dementia vs MCI, are presented in eTable 6 for late-life plasma Aβ (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Model-derived probabilities of normal, MCI, and dementia by late-life plasma Aβ predictors are presented in eFigure 2 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Full model results, including all covariate estimates for late-life plasma Aβ associations with MCI/dementia, are displayed in eTable 5 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33).
Midlife to Late-Life Change in Plasma Aβ
Results examining change in plasma Aβ predictors from midlife (visit 3) to late life (visit 5) in relation to visit 7 cognitive outcomes are displayed in eTable 7 (data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33). Associations of changes in plasma Aβ predictors with risks of MCI or dementia were not supported in any of the models examined, regardless of whether baseline plasma Aβ was adjusted for.
Sensitivity Analyses
Primary model results were unchanged in sensitivity analyses excluding participants assigned the lower limit of detection for plasma Aβ42 and Aβ40 at either midlife or late-life measurements (data not shown). In addition, removing APOE ε4 from the adjuster set did not significantly alter the estimates in the primary models. Estimates relating plasma Aβ42 and Aβ40 to cognitive outcomes did not differ by race for midlife or late-life models (eTables 8 and 9, respectively; data available from Dryad: doi.org/10.5061/dryad.m0cfxpp33).
Discussion
We investigated the association of midlife and late-life plasma Aβ42 and Aβ40 with risk of MCI or dementia classification in a large community-based sample followed up over 25 years. Lower plasma Aβ42 measured at midlife, but not late life, was associated with higher risk of dementia and marginally higher risk of MCI. Conversely, higher plasma Aβ40 was associated with higher risk of MCI and dementia, whether measured at midlife or late life. A lower midlife and late-life plasma Aβ42:Aβ40 ratio was associated with higher risk of MCI and dementia, but only up to a threshold of 0.20, after which an increasing ratio was not related to risk. A doubling of the Aβ42:Aβ40 ratio under this threshold at midlife was comparable to ≈5 years of younger age in terms of risk of MCI or dementia, whereas a doubling of the Aβ42:Aβ40 ratio under this threshold at late life was comparable to ≈3 years younger age. On average, plasma Aβ42 and Aβ40 levels increased significantly from midlife to late life, although changes were highly variable. However, we observed no association between change in plasma Aβ42, Aβ40, or Aβ42:Aβ40 and risk of MCI or dementia. All associations were independent of age, sex, education, race, APOE ε4, and cardiovascular risk factors. In addition, there was no evidence of effect moderation by race.
Our findings are consistent with previous reports associating lower levels of plasma Aβ42 and plasma Aβ42:Aβ40 ratio with all-cause and AD dementia,9,27 although not all studies have observed these associations.28,29 Differences in assay methods, study populations, and study time frames may explain much of the heterogeneity in reported effects of plasma Aβ related to cognitive impairment or dementia. This study reports these associations in a large community-based sample at midlife, with an average age of <60 years at the time of first plasma Aβ measurement, over a long follow-up period. Associations between plasma Aβ biomarkers and risk of MCI and dementia were comparable or stronger in the case of midlife plasma Aβ42, whether measured at midlife or late life. The advantage gained by a 20-year head start in screening has the potential to have considerable impact on future research on the epidemiology of dementia and AD. Associations between midlife plasma Aβ and MCI such as those reported here suggest particular utility in this regard because these biomarkers are associated with early-stage cognitive impairment.
The current study adds to existing literature by reporting the nonlinear relationship between plasma Aβ42:Aβ40 ratio and risk of cognitive impairment. While previous studies have reported that a higher Aβ42:Aβ40 ratio is associated with a lower risk of cognitive impairment, we observed this relationship only up to a threshold of 0.20 identified in midlife analyses. For every doubling of the ratio under this threshold, we reported 35% and 40% lower risk of MCI and dementia, respectively, with no additional protective association after 0.20. This same threshold was relevant in analyses of late-life plasma Aβ42:Aβ40; again, most of the relationship of increasing ratio with reduced risk of MCI and dementia was restrained to participants with values <0.20. These results may suggest that there is particular vulnerability in individuals with very low plasma Aβ42 in relation to plasma Aβ40 compared to individuals with average or high ratio values. Furthermore, while plasma Aβ42 and Aβ42:Aβ40 have garnered more attention as biomarkers for AD given their presumed relationship to CNS Aβ accumulation, we also report an association plasma Aβ40 and risk of MCI and dementia. Given the association between plasma Aβ40 and cerebrovascular pathologies, including white matter lesions11 and cerebral microbleeds,30 it is likely that this association is not specific to AD and may reflect multiple pathologic bases for cognitive impairment.
In contrast to other studies investigating change in plasma Aβ measurements over time in relation to cognitive function31 and AD diagnosis,32 we observed that change was not significantly associated with risk of MCI/dementia when accounting for baseline value. The Nurses’ Health Study reported that both baseline and 10-year change in Aβ42:Aβ40 ratio were related to greater decline in global cognition.31 In contrast to our findings, another previous study of dementia-free older adults suggested that higher baseline plasma Aβ42 was associated with higher 5-year risk of AD but that declines in plasma Aβ42 and plasma Aβ42:Aβ40 over this time period were also associated with incident AD.32 It is unclear whether either of these previous studies adjusted for baseline plasma Aβ values as was done in this study, although we observed no associations regardless of baseline adjustment.
There are some limitations in our study to consider. The analysis sample was conditionally selected for outcome status at visit 5 with retrospective ascertainment of the exposure at visit 3. The case-control methodology used was beneficial in assaying plasma in as many cases as possible, but this potentially contributed to some degree of survivor bias in that only the participants who attended visit 5 of the study had plasma Aβ assayed at midlife (visit 3). However, comparisons between the total ARIC cohort at visit 3 and the plasma Aβ sample did not suggest that this bias was large. This study used a commercially available immunoassay to estimate plasma Aβ concentrations. These relatively inexpensive and accessible assays are well suited to large-scale biomarker quantification at a population level, having been successfully implemented in large ARIC and Framingham Heart Study samples,9 but fall short in precision compared to measurements granted by newer mass spectrometry and single-molecule array methods.3,4,33 Of note, high-precision classification reported in other plasma Aβ assays is specific to Aβ(+) PET scans, not an adjudicated cognitive classification as is presented here. It is unknown whether potential imprecision in our assay method contributed to bias in our estimated effects in either direction. Last, like all plasma Aβ measurements, our estimates included circulating Aβ produced by platelets and cannot isolate plasma Aβ that has been cleared from the CNS.
Acknowledgment
The authors thank the staff and participants of the ARIC study for their important contributions.
Glossary
- Aβ
amyloid-β
- AD
Alzheimer disease
- ARIC
Atherosclerosis Risk in Communities
- BMI
body mass index
- CI
confidence interval
- MCI
mild cognitive impairment
- MMSE
Mini-Mental State Examination
- RRR
relative risk ratio
Appendix. Authors
Study Funding
The ARIC study has been funded in whole or in part with federal funds from the National Heart, Lung, and Blood Institute (NHLBI), NIH, Department of Health and Human Services, under contracts HHSN268201700001I, HHSN268201700002I, HHSN268201700003I, HHSN268201700005I, and HHSN268201700004I. Neurocognitive data are collected by U01 2U01HL096812, 2U01HL096814, 2U01HL096899, 2U01HL096902, 2U01HL096917, from the NIH (NHLBI, National Institute for Neurological Disorders and Stroke, National Institute on Aging, and National Institute on Deafness and Other Communication Disorders).
Disclosure
K.J. Sullivan, C. Blackshear, J. Simino, A. Tin, K.A. Walker, A.R. Sharrett, and S. Younkin report no disclosures relevant to the manuscript. R.F. Gottesman reports being a former associate editor for Neurology®. This article was prepared while R.F. Gottesman was employed at the Johns Hopkins University School of Medicine. The opinions expressed in this article are the author's own and do not reflect the view of the National Institutes of Health, the Department of Health and Human Services, or the United States Government. M.M. Mielke reports consulting for Biogen and Brain Protection Co. D. Knopman reports serving on a Data Safety Monitoring Board for the Dominantly Inherited Alzheimer’s Network (DIAN) study. He serves on a Data Safety Monitoring Board for a tau therapeutic for Biogen but receives no personal compensation. He is an investigator in clinical trials sponsored by Lilly Pharmaceuticals and the University of Southern California. He serves as a consultant for Samus Therapeutics, Third Rock, Roche, and Alzeca Biosciences but receives no personal compensation. B.G. Windham, M.E. Griswold, and T.H. Mosley report no disclosures relevant to the manuscript. Go to Neurology.org/N for full disclosures.
References
- 1.Jack CR Jr, Bennett DA, Blennow K, et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Verberk IMW, Slot RE, Verfaillie SCJ, et al. Plasma amyloid as prescreener for the earliest Alzheimer pathological changes. Ann Neurol. 2018;84(5):648-658. doi: 10.1002/ana.25334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nakamura A, Kaneko N, Villemagne VL, et al. High performance plasma amyloid-beta biomarkers for Alzheimer's disease. Nature. 2018;554(7691):249-254. [DOI] [PubMed] [Google Scholar]
- 4.Schindler SE, Bollinger JG, Ovod V, et al. High-precision plasma beta-amyloid 42/40 predicts current and future brain amyloidosis. Neurology. 2019;93(17):e1647–e1659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ovod V, Ramsey KN, Mawuenyega KG, et al. Amyloid beta concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimers Dement. 2017;13(8):841-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Doecke JD, Perez-Grijalba V, Fandos N, et al. Total Abeta42/Abeta40 ratio in plasma predicts amyloid-PET status, independent of clinical AD diagnosis. Neurology. 2020;94(15):e1580–e1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Graff-Radford NR, Crook JE, Lucas J, et al. Association of low plasma Abeta42/Abeta40 ratios with increased imminent risk for mild cognitive impairment and Alzheimer disease. Arch Neurol. 2007;64(3):354-362. [DOI] [PubMed] [Google Scholar]
- 8.Lambert JC, Schraen-Maschke S, Richard F, et al. Association of plasma amyloid beta with risk of dementia: the prospective Three-City Study. Neurology. 2009;73(11):847-853. [DOI] [PubMed] [Google Scholar]
- 9.Chouraki V, Beiser A, Younkin L, et al. Plasma amyloid-beta and risk of Alzheimer's disease in the Framingham Heart Study. Alzheimers Dement. 2015;11(3):249-257 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Wolf F, Ghanbari M, Licher S, et al. Plasma tau, neurofilament light chain and amyloid-beta levels and risk of dementia; a population-based cohort study. Brain. 2020;143(4):1220-1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Janelidze S, Stomrud E, Palmqvist S, et al. Plasma beta-amyloid in Alzheimer's disease and vascular disease. Sci Rep. 2016;6:26801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sperling RA, Aisen PS, Beckett LA, et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jack CR, Jr., Knopman DS, Jagust WJ, et al. Tracking pathophysiological processes in Alzheimer's disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 2013;12(2):207-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Babulal GM, Quiroz YT, Albensi BC, et al. Perspectives on ethnic and racial disparities in Alzheimer's disease and related dementias: update and areas of immediate need. Alzheimers Dement. 2019;15(2):292-312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.2021 Alzheimer's disease facts and figures. Alzheimers Dement. 2021;17(3):327-406. [DOI] [PubMed] [Google Scholar]
- 16.Launer LJ, Petrovitch H, Ross GW, Markesbery W, White LR. AD brain pathology: vascular origins? Results from the HAAS autopsy study. Neurobiol Aging. 2008;29(10):1587-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iadecola C. The overlap between neurodegenerative and vascular factors in the pathogenesis of dementia. Acta Neuropathol. 2010;120(3):287-296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Folstein MF, Folstein SE, McHugh PR. “Mini-Mental State": a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189-198. [DOI] [PubMed] [Google Scholar]
- 19.Schneider AL, Sharrett AR, Gottesman RF, et al. Normative data for 8 neuropsychological tests in older blacks and whites from the Atherosclerosis Risk in Communities (ARIC) study. Alzheimer Dis Assoc Disord. 2015;29(1):32-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rawlings AM, Bandeen-Roche K, Gross AL, et al. Factor structure of the ARIC-NCS Neuropsychological Battery: an evaluation of invariance across vascular factors and demographic characteristics. Psychol Assess. 2016;28(12):1674-1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Papp AC, Hatzakis H, Bracey A, Wu KK. ARIC hemostasis study, I: development of a blood collection and processing system suitable for multicenter hemostatic studies. Thromb Haemost. 1989;61(1):15-19. [PubMed] [Google Scholar]
- 22.Knopman DS, Gottesman RF, Sharrett AR, et al. Mild cognitive impairment and dementia prevalence: the Atherosclerosis Risk in Communities Neurocognitive Study (ARIC-NCS). Alzheimers Dement (Amst). 2016;2:1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412-2414. [DOI] [PubMed] [Google Scholar]
- 24.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40(9):771-781. [DOI] [PubMed] [Google Scholar]
- 25.Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559-564. [DOI] [PubMed] [Google Scholar]
- 26.ARIC: "Research With Heart" since 1987. Accessed February 22, 2021. sites.cscc.unc.edu/aric/.
- 27.Koyama A, Okereke OI, Yang T, Blacker D, Selkoe DJ, Grodstein F. Plasma amyloid-beta as a predictor of dementia and cognitive decline: a systematic review and meta-analysis. Arch Neurol. 2012;69(7):824-831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez OL, Kuller LH, Mehta PD, et al. Plasma amyloid levels and the risk of AD in normal subjects in the Cardiovascular Health Study. Neurology. 2008;70(19):1664-1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lovheim H, Elgh F, Johansson A, et al. Plasma concentrations of free amyloid beta cannot predict the development of Alzheimer's disease. Alzheimers Dement. 2017;13(7):778-782. [DOI] [PubMed] [Google Scholar]
- 30.Romero JR, Demissie S, Beiser A, et al. Relation of plasma beta-amyloid, clusterin, and tau with cerebral microbleeds: Framingham Heart Study. Ann Clin Transl Neurol. 2020;7(7):1083-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Okereke OI, Xia W, Selkoe DJ, Grodstein F. Ten-year change in plasma amyloid beta levels and late-life cognitive decline. Arch Neurol. 2009;66(10):1247-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schupf N, Tang MX, Fukuyama H, et al. Peripheral Abeta subspecies as risk biomarkers of Alzheimer's disease. Proc Natl Acad Sci USA. 2008;105(37):14052-14057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Li D, Mielke MM. An update on blood-based markers of Alzheimer's disease using the SiMoA platform. Neurol Ther. 2019;8(suppl 2):73-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The ARIC study data used here are available to qualified investigators on request. Further details regarding data availability and study protocols are available elsewhere.26