Key Points
Question
Are there differences in mortality and stroke between patients at low surgical risk who undergo transcatheter aortic valve replacement (TAVR) for bicuspid compared with tricuspid aortic stenosis?
Findings
In this registry-based cohort study that included 3168 propensity-matched pairs of patients at low surgical risk undergoing TAVR for bicuspid vs tricuspid aortic stenosis, there was no significant difference in death at 30 days (0.9% vs 0.8%), death at 1 year (4.6% vs 6.6%), stroke at 30 days (1.4% vs 1.2%), or stroke at 1 year (2.0% vs 2.1%).
Meaning
Patients at low surgical risk who underwent TAVR for bicuspid aortic stenosis compared with tricuspid aortic stenosis had no significant difference in mortality or stroke at 30 days or 1 year. Because of the potential for selection bias, randomized trials would be needed to adequately assess the efficacy and safety of TAVR for bicuspid aortic stenosis.
Abstract
Importance
There are limited data on outcomes of transcatheter aortic valve replacement (TAVR) for bicuspid aortic stenosis in patients at low surgical risk.
Objective
To compare the outcomes of TAVR with a balloon-expandable valve for bicuspid vs tricuspid aortic stenosis in patients who are at low surgical risk.
Design, Setting, and Participants
Registry-based cohort study of patients undergoing TAVR at 684 US centers. Participants were enrolled in the Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapies Registry from June 2015 to October 2020. Among 159 661 patients (7058 bicuspid, 152 603 tricuspid), 37 660 patients (3243 bicuspid and 34 417 tricuspid) who were at low surgical risk (defined as STS risk score <3%) were included in the analysis.
Exposures
TAVR for bicuspid vs tricuspid aortic stenosis.
Main Outcomes and Measures
Coprimary outcomes were 30-day and 1-year mortality and stroke. Secondary outcomes included procedural complications and valve hemodynamics.
Results
Among 159 661 patients (7058 bicuspid; 152 603 tricuspid), 3168 propensity-matched pairs of patients with bicuspid and tricuspid aortic stenosis at low surgical risk were analyzed (mean age, 69 years; 69.8% men; mean [SD] STS-predicted risk of mortality, 1.7% [0.6%] for bicuspid and 1.7% [0.7%] for tricuspid). There was no significant difference between the bicuspid and tricuspid groups’ rates of death at 30 days (0.9% vs 0.8%; hazard ratio [HR], 1.18 [95% CI, 0.68-2.03]; P = .55) and at 1 year (4.6% vs 6.6%; HR, 0.75 [95% CI, 0.55-1.02]; P = .06) or stroke at 30 days (1.4% vs 1.2%; HR, 1.14 [95% CI, 0.73-1.78]; P = .55) and at 1 year (2.0% vs 2.1%; HR 1.03 [95% CI, 0.69-1.53]; P = .89).There were no significant differences between the bicuspid and tricuspid groups in procedural complications, valve hemodynamics (aortic valve gradient: 13.2 mm Hg vs 13.5 mm Hg; absolute risk difference [RD], 0.3 mm Hg [95% CI, −0.9 to 0.3 mm Hg]), and moderate or severe paravalvular leak (3.4% vs 2.1%; absolute RD, 1.3% [95% CI, −0.6% to 3.2%]).
Conclusions and Relevance
In this preliminary, registry-based study of propensity-matched patients at low surgical risk who had undergone TAVR for aortic stenosis, patients treated for bicuspid vs tricuspid aortic stenosis had no significant difference in mortality or stroke at 30 days or 1 year. Because of the potential for selection bias and absence of a control group treated surgically for bicuspid aortic stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis in patients at low surgical risk.
This registry-based cohort study compared rates of mortality and stroke at 30 days and 1 year among patients at low surgical risk with bicuspid vs tricuspid aortic stenosis undergoing transcatheter aortic valve replacement (TAVR).
Introduction
In 2018, bicuspid aortic valve was estimated to be present in as much as 1% of the population1,2 and was associated with early degeneration leading to aortic stenosis or regurgitation.3 Of patients undergoing surgery for aortic stenosis, 15% to 29% have been reported to have bicuspid aortic valve.4,5,6 The recent pivotal low-risk randomized clinical trials expanded the indication of transcatheter aortic valve replacement (TAVR) toward aortic stenosis patients who have lower surgical risk and are younger7,8; however, these trials excluded bicuspid anatomy. Previously published registries on outcomes of TAVR in bicuspid aortic stenosis were limited to small sample size9 or older patients who were at higher risk for surgery.10,11 Given that bicuspid anatomy was frequently present in younger and low-risk patients undergoing surgery, the data on outcomes of TAVR in bicuspid aortic stenosis are crucial to guide the treatment of bicuspid aortic stenosis in these low-risk patients.
Since the US Food and Drug Administration’s 2019 approval of TAVR in patients at low surgical risk, an increasing number of patients at low surgical risk and bicuspid aortic stenosis have undergone TAVR. The Society of Thoracic Surgeons (STS)/American College of Cardiology (ACC) Transcatheter Valve Therapy (TVT) Registry includes all TAVR procedures performed in the US. The present study evaluated the outcomes of TAVR with contemporary balloon-expandable transcatheter heart valves for bicuspid aortic stenosis in this national registry, with a focus on patients at low surgical risk.
Methods
Study Population
All consecutive patients undergoing TAVR with contemporary balloon-expandable transcatheter heart valves (third-generation SAPIEN 3 and fourth-generation SAPIEN 3 Ultra valve [Edwards Lifesciences]) in the US, since commercial approval in June 2015 through October 2020, were evaluated in the present study. Patient follow-up ended on October 16, 2020. Clinical data was site-reported, according to the TVT Registry data dictionary. The registry protocol was granted a waiver of informed consent by Advarra, a centralized institutional review board (https://www.advarra.com/).
The primary population of interest comprised patients at low surgical risk undergoing TAVR (Figure 1). Low surgical risk was defined as having an STS predicted risk of mortality (STS-PROM) score of less than 3% (score range, 0%-100% [higher scores indicate higher risk of death within 30 days after surgery]). The secondary population comprised all patients, irrespective of surgical risk, undergoing TAVR for aortic stenosis (overall cohort). The outcomes of TAVR for bicuspid and tricuspid aortic stenosis in patients at low surgical risk, as well as patients in the overall cohort were compared.
Figure 1. Development of the Study Cohort.
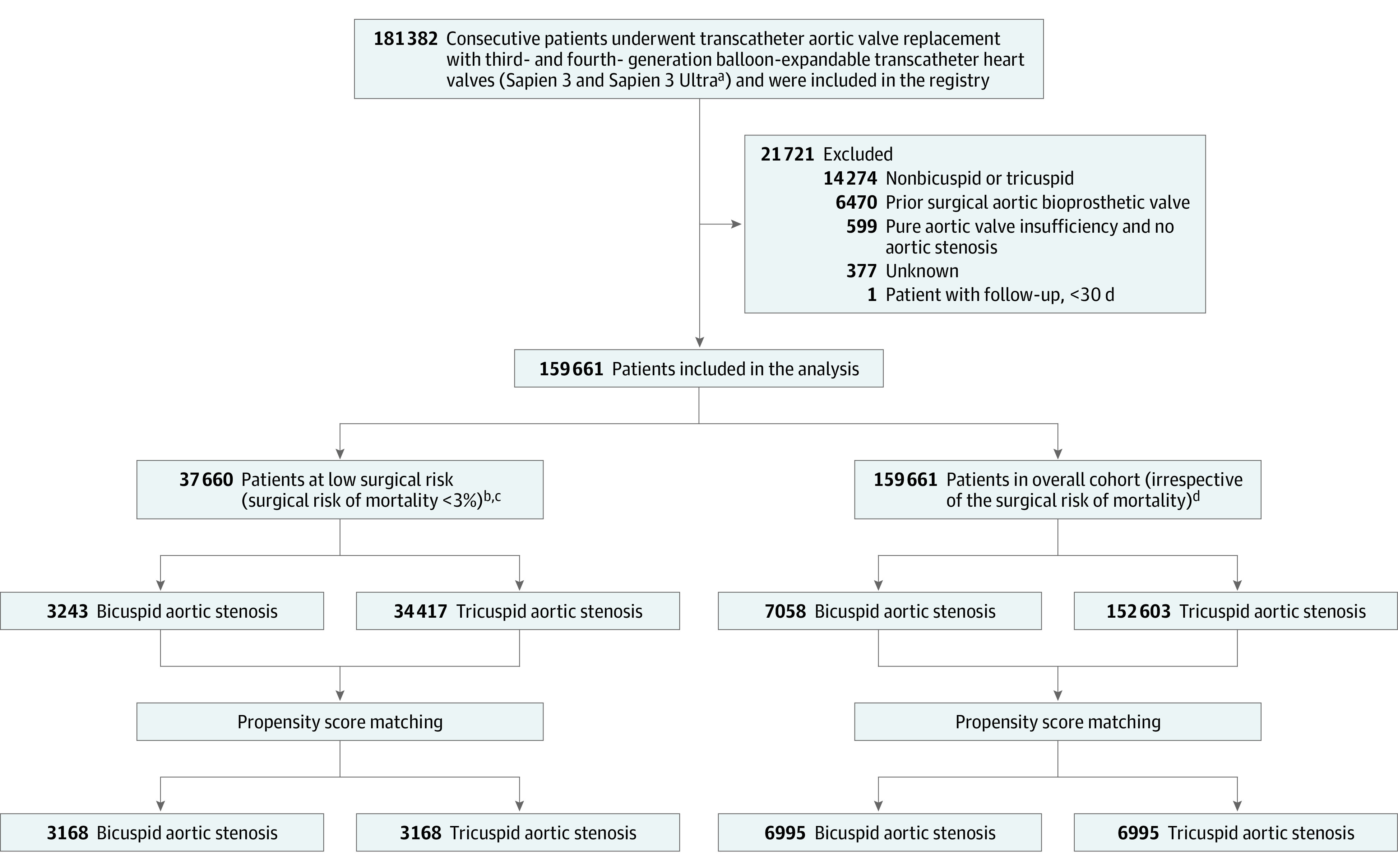
Patients with aortic stenosis undergoing transcatheter aortic valve replacement with the contemporary balloon-expandable transcatheter heart valves were enrolled in the Society of Thoracic Surgeons (STS)/American College of Cardiology Transcatheter Valve Therapy Registry.
aCompared with the third-generation balloon-expandable valves, the fourth-generation balloon-expandable transcatheter heart valve has a textured polyethylene terephthalate outer skirt with 40% increased height, which was designed to promote enhanced healing and endothelialization.
bSurgical risk of mortality was assessed with the STS Predicted Risk of Mortality score (score range, 0%-100% [higher scores indicate higher risk of death within 30 days after surgery]).
cThe mean (SD) risk of death at 30 days for patients in the low-risk cohort was 1.7% (0.6%).
dThe mean (SD) risk of death at 30 days in the overall cohort was 4.0% (3.6%).
Outcomes
The primary outcomes were the rates of death and stroke at 30 days and 1 year. The secondary outcomes included procedural and in-hospital outcomes, echocardiographic outcomes, functional status (New York Heart Association [NYHA] heart failure class), and health status (the Kansas City Cardiomyopathy Questionnaire overall summary score [KCCQ-OS]; score range, 0-100 [higher scores indicate less symptom burden and better quality of life]).12,13
Statistical Analysis
A detailed statistical analysis methodology is summarized in the eAppendix in the Supplement. Continuous variables were presented as mean (SD) or median interquartile ranges (IQR) and were compared between groups using 2-sample t tests or Wilcoxon rank-sum tests. Categorical variables were given as frequencies and percentages and were compared using χ2 or 2-tailed Fisher exact tests. The 30-day and 1-year adverse event rates were based on Kaplan-Meier estimates and all comparisons were made using the log-rank test.
It was anticipated that patients with bicuspid and tricuspid aortic stenosis would have significantly different baseline and procedural characteristics. The statistical analysis plan prespecified the use of propensity score–based matching to account for these differences between patients with bicuspid and tricuspid aortic stenosis.14 Propensity scores were calculated using a logistic regression model based on 29 baseline patient characteristics (covariates) with aortic valve type (bicuspid or tricuspid) as the dependent variable. The covariates included age, male sex, body mass index (calculated as weight in kilograms divided by height in meters squared), access site, prior percutaneous coronary intervention, prior coronary artery bypass graft surgery, prior stroke, carotid stenosis, peripheral arterial disease, hypertension, diabetes, chronic lung disease, immunocompromised status, porcelain aorta, atrial fibrillation, creatinine level, hemoglobin level, estimated glomerular filtration ratio, aortic valve mean gradient, left ventricular ejection fraction, mitral regurgitation, tricuspid regurgitation, NYHA functional class III/IV, 5-meter walk test time, STS-PROM score, shock within 24 hours prior to TAVR, hemodialysis, hostile chest, and KCCQ-OS score. The missing baseline values were imputed using the Markov Chain Monte Carlo method prior to modeling with a single imputation (eTable 1 in the Supplement). The primary analysis was performed with imputed data. Patients with bicuspid aortic stenosis were matched 1 to 1 to those with tricuspid aortic stenosis using a greedy matching strategy with a caliper of 0.01, producing 2 patient cohorts. Balance between the groups was assessed by calculating standardized differences for which an absolute difference of less than 0.10 was considered to indicate good balance.
Sensitivity analyses were performed to investigate the robustness of the results. The outcomes were assessed in 3 additional propensity score–matched cohorts: (1) patients determined to be at low surgical risk by the multidisciplinary heart team at the treating sites; (2) patients younger than 65 years; and (3) patients who reached 1 year prior to the data collection date. The sensitivity analyses were performed without imputation. The outcomes were also compared between propensity-matched patient pairs undergoing TAVR with the third- and fourth-generation balloon-expandable transcatheter heart valves for bicuspid aortic stenosis.
Multiple imputation was performed to account for missing outcomes data with the following assumptions15: (1) all patients with bicuspid aortic stenosis from the matched population who did not have a primary end point event and were missing 1-year follow-up were imputed based on missing data at random assumption (ie, they were considered to be similar to the observed patients with bicuspid aortic stenosis who either had primary end point events or completed the 1-year follow-up); and (2) all patients with tricuspid aortic stenosis from the matched population who did not have a primary end point event and were missing 1-year follow-up were imputed based on missing data at random assumption (ie, they were considered to be similar to the observed patients with tricuspid aortic stenosis who either had primary end point events or completed the 1-year follow-up). For missing follow-up primary end points (death and stroke), the imputed results (event and day of event) were generated from a uniform distribution probability in comparison with the observed conditional cumulative hazard function following the missing assumptions defined previously. First, a random number x from the uniform distribution between 0 and 1 was generated. If the value of x was less than the cumulative hazard function of patients with complete information at the end of follow-up time (1 year) then the event binary variable was imputed as yes; if otherwise, it was imputed as no. If the imputed event binary variable was yes, then the failure time of event was further imputed based on the value of x (impute failure time as t if value of x was between cumulative hazard function of time t and t +1). The overall multiple imputation estimate event rates and rate difference between patients with bicuspid and tricuspid aortic stenosis were obtained by averaging the event rate for each imputation. The overall estimated variance was the mean of the sum of all the estimated variances of each imputation. The statistical inference of the planned primary analysis was applied to estimate event rate and hazard ratio (HR) with 95% CI. For missing data on secondary end points (KCCQ score, NYHA functional class, and perivalvular leak), the missing baseline (discharge), 30-day and 1-year values were imputed using the Markov Chain Monte Carlo method based on missing data at random assumption.
Cox regression analysis was performed to assess the adjusted HR of patients with bicuspid vs tricuspid aortic stenosis on primary end points. The candidate covariates were identical to those used in the propensity score model (except the STS PROM score). The model was checked for violation of the proportional hazard assumption by Kolmogorov-type supremum test, and no relevant violations were found.
All P values were 2-sided, and P < .05 was considered significant for all tests. No adjustment for multiple testing was undertaken. Because of the potential for type I error due to multiple comparisons, all findings of this study should be interpreted as exploratory. All statistical analyses were performed using SAS version 9.4 (SAS Institute Inc). SAS Proc MI was used for multiple imputation, Proc Phreg for Cox regression model, and Proc logistics for propensity score calculation.
Results
Patient Population
Between June 2015 and October 2020, a total of 159 661 (7058 bicuspid, 152 603 tricuspid) patients underwent TAVR for aortic stenosis with balloon-expandable transcatheter heart valves at 684 institutions in the US. A total of 37 660 patients (3243 with bicuspid aortic stenosis and 34 417 with tricuspid aortic stenosis) were included in the primary analysis. Propensity-score matching between patients with bicuspid and tricuspid aortic stenosis was performed, generating 3168 low surgical-risk pairs and 6995 all-risk pairs (Figure 1). The temporal trends in the use of TAVR for bicuspid aortic stenosis are summarized in eFigures 1 and 2 in the Supplement. The proportion of patients at low surgical risk who underwent TAVR increased from 9.6% in 2015 to 43.8% in 2020 (eFigure 1 in the Supplement). The proportion of patients with bicuspid aortic stenosis undergoing TAVR increased from 2.8% in 2015 to 6.8% in 2020 (eFigure 2 in the Supplement). The proportion of patients with bicuspid aortic stenosis undergoing TAVR was 8.6% in the low-surgical risk group and 20.7% in patients younger than 65 years.
There were significant differences in baseline and procedural characteristics in unmatched patients with bicuspid and tricuspid aortic stenosis (Table 1). In the propensity score–matched cohorts, baseline and procedural characteristics were balanced with mean age of 69 years and STS-PROM score of 1.7% in both groups (Table 1).
Table 1. Baseline Demographic, Echocardiographic, and Procedural Characteristics of Patients at Less Than 3% Risk of 30-Day Surgical Mortality Who Underwent Transcatheter Aortic Valve Replacement.
| Characteristica | Unadjusted cohortb | Propensity score–matched cohortb,c | ||||
|---|---|---|---|---|---|---|
| Bicuspid aortic stenosis (n = 3243) | Tricuspid aortic stenosis (n = 34 417) | Absolute standardized difference | Bicuspid aortic stenosis (n = 3168) | Tricuspid aortic stenosis (n = 3168) | Absolute standardized difference | |
| Demographic | ||||||
| Age, mean (SD), y | 68.4 (9.1) | 75.6 (7.7) | 0.862 | 68.8 (8.7) | 68.7 (9.0) | 0.014 |
| Men | 2246/3242 (69.3) | 23 689/34 412 (68.8) | 0.009 | 2190/3167 (69.2) | 2230/3168 (70.4) | 0.027 |
| Women | 996/3242 (30.7) | 10 723/34 412 (31.2) | 977/3167 (30.8) | 938/3168 (29.6) | ||
| Body mass index, mean (SD)d | 30.0 (7.3) | 30.3 (6.8) | 0.044 | 30.1 (7.3) | 30.1 (6.5) | 0.0003 |
| Racee | ||||||
| American Indian or Alaska Native | 8/3243 (0.2) | 87/34 417 (0.3) | 0.001 | 8/3168 (0.3) | 8/3168 (0.3) | 0 |
| Asian | 89/3243 (2.7) | 1152/34 417 (3.3) | 0.035 | 85/3168 (2.7) | 124/3168 (3.9) | 0.069 |
| Black or African American | 62/3243 (1.9) | 343/34 417 (1.0) | 0.077 | 61/3168 (1.9) | 26/3168 (0.8) | 0.095 |
| Native Hawaiian or Pacific Islander | 7/3243 (0.2) | 46/34 417 (0.1) | 0.020 | 7/3168 (0.2) | 3/3168 (0.1) | 0.032 |
| White | 3003/3243 (92.6) | 32 256/34 417 (93.7) | 0.044 | 2937/3168 (92.7) | 2956/3168 (93.3) | 0.024 |
| Hispanic or Latino ethnicityf | 181/3170 (5.7) | 1471/33 850 (4.3) | 0.062 | 173/3097 (5.6) | 167/3114 (5.4) | 0.01 |
| Clinical | ||||||
| NYHA class III/IV heart failureg | 1761/3212 (54.8) | 19 609/34 140 (57.4) | 0.053 | 1727/3137 (55.1) | 1743/3139 (55.5) | 0.01 |
| STS risk score, mean (SD)h | 1.7 (0.7) | 2.0 (0.6) | 0.494 | 1.7 (0.6) | 1.7 (0.7) | 0.015 |
| KCCQ-OS score, mean (SD)i | 55.6 (25.3) | 55.1 (24.7) | 0.02 | 55.6 (25.3) | 55.7 (25.5) | 0.003 |
| 5-m walk test, mean (SD), sj | 6.4 (2.8) | 7.0 (5.3) | 0.142 | 6.4 (2.9) | 6.4 (3.0) | 0.007 |
| Comorbidities | ||||||
| Hypertension | 2546/3241 (78.6) | 30 109/34 403 (87.5) | 0.241 | 2510/3166 (79.3) | 2535/3167 (80.0) | 0.019 |
| Diabetes | 877/3239 (27.1) | 11 472/34 388 (33.4) | 0.137 | 866/3164 (27.4) | 849/3165 (26.8) | 0.012 |
| Current dialysis | 11/3242 (0.3) | 194/34 402 (0.6) | 0.034 | 11/3167 (0.3) | 10/3167 (0.3) | 0.005 |
| Atrial fibrillation or flutter | 529/3241 (16.3) | 8386/34 373 (24.4) | 0.202 | 523/3166 (16.5) | 554/3163 (17.5) | 0.027 |
| Prior stroke | 252/3242 (7.8) | 2934/34 400 (8.5) | 0.028 | 240/3167 (7.6) | 257/3167 (8.1) | 0.02 |
| Prior transient ischemic attack | 150/3234 (4.6) | 2196/34 351 (6.4) | 0.077 | 149/3159 (4.7) | 129/3162 (4.1) | 0.031 |
| Carotid stenosis | 241/2275 (10.6) | 4687/25 250 (18.6) | 0.2271 | 241/2222 (10.8) | 242/2309 (10.5) | 0.012 |
| Prior coronary stenting | 611/3240 (18.9) | 9026/34 381 (26.3) | 0.178 | 608/3165 (19.2) | 606/3165 (19.1) | 0.002 |
| Prior bypass graft surgery | 203/3240 (6.3) | 3245/34 387 (9.4) | 0.118 | 202/3165 (6.4) | 222/3167 (7.0) | 0.025 |
| Peripheral vascular disease | 542/3241 (16.7) | 5804/34 392 (16.9) | 0.004 | 527/3166 (16.6) | 535/3167 (16.9) | 0.007 |
| Chronic lung diseasek | 896/3224 (27.8) | 8600/34 213 (25.1) | 0.06 | 874/3149 (27.8) | 918/3150 (29.1) | 0.031 |
| Immunocompromised status | 204/3237 (6.3) | 1803/34 390 (5.2) | 0.045 | 200/3162 (6.3) | 209/3165 (6.6) | 0.011 |
| Hostile chestl | 139/3242 (4.3) | 1479/34 390 (4.3) | 0.001 | 137/3167 (4.3) | 160/3167 (5.1) | 0.034 |
| Porcelain aortam | 60/3241 (1.9) | 861/34 370 (2.5) | 0.045 | 59/3166 (1.9) | 62/3160 (2.0) | 0.007 |
| Creatinine, mean (SD), mg/dL | 1.0 (0.5) | 1.0 (0.6) | 0.048 | 0.9 (0.8-1.1) | 1.0 (0.8-1.1) | 0.027 |
| Estimated GFR, mean (SD), mL/min/1.73 m2 | 74.6 (22.7) | 72.0 (23.0) | 0.116 | 74.5 (22.6) | 73.9 (22.6) | 0.028 |
| Hemoglobin level, mean (SD), g/dL | 13.4 (1.9) | 13.0 (1.9) | 0.189 | 13.4 (1.9) | 13.4 (2.3) | 0.001 |
| Albumin level, mean (SD), g/dL | 3.9 (0.5) | 3.9 (0.5) | 0.023 | 3.9 (0.5) | 3.9 (0.5) | 0.06 |
| Echocardiographic | ||||||
| Aortic valve area, mean (SD), cm2 | 0.7 (0.2) | 0.8 (0.2) | 0.113 | 0.7 (0.2) | 0.8 (0.2) | 0.058 |
| Mean gradient, mean (SD), mm Hg | 46.9 (14.8) | 44.0 (13.2) | 0.209 | 46.8 (14.6) | 46.3 (14.4) | 0.034 |
| Ejection fraction, mean (SD), % | 56.5 (12.9) | 58.5 (10.7) | 0.171 | 56.6 (12.7) | 56.6 (12.6) | 0.0003 |
| Mitral insufficiency (moderate to severe) | 247/2301 (10.7) | 4239/26 724 (15.9) | 0.151 | 242/2245 (10.8) | 277/2399 (11.5) | 0.024 |
| Tricuspid insufficiency (moderate to severe) | 186/3201 (5.8) | 2737/34 081 (8.0) | 0.088 | 182/3128 (5.8) | 169/3138 (5.4) | 0.019 |
| Procedural | ||||||
| Procedure status | ||||||
| Elective | 3050/3242 (94.1) | 33 023/34 396 (96.0) | 0.089 | 2985/3167 (94.3) | 2984/3166 (94.3) | 0.00007 |
| Urgent | 186/3242 (5.7) | 1358/34 396 (3.9) | 0.084 | 177/3167 (5.6) | 178/3166 (5.6) | 0.001 |
| Emergent | 6/3242 (0.2) | 13/34 396 (0.04) | 0.044 | 5/3167 (0.2) | 4/3166 (0.1) | 0.008 |
| Salvage | 0/3242 (0) | 2/34 396 (0.01) | 0.011 | 0/3167 (0) | 0/3166 (0) | - |
| Cardiogenic shock <24 hn | 21/3243 (0.6) | 72/34 394 (0.2) | 0.067 | 17/3168 (0.5) | 21/3165 (0.7) | 0.016 |
| Access site | ||||||
| Transfemoral | 3133/3241 (96.7) | 33 382/34 399 (97.0) | 0.022 | 3061/3167 (96.7) | 3058/3165 (96.6) | 0.002 |
| Subclavian | 38/3241 (1.2) | 277/34 399 (0.8) | 0.037 | 38/3167 (1.2) | 22/3165 (0.7) | 0.052 |
| Axillary | 22/3241 (0.7) | 208/34 399 (0.6) | 0.009 | 22/3167 (0.7) | 31/3165 (1.0) | 0.031 |
| Type of anesthesia | ||||||
| Moderate sedation | 1841/3239 (56.8) | 21 113/34 355 (61.5) | 0.094 | 1799/3164 (56.9) | 1967/3164 (62.2) | 0.108 |
| General anesthesia | 1372/3239 (42.4) | 12 969/34 355 (37.7) | 0.094 | 1340/3164 (42.4) | 1172/3164 (37.0) | 0.109 |
| Combination | 26/3239 (0.8) | 264/34 355 (0.8) | 0.004 | 25/3164 (0.8) | 24/3164 (0.8) | 0.004 |
Abbreviations: GFR, glomerular filtration rate; KCCQ-OS, Kansas City Cardiomyopathy Questionnaire overall summary; NYHA, New York Heart Association; STS, Society of Thoracic Surgeons.
See Statistical Analysis Plan in Supplement 1 for the entire list of covariates and details of matching.
Numeric values are reported as No./total No. (%) unless otherwise indicated.
To account for baseline differences between patients with bicuspid vs tricuspid aortic stenosis, propensity score–based matching was used (calculated using a logistic regression model based on 29 baseline patient characteristics [covariates] with aortic valve type [bicuspid or tricuspid aortic stenosis] as the dependent variable). Model covariates included patient demographics and baseline characteristics (echocardiographic findings, health status, and functional status). Patients with bicuspid aortic stenosis were matched 1:1 with those with tricuspid aortic stenosis, producing 2 balanced cohorts (n = 3168 for each group). An explanatory presentation of data for the full cohort can be found in the eAppendix in Supplement 1.
Calculated as weight in kilograms divided by height in meters squared.
Race was reported by the treating centers based on a closed set of options.
Ethnicity was reported by the treating centers based on a closed set of options.
A categorization of patients based on how much they are limited during physical activity (range, I [no limitation] to IV [symptoms at rest]).
Estimates the potential risk for operative mortality of isolated aortic valve replacement (range, 0% to 100%; a higher score indicates an increased risk). Risk models were developed and validated using STS data from 2002 to 2006.
Health status was evaluated with the 12-item KCCQ-12, a patient-reported disease-specific status survey used to describe symptoms, functional status, and quality of life in patients with heart failure and collected by sites at baseline, 30 days, and 1 year after transcatheter aortic valve replacement (assesses 4 domains: physical limitation, symptoms, quality of life, and social limitation; combined into an overall summary score [KCCQ-OS]).
Indicates the time in seconds it takes the patient to walk 5 meters.
Category includes chronic obstructive pulmonary disease, chronic bronchitis, or emphysema. It can also include a medical condition requiring inhaled or oral pharmacological therapy (eg, β-adrenergic agonist, anti-inflammatory agent, leukotriene receptor antagonist, or steroid). Bronchial asthma or seasonal allergies are not included in chronic lung disease.
A medical condition that precludes an open chest procedure, which includes but is not limited to abnormal chest wall anatomy, severe kyphoscoliosis or other skeletal abnormality, complications from prior surgery, prior radiation involving the mediastinum or thorax, evidence of severe radiation damage, history of multiple recurrent pleural effusions with internal adhesions, chronic and ongoing skin defects or extremely severe soft tissue atrophy, and complete absence of reconstructive options based on plastic surgery consult.
Indicates severe atherosclerosis of the aorta; calcification may be severe and diffuse, causing an eggshell appearance seen on chest x-ray on computed tomography.
Indicates a clinical state of end-organ hypoperfusion due to cardiac failure according to the following criteria: persistent hypotension (systolic blood pressure <80-90 mm Hg or mean atrial pressure 30 mm Hg lower than baseline) and severe reduction in cardiac index (<1.8 L/min/m2 without support or <2.2 with support).
Primary Outcomes: Death and Stroke
In the propensity score–matched population, there was no significant difference in the rates of death between bicuspid and tricuspid populations at 30 days (0.9% vs 0.8%; HR, 1.18 [95% CI, 0.68 to 2.03]; P = .55) or 1 year (4.6% vs 6.6%; HR, 0.75 [95% CI, 0.55 to 1.02]; P = .06) (Figure 2A; Table 2). There was no significant difference in the rates of stroke between bicuspid and tricuspid populations at 30 days (1.4% vs 1.2%; HR, 1.14 [95% CI, 0.73 to 1.78]; P = .55) or 1 year (2.0% vs 2.1%; HR, 1.03 [95% CI, 0.69 to 1.53]; P = .89) (Figure 2B; Table 2). There was no significant difference in the rates of death or stroke between bicuspid and tricuspid populations at 30 days (2.2% vs 1.9%; HR, 1.14 [95% CI, 0.81 to 1.62]; P = .45) or 1 year (6.4% vs 8.4%; HR, 0.85 [95% CI, 0.66 to 1.08]; P = .18) (Figure 2C; Table 2). In the unadjusted population of patients at low surgical risk, mortality at 1 year was significantly lower in patients with bicuspid aortic stenosis compared with tricuspid aortic stenosis, and stroke rates were not significantly different between the 2 groups (eTable 2 in the Supplement). These rates were consistent, when adjusted for missing data by multiple imputation (eTable 3 in the Supplement). The in-hospital and clinical outcomes in the unmatched population are summarized in eTables 4 and 5 in the Supplement.
Figure 2. Cumulative Event Rates of All-Cause Mortality or Stroke Among Patients at Low Surgical Risk With Bicuspid and Tricuspid Aortic Stenosis Who Underwent Transcatheter Aortic Valve Replacement.
Cumulative event rates of all-cause mortality, stroke, and combined all-cause mortality or stroke after transcatheter aortic valve replacement in propensity-matched patients with bicuspid and tricuspid aortic stenosis. Median follow-up for the bicuspid aortic stenosis group was 43 days (IQR, 31-363) and for the tricuspid aortic stenosis group, 48 days (IQR, 33-365). P values were obtained from Cox proportional hazards models. Data for the full cohort are in eFigure 10 in the Supplement.
Table 2. Primary and Secondary Outcomes of Transcatheter Aortic Valve Replacement in Propensity-Matched Low Surgical-Risk Patients With Bicuspid or Tricuspid Aortic Stenosis.
| Patients with aortic valve stenosis, No. (%)a | Absolute difference (95% CI), % | Hazard ratio (95% CI) | P value | ||
|---|---|---|---|---|---|
| Bicuspid (n = 3168) | Tricuspid (n = 3168) | ||||
| Primary outcomes | |||||
| At 30 d | |||||
| Death from any cause | 28 (0.9) | 24 (0.8) | 0.14 (−0.34 to 0.61) | 1.18 (0.68 to 2.03) | .55 |
| Stroke | 42 (1.4) | 37 (1.2) | 0.16 (−0.41 to 0.72) | 1.14 (0.73 to 1.78) | .55 |
| Death from any cause or stroke | 67 (2.2) | 59 (1.9) | 0.26 (−0.46 to 0.97) | 1.14 (0.81 to 1.62) | .45 |
| At 1 y | |||||
| Death from any cause | 70 (4.6) | 101 (6.6) | 2.05 (0.31 to 3.78) | 0.75 (0.55 to 1.02) | .06 |
| Stroke | 49 (2.0) | 49 (2.1) | 0.14 (−0.77 to 1.04) | 1.03 (0.69 to 1.53) | .89 |
| Death from any cause or stroke | 115 (6.4) | 144 (8.4) | 2.00 (0.11 to 3.89) | 0.85 (0.66 to 1.08) | .18 |
| Secondary outcomes | |||||
| At 30 d | |||||
| Aortic valve gradient, mean (SD), mm Hg | 12.7 (5.4) | 12.9 (5.6) | 0.1 (−0.4 to 0.2) | .43 | |
| Moderate or severe paravalvular leak, No./total No. (%) | 38/2108 (1.8) | 24/2131 (1.1) | 0.7 (−0.1 to 1.4) | .07 | |
| NYHA functional class I or II, No./total No. (%) | 2385/2485 (96.0) | 2449/2548 (96.1) | 0.1 (−1.3 to 1.0) | .80 | |
| KCCQ-OS score, change from baseline, mean (SD) | 27.9 (24.8) | 28.1 (25.0) | 0.2 (−1.6 to 1.2) | .81 | |
| At 1 y | |||||
| Aortic valve gradient, mean (SD), mm Hg | 13.2 (6.0) | 13.5 (6.1) | 0.3 (−0.9 to 0.3) | .33 | |
| Moderate or severe paravalvular leak, No./total No. (%) | 22/642 (3.4) | 16/753 (2.1) | 1.3 (−0.6 to 3.2) | .14 | |
| NYHA functional class I/II, No./total No. (%) | 762/808 (94.3) | 874/903 (96.8) | 2.5 (−4.6 to −0.4) | .01 | |
| KCCQ-OS score, change from baseline, mean (SD) | 30.2 (25.6) | 31.7 (26.4) | 1.5 (−4.0 to 1.1) | .26 | |
Abbreviations: KCCQ-OS, Kansas City Cardiomyopathy Questionnaire overall summary score; NYHA, New York Heart Association.
Values are reported as No. (%) unless otherwise indicated.
In 1310 propensity score–matched pairs of patients deemed low surgical risk by the multidisciplinary heart team at the treating sites (eTables 6, 7, 8, and 9 and eFigure 3 in the Supplement) as well as in 1573 propensity score–matched pairs of patients younger than 65 years (eTables 10, 11, 12, and 13 and eFigure 4 in the Supplement), no significant differences were noted in death and stroke rates at 30 days and 1 year. Findings were consistent in 1803 propensity score–matched pairs of patients who underwent TAVR 1 year prior to data extraction date (eTables 14, 15, 16, and 17 and eFigure 5 in the Supplement). By multivariable analysis, death and stroke were not significantly different between the bicuspid and tricuspid groups (eTables 18, 19, 20, 21, and 22 in the Supplement).
Secondary Clinical Outcomes
There was no significant difference between groups in in-hospital death (0.6% bicuspid vs 0.4% tricuspid) or stroke (1.1% bicuspid vs 0.9% tricuspid) (Table 3). Serious procedural complications were uncommon in the bicuspid and tricuspid groups: conversion to surgery (0.4% vs 0.4%), coronary compression or obstruction (0.2% vs 0.1%), need for second valve implantation (0.3% vs 0.1%), perforation with or without tamponade (0.8% vs 0.5%), device embolization into aorta/ventricle (0.03% vs 0.1%), or new permanent pacemaker implantation (6.2% vs 5.2%). The 30-day and 1-year secondary clinical outcomes are summarized in eTable 23 in the Supplement.
Table 3. Procedural and In-Hospital Outcomes of Transcatheter Aortic Valve Replacement in Propensity-Matched Patients at Low Surgical Risk Patients and Bicuspid or Tricuspid Aortic Stenosis.
| Patients with aortic valve stenosis, No. (%)a | Absolute difference (95% CI), % | P value | ||
|---|---|---|---|---|
| Bicuspid (n = 3168) | Tricuspid (n = 3168) | |||
| Procedural outcomes | ||||
| Implant success | 3136 (99.0) | 3145/3166 (99.3) | −0.3 (−0.8 to 0.1) | .13 |
| Device success | 3044/3156 (96.5) | 3066/3152 (97.3) | −0.8 (−1.7 to 0.1) | .06 |
| Conversion to open heart surgery | 14/3167 (0.4) | 13/3164 (0.4) | 0 (−0.3 to 0.4) | .85 |
| Annular dissection | 6 (0.2) | 5 (0.2) | 0 (−0.2 to 0.3) | .76 |
| Aortic dissection | 6 (0.2) | 4 (0.1) | 0.1 (−0.2 to 0.3) | .75 |
| Coronary compression or obstruction | 7 (0.2) | 3 (0.1) | 0.1 (−0.1 to 0.4) | .34 |
| Device embolization to aorta | 0 | 3 (0.1) | −0.1 (−0.2 to 0) | .25 |
| Device embolization to ventricle | 1 | 0 | 0 (−0.1 to 0.1) | >.99 |
| Perforation | 26/3168 (0.8) | 15 (0.5) | 0.3 (−0.1 to 0.8) | .08 |
| Need for second valve | 10 (0.3) | 4 (0.1) | 0.2 (−0.1 to 0.5) | .11 |
| In-hospital event | ||||
| Death | 20 (0.6) | 13 (0.4) | 0.2 (−0.2 to 0.6) | .22 |
| Stroke | 35 (1.1) | 27 (0.9) | 0.3 (−0.3 to 0.8) | .31 |
| Death or stroke | 53 (1.7) | 39 (1.2) | 0.4 (−0.2 to 1.1) | .14 |
| Myocardial infarction | 7 (0.2) | 0 | 0.2 (0 to 0.4) | .02 |
| Life-threatening bleeding | 0 | 0 | ||
| Major vascular complication | 15 (0.5) | 17 (0.5) | −0.1 (−0.4 to 0.3) | .70 |
| New requirement for dialysis | 4 (0.1) | 7 (0.2) | −0.1 (−0.3 to 0.1) | .37 |
| New permanent pacemaker | 189/3035 (6.2) | 156/3028 (5.2) | 1.1 (−0.1 to 2.3) | .07 |
| New-onset atrial fibrillation | 43 (1.4) | 31 (1.0) | 0.4 (−0.2 to 0.9) | .16 |
Values are reported as No. (%) or as No./total No. (%). The denominator, if not shown, is 3168.
Echocardiographic Findings
Aortic valve hemodynamics improved significantly in patients with bicuspid and tricuspid aortic stenosis (eTables 24 and 25 in the Supplement). The mean aortic valve gradients did not significantly differ between the 2 groups at 30 days (12.7 mm Hg for bicuspid vs 12.9 mm Hg for tricuspid) and 1 year (13.2 mm Hg for bicuspid vs 13.5 mm Hg for tricuspid) (eFigure 6 in the Supplement). Moderate or severe paravalvular regurgitation was uncommon in both groups without significant difference between the groups at 30 days (1.8% for bicuspid vs 1.1% for tricuspid; absolute risk difference [RD], 0.7% [95% CI, −0.1 to 1.4%]) and 1 year (3.4% for bicuspid vs 2.1% for tricuspid; absolute RD, 1.3% [95% CI, −0.6 to 3.2%]) (eFigure 7 in the Supplement). Among patients with bicuspid aortic stenosis, the incidence of mild paravalvular regurgitation at 30 days was significantly less with the fourth-generation balloon-expandable valves compared with the third-generation balloon-expandable valves (13.1% for fourth-generation balloon-expandable valves vs 24.4% for third-generation balloon-expandable valves) (eTables 26, 27, and 28 in the Supplement).
Functional Status and Quality of Life
Both bicuspid and tricuspid groups had significant improvements in NYHA functional class I or II symptoms at 30 days (96.0% vs 96.1%; absolute RD, 0.1% [95% CI, −1.3 to 1.0%]) and 1 year (94.3% vs 96.8%; absolute RD, 2.5% [95% CI, −4.6 to −0.4%]) (eFigure 8 in the Supplement). Health status had improved in both groups at 30 days (mean increase from baseline in KCCQ-OS score, 27.9 for bicuspid vs 28.1 for tricuspid; absolute RD, 0.2 [95% CI, −1.6 to 1.2]), which persisted up to 1 year without significant difference between the groups (30.2 for bicuspid vs 31.7 for tricuspid; absolute RD, 1.5 [95% CI, −4.0 to 1.1]) (eFigure 9 and eTables 29 and 30 in the Supplement).
Outcomes in the Overall Cohort
The baseline characteristics and clinical outcomes in the unmatched and matched population in the overall cohort are summarized in eTables 31, 32, 33, 34, 35, and 36 in the Supplement). In the 6995 propensity-matched pairs of bicuspid and tricuspid patients undergoing TAVR, irrespective of the surgical risk, there was no significant difference in the incidence of death between bicuspid and tricuspid populations at 30 days (2.0% vs 1.7%; HR, 1.17 [95% CI, 0.91 to 1.51]; P = .21) or 1 year (8.6% vs 9.8%; HR, 0.90 [95% CI, 0.78 to 1.04]; P = .16) (eFigure 10A in the Supplement); or stroke at 30 days (1.9% vs 1.7%; HR, 1.10 [95% CI, 0.85 to 1.41]; P = .46) or 1 year (2.8% vs 3.1%; HR, 0.96 [95% CI, 0.78 to 1.20]; P = .75) (eFigure 10B in the Supplement). In the unadjusted overall cohort, mortality at 1 year was significantly lower in patients with bicuspid aortic stenosis compared with tricuspid aortic stenosis, and stroke rates were not significantly different between the 2 groups (eFigure 11 in the Supplement).
Discussion
In this registry-based study of propensity-matched patients at low surgical risk who had undergone TAVR for aortic stenosis, patients who had bicuspid aortic stenosis, compared with tricuspid aortic stenosis, had no significant difference in 30-day or 1-year mortality or stroke. There were no significant differences in valve hemodynamics (aortic valve areas and gradients) or the rates of moderate or severe paravalvular regurgitation between the 2 groups at 30 days and 1 year. Patients with bicuspid and tricuspid aortic stenosis had significant and comparable improvement in functional status and quality of life measures.
This is the first report from the STS/ACC TVT Registry on outcomes of TAVR in patients at low surgical risk and bicuspid aortic stenosis. There are currently no ongoing randomized trials of TAVR vs surgery for the treatment of bicuspid aortic stenosis. Given the paucity of data, patients with bicuspid aortic stenosis, especially those at low surgical risk, are often considered more suitable for surgery. The present study included patients at low surgical risk who underwent TAVR for bicuspid aortic stenosis (mean age, 69 years), had an STS-PROM predicted 30-day mortality of 1.7%, and who demonstrated a 30-day mortality of 0.9%. Other procedural outcomes such as conversion to surgery, aortic root injury, aortic dissection, and significant paravalvular regurgitation occurred less than 1% of the time and were comparable to the outcomes of TAVR for tricuspid aortic stenosis. These outcomes were also comparable with published surgical series on bicuspid aortic stenosis and reported 30-day mortality ranging from 0.4% to 2.5% in younger patients with a mean age of 55 to 61 years.16,17,18,19,20,21 However, the surgical series were from select medical centers in contrast to all consecutive patients undergoing TAVR in the United States included in this analysis. The largest series of surgery for bicuspid aortic stenosis in 1042 consecutive patients from 3 Swedish hospitals had a median age of 63 years and a 30-day mortality of 1%.21 The 30-day death and stroke rates in the present study were also comparable to the outcomes of surgery and TAVR in the pivotal low-risk randomized trials of tricuspid aortic stenosis.7,8 Mortality rates at 1 year of 4% in patients with bicuspid aortic stenosis and 6% in patients with tricuspid aortic stenosis were higher than the observed rates in the pivotal low-risk studies and are reflective of the all-comer nature of this registry, which enrolled all consecutive patients undergoing TAVR in the US, irrespective of coexisting clinical conditions and challenging anatomic considerations, many of which were exclusions in the pivotal low-risk studies. Similarly, higher mortality rates were observed at 1 year in patients at low surgical risk (defined as having an STS-PROM score <4%) who underwent surgery or transcatheter aortic valve replacement for severe aortic stenosis in an all-inclusive German national registry, compared with the pivotal randomized trials of patients at low surgical risk.22
Prior reports from the same registry in patients at higher surgical risk have reported higher rates of stroke, paravalvular regurgitation, and permanent pacemaker implantation after TAVR in patients with bicuspid aortic stenosis. In the present study of patients at low surgical risk and severe aortic stenosis, the rates of stroke, paravalvular regurgitation, and permanent pacemaker implantation were not significantly different between patients with bicuspid vs tricuspid aortic stenosis. Possible explanations for better outcomes in this population include better patient selection due to availability of surgical alternative in patients at low surgical risk, evolution of device technology (fourth-generation balloon-expandable transcatheter heart valves associated with decreased paravalvular regurgitation), and procedural technique (higher valve implantation associated with decreased pacemaker rates).
The present study used an STS-PROM score of less than 3%, instead of the local multidisciplinary heart team assessment, as an objective measure to define low surgical risk. The multidisciplinary heart team assessment of surgical risk in a clinical setting may differ across sites, depending on the local surgical and TAVR expertise.23 The results were consistent in sensitivity analyses evaluating outcomes in patients deemed low risk by the multidisciplinary heart team at the treating sites, as well as in patients younger than 65 years of age.
A recent multicenter study in patients undergoing TAVR for bicuspid aortic stenosis with central computed tomography core laboratory assessment demonstrated that anatomic phenotyping of the bicuspid valve was predictive of procedural and intermediate-term outcomes.24 The patients with a combination of calcified raphe and excess leaflet calcium had higher rates of aortic root injury, paravalvular regurgitation, and death at 2 years compared with patients with none or 1 of these features. Unfavorable computed tomography phenotype was present in approximately one-quarter of patients. Despite an increase in the use of TAVR in patients with bicuspid aortic stenosis, bicuspid aortic stenosis accounted for only 10% of patients in the current low surgical–risk cohort. Since a bicuspid aortic valve is present in 30% of patients undergoing aortic valve surgery,6,25 the patients with bicuspid aortic stenosis included in this study may represent a select group with more favorable anatomy or a referral bias against TAVR in patients with bicuspid aortic stenosis in the absence of meaningful clinical outcomes data. Hence, the findings of the current analysis cannot be generalized to the entire population with bicuspid aortic stenosis.
Younger patients with bicuspid aortic stenosis are likely to require more than 1 aortic valve procedure due to limited durability of bioprosthetic valves. In selecting TAVR vs surgery in patients at low surgical risk and bicuspid aortic stenosis, anatomic suitability for index procedure, feasibility of repeat TAVR or repeat surgery, coronary access for future interventions, pacemaker rates, and valve thrombosis should be carefully considered to optimize lifetime management of treatment for these patients. Further, factors such as presence of extensive coronary artery disease, concomitant aortopathy, or atrial fibrillation requiring surgical treatment may determine the choice of surgical aortic valve replacement over TAVR in some patients. While the short-term outcomes with TAVR for bicuspid aortic stenosis in this study were not significantly different from those for tricuspid aortic stenosis, randomized clinical trials comparing TAVR vs surgery with longer-term follow-up data are needed to direct the optimal interventional or surgical management of aortic stenosis in these patients.
Limitations
This study has several limitations. First, it had the inherent limitations of an observational study, including lack of center-independent adjudication of adverse events, lack of an independent imaging core laboratory to confirm bicuspid anatomy or valve function, potential underreporting of adverse events, and incomplete follow-up. The incomplete follow-up was likely exaggerated by the COVID-19 pandemic. Second, details from computed tomography of the aortic valve anatomy were not collected in the registry; thus, the study does not provide guidance on patient selection based on anatomic characteristics of the aortic valve. Third, the study did not assess the relationship between the presence of aortopathy and procedural complications. Fourth, the results of the study cannot be generalized to other valve types since the study only included balloon-expandable valves. Fifth, since the study had 4 primary end points, all findings in the present study should be interpreted as exploratory.
Conclusions
In this preliminary, registry-based study of propensity-matched patients at low surgical risk who had undergone TAVR for aortic stenosis, patients treated for bicuspid vs tricuspid aortic stenosis had no significant difference in mortality or stroke at 30 days or 1 year. Because of the potential for selection bias and absence of a control group treated surgically for bicuspid stenosis, randomized trials are needed to adequately assess the efficacy and safety of transcatheter aortic valve replacement for bicuspid aortic stenosis in patients at low surgical risk.
eAppendix. Statistical Analysis Plan
eTable 1. Missing Baseline Characteristic Values
eTable 2. 30-Day and 1-Year Clinical Outcomes in Unmatched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Who Underwent Transcatheter Aortic Valve Replacement
eTable 3. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Patients With Multiple Imputation
eTable 4. Procedural Characteristics and In-Hospital Outcomes in Unmatched Low Surgical–Risk Patients (STS <3%)
eTable 5. Procedural Characteristics in Matched Low Surgical–Risk Patients (STS <3%)
eTable 6. Baseline Characteristics in Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 7. Procedural Characteristics and In-Hospital Outcomes in Unmatched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 8. Procedural Characteristics and In-Hospital Outcomes in Matched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 9. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 10. Baseline Characteristics in Patients Younger Than 65 Years
eTable 11. Procedural Characteristics and In-Hospital Outcomes in Unmatched Patients Younger Than 65 Years
eTable 12. Procedural Characteristics and In-Hospital Outcomes in Matched Patients Younger Than 65 Years
eTable 13. 30-Day and 1-Year Clinical Outcomes in Matched Patients Younger Than 65 Years
eTable 14. Baseline Characteristics in Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 15. Procedural Characteristics and In-Hospital Outcomes in Unmatched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 16. Procedural Characteristics and In-Hospital Outcomes in Matched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 17. 30-Day and 1-Year Clinical Outcomes in Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 18. Adjusted Hazard Ratios for Adverse Outcomes of TAVR in Bicuspid AS Compared With Tricuspid AS in Low Surgical–Risk Patients
eTable 19. Adjusted Hazard Ratios for 30-Day Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 20. Adjusted Hazard Ratios for 30-Day Stroke of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 21. Adjusted Hazard Ratios for 1-Year Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 22. Adjusted Hazard Ratios for 1-Year Stroke of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 23. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Cohort
eTable 24. Postprocedural Echocardiographic Data in Low Surgical–Risk Matched Patients (STS <3%)
eTable 25. Postprocedural Echocardiographic Data in Low Surgical–Risk Matched Patients (STS <3%) With Multiple Imputation
eTable 26. Baseline Characteristics in Matched Patients with Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 27. Procedural Characteristics and In-Hospital Outcomes in Matched Patients With Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 28. Postprocedural Echocardiographic Data in Matched Patients With Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 29. Functional and Health Status in the Low Surgical–Risk Matched Patients (STS <3%) and Matched Overall Cohort
eTable 30. Functional and Health Status in the Low Surgical–Risk Matched Patients (STS <3%) With Multiple Imputation
eTable 31. Baseline Characteristics of Patients With Bicuspid and Tricuspid Aortic Stenosis in Unadjusted and Propensity Score–Matched Overall Cohort
eTable 32. Procedural Characteristics and In-Hospital Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Unmatched Patients in the Overall Cohort
eTable 33. Procedural Characteristics and In-Hospital Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eTable 34. 30-Day and 1-Year Clinical Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Unmatched Patients in the Overall Cohort
eTable 35. 30-Day and 1-Year Clinical Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eTable 36. Postprocedural Echocardiographic Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eFigure 1. Temporal Trends of Low Surgical–Risk and Younger Patients Who Underwent TAVR
eFigure 2. Temporal Trends of TAVR for Bicuspid Aortic Stenosis
eFigure 3. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients With Low Surgical Risk Based on Heart Team Assessment
eFigure 4. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients Younger Than 65 Years
eFigure 5. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eFigure 6. Valve Hemodynamics in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 7. Paravalvular Regurgitation in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 8. Functional Status in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 9. Quality of Life in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 10. Cumulative Event Rate of All-Cause Mortality and Stroke After TAVR in Propensity-Matched Patients With Bicuspid and Tricuspid Aortic Stenosis in Overall Cohort
eFigure 11. Cumulative Event Rate of All-Cause Mortality and Stroke After TAVR in Unmatched Patients With Bicuspid and Tricuspid Aortic Stenosis in Overall Cohort
References
- 1.Sillesen AS, Vøgg O, Pihl C, et al. Prevalence of bicuspid aortic valve and associated aortopathy in newborns in copenhagen, denmark. JAMA. 2021;325(6):561-567. doi: 10.1001/jama.2020.27205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Curfman G. Bicuspid aortic valve—a common form of structural heart disease. JAMA. 2021;325(6):540-541. doi: 10.1001/jama.2021.0109 [DOI] [PubMed] [Google Scholar]
- 3.Masri A, Svensson LG, Griffin BP, Desai MY. Contemporary natural history of bicuspid aortic valve disease: a systematic review. Heart. 2017;103(17):1323-1330. doi: 10.1136/heartjnl-2016-309916 [DOI] [PubMed] [Google Scholar]
- 4.Husso A, Airaksinen J, Juvonen T, et al. Transcatheter and surgical aortic valve replacement in patients with bicuspid aortic valve. Clin Res Cardiol. 2021;110(3):429-439. doi: 10.1007/s00392-020-01761-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elbadawi A, Saad M, Elgendy IY, et al. Temporal trends and outcomes of transcatheter versus surgical aortic valve replacement for bicuspid aortic valve stenosis. JACC Cardiovasc Interv. 2019;12(18):1811-1822. doi: 10.1016/j.jcin.2019.06.037 [DOI] [PubMed] [Google Scholar]
- 6.Bavaria JE, Desai ND, Cheung A, et al. The St Jude Medical Trifecta aortic pericardial valve: results from a global, multicenter, prospective clinical study. J Thorac Cardiovasc Surg. 2014;147(2):590-597. doi: 10.1016/j.jtcvs.2012.12.087 [DOI] [PubMed] [Google Scholar]
- 7.Mack MJ, Leon MB, Thourani VH, et al. ; PARTNER 3 Investigators . Transcatheter aortic-valve replacement with a balloon-expandable valve in low-risk patients. N Engl J Med. 2019;380(18):1695-1705. doi: 10.1056/NEJMoa1814052 [DOI] [PubMed] [Google Scholar]
- 8.Popma JJ, Deeb GM, Yakubov SJ, et al. ; Evolut Low Risk Trial Investigators . Transcatheter aortic-valve replacement with a self-expanding valve in low-risk patients. N Engl J Med. 2019;380(18):1706-1715. doi: 10.1056/NEJMoa1816885 [DOI] [PubMed] [Google Scholar]
- 9.Forrest JK, Ramlawi B, Deeb GM, et al. Transcatheter aortic valve replacement in low-risk patients with bicuspid aortic valve stenosis. JAMA Cardiol. 2021;6(1):50-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Makkar RR, Yoon SH, Leon MB, et al. Association between transcatheter aortic valve replacement for bicuspid vs tricuspid aortic stenosis and mortality or stroke. JAMA. 2019;321(22):2193-2202. doi: 10.1001/jama.2019.7108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Halim SA, Edwards FH, Dai D, et al. Outcomes of transcatheter aortic valve replacement in patients with bicuspid aortic valve disease: a report from the Society of Thoracic Surgeons/American College of Cardiology Transcatheter Valve Therapy Registry. Circulation. 2020;141(13):1071-1079. doi: 10.1161/CIRCULATIONAHA.119.040333 [DOI] [PubMed] [Google Scholar]
- 12.Green CP, Porter CB, Bresnahan DR, Spertus JA. Development and evaluation of the Kansas City Cardiomyopathy Questionnaire: a new health status measure for heart failure. J Am Coll Cardiol. 2000;35(5):1245-1255. doi: 10.1016/S0735-1097(00)00531-3 [DOI] [PubMed] [Google Scholar]
- 13.Pettersen KI, Reikvam A, Rollag A, Stavem K. Reliability and validity of the Kansas City cardiomyopathy questionnaire in patients with previous myocardial infarction. Eur J Heart Fail. 2005;7(2):235-242. doi: 10.1016/j.ejheart.2004.05.012 [DOI] [PubMed] [Google Scholar]
- 14.D’Agostino RB Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17(19):2265-2281. doi: [DOI] [PubMed] [Google Scholar]
- 15.Zhao Y, Herring AH, Zhou H, Ali MW, Koch GG. A multiple imputation method for sensitivity analyses of time-to-event data with possibly informative censoring. J Biopharm Stat. 2014;24(2):229-253. doi: 10.1080/10543406.2013.860769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Borger MA, Preston M, Ivanov J, et al. Should the ascending aorta be replaced more frequently in patients with bicuspid aortic valve disease? J Thorac Cardiovasc Surg. 2004;128(5):677-683. doi: 10.1016/j.jtcvs.2004.07.009 [DOI] [PubMed] [Google Scholar]
- 17.Girdauskas E, Disha K, Borger MA, Kuntze T. Long-term prognosis of ascending aortic aneurysm after aortic valve replacement for bicuspid versus tricuspid aortic valve stenosis. J Thorac Cardiovasc Surg. 2014;147(1):276-282. doi: 10.1016/j.jtcvs.2012.11.004 [DOI] [PubMed] [Google Scholar]
- 18.Itagaki S, Chikwe JP, Chiang YP, Egorova NN, Adams DH. Long-term risk for aortic complications after aortic valve replacement in patients with bicuspid aortic valve versus marfan syndrome. J Am Coll Cardiol. 2015;65(22):2363-2369. doi: 10.1016/j.jacc.2015.03.575 [DOI] [PubMed] [Google Scholar]
- 19.Andrei AC, Yadlapati A, Malaisrie SC, et al. Comparison of outcomes and presentation in men-versus-women with bicuspid aortic valves undergoing aortic valve replacement. Am J Cardiol. 2015;116(2):250-255. doi: 10.1016/j.amjcard.2015.04.017 [DOI] [PubMed] [Google Scholar]
- 20.Goland S, Czer LS, De Robertis MA, et al. Risk factors associated with reoperation and mortality in 252 patients after aortic valve replacement for congenitally bicuspid aortic valve disease. Ann Thorac Surg. 2007;83(3):931-937. doi: 10.1016/j.athoracsur.2006.10.047 [DOI] [PubMed] [Google Scholar]
- 21.Holmgren A, Enger TB, Naslund U, et al. Long-term results after aortic valve replacement for bicuspid or tricuspid valve morphology in a Swedish population. Eur J Cardiothoracic Surg. 2021;59(3):570-576. doi: 10.1093/ejcts/ezaa348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bekeredjian R, Szabo G, Balaban Ü, et al. Patients at low surgical risk as defined by the Society of Thoracic Surgeons Score undergoing isolated interventional or surgical aortic valve implantation: in-hospital data and 1-year results from the German Aortic Valve Registry (GARY). Eur Heart J. 2019;40(17):1323-1330. doi: 10.1093/eurheartj/ehy699 [DOI] [PubMed] [Google Scholar]
- 23.Vemulapalli S, Carroll JD, Mack MJ, et al. Procedural volume and outcomes for transcatheter aortic-valve replacement. N Engl J Med. 2019;380(26):2541-2550. doi: 10.1056/NEJMsa1901109 [DOI] [PubMed] [Google Scholar]
- 24.Yoon SH, Kim WK, Dhoble A, et al. ; Bicuspid Aortic Valve Stenosis Transcatheter Aortic Valve Replacement Registry Investigators . Bicuspid aortic valve morphology and outcomes after transcatheter aortic valve replacement. J Am Coll Cardiol. 2020;76(9):1018-1030. doi: 10.1016/j.jacc.2020.07.005 [DOI] [PubMed] [Google Scholar]
- 25.Puskas JD, Bavaria JE, Svensson LG, et al. ; COMMENCE Trial Investigators . The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg. 2017;52(3):432-439. doi: 10.1093/ejcts/ezx158 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
eAppendix. Statistical Analysis Plan
eTable 1. Missing Baseline Characteristic Values
eTable 2. 30-Day and 1-Year Clinical Outcomes in Unmatched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Who Underwent Transcatheter Aortic Valve Replacement
eTable 3. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Patients With Multiple Imputation
eTable 4. Procedural Characteristics and In-Hospital Outcomes in Unmatched Low Surgical–Risk Patients (STS <3%)
eTable 5. Procedural Characteristics in Matched Low Surgical–Risk Patients (STS <3%)
eTable 6. Baseline Characteristics in Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 7. Procedural Characteristics and In-Hospital Outcomes in Unmatched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 8. Procedural Characteristics and In-Hospital Outcomes in Matched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 9. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Patients Based on Heart Team Assessment
eTable 10. Baseline Characteristics in Patients Younger Than 65 Years
eTable 11. Procedural Characteristics and In-Hospital Outcomes in Unmatched Patients Younger Than 65 Years
eTable 12. Procedural Characteristics and In-Hospital Outcomes in Matched Patients Younger Than 65 Years
eTable 13. 30-Day and 1-Year Clinical Outcomes in Matched Patients Younger Than 65 Years
eTable 14. Baseline Characteristics in Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 15. Procedural Characteristics and In-Hospital Outcomes in Unmatched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 16. Procedural Characteristics and In-Hospital Outcomes in Matched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 17. 30-Day and 1-Year Clinical Outcomes in Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eTable 18. Adjusted Hazard Ratios for Adverse Outcomes of TAVR in Bicuspid AS Compared With Tricuspid AS in Low Surgical–Risk Patients
eTable 19. Adjusted Hazard Ratios for 30-Day Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 20. Adjusted Hazard Ratios for 30-Day Stroke of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 21. Adjusted Hazard Ratios for 1-Year Mortality of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 22. Adjusted Hazard Ratios for 1-Year Stroke of TAVR in Bicuspid AS Compared With Tricuspid AS
eTable 23. 30-Day and 1-Year Clinical Outcomes in Matched Low Surgical–Risk Cohort
eTable 24. Postprocedural Echocardiographic Data in Low Surgical–Risk Matched Patients (STS <3%)
eTable 25. Postprocedural Echocardiographic Data in Low Surgical–Risk Matched Patients (STS <3%) With Multiple Imputation
eTable 26. Baseline Characteristics in Matched Patients with Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 27. Procedural Characteristics and In-Hospital Outcomes in Matched Patients With Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 28. Postprocedural Echocardiographic Data in Matched Patients With Bicuspid AS Treated With SAPIEN 3 or SAPIEN 3 Ultra
eTable 29. Functional and Health Status in the Low Surgical–Risk Matched Patients (STS <3%) and Matched Overall Cohort
eTable 30. Functional and Health Status in the Low Surgical–Risk Matched Patients (STS <3%) With Multiple Imputation
eTable 31. Baseline Characteristics of Patients With Bicuspid and Tricuspid Aortic Stenosis in Unadjusted and Propensity Score–Matched Overall Cohort
eTable 32. Procedural Characteristics and In-Hospital Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Unmatched Patients in the Overall Cohort
eTable 33. Procedural Characteristics and In-Hospital Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eTable 34. 30-Day and 1-Year Clinical Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Unmatched Patients in the Overall Cohort
eTable 35. 30-Day and 1-Year Clinical Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eTable 36. Postprocedural Echocardiographic Outcomes of TAVR for Bicuspid and Tricuspid Aortic Stenosis in Propensity-Matched Patients in the Overall Cohort
eFigure 1. Temporal Trends of Low Surgical–Risk and Younger Patients Who Underwent TAVR
eFigure 2. Temporal Trends of TAVR for Bicuspid Aortic Stenosis
eFigure 3. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients With Low Surgical Risk Based on Heart Team Assessment
eFigure 4. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients Younger Than 65 Years
eFigure 5. Cumulative Event Rates of All-Cause Mortality or Stroke in Matched Patients Who Underwent TAVR 1 Year Prior to Data Extraction Date
eFigure 6. Valve Hemodynamics in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 7. Paravalvular Regurgitation in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 8. Functional Status in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 9. Quality of Life in Propensity-Matched Low Surgical–Risk Patients With Bicuspid and Tricuspid Aortic Stenosis Undergoing Transcatheter Aortic Valve Replacement
eFigure 10. Cumulative Event Rate of All-Cause Mortality and Stroke After TAVR in Propensity-Matched Patients With Bicuspid and Tricuspid Aortic Stenosis in Overall Cohort
eFigure 11. Cumulative Event Rate of All-Cause Mortality and Stroke After TAVR in Unmatched Patients With Bicuspid and Tricuspid Aortic Stenosis in Overall Cohort



