Abstract
Background
Intravenous prostaglandin E2 and F2 alpha can be used to induce labour, however their use is limited by unacceptable maternal side‐effect profiles. This is one of a series of reviews of methods of cervical ripening and labour induction using standardised methodology.
Objectives
To determine the effects of intravenous prostaglandin for third trimester cervical ripening or induction of labour.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group Trials Register (31 January 2004) and bibliographies of relevant papers. We updated this search on 7 May 2010 and added the results to the awaiting classification section.
Selection criteria
Randomised controlled trials comparing intravenous prostaglandin used for third trimester cervical ripening or labour induction with placebo/no treatment or other methods listed above it on a predefined list of labour induction methods.
Data collection and analysis
A generic strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. This involved a two‐stage method of data extraction. The initial data extraction was done centrally.
Main results
Thirteen trials (1165 women) were included in the review.
The use of intravenous prostaglandin was associated with higher rates of uterine hyperstimulation both with changes in the fetal heart rate (relative risk (RR) 6.76, 95% confidence interval (CI) 1.23 to 37.11) and without (RR 4.25, 95% CI 1.48 to 12.24) compared to oxytocin. Use of prostaglandin was also associated with significantly more maternal side‐effects (gastrointestinal, thrombophlebitis and pyrexia, RR 3.75, 95% CI 2.46 to 5.70) than oxytocin. Prostaglandin was no more likely to result in vaginal delivery than oxytocin (RR 0.85, 95% CI 0.61 to 1.18).
No significant differences emerged from subgroup analysis or from the trials comparing combination oxytocin/prostaglandin F2 alpha and oxytocin or extra‐amniotic versus intravenous prostaglandin E2.
Authors' conclusions
Intravenous prostaglandin is no more efficient than intravenous oxytocin for the induction of labour but its use is associated with higher rates of maternal side‐effects and uterine hyperstimulation.
No conclusions can be drawn form the comparisons of combination of prostaglandin F2 alpha and oxytocin compared to oxytocin alone or extra‐amniotic and intravenous prostaglandin E2.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
Keywords: Female; Humans; Pregnancy; Clinical Trials as Topic; Dinoprost; Dinoprost/administration & dosage; Dinoprostone; Dinoprostone/administration & dosage; Injections, Intravenous; Labor, Induced; Labor, Induced/methods; Oxytocics; Oxytocics/administration & dosage; Oxytocin; Oxytocin/administration & dosage
Plain language summary
Intravenous prostaglandin for induction of labour
Inducing labour with intravenous prostaglandin is effective but has adverse effects. Induction of labour is common when continuing the pregnancy poses a greater risk to the mother or her unborn child. Prostaglandins, produced naturally by the body, can ripen the cervix and stimulate contractions. This review found that giving prostaglandins intravenously during the third trimester of pregnancy is effective in inducing labour, but is more expensive and causes more adverse effects than oxytocin (another hormone for inducing labour).
Background
Induction of labour is a common obstetric practice performed when continuing the pregnancy is felt to be of greater risk to the physical or mental wellbeing of either the mother or her unborn child.
Prostaglandins are produced naturally by the body and are implicated in the onset and progression of spontaneous labour (Johnston 1993). Prostaglandins are capable not only of inducing cervical ripening but also stimulating uterine contractions resulting in labour, hence they may be more efficient than other agents such as oxytocin which rely mainly on stimulating uterine contractions. The use of prostaglandins for cervical ripening appears to reduce analgesic requirements, operative delivery and non‐delivery within 12 to 24 hours at the expense of increased uterine hyperstimulation and maternal gastrointestinal side‐effects in up to 7% of cases (Keirse 1993). The two most widely used types of prostaglandins are prostaglandin E2 and prostaglandin F2 alpha. Routes of administration include oral, extra‐amniotic and intravenous. Despite no evidence of increased fetal side‐effects (Lindmark 1976), use of intravenous prostaglandin has diminished in recent years because of perceived high rates of maternal gastrointestinal and cutaneous side‐effects compared to intravenous oxytocin (MacKenzie 1999).
This review is one of a series of reviews of methods of labour induction using a standardised protocol. For more detailed information on the rationale for this methodological approach, please refer to the currently published protocol (Hofmeyr 2000).
Objectives
To determine, from the best available evidence, the effectiveness and safety of intravenous prostaglandin for third trimester cervical ripening and induction of labour.
Methods
Criteria for considering studies for this review
Types of studies
Clinical trials comparing intravenous prostaglandin for cervical ripening or labour induction, with placebo/no treatment or other methods listed above it on a predefined list of methods of labour induction (see 'Methods of the review'); the trials included some form of random allocation to either group; and they reported one or more of the prestated outcomes.
Types of participants
Pregnant women due for third trimester induction of labour, carrying a viable fetus.
Types of interventions
Intravenous prostaglandin compared with placebo/no treatment or any other method above it on a predefined list of methods of labour induction. It was intended that comparisons with 12 methods, which are placed above intravenous prostaglandin on a predefined list, would be included in this review as follows:
placebo/no treatment;
vaginal prostaglandins;
intracervical prostaglandins;
intravenous oxytocin;
amniotomy;
intravenous oxytocin with amniotomy;
vaginal misoprostol;
oral misoprostol;
oral misoprostol with amniotomy;
mechanical methods including extra‐amniotic Foley catheter;
membrane sweeping;
extra‐amniotic prostaglandins.
Analysis was performed on intravenous prostaglandins as a single entity and also on intravenous prostaglandin E2 and F2 alpha separately. In the studies included, a wide variety of dosage regimens for both intravenous oxytocin and intravenous prostaglandins were used. Subgroup analysis on individual dosage regimens has not therefore been performed. For details of the predefined list of induction methods please refer to 'Methods of the review'.
Types of outcome measures
Clinically relevant outcomes for trials of methods of cervical ripening/labour induction have been prespecified by two authors of labour induction reviews (Justus Hofmeyr and Zarko Alfirevic). Differences were settled by discussion.
Five primary outcomes were chosen as being most representative of the clinically important measures of effectiveness and complications. Subgroup analyses were limited to the primary outcomes:
(1) vaginal delivery not achieved within 24 hours; (2) uterine hyperstimulation with fetal heart rate (FHR) changes; (3) caesarean section; (4) serious neonatal morbidity or perinatal death (e.g. seizures, birth asphyxia defined by trialists, neonatal encephalopathy, disability in childhood); (5) serious maternal morbidity or death (e.g. uterine rupture, admission to intensive care unit, septicaemia).
Perinatal and maternal morbidity and mortality are composite outcomes. This is not an ideal solution because some components are clearly less severe than others. It is possible for one intervention to cause more deaths but less severe morbidity. However, in the context of labour induction at term this was unlikely. Composite outcomes also provide heightened sensitivity to a change in the incidence of these rare events. The incidence of individual components was explored as secondary outcomes (see below).
Secondary outcomes relate to measures of effectiveness, complications and satisfaction:
Measures of effectiveness: (6) cervix unfavourable/unchanged after 12 to 24 hours; (7) oxytocin augmentation.
Complications: (8) uterine hyperstimulation without FHR changes; (9) uterine rupture; (10) epidural analgesia; (11) instrumental vaginal delivery; (12) meconium stained liquor; (13) Apgar score less than seven at five minutes; (14) neonatal intensive care unit admission; (15) neonatal encephalopathy; (16) perinatal death; (17) disability in childhood; (18) maternal side‐effects (all); (19) maternal nausea; (20) maternal vomiting; (21) maternal diarrhoea; (22) other maternal side‐effects; (23) postpartum haemorrhage (as defined by the trial authors); (24) serious maternal complications (e.g. intensive care unit admission, septicaemia but excluding uterine rupture); (25) maternal death.
Measures of satisfaction: (26) woman not satisfied; (27) caregiver not satisfied.
While all the above outcomes were sought, only those with data appear in the analysis tables.
The terminology of uterine hyperstimulation is problematic (Curtis 1987). In the reviews we used the term 'uterine hyperstimulation without FHR changes' to include uterine tachysystole (more than five contractions per 10 minutes for at least 20 minutes) and uterine hypersystole/hypertonus (a contraction lasting at least two minutes) and 'uterine hyperstimulation with FHR changes' to denote uterine hyperstimulation syndrome (tachysystole or hypersystole with fetal heart rate changes such as persistent decelerations, tachycardia or decreased short term variability).
Outcomes were included in the analysis if reasonable measures were taken to minimise observer bias and data were available for analysis according to original allocation.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2004). We updated this search on 7 May 2010 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
quarterly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL and MEDLINE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
Searching other resources
The reference lists of trial reports and reviews were searched by hand.
The original search was performed simultaneously for all reviews of methods of inducing labour, as outlined in the generic protocol for these reviews (Hofmeyr 2000).
We did not apply any language restrictions.
Data collection and analysis
A strategy was developed to deal with the large volume and complexity of trial data relating to labour induction. Many methods have been studied, in many different categories of women undergoing labour induction. Most trials are intervention‐driven, comparing two or more methods in various categories of women. Clinicians and parents need the data arranged by category of woman, to be able to choose which method is best for a particular clinical scenario. To extract these data from several hundred trial reports in a single step would be very difficult. We therefore developed a two‐stage method of data extraction. The initial data extraction was done in a series of primary reviews arranged by methods of induction of labour, following a standardised methodology. The data will then be extracted from the primary reviews into a series of secondary reviews, arranged by category of woman.
To avoid duplication of data in the primary reviews, the labour induction methods have been listed in a specific order, from one to 25. Each primary review includes comparisons between one of the methods (from two to 25) with only those methods above it on the list. Thus, the review of intravenous oxytocin (4) will include only comparisons with intracervical prostaglandins (3), vaginal prostaglandins (2), or placebo (1). Methods identified in the future will be added to the end of the list. The current list is as follows:
placebo/no treatment;
vaginal prostaglandins;
intracervical prostaglandins;
intravenous oxytocin;
amniotomy;
intravenous oxytocin with amniotomy;
vaginal misoprostol;
oral misoprostol;
mechanical methods including extra‐amniotic Foley catheter;
membrane sweeping;
extra‐amniotic prostaglandins;
intravenous prostaglandins;
oral prostaglandins;
mifepristone;
oestrogens with/without amniotomy;
corticosteroids;
relaxin;
hyaluronidase;
castor oil, bath, and/or enema;
acupuncture;
breast stimulation;
sexual intercourse;
homeopathic methods;
nitric oxide;
buccal or sublingual misoprostol;
hypnosis.
The primary reviews will be analysed by the following subgroups:
previous caesarean section or not;
nulliparity or multiparity;
membranes intact or ruptured;
cervix favourable, unfavourable or undefined.
The secondary reviews will include all methods of labour induction for each of the categories of women for which subgroup analysis has been done in the primary reviews, and will include only five primary outcome measures. There will thus be six secondary reviews of methods of labour induction in the following groups of women:
nulliparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
nulliparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined);
multiparous, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
multiparous, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined);
previous caesarean section, intact membranes (unfavourable cervix, favourable cervix, cervix not defined);
previous caesarean section, ruptured membranes (unfavourable cervix, favourable cervix, cervix not defined).
Each time a primary review is updated with new data, those secondary reviews which include data which have changed will also be updated.
The trials included in the primary reviews were extracted from an initial set of trials covering all interventions used in induction of labour (see above for details of search strategy). The data extraction process was conducted centrally. This was co‐ordinated from the Clinical Effectiveness Support Unit (CESU) at the Royal College of Obstetricians and Gynaecologists, UK, in co‐operation with the Pregnancy and Childbirth Group of The Cochrane Collaboration. This process allowed the data extraction process to be standardised across all the reviews.
The trials are initially reviewed on eligibility criteria, using a standardised form and the basic selection criteria specified above. Following this, data were extracted to a standardised data extraction form which was piloted for consistency and completeness. The pilot process involved the researchers at the CESU and previous review authors in the area of induction of labour.
Information was extracted regarding the methodological quality of trials on a number of levels. This process was completed without consideration of trial results. Assessment of selection bias examined the process involved in the generation of the random sequence and the method of allocation concealment separately. These were then judged as adequate or inadequate using the criteria described in Table 1 for the purpose of the reviews.
1. Methodological quality of trials.
| Methodological item | Adequate | Inadequate |
| Generation of random sequence | Computer generated sequence, random number tables, lot drawing, coin tossing, shuffling cards, throwing dice. | Case number, date of birth, date of admission, alternation. |
| Concealment of allocation | Central randomisation, coded drug boxes, sequentially sealed opaque envelopes. | Open allocation sequence, any procedure based on inadequate generation. |
We examined performance bias with regards to whom was blinded in the trials i.e. participant, caregiver, outcome assessor or analyst. In many trials the caregiver, assessor and analyst were the same party. We sought details of the feasibility and appropriateness of blinding at all levels.
Predefined subgroup analyses are: previous caesarean section or not; nulliparity or multiparity; membranes intact or ruptured, and cervix unfavourable, favourable or undefined. Only those outcomes with data will appear in the analysis tables.
Individual outcome data are included in the analysis if they meet the prestated criteria in 'Types of outcome measures'. Included trial data are processed as described in the Cochrane Reviewers' Handbook (Clarke 1999). Data extracted from the trials are analysed on an intention‐to‐treat basis (when this was not done in the original report, re‐analysis is performed if possible). Where data are missing, clarification is sought from the original authors. If the attrition was such that it might significantly affect the results, these data are excluded from the analysis. This decision rests with the review authors of primary reviews and is clearly documented. Once missing data become available, they will be included in the analyses.
We extracted data from all eligible trials to examine how issues of quality influence effect size in a sensitivity analysis. In trials where reporting is poor, methodological issues are reported as unclear or clarification sought.
Due to the large number of trials, double data extraction was not feasible and agreement between the three data extractors is therefore assessed on a random sample of trials.
Once the data have been extracted, they were distributed to individual review authors for entry onto the Review Manager computer software (RevMan 1999), checked for accuracy, and analysed as above using the RevMan software. For dichotomous data, relative risks and 95% confidence intervals are calculated, and in the absence of heterogeneity, results are pooled using a fixed‐effect model.
The predefined criteria for sensitivity analysis include all aspects of quality assessment as mentioned above, including aspects of selection, performance and attrition bias.
Primary analysis is limited to the prespecified outcomes and subgroup analyses. In the event of differences in unspecified outcomes or subgroups being found, these are analysed post hoc, but clearly identified as such to avoid drawing unjustified conclusions.
Results
Description of studies
In total, 29 studies were considered. Sixteen have been excluded and 13 have been included.
(Two reports from an updated search in May 2010 have been added to Studies awaiting classification.)
Excluded trials
Two trial reports (Kanhai 1988a; Kanhai 1988b) contained preliminary data that were published subsequently. However, this work reported data collected from women who had suffered intrauterine fetal death and were therefore not suitable for inclusion (see 'Characteristics of excluded studies'). A further trial (Spellacy 1972) contained data included in another report (Spellacy 1973 ‐ see included trials) and hence was excluded.
In a further nine trials, primary or secondary outcome data were either not reported directly or were not extractable (Bremme 1980; Calder 1974; Le Maire 1972; Lindmark 1976; Lipshitz 1984; Reichel 1985; Rosa 1974; Scher 1972; Spellacy 1971). The trial reported by Thomsen (Thomsen 1987) consisted of design details only with no results. One study (Van Heerden 1992) reported a comparison of induction of labour with intravenous oxytocin and intravenous prostaglandin (if required) immediately or after 24 hours in women presenting with ruptured membranes. No appropriate data were available.
One study (Amano 1999) reported a comparison of nulliparous women with unfavourable cervices randomised to either laminara tents or oral prostaglandin E2 and those with favourable cervices being randomised to intravenous oxytocin or intravenous prostaglandin F2 alpha. It was not possible to extract outcome data by actual method of induction. The trial reported by Anderson et al (Anderson 1972) was described as a double blind study with women being assigned to receive intravenous oxytocin, intravenous prostaglandin E2 or F2 alpha, however the administration protocol for prostaglandin E2 was changed after 21 women had been recruited because of poor response. The women do not appear to have been allocated in a random fashion to each of the three treatment arms. One further study (Brandel 1998) is reported as a randomised controlled trial, however no details are given about the method of randomisation, in addition, significantly more women were assigned to receive intravenous prostaglandin E2 (n = 43) than intravaginal prostaglandin (n = 36) implying selection bias.
Included trials
One trial (Iskander 1978) compared extra‐amniotic prostaglandin E2 with intravenous prostaglandin E2 in 40 women. Naismith et al (Naismith 1972) report a trial comparing intravenous oxytocin and prostaglandin E2 against intravenous oxytocin alone in 20 nulliparous women. All other included trials report a comparison of either prostaglandin E2 or F2 alpha with intravenous oxytocin (see 'Characteristics of included studies').
Two trials randomised women to receive either intravenous oxytocin, prostaglandin E2 or F2 alpha as part of a three armed study (Gowenlock 1975; Naismith 1973). Two trials report a comparison of intravenous prostaglandin E2 and intravenous oxytocin (Beazley 1970; Calder 1974). In one of these studies (Beazley 1970), 11 women with an intrauterine fetal death were randomised; however, these have been excluded from the analysis. Seven studies compare intravenous prostaglandin F2 alpha with intravenous oxytocin (Baxi 1980; Moller 1987; Rangarajan 1971; Spellacy 1973; Vakhariya 1972; Vroman 1972; Wildemeersch 1976).
Risk of bias in included studies
Randomisation
Two studies utilised random lot drawing using pre‐prepared cards (Naismith 1972; Naismith 1973) and three studies used random number tables (Spellacy 1973; Vroman 1972; Wildemeersch 1976). The method of randomisation in eight studies remains unclear (Baxi 1980; Beazley 1970; Calder 1974; Gowenlock 1975; Iskander 1978; Moller 1987; Rangarajan 1971; Vakhariya 1972); hence these studies should be interpreted with caution.
Blinding
No attempt was made at blinding in five studies (Gowenlock 1975; Iskander 1978; Moller 1987; Naismith 1973; Rangarajan 1971). Under those circumstances, there is a real possibility of bias, both in the clinical decision‐making and assessment of outcomes. A clinician with a prior belief that a new treatment is effective and safe might be less likely to perform a caesarean section in case of fetal distress or slow labour. On the other hand, a clinician who is anxious about possible risks of the new treatment may be more likely to intervene. In eight studies, adequate concealment of allocation sequence and blinding to both clinician and woman was achieved by preparation of coded drug boxes (Baxi 1980; Beazley 1970; Calder 1974; Naismith 1972; Spellacy 1973; Vakhariya 1972; Vroman 1972; Wildemeersch 1976).
Effects of interventions
Thirteen trials involving 1165 women were included.
Intravenous prostaglandin versus intravenous oxytocin ‐ all women
(i) Primary outcomes
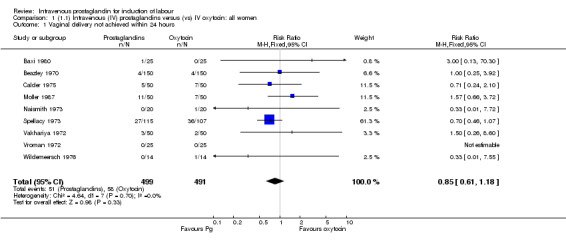
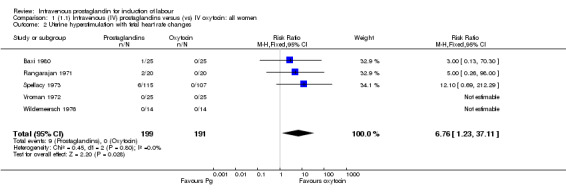
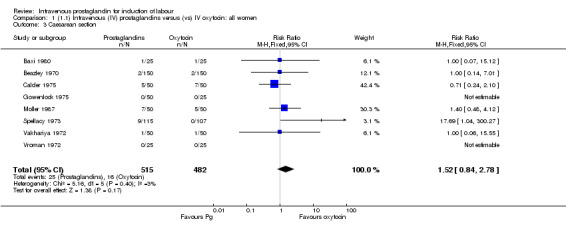
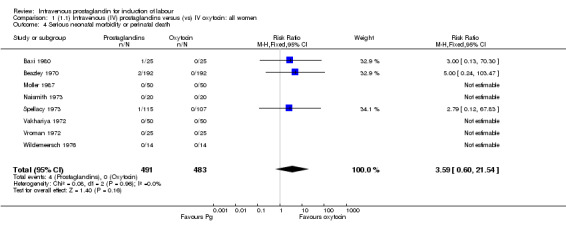
There were no significant differences in women failing to achieve a vaginal delivery within 24 hours in either the prostaglandin group (10.2%) or the oxytocin group (11.8% relative risk (RR) 0.85, 95% confidence interval (CI) 0.61 to 1.18). Significantly more episodes of uterine hypertonus associated with fetal heart rate (FHR) changes were seen in the intravenous prostaglandin group (RR 6.76, 95% CI 1.23 to 37.11). It is, however, surprising that no episodes of FHR changes in association with uterine hyperstimulation were seen in the oxytocin group. Caesarean section was performed for 4.9% of women receiving prostaglandin compared to 3.3% of women receiving oxytocin (RR 1.52, 95% CI 0.84 to 2.78). No serious maternal morbidity was reported in 449 women receiving intravenous prostaglandin or 441 women receiving oxytocin. There were four reports of serious adverse neonatal outcomes accounted for by four perinatal deaths in 491 women receiving prostaglandin compared to no perinatal deaths in 483 women treated with oxytocin (RR 3.59, 95% CI 0.60 to 21.54). Two perinatal deaths were in women treated with prostaglandin E2 (Beazley 1970), one was an intrapartum death in a woman induced for a prolonged pregnancy and a previous stillbirth and the second was a neonatal death occurring after delivery of a depressed infant (five minute Apgar score three) with the cord tightly around the neck. Two neonatal deaths occurred in women treated with prostaglandin F2 alpha, one occurred in an infant delivered by caesarean section for fetal heart bradycardia in association with uterine hyperstimulation (Spellacy 1973) and one was attributed to fulminating group B haemolytic streptococcus infection in a term infant born after a six hour labour, the membranes being ruptured two hours prior to delivery (Baxi 1980).
(ii) Other outcomes
Significantly more women (10.7%) receiving prostaglandin developed uterine hyperstimulation without FHR changes than those receiving oxytocin (2.5% RR 4.25, 95% CI 1.48 to 12.24). Maternal side‐effects were more common in women treated with prostaglandin compared to those receiving oxytocin (RR 3.75, 95% CI 2.46 to 5.70) including nausea (RR 2.00, 95% CI 0.74 to 5.41), vomiting (RR 1.88, 95% CI 0.91 to 3.86) and diarrhoea (RR 4.66, 95% CI 0.23 to 95.88). Significantly more women (9.2%) treated with prostaglandin developed thrombophlebitis compared to oxytocin (0.3% RR 10.67, 95% CI 3.3 to 34.45). Similarly more women (37.0%) in the prostaglandin group developed a pyrexia compared to those receiving oxytocin (11.4% RR 3.25, 95% 1.59 to 6.62).
No other significant differences were found. As stated above four perinatal deaths were encountered in women treated with prostaglandin compared to none amongst women treated with oxytocin (RR 3.59, 95% CI 0.60 to 21.54).
Intravenous prostaglandin versus intravenous oxytocin ‐ subgroup analysis
In primiparous women with intact membranes, there was no significant difference between prostaglandin and oxytocin treated women in terms of failure to achieve vaginal delivery in under 24 hours, delivery by caesarean section or serious maternal morbidity. Similar findings were made in multiparous women with intact membranes. In addition, in this group there were no significant differences in serious adverse neonatal outcomes or uterine hyperstimulation with FHR changes. Care must be taken in interpreting these results as the numbers are small.
Intravenous prostaglandin F2 alpha versus intravenous oxytocin ‐ all women
(i) Primary outcomes
Women treated with intravenous prostaglandin again had significantly higher rates of uterine hyperstimulation with FHR changes than women receiving oxytocin (4.5% versus 0% RR 6.76, 95% CI 1.23 to 37.11). No significant differences in women failing to achieve vaginal delivery within 24 hours were found (14.5% versus 16.2% RR 0.87, 95% CI 0.60 to 1.24). No major maternal morbidity was reported in either group. As described above, there were two perinatal deaths in the prostaglandin F2 alpha treated women and none amongst those women induced with intravenous oxytocin.
(ii) Other outcomes
Significantly more episodes of uterine hyperstimulation in the absence of FHR changes were noted in women receiving prostaglandin F2 alpha compared to oxytocin (13.8% versus 1.8% RR 7.5, 95% CI 1.79 to 31.36). More maternal side‐effects were noted in women receiving prostaglandin F2 alpha (RR 2.41, 95% CI 1.39 to 4.18), these included pyrexia, thrombophlebitis, nausea, vomiting and diarrhoea. There were no other significant differences.
Intravenous prostaglandin F2 alpha versus intravenous oxytocin ‐ subgroup analysis
No differences emerged from the reported data in multiparous and nulliparous women receiving either intravenous prostaglandin F2 alpha or oxytocin although caution has to be employed as the numbers in these subgroups are small.
Intravenous prostaglandin E2 versus intravenous oxytocin ‐ all women
(i) Primary outcomes
No significant differences in any of the primary outcome measure were found between the two groups of women. Two perinatal deaths occurred in women treated with prostaglandin E2 compared to none in the women induced with oxytocin.
(ii) Other outcomes
Significantly more maternal side‐effects were encountered in women receiving prostaglandin E2 compared to oxytocin (23.0% versus 4.1% RR 5.92, 95% CI 3.17 to 11.07) the reported side‐effects were thrombophlebitis (RR 16.33, 95% CI 3.19 to 83.66) and pyrexia (RR 3.57, 95% CI 1.77 to 7.18). No other significant differences emerged from the analysed trials.
Intravenous prostaglandin E2 versus intravenous oxytocin ‐ subgroup analysis
No differences emerged from the reported data in nulliparous women receiving either intravenous prostaglandin E2 or oxytocin although caution is needed as the numbers in these subgroups are small.
Intravenous oxytocin and prostaglandin F2 alpha versus intravenous oxytocin alone
No significant differences emerged in either primary or secondary outcome data although numbers are too small for meaningful analysis.
Intravenous prostaglandin E2 versus extra‐amniotic prostaglandin E2
No significant differences emerged in either primary or secondary outcome data although numbers are too small for meaningful analysis.
Discussion
Currently available data indicate that the use of intravenous prostaglandins to induce labour is associated with significantly higher rates of maternal side‐effects (gastrointestinal disturbance, thrombophlebitis and pyrexia) compared with oxytocin. There is no evidence to suggest that intravenous prostaglandins are more efficient in inducing labour, indeed they are associated with higher rates of uterine hyperstimulation both with and without fetal heart rate (FHR) changes (although it is surprising that no episodes of hyperstimulation with FHR changes were reported amongst 191 women receiving oxytocin).
It is of concern that four perinatal deaths occurred in infants of women treated with prostaglandin and none in women treated with oxytocin however the death reported by Baxi (Baxi 1980) was secondary to beta haemolytic streptococcae infection and respiratory distress. It should be stressed that these deaths constitute only a trend and are not statistically significant. Use of intravenous prostaglandins was not associated with lower Apgar scores than intravenous oxytocin, however none of the included trials reported data on admissions to neonatal intensive care units, umbilical cord blood sampling or long term neurological sequelae and so these excess deaths must be interpreted with caution. The data are not robust enough to allow comparisons of severe adverse maternal outcomes.
Analysis of trials reporting the use of prostaglandin E2 and/or prostaglandin F2 alpha gave similar findings. As stated in the methodology, analysis was not performed according to individual infusion dosage regime and this compromises the heterogeneity of included trials.
Insufficient data exist to assess the combination of intravenous prostaglandins with oxytocin or to compare extra‐amniotic prostaglandin with intravenous prostaglandin.
Authors' conclusions
Implications for practice.
Intravenous prostaglandin is more expensive than intravenous oxytocin and the existing data do not support its use as it is no more successful than oxytocin and is associated with higher rates of maternal side‐effects and episodes of uterine hyperstimulation.
Implications for research.
Large scale randomised trials (recruiting thousands of women) would be needed to answer the concerns over fetal wellbeing and rare adverse maternal outcomes. In the absence of compelling advantages associated with the use of intravenous prostaglandins it is to be doubted that such trials should ever take place.
[Note: The two citations in the awaiting classification section of the review may alter the conclusions of the review once assessed.]
What's new
| Date | Event | Description |
|---|---|---|
| 30 January 2013 | Amended | Contact details updated. |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 7 May 2010 | Amended | Search updated. Two new reports added to Studies awaiting classification (Craft 1971;Roberts 1970). |
| 13 August 2008 | Amended | Converted to new review format. |
Notes
We are currently looking for a new review team to update this review. Please contact Sonja Henderson (sonjah@liv.ac.uk) if you are interested in preparing the update.
Acknowledgements
The authors thank Tony Kelly and Jo Kavanagh ‐ Research fellows of the Clinical Effectiveness Support Unit, RCOG, UK and the team involved in co‐ordinating the induction of labour primary reviews. We also thank Zarko Alfirevic for expert and much needed advice in compiling the review.
Data and analyses
Comparison 1. (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 9 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.61, 1.18] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.76 [1.23, 37.11] |
| 3 Caesarean section | 8 | 997 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.52 [0.84, 2.78] |
| 4 Serious neonatal morbidity or perinatal death | 8 | 974 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [0.60, 21.54] |
| 5 Serious maternal morbidity or death | 8 | 890 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 2 | 392 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.74, 1.55] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 5 | 318 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.25 [1.48, 12.24] |
| 11 Instrumental vaginal delivery | 3 | 240 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.04 [0.81, 1.34] |
| 13 Apgar score < 7 at 5 minutes | 9 | 876 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.47, 2.34] |
| 14 Neonatal intensive care unit admission | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.89] |
| 16 Perinatal death | 9 | 990 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.59 [0.60, 21.53] |
| 18 Maternal side‐effects (all) | 8 | 940 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.75 [2.46, 5.70] |
| 19 Nausea | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.74, 5.41] |
| 20 Vomiting | 3 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.91, 3.86] |
| 21 Diarrhoea | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [0.23, 95.87] |
| 22 Thrombophlebitis | 5 | 650 | Risk Ratio (M‐H, Fixed, 95% CI) | 10.67 [3.30, 34.45] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.55] |
| 24 Serious maternal complication | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 28 Maternal pyrexia | 2 | 140 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.25 [1.59, 6.62] |
1.1. Analysis.
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
1.2. Analysis.
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
1.3. Analysis.
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 3 Caesarean section.
1.4. Analysis.
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
1.5. Analysis.
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 5 Serious maternal morbidity or death.
1.6. Analysis.
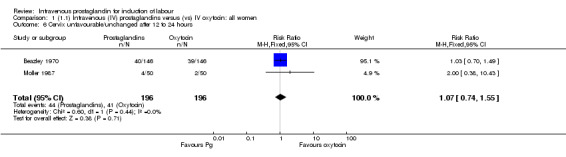
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
1.8. Analysis.
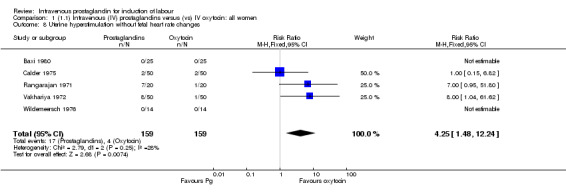
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
1.11. Analysis.
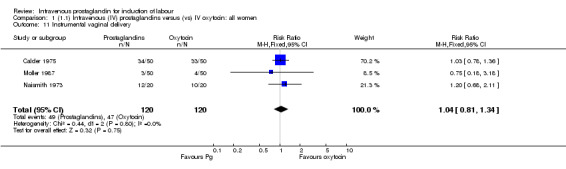
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
1.13. Analysis.
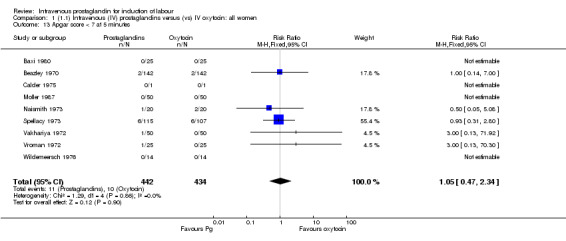
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
1.14. Analysis.
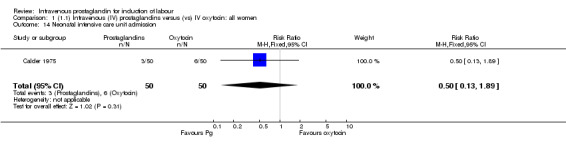
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 14 Neonatal intensive care unit admission.
1.16. Analysis.
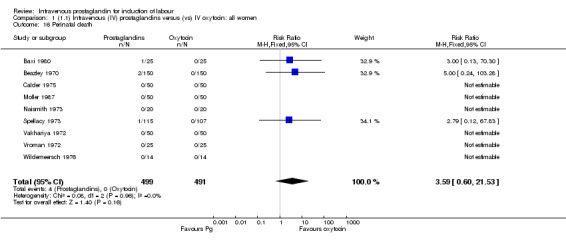
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 16 Perinatal death.
1.18. Analysis.
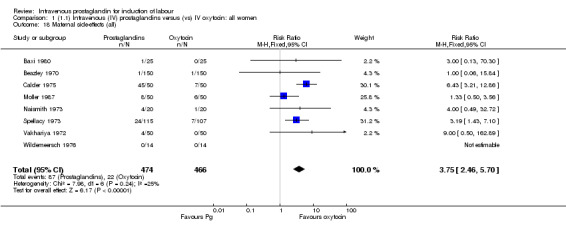
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 18 Maternal side‐effects (all).
1.19. Analysis.
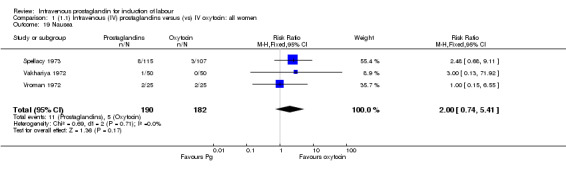
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 19 Nausea.
1.20. Analysis.
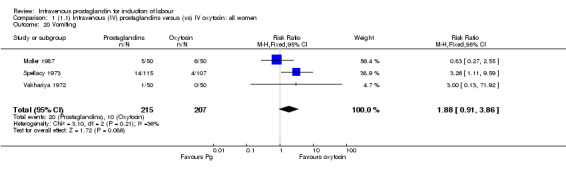
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 20 Vomiting.
1.21. Analysis.
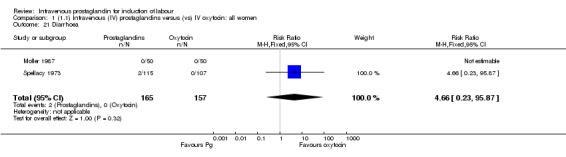
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 21 Diarrhoea.
1.22. Analysis.
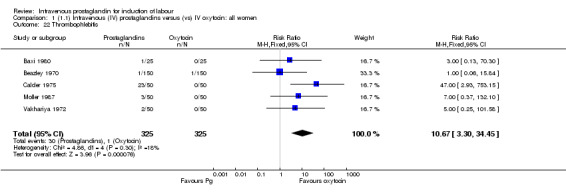
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 22 Thrombophlebitis.
1.23. Analysis.
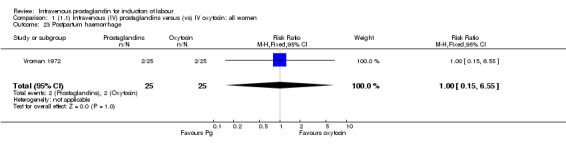
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 23 Postpartum haemorrhage.
1.24. Analysis.
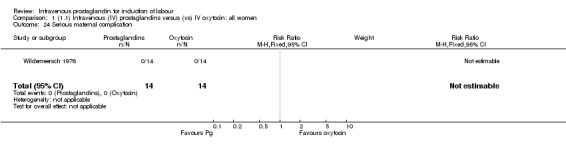
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 24 Serious maternal complication.
1.28. Analysis.
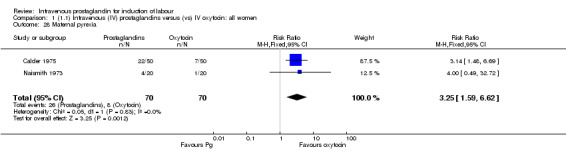
Comparison 1 (1.1) Intravenous (IV) prostaglandins versus (vs) IV oxytocin: all women, Outcome 28 Maternal pyrexia.
Comparison 2. IV prostaglandin vs IV oxytocin: primiparous, membranes intact, cervix unfavourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
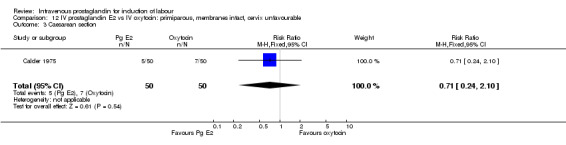
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.10] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.10] |
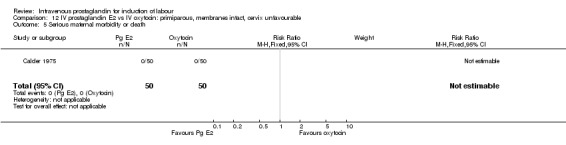
| 5 Serious maternal morbidity or death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
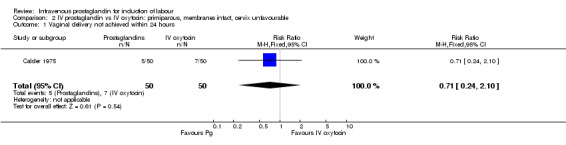
Comparison 2 IV prostaglandin vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
2.3. Analysis.
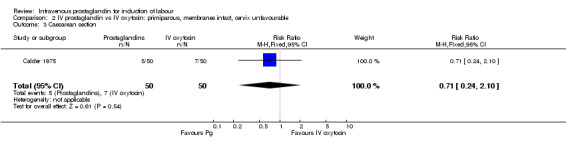
Comparison 2 IV prostaglandin vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 3 Caesarean section.
2.5. Analysis.
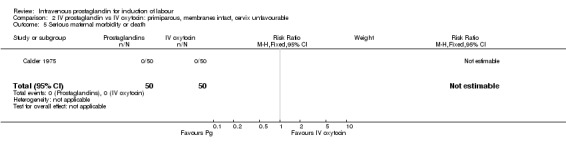
Comparison 2 IV prostaglandin vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 5 Serious maternal morbidity or death.
Comparison 3. IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix unfavourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
3.1. Analysis.
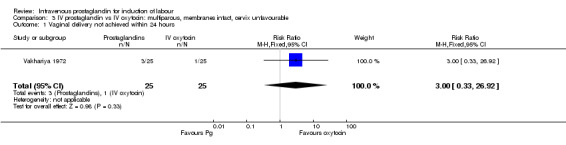
Comparison 3 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix unfavourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 4. IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix favourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
4.1. Analysis.
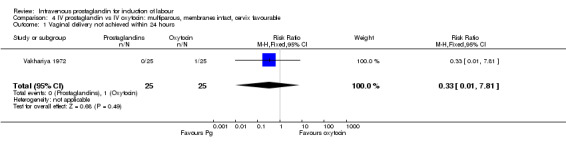
Comparison 4 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix favourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 5. IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
5.1. Analysis.
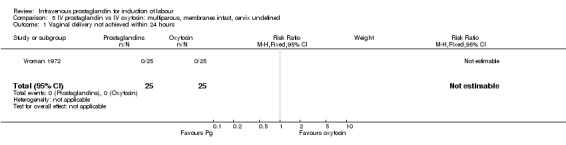
Comparison 5 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 1 Vaginal delivery not achieved within 24 hours.
5.2. Analysis.
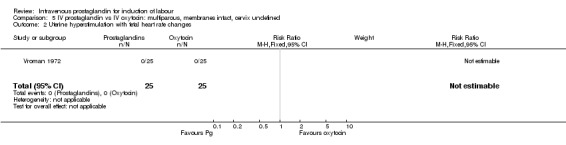
Comparison 5 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
5.3. Analysis.
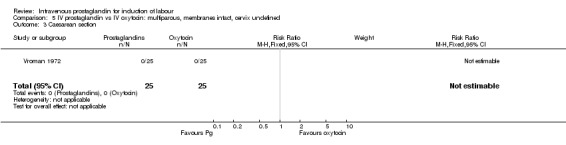
Comparison 5 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 3 Caesarean section.
5.4. Analysis.
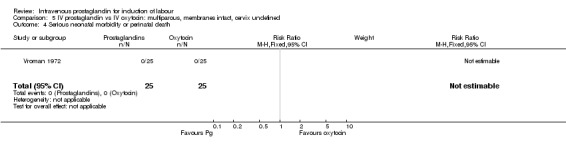
Comparison 5 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 4 Serious neonatal morbidity or perinatal death.
5.5. Analysis.
Comparison 5 IV prostaglandin vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 5 Serious maternal morbidity or death.
Comparison 6. IV prostaglandin F2 alpha vs IV oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 7 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.60, 1.24] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 5 | 390 | Risk Ratio (M‐H, Fixed, 95% CI) | 6.76 [1.23, 37.11] |
| 3 Caesarean section | 6 | 572 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.07 [0.95, 4.49] |
| 4 Serious neonatal morbidity or perinatal death | 7 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.31, 27.26] |
| 5 Serious maternal morbidity or death | 6 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 2 | 128 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.33, 5.01] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 4 | 218 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.5 [1.79, 31.35] |
| 11 Instrumental vaginal delivery | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.73, 2.24] |
| 13 Apgar score < 7 at 5 minutes | 7 | 580 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.14 [0.46, 2.86] |
| 16 Perinatal death | 6 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.89 [0.31, 27.26] |
| 18 Maternal side‐effects (all) | 6 | 550 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.41 [1.39, 4.18] |
| 19 Nausea | 3 | 372 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [0.74, 5.41] |
| 20 Vomiting | 3 | 422 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.88 [0.91, 3.86] |
| 21 Diarrhoea | 2 | 322 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.66 [0.23, 95.87] |
| 22 Thrombophlebitis | 3 | 250 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.88, 28.37] |
| 23 Postpartum haemorrhage | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.55] |
| 24 Serious maternal complication | 1 | 28 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
6.1. Analysis.
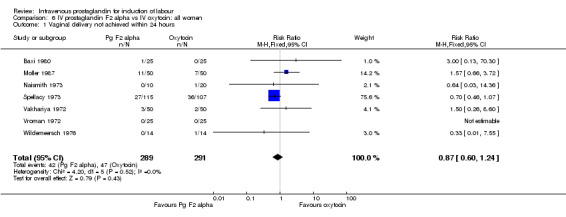
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
6.2. Analysis.
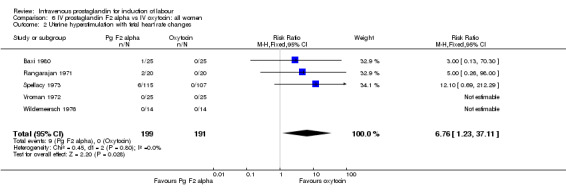
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
6.3. Analysis.
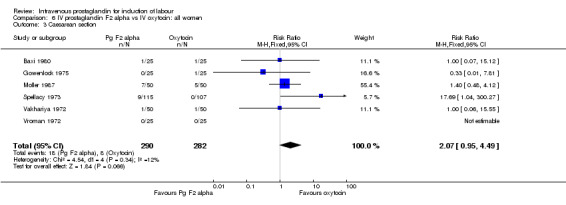
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 3 Caesarean section.
6.4. Analysis.
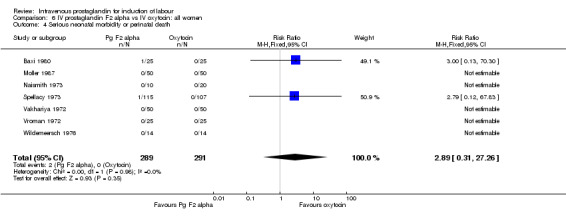
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
6.5. Analysis.
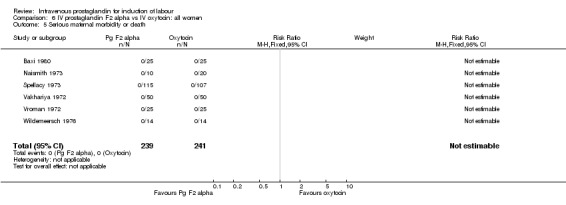
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 5 Serious maternal morbidity or death.
6.6. Analysis.
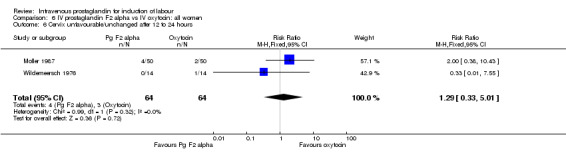
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
6.8. Analysis.
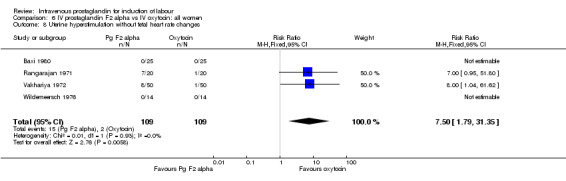
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
6.11. Analysis.
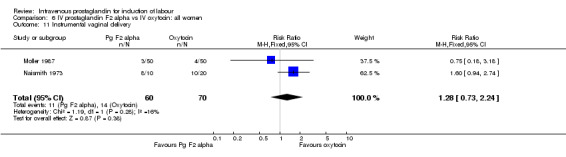
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
6.13. Analysis.
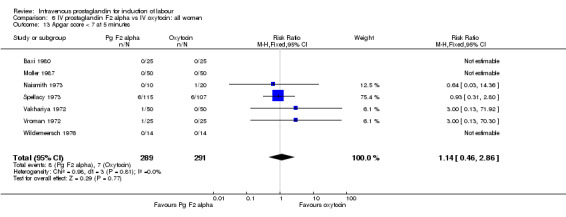
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
6.16. Analysis.
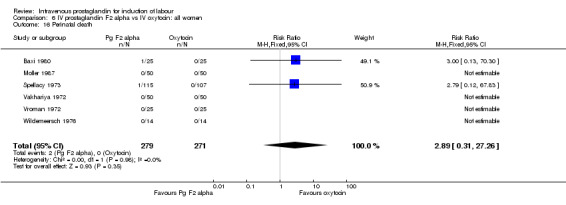
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 16 Perinatal death.
6.18. Analysis.
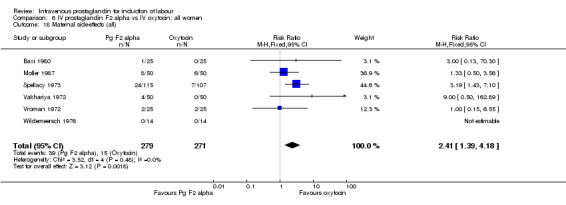
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 18 Maternal side‐effects (all).
6.19. Analysis.
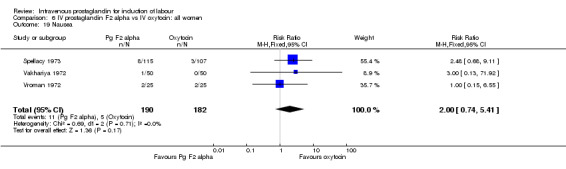
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 19 Nausea.
6.20. Analysis.
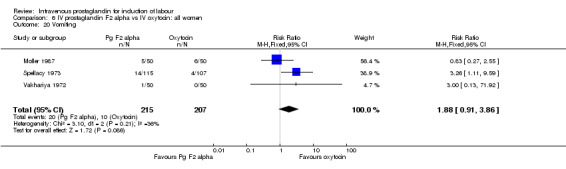
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 20 Vomiting.
6.21. Analysis.
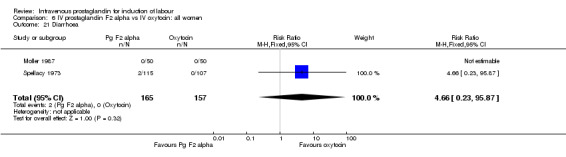
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 21 Diarrhoea.
6.22. Analysis.
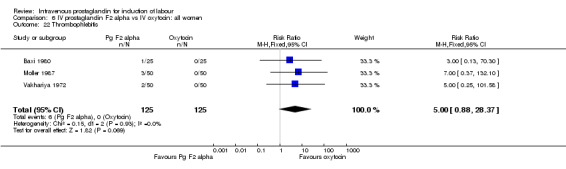
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 22 Thrombophlebitis.
6.23. Analysis.
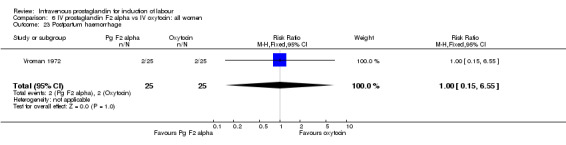
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 23 Postpartum haemorrhage.
6.24. Analysis.
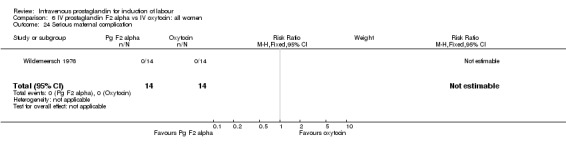
Comparison 6 IV prostaglandin F2 alpha vs IV oxytocin: all women, Outcome 24 Serious maternal complication.
Comparison 7. IV prostaglandin F2 alpha vs IV oxytocin: primiparous, membranes intact, cervix undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.03, 14.36] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
7.1. Analysis.
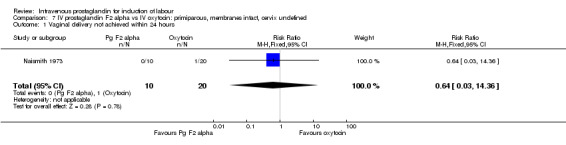
Comparison 7 IV prostaglandin F2 alpha vs IV oxytocin: primiparous, membranes intact, cervix undefined, Outcome 1 Vaginal delivery not achieved within 24 hours.
7.4. Analysis.
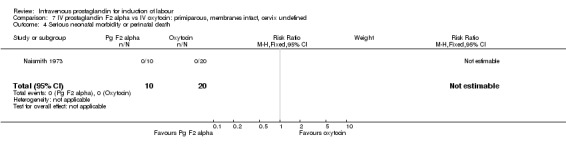
Comparison 7 IV prostaglandin F2 alpha vs IV oxytocin: primiparous, membranes intact, cervix undefined, Outcome 4 Serious neonatal morbidity or perinatal death.
7.5. Analysis.
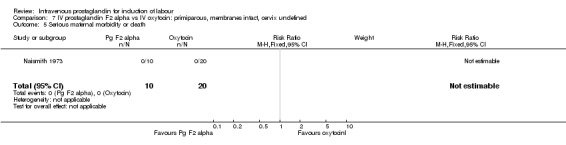
Comparison 7 IV prostaglandin F2 alpha vs IV oxytocin: primiparous, membranes intact, cervix undefined, Outcome 5 Serious maternal morbidity or death.
Comparison 8. IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix unfavourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.33, 26.92] |
8.1. Analysis.
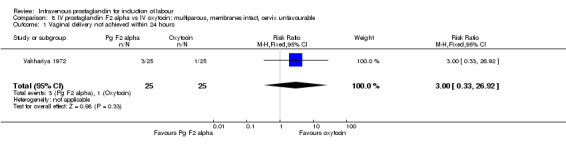
Comparison 8 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix unfavourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 9. IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix favourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.81] |
9.1. Analysis.
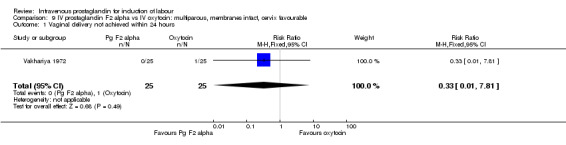
Comparison 9 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix favourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
Comparison 10. IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Uterine hyperstimulation with fetal heart rate changes | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 50 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
10.1. Analysis.
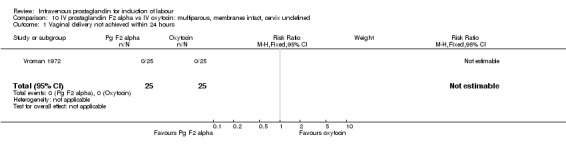
Comparison 10 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 1 Vaginal delivery not achieved within 24 hours.
10.2. Analysis.
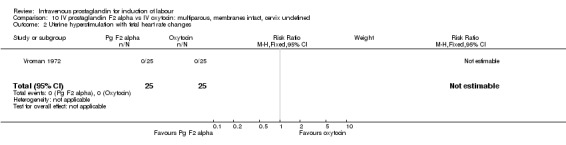
Comparison 10 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 2 Uterine hyperstimulation with fetal heart rate changes.
10.3. Analysis.
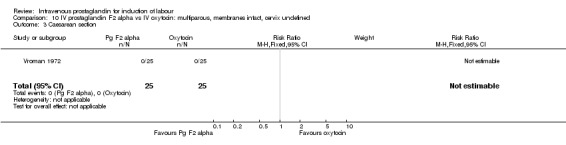
Comparison 10 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 3 Caesarean section.
10.4. Analysis.
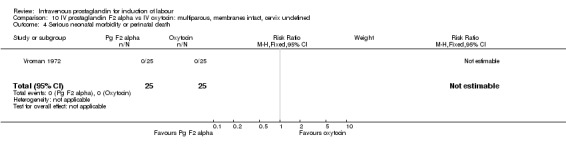
Comparison 10 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 4 Serious neonatal morbidity or perinatal death.
10.5. Analysis.
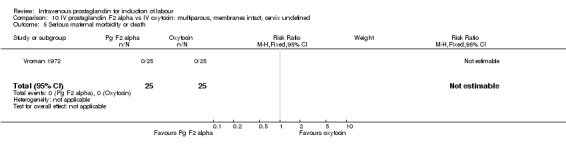
Comparison 10 IV prostaglandin F2 alpha vs IV oxytocin: multiparous, membranes intact, cervix undefined, Outcome 5 Serious maternal morbidity or death.
Comparison 11. IV prostaglandin E2 vs IV oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.35, 1.90] |
| 3 Caesarean section | 4 | 480 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.30, 1.68] |
| 4 Serious neonatal morbidity or perinatal death | 2 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.06 [0.30, 14.23] |
| 5 Serious maternal morbidity or death | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 292 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.03 [0.70, 1.49] |
| 8 Uterine hyperstimulation without fetal heart rate changes | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.15, 6.82] |
| 11 Instrumental vaginal delivery | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.76, 1.30] |
| 13 Apgar score < 7 at 5 minutes | 3 | 414 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.68 [0.26, 1.80] |
| 14 Neonatal intensive care unit admission | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.5 [0.13, 1.89] |
| 16 Perinatal death | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.24, 103.28] |
| 17 Maternal side‐effects (all) | 3 | 430 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.92 [3.17, 11.07] |
| 22 Thrombophlebitis | 2 | 400 | Risk Ratio (M‐H, Fixed, 95% CI) | 16.33 [3.19, 83.65] |
| 28 Maternal pyrexia | 2 | 130 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.57 [1.77, 7.18] |
11.1. Analysis.
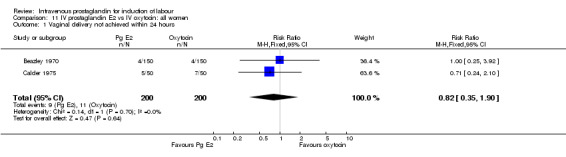
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
11.3. Analysis.
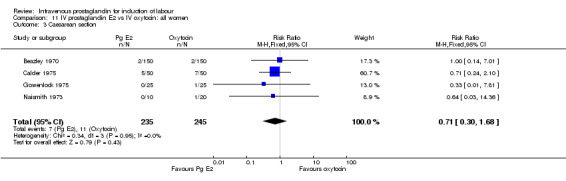
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 3 Caesarean section.
11.4. Analysis.
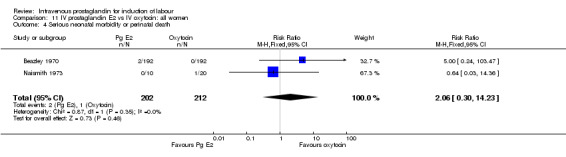
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
11.5. Analysis.
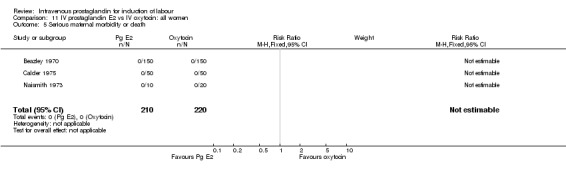
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 5 Serious maternal morbidity or death.
11.6. Analysis.
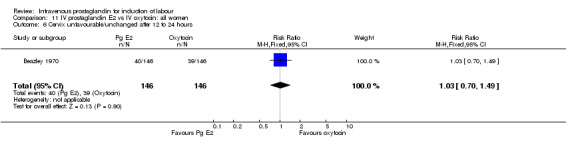
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
11.8. Analysis.
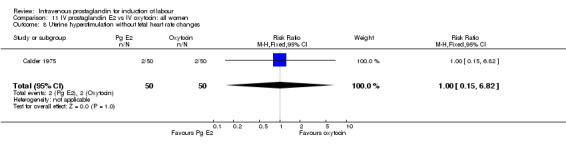
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 8 Uterine hyperstimulation without fetal heart rate changes.
11.11. Analysis.
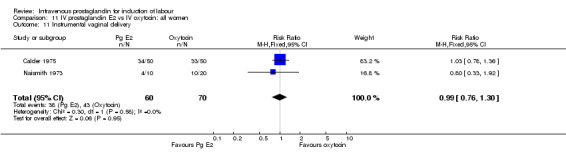
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
11.13. Analysis.
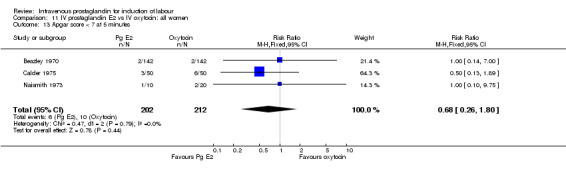
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
11.14. Analysis.
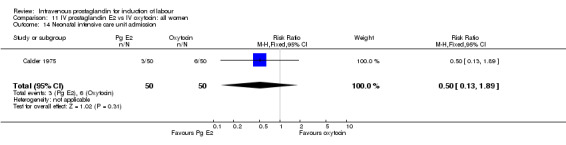
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 14 Neonatal intensive care unit admission.
11.16. Analysis.
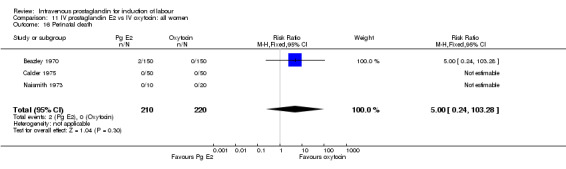
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 16 Perinatal death.
11.17. Analysis.
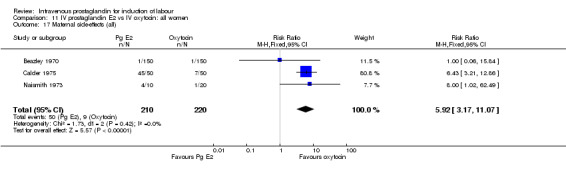
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 17 Maternal side‐effects (all).
11.22. Analysis.
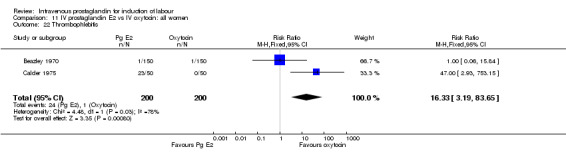
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 22 Thrombophlebitis.
11.28. Analysis.
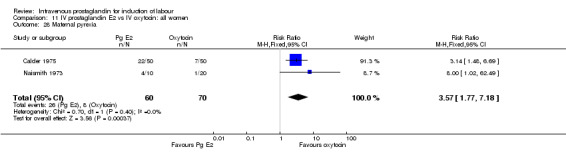
Comparison 11 IV prostaglandin E2 vs IV oxytocin: all women, Outcome 28 Maternal pyrexia.
Comparison 12. IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix unfavourable.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.10] |
| 3 Caesarean section | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.24, 2.10] |
| 5 Serious maternal morbidity or death | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
12.1. Analysis.
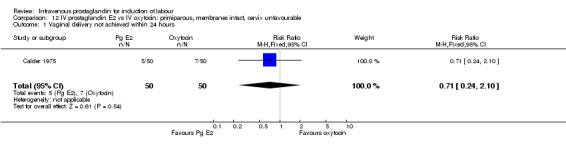
Comparison 12 IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 1 Vaginal delivery not achieved within 24 hours.
12.3. Analysis.
Comparison 12 IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 3 Caesarean section.
12.5. Analysis.
Comparison 12 IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix unfavourable, Outcome 5 Serious maternal morbidity or death.
Comparison 13. IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Caesarean section | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.03, 14.36] |
| 5 Serious maternal morbidity or death | 1 | 30 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
13.3. Analysis.
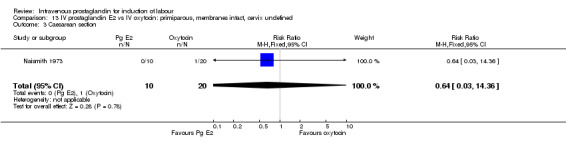
Comparison 13 IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix undefined, Outcome 3 Caesarean section.
13.5. Analysis.
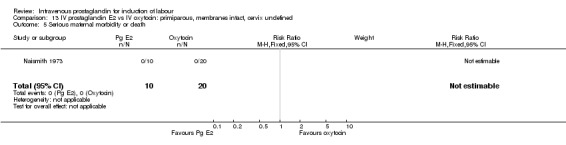
Comparison 13 IV prostaglandin E2 vs IV oxytocin: primiparous, membranes intact, cervix undefined, Outcome 5 Serious maternal morbidity or death.
Comparison 14. IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cervix unfavourable/unchanged after 12 to 24 hours | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.4 [0.67, 2.94] |
| 13 Apgar score < 7 at 5 minutes | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| 14 Neonatal intensive care unit admission | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.27, 92.62] |
| 18 Maternal side‐effects (all) | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
14.1. Analysis.
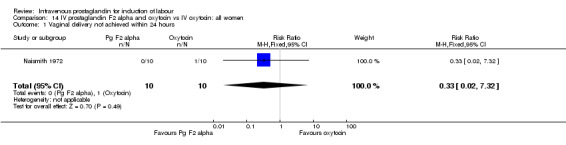
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
14.3. Analysis.
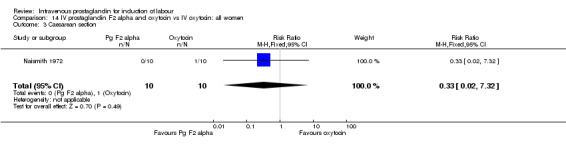
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 3 Caesarean section.
14.4. Analysis.
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
14.6. Analysis.
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 6 Cervix unfavourable/unchanged after 12 to 24 hours.
14.11. Analysis.
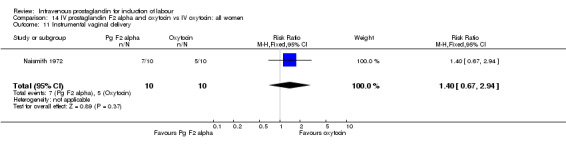
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 11 Instrumental vaginal delivery.
14.13. Analysis.
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 13 Apgar score < 7 at 5 minutes.
14.14. Analysis.
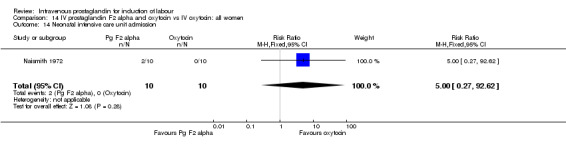
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 14 Neonatal intensive care unit admission.
14.18. Analysis.
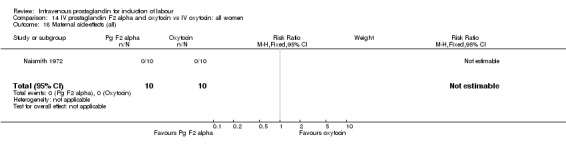
Comparison 14 IV prostaglandin F2 alpha and oxytocin vs IV oxytocin: all women, Outcome 18 Maternal side‐effects (all).
Comparison 15. IV prostaglandin F2 a and oxytocin vs IV oxytocin: primiparous, membranes ruptured, cervix variable/undefined.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 3 Caesarean section | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.02, 7.32] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
15.1. Analysis.
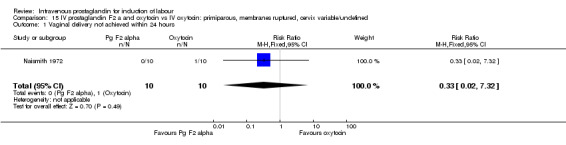
Comparison 15 IV prostaglandin F2 a and oxytocin vs IV oxytocin: primiparous, membranes ruptured, cervix variable/undefined, Outcome 1 Vaginal delivery not achieved within 24 hours.
15.3. Analysis.
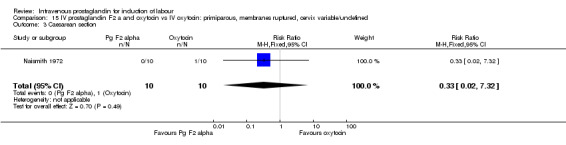
Comparison 15 IV prostaglandin F2 a and oxytocin vs IV oxytocin: primiparous, membranes ruptured, cervix variable/undefined, Outcome 3 Caesarean section.
15.4. Analysis.
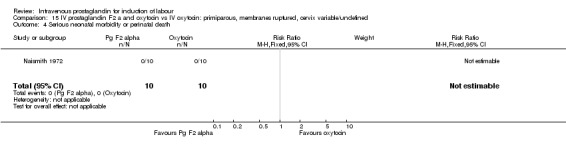
Comparison 15 IV prostaglandin F2 a and oxytocin vs IV oxytocin: primiparous, membranes ruptured, cervix variable/undefined, Outcome 4 Serious neonatal morbidity or perinatal death.
Comparison 29. IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Vaginal delivery not achieved within 24 hours | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 3 Caesarean section | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.72] |
| 4 Serious neonatal morbidity or perinatal death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Serious maternal morbidity or death | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 11 Instrumental vaginal delivery | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.0 [0.20, 20.33] |
| 18 Maternal side‐effects (all) | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 7.0 [0.38, 127.32] |
| 20 Vomiting | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.0 [0.13, 69.52] |
| 22 Erythema | 1 | 40 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.26, 98.00] |
29.1. Analysis.
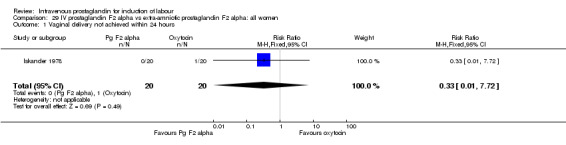
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 1 Vaginal delivery not achieved within 24 hours.
29.3. Analysis.
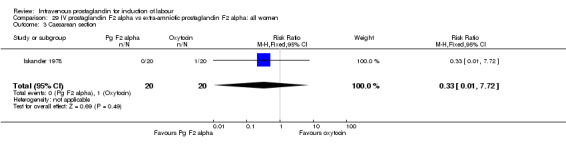
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 3 Caesarean section.
29.4. Analysis.
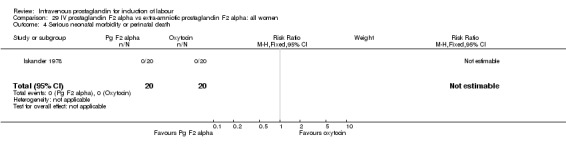
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 4 Serious neonatal morbidity or perinatal death.
29.5. Analysis.
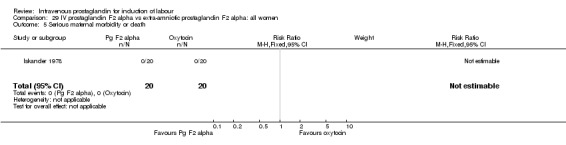
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 5 Serious maternal morbidity or death.
29.11. Analysis.
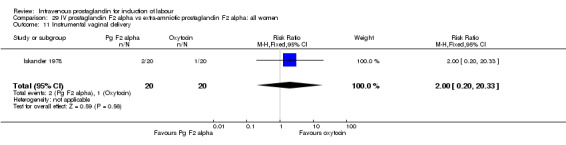
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 11 Instrumental vaginal delivery.
29.18. Analysis.
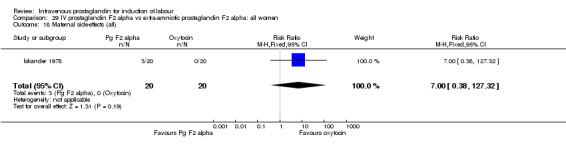
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 18 Maternal side‐effects (all).
29.20. Analysis.
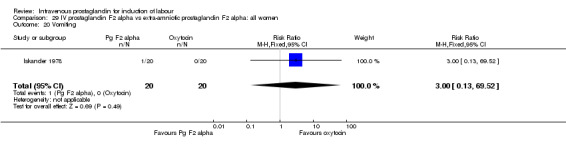
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 20 Vomiting.
29.22. Analysis.
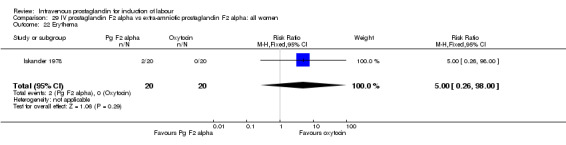
Comparison 29 IV prostaglandin F2 alpha vs extra‐amniotic prostaglandin F2 alpha: all women, Outcome 22 Erythema.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Baxi 1980.
| Methods | Randomisation method not reported. | |
| Participants | 50 women between 37 and 43 weeks. Bishop score 0‐6 difficult and 7‐13 easy. | |
| Interventions | Intravenous oxytocin (maximum 16 mU/min) versus intravenous prostaglandin F2alpha (maximum 20 ug/min). Infusions doubled every hour until labour was established (maximum of three doublings). If after 10 hours, labour did not ensue, other unspecified methods were used. Amniotomy was performed after 90 minutes or when possible. | |
| Outcomes | Primary outcome data reported. Unable to extract data for subgroup analysis. | |
| Notes | 1 episode of tachysystole with FHR changes after administration of promethazine and 1 neonatal death secondary to B haemolytic Strep. infection in the prostaglandin F2alpha group. 1 episode of tachysystole with FHR changes in the Prostaglandin F2alpha group after administration of promethazine and meperidine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Beazley 1970.
| Methods | Randomisation method not reported. | |
| Participants | 300 women, 35 to 43 weeks. 11 cases of intrauterine death. | |
| Interventions | Intravenous oxytocin (maximum 67 mU/min) versus intravenous prostaglandin E2 (maximum 6.7 ug/min). Infusions doubled every hour until labour was established. Successful induction was deemed as a cervical dilatation of 6 cm or delivery was achieved within 12 hours. Amniotomy performed at an unspecified time. | |
| Outcomes | Primary outcome data available for serious neonatal morbidity only. Secondary data available for low Apgar scores. Failed induction reported by 12 hours but this is defined as a cervix less than 6 cm dilated. | |
| Notes | 1 neonatal death (cord around the neck) and one intrapartum death in the prostaglandin E2 group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Calder 1975.
| Methods | Automatic 'machine' randomisation. | |
| Participants | 100 primigravidas with bishop score 6 or less at 37‐42 weeks. | |
| Interventions | All participants had amniotomy at the start. Intravenous oxytocin doubling hourly to a maximum of 64 mu/min vs prostaglandin E2 intravenously doubling hourly to a maximum of 4 ug/min. Half of each group received the infusion via an automatic infusor correlated to intrauterine pressures. All women had epidurals. Amniotomy was performed when possible (not specified). | |
| Outcomes | Caesarean sections reported but unable to extract the failure to deliver within 24 hours data. | |
| Notes | Very high forceps delivery rate probably accounted for by the use of epidurals. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Gowenlock 1975.
| Methods | Method of randomisation not reported. | |
| Participants | 75 women at or over 35 weeks of gestation. | |
| Interventions | 3 groups of 25 women each receiving 1 mU/min oxytocin increasing every 30 minutes until labour commenced versus prostaglandin E2 (n = 25) to a maximum of 1 ug/min or prostaglandin F2alpha (n = 25) to a maximum of 10 ug/min. Amniotomy was performed when possible. | |
| Outcomes | Primarily reporting biochemical and haematological outcomes. Caesarean section in each group are reported. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Iskander 1978.
| Methods | Method of randomisation not reported. | |
| Participants | 40 women with Bishop score of 6 or less. | |
| Interventions | Extra amniotic infusion (control) up to a maximum of 150 ug/hour versus intravenous prostaglandin E2 up to a maximum of 1 ug/minute. Amniotomy was performed when possible. | |
| Outcomes | Caesarean section data and instrumental delivery data extractable. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Moller 1987.
| Methods | Method or randomisation not reported. | |
| Participants | 100 women with prelabour rupture of the membranes at 37‐42 weeks gestation. | |
| Interventions | Intravenous oxytocin (maximum 30 mU/min) versus intravenous prostaglandin F2 alpha (maximum of 6 ug/min). | |
| Outcomes | Caesarean section data readily available, but no other primary outcome data reported. | |
| Notes | Analgesic requirement less in the oxytocin group (18/50 vs 8/50) however overall, the use of analgesia seems very low. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Naismith 1972.
| Methods | Randomisation by 'random lottery draw'. | |
| Participants | 20 primigravaed women who were post term with singleton cephalically presenting fetuses. | |
| Interventions | Oxytocin (maximum of 300 mU/min) versus oxytocin (maximum of 300 mU/min) and intravenous prostaglandin E2 (0.5 ug/min fixed rate). Amniotomy was performed at the start in each case. | |
| Outcomes | Caesarean and instrumental delivery data available. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Naismith 1973.
| Methods | Randomisation by 'random lottery draw'. | |
| Participants | 40 women with singleton gestations and intact membranes all of whom were post term. | |
| Interventions | Oxytocin (n = 20) increasing to a maximum of 340 mU/minute versus prostaglandin F2 alpha (n = 10) increasing to a maximum of 80 ug/min versus prostaglandin E2 (n = 10) to a maximum of 8 ug/min. Amniotomy being performed when labour was established. | |
| Outcomes | Caesarean data and instrumental delivery data available. | |
| Notes | High operative delivery rate influenced by the use of epidural analgesia which was associated with instrumental delivery in 21 out of 23 cases (91%). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Rangarajan 1971.
| Methods | Alternate allocation sequence. | |
| Participants | 40 women in the third trimester. Group A with an unfavourable cervix and group B with a favourable cervix. | |
| Interventions | Oxytocin (maximum 10 mu/minute) versus intravenous prostaglandin F2alpha. Group A oxytocin (n = 10) vs prostaglandin (n = 8), in group B oxytocin (n = 10) versus prostaglandin (n = 12). Amniotomy performed when labour was established. | |
| Outcomes | Hyperstimulation with and without fetal heart rate changes were the only outcome measure extractable. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
Spellacy 1973.
| Methods | Table of random numbers. | |
| Participants | 222 woman between 36 and 43 weeks. Previous caesarean section and intrauterine fetal deaths were exclusion criteria. | |
| Interventions | Oxytocin increasing to a maximum of 8 mU/min (n = 107) and prostaglandin F 2 alpha increasing to a maximum of 40 ug/min (n = 115). Amniotomy was performed 90 minutes after labour was judged to have commenced. | |
| Outcomes | Failure to deliver after 10 hours resulted in abandoning the procedure and discharge the following day. | |
| Notes | A relatively high dosage regimen of prostaglandin F2 alpha is compared to a low dose regimen of oxytocin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Vakhariya 1972.
| Methods | Unclear method of randomisation. | |
| Participants | 100 multiparous women at 36‐43 weeks of gestation. Exclusion criteria were grand muliparity and previous caesarean section. Bishop score less than 6 was deemed a difficult induction. | |
| Interventions | Oxytocin increasing to a maximum of 16 mU/min (n = 50) versus intravenous prostaglandin F2 alpha increasing to a maximum of 40 ug/minute (n = 50). Amniotomy was performed when possible (not specified). | |
| Outcomes | Failure to deliver after 10 hours resulted in abandoning the procedure and discharge the following day. | |
| Notes | A relatively high dosage regimen of prostaglandin F2 alpha is compared to a low dose regimen of oxytocin. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Vroman 1972.
| Methods | Prepared table of random numbers. | |
| Participants | 50 multigravid women at 38‐42 weeks of gestation. Exclusion criteria included previous caesarean section, grand multiparity and obstetric complications. | |
| Interventions | Oxytocin to a maximum of 16 mU/minute (n = 50) versus intravenous prostaglandin F2alpha to a maximum of 40 ug/min (n = 50). Amniotomy was performed 90 minutes after the onset of labour. | |
| Outcomes | All primary outcome data extractable. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Wildemeersch 1976.
| Methods | Random number tables | |
| Participants | 28 women at 37‐42 weeks with no complications. | |
| Interventions | Oxytocin increasing to 8 mU/minute (n = 14) versus prostaglandin F2 alpha increasing to 20 ug/minute (n = 14). Amniotomy when possible. | |
| Outcomes | ||
| Notes | 3 caesarean sections, induction agent not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | D ‐ Not used |
FHR: fetal heart rate min: minute vs: versus
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Amano 1999 | Induction at 39 weeks vs conservative management until 42 weeks in nulliparas. Method of randomisation and blinding unclear. Induction by oral prostaglandin or laminara tent if under 3 cm dilated or by intravenous oxytocin or prostaglandin F2 alpha if over 3 cm. Unable to extract data for each method of induction. |
| Anderson 1972 | Unclear if randomised or not. Protocol changed after 21 patients therefore data on 79 patients only. Allocated to intravenous oxytocin (n = 27), intravenous prostaglandin F2alpha (n = 46) or prostaglandin E2 (n = 6). Reported as double blind but no details given. Multips only. No primary outcome data reported but uterine hyperstimulation without fetal heart rate changes more common in the prostaglandin F2pha group (6/46 vs 1/27). |
| Brandel 1998 | Randomised trial of intravaginal prostaglandin E2 versus intravenous prostaglandin E2. No details of method of randomisation nor of treatment blinding. Out of 78 patients enrolled 43 received prostaglandin by the intravenous route whereas only 36 received it intravaginally implying some allocation bias. Primary outcome statistics not adequately reported. |
| Bremme 1980 | Only biochemical outcomes reported. |
| Calder 1974 | Neonatal follow up study to 1975. No appropriate outcome data. |
| Kanhai 1989 | 85 women all with an intrauterine fetal death. Gestations ranging from 14 to 42 weeks of gestation. Randomised to 2 different intravenous regimes of prostaglandin E2. |
| Le Maire 1972 | Biochemical outcomes reported. |
| Lindmark 1976 | Non‐randomised observational study comparing intravenous oxytocin and prostaglandin F2 alpha and the resultant uterine contraction pressure profiles. No primary or secondary outcome data reported. |
| Lipshitz 1984 | Observational study examining the effects of two tocolytics on prostaglandin F2 alpha stimulated uterine contractions. |
| Reichel 1985 | Plasma prostaglandin levels were the only outcomes reported |
| Rosa 1974 | No primary or secondary outcome data reported. |
| Scher 1972 | No extractable outcome data. |
| Spellacy 1971 | Biochemical data only. No useful outcome data |
| Spellacy 1972 | Duplicate study of Spellacy 1973. |
| Thomsen 1987 | No data. |
| Van Heerden 1992 | A trial of induction versus conservative management in women presenting with ruptured membranes at 34 weeks or more. The induction regimen was 12 hours of oxytocin and then intravenous prostaglandin if required. The conservative management group underwent the same regimen after 24 hours of conservative management if labour had not ensued. |
vs: versus
Contributions of authors
Murray Luckas entered the data into RevMan and this was validated by Leanne Bricker. Both authors wrote the text.
Declarations of interest
None known.
Edited (no change to conclusions)
References
References to studies included in this review
Baxi 1980 {published data only}
- Baxi LV, Petrie RH, Caritis SN. Induction of labor with low‐dose prostaglandin F2alpha and oxytocin. American Journal of Obstetrics and Gynecology 1980;136:28‐31. [DOI] [PubMed] [Google Scholar]
Beazley 1970 {published data only}
- Beazley JM, Gillespie A. Double‐blind trial of prostaglandin E2 and oxytocin in induction of labour. Lancet 1971;1:152‐5. [DOI] [PubMed] [Google Scholar]
Calder 1975 {published data only}
- Calder AA, Embrey MP. Comparison of intravenous oxytocin and prostaglandin E2 for induction of labour using automatic and non‐automatic infusion techniques. British Journal of Obstetrics and Gynaecology 1975;82:728‐33. [DOI] [PubMed] [Google Scholar]
Gowenlock 1975 {published data only}
- Gowenlock AH, Taylor DS, Sanderson JH. Biochemical and haematological changes during the induction of labour at term with oxytocin, prostaglandin E2 and prostaglandin F2alpha. British Journal of Obstetrics and Gynaecology 1975;82:215‐20. [DOI] [PubMed] [Google Scholar]
Iskander 1978 {published data only}
- Iskander MN. A comparison of the efficacy and safety of extra‐amniotic prostaglandin E2 and intravenous prostaglandin E2 for the induction of labour in patients with unripe cervices. Journal of International Medical Research 1978;6:144‐6. [DOI] [PubMed] [Google Scholar]
Moller 1987 {published data only}
- Moller M, Thomsen AC, Sorensen J, Forman A. Oxytocin‐ or low‐dose prostaglandin F2alpha‐infusion for stimulation of labor after primary rupture of membranes. Acta Obstetricia et Gynecologica Scandinavica 1987;66:103‐6. [DOI] [PubMed] [Google Scholar]
Naismith 1972 {published data only}
- Naismith WCMK, Barr W, MacVicar J. Induction of labour by simultaneous intravenous administration of prostaglandin E2 and oxytocin. BMJ 1972;4:461‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Naismith 1973 {published data only}
- Naismith WCMK, Barr W, MacVicar J. Comparison of intravenous prostaglandins F2alpha and E2 with intravenous oxytocin in the induction of labour. Journal of Obstetrics and Gynaecology of the British Commonwealth 1973;80:531‐5. [DOI] [PubMed] [Google Scholar]
Rangarajan 1971 {published data only}
- Rangarajan NS, Croix GE, Moghissi KS. Induction of labor with prostaglandin. Obstetrics & Gynecology 1971;38:546‐50. [DOI] [PubMed] [Google Scholar]
Spellacy 1973 {published data only}
- Spellacy WN, Gall SA, Shevach AB, Holsinger KK. The induction of labor at term. Comparisons between prostaglandin F2alpha and oxytocin infusion. Obstetrics & Gynecology 1973;41:14‐21. [PubMed] [Google Scholar]
Vakhariya 1972 {published data only}
- Vakhariya VR, Sherman AI. Prostaglandin F2alpha for induction of labor. American Journal of Obstetrics and Gynecology 1972;113:212‐22. [DOI] [PubMed] [Google Scholar]
Vroman 1972 {published data only}
- Vroman S, Thiery M, Yo Le Sian A, Depiere M, Vanderheyden C, Derom R, et al. A double blind comparative study of prostaglandin F2alpha and oxytocin for the elective induction of labor. European Journal of Obstetrics & Gynecology and Reproductive Biology 1972;4S:115‐23. [Google Scholar]
Wildemeersch 1976 {published data only}
- Wildemeersch DA, Schellen AMCM. Double‐blind trial of prostaglandin F2alpha and oxytocin in the induction of labour. Current Medical Research and Opinion 1976;4:263‐6. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Amano 1999 {published data only}
- Amano K, Saito K, Shoda T, Tani A, Yoshihara H, Nishijima M. Elective induction of labor at 39 weeks of gestation: a prospective randomized trial. Journal of Obstetrics and Gynaecology Research 1999;25:33‐7. [DOI] [PubMed] [Google Scholar]
Anderson 1972 {published data only}
- Anderson GG, Hobbins JC, Speroff L. Intravenous prostaglandins E2 and F2alpha for the induction of term labor. American Journal of Obstetrics and Gynecology 1972;112:382‐6. [DOI] [PubMed] [Google Scholar]
Brandel 1998 {published data only}
- Brandel E, Bascou V, Meeus JB, Magnin G. Results of a randomised trial of cervical ripening in premature rupture of membranes at term: intravenous prostaglandin E2 versus intravaginal prostaglandin E2 [Resultats d'un essai randomise de maturation cervicale dans les ruptures prematurees de membranes a terme: prostine E2 intraveineuse versus prostine E2 en gel vaginal]. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1998;27:111. [Google Scholar]
Bremme 1980 {published data only}
- Bremme K, Eneroth P. Changes in serum hormone levels during labor induced by oral PGE2 or oxytocin infusion. Acta Obstetricia et Gynecologica Scandinavica 1980;92 Suppl:31‐43. [DOI] [PubMed] [Google Scholar]
Calder 1974 {published data only}
- Calder AA, Moar VA, Ounsted MK, Turnbull AC. Increased bilirubin levels in neonates after induction of labour by intravenous prostaglandin E2 or oxytocin. Lancet 1974;2:1339‐42. [DOI] [PubMed] [Google Scholar]
Kanhai 1989 {published data only}
- Kanhai HHH, Keirse MJNC. Induction of labour after fetal death: a randomized controlled trial of two prostaglandin regimens. British Journal of Obstetrics and Gynaecology 1989;96:1400‐4. [DOI] [PubMed] [Google Scholar]
- Kanhai HHH, Keirse MJNC. Intravenous administration of sulfprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil; 1988:201‐2.
- Kanhai HHH, Keirse MJNC. Intravenous administration of sulprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. Proceedings of 1st European Congress on Prostaglandins in Reproduction, Vienna, Austria, 1988.
Le Maire 1972 {published data only}
- Maire WJ, Spellacy WN, Shevach AB, Gall SA. Changes in plasma estriol and progesterone during labor induced with prostaglandin F2alpha or oxytocin. Prostaglandins 1972;2:93‐101. [DOI] [PubMed] [Google Scholar]
Lindmark 1976 {published data only}
- Lindmark G, Nilsson BA. A comparative study of uterine activity in labour induced with prostaglandin F2alpha or oxytocin and in spontaneous labour I. Pattern of uterine contractions. Acta Obstetricia et Gynecologica Scandinavica 1976;55:453‐60. [DOI] [PubMed] [Google Scholar]
Lipshitz 1984 {published data only}
- Lipshitz J, Lipshitz EM. Uterine and cardiovascular effects of fenoterol and hexoprenaline in prostaglandin F2alpha‐induced labor in humans. Obstetrics & Gynecology 1984;63:396‐400. [PubMed] [Google Scholar]
Reichel 1985 {published data only}
- Reichel R, Husslein P, Goschen K, Rasche M, Sinzinger H. Studies of the uptake of prostaglandin e2 administered locally by various means to achieve ripening of the cervix and induction of labour [Untersuchungen zur Resorption von Prostaglandin E2 nach unterschiedlicher lokaler Applikation zur Zervixreifung und zur Geburtseinleitung]. Wiener Klinische Wochenschrift 1985;97:500‐3. [PubMed] [Google Scholar]
Rosa 1974 {published data only}
- Rosa P. A comparison of the efficiency of oxytocin and prostaglandin F2alpha in the treatment of dystocia in the primiparous woman at term. Journal de Gynecologie, Obstetrique et Biologie de la Reproduction (Paris) 1974;6:571‐80. [PubMed] [Google Scholar]
Scher 1972 {published data only}
- Scher J, Davey DA, Baillie P, Friend J, Friend DM. A comparison of prostaglandin F2alpha and oxytocin in the induction of labour. South African Medical Journal 1972;46:2009‐12. [PubMed] [Google Scholar]
Spellacy 1971 {published data only}
- Spellacy WN, Buhi WC, Holsinger KK. The effect of prostaglandin F2alpha and E2 on blood glucose and plasma insulin levels during pregnancy. American Journal of Obstetrics and Gynecology 1971;111:239‐43. [DOI] [PubMed] [Google Scholar]
Spellacy 1972 {published data only}
- Spellacy WN, Gall SA. Prostaglandin F2alpha and oxytocin for term labor induction. The prostaglandins. Clinical applications in human reproduction. New York: Futura, 1972:107‐13. [Google Scholar]
Thomsen 1987 {published data only}
- Thomsen AC. Induction of labour by low‐dose PGF2alpha and oxytocin. A randomised double‐blind study. Personal communication 1987.
Van Heerden 1992 {published data only (unpublished sought but not used)}
- Heerden J, Steyn DW. Management of prelabour rupture of the membranes after 34 weeks gestation: early vs delayed induction of labour. Eleventh Conference on Priorities in Perinatal Care in South Africa. 1992:98‐100.
References to studies awaiting assessment
Craft 1971 {published data only}
- Craft IL, Cullum AR, May DTL, Noble AD, Thomas DJ. Prostaglandin E2 compared with oxytocin for the induction of labour. BMJ 1971;3:276‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roberts 1970 {published data only}
- Roberts G. Induction of labour using prostaglandins. Journal of Reproduction and Fertility 1970;23:370‐1. [DOI] [PubMed] [Google Scholar]
Additional references
Clarke 1999
- Clarke M, Oxman AD, editors. Cochrane Reviewers' Handbook 4.0 [updated July 1999]. In: Review Manager (RevMan) [Computer program]. Version 4.0 Oxford, England: The Cochrane Collaboration, 1999.
Hofmeyr 2000
- Hofmeyr GJ, Alfirevic Z, Kelly AJ, Kavanagh J, Thomas J, Brocklehurst P, et al. Methods for cervical ripening and labour induction in late pregnancy: generic protocol. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD002074] [DOI] [PubMed] [Google Scholar]
Johnston 1993
- Johnston TA, Greer IA, Kelly RW, Calder AA. Plasma prostaglandin metabolite concentrations in normal and dysfunctional labour. British Journal of Obstetrics and Gynaecology 1993;100:483‐8. [DOI] [PubMed] [Google Scholar]
Kanhai 1988a
- Kanhai HHH, Keirse MJNC. Intravenous administration of sulprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. Proceedings of 1st European Congress on Prostaglandins in Reproduction, Vienna, Austria, 1988.
Kanhai 1988b
- Kanhai HHH, Keirse MJNC. Intravenous administration of sulfprostone for the induction of labour after fetal death: a randomized comparison of two dose schedules. World Congress of Gynecology and Obstetrics; 1988 October 23‐28; Brazil; 1988:201‐2.
Keirse 1993
- Keirse MJNC. Any prostaglandin (by any route) vs oxytocin (any route) for induction of labour. [revised 22 April 1993]. In: Enkin MW, Keirse MJNC, Renfrew MJ, Neilson JP, Crowther C (eds.) Pregnancy and Childbirth Module. In: The Cochrane Pregnancy and Childbirth Database [database on disk and CDROM]. The Cochrane Collaboration; Issue 2, Oxford: Update Software; 1995.
MacKenzie 1999
- MacKenzie AZ. Labor induction including pregnancy termination for fetal anomaly. In: James DK, Steer PJ, Weiner CP, Gonik B editor(s). High risk pregnancy, management options. 2nd Edition. London: WB Saunders, 1999:1079‐102. [Google Scholar]
RevMan 1999 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.0 for Windows. Oxford, England: The Cochrane Collaboration, 1999.


