Abstract
Background:
Arginine (Arg) 14 deletion (R14del) in the calcium regulatory protein phospholamban (hPLNR14del) has been identified as a disease-causing mutation in patients with an inherited cardiomyopathy. Mechanisms underlying the early arrhythmogenic phenotype that predisposes carriers of this mutation to sudden death with no apparent structural remodeling remain unclear.
Methods:
To address this, we performed high spatio-temporal resolution optical mapping of intact hearts from adult knock-in mice harboring the human PLNWT (WT, N=12) or the heterozygous human PLNR14del mutation (R14del, N=12) before and after ex-vivo challenge with isoproterenol and rapid pacing.
Results:
Adverse electrophysiological remodeling was evident in the absence of significant structural or hemodynamic changes. R14del hearts exhibited increased arrhythmia susceptibility compared to WT. Underlying this susceptibility was preferential right ventricular (RV) action potential prolongation that was unresponsive to β-adrenergic stimulation. A steep repolarization gradient at the LV/RV interface provided the substrate for inter-ventricular activation delays and ultimately local conduction block during rapid pacing. This was followed by the initiation of macroreentrant circuits supporting the onset of VT. Once sustained, these circuits evolved into high frequency rotors, which in their majority were pinned to the RV. Importantly, these rotors exhibited unique spatio-temporal dynamics that promoted their increased stability in R14del compared to WT hearts.
Conclusions:
Our findings highlight the crucial role of primary electrical remodeling caused by the hPLNR14del mutation. These inherently arrhythmogenic features form the substrate for adrenergic-mediated VT at early stages of PLNR14del induced cardiomyopathy.
Keywords: Arrhythmogenic cardiomyopathy, dilated cardiomyopathy, sudden death, phospholamban, arrhythmia, ventricular tachycardia, spiral wave reentry, dynamics
Introduction
Inherited cardiomyopathies (CM) represent a heterogeneous class of cardiac disorders that frequently predispose to sudden cardiac death (SCD) on the one hand or culminate in end-stage heart failure (HF) on the other hand.1, 2 Over the past 3 decades, the discovery of numerous disease-causing mutations and modifier genes has improved our understanding of the molecular genetics of CM-related SCD and highlighted the importance of clinical genetic testing and counseling for affected patients and their relatives.1 The global burden of CM is substantial and poses a particularly challenging problem during its early concealed phase when heightened risk of arrhythmias leading to SCD precedes overt remodeling of cardiac structure or mechanical function. Indeed, this heightened risk can often go undetected until the first manifestation of the disease (usually in the form of SCD) occurs.3 Hence, early identification of asymptomatic but genotype-positive carriers of disease-causing mutations has vital prognostic implications in deterring the risk of SCD before onset of the initial symptoms.1
In this report, we focus on a naturally occurring heterozygous mutation, known as hPLNR14del, which is a deletion of Arginine14 in the coding region of the human phospholamban (hPLN) gene.2, 4 PLN is a crucial SR-Ca2+ regulatory protein and a key modulator of cardiac performance by mediating the primary ß-adrenergic effects on excitation-contraction coupling.5 The role of PLN in the regulation of cardiac contraction and its impact on relaxation has been highlighted in different disease models for which PLN mutations are implicated.6, 7 In the study by Van der Zwaag et al., considerable overlap between dilated cardiomyopathy (DCM) and arrhythmogenic cardiomyopathy (ACM) was found in patients harboring the R14del mutation.8 In addition, carriers of the mutation were far more likely to have a positive family history of SCD and to experience appropriate ICD therapy compared to their non-carrier counterparts.8 Moreover, the onset of ventricular tachycardia (VT) in these patients occurred as early as 20-years of age highlighting a potentially primary arrhythmogenic nature of this mutation.9 Therefore, the prognostic stratification of PLN-R14del patients for SCD in the early concealed stage of the disease when signs-and-symptoms of heart failure are absent is of utmost clinical importance. In this regard, Denis et al.10 reported that adrenergic-mediated VT could be readily induced in 90% of patients suspected of having ACM;10 thereby unmasking early arrhythmogenicity before onset of overt cardiac disease. Despite the recognition of a link between R14del-related CM and SCD, the detailed electrophysiological mechanisms by which R14del promotes the onset, and more importantly, the maintenance of VT that underlie SCD in the absence of overt structural remodeling remain fully unknown.
We developed a mouse model with a knock-in of the human wild type PLN (hPLNWT or WT) and the heterozygous hPLNR14del (R14del) mutation with the goal of studying the early electrophysiological (EP) phenotype in the young adult murine heart. Using high-resolution optical action potential mapping, we reveal key features of the electrophysiological substrate that promote the initiation of R14del-related arrhythmias. We demonstrate how predominantly RV-sided rotors underlie the maintenance of adrenergic-mediated VT in this model. Finally, we discuss how electrical properties of R14del hearts influence the stability of pinned spirals in this inherently arrhythmogenic substrate. These findings could contribute to new approaches for arrhythmia prediction, prevention and therapy during the early concealed phase of the disease.
Materials and Methods
The data, analytic methods, and study materials that support the findings of this work will be made available upon reasonable request. A comprehensive Methods section is included as Supplementary material. It provides important experimental and analytic details regarding the generation of hPLNWT and hPLNR14del knock-in mice, optical action potential mapping and arrhythmia induction protocols, algorithms for the analysis of arrhythmia dynamics and other molecular and statistical methods.
Optical Mapping and Arrhythmia Induction Protocol
All procedures were approved by the Institutional Animal Care and Use Committee of ISMMS. Hearts from 12–16week old WT (n=12) and R14del (n=12) mice were studied using optical mapping as previously described.11, 12 The experimental protocol is detailed in the supplemental material and illustrated in Fig. 1b. EP recordings were obtained before-and-after ISO bolus infusion at progressively increasing pacing frequencies until induction of sustained VT at the so-called critical frequency (fCrit). We define sustained VT as episodes lasting >30sec. We define VT-inducibility as the percentage of hearts that exhibited sustained VT before reaching a cut-off pacing frequency (fcut-off) of 22.5Hz. Once sustained VT was confirmed, two consecutive optical measurements (2sec each) were recorded per heart (WT, N=24; R14del, N=24). Since the majority of VT episodes were demonstrably caused by reentrant circuits in our field of view (WT, N=18/24; R14del, N=20/24), we focused our analysis on the study of reentry.
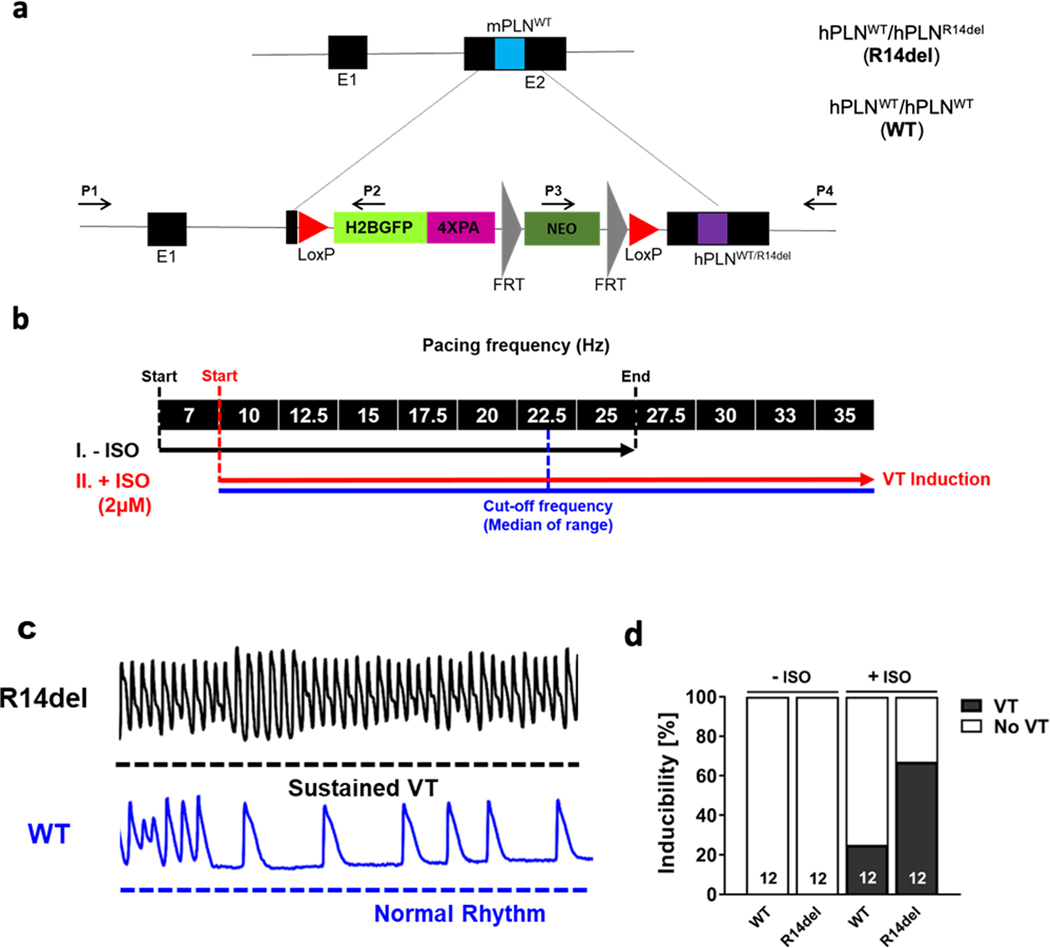
Figure 1: Arrhythmia induction in ex-vivo retrograde perfused hPLNR14del (R14del) and hPLNWT (WT) murine hearts.
a. Schematic describing the generation of the mouse models with knock-in hPLNWT and hPLNR14del. H2BGFP expressed under the control of endogenous mouse PLN promoter. b. Pacing protocol used on the ex-vivo perfused hearts. The median of the pacing frequency range in the second segment of the protocol (with ISO) was chosen as the cut-off frequency (i.e. at 22.5Hz). c. Representative 2-sec optical AP recordings showing the initiation of sustained VT in R14del but not WT hearts in response to an identical challenge. d. Up to a cut-off frequency of 22.5Hz, sustained VT was induced in 67% of R14del hearts vs. 25% of WT hearts, exclusively upon exposure to ISO.
Arrhythmia Dynamics Analysis
As detailed by Berenfield et al,13 dominant frequency (fDom) maps were generated by computing a Fast Fourier Transform of the optical mapping signal at each spatial location. To characterize arrhythmia dynamics in the presence of rotors, we converted AP activation patterns to a phase representation14 and calculated phase singularities (PS), which represent the organizing centers of rotating waves. For each pixel, we subtracted the mean optical signal from the time-series and used the MATLAB™ hilbert function to obtain an analytical (complex-valued) signal centered upon the origin in the complex plane via the Hilbert Transform (HT).15 In each frame, PS were detected from the phase map using the 4-point integration path method.16 Detected PS locations were processed in two ways: First, as a measure of local meandering, PS velocity drift (VDrift) was calculated from the frame-to-frame change in position of a given PS and averaged over the course of the VT episode. Second, from all the PS positions throughout a given episode, a PS-density map was constructed, indicating, at every position, the probability of encountering a PS within a 5-pixel radius at a random time-point within the episode. The “meandering range” of the spiral was defined as the maximal diameter of the roughly circular area around the pinning location of the rotor with a PS-density threshold >0.4. By integrating the PS-density over the LV and RV masks, LV and RV occupancy rates indicating the preferential location of the rotor center were computed.
We calculated instantaneous CV in the tissue using the same method as for periodically-paced activation patterns, only based on the phase rather than activation maps. Plane-fitting after local phase unwinding in a 5×5 neighborhood yielded a phase-gradient in [rad.mm−1] at each spatial location, which was converted to a time-gradient [ms.mm−1] (inverse velocity) using the local instantaneous frequency in [rad.ms−1].
For stable rotors (defined by VDrift≤0.10mm.ms−1), the average CV approaches zero towards the center of rotation, coinciding closely with the peak of the phase density map. Similar to the study by Noujaim et al.,17 average CVs were plotted as a function of distance from this reference point, restricted to a circular area 2.0mm in diameter where linear behavior was observed. The slope sV was obtained from a least-squares linear fit to this plot, which depends on the shape and behavior of the rotating wave near its tip, and on the local tissue properties where the spiral is anchored. To put sV into context, we converted it to a pseudofrequency (fPseudo=sV/2π). For a rigidly rotating object, i.e. perfectly tangential velocity vectors with magnitude 2πrf (where r is the distance from the center), fPseudo equals the rotation frequency f. Deviations from f therefore indicate altered propagation properties near the core due to radial velocity components or time-averaging of a micro-meandering pattern. Please refer to the Supplemental material for an expanded description.
Statistical Analyses
Data are presented as mean ±standard deviation of the mean. Baseline values were compared to those with ISO using the one-way, two-way analysis of variance (ANOVA) followed by Tukey’s post-hoc or repeated two-way ANOVA where appropriate. The Student’s t-test (unpaired) was applied where necessary. The level of statistical significance was taken as p<0.05.
Results
VT susceptibility in a new knock-in mouse model harboring the human PLNR14del mutation.
To assess arrhythmia propensity hearts from knock-in mice expressing human mutant (PLNR14del) or human WT (PLNWT) PLN as controls (Fig. 1a) were studied ex-vivo before-and-after challenge with a bolus infusion of ISO (Fig. 1b), which was found clinically to unmask arrhythmia risk in patients with suspected ACM.10 While neither group was prone to pacing-induced VT at baseline (i.e before ISO), β-adrenergic stimulation unmasked a higher propensity for sustained VT at the pre-defined cutoff pacing frequency (median of the maximal pacing frequency range) of 22.5Hz in R14del relative to WT hearts. Representative responses to rapid stimulation for R14del and WT hearts documenting the genesis of sustained VT in the former but not the latter are shown in Fig. 1c. Of note, the increased vulnerability of R14del hearts to adrenergic-mediated VT (Fig. 1d) was independent of any changes in hemodynamic function or structural remodeling by echocardiography (Fig. I in the Supplement), highlighting R14del as potentially a primary electrical disorder.
Interventricular APD gradient in R14del hearts.
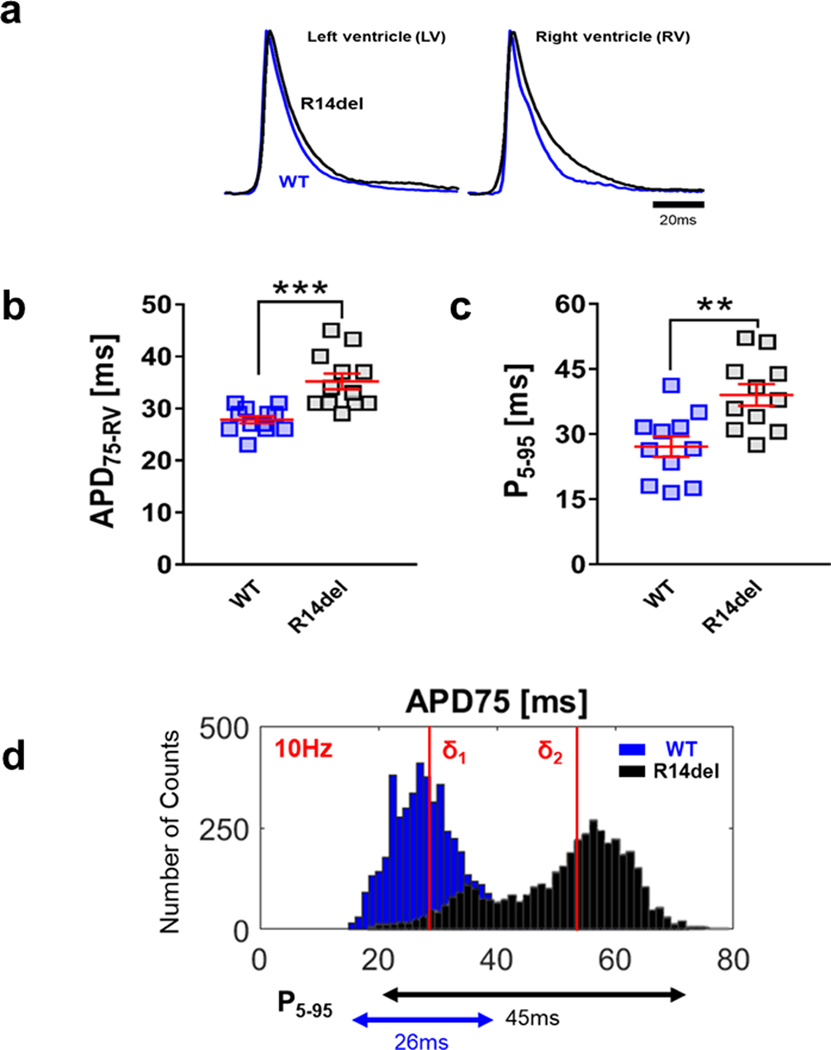
We proceeded to study the tissue-level EP substrate that promoted the onset of adrenergic-mediated VT in R14del hearts. At baseline, we found clear evidence of preferential action potential duration (APD) prolongation in the RV of the R14del heart, causing a marked increase in the spatial inter-ventricular APD gradient relative to WT. Fig. 2a shows averaged AP traces recorded from the RV and LV of representative WT (blue) and R14del (black) hearts at 10Hz. Substantial action potential prolongation is clearly evident in the RV but not the LV of R14del hearts relative to WT. Indeed, R14del hearts exhibited significant right ventricular APD75 prolongation compared to WT as quantified in each heart from both groups (WT = 28.0±0.7ms (n=12), R14del = 35.2±1.5ms (n=12), p < 0.001, Fig. 2b, left). The extent of spatial APD75 dispersion was also quantified by examining epicardial APD75 histograms, which exhibited a rightward shift with an almost 2-fold increase in the dispersion metric P5–95 (Fig. 2d). This was indicative of increased epicardial APD75 heterogeneity which again was documented in individual hearts from both groups (WT = 27.1±2.4ms, R14del = 39.0±2.5ms, p < 0.01, Fig. 2c).
Figure 2: Preferential APD prolongation in the RV and increased APD dispersion in R14del hearts.
a. Representative AP traces measured from the RV and LV of ex-vivo R14del (black) and WT (blue) hearts, showing preferential APD prolongation in the RV vs LV of mutant hearts at 10Hz. b. Summary data showing the average right ventricular APD75 and c. P5–95, an index of the epicardial APD dispersion, in all WT and R14del hearts. *** p <0.001, ** p <0.01. d. R14del hearts exhibited a rightward shift in APD histograms relative to WT, with a bimodal distribution resulting in a marked increase in APD dispersion. δ1 and δ2 indicate the median of the distribution for WT and R14del hearts, respectively.
Loss of biventricular APD adaptation in response to acute ß-adrenergic stress.
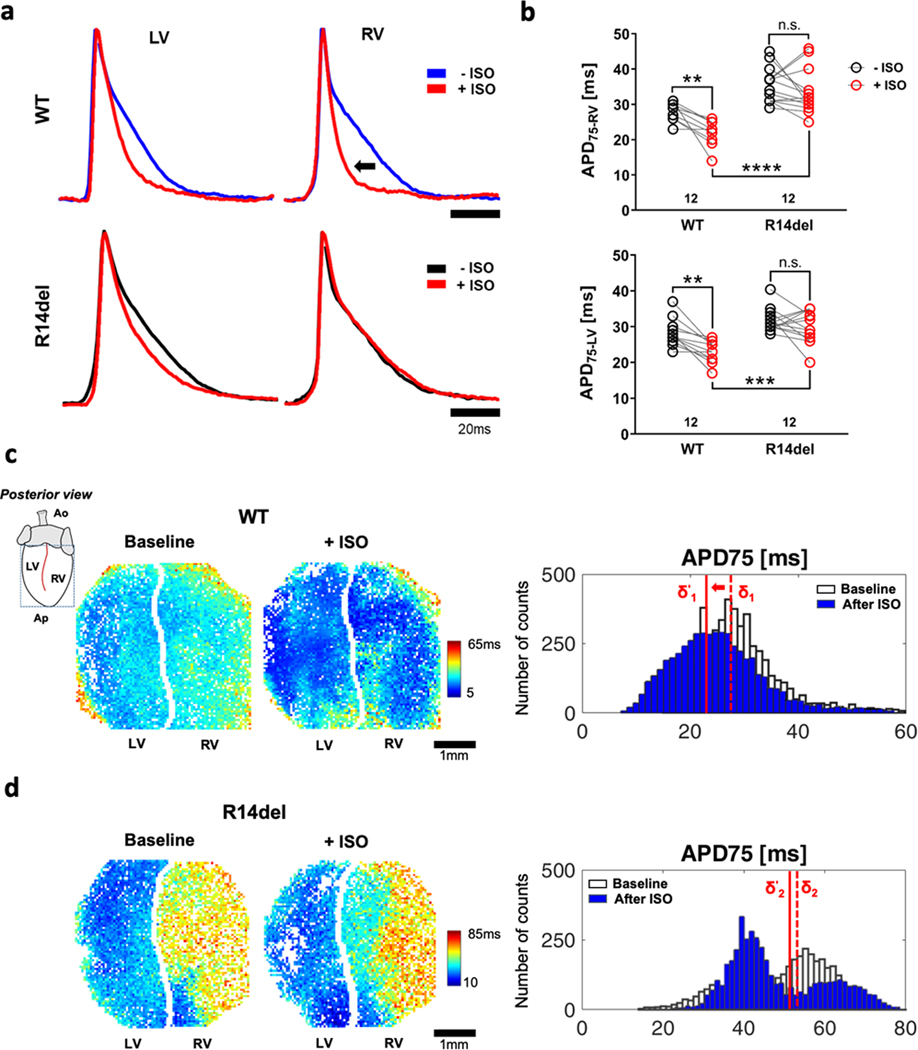
A key adaptive electrophysiological response to β-adrenergic stimulation is APD shortening, which is clearly evident by the representative AP recordings from the RV and LV of control knock-in mice with human-WT PLN (Fig. 3a, top) and native WT mice (murine-WT PLN, Fig. II in the Supplement). As expected, in both cases, APs shorten upon exposure to ISO (red). In the R14del heart, on the other hand, ISO-induced APD shortening was blunted in the LV and RV (Fig. 3). In hearts from WT mice, we calculated an overall decrease in APD of 21.7% and 19.2% in the RV and LV, respectively. In contrast, the corresponding decrease in APD of R14del mice was insignificant in either chamber (Fig. 3b). The blunted APD shortening in response to ISO in R14del compared to WT is documented in the epicardial APD75 maps, which also reveal the marked APD gradient in the former versus the latter, both before and after ISO (Fig. 3c). Also shown are the epicardial APD75 histograms at baseline (white) and after ISO (blue) in representative WT (top) and R14del (bottom) hearts (Fig. 3d). These data illustrate the leftward shift in the median of the WT APD distribution by ISO, an effect which was blunted in R14del.
Figure 3: Impaired APD adaptation to β-adrenergic stimulation in R14del hearts.
a. Representative AP traces recorded from the LV and RV before (blue for WT, black for R14del) and after (red) challenging hearts with ISO at 10Hz. b. A blunted response to ISO is observed across all R14del hearts in both LV and RV. c. Colormaps showing APD75 values of individual pixels from the segmented left and right masks over the posterior epicardium of WT (top) and R14del (bottom) hearts before and after ISO showing a relatively static response to ß-adrenergic stimulation in R14del compared to WT as well as marked APD dispersion in the former vs latter. d. Representative histograms showing the epicardial APD75 distribution in WT and R14del hearts at baseline (white) and after isoproterenol (blue). A leftward shift in the WT (median shift δ1 → δ’1) indicates an overall decrease in average APD75 vs. a much-reduced response in R14del hearts (δ2 → δ’2). ** p <0.01, *** p <0.001, **** p <0.0001.
Interventricular dispersion of repolarization produces local conduction block in R14del hearts.
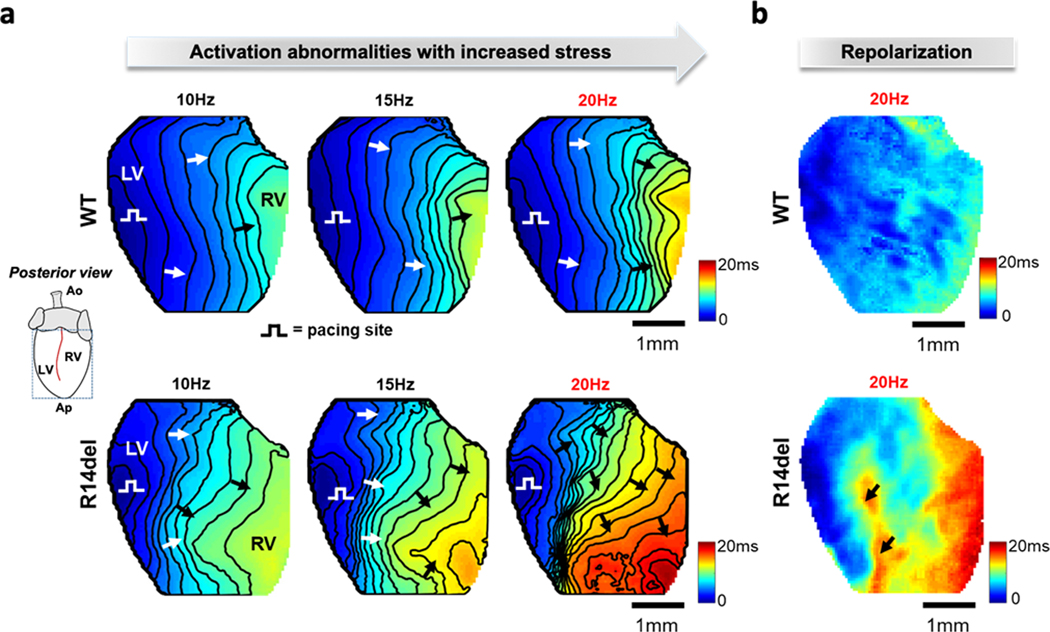
Compared to WT, conduction velocity (CV) was 15% decreased overall (WT 0.45±0.02 m.s−1 vs R14del 0.38±0.04 m.s−1, p < 0.05), and a 20% decrease was recorded in the RV of R14del hearts alone (WT CVRV = 0.43±0.02 m.s−1, R14del CVRV = 0.34±0.04 m.s−1, p < 0.05). In Fig. 4a, representative activation maps illustrate the asymmetric changes in electrophysiological properties, as the pacing frequency is increased from 10-to-20Hz, prior to arrhythmia induction in R14del. At 10Hz, a planar wave propagates across the posterior epicardium from the LV to the RV in both groups. Clustering of isochronal lines at 15Hz indicates local conduction slowing at the LV/RV interface in R14del (bottom). At 20Hz, discrete regions of conduction block in R14del but not WT formed a concavity in the orthodromic wave-front, which only reached the contralateral side after 20ms [Movie I in the Supplement]. The wide functional dispersion observed in R14del at 20Hz formed a substrate for subsequent conduction block as successive wavefronts impinged on the refractoriness of previous beats. Functional lines of block (Fig. 4a) that emerged at 20Hz coincided closely with the inter-ventricular area of highest spatial repolarization gradient (Fig. 4b, black arrows).
Figure 4: Mechanism of arrhythmia initiation and maintenance in the R14del heart.
a. Representative activation maps measured during pacing at 10, 15, and 20Hz that illustrate the rate-dependent development of severe local conduction slowing/block in R14del but not WT hearts. b. Repolarization maps for the same WT and R14del hearts. R14del hearts exhibit a marked interventricular repolarization gradient compared to WT. Arrows indicate site of high repolarization gradient that coincides with the loci of severe conduction slowing/block in R14del.
Sustained rotors preferentially anchor to the RV in R14del hearts.
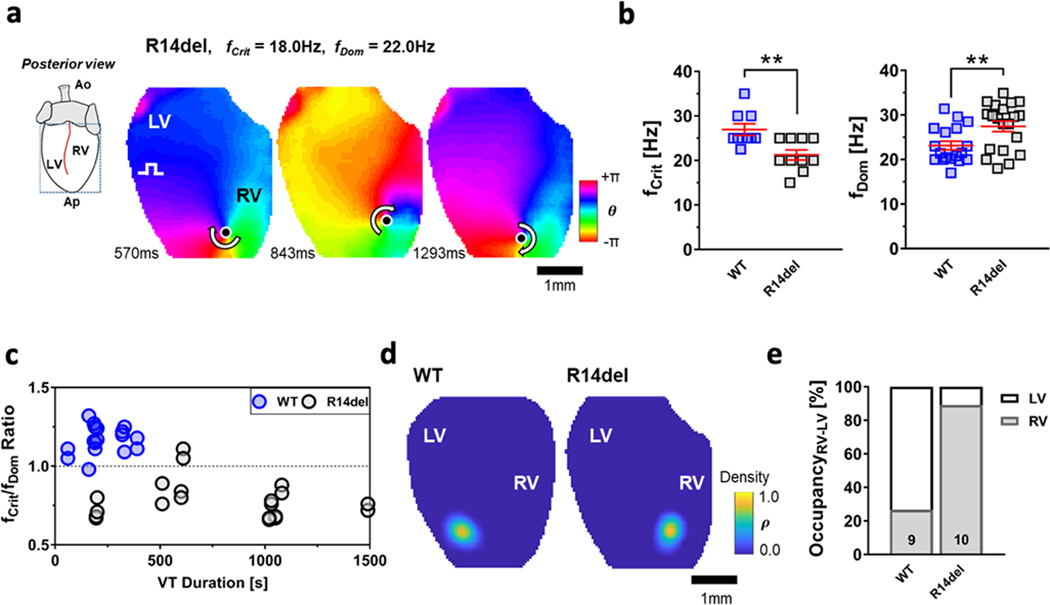
We identified the active sources of reentry during sustained VT (Fig. 5a). In the majority of cases (6/9 WT, 7/10 R14del), the wave patterns were characterized by a single stable reentrant source rotating around a PS anchored to either ventricle [Movies showing spiral waves anchored to the LV and RV in the WT and R14del hearts, respectively, can be found in Movie II & III in the Supplement]. The remaining reentry episodes (3/9 WT, 3/10 R14del) were due to macro-meandering spiral waves or fibrillatory activity caused by multiple simultaneous rotors (Fig. III and Movies IV & V in the Supplement). In Fig. 5b, the lower VT-threshold (fCrit) documented the heightened propensity of the R14del group for arrhythmia (WT fCrit = 26.9±1.3Hz, R14del fCrit = 21.3±1.1Hz, p < 0.01). For the R14del heart illustrated in Fig. 5a, fDom map revealed a peak centered around 22Hz, which exceeded the pacing frequency at which the spiral was originally created (fCrit = 18Hz). The ratio fCrit/fDom was found to be consistently <1 (0.79±0.03) in the R14del group as the rotational frequency of the spirals was faster than fCrit (Fig. 5c). Interestingly, the opposite was true in WT hearts, where fCrit was either equal to or greater than fDom, hence fCrit/fDom >1 (1.17±0.02) (Fig. 5c). Once induced, spirals were sustained for almost twice as long in R14del compared to WT hearts before self-terminating (WT VT Duration = 214±37s, R14del VT Duration = 780±133s, p<0.01). None of the WT hearts accommodated a sustained reentry for more than 400sec (Fig. 5c). PS-density maps in Fig. 5d revealed probability maxima located in the LV for WT and RV for R14del hearts. The probability of finding a PS at more distal locations was 0. This probability distribution was typically observed for all stationary and minimally-meandering spirals in both groups. An exceeding majority (89.1±3.2%) of all reentrant VT episodes was predominantly RV-sided in the R14del group, whereas only 26.7±8.7% was so in WT (Fig. 5e). Averaging of these metrics (fCrit, fDom, VT-duration and LV/RV occupancy) across all VT episodes, regardless of whether they were reentrant or not, led to consistent findings (data not shown). In a minority of R14del hearts (<20%) VT was driven by focally-mediated activation patterns emanating from the RV (Fig. IV in the Supplement) with clear evidence of wavebreaks in the LV that gave rise to sporadic PS formation and annihilation. Unlike the sustained and stable RV-sided rotors that acted as the VT driver in the majority of R14del hearts, PS formation in the LV was diffuse and intermittent (Fig. IV in the Supplement).
Figure 5: Sustained rotors preferentially pin to the RV in R14del hearts.
a. Snapshots showing the instantaneous phase for all pixels. These sequential maps show rotation around a PS (filled circle) with the arrow showing the direction of rotation (chirality). b. A lower fCrit in R14del vs WT hearts (left) documents their significantly higher vulnerability to arrhythmia. Spirals entrained the ventricles at higher fDom in R14del vs WT hearts (right). c. Composite plot of the ratio of fCrit/fDom and the VT duration of 9 WT and 10 R14del hearts. The ratio fCrit/fDom is < 1 in R14del hearts and > 1 in WT hearts, resulting in a clear separation between the two groups. d. Phase density maps showing the probability of finding a PS at a certain location at a random time point during the VT episode in representative WT and R14del hearts. e. In R14del hearts, PS were located within the RV in more than 80% of the time vs. less than 25% of the time in WT hearts. ** p <0.01.
In an additional set of hearts (WT, n=3, R14del, n=3), we tested the implications of the interventricular APD gradient by pacing from the RV instead. Although VT was induced at comparable pacing rates in R14del vs WT hearts (WT fCrit=25.8±0.9Hz, R14del fCrit=27.5±1.4Hz, p=0.374), AP alternans were observed selectively in the RV of the R14del group starting at frequencies in the 15–25Hz range, which neighbor the fCrit recorded when hearts were paced from the LV (Fig. V in the Supplement).
Spatio-temporal dynamics of anchored rotors in WT and R14del hearts.
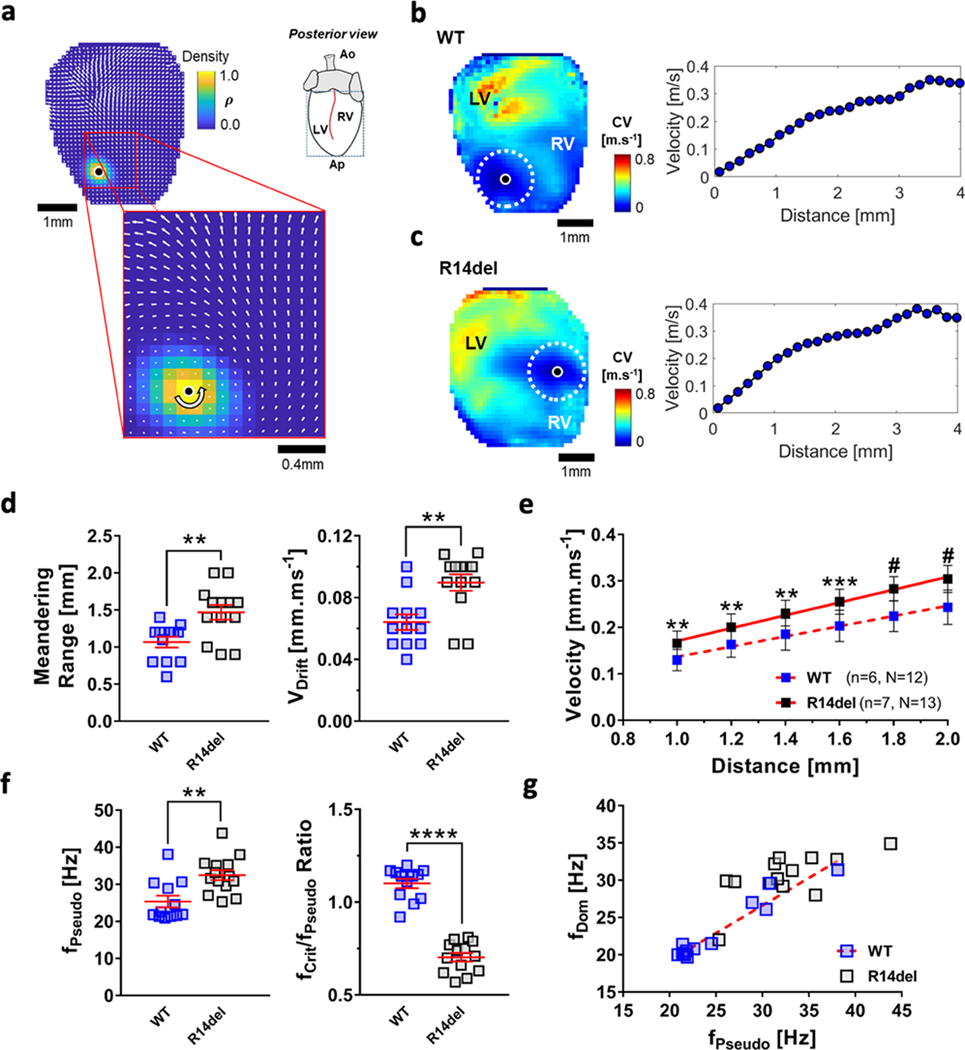
Fig. 6a shows a velocity field superimposed on the WT PS-density map, where the local curvature of the wavefront towards the PS increases and the velocity decreases. In Figs. 6b & 6c, the average velocity values over the entire 2-sec epoch are color-coded (0–0.8m.s−1) in the maps of the WT and R14del hearts, respectively. Also shown is the velocity as a function of distance away from the PS for WT and R14del hearts. R14del rotors exhibited a 40.2% increase in their meandering range (R14del=1.50±0.12mm, WT=1.07±0.07mm, p<0.01) with a 50% faster drift velocity (VDrift: R14del=0.09±0.01mm.ms−1, WT=0.06±0.01mm.ms−1, p<0.01), indicating less stationary behavior than their WT counterparts (Fig. 6d). The slopes of the velocity-vs.-distance plots were estimated from a least-squares fit spanning the radius from 1–2mm from the reference point (Fig. 6e); thereby, excluding the meandering range (i.e. radial distance 0–1mm from the tip). The rate of increase was greater in R14del (sV =0.138±0.006 ms−1, R2=0.99) vs WT hearts (sV=0.109±0.006 ms−1, R2 =0.99). Fig. 6f shows that spiral waves in R14del hearts exhibited a significantly higher fPseudo as they anchored to the RV (fPseudo=32.5±1.3Hz), compared to WT, the majority of which were pinned to the LV (fPseudo =25.4±1.6Hz). While the frequency of the spiral wave after attachment remained in the vicinity of fCrit in WT hearts (fCrit/fPseudo=1.10±0.03), this property was entirely lost in R14del hearts (fCrit/fPseudo =0.70±0.02). The correlation between the two functional parameters fDom and fPseudo remained consistent in WT hearts (Fig. 6g): as fPseudo increases, an increase in fDom is observed (slope =0.75±0.06, y-intercept =4.4±1.6, R2 =0.94). This property was not conserved in R14del hearts (R2 =0.44), where the dynamics observed locally (characterized by fPseudo) and those observed globally (characterized by fDom) were decoupled.
Figure 6: CV as a function of distance from the tip during reentry in WT and R14del hearts.
a. Velocity field calculated from the phase gradient in a WT heart during VT. Inset shows a magnified view around the PS. b.& c. Velocity maps in the WT (top) and R14del hearts (bottom). Lowest velocities are recorded in the vicinity of the PS, progressively increasing in magnitude away from it. Corresponding velocity vs. distance (0 to 4mm) plots are also shown. d. Increased meandering range and faster VDrift were calculated across R14del vs WT hearts. ** p <0.01. e. Weighted slopes (±95% confidence interval) of the velocity vs distance relationships for all WT and R14del hearts are 0.105±0.005 (R2 = 0.99) and 0.137±0.006 (R2 = 0.99), respectively. ** p <0.01, *** p <0.001, # p <0.0001. f. Spiral waves in R14del hearts exhibited a considerably faster fPseudo compared to WT. The ratio of fCrit/fPseudo is significantly lower in R14del hearts. ** p <0.01, **** p <0.0001. g. fDom vs. fPseudo plot. The red dotted line represents the best fit (slope = 0.81±0.06, R2 = 0.94), as fDom correlates nicely with fPseudo in WT hearts. Such property is lost in R14del hearts (R2 = 0.44).
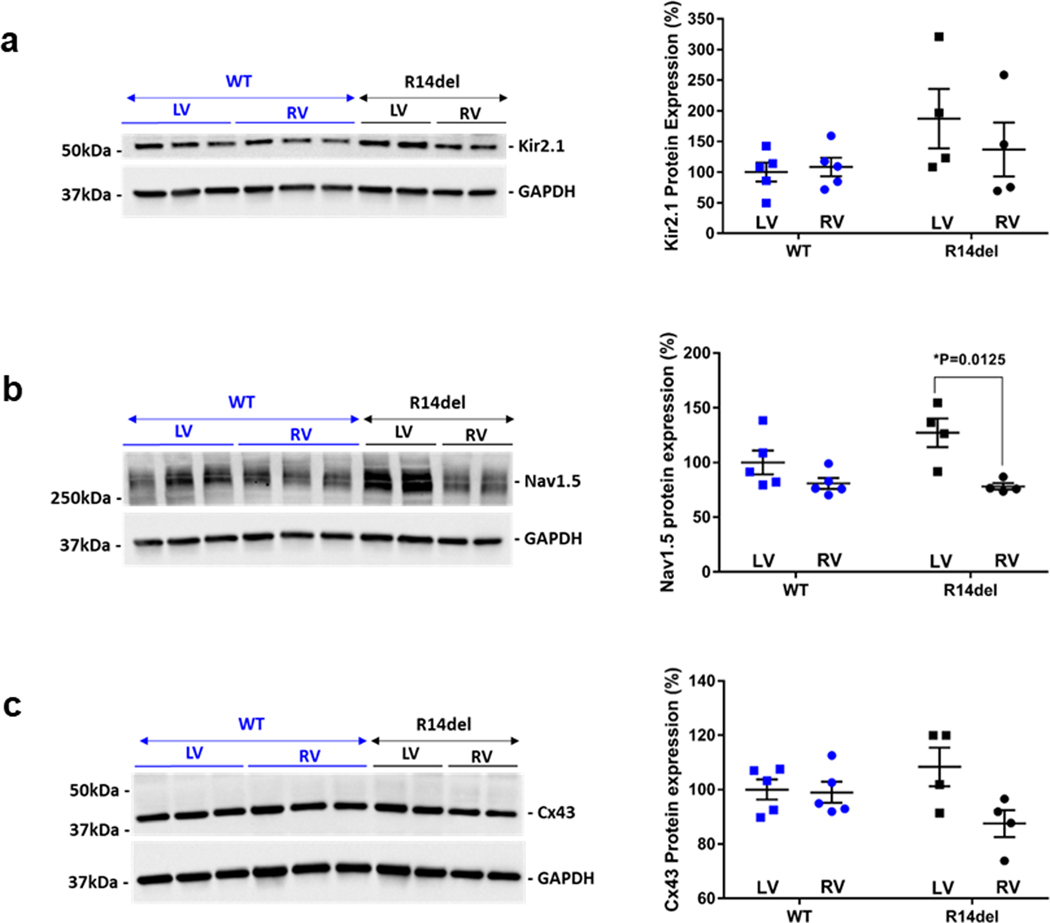
Finally, to gain insight into potential molecular mechanisms underlying the unique electrophysiological properties that promoted the maintenance of VT in R14del hearts, we measured the expression levels of key excitability proteins that are critical for AP propagation and resting membrane stability, with particular focus on inter-ventricular (RV-vs-LV) gradients in the expression of these proteins. As shown in Fig. 7, we found no inter-ventricular differences in the expression of Kir2.1, neither in WT nor in R14del hearts. In sharp contrast, Nav1.5 and Cx43 exhibited either a significant or a strong trend towards reduced expression in the RV vs LV of R14del hearts (Fig. 7).
Figure 7: RV and LV expression of Kir2.1, Nav1.5, and Cx43 in WT (n=5) and R14del (n=4) hearts.
Representative western blots and quantification of RV vs LV protein expression of Kir2.1 (a), Nav1.5 (b), and Cx43 (c) in WT and R14del. GAPDH was used as an internal loading control.
Discussion
The present study elucidates the tissue-level mechanism by which a mutation in the coding region of the human PLN gene, known as R14del, promotes the initiation and maintenance of VT in response to ß-adrenergic stimulation. The sequelae of electrophysiological events involve the emergence of local conduction abnormalities at rapid pacing rates as successive activation wavefronts encounter a steep interventricular repolarization gradient. This gradient, we found, was consistently driven by preferential APD prolongation in the RV of R14del hearts that failed to adapt to ß-adrenergic stimulation. Discrete lines of conduction block at the RV/LV interface set the stage for reentrant activation patterns, which rapidly evolved into high-frequency rotors anchored within the RV. R14del rotors differed considerably in terms of their spatio-temporal dynamics, localization and stability from their LV-predominant counterparts in WT. Our findings highlight a primary arrhythmogenic role of the R14del mutation in which the substrate for dynamic instability and arrhythmogenesis occurred in the absence of structural or hemodynamic dysfunction. As such, our current findings which explain the heightened risk of malignant VT at a young age in PLNR14del patients,18 highlight the importance of not relying strictly on echocardiography or even cardiac MR imaging with late gadolinium enhancement as reliable measures for predicting risk of SCD due to this mutation. Rather, an ISO bolus challenge may uncover impaired ECG surrogates of repolarization as a potentially powerful non-invasive tool for predicting arrhythmia risk in patients and family members harboring this mutation.
hPLNR14del-related CM as an inherited arrhythmia syndrome
Our study is the first to examine the primary electrophysiological abnormalities caused by the human PLNR14del mutation in a humanized heterozygous mouse model that recapitulates the early pro-arrhythmic phenotype observed in patients. The R14del mutation has been identified in patients diagnosed with severe forms of CM and SCD.4, 9, 18, 19 Models harboring this mutation have been previously described in the mouse. Haghighi et al.4 created a heterozygous transgenic mouse by overexpressing the mutant mouse PLNR14del. This mutant mouse died between 2 and 16 weeks of age and exhibited cardiac hypertrophy, ventricular dilatation and fibrosis.4 In a follow-up study, the same group introduced the human R14del mutation into the PLN-null mouse revealing evidence of increased wall thickness, myocyte disarray and fibrosis relative to PLN-KO hearts.20 While these studies focused on the advanced phenotype associated with chronic structural remodeling due to R14del, mechanisms by which the early pro-arrhythmic risk that marks the concealed phase of the disease remained largely unknown. To fill this knowledge gap, we performed a comprehensive study that examined in detail the electrophysiological sequelae of events that culminates in the pathogenesis of sustained VT in the absence of overt structural or mechanical remodeling. The knock-in mouse model, which we investigated here, harbors the human PLNR14del mutation in the presence of an endogenous expression of the human PLNWT to mimic the genotype observed in patients. The clinical relevance of this model is highlighted by the fact that, to the best of our knowledge, there are no reports of a homozygous manifestation of this mutation in humans. Our ex-vivo study revealed that sustained VT was readily inducible upon challenge with ISO and rapid pacing despite the absence of detectable mechanical abnormalities or gross structural remodeling. This arrhythmogenic response to ISO is in concordance with the clinical report by Denis et al.10 Their data showed a high sensitivity to ISO in triggering arrhythmias in patients suspected of having ACM.10, 21 This emphasizes the importance of primary electrical remodeling, a feature that is faithfully accounted for in our model, which seems to mimic the slow clinical progression observed in PLNR14del patients.9 Another key feature of our model was the mode of arrhythmia initiation in PLNR14del hearts, which was dependent on β-adrenergic stimulation. Indeed, we uncovered a fundamental impairment in the APD adaptation response of R14del hearts to ISO, which is akin to the observation by Ceholski et al. who demonstrated impaired ability of this mutation to undergo PKA-mediated phosphorylation in-vitro.22 Failure of the APD to adapt to ISO provided a substrate where successive activation wavefronts encountered a substantial spatial repolarization gradient at the RV/LV interface causing local conduction slowing and wavebreak formation. Such destabilizing factors led to the early onset of VT in this model. As such, our findings provide an electrophysiological basis for the typical mode of SCD in these patients, which is invariably related to adrenergic stimulation and/or strenuous exercise.23, 24 Underscoring the importance of sympathetic activation are clinical reports that document the utility of bilateral cardiac sympathectomy in treatment of refractory VT in ACM patients.25, 26
The RV as a Substrate for Reentry and Arrhythmia
This is the first study to provide direct evidence of how reentrant circuits arising in the RV can sustain VT in a humanized mouse model of PLNR14del-related cardiomyopathy. In the presence of a high interventricular gradient, Speerschneider et al. showed that VT is readily inducible when pacing from the site of shorter APD, and the heart becomes relatively protected when pacing from the site of longer APD,27 which is consistent with our results. Despite higher VT-thresholds, alternans were selectively induced in the RV of R14del hearts at frequencies neighboring those recorded when hearts were paced from the LV. The emergence of action potential alternans at lower heart rates has been mechanistically linked to arrhythmia susceptibility.12, 28 R14del-related electrophysiological remodeling facilitated the formation of wave-breaks at frequencies that do not necessarily alter propagation in otherwise healthy hearts, accounting for the decrease in fCrit. PS-density maps revealed the RV-predominant loci of PS in R14del hearts. This finding is consistent with multiple clinical reports that document reentrant VT as arising from the RV.11, 18, 24
As rapidly succeeding wavefronts emanate from rotors, the tissue becomes entrained with a dominant frequency (fDom) imposed by the rotational frequency of the single rotor, which is usually close to but lower than the frequency at which the initial wave-break was generated (i.e. fCrit).29 In WT hearts, the site of the highest source-sink mismatch is near the pacing site in the LV, where the majority of spirals were pinned, and the ratio fCrit/fDom >1 was conserved. The spiral waves in R14del hearts exhibited aberrant behavior. For one, the spiral waves entrained the heart at higher rotational frequencies than fCrit, hence fCrit/fDom <1. Secondly, the spiral did not anchor to the areas of slowest conduction (i.e. at the LV/RV interface shown in Fig. 4a) but rather was localized within a region that exhibited a relatively long basal APD. Thirdly, the anchoring in a region of longer APD paradoxically facilitated longer episodes of sustained VT with faster rotational frequencies than in WT hearts, which would normally encroach on the refractory period, increasing the likelihood of fractionation and wave-break formation. With regards to the first aberrancy, the RV in the R14del heart can evidently sustain higher frequencies than the LV, as observed with the higher VT-thresholds (fCrit) measured when these hearts were paced from the RV. As a result, fDom is not necessarily limited by fCrit. As such, the unique spiral waves in R14del hearts, where fCrit/fDom <1, are not likely to be responsive to anti-tachycardia pacing, since exogenous sources of entrainment would fail to unpin or perturb the spiral. The presence of a considerable APD gradient may account for the second aberrancy. Using the Luo-Rudy-Model-1, TenTusscher and Panfilov showed that gradients in electrophysiological properties can mobilize spiral waves toward the longer APD region in heterogeneous tissue.30 This is consistent with our experimental findings regarding the ultimate position of rotors in R14del hearts. Despite reported cases of anomalous drift,31 we speculate that spiral waves in our model likely followed a “normal” pattern from the location of the wavebreak to the RV, where the APD is longest. Investigating the transient dynamics from initiation to sustenance could help clarify this point in future studies.
Spatiotemporal Complexity Underlying Spiral Wave Activity in R14del Hearts
For sustained arrhythmias in both groups, we characterized the spatiotemporal dynamics of the spiral waves in the vicinity of the PS by examining the local velocity as a function of distance from the tip (Fig. 6). This analysis was modified from the elegant method presented by Noujaim et al.17 in two key aspects. First, we calculated velocities from the phase maps and averaged them over the entire VT epoch, eliminating dependence on activation times and limitation to one single spiral rotation around its tip. Second, we defined a pseudofrequency metric (fPseudo), which corresponds to the frequency of rigidly rotating objects with the same velocity-vs-distance dependence. The linear correlation between fDom and fPseudo in the WT heart (Fig. 6) indicates that under normal physiological conditions, the rigid-rotation analogy holds true. The observed decoupling of these two frequencies in mutant hearts and the loss of correlation in a sample-by-sample comparison (Fig. 6g) indicate a change in R14del vs WT hearts that went beyond a simple increase in arrhythmia propensity. Unlike fDom, fPseudo is sensitive to the dynamics close to the PS, such as local wavefront curvature, micro-meandering of the spiral tip, core size, and local excitability. Even though not directly accessible experimentally, we speculate that one or more of these local properties are significantly altered in R14del hearts, which would favor the initiation and stability of VT. On a more general level, the fact that our velocity field quantification near the PS is capable of detecting these subtle changes also makes it an interesting tool for the characterization of pinned or minimally meandering rotors in other cardiac diseases. Further developed, it could reveal additional aspects of the non-trivial link between molecular changes, structural remodeling properties and emergent excitation wave dynamics.
Limitations of the study
The mouse is important for modeling human disease. However, significant differences exist in channel function and mechanisms of murine and human AP, particularly in terms of heart rate and repolarization. Therefore, direct extrapolation of results from mouse models to humans is not straightforward. For instance, a complete knock-out of PLN in the mouse was not associated with structural abnormalities or adverse outcomes.20 To our knowledge, such a mutation has not been reported in humans, potentially due to its embryonic lethality. Another relevant difference between mice and humans is that mice rely more on SR Ca2+ uptake for removal of cytosolic Ca2+ than humans,32 which could potentially alter the manifestation of mutations affecting Ca2+ homeostasis. Nevertheless, studying this mouse model provided useful insights into the role that a PLN-mutation plays in maintaining reentry. The extent of Ca2+ dysregulation in modulating the different frequency metrics seen in this study will require further evaluation. We were only able to observe the established arrhythmia, which does not consider the transient spiral dynamics from initiation to sustenance. Finally, we did not address the reason for the preferential involvement of the RV in this model. We speculate that the mechanisms underlying chamber-specific EP-remodeling might arise from developmental perturbations or transcriptional changes in the context of the hPLNR14del mutation. Despite these limitations, our study provides useful insights into mechanisms underlying increased arrhythmia susceptibility in this particular cardiomyopathy. It is important to note that inherited arrhythmias arising in the context of various disease-causing mutations may be mechanistically distinct from one another, both in terms of their etiologies, mode of initiation, pathogenesis and maintenance. Therefore, caution must be exercised when extrapolating findings from this study to other forms of inherited cardiomyopathies, whether classified as ACM, DCM or of a mixed phenotype. Nonetheless, the experimental and analytical tools that we report in this paper will be useful in determining the applicability of our current findings to other disease-causing mutations.
Conclusion
In the present study, we showed how the observed electrical heterogeneity and perturbations of electrophysiological parameters were sufficient to predispose the cardiac tissue to conduction defects, to initiate reentry, and to increase arrhythmia vulnerability in hearts harboring the heterozygous hPLNR14del mutation. We provided direct evidence that these adrenergic-driven organized sources of arrhythmia could be easily sustained in the RV in the absence of structural or mechanical changes. This supports the emerging hypothesis that this disease is inherently arrhythmogenic, independently of fatty infiltration, fibrosis or mechanical dysfunction. This increased susceptibility prompts the implementation of more aggressive SCD risk stratification to improve prognosis in PLNR14del patients.
Supplementary Material
Clinical Perspective.
What’s New?
A mutation in a calcium regulatory protein identified in patients with an inherited cardiomyopathy causes primary electrical remodeling and increased arrhythmia susceptibility in the absence of overt mechanical dysfunction when expressed in a knock-in mouse model.
These humanized mice exhibit inter-ventricular repolarization gradients that form lines of conduction block leading to ventricular tachycardia upon β-adrenergic stimulation.
Inherited arrhythmias caused by this heterozygous mutation are largely sustained by high-frequency right ventricular rotors with unique spatio-temporal dynamics that promote their stability.
Clinical Implications?
Collectively, the results of this study provide evidence for heightened risk of sustained arrhythmias in PLN-R14del patients at early stages of the disease.
Implementing a risk stratification strategy by potentially examining how surrogates of repolarization such as the corrected QT-interval respond to acute β-adrenergic stimulation may identify appropriate candidates for implantable cardioverter defibrillators.
If successfully translated to humans, our findings may help prevent sudden cardiac death during the early concealed phase of the disease, which predominantly affects young individuals.
Acknowledgements
NR and FGA designed research protocols; FS, DJ, DC, EK, RJH, LZ, and CC designed the mouse model; NR, MC, and ZI performed experiments; NR and PB designed the analysis; PB implemented the algorithms; NR analyzed the data; NR, PB, and FGA wrote the paper.
Sources of Funding
This work was supported in part by grants from the National Institutes of Health [1R01HL149344, 1R01HL148008 & 1R01HL137259 to F.G.A.] and the Leducq Foundation [18CVD01 to E.G.K & F.S.].
Non-standard Abbreviations and Acronyms
- R14del
Argenine 14 deletion
- PLN
Phospholamban
- hPLN
human phospholamban
- hPLNR14del
deletion of R14 in coding region of human PLN
- WT
wildtype
- RV
right ventricular
- LV
left ventricular
- VT
ventricular tachycardia
- CM
cardiomyopathies
- HF
heart failure
- SCD
sudden cardiac death
- ACM
arrhythmogenic cardiomyopathy
- DCM
dilated cardiomyopathy
- ICD
implantable cardioverter defibrillator
- fDom
dominant frequency
- fCrit
critical frequency
- PS
phase singularity
- VDrift
drift velocity of phase singularity
- CV
conduction velocity
- APD
action potential duration
- Sv
slope of the velocity vs distance relationship
- fpseudo
pseudo frequency
- ISO
isoproterenol
- CVRV
right ventricular conduction velocity
- Nav1.5
pore forming subunit of cardiac voltage gated sodium channel
- Cx43
connexin 43
- Kir2.1
inward rectifier potassium channel subunit
- δ
median of the distribution
Footnotes
Disclosures
None.
References
- 1.Jacoby D and McKenna WJ. Genetics of inherited cardiomyopathy. Eur Heart J. 2012;33:296–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cerrone M, Remme CA, Tadros R, Bezzina CR and Delmar M. Beyond the One Gene-One Disease Paradigm: Complex Genetics and Pleiotropy in Inheritable Cardiac Disorders. Circulation. 2019;140:595–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braunwald E. Cardiomyopathies: An Overview. Circ Res. 2017;121:711–721. [DOI] [PubMed] [Google Scholar]
- 4.Haghighi K, Kolokathis F, Gramolini AO, Waggoner JR, Pater L, Lynch RA, Fan GC, Tsiapras D, Parekh RR, Dorn GW 2nd, MacLennan DH, Kremastinos DT and Kranias EG. A mutation in the human phospholamban gene, deleting arginine 14, results in lethal, hereditary cardiomyopathy. Proc Natl Acad Sci U S A. 2006;103:1388–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bers DM. Calcium cycling and signaling in cardiac myocytes. Annu Rev Physiol. 2008;70:23–49. [DOI] [PubMed] [Google Scholar]
- 6.Luo W, Wolska BM, Grupp IL, Harrer JM, Haghighi K, Ferguson DG, Slack JP, Grupp G, Doetschman T, Solaro RJ and Kranias EG. Phospholamban gene dosage effects in the mammalian heart. Circ Res. 1996;78:839–47. [DOI] [PubMed] [Google Scholar]
- 7.Kadambi VJ, Ponniah S, Harrer JM, Hoit BD, Dorn GW, 2nd, Walsh RA and Kranias EG. Cardiac-specific overexpression of phospholamban alters calcium kinetics and resultant cardiomyocyte mechanics in transgenic mice. J Clin Invest. 1996;97:533–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van der Zwaag PA, van Rijsingen IA, Asimaki A, Jongbloed JD, van Veldhuisen DJ, Wiesfeld AC, Cox MG, van Lochem LT, de Boer RA, Hofstra RM, Christiaans I, van Spaendonck-Zwarts KY, Lekanne dit Deprez RH, Judge DP, Calkins H, Suurmeijer AJ, Hauer RN, Saffitz JE, Wilde AA, van den Berg MP and van Tintelen JP. Phospholamban R14del mutation in patients diagnosed with dilated cardiomyopathy or arrhythmogenic right ventricular cardiomyopathy: evidence supporting the concept of arrhythmogenic cardiomyopathy. Eur J Heart Fail. 2012;14:1199–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Rijsingen IA, van der Zwaag PA, Groeneweg JA, Nannenberg EA, Jongbloed JD, Zwinderman AH, Pinto YM, Dit Deprez RH, Post JG, Tan HL, de Boer RA, Hauer RN, Christiaans I, van den Berg MP, van Tintelen JP and Wilde AA. Outcome in phospholamban R14del carriers: results of a large multicentre cohort study. Circ Cardiovasc Genet. 2014;7:455–65. [DOI] [PubMed] [Google Scholar]
- 10.Denis A, Sacher F, Derval N, Lim HS, Cochet H, Shah AJ, Daly M, Pillois X, Ramoul K, Komatsu Y, Zemmoura A, Amraoui S, Ritter P, Ploux S, Bordachar P, Hocini M, Jais P and Haissaguerre M. Diagnostic value of isoproterenol testing in arrhythmogenic right ventricular cardiomyopathy. Circ Arrhythm Electrophysiol. 2014;7:590–7. [DOI] [PubMed] [Google Scholar]
- 11.Ilkan Z, Strauss B, Campana C and Akar FG. Optical Action Potential Mapping in Acute Models of Ischemia-Reperfusion Injury: Probing the Arrhythmogenic Role of the Mitochondrial Translocator Protein. Methods Mol Biol. 2018;1816:133–143. [DOI] [PubMed] [Google Scholar]
- 12.Cacheux M, Strauss B, Raad N, Ilkan Z, Hu J, Benard L, Feske S, Hulot JS and Akar FG. Cardiomyocyte-Specific STIM1 (Stromal Interaction Molecule 1) Depletion in the Adult Heart Promotes the Development of Arrhythmogenic Discordant Alternans. Circ Arrhythm Electrophysiol. 2019;12:e007382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berenfeld O, Zaitsev AV, Mironov SF, Pertsov AM and Jalife J. Frequency-dependent breakdown of wave propagation into fibrillatory conduction across the pectinate muscle network in the isolated sheep right atrium. Circ Res. 2002;90:1173–80. [DOI] [PubMed] [Google Scholar]
- 14.Umapathy K, Nair K, Masse S, Krishnan S, Rogers J, Nash MP and Nanthakumar K. Phase mapping of cardiac fibrillation. Circ Arrhythm Electrophysiol. 2010;3:105–14. [DOI] [PubMed] [Google Scholar]
- 15.Bray MA, Lin SF, Aliev RR, Roth BJ and Wikswo JP Jr., Experimental and theoretical analysis of phase singularity dynamics in cardiac tissue. J Cardiovasc Electrophysiol. 2001;12:716–22. [DOI] [PubMed] [Google Scholar]
- 16.Iyer AN and Gray RA. An experimentalist’s approach to accurate localization of phase singularities during reentry. Ann Biomed Eng. 2001;29:47–59. [DOI] [PubMed] [Google Scholar]
- 17.Noujaim SF, Pandit SV, Berenfeld O, Vikstrom K, Cerrone M, Mironov S, Zugermayr M, Lopatin AN and Jalife J. Up-regulation of the inward rectifier K+ current (I K1) in the mouse heart accelerates and stabilizes rotors. J Physiol. 2007;578:315–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groeneweg JA, van der Zwaag PA, Jongbloed JD, Cox MG, Vreeker A, de Boer RA, van der Heijden JF, van Veen TA, McKenna WJ, van Tintelen JP, Dooijes D and Hauer RN. Left-dominant arrhythmogenic cardiomyopathy in a large family: associated desmosomal or nondesmosomal genotype? Heart Rhythm. 2013;10:548–59. [DOI] [PubMed] [Google Scholar]
- 19.Posch MG, Perrot A, Geier C, Boldt LH, Schmidt G, Lehmkuhl HB, Hetzer R, Dietz R, Gutberlet M, Haverkamp W and Ozcelik C. Genetic deletion of arginine 14 in phospholamban causes dilated cardiomyopathy with attenuated electrocardiographic R amplitudes. Heart Rhythm. 2009;6:480–6. [DOI] [PubMed] [Google Scholar]
- 20.Haghighi K, Pritchard T, Bossuyt J, Waggoner JR, Yuan Q, Fan GC, Osinska H, Anjak A, Rubinstein J, Robbins J, Bers DM and Kranias EG. The human phospholamban Arg14-deletion mutant localizes to plasma membrane and interacts with the Na/K-ATPase. J Mol Cell Cardiol. 2012;52:773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T and Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the task force criteria. Circulation. 2010;121:1533–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ceholski DK, Trieber CA, Holmes CF and Young HS. Lethal, hereditary mutants of phospholamban elude phosphorylation by protein kinase A. J Biol Chem. 2012;287:26596–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Thiene G, Nava A, Corrado D, Rossi L and Pennelli N. Right ventricular cardiomyopathy and sudden death in young people. N Engl J Med. 1988;318:129–33. [DOI] [PubMed] [Google Scholar]
- 24.Corrado D, Basso C, Thiene G, McKenna WJ, Davies MJ, Fontaliran F, Nava A, Silvestri F, Blomstrom-Lundqvist C, Wlodarska EK, Fontaine G and Camerini F. Spectrum of clinicopathologic manifestations of arrhythmogenic right ventricular cardiomyopathy/dysplasia: a multicenter study. J Am Coll Cardiol. 1997;30:1512–20. [DOI] [PubMed] [Google Scholar]
- 25.Assis FR, Krishnan A, Zhou X, James CA, Murray B, Tichnell C, Berger R, Calkins H, Tandri H and Mandal K. Cardiac sympathectomy for refractory ventricular tachycardia in arrhythmogenic right ventricular cardiomyopathy. Heart Rhythm. 2019;16:1003–1010. [DOI] [PubMed] [Google Scholar]
- 26.Te Riele AS, Ajijola OA, Shivkumar K and Tandri H. Role of Bilateral Sympathectomy in the Treatment of Refractory Ventricular Arrhythmias in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ Arrhythm Electrophysiol. 2016;9:e003713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Speerschneider T, Grubb S, Olesen SP, Calloe K and Thomsen MB. Ventricular repolarization time, location of pacing stimulus and current pulse amplitude conspire to determine arrhythmogenicity in mice. Acta Physiol (Oxf). 2017;219:660–668. [DOI] [PubMed] [Google Scholar]
- 28.Pastore JM, Girouard SD, Laurita KR, Akar FG and Rosenbaum DS. Mechanism linking T-wave alternans to the genesis of cardiac fibrillation. Circulation. 1999;99:1385–94. [DOI] [PubMed] [Google Scholar]
- 29.Majumder R, Pandit R and Panfilov AV. Turbulent electrical activity at sharp-edged inexcitable obstacles in a model for human cardiac tissue. Am J Physiol Heart Circ Physiol. 2014;307:H1024–35. [DOI] [PubMed] [Google Scholar]
- 30.Ten Tusscher KH and Panfilov AV. Reentry in heterogeneous cardiac tissue described by the Luo-Rudy ventricular action potential model. Am J Physiol Heart Circ Physiol. 2003;284:H542–8. [DOI] [PubMed] [Google Scholar]
- 31.Sridhar S, Sinha S and Panfilov AV. Anomalous drift of spiral waves in heterogeneous excitable media. Phys Rev E Stat Nonlin Soft Matter Phys. 2010;82:051908. [DOI] [PubMed] [Google Scholar]
- 32.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.