To the Editor:
Hyperkalemia is common in critically ill patients and is associated with an increased risk of cardiac arrhythmia, cardiac arrest, and death (1). Intravenous fluids affect plasma electrolyte concentrations (2). Saline (0.9% sodium chloride) causes hyperchloremia and metabolic acidosis, which may move potassium from the intracellular space into the interstitial fluid and plasma (3). Balanced crystalloids, such as lactated Ringer’s solution or Plasma-Lyte A, contain 4.0–5.0 mmol/L of potassium—a concentration similar to that of normal human plasma. The theoretical risk of hyperkalemia from supplemental potassium is a frequently cited concern with balanced crystalloids (4). However, balanced crystalloids also contain buffers such as lactate and acetate, which may prevent acidosis-induced potassium shifts (3). To evaluate the effect of fluid composition on the incidence of hyperkalemia and renal replacement therapy (RRT), we performed a secondary analysis of a large, pragmatic trial comparing balanced crystalloids with saline among critically ill adults (2).
Some of the results of this study have been previously reported in the form of an abstract (5).
Methods
Patient populations
SMART (Isotonic Solutions and Major Adverse Renal Events Trial) compared balanced crystalloids versus saline among 15,802 critically ill adults (2). We identified the following two cohorts of patients in SMART at risk of severe hyperkalemia: patients with hyperkalemia at ICU admission and patients with acute kidney injury (AKI) at ICU admission. Hyperkalemia at baseline was defined as a plasma potassium concentration of ≥6.5 mmol/L in the 24 hours before ICU admission (or in the 6 h after ICU admission if no potassium values were available before ICU admission). A potassium concentration of ≥6.5 mmol/L was used to define hyperkalemia in the original SMART and other prior trials of balanced crystalloids (2, 6). AKI at baseline was defined as a plasma creatinine concentration in the 24 hours before ICU admission (or in the 6 h after ICU admission if no creatinine values were available before ICU admission) meeting criteria for stage 2 or greater kidney injury according to the Kidney Disease: Improving Global Outcomes creatinine criteria (7). The method for identifying baseline creatinine and calculating a value when a measured value was unavailable has been previously described (2).
Study outcomes
Outcomes included severe hyperkalemia, defined as a plasma potassium concentration ≥7.0 mmol/L (8), between ICU admission and hospital discharge; highest potassium concentration from ICU admission to hospital discharge; death; new receipt of RRT; and incident AKI by the Kidney Disease: Improving Global Outcomes creatinine criteria. All outcomes were censored at the first of hospital discharge or 30 days. Patients who had received RRT before ICU admission were ineligible to meet criteria for incident AKI or new receipt of RRT but were eligible for other outcomes.
Statistical analysis
Analyses used the same approach to modeling as the original SMART (2). Binary outcomes were analyzed with a generalized, linear, mixed-effects model that included group assignment as a fixed effect and the ICU to which the patient was admitted as a random effect. Continuous outcomes were analyzed using a proportional odds logistic regression model that included group assignment as an independent variable and used robust covariance matrix estimates to adjust for within-ICU correlation. For outcomes that included potassium, models included baseline potassium as a fixed effect.
Results
A total of 254 patients had a baseline potassium concentration of ≥6.5 mmol/L, of whom 67 were excluded for artifactual hyperkalemia from hemolysis, and 187 were included in the analysis (94 in the balanced crystalloids group and 93 in the saline group). Among 15,016 patients in SMART who had not received RRT before ICU admission, 1,324 patients had AKI at baseline (681 in the balanced crystalloid group and 643 in the saline group). The characteristics of patients randomized to the balanced crystalloid and saline groups in each cohort are displayed in Table 1.
Table 1.
Patient Characteristics, Volume of Isotonic Crystalloid, and Outcomes
| Patient Characteristics | Hyperkalemia at Enrollment |
AKI at Enrollment |
||||
|---|---|---|---|---|---|---|
| Balanced (n = 94) | Saline (n = 93) | P Value | Balanced (n = 681) | Saline (n = 643) | P Value | |
| Baseline characteristics | ||||||
| Age, yr | 60 (43.0–70.0) | 55 (44.0–65.0) | 0.21 | 59 (45–69) | 60 (49–69) | 0.51 |
| Sex, M, n (%) | 58 (61.7) | 57 (61.3) | 0.95 | 407 (59.8) | 387 (60.2) | 0.88 |
| Race, white, n (%) | 74 (78.7) | 59 (63.4) | 0.02 | 509 (74.7) | 489 (76.0) | 0.58 |
| Weight, kg | 79.8 (69.9–92.5) | 77.1 (68.0–92.1) | 0.53 | 82.6 (68.0–99.8) | 82.1 (68.9–99.8) | 0.85 |
| CKD or prior RRT,*n (%) | 54 (57.4) | 50 (53.8) | 0.61 | 107 (15.7) | 106 (16.5) | 0.70 |
| CKD | 39 (41.5) | 26 (28.0) | 0.05 | 107 (15.7) | 106 (16.5) | 0.70 |
| Prior RRT | 15 (16.0) | 24 (25.8) | 0.01 | NA† | NA† | |
| Sepsis or septic shock, n (%) | 27 (28.7) | 23 (24.7) | 0.54 | 250 (36.7) | 270 (42.0) | 0.05 |
| Mechanical ventilation, n (%) | 31 (33.0) | 35 (37.6) | 0.51 | 294 (43.2) | 280 (43.5) | 0.89 |
| Vasopressors, n (%) | 25 (26.6) | 27 (29.0) | 0.71 | 286 (42.0) | 291 (45.3) | 0.23 |
| AKI, stage 2 or greaterठ| 33 (41.8) | 35 (50.7) | 0.28 | 681 (100.0) | 643 (100.0) | 1.00 |
| University health consortium expected mortality, meanc ± SD, %‖ | 17.1 ± 26.2 | 17.7 ± 22.9 | 0.83 | 20.4 ± 25.0 | 21.1 ± 24.9 | 0.31 |
| Potassium on ICU admission, mmol/L | 7.0 (6.7–7.4) | 7.0 (6.8–7.5) | 0.61 | 4.5 (3.9–5.3) | 4.5 (3.9–5.1) | 0.27 |
| Bicarbonate on ICU admission, mmol/L | 16.0 (10.0–19.0) | 16.5 (12.2–20.0) | 0.22 | 19.0 (15.0–23.0) | 19.0 (15.0–22.0) | 0.87 |
| Creatinine on ICU admission, mg/dl‡ | 3.3 (2.0–4.9) | 2.7 (1.6–4.1) | 0.29 | 2.5 (1.7–3.7) | 2.4 (1.7–3.4) | 0.52 |
| Volume of intravenous fluid through Day 30¶, mean ± SD, ml | ||||||
| 0.9% sodium chloride | 1,917 ± 3,518 | 3,713 ± 5,741 | <0.001 | 1,260 ± 3,362 | 3,148 ± 4,739 | <0.001 |
| Balanced crystalloid | 2,608 ± 4,046 | 226 ± 855 | <0.001 | 2,755 ± 4,113 | 654 ± 2,314 | <0.001 |
| Total isotonic crystalloid | 4,524 ± 5,913 | 3,939 ± 5,858 | 0.82 | 4,015 ± 5,679 | 3,802 ± 5,570 | 0.12 |
| Receipt of potassium-lowering treatments, n (%) | ||||||
| Kayexalate | 35 (37.2) | 38 (40.9) | 0.61 | NA** | NA** | NA |
| Sodium bicarbonate | 26 (27.7) | 31 (33.3) | 0.4 | NA** | NA** | NA |
| Intravenous insulin | 36 (38.3) | 48 (51.6) | 0.07 | NA** | NA** | NA |
| Clinical outcomes | ||||||
| Severe hyperkalemia (K ≥ 7.0 mmol/L), n (%) | 8 (8.5) | 13 (14) | 0.24 | 3 (0.4) | 9 (1.4) | 0.10 |
| Highest potassium concentration, mmol/L | 6.2 (5.3–6.8) | 6.3 (5.5–6.9) | 0.20 | 5.0 (4.4–5.7) | 5.1 (4.5–5.7) | 0.001 |
| Lowest bicarbonate concentration, mmol/L | 16.0 (12.0–20.0) | 17.0 (13.0–20.0) | 0.84 | 17.0 (14.0–20.0) | 16.0 (13.0–19.0) | <0.001 |
| Stage 2 or greater AKI developing after enrollment, n (%)ठ| 20/79 (25.3) | 29/69 (42.0) | 0.03 | 304 (44.6) | 332 (51.6) | 0.01 |
| New RRT, n (%)‡ | 11/79 (13.9) | 20/69 (29.0) | 0.03 | 97 (14.2) | 125 (19.4) | 0.01 |
| In-hospital mortality, n (%) | 17 (18.1) | 18 (19.4) | 0.82 | 188 (27.6) | 180 (28.0) | 0.88 |
Definition of abbreviations: AKIc = cacute kidney injury; CKDc = cchronic kidney disease (stage 3 or greater) N/A = not applicable; RRTc = crenal replacement therapy.
Continuous data are presented as median (25th percentile–75th percentile) unless otherwise noted.
Chronic kidney disease stage 3 or greater is defined as a glomerular filtration rate of <60 ml/min per 1.73 m2 as calculated by the Chronic Kidney Disease Epidemiology Collaboration equation using the patient’s baseline creatinine concentration.
Patients who had received RRT before admission were excluded from the cohort of patients with AKI on admission.
Patients who had received RRT before ICU admission were excluded from calculations of AKI and creatinine at baseline and were ineligible to meet the clinical outcome criteria for new RRT or new AKI developing after enrollment.
Stage 2 or greater AKI is defined using the Kidney Disease Improving Global Outcomes creatinine criteria (7) as any creatinine value between ICU admission and discharge or 30 days that is 1) increased at least 0.3 mg/dl from a preceding post–ICU admission value and 2) at least 200% of the baseline value, at least 200% of a preceding post–ICU admission value, or at least 4.0 mg/dl; or new receipt of RRT.
The University Health Consortium index is a severity-of-illness score calculated using variables available on hospital admission to provide an estimation of mortality from 0% to 100%.
Cumulative volume of fluid ordered from ICU admission through Days 30, includes fluid ordered both in the ICU and after transfer out of the ICU. Balanced crystalloid includes lactated Ringer’s and Plasma-Lyte A.
Data on potassium-lowering therapies only available among patients with hyperkalemia on ICU admission.
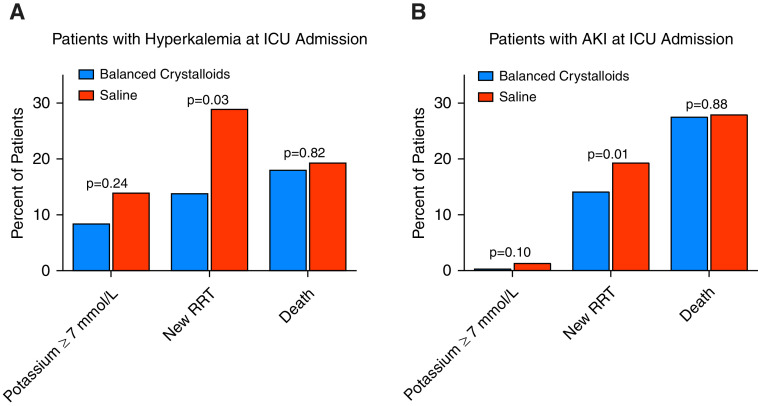
Among patients with hyperkalemia at baseline, eight patients (8.5%) in the balanced crystalloid group and 13 patients (14.0%) in the saline group went on to experience severe hyperkalemia (adjusted odds ratio, 0.57; 95% confidence interval [CI], 0.22–1.46; P = 0.24) (Figure 1 and Table 1). New or worsening AKI (25.3% vs. 42.0%; adjusted odds ratio, 0.47; 95% CI, 0.23–0.94; P = 0.03) and new receipt of RRT (13.9% vs. 29.0%; adjusted odds ratio, 0.40; 95% CI, 0.17–0.90; P = 0.03) occurred less frequently in patients in the balanced crystalloid group compared with those in the saline group (Figure 1 and Table 1). The receipt of potassium-lowering therapies, including sodium polystyrene, insulin/dextrose, sodium bicarbonate, and calcium gluconate/chloride, was similar between the two groups (Table 1).
Figure 1.
Incidence of severe hyperkalemia, receipt of new RRT, and death in the balanced crystalloid group versus saline group among patients with hyperkalemia at ICU admission (A) or AKI at ICU admission (B). Vertical bars represent the overall incidence of the designated outcome in each group. The P value represents the between-group difference in the designated outcome as calculated using a generalized, linear, mixed-effects model that included group assignment as a fixed effect and the ICU to which the patient was admitted as a random effect. For severe hyperkalemia, the model also included baseline potassium as a fixed effect. AKI = acute kidney injury; RRT = renal replacement therapy.
Among patients with AKI at baseline, three patients (0.4%) in the balanced crystalloid group and nine patients (1.4%) in the saline group experienced severe hyperkalemia (adjusted odds ratio, 0.33; 95% CI, 0.09–1.25; P = 0.10) (Figure 1 and Table 1). A total of 97 patients (14.2%) in the balanced crystalloid group received new RRT compared with 125 patients (19.4%) in the saline group (adjusted odds ratio, 0.69; 95% CI, 0.51–0.92; P = 0.01) (Figure 1 and Table 1).
Results were similar in sensitivity analyses that used alternative definitions of hyperkalemia at ICU admission. Of the 491 patients with a potassium concentration of ≥5.5 mmol/L in the balanced crystalloid group, a total of 23 (4.7%) experienced severe hyperkalemia, compared with 22 of 455 (4.8%) in the saline group. Of the 258 patients with a potassium concentration of ≥6.0 mmol/L in the balanced crystalloid group, a total of 16 (6.2%) experienced severe hyperkalemia, compared with 16 of 242 (6.6%) in the saline group.
Discussion
In this secondary analysis of a clinical trial, the use of balanced crystalloids was not associated with an increased incidence of severe hyperkalemia compared with saline and was associated with a significantly lower incidence of RRT among patients with hyperkalemia at ICU admission and patients with AKI at ICU admission. These results are consistent with randomized trials among patients undergoing renal transplantation, in whom the administration of balanced crystalloids resulted in similar or lower rates of hyperkalemia compared with saline (9).
Patients with hyperkalemia at ICU admission received a mean of approximately 4 L of isotonic crystalloid during their critical illness. This volume of balanced crystalloid solution contains 16–20 mmol of potassium, or approximately 0.5% of total body potassium for a 70-kg person (10). Our results suggest that the acid–base effects of isotonic crystalloids are more important for potassium homeostasis than the relatively small amount of potassium in these fluids.
Our study has several limitations, including conduct at a single center, lack of blinding, initiation of RRT at the discretion of the treating clinicians, and limited power for subgroup analyses among patients with hyperkalemia and AKI. Based on prior guidelines (8), our analysis defined severe hyperkalemia as a serum potassium ≥7.0 mmol/L, but there is no standard definition for severe hyperkalemia, and the threshold at which complications from hyperkalemia may occur is likely to vary on the basis of patient characteristics. Because the analysis focused on a subgroup of patients from SMART identified by laboratory values obtained after presentation, group assignment is not protected by randomization, and imbalances in baseline characteristics could contribute to observed differences in outcomes. However, baseline demographics, severity of illness, and potassium concentrations were similar between groups. Saline administration in the balanced crystalloid group was more common in this secondary analysis of patients with hyperkalemia than in SMART overall. Such crossover, however, would bias our results toward the null hypothesis and would not explain a numerically lower incidence of hyperkalemia and new RRT in the balanced crystalloid group.
In conclusion, in this secondary analysis of patients with hyperkalemia or AKI in a large randomized trial, use of balanced crystalloids was not associated with a higher incidence of severe hyperkalemia and appeared to be associated with a lower incidence of new RRT.
Footnotes
Supported in part by Vanderbilt Clinical and Translational Science Award grant UL1TR002243 from the National Center for Advancing Translational Sciences/NIH. J.D.C. was supported in part by the NIH (K12HL133117 and K23HL153584). M.W.S. was supported in part by the NHLBI (K12HL133117 and K23HL143053). T.W.R. was supported in part by the NIH (R34HL105869). E.D.S. was supported in this work by the Vanderbilt Center for Kidney Diseases. Data collection used the Research Electronic Data Capture tool developed and maintained with Vanderbilt Institute for Clinical and Translational Research grant support (UL1 TR000445 from the National Center for Advancing Translational Sciences/NIH).
Authors Contributions: Study concept and design: A.H.T., M.W.S., T.W.R., and J.D.C. Acquisition of data: A.H.T., M.W.S., J.P.W., and J.D.C.; Analysis and interpretation of data: A.H.T., M.W.S., L.W., and J.D.C. Drafting of the manuscript: A.H.T., M.W.S., and J.D.C. Critical revision of the manuscript for important intellectual content: A.H.T., M.W.S., W.H.S., G.R.B., L.W., E.D.S., J.L.S., J.P.W., T.W.R., and J.D.C.
Originally Published in Press as DOI: 10.1164/rccm.202011-4122LE on January 27, 2021
Author disclosures are available with the text of this letter at www.atsjournals.org.
Contributor Information
Collaborators: for the SMART Investigators and the Pragmatic Critical Care Research Group
References
- 1. Goyal A, Spertus JA, Gosch K, Venkitachalam L, Jones PG, Van den Berghe G, et al. Serum potassium levels and mortality in acute myocardial infarction. JAMA. 2012;307:157–164. doi: 10.1001/jama.2011.1967. [DOI] [PubMed] [Google Scholar]
- 2. Semler MW, Self WH, Rice TW. Balanced crystalloids versus saline in critically ill adults. N Engl J Med. 2018;378:1951. doi: 10.1056/NEJMc1804294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Pfortmueller CA, Fleischmann E. Acetate-buffered crystalloid fluids: current knowledge, a systematic review. J Crit Care. 2016;35:96–104. doi: 10.1016/j.jcrc.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 4. O’Malley CMN, Frumento RJ, Bennett-Guerrero E. Intravenous fluid therapy in renal transplant recipients: results of a US survey. Transplant Proc. 2002;34:3142–3145. doi: 10.1016/s0041-1345(02)03593-5. [DOI] [PubMed] [Google Scholar]
- 5. Toporek AH, Casey JD, Siew ED, Rice TW, Semler MW. Effects of balanced crystalloid versus saline in patients with hyperkalemia, a retrospective analysis of the SMART trial [abstract] Am J Respir Crit Care Med. 2019;199:A5991. [Google Scholar]
- 6. Young P, Bailey M, Beasley R, Henderson S, Mackle D, McArthur C, et al. SPLIT Investigators; ANZICS CTG. Effect of a buffered crystalloid solution vs saline on acute kidney injury among patients in the intensive care unit: the SPLIT randomized clinical trial. JAMA. 2015;314:1701–1710. doi: 10.1001/jama.2015.12334. [DOI] [PubMed] [Google Scholar]
- 7. Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120:c179–c184. doi: 10.1159/000339789. [DOI] [PubMed] [Google Scholar]
- 8. American Heart Association. Part 8: advanced challenges in resuscitation. Section 1: life-threatening electrolyte abnormalities. European Resuscitation Council. Resuscitation . 2000;46:2530–259. [PubMed] [Google Scholar]
- 9. Potura E, Lindner G, Biesenbach P, Funk G-C, Reiterer C, Kabon B, et al. An acetate-buffered balanced crystalloid versus 0.9% saline in patients with end-stage renal disease undergoing cadaveric renal transplantation: a prospective randomized controlled trial. Anesth Analg. 2015;120:123–129. doi: 10.1213/ANE.0000000000000419. [DOI] [PubMed] [Google Scholar]
- 10. Palmer BF, Clegg DJ. Physiology and pathophysiology of potassium homeostasis. Adv Physiol Educ. 2016;40:480–490. doi: 10.1152/advan.00121.2016. [DOI] [PubMed] [Google Scholar]