Abstract
The Sphaeropsidales, coelomycetous fungi producing asexual conidia within enclosed conidiomata (pycnidia), are saprobic on numerous vascular plants. Despite their ubiquitous nature, only a limited number of genera have been documented as causing human disease. We report what we believe to be the first human case of osteomyelitis due to a Phomopsis species in a chronically immunosuppressed female. The patient developed a subcutaneous abscess on the distal phalanx of the right fourth finger complicated by osteomyelitis. Operative specimens revealed fungal hyphae and a pure culture of mould. The patient was treated with a 6-month course of itraconazole. At 16 months of follow-up, she remained free of recurrence. Phomopsis species differ from the similar, more frequently reported Phoma species by having immersed, thick-walled, multiloculate conidiomata and by the production of alpha (short, ellipsoidal) and beta (long, filamentous) conidia.
Coelomycetous fungi, those that produce their conidia (mitospores) within asexual fruiting structures known as conidiomata, are ubiquitous in nature, inhabiting the twigs, branches, and leaves of various host plants. Despite their prevalence, only a limited number of genera have been documented as agents of human disease, including Colletotrichum species, Coniothyrium fuckelii, Lasiodiplodia theobromae, Nattrassia mangiferae, Phoma species, Phomopsis species, Pleurophoma pleurospora, Pleurophomopsis lignicola, Pseudochaetosphaeronema larense, Pyrenochaeta species, and Sphaeropsis species, to name a few (1–4, 7, 10, 13, 15, 18, 22, 23; this study). Coelomycetes previously referred to as the Melanconiales or Sphaeropsidales produce conidiomata known either as acervuli (open and cup-shaped) or pycnidia (closed with ostioles), respectively, or structures sharing characteristics between the two. We report a pycnidial fungus, a Phomopsis species, that was isolated from the finger of a diabetic female undergoing chronic corticosteroid therapy for rheumatoid arthritis. The fungus was most likely introduced through frequent contact with plants and the soil. Salient features of this fungus will be reviewed and compared to those of the similar, more common coelomycetous taxon, Phoma.
Case report.
At the time of clinical presentation, the patient was a 61-year-old African-American female with a history of diabetes and rheumatoid arthritis. She had been treated with 5 to 30 mg of prednisone per day and with weekly doses of methotrexate. She was also an urban gardener. In July or August 1996, she noted several small, painless, red lumps on the palmar aspect of the third and fourth fingers of her right hand. Most of these lumps resolved spontaneously after several weeks, but a lump on the distal palmar surface of the fourth finger of her right hand gradually became larger. She came to medical attention in December 1996 after this nodule had enlarged and had become quite erythematous and extremely painful. She was seen by her primary care physician, who detected a dime-sized pustular lesion. This abscess was incised and drained, and a large amount of purulent material was produced. Exploration of the wound revealed extension of the abscess down to the bone. Gram stain of this material revealed abundant polymorphonuclear neutrophils, moderate fungal hyphae, and no bacteria. Subsequent cultures grew abundant colonies of a mould that was initially identified as a Chrysosporium species. The patient was started empirically on 400 mg of fluconazole (FLU) daily. In addition to the clinical findings, osteomyelitis was confirmed radiographically by the presence of lytic bone changes on plain X-rays and abnormally increased radiotracer uptake in the distal phalanx on bone scans (Fig. 1). Despite 4 weeks of therapy, she had persistent erythema and a tender pea-sized nodule over the old surgical site. Treatment with FLU was discontinued, and the patient was begun on 200 mg of itraconazole (ITRA) per os twice a day. The isolate from the site was sent to the Fungus Testing Laboratory, Department of Pathology, University of Texas Health Science Center at San Antonio, under UTHSC accession no. 97-62, for identification and antifungal susceptibility testing. After 3 weeks of ITRA therapy, the patient experienced significant clinical improvement, with resolution of the painful nodule over the distal phalanx. She completed a 6-month course of ITRA without incident while continuing daily prednisone and weekly methotrexate therapy. There was no evidence of recurrence at 16 months of follow-up.
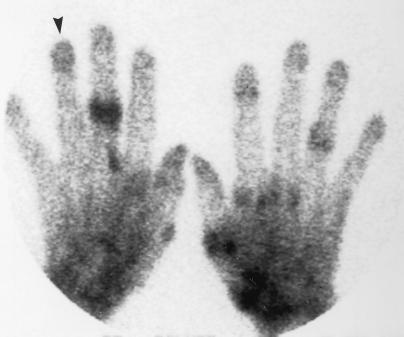
FIG. 1.
X-ray revealing osteopenia of the right fourth distal phalanx (arrow).
Mycological studies.
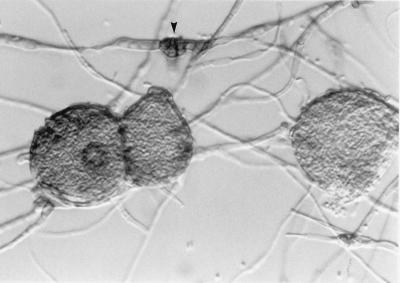
The culture, which was submitted to the Fungus Testing Laboratory on Sabouraud dextrose agar (Becton Dickinson Microbiology Systems, Cockeysville, Md.), was initially white and woolly; however, after being maintained at room temperature (25°C) for 4 weeks, slightly greenish areas developed. Subcultures onto potato flakes agar (PFA) (17), prepared in-house, were buff and sterile with a few black sclerotia after 4 weeks at 25°C. The prominent microscopic features of the culture on PFA were the chains of chlamydoconidia that formed throughout the culture. Temperature studies on PFA revealed good growth at 25 and 35°C but no growth at 42°C after 10 days of incubation. Subcultures onto Czapek Dox agar (Remel, Lenexa, Kans.) also remained sterile after 4 weeks. Subcultures onto carnation leaf agar (12) used for Fusarium identification produced dark brown to black pycnidia after 2 weeks of incubation at 25°C and are shown in Fig. 2 at 4 weeks. Mature pycnidia were teased from the carnation leaves, placed in 10% formalin, sectioned, and stained with a fungal periodic acid-Schiff (PAS) stain. Stained sections revealed conidiomata that were ostiolate (had openings at the top), thick walled (Fig. 3a), immersed (Fig. 3b), multilocular (had more than one cavity) (Fig. 3a and c), and measured 101 to 106 μm long by 196 to 274 μm wide. Crushed conidiomata revealed short, ellipsoidal alpha conidia that were 2.0 to 2.5 μm long by 5.8 to 6.0 μm wide (Fig. 4) and long, filamentous beta conidia that were 0.4 to 0.5 μm long by 18 to 22 μm wide (Fig. 5). For comparison, a thin-walled pycnidium from a Phoma species is shown in Fig. 6. It is important to also note in Fig. 6 the alternarioid, intercalary chlamydoconidium, which is frequently associated with Phoma species.
FIG. 2.
Pycnidial formation on carnation leaf agar, 25°C, 4 weeks.
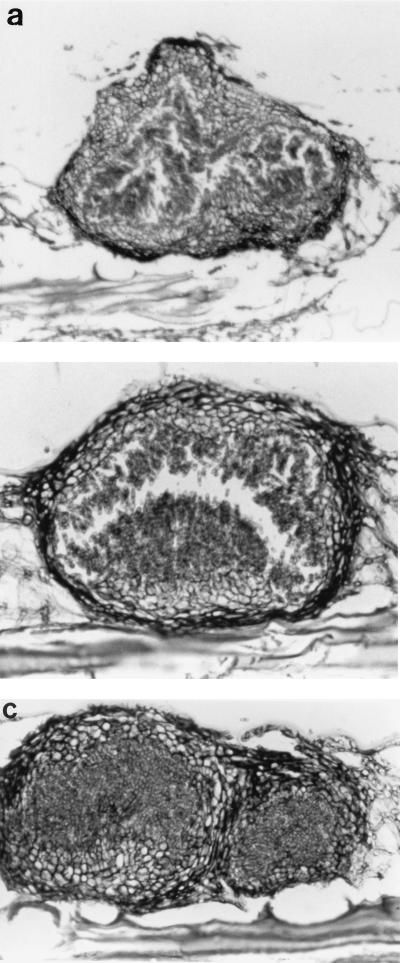
FIG. 3.
(a) Thick-walled, multilocular pycnidium. PAS stain. Magnification, ×230. (b) Thick-walled, immersed pycnidium with conidiogenous cells lining the cavity. PAS stain. Magnification, ×430. (c) Thick-walled, multilocular pycnidium. PAS stain. Magnification, ×360.
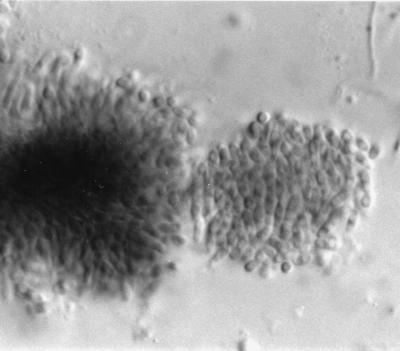
FIG. 4.
Alpha conidia and phialides of Phomopsis. Magnification, ×920.
FIG. 5.
Ellipsoidal alpha and hamate beta conidia of Phomopsis. Magnification, ×920.
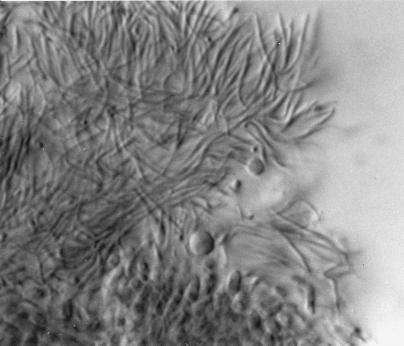
FIG. 6.
Phoma species showing thin-walled pycnidia, as well as an alternarioid, intercalary chlamydoconidium (arrow). Magnification, ×370.
In vitro antifungal susceptibility testing.
The case isolate was tested to determine its susceptibilities to antifungal agents. Tests were performed by previously described macrodilution methods (11). Briefly, the case isolate and the Paecilomyces control strain (UTHSC 90-459) were grown on PFA, which was prepared in-house, for 14 days at 25°C. Because no conidia were produced on PFA after 2 weeks of incubation, the inoculum was standardized spectrophotometrically. The PFA slant was flooded with sterile distilled H2O, adjusted to 95% transmission at 530 nm, and then diluted 1:10 in medium to provide a final inoculum concentration of 1.0 × 104. Final drug concentration ranges were as follows: for amphotericin B (AMB; E. R. Squibb & Sons, Princeton, N.J.), 0.03 to 16 μg/ml; for FLU (Pfizer, Inc., New York, N.Y.), 0.125 to 64 μg/ml; and for ITRA (Janssen Pharmaceutica, Titusville, N.J.), 0.015 to 8 μg/ml. AMB was tested in antibiotic medium 3 (Difco, Detroit, Mich.); other antifungal agents were tested in RPMI 1640 with l-glutamine and morpholinepropanesulfonic acid (MOPS) buffer at a concentration of 165 mM and without sodium bicarbonate (American Biorganics, Inc., Niagara Falls, N.Y.). Previously prepared, frozen drug tubes containing 0.1 ml of drug were allowed to thaw and were inoculated with 0.9 ml of the hyphal medium suspension. A drug-free growth control tube was included with the tubes containing the case isolate and control organism. The tubes were incubated at 35°C, and MICs were determined at the first 24-h interval when growth was observed in the drug-free growth control tube (24 and 48 h). MICs were defined in terms of the first tube that gave a score of 0 (optically clear) for AMB and a score of 2 (reduction in turbidity of ≥80% in contrast to that of the drug-free control tube) for FLU and ITRA. The results of in vitro susceptibility tests, based on drug concentrations normally achievable in patients receiving recommended dosages, indicated that the isolate appeared susceptible to AMB, FLU, and ITRA. These data are shown in Table 1.
TABLE 1.
In vitro antifungal susceptibility data for a Phomopsis species (UTHSC accession no. 97-62)
| Antifungal agent | MIC (μg/ml)
|
MLCa (μg/ml)
|
||
|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | |
| AMBb | 0.25 | 0.25 | 0.25 | 0.5 |
| FLUc | 2 | 4 | ||
| ITRAc | 0.125 | 0.125 | ||
Minimum lethal concentration.
Tested in antibiotic medium 3 (Difco).
Tested in RPMI 1640 (American Biorganics, Inc.).
Discussion.
Coelomycetous fungi have been traditionally referred to as the Melanconiales (producing acervuli or open cup-shaped conidiomata) and the Sphaeropsidales (producing pycnidia or closed conidiomata with ostioles), although these distinctions are losing favor (20). These fungi are being identified with increasing frequency in the Fungus Testing Laboratory, particularly in immunosuppressed hosts. The most common presentation has been skin and soft tissue infection (1, 18, 23), although one report suggests their role in systemic disease (10). These fungi are presumably introduced through some type of traumatic implantation, and this appears to be the method of acquisition of the Phomopsis species in the present case. The patient was an urban gardener with frequent contact with plants and soil. Her history of chronic immunosuppression due to diabetes and treatment of rheumatoid arthritis with prednisone and methotrexate was a likely risk factor for opportunistic phaeohyphomycotic osteomyelitis.
Although standardization in antifungal susceptibility testing of filamentous fungi is only just commencing (5), parameters that were previously defined for yeast testing, with some modification also appear to be useful for mould testing. The case isolate, tested by a modified standard M27-A (11), appeared susceptible, in vitro, to FLU, with MICs of 2 and 4 μg/ml at 24 and 48 h, respectively. A 400-mg/day empirically started FLU regimen had to be discontinued, however, due to clinical failure. Factors in addition to in vitro susceptibility which may have influenced the outcome, as outlined by Rex et al., include the pharmacokinetics of the drug, general host factors, site of infection, and virulence of the pathogen (16). Significant clinical improvement was observed after the patient begun treatment with 200 mg of ITRA twice daily. She received 6 months of therapy without difficulty. The patient has continued her immunosuppressive regimen posttreatment and now remains free of recurrence after 16 months of follow-up.
The genus Phomopsis (Sacc.) Sacc., Ann. mycol. 3:166 (1905), is a large, diverse one with over 400 described species (20). Being parasitic, and causing spots on various plant parts, the species have been described mostly as they appear on the hosts they infect. Some species, particularly Phomopsis leptostromiformis, which is found on lupin plants (Lupinus species), also produce the potent mycotoxins phomopsin A and phomopsin B, causing lupinosis, a mycotoxicosis in various animals following ingestion of the toxin (6, 19). Several older, obsolete names predate Phomopsis, including Phoma Westd. subgen. Phomopsis Sacc., Syll. fung. 3:66 (1884) and Myxolibertella Höhn., Ann. mycol. 1:526 (1903). Thus far, the generic name has not be correctly typified; however, the two original species, Phomopsis lamii Sacc. et D. Sacc., and Phomopsis pritchardiae (Cke et Harkn.) Sacc., appear to be likely candidates. Neither, however, has been reexamined since its original description, so the assignment of either one or the other, should they not be congeneric, would be unfortunate, since many taxa in the genus are now accepted. Additionally, the genus Myxolibertella predates Phomopsis, so Phomopsis will have to be conserved against Myxolibertella, as long as the lectotypification problem can be solved. The extensive literature concerning the genus point in favor of conservation of the generic name. The genus Diaporthe Nitschke 1870 is the teleomorph of Phomopsis, and correlation of these teleomorphs and anamorphs is mandatory for future delimitation of taxa in the genus (20).
Despite the fact that the genus is in need of revision, and that it is almost impossible to identify isolates to the species level without knowledge of the plant host (which we did not attempt to determine, nor did we try to recover the same isolate from the patient’s environment), several salient features recognizable in the routine laboratory, in an artificial cultural environment, may be used to differentiate this genus from the more common coelomycetous taxon Phoma. These features are shown in Table 2. The most diagnostic characteristics include dark, thick-walled, immersed, ostiolate, stromatic conidiomata, either unilocular or multilocular (single or several cavities), producing both alpha conidia (fusiform, straight, usually greater than 5 μm in length) and beta conidia (filiform to hamate [hooked], usually greater than 15 μm in length). The similar genus, Phoma, containing several human pathogenic species, is characterized by thin-walled, ostiolate conidiomata and usually produces only a single type of smaller conidia (often in the range of 1.5 to 2.5 by 4 to 6 μm). Phomopsis species described that fail to produce beta conidia would present a diagnostic dilemma; however, other characteristics, such as features of the conidiomata and the size and shape of alpha conidia, may lead to a tentative identification. Other dematiaceous (14) genera which may resemble Phoma species include Pleurophoma, Pleurophomopsis, Coniothyrium, Microsphaeropsis, Pseudochaetosphaeroma, and some Pyrenochaeta species. Some of these genera appear in need of more thorough taxonomic scrutiny (8, 9).
TABLE 2.
Microscopic features of Phomopsis and Phomaa
| Feature | Characteristics of:
|
|
|---|---|---|
| Phomopsis species | Phoma species | |
| Mycelium | Immersed, branched, septate, hyaline to pale brown | Immersed, branched, septate, hyaline to pale brown |
| Conidiomata | Eustromatic (true stroma—host tissue incorporated into the pycnidium), immersed, brown to dark brown, separate or aggregated and confluent, globose, ampulliform or applanate (flattened), unilocular to multilocular | Immersed, or semi-immersed, sometimes erumpent, unilocular, brown, globose, separate or aggregated, mostly thin-walled pale- to medium-brown textura angularis |
| Ostioles | Single or several in complex conidiomata, circular, often papillate | Single or several, central, not papillate |
| Conidiophores | Branched and septate at the base, occasionally short, more frequently multiseptate and filiform, hyaline, formed from inner cells of the locular walls | Present in only two species |
| Conidiogenous cells | Enteroblastic, phialidic, determinate, integrated, rarely discrete, hyaline, cylindrical, aperature apical on long or short lateral and main branches of the conidiophores, collarette, channel and periclinal thickening minuteb | Enteroblastic, phialidic, integrated or discrete, ampulliform to doliform (barrel-shaped), hyaline, collarette minute, periclinal wall markedly thickened |
| Conidia | Two basic types, but in some species with intermediates between the two; alpha conidia hyaline, fusiform straight, usually biguttulate; beta conidia hyaline, filiform, straight or more often hamate (hooked), egutullate (without guttules), aseptatec | Hyaline, aseptate or occasionally singly septate, thin-walled, often guttulate (with oil droplets), ellipsoid, cylindrical, fusiform, pyriform or globose |
Adapted from the work of Sutton (20).
Periclinal wall thickening = a zone of increased material surrounding the opening of a phialide.
Some species lack beta conidia.
The presently described case of invasive Phomopsis disease does not differ clinically from other cases of subcutaneous phaeohyphomycotic infections. In vitro data suggest that ITRA may be an efficacious regimen for coelomycetous fungi (21), which is supported by the rapid clinical response in our patient after initiation of ITRA therapy. The difficulty in identification of this isolate, which led to a delay in appropriate antifungal therapy, underscores the importance of referring such isolates to reference laboratories. Phomopsis species should be added to the list of coelomycetous fungi capable of causing human disease, particularly subcutaneous mycoses in immunocompromised hosts. Those that grow at 35°C should also be considered potentially invasive. Further studies are under way to more fully characterize the growth habits of human pathogenic coelomycetous fungi observed under laboratory conditions.
REFERENCES
- 1.Baker J, Salkin I F, Forgacs P, Haines J H, Kemma M E. First report of subcutaneous phaeohyphomycosis of the foot caused by Phoma minutella. J Clin Microbiol. 1987;25:2395–2397. doi: 10.1128/jcm.25.12.2395-2397.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chabasse D, de Bievre C, Legrand E, Saint-Andre J P, de Gentile L, Cimon B, Bouchara J P. Subcutaneous abscess caused by Pleurophomopsis lignicola Petr: first case. J Med Vet Mycol. 1995;33:415–417. [PubMed] [Google Scholar]
- 3.de Hoog G S, Guarro J. Atlas of clinical fungi. Baarn, The Netherlands: Centraalbureau voor Schimmelcultures; 1995. [Google Scholar]
- 4.Dooley D P, Beckius M L, Jeffery B S, McAllister C K, Radentz W H, Feldman A R, Rinaldi M G, Bailey S R, Keeling J H. Phaeohyphomycotic cutaneous disease caused by Pleurophoma in a cardiac transplant patient. J Infect Dis. 1989;159:503–507. doi: 10.1093/infdis/159.3.503. [DOI] [PubMed] [Google Scholar]
- 5.Espinel-Ingroff A, Dawson K, Pfaller M, Anaissie E, Breslin B, Dixon D, Fothergill A, Paetznick V, Peter J, Rinaldi M, Walsh T. Comparative and collaborative evaluation of standardization of antifungal susceptibility testing for filamentous fungi. Antimicrob Agents Chemother. 1995;39:314–319. doi: 10.1128/aac.39.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Luduena R F, Prasad V, Roach M C, Lacey E. The interaction of phomopsin A with bovine brain tubulin. Arch Biochem Biophys. 1989;272:32–38. doi: 10.1016/0003-9861(89)90191-4. [DOI] [PubMed] [Google Scholar]
- 7.Matsumoto T, Ajello L, Matsuda T, Szaniszlo P J, Walsh T J. Developments in hyalohyphomycosis and phaeohyphomycosis. J Med Vet Mycol. 1994;32(Suppl. 1):329–349. doi: 10.1080/02681219480000951. [DOI] [PubMed] [Google Scholar]
- 8.Morgan-Jones G. Concerning some species of Microsphaeropsis. Can J Bot. 1974;52:2575–2579. [Google Scholar]
- 9.Morgan-Jones G. Notes on coelomycetes. III. Concerning Microsphaeropsis concentrica: morphology and ultrastructure. Mycotaxon. 1987;30:177–187. [Google Scholar]
- 10.Morris J T, Beckius M L, Jeffwry B S, Longfeld R N, Heaven R F, Baker W J. Lung mass caused by Phoma species. Infect Dis Clin Pract. 1995;4:58–59. [Google Scholar]
- 11.National Committee for Clinical Laboratory Standards. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard M27-A. Wayne, Pa: National Committee for Clinical Laboratory Standards; 1997. [Google Scholar]
- 12.Nelson P E, Toussoun T A, Marasas W F O. Fusarium species. An illustrated manual for identification. University Park: The Pennsylvania State University Press; 1983. [Google Scholar]
- 13.Padhye A A, Gutekunst R W, Smith D J, Punithalingam E. Maxillary sinusitis caused by Pleurophomopsis lignicola. J Clin Microbiol. 1997;35:2136–2141. doi: 10.1128/jcm.35.8.2136-2141.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pappagianis D, Ajello L. Dematiaceous—a mycologic misnomer? J Med Vet Mycol. 1994;32:319–321. doi: 10.1080/02681219480000401. [DOI] [PubMed] [Google Scholar]
- 15.Punithalingam E. Sphaeropsidales in culture from humans. Nova Hedwigia. 1979;31:119–158. [Google Scholar]
- 16.Rex J H, Pfaller M A, Galgiani J N, Bartlett M S, Espinel-Ingroff A, Ghannoum M A, Lancaster M, Odds F C, Rinaldi M G, Walsh T J, Barry A L Subcommittee on Antifungal Susceptibility Testing of the National Committee for Clinical Laboratory Standards. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro-in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin Infect Dis. 1997;24:235–247. doi: 10.1093/clinids/24.2.235. [DOI] [PubMed] [Google Scholar]
- 17.Rinaldi M G. Use of potato flakes agar in clinical mycology. J Clin Microbiol. 1982;15:1159–1160. doi: 10.1128/jcm.15.6.1159-1160.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shukla N, Agarwal G P, Gupta D K. Phoma as a human pathogen. Mykosen. 1984;27:255–258. doi: 10.1111/j.1439-0507.1984.tb02027.x. [DOI] [PubMed] [Google Scholar]
- 19.Soler Rodriguez F, Miguez Santiyan M P, Pedrera Zamorano J D, Roncero Cordero V. An outbreak of lupinosis in sheep. Vet Human Toxicol. 1991;33:492–494. [PubMed] [Google Scholar]
- 20.Sutton B C. The Coelomycetes. Fungi Imperfecti with pycnidia, acervuli, and stromata. Kew, United Kingdom: Commonwealth Mycological Institute; 1980. [Google Scholar]
- 21.Sutton D A, Fothergill A W, Rinaldi M G. Guide to clinically significant fungi. Baltimore, Md: The Williams & Wilkins Co.; 1998. [Google Scholar]
- 22.Sutton D A, Timm W D, Morgan-Jones G, Rinaldi M G. Abstracts of the 98th General Meeting of the American Society for Microbiology 1998. Washington, D.C: American Society for Microbiology; 1998. Human phaeohyphomycotic osteomyelitis caused by the coelomycete Phompsis: criteria for identification, case history, and therapy, abstr. F-94; p. 269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Young N A, Kwon-Chung K J, Freeman J. Subcutaneous abscess caused by Phoma sp. resembling Pyrenochaeta romeroi: unique fungal infection occurring in immunosuppressed recipient of renal allograft. Am J Clin Pathol. 1973;59:810–816. doi: 10.1093/ajcp/59.6.810. [DOI] [PubMed] [Google Scholar]