Abstract
MicroRNAs (miRs) and inflammatory cytokines can induce acute lung injury (ALI), which can develop into acute respiratory distress syndrome in severe cases. Previous research has revealed that miR-122-5p participates in the development of ALI, and that its expression is positively associated with ALI. However, the mechanism by which miR-122-5p contributes to ALI remains to be determined. In the current study, TargetScan and dual luciferase reporter gene assays were used to confirm that IL-1 receptor antagonist (IL1RN) was a target of miR-122-5p. Subsequently, by referring to previous literature, a lipopolysaccharide (LPS)-induced ALI cell model was established. A549 cells were transfected with mimic control or miR-122-5p mimics for 24 h, and 10 µg LPS was used to treat the transfected cells for 12 h. The results revealed that miR-122-5p mimics decreased cell viability and promoted apoptosis. Lactate dehydrogenase (LDH) release assays indicated that miR-122-5p mimics increased LDH release. ELISA demonstrated that miR-122-5p mimics promoted TNF-α, IL-1β and IL-6 expression levels. A549 cells were transfected with inhibitor control, miR-122-5p inhibitor, miR-122-5p inhibitor + control-small interfering (si)RNA or miR-122-5p inhibitor + IL1RN-siRNA for 24 h, after which the cells were treated with 10 µg LPS for 12 h. The results revealed that the effects of the miR-122-5p inhibitor were the opposite of those of the miR-122-5p mimic. All the effects of miR-122-5p inhibitor on LPS-treated A549 cells were significantly reversed by IL1RN-siRNA. Overall, the results highlighted miR-122-5p as a potential novel target for the treatment of ALI.
Keywords: acute lung injury, microRNA-122-5p, IL-1 receptor antagonist, lipopolysaccharide
Introduction
Acute lung injury (ALI) is caused by epithelial and capillary endothelial cell damage that is induced by various direct and indirect injurious factors, such as increased vascular permeability, overproduction of cytokines, leukocyte recruitment and dysfunction of surfactant, resulting in diffuse lung interstitial and alveolar edema and acute hypoxic respiratory insufficiency (1). ALI is a disease that can threaten an individual's life. In severe cases, it can lead to acute respiratory distress syndrome (ARDS) and respiratory failure, which has a mortality rate of >30% (2,3). The primary features of ALI and ARDS are rapid onset of respiratory failure, severe hypoxemia and reduced static respiratory system compliance; however, ALI and ARDS are primarily caused by uncontrolled acute inflammation (4,5). Previous studies have also demonstrated that the inhibition of inflammation and oxidative stress can prevent ALI (6,7).
Lipopolysaccharide (LPS) is an endotoxin and a constituent of the membrane of gram-negative bacteria (8). When gram-negative bacteria infect the lungs, LPS is the primary pathogenic factor that causes ALI (9). LPS can activate pattern recognition receptors, thereby activating downstream NF-κB and MAPK signaling molecules, which in turn promote the synthesis and release of inflammatory factors, leading to excessive oxidative stress (8,9). When endothelial and epithelial cells are damaged, the endothelial barrier is destroyed, which increases capillary permeability and promotes alveolar edema (10). Thus far, drug treatments for ALI have not produced favorable results. Therefore, novel methods to treat ALI using different approaches are required.
At present, an increasing body of evidence has revealed that microRNAs (miRNAs/miRs) serve an important role in various diseases (11), including cancer (12), atherosclerosis (13) and cardiovascular diseases (14). miRNAs are a general term for a class of small-molecule non-coding RNAs that are 20-22 nucleotides in length, as opposed to mRNA-transcribed proteins. miRNAs do not encode proteins, but instead inhibit target gene expression (15-17). miR-122-5p is located at chr18q21.31, and it has been demonstrated to participate in the development of several diseases, including cancer (18), skeletal muscle myogenesis (19), drug-induced liver injury (20) and radiation-induced rectal injury (21). In addition, Lu et al (22) revealed that miR-122-5p was involved in ALI development; miR-122-5p expression in ALI was notably upregulated, and it was demonstrated to regulate pulmonary microvascular endothelial cells by affecting the dual specificity phosphatase 4 (DUSP4)/ERK signaling pathway to aggravate ALI (22). However, its specific mechanism of action is yet to be fully elucidated.
IL-1 receptor antagonist (IL1RN) is a member of the IL-1 family, which is an immune and pro-inflammatory cytokine that competitively binds to IL1R1 and prevents it from binding to the co-receptor IL-1 receptor accessory protein (23). Therefore, IL1RN suppresses IL-1 activity, which regulates several immune and inflammatory responses associated with IL-1(24). It has been reported that IL1RN serves a protective role in numerous types of lung injuries (25,26). Preliminary bioinformatics analysis for the present study revealed that IL1RN is a target gene of miR-122-5p. Therefore, it was hypothesized that miR-122-5p may be involved in ALI through the modulation of IL1RN to regulate alveolar epithelial cell injury.
Materials and methods
Cell culture and LPS exposure
The A549 human pulmonary epithelial cell line was acquired from the American Type Culture Collection. Cells were cultured in DMEM (Gibco; Thermo Fisher Scientific, Inc.) supplemented with 10% FBS (Gibco; Thermo Fisher Scientific, Inc.) and 1% penicillin-streptomycin at 37˚C in a humidified incubator with 5% CO2. A549 was induced using 0.1, 1, 10 and 100 µg LPS (Sigma-Aldrich; Merck KGaA) for 12 h, or induced using 10 µg LPS (Sigma) for 4, 8, 12 and 24 h. An ALI cell model was established through treatment with 10 µg LPS (Sigma-Aldrich; Merck KGaA) for 12 h (27).
Cell transfection
A549 cells were transfected with 50 nM miR-122-5p mimic (5'-UGGAGUGUGACAAUGGUGUUUG-3') 50 nM mimic control (5'-UUCUCCGAACGUGUCACGUTT-3'), 100 nM miR-122-5p inhibitor (miR-122-5p antagomir; 5'-CAAACACCAUUGUCACACUCCA-3') and 100 nM inhibitor control (the negative control of miR-122-5p antagomir; 5'-CAGUACUUUUGUGUAGUACAAA-3'), 0.2 µM scrambled control small interfering (si)RNA (cat no. sc-36869; Santa Cruz Biotechnology, Inc.), 0.2 µM IL1RN-siRNA (cat no. sc-39617; Santa Cruz Biotechnology, Inc.), 100 nM miR-122-5p inhibitor + 0.2 µM control-siRNA, or 100 nM miR-122-5p inhibitor + 0.2 µM IL1RN-siRNA using Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) at 37˚C for 24 h, according to the manufacturer's instructions. After 24 h, RT-qPCR analysis was conducted to measure the efficiency of transfection.
Bioinformatics
TargetScan version 7.2 (http://www.targetscan.org/vert_72/) was used to predict the potential targets of miR-122-5p.
Dual luciferase reporter assay
TargetScan was used to predict the potential targets of miR-122-5p. The results revealed that IL1RN was identified as a potential target of miR-122-5p. Therefore, the wild-type (IL1RN-WT; 5'-UCCAAGCUCCAUCUCCACUCCAG-3') and mutant (IL1RN-MUT; 3'UCCAAGCUCCAUCUCGUGAGGUG-5') 3'untranslated regions (UTRs) of IL1RN, containing miR-122-5p-binding elements, were generated by reverse transcription (RT). RT was conducted using a HiScript 1st Strand cDNA Synthesis kit (Vazyme Biotech Co., Ltd.) for 5 min at 25˚C followed by 60 min at 42˚C from total RNA preparations extracted from A549 cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.). Samples were then cloned into BamHI and AscI sites of the pmiRGLO vector (Promega Corporation). The recombinant plasmids were acquired by using an EndoFree Plasmid Maxi kit (Vazyme Biotech Co., Ltd.). Subsequently, 293T cells (American Type Culture Collection) were seeded (5x104 cells/well) in 24-well plates and co-transfected with miR-122-5p mimics (5'-UGGAGUGUGACAAUGGUGUUUG-3'; Guangzhou RiboBio Co., Ltd.) or mimic control (5'-UUCUCCGAACGUGUCACGUTT-3'; Guangzhou RiboBio Co., Ltd.) and the indicated luciferase reporter constructs using Lipofectamine 2000® (Thermo Fisher Scientific, Inc.) according to the manufacturer's instructions at 37˚C for 48 h. After transfection for 48 h, luciferase activity was assessed using Dual Luciferase Reporter assay system (Promega Corporation) and normalized to Renilla luciferase activity.
RT-quantitative (q)PCR assay
Total RNA was extracted from A549 cells using TRIzol® reagent (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. RNA concentration was detected using a NanoDrop™ 2000 spectrophotometer (Thermo Fisher Scientific, Inc.). Total RNA was reverse transcribed into cDNA using a HiScript 1st Strand cDNA Synthesis kit (Vazyme Biotech Co., Ltd.) according to the manufacturer's protocol. Subsequently, qPCR was performed using a SYBR® Green PCR kit (Vazyme Biotech Co., Ltd.) according to the manufacturer's protocol. The following thermocycling conditions were used for qPCR: Initial denaturation at 95˚C for 5 min; followed by 38 cycles of 15 sec at 95˚C, 1 min at 60˚C and 30 sec at 72˚C; and a final extension for 10 min at 72˚C. GAPDH (for IL1RN mRNA) or U6 (for miR-122-5p) were used as the endogenous controls. Gene expression was calculated using the 2-ΔΔCq method (28). The primer sequences for qPCR were as follows: GAPDH Forward, 5'-CTTTGGTATCGTGGAAGGACTC-3', and reverse, 5'-GTAGAGGCAGGGATGATGTTCT-3'; U6 forward, 5'-GCTTCGGCAGCACATATACTAAAAT-3', and reverse, 5'-CGCTTCACGAATTTGCGTGTCAT-3'; miR-122-5p forward 5'-GTGACAATGGTGGAATGTGG-3', and reverse, 3'-CAGAACCGTAGCAAACGAAA-5'; IL1RN forward 5'-AACAGAAAGCAGGACAAGCG-3', and reverse, 5'-CCTTCGTCAGGCATATTGGT-3'.
Western blotting
Total protein was obtained from A549 cells using RIPA lysis buffer (Beijing Solarbio Science & Technology Co., Ltd.). A BCA assay kit (Thermo Fisher Scientific, Inc.) was used to quantify the total protein concentration. Equal amounts of proteins (40 µg per lane) were isolated using 12% SDS-PAGE, and then transferred to PVDF membranes. The membranes were blocked with 5% non-fat milk to prevent non-specific binding at room temperature for 1.5 h, and then incubated with primary antibodies against anti-IL1RN (cat. no. ab124962; 1:1,000; Abcam), cleaved-caspase 3 (cat. no. ab32042; 1:1,000; Abcam), caspase 3 (cat. no. ab32351; 1:1,000; Abcam), and GAPDH (cat. no. ab9485; 1:2,500; Abcam) at 4˚C overnight. The following day, membranes were incubated with goat anti-rabbit IgG H&L (HRP)-conjugated secondary antibody (1:5,000; cat. no. ab7090; Abcam) at room temperature for 2 h. Protein signals were visualized using an ECL reagent (Cytiva). ImageJ v.2.0 software (National Institutes of Health) was used to quantify band intensity.
ELISA
ELISA was performed to examine the levels of TNF-α (cat. no. PT518), IL-1β (cat. no. PI305) and IL-6 (cat. no. PI330) in the supernatant of transfected A549 cells. Specific ELISA kits (Beyotime Institute of Biotechnology) were used to detect the expression of associated markers according to the manufacturer's protocol.
Lactate dehydrogenase (LDH) release assay
LDH release was determined using an LDH Release Assay kit (cat. no. C0016; Beyotime Institute of Biotechnology) according to the manufacturer's protocol. After treatment with LPS, 150 µl LDH release reagent was added to DMEM and cells were incubated at 37˚C for a total of 2 h. Subsequently, samples were centrifuged at 4˚C and 500 x g for 5 min, and 120 µl supernatant from each well was used to measure the LDH release according to the requirements of the kit. Absorbance was measured at a wavelength of 490 nm using a microplate reader.
Flow cytometry
Transfected cells were processed using an Annexin-V/PI Apoptosis Detection kit (BD Biosciences). After cell transfection, cells were induced with LPS for 12 h, collected through centrifugation at 1,000 x g at 4˚C for 5 min and then resuspended in 100 µl FITC-binding buffer. Subsequently, ~5 µl ready-to-use Annexin V-FITC and 5 µl PI were added to the buffer and incubated in the dark for 30 min at room temperature. Annexin V-FITC and PI fluorescence were assessed using a BD FACSCalibur flow cytometer (BD Biosciences) and the results were analyzed using Kaluza Analysis software (v2.1.1.20653; Beckman Coulter, Inc.).
MTT assay
Transfected cells were treated with 10 µg LPS for 12 h and then plated in a 96-well plate and incubated at 37˚C for 24 h. Subsequently, 20 µl MTT (5 mg/ml; Sigma-Aldrich; Merck KGaA) was added to each well, and the wells were further cultured at 37˚C for 4 h. The absorbance was measured at 570 nm using a multifunctional plate reader (BioTek Instruments, Inc.).
Statistical analysis
Results were analyzed using GraphPad Prism v6.0 (GraphPad Software, Inc.). Differences between two groups were compared using an unpaired Student's t-test. The differences among multiple groups were calculated using one-way ANOVA followed by Tukey's post hoc test. Data are presented as the mean ± SD of three independent experiments. P<0.05 was considered to indicate a statistically significant difference.
Results
IL1RN is the direct target gene of miR-122-5p
The target gene downstream of miR-122-5p was searched using the bioinformatics tool, TargetScan. TargetScan results revealed that there were thousands of target genes of miR-122-5p including IL1RN (Fig. 1A). Based on software prediction, miR-122-5p was partially complementary with the IL1RN 3'-UTR. IL1RN is an antagonist of IL1, which is a pro-inflammatory cytokine (24). It has been reported that IL1RN serves a protective role in numerous types of lung injury (25,26). Thus, the present study hypothesized that miR-122-5p may be involved in ALI via the modulation of IL1RN expression. Therefore, IL1RN was selected for further study. Subsequently, 293T cells were co-transfected with IL1RN-WT or IL1RN-MUT and miR-122-5p mimic or mimic control for 48 h, after which a dual-luciferase reporter assay was performed to detect luciferase activity. Dual-luciferase reporter assays indicated that miR-122-5p mimics could significantly inhibit the luciferase activity of IL1RN-WT. However, miR-122-5p mimic did not inhibit the luciferase activity of IL1RN-MUT (Fig. 1B). Overall, IL1RN was revealed to be a target gene of miR-122-5p.
Figure 1.

Relationship between IL1RN and miR-122-5p. (A) Interactions between miR-122-5p and the 3'UTR of IL1RN were predicted using TargetScan software. (B) A dual luciferase reporter gene assay was conducted for the verification of miR-122-5p and IL1RN interactions. **P<0.01 vs. mimic control. IL1RN, IL-1 receptor antagonist; miR, microRNA; UTR, untranslated region; WT, wild-type; MUT, mutant.
Expression of IL1RN and miR-122-5p in the LPS-induced ALI cell model
To detect the expression of IL1RN and miR-122-5p in the in vitro model of ALI, A549 cells were treated with LPS. A549 was induced using 0.1, 1, 10 and 100 µg LPS for 12 h. RT-qPCR and western blotting were used to detect miR-122-5p and IL1RN expression. The results revealed that LPS dose-dependently increased miR-122-5p expression in A549 cells (Fig. 2A), and reduced IL1RN expression at both the protein and mRNA levels (Fig. 2B and C); these results were significant at a dosage of ≥1 µg. The effects of treatment with LPS for different time periods were subsequently assessed. A549 cells were induced using 10 µg LPS for 4, 8, 12 and 24 h. The results indicated that LPS significantly increased miR-122-5p expression in A549 cells (Fig. 2D) and significantly reduced the protein and mRNA expression levels of IL1RN in a time-dependent manner (Fig. 2E and F). For subsequent experiments, A549 cells were induced with 10 µg LPS for 12 h to establish the ALI cell model (27). The successful establishment of the LPS-induced ALI cell model was confirmed through the reduction of A549 cell viability, enhanced LDH activity, increased apoptosis and enhanced inflammatory cytokine levels.
Figure 2.
miR-122-5p expression is upregulated and IL1RN is downregulated in the LPS-treated acute lung injury cell model. Different concentrations of LPS (0.1, 1, 10 or 100 µg) were used to induce A549 cells for 12 h. (A) RT-qPCR analysis of miR-122-5p expression. (B) Western blotting of IL1RN expression. (C) RT-qPCR analysis of IL1RN expression. (D) RT-qPCR analysis of miR-122-5p expression when A549 cells were treated with 10 µg LPS for 4, 8, 12 or 24 h. (E) Protein and (F) mRNA expression analysis of IL1RN expression in A549 cells treated with 10 µg LPS for 4, 8, 12 or 24 h. *P<0.05 and **P<0.01 vs. control. miR, microRNA; IL1RN, IL-1 receptor antagonist; LPS, lipopolysaccharide; RT-qPCR, reverse transcription-quantitative PCR.
Effects of miR-122-5p mimics on the LPS-induced ALI cell model
To investigate the effects of miR-122-5p mimics on LPS-induced ALI cells. A549 cells were transfected with mimic control or miR-122-5p mimic for 24 h. RT-qPCR was performed to confirm the transfection efficiency. Compared with the mimic control group, miR-122-5p mimic significantly increased miR-122-5p expression in A549 cells (Fig. 3A). After 24 h, transfected cells were induced with 10 µg LPS for 12 h, and subsequent analysis was performed. RT-qPCR analysis revealed that miR-122-5p expression levels were significantly increased in LPS-induced cells compared with their respective control (Fig. 3B). By contrast, MTT analysis indicated that LPS significantly decreased cell viability in the LPS and LPS + miR-122-5p mimic groups compared with the control and LPS + mimic control, respectively (Fig. 3C). Additionally, LPS significantly promoted LDH release in these groups (Fig. 3D). Flow cytometry analysis indicated that LPS significantly increased the rate of apoptosis (Fig. 3E and F); similarly, western blotting revealed that cleaved-caspase 3 expression (Fig. 3G) and the cleaved-caspase 3/caspase 3 ratio were significantly increased (Fig. 3H). All these changes were reinforced by miR-122-5p mimic.
Figure 3.
miR-122-5p mimic further promotes LPS-treated acute lung injury cell injury. (A) RT-qPCR analysis of miR-122-5p expression in A549 cells transfected with mimic control or miR-122-5p mimic for 24 h. (B) RT-qPCR analysis of miR-122-5p expression in A549 cells in the different groups. (C) MTT assay of cell viability in the different groups. (D) LDH release analysis in the different groups of cells. (E) Flow cytometry analysis of apoptosis and (F) the apoptotic ratio. (G) Western blotting of cleaved-caspase 3 protein expression and the (H) cleaved-caspase 3/caspase 3 ratio. **P<0.01 vs. mimic control; ##P<0.01 vs. control; &&P<0.01 vs. LPS + mimic control. miR, microRNA; LPS, lipopolysaccharide; RT-qPCR, reverse transcription-quantitative PCR; LDH, lactate dehydrogenase.
Effect of miR-122-5p mimic on expression of inflammatory cytokines
ELISA was performed to detect the expression of inflammatory cytokines. The results revealed that TNF-α, IL-1β and IL-6 levels were all significantly increased in the cell supernatant of the LPS-treated group compared with the control. The miR-122-5p mimic further significantly increased the levels of TNF-α (Fig. 4A), IL-1β (Fig. 4B) and IL-6 (Fig. 4C) compared with the LPS + mimic control. Western blotting and RT-qPCR analysis were subsequently used to detect IL1RN expression. The results revealed that LPS significantly decreased IL1RN expression compared with the control group; while miR-122-5p mimics further significantly decreased IL1RN expression in LPS-induced A549 cells compared with the LPS + mimic control group (Fig. 4D and E).
Figure 4.
miR-122-5p mimic further promotes the release of inflammatory cytokines in LPS-treated A549 cells. ELISA assessment of (A) TNF-α, (B) IL-1β (C) and IL-6 levels. (D) Western blotting of IL1RN expression. (E) Reverse transcription-quantitative PCR analysis of IL1RN expression at the mRNA level. **P<0.01 vs. control; ##P<0.01 vs. LPS + mimic control. miR, microRNA; LPS, lipopolysaccharide.
miR-122-5p negatively regulates IL1RN expression in A549 cells
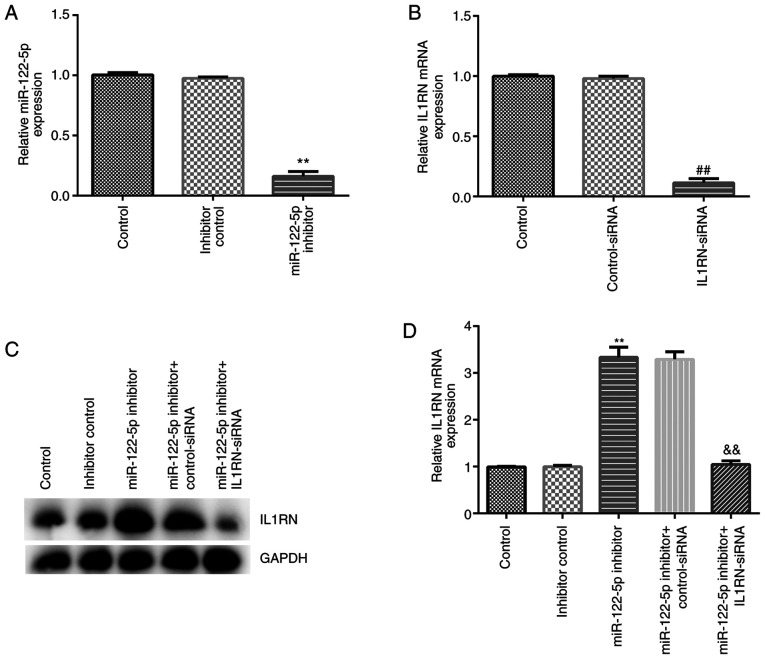
The effect of miR-122-5p inhibition on the LPS-induced ALI cell model was assessed. A549 cells were transfected with inhibitor control, miR-122-5p inhibitor, control-siRNA, IL1RN-siRNA, miR-122-5p inhibitor + control-siRNA or miR-122-5p inhibitor + IL1RN-siRNA for 24 h, after which RT-qPCR was performed to evaluate transfection efficiency. Compared with the inhibitor control group, the miR-122-5p inhibitor significantly reduced miR-122-5p expression in A549 cells (Fig. 5A). Compared with the control-siRNA group, IL1RN-siRNA significantly decreased IL1RN mRNA expression in A549 cells (Fig. 5B). In addition, the miR-122-5p inhibitor significantly increased IL1RN expression compared with the inhibitor control, and this increase was significantly reversed by IL1RN-siRNA (Fig. 5C and D).
Figure 5.
Transfection efficiency of miR-122-5p inhibitor and IL1RN-siRNA in A549 cells. (A) RT-qPCR analysis of miR-122-5p expression following A549 cell transfection with the inhibitor control or miR-122-5p inhibitor. (B) RT-qPCR analysis of IL1RN expression of A549 cells following transfection with control-siRNA or IL1RN-siRNA. (C) Western blotting and (D) RT-qPCR analysis of IL1RN expression in A549 cells following transfection with miR-122-5p inhibitor + control-siRNA or miR-122-5p inhibitor + IL1RN-siRNA. **P<0.01 vs. inhibitor control; ##P<0.01 vs. control-siRNA; &&P<0.01 vs. miR-122-5p inhibitor + control-siRNA. miR, microRNA; siRNA or si, small interfering RNA; IL1RN, IL-1 receptor antagonist; RT-qPCR, reverse transcription-quantitative PCR.
Effect of the miR-122-5p inhibitor on the LPS-induced ALI cell model
A549 cells were transfected with inhibitor control, miR-122-5p inhibitor, miR-122-5p inhibitor + control-siRNA or miR-122-5p inhibitor + IL1RN-siRNA for 24 h, and then subsequently induced with 10 µg LPS for 12 h. MTT assays revealed that LPS significantly decreased cell viability compared with the control (Fig. 6A). The LDH release assay indicated that LDH release was significantly increased in the LPS group compared with the control (Fig. 6B). The results from the flow cytometry assays demonstrated that LPS significantly promoted apoptosis compared with the control (Fig. 6C and D). Western blotting revealed that LPS significantly increased cleaved-caspase 3 expression (Fig. 6E) and the cleaved-caspase 3/caspase 3 ratio compared with the control (Fig. 6F). When compared with the LPS + inhibitor control group, the cell viability of the LPS + miR-122-5p inhibitor group was significantly increased (Fig. 6A), LDH release was significantly reduced (Fig. 6B), apoptosis was significantly decreased (Fig. 6C and D), the cleaved-caspase 3 protein expression was significantly decreased (Fig. 6E) and the cleaved-caspase 3/caspase 3 ratio was significantly decreased in the LPS + miR-122-5p inhibitor group (Fig. 6F). All these changes were significantly reversed by IL1RN-siRNA.
Figure 6.
miR-122-5p inhibitor protects A549 cells from LPS-induced injury by targeting IL1RN. (A) MTT assay of cell viability. (B) LDH release assay. (C) Flow cytometry analysis of apoptosis and (D) the apoptotic ratio. (E) Western blotting of cleaved-caspase 3 expression and (F) the cleaved-caspase 3/caspase 3 ratio. **P<0.01 vs. control; ##P<0.01 vs. LPS + inhibitor control; &&P<0.01 vs. LPS + miR-122-5p inhibitor + control-siRNA. miR, microRNA; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; IL1RN, IL-1 receptor antagonist; si, small interfering RNA.
Effects of miR-122-5p inhibitor on the expression of inflammatory cytokines
The effects of the miR-122-5p inhibitor on the expression of inflammatory cytokines were assessed. Compared with the control group, the secretion profiles of TNF-α, IL-1β and IL-6 in the cell supernatant of the LPS group were significantly increased (Fig. 7A-C), and the expression of IL1RN was significantly reduced (Fig. 7D and E). Compared with the LPS + inhibitor control group, the secretion profiles of TNF-α, IL-1β and IL-6 were significantly reduced in the LPS + miR-122-5p inhibitor group (Fig. 7A-C), and IL1RN expression was significantly increased (Fig. 7D and E). All of these changes were significantly reversed by IL1RN-siRNA.
Figure 7.
miR-122-5p inhibitor decreases inflammatory cytokine expression in the LPS-induced acute lung injury cell model. ELISA of (A) TNF-α, (B) IL-1β (C) and IL-6 levels. (D) Western blotting of IL1RN expression. (E) Reverse transcription-quantitative PCR analysis of IL1RN expression at the mRNA level. **P<0.01 vs. control; ##P<0.01 vs. LPS + inhibitor control; &&P<0.01 vs. LPS + miR-122-5p inhibitor + control-siRNA. miR, microRNA; IL1RN, IL-1 receptor antagonist; LPS, lipopolysaccharide; si, small interfering RNA.
Discussion
ALI can lead to the development of ARDS, which severely impacts an individual's life (2,3). The primary feature of ALI is abnormal respiratory function (4,5). Although treatment methods for ARDS are constantly improving, the mortality rate of patients with ARDS remains high at ~30% (29). Recently, miRNAs have been demonstrated to serve a notable role in the development of several types of diseases and have attracted significant attention. Yang and Zhao (30) revealed that miR-490-3p upregulation suppressed LPS-induced ALI via the IL-1 receptor-associated kinase 1/TNF receptor-associated factor 6 pathway. Furthermore, Li et al (31) demonstrated that miR-150 expression was downregulated in an LPS-induced ALI cell model, and that miR-150-overexpression inhibited LPS-induced ALI. Suo et al (32) demonstrated that miR-1246 inhibited ALI-induced inflammation via the NF-κB and Wnt/β-catenin signaling pathways. Results of a previous study indicated that miR-122-5p participates in the development of ALI via the DUSP4/ERK signaling axis (22). In the present study, another potential regulatory mechanism involving miR-122-5p in the development of ALI was assessed.
Several studies have demonstrated that miR-122-5p participates in the development of various diseases (19,20,33). Ma et al (33) revealed that miR-122-5p inhibited osteosarcoma cell proliferation. Ding et al (19) demonstrated that miR-122-5p promoted skeletal muscle myogenesis via transforming growth factor β receptor 2. Yang et al (20) demonstrated that miR-122-5p downregulation can protect against acetaminophen-mediated liver damage by upregulating NDRG family member 3 expression. The results of the present study indicated that the target gene downstream of miR-122-5p was IL1RN, and that there was a direct interaction between IL1RN mRNA and miR-122-5p. However, to validate IL1RN as the direct target gene of miR-122-5p, further investigations into the effects of an miR-122-5p inhibitor on the luciferase activity of IL1RN-WT are required. This was a limitation of the current study.
The IL-1 family consists of three members, IL-1α, IL-1β and IL1RN (34). IL1RN is an anti-inflammatory molecule that exhibits homology with IL-1α and IL-1β. IL1RN participates in the development of several types of cancer, such as prostate cancer (34), non-cardia gastric carcinoma (35), glioma (36) and bladder cancer (37). miRNAs are endogenous, non-coding, single-stranded small RNAs, which negatively regulate gene expression by interacting with the 3'-UTRs of target mRNAs at the posttranscriptional or translational level (38). The present study revealed that miR-122-5p negatively regulated IL1RN expression in A549 cells.
ALI is characterized by an inflammatory process that is associated with the upregulation of chemokines and inflammatory cytokines (39). Niu et al (40) indicated that when the lungs are infected, LPS binds to molecules on the surface of endothelial cells, thereby promoting the expression of various inflammatory cytokines, including IL-1β, IL-6, IL-8 and TNF-α. Overproduction of pro-inflammatory cytokines leads to severe lung damage, and the abnormal apoptosis of pulmonary cells is a pathophysiological feature of ALI (41,42). In the present study, miR-122-5p mimics promoted the expression of inflammatory cytokines. However, the miR-122-5p inhibitor suppressed TNF-α, IL-1β and IL-6 levels in LPS-treated A549 cells. Furthermore, miR-122-5p mimic suppressed cell viability and promoted apoptosis in LPS-treated A549 cells, with the miR-122-5p inhibitor demonstrating the opposite effect.
Caspase 3, a member of the caspase family (key effector molecules of apoptosis), is considered to serve an important role in the cascade of apoptosis and is regarded as an executor and terminator of multiple apoptotic pathways (43). After cleaved activation (cleaved-caspase 3), caspase 3 exerts a pro-apoptotic effect, and the increase of the cleaved-caspase 3/caspase 3 ratio reflects the activation of caspase 3 (43,44). The present study also analyzed caspase 3, and the data indicated that miR-122-5p mimic increased cleaved-caspase 3 protein expression and the cleaved-caspase 3/caspase 3 ratio in LPS-treated A549 cells, while the miR-122-5p inhibitor had the opposite effect.
In conclusion, downregulation of miR-122-5p reduced LPS-induced ALI by targeting IL1RN. However, the present study was only a preliminary in vitro study of the effect of miR-122-5p on an LPS-induced ALI cell model. In order to make the role of miR-122-5p in ALI more convincing, more in-depth research is required. For example, the role of miR-122-5p and IL1RN alone in A549 cells in the absence of LPS should be clarified, and the effect of miR-122-5p/IL1RN in an animal model of ALI should be explored. Moreover, the expression of miR-122-5p/IL1RN in patients with ALI and its association with the clinicopathological parameters of patients with ALI should be further studied.
Acknowledgements
Not applicable.
Funding Statement
Funding: No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JL and XZ contributed to the conception and design of the study, in addition to data acquisition, analysis and interpretation. JZ and XZ also drafted and critically revised the manuscript. WW contributed to data collection, statistical analysis and manuscript preparation. JL and XZ confirm the authenticity of all the raw data. All authors have read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Chen H, Bai C, Wang X. The value of the lipopolysaccharide-induced acute lung injury model in respiratory medicine. Expert Rev Respir Med. 2010;4:773–783. doi: 10.1586/ers.10.71. [DOI] [PubMed] [Google Scholar]
- 2.Avecillas JF, Freire AX, Arroliga AC. Clinical epidemiology of acute lung injury and acute respiratory distress syndrome: Incidence, diagnosis, and outcomes. Clin Chest Med. 2006;27:549–557. doi: 10.1016/j.ccm.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Bellani G, Laffey JG, Pham T, Fan E, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, et al. Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 4.Jongerius I, Porcelijn L, van Beek AE, Semple JW, van der Schoot CE, Vlaar APJ, Kapur R. The role of complement in transfusion-related acute lung injury. Transfus Med Rev. 2019;33:236–242. doi: 10.1016/j.tmrv.2019.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murray DD, Itenov TS, Sivapalan P, Eklöf JV, Holm FS, Schuetz P, Jensen JU. Biomarkers of acute lung injury the individualized approach: For phenotyping, risk stratification and treatment surveillance. J Clin Med. 2019;8(1163) doi: 10.3390/jcm8081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu H, Yu X, Yu S, Kou J. Molecular mechanisms in lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Int Immunopharmacol. 2015;29:937–946. doi: 10.1016/j.intimp.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 7.Lei J, Wei Y, Song P, Li Y, Zhang T, Feng Q, Xu G. Cordycepin inhibits LPS-induced acute lung injury by inhibiting inflammation and oxidative stress. Eur J Pharmacol. 2018;818:110–114. doi: 10.1016/j.ejphar.2017.10.029. [DOI] [PubMed] [Google Scholar]
- 8.Nova Z, Skovierova H, Calkovska A. Alveolar-capillary membrane-related pulmonary cells as a target in endotoxin-induced acute lung injury. Int J Mol Sci. 2019;20(831) doi: 10.3390/ijms20040831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reiss LK, Uhlig U, Uhlig S. Models and mechanisms of acute lung injury caused by direct insults. Eur J Cell Biol. 2012;91:590–601. doi: 10.1016/j.ejcb.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 10.Rebetz J, Semple JW, Kapur R. The pathogenic involvement of neutrophils in acute respiratory distress syndrome and transfusion-related acute lung injury. Transfus Med Hemother. 2018;45:290–298. doi: 10.1159/000492950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Zhang M, Zhong M, Suo Q, Lv K. Expression profiles of miRNAs in polarized macrophages. Int J Mol Med. 2013;31:797–802. doi: 10.3892/ijmm.2013.1260. [DOI] [PubMed] [Google Scholar]
- 12.Chen X, Slack FJ, Zhao H. Joint analysis of expression profiles from multiple cancers improves the identification of microRNA-gene interactions. Bioinformatics. 2013;29:2137–2145. doi: 10.1093/bioinformatics/btt341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei Y, Nazari-Jahantigh M, Chan L, Zhu M, Heyll K, Corbalán-Campos J, Hartmann P, Thiemann A, Weber C, Schober A. The microRNA-342-5p fosters inflammatory macrophage activation through an Akt1- and microRNA-155-dependent pathway during atherosclerosis. Circulation. 2013;127:1609–1619. doi: 10.1161/CIRCULATIONAHA.112.000736. [DOI] [PubMed] [Google Scholar]
- 14.Ono K, Kuwabara Y, Han J. MicroRNAs and cardiovascular diseases. FEBS J. 2011;278:1619–1633. doi: 10.1111/j.1742-4658.2011.08090.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ro S, Park C, Young D, Sanders KM, Yan W. Tissue-dependent paired expression of miRNAs. Nucleic Acids Res. 2007;35:5944–5953. doi: 10.1093/nar/gkm641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mallory AC, Vaucheret H. MicroRNAs: Something important between the genes. Curr Opin Plant Biol. 2004;7:120–125. doi: 10.1016/j.pbi.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 17.Garzon R, Calin GA, Croce CM. MicroRNAs in cancer. Annu Rev Med. 2009;60:167–179. doi: 10.1146/annurev.med.59.053006.104707. [DOI] [PubMed] [Google Scholar]
- 18.Meng L, Chen Z, Jiang Z, Huang T, Hu J, Luo P, Zhang H, Huang M, Huang L, Chen Y, et al. MiR-122-5p suppresses the proliferation, migration, and invasion of gastric cancer cells by targeting LYN. Acta Biochim Biophys Sin (Shanghai) 2020;521:49–57. doi: 10.1093/abbs/gmz141. [DOI] [PubMed] [Google Scholar]
- 19.Ding Z, Lin J, Sun Y, Cong S, Liu S, Zhang Y, Chen Q, Chen J. miR-122-5p negatively regulates the transforming growth factor-β/Smad signaling pathway in skeletal muscle myogenesis. Cell Biochem Funct. 2020;382:231–238. doi: 10.1002/cbf.3460. [DOI] [PubMed] [Google Scholar]
- 20.Yang Z, Wu W, Ou P, Wu M, Zeng F, Zhou B, Wu S. MiR-122-5p knockdown protects against APAP-mediated liver injury through up-regulating NDRG3. Mol Cell Biochem. 2021;476:1257–1267. doi: 10.1007/s11010-020-03988-0. [DOI] [PubMed] [Google Scholar]
- 21.Ge Y, Tu W, Li J, Chen X, Chen Y, Xu Y, Xu Y, Wang Y, Liu Y. MiR-122-5p increases radiosensitivity and aggravates radiation-induced rectal injury through CCAR1. Toxicol Appl Pharmacol. 2020;399(115054) doi: 10.1016/j.taap.2020.115054. [DOI] [PubMed] [Google Scholar]
- 22.Lu Z, Feng H, Shen X, He R, Meng H, Lin W, Geng Q. MiR-122-5p protects against acute lung injury via regulation of DUSP4/ERK signaling in pulmonary microvascular endothelial cells. Life Sci. 2020;256(117851) doi: 10.1016/j.lfs.2020.117851. [DOI] [PubMed] [Google Scholar]
- 23.Yazdi AS, Ghoreschi K. The interleukin-1 family. Adv Exp Med Biol. 2016;941:21–29. doi: 10.1007/978-94-024-0921-5_2. [DOI] [PubMed] [Google Scholar]
- 24.Lin SC, Lo YC, Wu H. Helical assembly in the MyD88-IRAK4-IRAK2 complex in TLR/IL-1R signalling. Nature. 2010;465:885–890. doi: 10.1038/nature09121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bui CB, Kolodziej M, Lamanna E, Elgass K, Sehgal A, Rudloff I, Schwenke DO, Tsuchimochi H, Kroon MAGM, Cho SX, et al. Interleukin-1 receptor antagonist protects newborn mice against pulmonary hypertension. Front Immunol. 2019;10(1480) doi: 10.3389/fimmu.2019.01480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun D, Zhao M, Ma D, Liao S, Di C. Protective effect of interleukin-1 receptor antagonist on oleic acid-induced lung injury. Chin Med J (Engl) 1996;109:522–526. [PubMed] [Google Scholar]
- 27.Zhou H, Wang X, Zhang B. Depression of lncRNA NEAT1 antagonizes LPS-evoked acute injury and inflammatory response in alveolar epithelial cells via HMGB1-RAGE signaling. Mediators Inflamm. 2020;2020(8019467) doi: 10.1155/2020/8019467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 29.Gill SE, Yamashita CM, Veldhuizen RA. Lung remodeling associated with recovery from acute lung injury. Cell Tissue Res. 2017;367:495–509. doi: 10.1007/s00441-016-2521-8. [DOI] [PubMed] [Google Scholar]
- 30.Yang G, Zhao Y. MicroRNA-490-3p inhibits inflammatory responses in LPS-induced acute lung injury of neonatal rats by suppressing the IRAK1/TRAF6 pathway. Exp Ther Med. 2021;21(152) doi: 10.3892/etm.2020.9584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Li P, Yao Y, Ma Y, Chen Y. MiR-150 attenuates LPS-induced acute lung injury via targeting AKT3. Int Immunopharmacol. 2019;75(105794) doi: 10.1016/j.intimp.2019.105794. [DOI] [PubMed] [Google Scholar]
- 32.Suo T, Chen GZ, Huang Y, Zhao KC, Wang T, Hu K. miRNA-1246 suppresses acute lung injury-induced inflammation and apoptosis via the NF-κB and Wnt/β-catenin signal pathways. Biomed Pharmacother. 2018;108:783–791. doi: 10.1016/j.biopha.2018.09.046. [DOI] [PubMed] [Google Scholar]
- 33.Ma W, Zhao X, Xue N, Gao Y, Xu Q. The LINC01410/miR-122-5p/NDRG3 axis is involved in the proliferation and migration of osteosarcoma cells. IUBMB Life. 2021;73:705–717. doi: 10.1002/iub.2452. [DOI] [PubMed] [Google Scholar]
- 34.Fan YC, Lee KD, Tsai YC. Roles of interleukin-1 receptor antagonist in prostate cancer progression. Biomedicines. 2020;8(602) doi: 10.3390/biomedicines8120602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rocha GA, Guerra JB, Rocha AM, Saraiva IE, da Silva DA, de Oliveira CA, Queiroz DM. IL1RN polymorphic gene and cagA-positive status independently increase the risk of noncardia gastric carcinoma. Int J Cancer. 2005;115:678–683. doi: 10.1002/ijc.20935. [DOI] [PubMed] [Google Scholar]
- 36.Wang K, Liu H, Liu J, Wang X, Teng L, Zhang J, Liu Y, Yao Y, Wang J, Qu Y, et al. IL1RN mediates the suppressive effect of methionine deprivation on glioma proliferation. Cancer Lett. 2019;54:146–157. doi: 10.1016/j.canlet.2019.04.004. [DOI] [PubMed] [Google Scholar]
- 37.Worst TS, Reiner V, Gabriel U, Weiß C, Erben P, Martini T, Bolenz C. IL1RN and KRT13 expression in bladder cancer: Association with pathologic characteristics and smoking status. Adv Urol. 2014;2014(184602) doi: 10.1155/2014/184602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartel DP. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–297. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 39.Li P, Zhou Y, Goodwin AJ, Cook JA, Halushka PV, Zhang XK, Wilson CL, Schnapp LM, Zingarelli B, Fan H. Fli-1 governs pericyte dysfunction in a murine model of sepsis. J Infect Dis. 2018;218:1995–2005. doi: 10.1093/infdis/jiy451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Niu X, Zang L, Li W, Xiao X, Yu J, Yao Q, Zhao J, Ye Z, Hu Z, Li W. Anti-inflammatory effect of yam glycoprotein on lipopolysaccharide-induced acute lung injury via the NLRP3 and NF-κB/TLR4 signaling pathway. Int Immunopharmacol. 2020;81(106024) doi: 10.1016/j.intimp.2019.106024. [DOI] [PubMed] [Google Scholar]
- 41.Li S, Zhang Y, Guan Z, Li H, Ye M, Chen X, Shen J, Zhou Y, Shi ZL, Zhou P, Peng K. SARS-CoV-2 triggers inflammatory responses and cell death through caspase-8 activation. Signal Transduct Target Ther. 2020;5(235) doi: 10.1038/s41392-020-00334-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yang L, Zhang Z, Zhuo Y, Cui L, Li C, Li D, Zhang S, Cui N, Wang X, Gao H. Resveratrol alleviates sepsis-induced acute lung injury by suppressing inflammation and apoptosis of alveolar macrophage cells. Am J Transl Res. 2018;10:1961–1975. [PMC free article] [PubMed] [Google Scholar]
- 43.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 44.Fan Y, Bergmann A. The cleaved-caspase-3 antibody is a marker of caspase-9-like DRONC activity in Drosophila. Cell Death Differ. 2010;17:534–539. doi: 10.1038/cdd.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.