Abstract
Rationale: Standard physiologic assessments of extubation readiness in patients with acute hypoxemic respiratory failure (AHRF) may not reflect lung injury resolution and could adversely affect clinical decision-making and patient outcomes.
Objectives: We hypothesized that elevations in inflammatory plasma biomarkers sST2 (soluble suppression of tumorigenicity-2) and IL-6 indicate ongoing lung injury in AHRF and better inform patient outcomes compared with standard clinical assessments.
Methods: We measured daily plasma biomarkers and physiologic variables in 200 patients with AHRF for up to 9 days after intubation. We tested the associations of baseline values with the primary outcome of unassisted breathing at Day 29. We analyzed the ability of serial biomarker measurements to inform successful ventilator liberation.
Measurements and Main Results: Baseline sST2 concentrations were higher in patients dead or mechanically ventilated versus breathing unassisted at Day 29 (491.7 ng/ml [interquartile range (IQR), 294.5–670.1 ng/ml] vs. 314.4 ng/ml [IQR, 127.5–550.1 ng/ml]; P = 0.0003). Higher sST2 concentrations over time were associated with a decreased probability of ventilator liberation (hazard ratio, 0.80 per log-unit increase; 95% confidence interval [CI], 0.75–0.83; P = 0.03). Patients with higher sST2 concentrations on the day of liberation were more likely to fail liberation compared with patients who remained successfully liberated (320.9 ng/ml [IQR, 181.1– 495.6 ng/ml] vs. 161.6 ng/ml [IQR, 95.8–292.5 ng/ml]; P = 0.002). Elevated sST2 concentrations on the day of liberation decreased the odds of successful liberation when adjusted for standard physiologic parameters (odds ratio, 0.325; 95% CI, 0.119–0.885; P = 0.03). IL-6 concentrations did not associate with outcomes.
Conclusions: Using sST2 concentrations to guide ventilator management may more accurately reflect underlying lung injury and outperform traditional measures of readiness for ventilator liberation.
Keywords: biomarkers, acute respiratory distress syndrome, mechanical ventilator weaning
At a Glance Commentary
Scientific Knowledge on the Subject
Clinicians are limited in their ability to predict extubation readiness in patients with acute hypoxemic respiratory failure (AHRF) often because of a reliance on physiologic parameters that may not reflect the degree of underlying lung injury. Plasma sST2 (soluble suppression of tumorigenicity-2) and IL-6 are biomarkers that are elevated in lung inflammation and may provide objective measures of ongoing lung injury.
What This Study Adds to the Field
In this study of 200 patients with AHRF, elevated baseline sST2 concentrations were associated with death or continued mechanical ventilation at Day 29. Measured longitudinally, each log-unit elevation in sST2 was associated with a 20% decrease in probability of liberation from mechanical ventilation. Patients with elevated sST2 concentrations on the day of liberation were more likely to fail liberation and be reintubated, even after adjustment for standard physiological variables. IL-6 concentrations were not associated with clinical outcomes. Measuring sST2 concentrations in patients with AHRF could provide real-time prognostic information about a patient’s clinical trajectory and readiness for ventilator liberation.
Patients with acute hypoxemic respiratory failure (AHRF) managed with mechanical ventilation account for a majority of ICU admissions, and AHRF is associated with increased short- and long-term ICU mortality (1–3). However, clinicians remain limited in their ability to predict the duration of mechanical ventilation and determine readiness for ventilator liberation in patients with AHRF.
Lung-protective ventilatory strategies in patients with acute respiratory distress syndrome (ARDS) minimize the development of ventilator-induced lung injury and decrease mortality (4–10). However, increased duration of mechanical ventilation and the use of prolonged sedation to facilitate ventilator synchrony are associated with infection, longer ICU and hospital stays, increased resource utilization, and mortality (11, 12). Current assessments of liberation readiness involve daily weaning assessments and spontaneous awakening and breathing trials if clinical criteria are met (13–15). However, 10–20% of all patients extubated after passing such trials fail liberation and require reintubation within 48 hours, and these patients have increased mortality and are less likely to be discharged from the ICU (16). There remains a significant need for improved recognition of readiness for ventilator liberation and risk of reintubation beyond physiologic assessments alone.
Plasma biomarkers of lung injury are attractive tools for determining readiness for liberation. sST2 (soluble suppression of tumorigenicity-2) and IL-6 are cytokines elevated during pulmonary inflammation (17, 18); sST2 is also secreted by lung tissue (19). We previously studied the plasma concentrations of these two biomarkers in patients enrolled in FACTT (Fluids and Catheters Treatment Trial) (20, 21) and found that higher plasma concentrations of sST2 and IL-6 in patients with ARDS are associated with increased mortality and a decreased likelihood of passing a weaning assessment, passing a spontaneous breathing trial, or being successfully extubated (21, 22). We also showed that elevated biomarker concentrations are associated with reintubation (22). However, in the FACTT analysis, biomarkers were only cross-sectionally measured at baseline and on Day 3. We hypothesized that serial biomarker measurements over time might better inform clinical trajectory and ventilator management in patients with AHRF. In this study, we follow a prospectively enrolled validation cohort of patients with AHRF requiring mechanical ventilation and investigate longitudinal sST2 and IL-6 measurement relative to clinical outcomes, including successful liberation from mechanical ventilation.
Methods
Data Collection
We enrolled endotracheally intubated patients with hypoxemia, which was defined as a PaO2:FiO2 ratio of less than 300, that was not solely attributable to cardiogenic pulmonary edema. All patients were enrolled within 24 hours of meeting the inclusion criteria (see Figure E1 in the online supplement). Detailed inclusion and exclusion criteria can be found in Figure E2. Physiologic variables were collected daily. Patients underwent a daily weaning assessment with subsequent spontaneous breathing trial if indicated per ICU protocol (Figure E3) (4). All extubated patients passed a weaning assessment and spontaneous breathing trial. The primary outcome was breathing without assistance at Day 29, which was defined as breathing without the need for mechanical ventilation (Figure E3) (4). Patients with tracheostomy placement during the study period were excluded from the primary outcome. A secondary analysis was performed for successful ventilator liberation, which was defined as unassisted breathing without resumption of mechanical ventilation for the duration of the study period. Failed liberation was defined as any subsequent requirement for mechanical ventilation. Patients with a tracheostomy who were able to breathe unassisted for greater than 48 hours were included in the secondary analysis. The study protocol was approved by our institutional review board. Informed consent was waived, as samples were obtained from excess clinical specimens.
Blood Sample Collection and Analysis
Plasma samples were collected from excess clinical blood draws. Samples were collected daily after study enrollment for a maximum of 9 days. Biomarker concentrations on the day of ventilator liberation were available for sST2 in 127 patients and for IL-6 in 125 patients. The last available measurements were used for patients liberated after the 9-day collection period. Samples were stored in ethylenediaminetetraacetic acid–treated plasma at −80°C and examined using a commercially available IL-6 assay (Luminex; R&D Systems) and a highly sensitive sST2 assay (Presage; Critical Diagnostics).
Statistical Analysis
Summary statistics of patient demographics, characteristics, and outcomes were collected. The associations between biomarker concentrations and prespecified outcomes were determined at Day 29. Fisher’s exact test for categorical variables and the Student’s t test or the Kruskal-Wallis nonparametric test for continuous variables were used to assess the statistical significance of differences between groups. Biomarker concentrations were logarithmically transformed for further analyses. Mixed-model ANOVA was performed on serial biomarker measurements with Sidak’s correction for multiple comparisons.
We used a shared-parameter joint model for longitudinal and time-to-event data to estimate a longitudinal mixed-effects model for serial sST2 measurements and ventilator liberation, accounting for the competing risk of death. A proportional hazards approach modeled the time-to-event outcome. Estimates of event-free probabilities were calculated using the Markov chain Monte Carlo method. Information on sST2 was right-censored on the basis of the day of liberation, death before liberation, or last measurement taken.
We created a multivariable model for successful liberation using the log-transformed biomarker values together with covariates from the day of liberation. The optimal biomarker cutoff values were determined using the Youden Index summary statistic with receiver operating characteristic curve analysis (23, 24). We dichotomized biomarkers around the cutoff value and obtained odds ratios (ORs) and 95% confidence intervals (CIs) from multivariable logistic regressions.
Analyses were conducted using SAS version 9.4 (SAS Institute Inc.) and R version 4.0 (R Core Team). The JM package was used to construct our joint model. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Characteristics of the Study Population and Major Outcomes
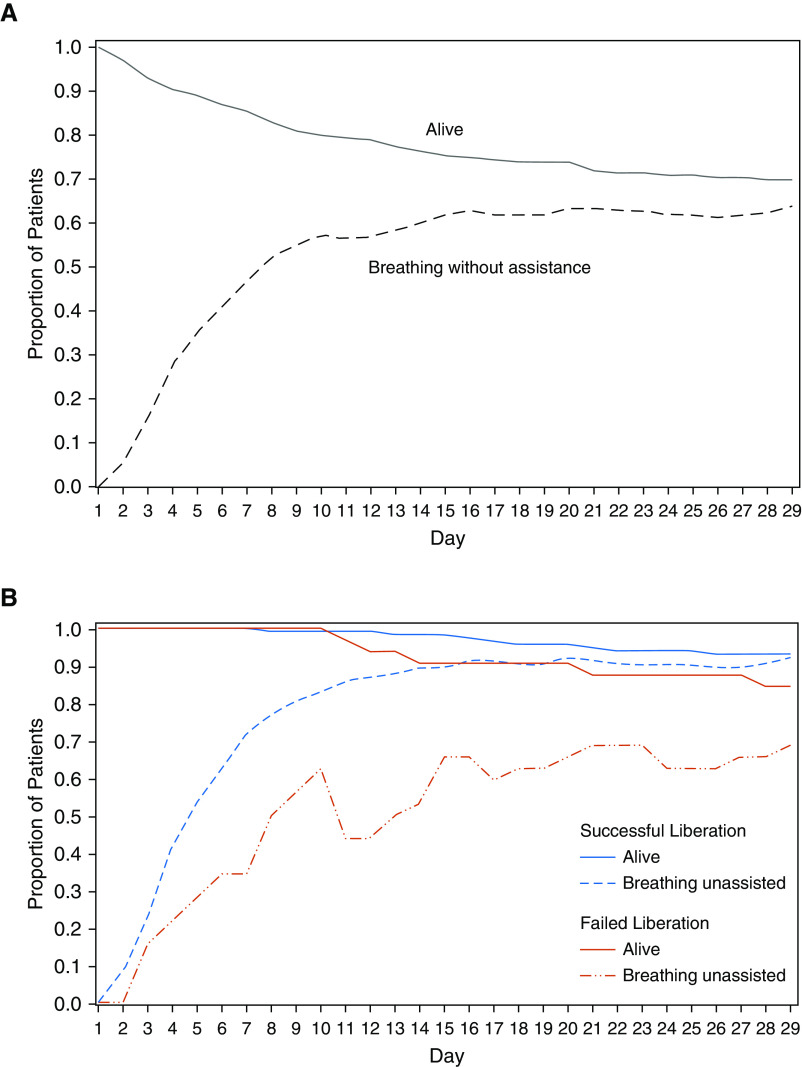
We prospectively enrolled 217 patients with AHRF within 24 hours of intubation at Massachusetts General Hospital in Boston, Massachusetts, from November 2015 to January 2017. Of the 217 enrolled patients, 209 patients met the inclusion criteria and had clinical plasma samples available for measurement (Figure E1). Demographic and baseline physiologic data for the 209 patients in the overall study population are shown in Table 1. On enrollment, patients had a mean modified Sequential Organ Failure Assessment (mSOFA) score of 9.2 and PaO2:FiO2 ratio of 187. Vasopressors were initiated in 183 patients (87.6%) within 24 hours of ICU admission. Of the 209 enrolled patients, 199 patients (95.2%) met Berlin criteria for ARDS (25). The distribution of baseline characteristics was similar for patients with successful and failed ventilator liberation, with exceptions for ethnicity, mSOFA score, and duration of mechanical ventilation. Nine patients were terminally extubated before Day 29 and were excluded from further downstream analyses. The overall rate of survival to hospital discharge and breathing without ventilator assistance during the first 29 days after enrollment is shown for the remaining 200 patients in Figure 1A, with 63.5% of all patients breathing without assistance by Day 29. The rate of breathing without assistance at Day 29 was 92.1% for patients who were successfully liberated from the ventilator and 68.8% for those who failed initial liberation (Figure 1B).
Table 1.
Baseline Characteristics and Physiological Factors at Study Enrollment
| Variable | Overall (n = 209) | Successful Liberation (n = 114) | Failed Liberation (n = 32) | P Value |
|---|---|---|---|---|
| Demographics | ||||
| Age, yr, mean ± SD | 59.7 ± 16.3 | 58.0 ± 17.0 | 57.2 ± 16.5 | 0.82 |
| Sex, n (%) | 1.00 | |||
| M | 139 (66.5) | 79 (69.3) | 22 (68.8) | |
| F | 70 (33.5) | 35 (30.7) | 10 (31.3) | |
| BMI, kg/m2, mean ± SD | 28.9 ± 7.6 | 28.6 ± 8.1 | 29.4 ± 6.2 | 0.59 |
| Ethnicity, n (%) | 0.02 | |||
| Non-Hispanic white | 151 (72.2) | 80 (70.2) | 27 (84.4) | |
| Non-Hispanic Black | 14 (6.7) | 9 (7.9) | 0 (0.0) | |
| Hispanic | 12 (5.7) | 10 (8.8) | 0 (0.0) | |
| Asian/Pacific Islander | 8 (3.8) | 3 (2.6) | 3 (9.4) | |
| American Indian/Alaska native | 1 (0.5) | 0 (0.0) | 1 (3.1) | |
| Other | 23 (11.0) | 12 (10.5) | 1 (3.1) | |
| Chest radiograph infiltrates* | ||||
| Bilateral, n (%) | 199 (95.2) | 107 (93.9) | 30 (93.8) | 1.00 |
| Number of quadrants, mean ± SD | 3 ± 1 | 3 ± 1 | 3 ± 1 | 0.39 |
| Baseline physiologic factors | ||||
| PaO2:FiO2 ratio, mean ± SD | 187.0 ± 92.8 | 189.7 ± 91.0 | 193.8 ± 93.9 | 0.83 |
| Vasopressors, n (%) | 183 (87.6) | 81 (71.1) | 24 (75.0) | 0.29 |
| mSOFA (no GCS), mean ± SD | 9.2 ± 3.4 | 8.2 ± 3.0 | 10.0 ± 4.2 | 0.03 |
| Outcome | ||||
| Ventilated days†, mean ± SD | 8.3 ± 6.7 | 6.0 ± 5.7 | 12.3 ± 8.0 | 0.0002 |
| In-hospital mortality, Day 29, n (%) | 69 (33.0) | 8 (7.1) | 5 (15.6) | 0.16 |
| In-hospital mortality, Day 60, n (%) | 75 (35.8) | 11 (9.6) | 6 (18.8) | 0.21 |
Definition of abbreviations: BMI = body mass index; GCS = Glasgow Coma Scale; mSOFA = modified Sequential Organ Failure Assessment.
Baseline physiologic factors were measured within 24 hours of ICU admission.
*Day 2 chest radiographs were read if enrollment chest radiographs were not present (n = 9).
†Total duration of mechanical ventilation in the first 29 days.
Figure 1.
Rate of survival to hospital discharge and of breathing without ventilator assistance during the first 29 days after study enrollment. (A) At Day 29, 127 of the overall cohort of 200 patients (63.5%) were breathing without assistance. (B) At Day 29, 105 of the 114 successfully liberated patients (92.1%) were breathing without assistance, whereas only 22 of the 32 patients who failed liberation (68.8%) were breathing unassisted.
Elevated Baseline sST2 Concentrations Are Associated with Mortality and Need for Mechanical Ventilation at Day 29
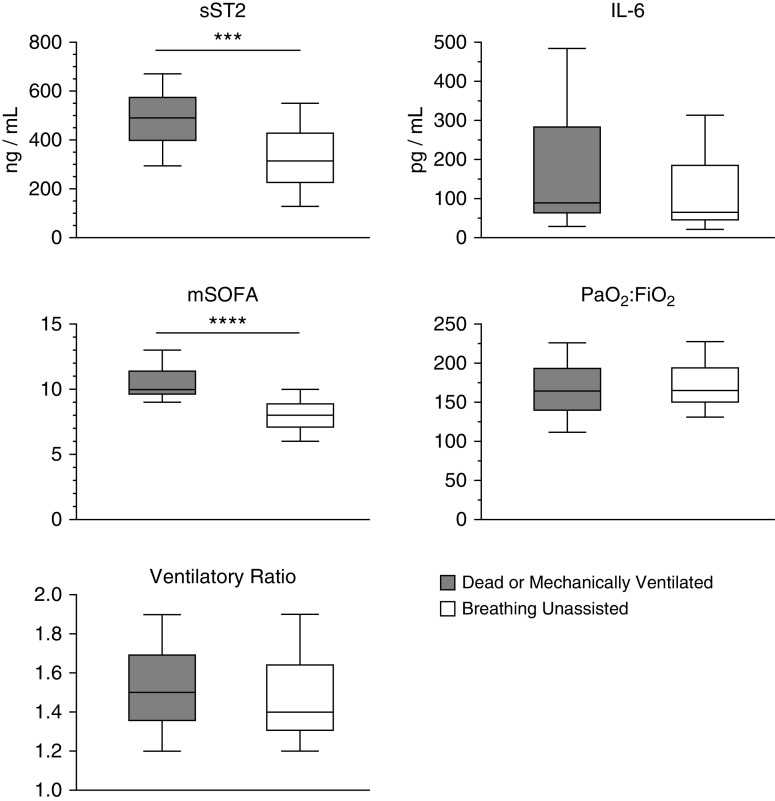
We measured average biomarker concentrations on Study Days 1–9 and tested the association between log-transformed values and the primary outcome (Figure 2). Thirty patients underwent tracheostomy placement during the study period and were not included in the primary outcome. sST2 concentrations were elevated on Days 1–9 in patients dead or mechanically ventilated at Day 29 compared with patients breathing unassisted. Median baseline sST2 concentrations were higher in patients who were dead or mechanically ventilated at Day 29 compared with patients who were breathing unassisted (491.7 ng/ml [interquartile range (IQR), 294.5–670.1 ng/ml] vs. 314.4 ng/ml [IQR, 127.5–550.1 ng/ml]; P = 0.0003; Figure 3). Median baseline IL-6 values were similar between patients dead or mechanically ventilated and those breathing unassisted at Day 29 (88.4 pg/ml [IQR, 28.5–483.7 pg/ml] vs. 63.4 pg/ml [IQR, 21.1–313.3 pg/ml]; P = 0.17).
Figure 2.
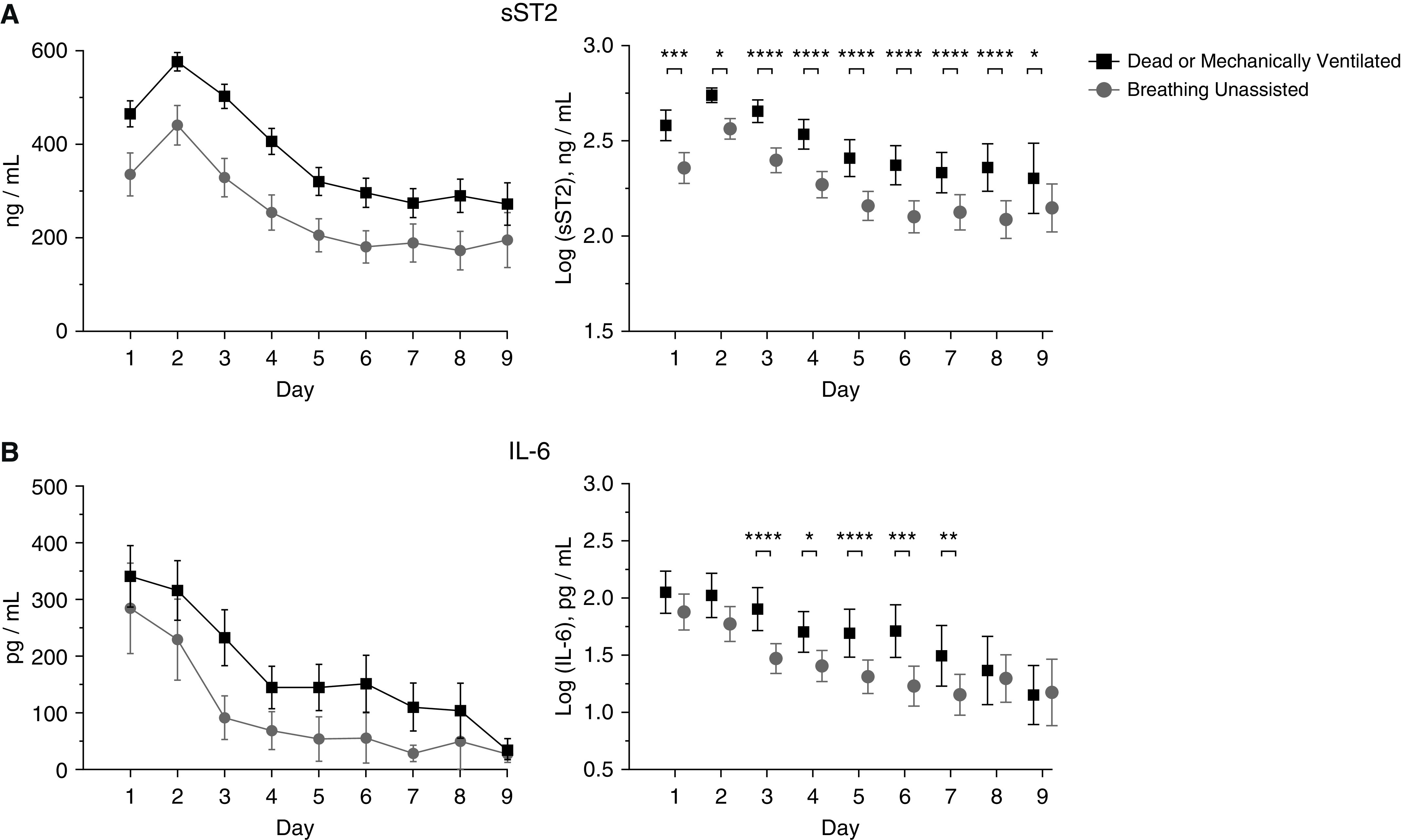
Biomarker trends by day and association with the primary outcome. (A) sST2 (soluble suppression of tumorigenicity-2) and IL-6 concentrations by day in patients dead or mechanically ventilated versus those breathing unassisted by Day 29. Data shown are mean and 95% confidence interval (CI). (B) Comparison of log-transformed sST2 and IL-6 concentrations by day in patients dead or mechanically ventilated versus those breathing unassisted by Day 29. Data shown are mean and 95% CI. Mixed-model ANOVA with Sidak’s test for multiple comparisons was used to assess the statistical significance of differences between groups. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001.
Figure 3.
Elevated baseline sST2 (soluble suppression of tumorigenicity-2) concentrations are associated with mortality and need for mechanical ventilation at Day 29. Baseline sST2 concentration and mSOFA score were higher in patients who were dead or mechanically ventilated at Day 29 compared with those breathing without assistance. Baseline IL-6 concentration was not different between groups. Data are represented as box plots of median and interquartile range, with whiskers indicating minimum and maximum values. Kruskal-Wallis nonparametric test for continuous variables was used to assess the statistical significance of differences between groups. ***P < 0.001 and ****P < 0.0001. mSOFA = modified Sequential Organ Failure Assessment.
Elevated sST2 Concentrations over Time Are Associated with Ventilator Dependence
Patients with higher sST2 concentrations over time were more likely to die before ventilator liberation (Figure E4A). Elevated baseline sST2 values were also associated with decreased cause-specific cumulative hazard for liberation over the first 9 days after intubation (Figure E4B). We therefore tested the utility of longitudinal sST2 measurement to inform ventilator management by jointly modeling sST2 over the first 9 days after intubation with ventilator liberation as the primary event of interest, adjusting for baseline covariates and accounting for the competing risk of death. The joint model showed that higher sST2 values at any given time point during the first 9 days after intubation were associated with decreased adjusted probability of liberation over time (hazard ratio, 0.80 per log-unit increase; 95% CI, 0.75–0.83; P = 0.03; Table 2).
Table 2.
Results of the Joint Model of the Association of sST2 Values over Time with Ventilator Liberation
| Variable* | Hazard Ratio | 95% CI | P Value |
|---|---|---|---|
| Log(sST2), ng/ml | 0.80 | 0.75–0.83 | 0.03 |
| Age, yr | 0.99 | 0.99–1.00 | 0.01 |
| PaO2:FiO2 | 1.81 | 0.41–8.08 | 0.15 |
| PEEP, cm H2O | 1.09 | 0.18–6.60 | 0.76 |
| e, L/min | 1.07 | 0.09–13.30 | 0.60 |
| mSOFA score | 0.85 | 0.77–0.95 | 0.01 |
Definition of abbreviations: CI = confidence interval; mSOFA = modified Sequential Organ Failure Assessment; PEEP = positive end-expiratory pressure; sST2 = soluble suppression of tumorigenicity-2.
Model adjusted for baseline covariates. For continuous variables, hazard ratios are for a 1-unit increase in the variable.
Elevated sST2 Concentrations on the Day of Ventilator Liberation Are Associated with Liberation Failure
To determine whether biomarkers could provide real-time information regarding liberation readiness, we compared biomarker concentrations on the day of ventilator liberation in patients who remained successfully liberated to those who failed. Of the 200 analyzed patients, 114 patients (57%) were successfully liberated, and 32 patients (16%) failed. Three patients who failed and resumed mechanical ventilation had a tracheostomy in place, and 29 patients were reintubated. The median time to initial ventilator liberation was 5 days (IQR, 4–8). Patients who failed liberation resumed mechanical ventilation within a median of 2 days (IQR, 1.0–5.25), with 11 patients reintubated within 24 hours. The primary indication for resumption of mechanical ventilation was high breathing effort (Table E1). Median sST2 concentrations on the day of ventilator liberation were higher in patients who failed liberation compared with those who remained successfully liberated (320.9 ng/ml [IQR, 181.1–495.6 ng/ml] vs. 161.6 ng/ml [IQR, 95.8–292.5 ng/ml]; P = 0.002; Figure 4A). Conventional physiologic measures, including PaO2:FiO2 ratio, positive end-expiratory pressure (PEEP), and e, were not associated with liberation success. Median IL-6 concentrations on the day of liberation were not different between patients who failed liberation compared with those who were successfully liberated (22.3 pg/ml [IQR, 10.8–45.2 pg/ml] vs. 14.9 pg/ml [IQR, 6.4–38.8 pg/ml]; P = 0.25). In addition, the median sST2 concentration decreased from baseline to the day of liberation in successfully liberated patients (307.3 ng/ml [IQR, 116.1–512.9 ng/ml] vs. 161.6 ng/ml [IQR, 95.8–292.5 ng/ml]; P = 0.0007; Figure 4B) but remained elevated in patients who failed liberation (497.1 ng/ml [IQR, 152.7–658.3 ng/ml] vs. 320.9 ng/ml [IQR, 178.4–505.2 ng/ml]; P = 0.15). Median IL-6 concentrations significantly decreased from baseline to the day of liberation both in patients successfully liberated (49.8 pg/ml [IQR, 17.3–296.1 pg/ml] vs. 14.9 pg/ml [IQR, 6.4–38.8 pg/ml]; P < 0.0001) and those who failed liberation (195.6 pg/ml [IQR, 29.8–531.3 pg/ml] vs. 22.3 pg/ml [IQR, 10.8–45.2 pg/ml]; P < 0.0001).
Figure 4.
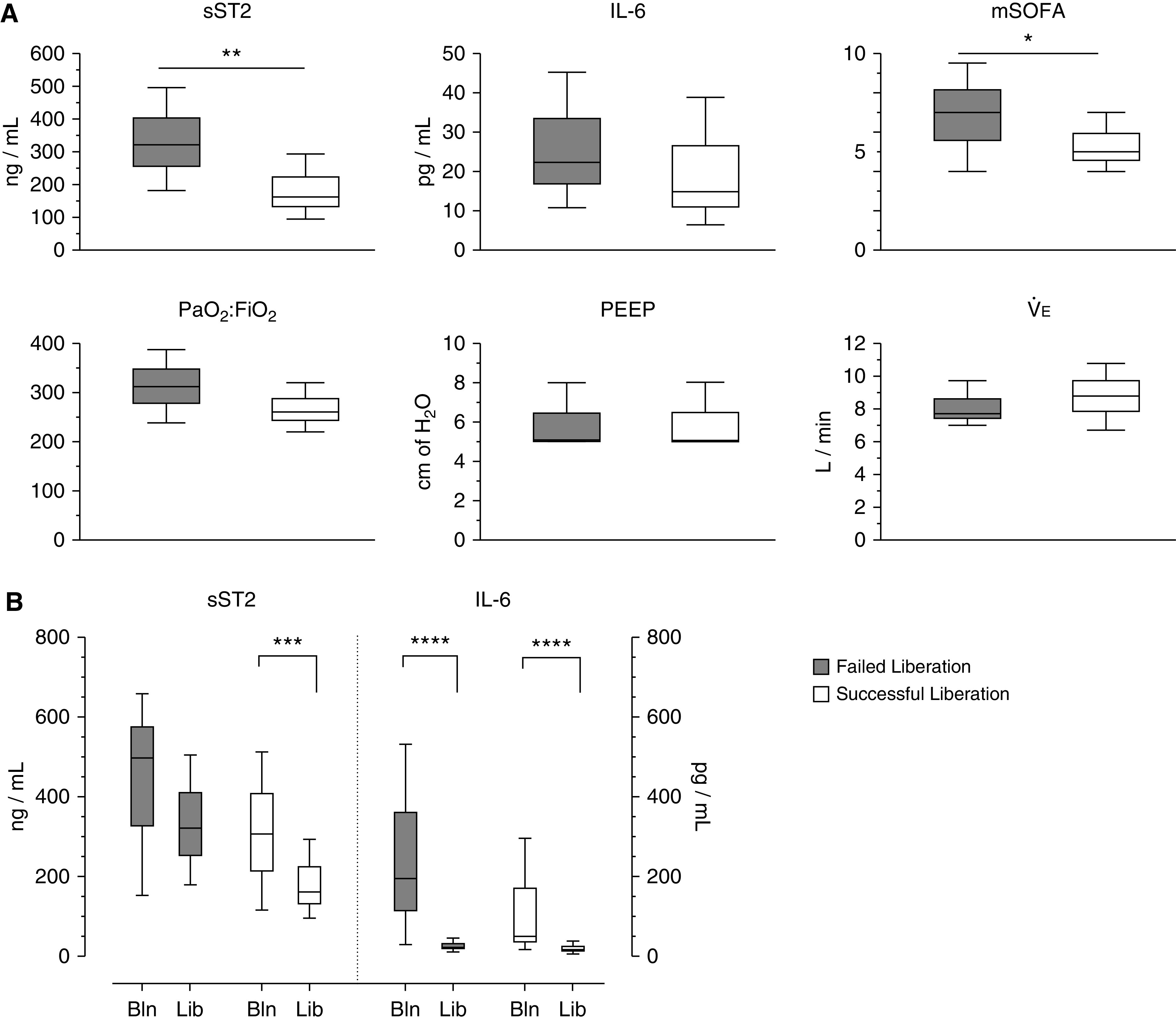
Elevated sST2 (soluble suppression of tumorigenicity-2) concentrations on the day of ventilator liberation are associated with liberation failure. (A) sST2 concentrations and mSOFA score on the day of liberation were higher in patients who failed liberation compared with those who remained successfully liberated. IL-6 concentration, PaO2:FiO2 ratio, PEEP, and e were not different on the day of liberation for patients who failed liberation compared with patients successfully liberated. (B) sST2 concentrations decreased from baseline (Bln) to the day of liberation (Lib) in successfully liberated patients but remained elevated in those who failed liberation. IL-6 concentrations decreased from Bln to the Lib in both successfully liberated patients and those who resumed mechanical ventilation. Data are represented as box plots of median and interquartile range, with whiskers indicating minimum and maximum values. Kruskal-Wallis nonparametric test for continuous variables was used to assess the statistical significance of differences between groups. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001. mSOFA = modified Sequential Organ Failure Assessment; PEEP = positive end-expiratory pressure.
sST2 Concentrations Are Associated with Liberation Success after Adjustment for Standard Clinical Measures
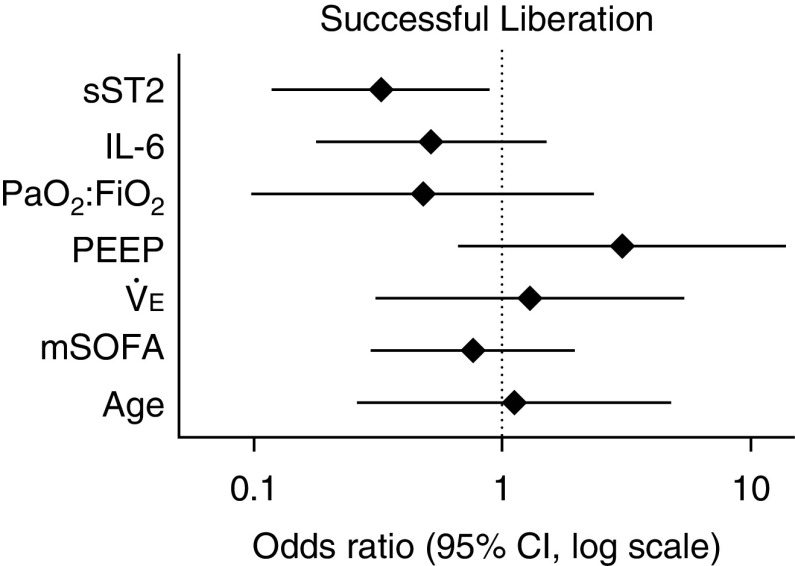
The optimal log-transformed biomarker cutoff value on the day of liberation that was associated with liberation success was 2.49 pg/ml for IL-6 and 5.68 ng/ml for sST2 (sensitivity = 0.73, specificity = 0.54, and C-statistic = 0.63). We created a multivariable regression model for successful liberation using the dichotomized sST2 and IL-6 values, age, PaO2:FiO2 ratio, PEEP, e, and mSOFA score on the day of liberation. Each variable was log-transformed, and adjusted ORs were calculated. Results from the multivariable logistic regression analysis indicated that only sST2 concentration was significantly associated with liberation success (Figure 5 and Table E2). Patients with log-transformed sST2 concentrations ⩾5.68 ng/ml on the day of liberation had a decreased likelihood of liberation success compared with patients with sST2 concentrations <5.68 ng/ml (adjusted OR, 0.325; 95% CI, 0.119–0.885; P = 0.03). Log-transformed IL-6 concentrations ⩾2.49 pg/ml compared with <2.49 pg/ml were not associated with liberation success (OR, 0.517; 95% CI, 0.179–1.489; P = 0.22).
Figure 5.
Elevated sST2 (soluble suppression of tumorigenicity-2) concentration on the day of ventilator liberation decreases the odds of liberation success after adjustment for standard clinical measures. Patients with log-transformed sST2 ⩾5.68 ng/ml on the day of liberation had significantly lower odds of liberation success compared with patients with sST2 <5.68 ng/ml (adjusted odds ratio [OR], 0.325; 95% confidence interval [CI], 0.119–0.885; P = 0.03). Log-transformed IL-6 values ⩾2.49 pg/ml at the time of liberation were not associated with liberation success (OR, 0.517; 95% CI, 0.179–1.489; P = 0.22). Patient age and physiologic measures on the day of liberation did not predict liberation success (ORs are reported per log-unit increase). Symbols indicate OR, and error bars indicate 95% CI. mSOFA = modified Sequential Organ Failure Assessment; PEEP = positive end-expiratory pressure.
Discussion
We have previously shown that in historical patient cohorts such as FACTT, elevated sST2 and IL-6 concentrations measured at the time of intubation were associated with a decreased rate of extubation and an increased need for reintubation (21, 22). In the present study, we hypothesized that longitudinally measured sST2 and IL-6 concentrations are better indicators of lung injury resolution and readiness for ventilator liberation compared with standard clinical assessments and that following serial biomarker concentrations can inform patient mortality and ventilator dependence. Our results demonstrate that sST2 concentrations strongly associate with mortality and ventilator outcomes and may be a prognostic marker of disease severity in patients with AHRF. A single measurement of sST2 upon intubation prognosticated future outcomes, but repeated measurements added considerable information regarding likelihood for ventilator liberation.
Joint modeling is a powerful method that combines random effects and survival models, thereby avoiding informative dropout bias. Using a joint model, we found that elevated sST2 concentrations over time were independently associated with ventilator dependence among patients with AHRF. The results show that for every log-unit increase in sST2 concentration, there was a 20% decrease in the probability of ventilator liberation. Thus, this study suggests that elevated sST2 values measured both cross-sectionally and longitudinally are associated with the need for mechanical ventilation even after adjusting for important baseline clinical factors such as age, mSOFA score, and PaO2:FiO2 ratio. The statistical adjustments for these covariates and the use of a joint model reinforce the association of sST2 with outcomes. Concentrations of IL-6 did not provide prognostic information on ventilator dependence.
These results also build on our prior work by demonstrating that elevated sST2 concentrations measured at the time of ventilator liberation strongly associate with failed liberation and return to mechanical ventilation. This association was not observed for standard physiologic measures of liberation readiness, including PaO2:FiO2 ratio, PEEP, and e, suggesting that elevated sST2 concentrations may signify ongoing unresolved respiratory disease at the time of ventilator liberation. Moreover, following serial sST2 concentrations over a patient’s ICU course was strongly associated with ventilator dependence over time, even when adjusted for common physiologic measurements. To our knowledge, this is the first study to investigate a biomarker-directed approach to bedside ventilator management in patients with AHRF, and it suggests that elevated sST2 concentrations may be a better indicator of ongoing lung injury than standard physiologic assessment (26–28).
sST2 is a member of the IL-1 receptor family of proteins, and the IL-33/ST2 signaling pathway is well described in allergic and inflammatory conditions and in the setting of myocardial strain among patients with heart failure (29). Although IL-33 binding to the transmembrane form of ST2 is protective, sST2 is believed to act as a “decoy receptor” that is associated with increased tissue fibrosis and remodeling (29, 30). Serum and BAL sST2 concentrations are increased in pulmonary inflammatory diseases, including chronic obstructive pulmonary disease, asthma, and pneumonia (18, 31, 32). Notably, the highest concentrations of sST2 are reported in patients with ARDS, and prior work from our group demonstrated that higher concentrations of plasma sST2 accurately discriminate between ARDS and heart failure (21, 22). Recent data have shown that human type II pneumocytes produce sST2 in response to mechanical stress and that sST2 concentrations measured in human bronchial aspirates correlate with serum values (19, 22). Accordingly, though measurement of sST2 has a prognostic role in cardiac diseases, our results further emphasize the value of sST2 measurement for detection of local epithelial cell injury in the lung; longitudinal sST2 measurement may provide readily accessible, real-time, and dynamic information to clinicians about the degree of ongoing lung injury in their patients with AHRF over the course of an ICU admission.
IL-6 is an important mediator of the systemic inflammatory response and is a pleiotropic cytokine secreted by multiple immune and stromal cell types into the circulation, where it exerts both proinflammatory and antiinflammatory effects (17, 33). Elevated plasma IL-6 concentrations have been associated with increased mortality in ARDS (34). IL-6 concentrations may also increase in response to lung overdistension, and higher plasma and BAL concentrations of IL-6 have been measured during conventional compared with lung-protective ventilation (4, 5, 35, 36). Although our prior work demonstrated that IL-6 values upon intubation were associated with mortality and likelihood of extubation in the FACTT cohort, these findings were not observed in this study (21). This difference may be due to our current cohort representing a sicker patient population, with 88% of enrolled patients on vasopressors compared with 33% in the FACTT trial (20). As IL-6 concentrations are known to be elevated in systemic inflammation, a sicker overall cohort may decrease this biomarker’s predictive value. We also collected serial biomarker measurements for a longer duration than in our prior studies, and this longitudinal collection revealed that IL-6 concentrations decreased rapidly over time in our patients with AHRF (Figures 2 and 4B). This rapid decline may limit the predictive value of IL-6 over the course of an ICU admission. Overall, IL-6 likely provides a more global measure of systemic inflammation in critically ill patients and thus lacks specificity as a biomarker for ongoing lung injury to predict liberation readiness.
An important finding in our work is that sST2 concentrations provide real-time information about a patient’s readiness for ventilator liberation beyond standard physiologic measures alone that could significantly inform treatment decisions and patient outcomes. sST2 concentration measured at the time of ventilator liberation was able to discriminate between patients who needed to resume mechanical ventilation and patients who remained successfully liberated. Importantly, sST2 performed better than conventional clinical assessments and current standard of care, as all extubated patients passed a spontaneous breathing trial before extubation. Despite this, sST2 measurement—both cross-sectionally and longitudinally—added unique information regarding a patient’s likelihood for successful liberation from mechanical ventilation.
The findings of this study support the potential for a biomarker-directed approach to ventilator management, as elevated sST2 concentrations could indicate the presence of ongoing lung injury that is not apparent using standard clinical measures. Although the clinical management of mechanically ventilated patients has advocated early and aggressive attempts at spontaneous breathing trials, conventional readiness protocols still use cutoff values based on the PaO2:FiO2 ratio, PEEP, and e. These physiologic measures of global lung function are unlikely to accurately reflect the degree of underlying lung injury, particularly when the injury is heterogeneous. Indeed, our results show that these physiologic measures are not well predictive of liberation readiness. We propose that sST2 and other tissue-specific biomarkers of ongoing lung epithelial injury, such as soluble receptor for advanced glycation end products, may be better predictors of weaning and liberation success than current methods and could guide the assessment of a patient’s readiness for spontaneous breathing (37, 38).
We acknowledge important limitations of our study. Although 95% of our enrolled patients met the criteria for ARDS, we did not separate our cohort into distinct ARDS subphenotypes, and this could affect the discriminatory value of our measurements (39). However, our study likely underestimates the predictive value of sST2 and IL-6, as we hypothesize that inflammatory biomarkers would be more predictive of patient outcomes in an enriched hyperinflammatory ARDS cohort. In addition, we only measured plasma, not BAL, biomarker concentrations. Although local measurements are likely to provide a more accurate assessment of ongoing lung injury, we designed our study to use samples that are readily available at the bedside. Moreover, serum sST2 concentrations have been shown to correlate with bronchial aspirate values (19). We acknowledge the potential for surveillance bias, as individuals who remained mechanically ventilated for a longer duration had more measurements taken. In addition, we adopted a pragmatic approach to liberation failure in our secondary analysis and included, for example, patients reintubated for a procedure (Table E1). Although unlikely to be associated with elevated biomarker concentrations, the observation that elevated sST2 concentrations on the day of liberation associate with liberation failure despite the inclusion of these patients only strengthens our findings. Finally, although earlier identification of patient readiness for liberation would decrease complications associated with mechanical ventilation, it remains an open question whether prolonging mechanical ventilation in patients with ongoing evidence of lung injury would improve patient outcomes. Although this area deserves further study, we argue that more objective measurements of lung injury are critical to advancing patient care and improving outcomes in mechanically ventilated patients.
Clinicians are currently limited in their ability to predict the duration of mechanical ventilation and likelihood of liberation success in their patients, often because of a reliance on physiologic parameters that may not reflect the true degree of underlying lung injury (40). With a growing population of spontaneously breathing patients with AHRF using noninvasive support devices, such as high-flow nasal cannulas, plasma biomarkers such as sST2 could provide meaningful bedside assessment of lung injury to help predict a patient’s clinical trajectory and need for intubation (41, 42). Ultimately, although this study remains observational, a randomized clinical trial comparing established biomarker thresholds versus standard physiologic criteria to guide ventilator management and determination of patient readiness for liberation from mechanical ventilation is needed.
Acknowledgments
Acknowledgment
The authors thank Kent Lewandrowski, M.D., for his assistance in obtaining clinical specimens.
Footnotes
Supported by NIH grants R01 HL119344 and T32 HL116275.
Author Contributions: Acquisition of data: J.A., S.D.L., and K.L.B. Conception and design: J.A., S.D.L., B.T.T., and E.K.B. Analysis and interpretation: J.A., J.L.C., S.R.R., A.C., B.D.M., J.L.J., B.T.T., and E.K.B. Wrote the manuscript: J.A., J.L.J., and B.T.T. Approved and edited the manuscript: S.D.L., J.L.C., K.A.H., R.S.H., B.D.M., J.L.J., B.T.T., and E.K.B.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202005-1951OC on January 5, 2021
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1. Vincent J-L, Akça S, De Mendonça A, Haji-Michael P, Sprung C, Moreno R, et al. SOFA Working Group. Sequntial organ failure assessment. The epidemiology of acute respiratory failure in critically ill patients. Chest. 2002;121:1602–1609. doi: 10.1378/chest.121.5.1602. [DOI] [PubMed] [Google Scholar]
- 2. Lewandowski K, Metz J, Deutschmann C, Preiss H, Kuhlen R, Artigas A, et al. Incidence, severity, and mortality of acute respiratory failure in Berlin, Germany. Am J Respir Crit Care Med. 1995;151:1121–1125. doi: 10.1164/ajrccm.151.4.7697241. [DOI] [PubMed] [Google Scholar]
- 3. Prescott HC, Sjoding MW, Langa KM, Iwashyna TJ, McAuley DF. Late mortality after acute hypoxic respiratory failure. Thorax. 2017;73:618–625. doi: 10.1136/thoraxjnl-2017-210109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Brower RG, Matthay MA, Morris A, Schoenfeld D, Thompson BT, Wheeler A. Acute Respiratory Distress Syndrome Network. Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med. 2000;342:1301–1308. doi: 10.1056/NEJM200005043421801. [DOI] [PubMed] [Google Scholar]
- 5. Determann RM, Royakkers A, Wolthuis EK, Vlaar AP, Choi G, Paulus F, et al. Ventilation with lower tidal volumes as compared with conventional tidal volumes for patients without acute lung injury: a preventive randomized controlled trial. Crit Care. 2010;14:R1. doi: 10.1186/cc8230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wilson JG, Matthay MA. Mechanical ventilation in acute hypoxemic respiratory failure: a review of new strategies for the practicing hospitalist. J Hosp Med. 2014;9:469–475. doi: 10.1002/jhm.2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Futier E, Constantin J-M, Paugam-Burtz C, Pascal J, Eurin M, Neuschwander A, et al. IMPROVE Study Group. A trial of intraoperative low-tidal-volume ventilation in abdominal surgery. N Engl J Med. 2013;369:428–437. doi: 10.1056/NEJMoa1301082. [DOI] [PubMed] [Google Scholar]
- 8. Serpa Neto A, Cardoso SO, Manetta JA, Pereira VGM, Espósito DC, Pasqualucci MdO, et al. Association between use of lung-protective ventilation with lower tidal volumes and clinical outcomes among patients without acute respiratory distress syndrome: a meta-analysis. JAMA. 2012;308:1651–1659. doi: 10.1001/jama.2012.13730. [DOI] [PubMed] [Google Scholar]
- 9. Slutsky AS, Ranieri VM. Ventilator-induced lung injury. N Engl J Med. 2013;369:2126–2136. doi: 10.1056/NEJMra1208707. [DOI] [PubMed] [Google Scholar]
- 10. Yoshida T, Fujino Y, Amato MBP, Kavanagh BP. Fifty years of research in ARDS. spontaneous breathing during mechanical ventilation: risks, mechanisms, and management. Am J Respir Crit Care Med. 2017;195:985–992. doi: 10.1164/rccm.201604-0748CP. [DOI] [PubMed] [Google Scholar]
- 11. Feng Y, Amoateng-Adjepong Y, Kaufman D, Gheorghe C, Manthous CA. Age, duration of mechanical ventilation, and outcomes of patients who are critically ill. Chest. 2009;136:759–764. doi: 10.1378/chest.09-0515. [DOI] [PubMed] [Google Scholar]
- 12. Sudarsanam TD, Jeyaseelan L, Thomas K, John G. Predictors of mortality in mechanically ventilated patients. Postgrad Med J. 2005;81:780–783. doi: 10.1136/pgmj.2005.033076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342:1471–1477. doi: 10.1056/NEJM200005183422002. [DOI] [PubMed] [Google Scholar]
- 14. Esteban A, Frutos F, Tobin MJ, Alía I, Solsona JF, Valverdú I, et al. Spanish Lung Failure Collaborative Group. A comparison of four methods of weaning patients from mechanical ventilation. N Engl J Med. 1995;332:345–350. doi: 10.1056/NEJM199502093320601. [DOI] [PubMed] [Google Scholar]
- 15. McConville JF, Kress JP. Weaning patients from the ventilator. N Engl J Med. 2012;367:2233–2239. doi: 10.1056/NEJMra1203367. [DOI] [PubMed] [Google Scholar]
- 16. Epstein SK, Ciubotaru RL, Wong JB. Effect of failed extubation on the outcome of mechanical ventilation. Chest. 1997;112:186–192. doi: 10.1378/chest.112.1.186. [DOI] [PubMed] [Google Scholar]
- 17. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol. 2015;16:448–457. doi: 10.1038/ni.3153. [DOI] [PubMed] [Google Scholar]
- 18. Bajwa EK, Mebazaa A, Januzzi JL. ST2 in pulmonary disease. Am J Cardiol. 2015;115(Suppl):44B–47B. doi: 10.1016/j.amjcard.2015.01.040. [DOI] [PubMed] [Google Scholar]
- 19. Pascual-Figal DA, Pérez-Martínez MT, Asensio-Lopez MC, Sanchez-Más J, García-García ME, Martinez CM, et al. Pulmonary production of soluble ST2 in heart failure. Circ Heart Fail. 2018;11:e005488. doi: 10.1161/CIRCHEARTFAILURE.118.005488. [DOI] [PubMed] [Google Scholar]
- 20. Wiedemann HP, Wheeler AP, Bernard GR, Thompson BT, Hayden D, deBoisblanc B, et al. National Heart, Lung, and Blood Institute Acute Respiratory Distress Syndrome (ARDS) Clinical Trials Network. Comparison of two fluid-management strategies in acute lung injury. N Engl J Med. 2006;354:2564–2575. doi: 10.1056/NEJMoa062200. [DOI] [PubMed] [Google Scholar]
- 21. Bajwa EK, Volk JA, Christiani DC, Harris RS, Matthay MA, Thompson BT, et al. National Heart, Lung and Blood Institute Acute Respiratory Distress Syndrome Network. Prognostic and diagnostic value of plasma soluble suppression of tumorigenicity-2 concentrations in acute respiratory distress syndrome. Crit Care Med. 2013;41:2521–2531. doi: 10.1097/CCM.0b013e3182978f91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alladina JW, Levy SD, Hibbert KA, Januzzi JL, Harris RS, Matthay MA, et al. Plasma concentrations of soluble suppression of tumorigenicity-2 and interleukin-6 are predictive of successful liberation from mechanical ventilation in patients with the acute respiratory distress syndrome. Crit Care Med. 2016;44:1735–1743. doi: 10.1097/CCM.0000000000001814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3:32–35. doi: 10.1002/1097-0142(1950)3:1<32::aid-cncr2820030106>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 24. Zweig MH, Campbell G. Receiver-operating characteristic (ROC) plots: a fundamental evaluation tool in clinical medicine. Clin Chem. 1993;39:561–577. [PubMed] [Google Scholar]
- 25. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, et al. ARDS Definition Task Force. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 26. Blondonnet R, Constantin J-M, Sapin V, Jabaudon M. A pathophysiologic approach to biomarkers in acute respiratory distress syndrome. Dis Markers. 2016;2016:3501373. doi: 10.1155/2016/3501373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. García-Laorden MI, Lorente JA, Flores C, Slutsky AS, Villar J. Biomarkers for the acute respiratory distress syndrome: how to make the diagnosis more precise. Ann Transl Med. 2017;5:283. doi: 10.21037/atm.2017.06.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Spadaro S, Park M, Turrini C, Tunstall T, Thwaites R, Mauri T, et al. Biomarkers for acute respiratory distress syndrome and prospects for personalised medicine. J Inflamm (Lond) 2019;16:1. doi: 10.1186/s12950-018-0202-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. McCarthy CP, Januzzi JL., Jr Soluble ST2 in heart failure. Heart Fail Clin. 2018;14:41–48. doi: 10.1016/j.hfc.2017.08.005. [DOI] [PubMed] [Google Scholar]
- 30. Sabatine MS, Morrow DA, Higgins LJ, MacGillivray C, Guo W, Bode C, et al. Complementary roles for biomarkers of biomechanical strain ST2 and N-terminal prohormone B-type natriuretic peptide in patients with ST-elevation myocardial infarction. Circulation. 2008;117:1936–1944. doi: 10.1161/CIRCULATIONAHA.107.728022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Watanabe M, Takizawa H, Tamura M, Nakajima A, Kurai D, Ishii H, et al. Soluble ST2 as a prognostic marker in community-acquired pneumonia. J Infect. 2015;70:474–482. doi: 10.1016/j.jinf.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 32. Zhao J, Zhao Y. Interleukin-33 and its receptor in pulmonary inflammatory diseases. Crit Rev Immunol. 2015;35:451–461. doi: 10.1615/CritRevImmunol.2016015865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Jawa RS, Anillo S, Huntoon K, Baumann H, Kulaylat M. Analytic review: interleukin-6 in surgery, trauma, and critical care: part I: basic science. J Intensive Care Med. 2011;26:3–12. doi: 10.1177/0885066610395678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Meduri GU, Headley S, Kohler G, Stentz F, Tolley E, Umberger R, et al. Persistent elevation of inflammatory cytokines predicts a poor outcome in ARDS: plasma IL-1 beta and IL-6 levels are consistent and efficient predictors of outcome over time. Chest. 1995;107:1062–1073. doi: 10.1378/chest.107.4.1062. [DOI] [PubMed] [Google Scholar]
- 35. Gurkan OU, He C, Zielinski R, Rabb H, King LS, Dodd-o JM, et al. Interleukin-6 mediates pulmonary vascular permeability in a two-hit model of ventilator-associated lung injury. Exp Lung Res. 2011;37:575–584. doi: 10.3109/01902148.2011.620680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Saito F, Tasaka S, Inoue K, Miyamoto K, Nakano Y, Ogawa Y, et al. Role of interleukin-6 in bleomycin-induced lung inflammatory changes in mice. Am J Respir Cell Mol Biol. 2008;38:566–571. doi: 10.1165/rcmb.2007-0299OC. [DOI] [PubMed] [Google Scholar]
- 37. Calfee CS, Ware LB, Eisner MD, Parsons PE, Thompson BT, Wickersham N, et al. NHLBI ARDS Network. Plasma receptor for advanced glycation end products and clinical outcomes in acute lung injury. Thorax. 2008;63:1083–1089. doi: 10.1136/thx.2008.095588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ware LB, Zhao Z, Koyama T, Brown RM, Semler MW, Janz DR, et al. Derivation and validation of a two-biomarker panel for diagnosis of ARDS in patients with severe traumatic injuries. Trauma Surg Acute Care Open. 2017;2:e000121. doi: 10.1136/tsaco-2017-000121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA. NHLBI ARDS Network. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Figueroa-Casas JB, Connery SM, Montoya R, Dwivedi AK, Lee S. Accuracy of early prediction of duration of mechanical ventilation by intensivists. Ann Am Thorac Soc. 2014;11:182–185. doi: 10.1513/AnnalsATS.201307-222OC. [DOI] [PubMed] [Google Scholar]
- 41. Roca O, Caralt B, Messika J, Samper M, Sztrymf B, Hernández G, et al. An index combining respiratory rate and oxygenation to predict outcome of nasal high-flow therapy. Am J Respir Crit Care Med. 2019;199:1368–1376. doi: 10.1164/rccm.201803-0589OC. [DOI] [PubMed] [Google Scholar]
- 42. Hill NS, Ruthazer R. Predicting outcomes of high-flow nasal cannula for acute respiratory distress syndrome: an index that ROX. Am J Respir Crit Care Med. 2019;199:1300–1302. doi: 10.1164/rccm.201901-0079ED. [DOI] [PMC free article] [PubMed] [Google Scholar]