Abstract
Oxidized low-density lipoprotein (ox-LDL) induces endothelial cell apoptosis and dysfunction. Statins are drugs that are clinically used to lower serum cholesterol levels, and they have been shown to exert vascular protective effects. In the present study, human umbilical vein endothelial cells were transfected with scramble control siRNA or siRNA specific for glutathione peroxidase (GPx)4 or cystine-glutamate antiporter (xCT). MTT, Matrigel and Transwell assays were used to evaluate cell proliferation, tube formation and migration, respectively. The levels of TNF-α, IL-α, 4-hydroxynonenal, GPx4 and xCT expression were detected by western blot analysis. It was demonstrated that ox-LDL promoted cytokine production and reduced the proliferation, migration and angiogenesis of endothelial cells. It was also observed that ox-LDL decreased GPx4 and xCT expression and induced ferroptosis. Furthermore, the inhibition of ferroptosis by deferoxamine mesylate attenuated ox-LDL-induced endothelial cell dysfunction and restored ox-LDL-decreased GPx4 and xCT expression. Consistent with these results, GPx4 and xCT knockdown by siRNA transfection aggravated ox-LDL-induced endothelial cell dysfunction and inhibition of proliferation. To the best of our knowledge, the present study was the first to discover that fluvastatin may protect endothelial cells from ox-LDL-induced ferroptosis and dysfunction. Furthermore, knockdown of GPx4 and xCT expression blunted the protective effects of fluvastatin on ox-LDL-treated endothelial cells. These data indicated a novel function of fluvastatin in the protection of endothelial cells from ox-LDL-induced ferroptosis, the mechanism of which involves the regulation of GPx4 and xCT.
Keywords: oxidized low-density lipoprotein, ferroptosis, fluvastatin
Introduction
The endothelium, which is an important part of the vasculature, forms the inner cell lining of all blood and lymphatic vessels in the body (1,2). Furthermore, mounting evidence suggests that endothelial dysfunction contributes to the development of various diseases, including cancers and cardiovascular diseases (3-5). Endothelial function is known to be impaired in hyperlipidemic patients (6), and elevated levels of plasma lipids, particularly oxidized low-density lipoprotein (ox-LDL), have been shown to induce endothelial injuries, such as senescence and cell death, resulting in endothelial dysfunction (7). The mechanisms underlying ox-LDL-induced endothelial injury have been reported in several studies. In endothelial cells, ox-LDL primarily signals through lectin-like ox-LDL receptor 1 (LOX-1) (8). The binding of ox-LDL to LOX-1 upregulates cytoplasmic adaptor protein TRAF3IP2 expression, which further activates downstream signaling pathway of IKK/NF-κB p65, resulting in apoptotic cell death (7,9,10).
Ferroptosis is a newly identified form of non-apoptotic cell death (11) characterized by the iron-dependent accumulation of lipid peroxides. Glutathione peroxidase (GPx)4, one of the GPxs present in mammals, has been shown to play an important role in reducing lipid peroxide levels (12). Wortmann et al (13) observed that the loss of endothelial GPx4 combined with limited access to vitamin E induces endothelial cell death in multiple organs and promotes thrombus formation. Although ferroptosis has been suggested as a possible pathway that mediates endothelial cell death and dysfunction, whether ox-LDL plays a pathophysiological role in endothelial cell ferroptosis has not been elucidated.
Statins, which are inhibitors of HMG-CoA reductase, have beneficial effects in reducing serum cholesterol levels (14). The results of recent studies suggest that statins exert pleiotropic effects apart from their serum lipid-lowering effects. One of the most extensively investigated cholesterol-independent functions of statins is cardiovascular protection (15), where the specific cardiovascular protective effects of statins are dose-dependent. Low doses of statins promote angiogenesis through activation of AKT, while high doses may exert anti-angiogenic effects and therefore inhibit tumor growth (16,17). Moreover, statins have been reported as inducers or sensitizers of ferroptosis in various cancer cells, including melanoma, colorectal and prostate cancer cells (18). However, whether statins have any effect on endothelial cell ferroptosis remains unclear, and there is no experimental evidence demonstrating a link between statins and the development of endothelial cell ferroptosis.
The present study investigated whether ox-LDL induces human endothelial cell ferroptosis and whether this effect is mediated through affecting the expression of GPx4 and cystine-glutamate antiporter (xCT) as well as the migration and angiogenesis of human endothelial cells. It was also investigated whether deferoxamine mesylate (DFOM) treatment could inhibit ferroptosis by reversing the effects of ox-LDL on the migration and angiogenesis of human endothelial cells and the expression of GPx4 and xCT. Furthermore, the protective effects of fluvastatin against ox-LDL-induced ferroptosis were investigated, hoping to uncover a novel function for fluvastatin in attenuating ox-LDL-induced ferroptosis and improving endothelial cell dysfunction.
Materials and methods
Cell culture
Human umbilical vein cells (HUVECs) were purchased from ScienCell Research Laboratory and cultured in endothelial culture medium [C-22010; Miaotong (Shanghai) Biotech Co., Ltd.] at 37˚C in a humidified incubator with 5% CO2. HUVECs cultured for 3-6 passages were used in the present study.
Tube formation assay
Before tube formation assays, HUVECs transfected with Scramble control siRNA or siRNA specific for GPx4 or xCT (JTS Scientific Ltd.) along with the transfection reagents of DharmaFECT-3 (DharmaconFECT3; T-2003; Thermo Fisher Scientific, Inc.) in reduced-serum medium (Gibco; Thermo Fisher Scientific, Inc.; 31985070) were transfected and incubated for 24 h in accordance with the manufacturer's protocol. HUVECs were incubated with reagents containing scramble control siRNA or siRNA specific for GPx4 or xCT at 37°C in 5% CO2 for 24 h. The sequences were as follows: xCT siRNA-1, forward, 5'-GGAGGUCAUUACACAUAUATT-3' and reverse, 5'-UAUAUGUGUAAUGACCUCCTT-3'; xCT siRNA-2 forward, 5'-GCCUACUUUACGACCAUUATT-3' and reverse, 5'-UAAUGGUCGUAAAGUAGGCTT-3'; xCT siRNA-3 forward, 5'-CACCCUUUGACAAUGAUAATT-3' and reverse, 5'-UUAUCAUUGUCAAAGGGUGTT-3'; GPX4 siRNA-1 forward, 5'-CAGGGAGUAACGAAGAGAUTT-3' and reverse, 5'-AUCUCUUCGUUACUCCCUGTT-3'; GPX4 siRNA-2 forward, 5'-GACCGAAGUAAACUACACUTT-3' and reverse, 5'-AGUGUAGUUUACUUCGGUCTT-3'; GPX4 siRNA-3 forward, 5'-UGGUGAUAGAGAAGGACCUTT-3' and reverse, 5'-AGGUCCUUCUCUAUCACCATT-3'; scramble control siRNA, forward, 5'-UUCUCCGAACGUGUCACGUTT-3' and reverse, 5'-ACGUGACACGUUCGGAGAATT-3'; These siRNA were all purchased from JTS Scientific Ltd. The transfection efficiencies of siRNA were estimated by RT-PCR after 24 h and Western blotting after 48 h. The effective siRNA delivery was >90% knockdown. the mRNA expression of GPX4/xCT was reduced to <10% of its previous level after transfecting GPX4/xCT siRNA for 24 h. Additionally, the protein level of GPX4/xCT was reduced to <10% of its previous level after transfecting GPX4/xCT siRNA for 48 h. GPX4 siRNA-1 and xCT siRNA-2 have the appropriate transfection efficiency and were carefully selected as the candidates. The mRNA expression of GPX4 was reduced to 5% of its previous level after transfecting GPX4 siRNA-1 for 24 h. Furthermore, the protein level of GPX4 was reduced to 7% of its previous level after transfecting GPX4 siRNA-1 for 48 h. Similarly, the mRNA expression of xCT was reduced to 4% of its previous level after transfecting xCT siRNA-2 for 24 h. Finally, the protein level of xCT reduced to ~7% of its previous level after transfecting xCT siRNA-2 for 48 h.
Then, 4x105 HUVECs/well were seeded in a 48-well plate that was pre-coated at 37°C for 12 h with growth factor-reduced Matrigel (BD Biosciences; 354230). The cells were assayed 24 h after incubation, with images acquired randomly from three different fields per well using microscope [SOPTOP EX21; infinity color corrected optical system with light splitting ratio R: T=8:2; Sunny Optical Technology (Group) Company Limited; magnification, x100]. The level of tube formation was analyzed using ImageJ software 1.46r (National Institutes of Health). HUVEs were divided into the following groups: i) Control; ii) ox-LDL; iii) si-GPx4 + ox-LDL; iv) si-GPx4 + ox-LDL + DFOM; v) si-xCT + ox-LDL; vi) si-xCT + ox-LDL + DFOM. Each group contained 8 wells and each experiment was repeated 5 times. In separate experiments, HUVECs were seeded in Matrigel with 100 µg/ml of ox-LDL or 125 µM DFOM. Tube formation was observed after 24 h of incubation. In the fluvastatin rescue experiment, HUVECs were seeded in Matrigel supplemented with 2.5,5 or 10 µmol/l fluvastatin. Tube formation was observed after 24 h of incubation.
MTT assay
Cell viability was assessed using the MTT assay. Briefly, HUVECs were seeded in 96-well plates at a density of 4x105 cells per well and were incubated at 37°C for 48 h. After being treated with ox-LDL, DFOM, or fluvastatin for 24 h, 20 µl of 5 mg/ml MTT (Sangon Biotech Co., Ltd.) was added to each well. Then, after incubating at 37˚C for 4 h, 100 µl of dimethyl sulfoxide was added and mixed thoroughly to dissolve the formazan crystals, after which time the absorbance was measured at 490 nm using a microplate reader (Awareness Technologies, Inc.; Stat 2100).
Transwell migration assay
Cell migration was evaluated using the Transwell migration assay. Briefly, HUVECs were seeded at 2x105 cells per well in the upper chamber of the Transwell plate with DMEM (Jianshunbio; 66001-077) containing 1% FBS (Shanghai ExCell Biology, Inc.; FCS500), and the lower chamber was filled with endothelial culture medium(MT-Bio, C-22010) with 20% fetal bovine serum (Excell Bio, FCS500). After incubating for 24 h, the non-migrated cells were removed from the top of the filter using cotton swabs, and cells that migrated to the bottom were fixed in 4% paraformaldehyde at room temperature for 15 min on a mild shaker (RCK 2D 200C; Dam industry Co., Ltd.). After three washes with PBS on the mild shaker, cells were stained with 0.01% crystal violet (Hzhxbio; HCY124) and incubated at room temperature for 20 min. Cells were then counted using microscope [SOPTOP EX21; infinity color corrected optical system with light splitting ratio R:T=8:2; Sunny Optical Technology (Group) Company Limited; magnification, x100]. As mentioned above, each group contains 8 wells and the experiment was repeated for 5 times.
Western blot analysis
Total protein from treated HUVECs was isolated using RIPA lysis buffer (Beyotime Institute of Biotechnology, P0013) and the protein concentration was determined with a Bio-Rad Protein Assay kit (Bio-Rad Laboratories, Inc.). Then, equal amounts of protein (50 µg) were separated by 12% SDS-PAGE. The separated proteins were then transferred to PVDF membranes (Merck KGaA, Germany) and blocked for 1 h at room temperature in PBS with 0.05% Tween-20 containing 3% non-fat powdered milk. The membranes were then incubated with primary antibodies overnight at 4˚C, followed by an incubation with HRP-conjugated Goat Anti-rabbit IgG secondary antibodies (1:3,000; A0181; Beyotime Institute of Biotechnology) at room temperature for 2 h. Subsequently, the protein bands were visualized with an enhanced chemiluminescence detection system (Amersham Cytiva, RPN2232). The following primary antibodies were used: 4-hydroxynonenal (4-HNE; 1:2,000; ab46544; Abcam), TNF-α (1:500; WL01581; Wanleibio; Co., Ltd.), IL-1α (1:2,000; ab134908; Abcam), GPx4 (1:1,000; 14432-1-AP; ProteinTech Group, Inc.), xCT (1:1,000; 26864-1-AP; ProteinTech Group, Inc.); Caspase-3/cleaved-caspase3 (1:500; WL02117; Wanleibio; Co., Ltd.), Bax (1:1,000; WL01637; Wanleibio; Co., Ltd.), Bcl-2 (1:1,000; WL01556; Wanleibio; Co., Ltd.), β-actin (1:2,000; WL01845 Wanleibio; Co., Ltd.).
Statistical analysis
All quantitative data are presented as the means ± standard deviation (SD). Statistical analyses were performed using SPSS 22.0 (IBM Corp.) using the unpaired Student's t-test or one-way ANOVA followed by a Fisher's post hoc comparison test. Two-sided tests were used throughout this study, and a P<0.05 was considered to indicate statistically significant difference.
Results
Ox-LDL promotes endothelial dysfunction by induction of ferroptosis
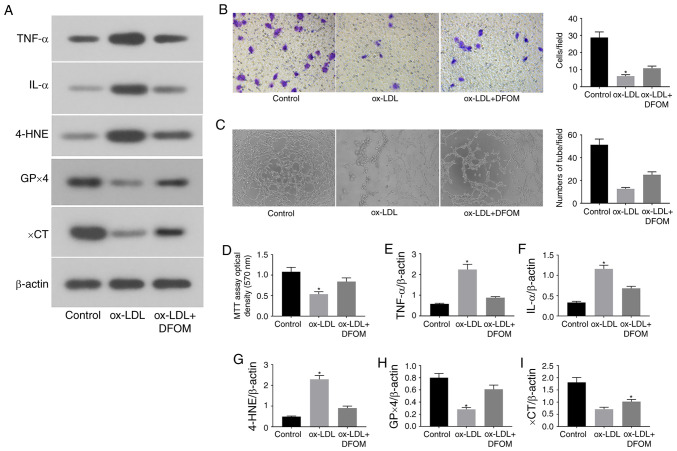
Accumulating evidence has demonstrated that endothelial dysfunction is a crucial and early step in the development of cardiovascular diseases, including atherosclerosis (5). Increased local and systemic levels of ox-LDL have been shown to induce endothelial injury and contribute to the development and progression of atherosclerosis (19). Wortmann et al observed that GPx4 deficiency combined with a vitamin E-depleted diet caused vascular dysfunction and early death in mice (13). The present study investigated whether ox-LDL-induced endothelial cell ferroptosis occurs through the regulation of GPx4 activity (Fig. 1). It was observed that treatment with ox-LDL (100 µg/ml) for 24 h significantly induced the expression of pro-inflammatory cytokines, including TNF-α and IL-1α (Fig. 1A, E and F). In addition, the migration and angiogenesis of human endothelial cells were examined after ox-LDL treatment. The data revealed that ox-LDL strongly decreased cell migration in Transwell assays (Fig. 1B) and disrupted tube formation in vitro (Fig. 1C), indicating that ox-LDL induces endothelial cell dysfunction.
Figure 1.
ox-LDL promotes endothelial cell dysfunction via induction of ferroptosis. (A) Immunoblots of TNF-α, IL-α, 4-HNE, GPx4 and xCT in the normal (control), ox-LDL and ox-LDL + DFOM groups. (B) Representative Transwell migration assay images of HUVECs treated with ox-LDL and ox-LDL + DFOM (magnification, x10). (C) Representative tube formation assay images of HUVECs treated with PBS, 100 µg/ml ox-LDL and 125 µM DFOM (magnification, x10). (D) Analysis of HUVEC viability using the MTT assay after ox-LDL treatment. Columns, means of three experiments; bars, SD. Histograms of (E) relative TNF-α/β-actin levels, (F) relative IL-α/β-actin levels, (G) relative 4-HNE/β-actin levels, (H) relative GPx4/β-actin levels and (I) relative xCT/β-actin levels. *P<0.05 vs. control group. ox-LDL, oxidized low-density lipoprotein; GPx4, glutathione peroxidase 4; xCT, cystine-glutamate antiporter; 4-HNE, 4-hydroxynonenal; DFOM, deferoxamine mesylate; HUVEC, human umbilical vein cell.
To further assess whether ox-LDL-induced endothelial dysfunction is mediated by ferroptosis, the expression of central ferroptosis regulators, including GPx4, xCT and 4-HNE, was evaluated after ox-LDL treatment. ox-LDL markedly reduced the expression of GPx4 and xCT, while increasing the expression of 4-HNE in human endothelial cells (Fig. 1A and G-I). Subsequently, the proliferation of human endothelial cells was assessed by MTT assay. As shown in Fig. 1D, ox-LDL reduced the proliferation of endothelial cells by 49.3%. DFOM, an iron chelator, has been shown to reduce unbound iron levels and inhibit the production of ROS and the occurrence of ferroptosis (11). It was observed that pre-treatment with DFOM (125 µM) increased the expression of GPx4 and xCT, while decreasing that of 4-HNE after ox-LDL treatment compared to that observed in the vehicle control (Fig. 1A and G-I). In addition, the ox-LDL-mediated inhibition of endothelial cell proliferation was also rescued by DFOM (Fig. 1D), indicating that DFOM inhibits ox-LDL-induced ferroptosis in human endothelial cells. The data further demonstrated that inhibition of ferroptosis by DFOM reduced ox-LDL-induced pro-inflammatory cytokine production (Fig. 1A, E and F) and increased ox-LDL-inhibited endothelial cell migration (Fig. 1B) and angiogenesis (Fig. 1C).
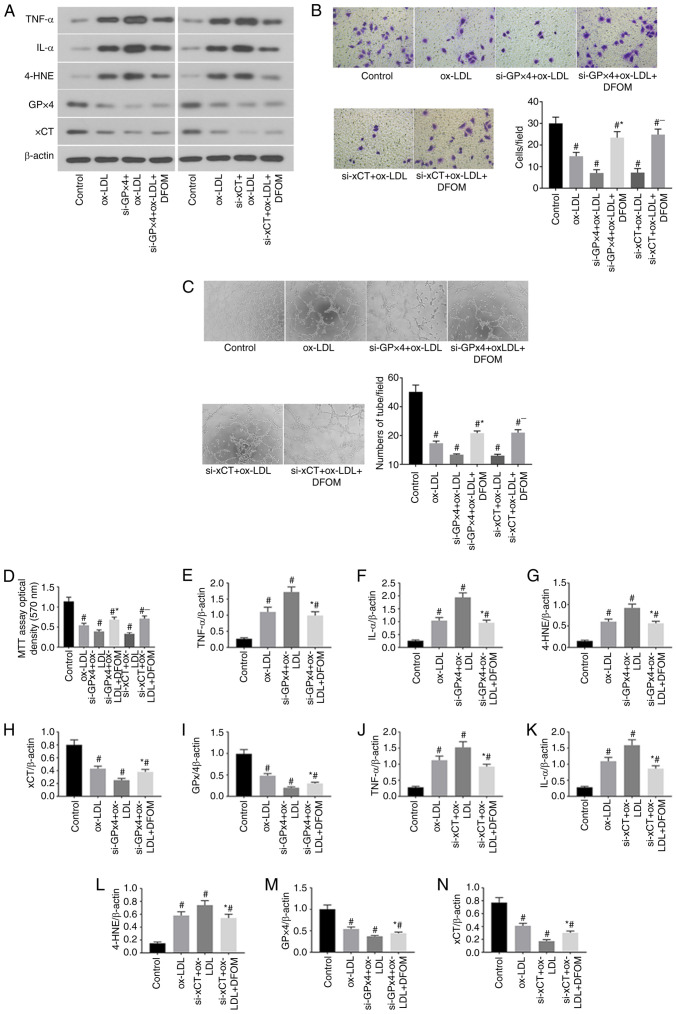
RNAi-mediated GPx4 and xCT knockdown (KD) promotes ox-LDL-induced ferroptosis in human endothelial cells
Previous studies have demonstrated that inhibition of GPx4 and xCT activity induces ferroptosis in cancer cells (20,21). The data of the present study demonstrated that ox-LDL significantly reduced CPx4 and xCT expression and induced ferroptosis in endothelial cells (Fig. 1A). To further confirm the role of GPx4 and xCT in the ox-LDL-mediated induction of endothelial cell ferroptosis, GPx4 and xCT expression was knocked down by siRNA transfection in human endothelial cells (Fig. 2). The results presented in Fig. 2A show successful Knockdown (KD) of GPx4 and xCT in human endothelial cells. It was observed that KD of either GPx4 or xCT increased pro-inflammatory cytokine production after ox-LDL treatment in endothelial cells compared to that observed in endothelial cells transfected with scramble control siRNA (Fig. 2A, E and F). Cell proliferation was further reduced after KD of either GPx4 or xCT in ox-LDL-treated endothelial cells (Fig. 2D). Additionally, decreased endothelial cell migration (Fig. 2B) and tube formation (Fig. 2C) were observed in ox-LDL-treated endothelial cells after KD of either GPx4 or xCT. Interestingly, the application of DFOM (125 µM) attenuated the increased the production of pro-inflammatory cytokines (Fig. 2A, E and F) and decreased cell proliferation (Fig. 2D), migration (Fig. 2B) and angiogenesis (Fig. 2C) after KD of GPx4 or xCT in ox-LDL-treated endothelial cells. These data demonstrate that ox-LDL-induced endothelial cell ferroptosis is mediated by GPx4 and xCT activity.
Figure 2.
RNAi-mediated GPx4 and xCT knockdown promotes ox-LDL-induced ferroptosis in human endothelial cells. (A) Immunoblots of TNF-α, IL-α, 4-HNE, GPx4 and xCT in the normal (control), ox-LDL, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + DFOM, si-xCT + ox-LDL and si-xCT + ox-LDL + DFOM treatment groups. (B) Representative Transwell migration assay images of HUVECs treated with ox-LDL, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + DFOM, si-xCT + ox-LDL and si-xCT + ox-LDL + DFOM (magnification, x10). #P<0.05 vs. control group; *P<0.05 vs. si-GPx4 + ox-LDL; -P<0.05 vs. si-xCT + ox-LDL. (C) Representative images showing HUVEC tube formation in the presence of PBS (control) or 100 µg/ml ox-LDL,si-GPx4 + 100 µg/ml ox-LDL, si-GPx4 + 100 µg/ml ox-LDL + 125 µM DFOM, si-xCT + 100 µg/ml ox-LDL or si-xCT + 100 µg/ml ox-LDL + 125 µM DFOM (all magnifications, x10). #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL, -P<0.05 vs. si-xCT + ox-LDL. (D) Analysis of cell viability using the MTT assay in HUVECs after ox-LDL, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + DFOM, si-xCT + ox-LDL and si-xCT + ox-LDL + DFOM treatments. Columns, mean of three experiments; bars, SD. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL, -P<0.05 vs. si-xCT + ox-LDL. (E) Histogram of relative TNF-α/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL. (F) Histogram of relative IL-α/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL. (G) Histogram of relative 4-HNE/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL. (H) Histogram of relative xCT/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL. (I) Histogram of relative GPx4/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-GPx4 + ox-LDL. (J) Histogram of relative TNF-α/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-xCT + ox-LDL. (K) Histogram of relative IL-α/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-xCT + ox-LDL. (L) Histogram of relative 4-HNE/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-xCT + ox-LDL. (M) Histogram of relative GPx4/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-xCT + ox-LDL. (N) Histogram of relative xCT/β-actin levels. #P<0.05 vs. control group, *P<0.05 vs. si-xCT + ox-LDL. ox-LDL, oxidized low-density lipoprotein; GPx4, glutathione peroxidase 4; xCT, cystine-glutamate antiporter; 4-HNE, 4-hydroxynonenal; DFOM, deferoxamine mesylate; HUVEC, human umbilical vein cell.
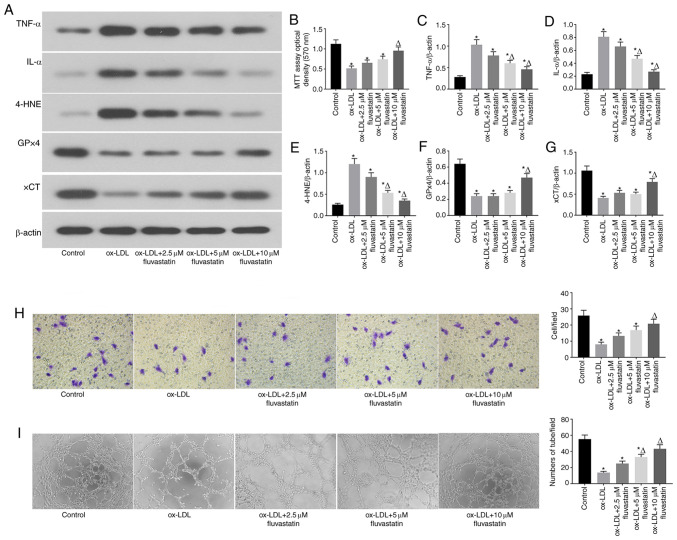
Fluvastatin attenuates ox-LDL-induced endothelial cell dysfunction and ferroptosis
The results of previous studies revealed that fluvastatin can reverse endothelial dysfunction in arthritis (22). To investigate whether fluvastatin could rescue ox-LDL-induced ferroptosis in endothelial cells, ox-LDL-induced human endothelial cells were treated with different concentrations of fluvastatin (Fig. 3). It was observed that fluvastatin ameliorated the ox-LDL-mediated decrease in GPx4 and xCT expression and the increased 4-HNE expression in a dose-dependent manner (Fig. 3A, E, F and G). Fluvastatin also restored ox-LDL-inhibited cell proliferation (Fig. 3B) and lowered the ox-LDL-induced increase in inflammatory cytokine levels (Fig. 3C and D), suggesting that fluvastatin attenuated ox-LDL-induced endothelial ferroptosis. Of note, fluvastatin treatment rescued the ox-LDL-mediated inhibition of endothelial cell migration (Fig. 3H) and angiogenesis (Fig. 3I). Taken together, these data indicated that fluvastatin may normalized ox-LDL-induced endothelial dysfunction via inhibition of ferroptosis.
Figure 3.
Fluvastatin attenuates ox-LDL-induced endothelial cell dysfunction and ferroptosis. (A) Immunoblots of TNF-α, IL-α, 4-HNE, GPx4 and xCT in the normal, ox-LDL, ox-LDL + 2.5 µM fluvastatin, ox-LDL + 5 µM fluvastatin, ox-LDL + 10 µM fluvastatin treatment groups. (B) Analysis of cell viability using the MTT assay in HUVECs after ox-LDL, ox-LDL + 2.5 µM fluvastatin, ox-LDL + 5 µM fluvastatin and ox-LDL + 10 µM fluvastatin treatments. Columns, means of three experiments; bars, SD. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (C) Histogram of relative TNF-α/β-actin levels. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (D) Histogram of relative IL-α/β-actin levels. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (E) Histogram of relative 4-HNE/β-actin levels. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (F) Histogram of relative GPx4/β-actin levels. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (G) Histogram of relative xCT/β-actin levels.*P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. (H) Representative Transwell migration assay images of HUVECs treated with ox-LDL, ox-LDL + 2.5 µM fluvastatin, ox-LDL + 5 µM fluvastatin, or ox-LDL + 10 µM fluvastatin. *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group (magnification, 100x). (I) Representative tube formation assay images of HUVEC in the presence of PBS (control) or with 100 µg/ml ox-LDL, 100 µg/ml ox-LDL + 2.5 µM fluvastatin, 100 µg/ml ox-LDL + 5 µM fluvastatin, or 100 µg/ml ox-LDL + 10 µM fluvastatin (magnification, 100x). *P<0.05 vs. control group, ΔP<0.05 vs. ox-LDL group. ox-LDL, oxidized low-density lipoprotein; GPx4, glutathione peroxidase 4; xCT, cystine-glutamate antiporter; 4-HNE, 4-hydroxynonenal; HUVEC, human umbilical vein cell.
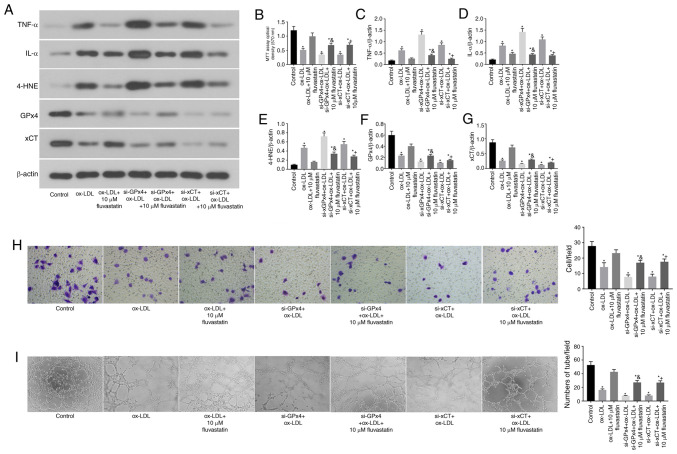
GPx4 and xCT are required for the fluvastatin-mediated attenuation of ferroptosis in human endothelial cells
Subsequently, it was assessed whether the fluvastatin-mediated attenuation of ferroptosis in ox-LDL-treated endothelial cells requires GPx4 and xCT (Fig. 4). To this end, GPx4 and xCT were knocked down in endothelial cells by transfection with specific siRNA (cells transfected with scramble siRNA were used as controls). It was observed that KD of either GPx4 or xCT partially blocked the fluvastatin-mediated attenuation of pro-inflammatory cytokine production in ox-LDL-treated endothelial cells (Fig. 4A, C and D). The KD of endothelial GPx4 or xCT also attenuated the protective effects of fluvastatin on cell proliferation (Fig. 4B), migration (Fig. 4H) and angiogenesis (Fig. 4I).
Figure 4.
GPx4 and xCT are required for the fluvastatin-mediated attenuation of ferroptosis in human endothelial cells. (A) Immunoblots of TNF-α, IL-α, 4-HNE, GPx4 and xCT in the normal, ox-LDL, ox-LDL + 10 µM fluvastatin, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + 10 µM fluvastatin, si-xCT + ox-LDL and si-xCT + ox-LDL + 10 µM fluvastatin treatment groups. (B) Analysis of cell viability using the MTT assay in HUVECs after ox-LDL, ox-LDL + 10 µM fluvastatin, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + 10 µM fluvastatin, si-xCT + ox-LDL or si-xCT + ox-LDL + 10 µM fluvastatin treatments. Columns, means of three experiments; bars, SD. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (C) Histogram of relative TNF-α/β-actin levels. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (D) Histogram of relative IL-α/β-actin levels. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (E) Histogram of relative 4-HNE/β-actin levels. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (F) Histogram of relative GPx4/β-actin levels. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (G) Histogram of relative xCT/β-actin levels. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. (H) Representative Transwell migration assay images of HUVECs treated with ox-LDL, ox-LDL + 10 µM fluvastatin, si-GPx4 + ox-LDL, si-GPx4 + ox-LDL + 10 µM fluvastatin, si-xCT + ox-LDL and si-xCT + ox-LDL + 10 µM fluvastatin. *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL (magnification, 100x). (I) Representative tube formation assay images of HUVECs in the presence of PBS or with 100 µg/ml ox-LDL, 100 µg/ml ox-LDL + 10 µM fluvastatin, si-GPx4 + 100 µg/ml ox-LDL, si-GPx4 + 100 µg/ml ox-LDL + 10 µM fluvastatin, si-xCT + 100 µg/ml ox-LDL or si-xCT + 100 µg/ml ox-LDL + 10 µM fluvastatin (magnification, 100x). *P<0.05 vs. control group, &P<0.05 vs. si-GPx4 + ox-LDL, +P<0.05 vs. si-xCT + ox-LDL. ox-LDL, oxidized low-density lipoprotein; GPx4, glutathione peroxidase 4; xCT, cystine-glutamate antiporter; 4-HNE, 4-hydroxynonenal; HUVEC, human umbilical vein cell.
Discussion
The endothelium is one of the largest organs in the body by area (23). Furthermore, the endothelium interacts with nearly every organ and system in the body, and dysregulation of endothelial function is implicated in a diverse range of diseases, including cancer, stroke and cardiovascular diseases (24-26). Moreover, the endothelium functions as one of the first lines of defense of the immune system by combating the invasion of microbes and endogenous substances (27). In the context of inflammatory diseases, such as the development of atherosclerotic lesions, endothelial cells are activated by ox-LDL and immune cell-derived cytokines, which is characterized by an increased expression of intercellular adhesion molecule-1, chemokines, cytokines and vascular cell adhesion molecule-1 (28,29). These changes in endothelial cells further promote the formation of atherosclerotic lesions. In the present study, it was observed that treatment with ox-LDL (100 µg/ml) significantly induced the production of pro-inflammatory cytokines, including TNF-α and IL-1α. The results of a previous study by Valente et al (7) suggested that ox-LDL may induce endothelial cell dysfunction and apoptosis via activation of TRAF3 interacting protein 2 (TRAF3IP2). It was observed that ox-LDL reduced the migration and angiogenesis of endothelial cells, indicating that ox-LDL caused endothelial injury. Additionally, MTT assays demonstrated that ox-LDL significantly inhibited endothelial cell proliferation, prompting further investigation of whether ox-LDL may promote endothelial cell death.
Ferroptosis, an iron-dependent form of regulated cell death, is distinct from apoptosis, and accumulating evidence indicates that ferroptosis is a significant type of cell death observed in various cell populations, including cardiomyocytes (18,30). In the present study, it was observed that ox-LDL significantly inhibited GPx4 expression in human endothelial cells. Inhibited GPx4 activity decreases the reduction of lipid peroxides, which is one of the major pathways that lead to ferroptosis (31). The expression of xCT, a specific cys2/glutamate antiporter that plays a role in negatively regulating ferroptosis, is also inhibited by ox-LDL (32). These findings suggest that ox-LDL-induced endothelial dysfunction occurs by promoting ferroptosis. To further confirm these results, ferroptosis in ox-LDL-treated endothelial cells was inhibited using the ferroptosis inhibitor DFOM, which blunted the ox-LDL-mediated decrease in GPx4 and xCT expression in endothelial cells. In addition, DFOM attenuated the ox-LDL-mediated induction of cytokine production and rescued the decreased proliferation, migration and angiogenesis of endothelial cells treated with ox-LDL. Moreover, KD of GPx4 and xCT by transfecting endothelial cells with specific siRNAs aggravated the ox-LDL-induced ferroptosis and dysfunction of endothelial cells.
Fluvastatin has been used as a drug to lower serum cholesterol levels (33). Haruna et al (22) showed that fluvastatin exerted protective effects against endothelial dysfunction by reducing the levels of p22phox mRNA. To investigate whether fluvastatin protects against endothelial ferroptosis, endothelial cells were treated with low doses of fluvastatin (2.5, 5 and 10 µM) in the presence of ox-LDL. The data demonstrated that 10 µM fluvastatin significantly blunted ox-LDL-induced endothelial cell dysfunction. Importantly, fluvastatin also reversed the ox-LDL-mediated decrease in GPx4 and xCT expression. Combined with its protective effect on cell proliferation, fluvastatin inhibited ox-LDL-induced ferroptosis in human endothelial cells. The expression of GPx4 and xCT was then knocked down in endothelial cells by siRNA transfection. It was observed that the suppression of GPx4 and xCT partly blocked the protective effects of fluvastatin against the ox-LDL-mediated decrease in endothelial cell proliferation, migration and angiogenesis, which further confirmed that fluvastatin functions by regulating GPx4 and xCT to inhibit endothelial cell dysfunction and ferroptosis.
Bioccaa et al (10) demonstrated that, in endothelial cells, ox-LDL functions through lectin-like ox-LDL receptor 1 (LOX-1), and all tested statins (atorvastatin, fluvastatin, lovastatin, pitavastatin and pravastatin) were able to displace the binding of ox-LDL to LOX-1 by directly interacting with LOX-1, leading to a significant loss of ox-LDL-induced LOX-1 function, including ox-LDL-induced ferroptosis. Based on the results of this previous study, it was inferred that the underlying mechanism through which fluvastatin alleviates endothelial cell ferroptosis involves counteracting the effects of ox-LDL by disrupting the interaction of ox-LDL with the C-type lectin-like recognition domain of LOX-1(10). As all statins can disrupt the binding of ox-LDL to the C-type lectin-like recognition domain of LOX-1, they all have the same effects of alleviating ox-LDL-induced endothelial ferroptosis (10). Previous studies have demonstrated that ox-LDL does not interact with xCT and GPx4 directly, but that it rather acts via LOX-1 (34-36). Similarly, all tested statins (atorvastatin, fluvastatin, lovastatin, pitavastatin and pravastatin) did not directly interact with xCT and GPx4 to improve ferroptosis (10), but protected endothelial cells from ferroptosis by disrupting the interaction of ox-LDL with LOX-1. Therefore, it was observed in the present study that fluvastatin sequentially activated GPx4 and xCT indirectly.
Recently, a number of studies reported various mechanisms through which statins modulate ox-LDL toxicity (10). For example, in endothelial cells, ox-LDL functions via binding LOX-1, which upregulates the expression of the cytoplasmic adaptor protein TRAF3IP2, further activating the downstream signaling pathway of IKK/NF-κB p65(10), resulting in endothelial dysfunction and endothelial cell death, as well as impaired vasorelaxation (7,9,10). Fluvastatin can inhibit this ox-LDL-mediated toxicity by displacing the binding of ox-LDL to LOX-1(10). Previous studies have also demonstrated that statins target the 3-hydroxy-3-methylglutaryl (HMG)-CoA/mevalonate/isopentenyl-pp/TRIT1 pathways to alleviate ferroptosis by inhibiting HMG-CoA reductase (37). In addition, fluvastatin can inhibit ferroptosis induced by erastin and RSL3 by inhibiting LOX-1 (10,38).
The present study further demonstrated that ox-LDL promotes endothelial cell ferroptosis by inhibiting GPx4 and xCT, and that fluvastatin may protect endothelial cells from ox-LDL-induced endothelial ferroptosis by counteracting the ox-LDL-induced inhibition of GPx4 and xCT expression. These findings revealed a new mechanism by which statins modulate ox-LDL toxicity in addition to other well-known mechanisms. Furthermore, Yu et al (32) and Friedmann Angeli et al (37) reported that statins inhibited HMG-CoA reductase, thereby regulating the activity of GPX4. Sui et al (31) found that inhibiting GPX4 may lead to ferroptosis by decreasing the reduction of lipid peroxide levels. Therefore, fluvastatin can alleviate ferroptosis via inhibiting HMG-CoA reductase to regulate GPX4, the function of which is also indirect.
The results of the present study demonstrated that fluvastatin exerts potent protective effects against ox-LDL-induced endothelial cell dysfunction through regulation of GPx4 and xCT, providing a scientific rationale for the clinical use of fluvastatin in atherosclerosis. Importantly, our data indicate that statins may have clinical benefits in patients beyond their lipid level-lowering properties, by improving endothelial cell function. However, supporting data from pre-clinical and clinical studies are required to confirm these hypotheses.
A limitation of the present study was that it primarily focused on the mechanisms through which fluvastatin regulates HUVC ferroptosis in vitro. However, in vivo experiments could further elucidate the function of fluvastatin in endothelial ferroptosis.
In the future, in vivo studies will aim to use Cre-LoxP technology to generate transgenic mice with vascular endothelial-specific knockout of GPx4/xCT and establish an ox-LDL overexpression animal model through high-fat diet.
Acknowledgements
Not applicable.
Funding Statement
Funding: The present study was funded by Shaanxi Social Development Funding (grant no. 2017SF-134) and Shaanxi Nature Science Funding (grant no. 2020JQ-553).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JD, QL, CL, EX, YT and ZC designed the experiments; analyzed and interpreted the data; and drafted the manuscript. JD, QL, CL, LD, EX, YT, ZC and NY were involved in the data acquisition and analysis. All authors revised the manuscript critically for important intellectual content and approved the final version to be published. JD is responsible for the integrity of the work as a whole. JD, QL, CL, LD, EX, YT and ZC confirm the authenticity of all the raw data.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Khaddaj Mallat R, Mathew John CM, Kendrick DJ, Braun AP. The vascular endothelium: A regulator of arterial tone and interface for the immune system. Crit Rev Clin Lab Sci. 2017;54:458–470. doi: 10.1080/10408363.2017.1394267. [DOI] [PubMed] [Google Scholar]
- 2.Rajendran P, Rengarajan T, Thangavel J, Nishigaki Y, Sakthisekaran D, Sethi G, Nishigaki I. The vascular endothelium and human diseases. Int J Biol Sci. 2013;9:1057–1069. doi: 10.7150/ijbs.7502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beerepoot LV, Mehra N, Vermaat JS, Zonnenberg BA, Gebbink MF, Voest EE. Increased levels of viable circulating endothelial cells are an indicator of progressive disease in cancer patients. Ann Oncol. 2004;15:139–145. doi: 10.1093/annonc/mdh017. [DOI] [PubMed] [Google Scholar]
- 4.Furstenberger G, von Moos R, Lucas R, Thürlimann B, Senn HJ, Hamacher J, Boneberg EM. Circulating endothelial cells and angiogenic serum factors during neoadjuvant chemotherapy of primary breast cancer. Br J Cancer. 2006;94:524–531. doi: 10.1038/sj.bjc.6602952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Park KH, Park WJ. Endothelial dysfunction: Clinical implications in cardiovascular disease and therapeutic approaches. J Korean Med Sci. 2015;30:1213–1225. doi: 10.3346/jkms.2015.30.9.1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li TB, Zhang YZ, Liu WQ, Zhang JJ, Peng J, Luo XJ, Ma QL. Correlation between NADPH oxidase-mediated oxidative stress and dysfunction of endothelial progenitor cell in hyperlipidemic patients. Korean J Intern Med. 2018;33:313–322. doi: 10.3904/kjim.2016.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Valente AJ, Irimpen AM, Siebenlist U, Chandrasekar B. OxLDL induces endothelial dysfunction and death via TRAF3IP2: Inhibition by HDL3 and AMPK activators. Free Radic Biol Med. 2014;70:117–128. doi: 10.1016/j.freeradbiomed.2014.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morawietz H. LOX-1 receptor as a novel target in endothelial dysfunction and atherosclerosis. Dtsch Med Wochenschr. 2010;135:308–312. doi: 10.1055/s-0029-1244854. (In German) [DOI] [PubMed] [Google Scholar]
- 9.Wahyudi S, Sargowo D. Green tea polyphenols inhibit oxidized LDL-induced NF-KB activation in human umbilical vein endothelial cells. Acta Med Indones. 2007;39:66–70. [PubMed] [Google Scholar]
- 10.Biocca S, Iacovelli F, Matarazzo S, Vindigni G, Oteri F, Desideri A, Falconi M. Molecular mechanism of statin-mediated LOX-1 inhibition. Cell Cycle. 2015;14:1583–1595. doi: 10.1080/15384101.2015.1026486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li J, Cao F, Yin HL, Huang ZJ, Lin ZT, Mao N, Sun B, Wang G. Ferroptosis: Past, present and future. Cell Death Dis. 2020;11(88) doi: 10.1038/s41419-020-2298-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maiorino M, Conrad M, Ursini F. GPx4, lipid peroxidation, and cell death: Discoveries, rediscoveries, and open issues. Antioxid Redox Signal. 2018;29:61–74. doi: 10.1089/ars.2017.7115. [DOI] [PubMed] [Google Scholar]
- 13.Wortmann M, Schneider M, Pircher J, Hellfritsch J, Aichler M, Vegi N, Kölle P, Kuhlencordt P, Walch A, Pohl U, et al. Combined deficiency in glutathione peroxidase 4 and vitamin E causes multiorgan thrombus formation and early death in mice. Circ Res. 2013;113:408–417. doi: 10.1161/CIRCRESAHA.113.279984. [DOI] [PubMed] [Google Scholar]
- 14.Ridker PM, Mora S, Rose L, Group JTS. Percent reduction in LDL cholesterol following high-intensity statin therapy: Potential implications for guidelines and for the prescription of emerging lipid-lowering agents. Eur Heart J. 2016;37:1373–1379. doi: 10.1093/eurheartj/ehw046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Correction to. Pleiotropic effects of statins on the cardiovascular system. Circ Res. 2018;123(e20) doi: 10.1161/RES.0000000000000228. [DOI] [PubMed] [Google Scholar]
- 16.Wang J, Tokoro T, Matsui K, Higa S, Kitajima I. Pitavastatin at low dose activates endothelial nitric oxide synthase through PI3K-AKT pathway in endothelial cells. Life Sci. 2005;76:2257–2268. doi: 10.1016/j.lfs.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 17.Weis M, Heeschen C, Glassford AJ, Cooke JP. Statins have biphasic effects on angiogenesis. Circulation. 2002;105:739–745. doi: 10.1161/hc0602.103393. [DOI] [PubMed] [Google Scholar]
- 18.Stockwell BR, Friedmann Angeli JP, Bayir H, Bush AI, Conrad M, Dixon SJ, Fulda S, Gascón S, Hatzios SK, Kagan VE, et al. Ferroptosis: A regulated cell death nexus linking metabolism, redox biology, and disease. Cell. 2017;171:273–285. doi: 10.1016/j.cell.2017.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Di Pietro N, Formoso G, Pandolfi A. Physiology and pathophysiology of oxLDL uptake by vascular wall cells in atherosclerosis. Vascul Pharmacol. 2016;84:1–7. doi: 10.1016/j.vph.2016.05.013. [DOI] [PubMed] [Google Scholar]
- 20.Yang WS, SriRamaratnam R, Welsch ME, Shimada K, Skouta R, Viswanathan VS, Cheah JH, Clemons PA, Shamji AF, Clish CB, et al. Regulation of ferroptotic cancer cell death by GPX4. Cell. 2014;156:317–331. doi: 10.1016/j.cell.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang Z, Ding Y, Wang X, Lu S, Wang C, He C, Wang L, Piao M, Chi G, Luo Y, Ge P. Pseudolaric acid B triggers ferroptosis in glioma cells via activation of Nox4 and inhibition of xCT. Cancer Lett. 2018;428:21–33. doi: 10.1016/j.canlet.2018.04.021. [DOI] [PubMed] [Google Scholar]
- 22.Haruna Y, Morita Y, Yada T, Satoh M, Fox DA, Kashihara N. Fluvastatin reverses endothelial dysfunction and increased vascular oxidative stress in rat adjuvant-induced arthritis. Arthritis Rheum. 2007;56:1827–1835. doi: 10.1002/art.22632. [DOI] [PubMed] [Google Scholar]
- 23.Ait-Oufella H, Maury E, Lehoux S, Guidet B, Offenstadt G. The endothelium: Physiological functions and role in microcirculatory failure during severe sepsis. Intensive Care Med. 2010;36:1286–1298. doi: 10.1007/s00134-010-1893-6. [DOI] [PubMed] [Google Scholar]
- 24.Cosentino F, Rubattu S, Savoia C, Venturelli V, Pagannonne E, Volpe M. Endothelial dysfunction and stroke. J Cardiovasc Pharmacol. 2001;38 (Suppl 2):S75–S78. doi: 10.1097/00005344-200111002-00018. [DOI] [PubMed] [Google Scholar]
- 25.Franses JW, Drosu NC, Gibson WJ, Chitalia VC, Edelman ER. Dysfunctional endothelial cells directly stimulate cancer inflammation and metastasis. Int J Cancer. 2013;133:1334–1344. doi: 10.1002/ijc.28146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Widmer RJ, Lerman A. Endothelial dysfunction and cardiovascular disease. Glob Cardiol Sci Pract. 2014;2014:291–308. doi: 10.5339/gcsp.2014.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young MR. Endothelial cells in the eyes of an immunologist. Cancer Immunol Immunother. 2012;61:1609–1616. doi: 10.1007/s00262-012-1335-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stroka KM, Levitan I, Aranda-Espinoza H. OxLDL and substrate stiffness promote neutrophil transmigration by enhanced endothelial cell contractility and ICAM-1. J Biomech. 2012;45:1828–1834. doi: 10.1016/j.jbiomech.2012.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Feng Y, Cai ZR, Tang Y, Hu G, Lu J, He D, Wang S. TLR4/NF-κB signaling pathway-mediated and oxLDL-induced up-regulation of LOX-1, MCP-1, and VCAM-1 expressions in human umbilical vein endothelial cells. Genet Mol Res. 2014;13:680–695. doi: 10.4238/2014.January.28.13. [DOI] [PubMed] [Google Scholar]
- 30.Fang X, Wang H, Han D, Xie E, Yang X, Wei J, Gu S, Gao F, Zhu N, Yin X, et al. Ferroptosis as a target for protection against cardiomyopathy. Proc Natl Acad Sci USA. 2019;116:2672–2680. doi: 10.1073/pnas.1821022116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sui X, Zhang R, Liu S, Duan T, Zhai L, Zhang M, Han X, Xiang Y, Huang X, Lin H, Xie T. RSL3 drives ferroptosis through GPX4 inactivation and ROS production in colorectal cancer. Front Pharmacol. 2018;9(1371) doi: 10.3389/fphar.2018.01371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yu H, Guo P, Xie X, Wang Y, Chen G. Ferroptosis, a new form of cell death, and its relationships with tumourous diseases. J Cell Mol Med. 2017;21:648–657. doi: 10.1111/jcmm.13008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams SP, Sekhon SS, Tsang M, Wright JM. Fluvastatin for lowering lipids. Cochrane Database Syst Rev. 2018;3(CD012282) doi: 10.1002/14651858.CD012282.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lubrano V, Balzan S. Role of oxidative stress-related biomarkers in heart failure: Galectin 3, α1-antitrypsin and LOX-1: New therapeutic perspective? Mol Cell Biochem. 2020;464:143–152. doi: 10.1007/s11010-019-03656-y. [DOI] [PubMed] [Google Scholar]
- 35.Singh S, Gautam AS. Upregulated LOX-1 receptor: Key player of the pathogenesis of atherosclerosis. Curr Atheroscler Rep. 2019;21(38) doi: 10.1007/s11883-019-0801-y. [DOI] [PubMed] [Google Scholar]
- 36.Zeya B, Chandra NC. LOX-1: Its cytotopographical variance and disease stress. J Biochem Mol Toxicol. 2019;33(e22375) doi: 10.1002/jbt.22375. [DOI] [PubMed] [Google Scholar]
- 37.Friedmann Angeli JP, Conrad M. Selenium and GPX4, a vital symbiosis. Free Radic Biol Med. 2018;127:153–159. doi: 10.1016/j.freeradbiomed.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Shintoku R, Takigawa Y, Yamada K, Kubota C, Yoshimoto Y, Takeuchi T, Koshiishi I, Torii S. Lipoxygenase-mediated generation of lipid peroxides enhances ferroptosis induced by erastin and RSL3. Cancer Sci. 2017;108:2187–2194. doi: 10.1111/cas.13380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.