Abstract
Background:
Depression is common among people living with HIV. Multiple studies demonstrate a link between depression and cognitive dysfunction in adults with HIV, but the association has been minimally investigated in children and adolescents with HIV in Africa.
Methods:
We conducted a cross-sectional analysis as part of the HIV-associated Neurocognitive Disorders in Zambia (HANDZ) study, a prospective cohort study in Lusaka, Zambia. We included 208 perinatally-infected children with HIV ages 8-17 taking antiretroviral therapy and 208 HIV-exposed uninfected (HEU) controls. Cognition was assessed with a comprehensive neuropsychological battery. Depressive symptoms were evaluated using self-report and parent-report versions of the NIH Toolbox Sadness module and the Patient Health Questionnaire-9 (PHQ-9). Risk factors for depression and associations between depressive symptoms and cognition were evaluated in bivariable and multivariable regression models.
Results:
Participants with HIV demonstrated higher levels of depressive symptoms than controls (mean NIH Toolbox Sadness T-Score 50 vs. 44, p<0.01; mean PHQ-9 score 2.0 vs. 1.5, p=0.03), and were more likely to have cognitive impairment (30% vs. 13%, p<0.001). Risk factors for depressed mood included self-reported poor health (OR 7.8, p<0.001) and negative life events (OR 1.3, p=0.004) Depressed mood was associated with cognitive impairment in participants with HIV (OR=2.9, 95% CI 1.2-7.2, p=0.02) but not in HEU participants (OR 1.7, 95% CI 0.18-15.7, p=0.6).
Conclusion:
Depressed mood is common among youth with HIV in Zambia, and is associated with cognitive impairment. Depression may be a result of HIV-related stress and stigma, or may be part of the spectrum of HIV-associated neurocognitive disorders. The causal relationship between depressed mood and cognitive impairment is unclear and should be evaluated in future longitudinal studies.
Keywords: HIV, depression, HIV-associated Neurocognitive Disorders, Pediatric Neurology, Zambia
Introduction
Antiretroviral therapy (ART) has transformed Human Immunodeficiency Virus (HIV) infection from a nearly universally fatal disease into a potentially manageable chronic condition.1-6 As fewer patients with HIV die from opportunistic infections, concern has increased regarding chronic complications and comorbidities associated with HIV.1,6 People with HIV are two to three times more likely to have depression compared to the general population7,8. Depression is one of the most common comorbidities in people with HIV, and is one of the largest contributors to decreased quality of life.7,8 Depression may negatively affect cognitive function both through direct effects and indirect effects mediated through poor adherence9-12. Neurobiological effects of HIV on the brain could contribute to depression and cognitive impairment through chronic neuroinflammation and alterations in dopamine and other neurotransmitters.11-19
Children and adolescents with perinatally-acquired HIV are at particularly high risk of depression.20 Factors potentially contributing to depression in this population include effects of chronic illness, worries about the future, and HIV-related stigma.21-23 In addition, adverse childhood experiences and negative life events such as illness and death in family members, and exposure to violence may be more common in children with HIV and may contribute to depressed mood.24-25 As children with HIV age into adulthood, potential effects of both depression and cognitive impairment on quality of life, adherence, and sexual decision-making are a major concern.6-8,11,12 However, there is little data on the relationship between depressive symptoms and cognition specifically among children and adolescents. In addition, there is even less data on this relationship within Sub-Saharan Africa, which is where more than 90% of children with HIV reside.1 Our current project was designed to address these knowledge gaps and was conducted as a sub-study of the HIV-associated Neurocognitive Disorders in Zambia (HANDZ) study26. Zambia is a Sub-Saharan nation with 1.1 million people living with HIV, including more than 72,000 children. The majority of HIV-infected youth in Zambia are treated with ART.27-28
The goal of this study is to evaluate the relationship between HIV status, depressed mood, and cognition in children and adolescents with HIV in Zambia, and to identify risk factors for depressed mood in this population. We hypothesized that depressed mood would be more common in children with HIV than in HIV-exposed uninfected (HEU) controls, and that depressive symptoms would be associated with cognitive impairment. We further hypothesized that risk factors for depression in children with HIV would include health related factors, HIV-specific factors (e.g. CD4 count and WHO Stage), and negative life events.
Methods
Overview
Full details of the methods for the parent HIV-associated Neurocognitive Disorders in Zambia (HANDZ) study have previously been described.26 Briefly, the HANDZ study is a prospective, longitudinal study of neurocognition and psychiatric comorbidities in children and adolescents with HIV living in Zambia. In this paper, we present cross-sectional results on depression and cognition collected at the baseline visit of the HANDZ study.
Study Site and Participants
All research activities were conducted at the Pediatric HIV/AIDS Center of Excellence (PCOE) at the University Teaching Hospital (UTH) in Lusaka, the capital city of Zambia. Recruitment was conducted from November 2017 to November 2018. HIV-infected youth were recruited at routine outpatient medication refill visits to PCOE. Youth in the HIV-exposed uninfected (HEU) group were recruited from the Lusaka community by a community health worker from the same neighborhoods in which participants with HIV resided. Stratified sampling was used to ensure comparable between-group distribution of the participants according to age, sex, and neighborhood of residence in Lusaka.
Inclusion and Exclusion Criteria
Eligible participants were 8-17 years old at time of enrollment with at least one living biological parent available to give consent and participate in the study with their child. Inclusion criteria for participants with HIV included perinatally acquired HIV with infection status confirmed by Western blot or DNA PCR, current treatment with ART, and treatment with ART initiated at least one year prior to enrollment. For the HEU control group, inclusion criteria included prenatal HIV exposure documented through maternal or child medical records, and current HIV-negative status confirmed by immunoassay. Exclusion criteria for both groups included: history of central nervous system infection with an organism other than HIV; HIV infection known to be secondary to sexual contact or blood transfusion; and inability of child to understand and communicate basic concepts in English.
Sample Size Estimation
A sample size of 200 HIV-infected participants and 200 HEU controls was planned based on simulation studies for each aim of the parent study, with the goal of ensuring model stability and avoiding overfitting for regression models. Assuming rates of depressed mood of at least 20% in the population of children with HIV, the study had 80% power to detect odds ratios of 1.5 or greater in the logistic regression analysis. Participants were over-enrolled by 4% in order to guarantee appropriate power remained despite potential losses to follow up.
Data Collected:
Data were collected using a combination of standardized participant and parent interviews, chart review, and standardized neuropsychological testing. General physical health was evaluated by self-report using a 5-point scale ranging from very good to very bad. Participants were considered to have poor health if they answered that their general physical health was either “somewhat bad” or “very bad.” Socioeconomic status (SES) was evaluated using an adapted version of the Multiple Indicator Cluster Survey-5 (MICS5).29 The questionnaire evaluates access to running water and electricity, toilet facilities, food security, and review of household items that are markers of wealth (e.g., radio, mobile phone, television, etc.). Measures of socioeconomic status are combined into a socioeconomic status index (SESI). History of malnutrition was determined using growth curves abstracted from medical records and comparing them against the WHO definition of malnutrition and severe malnutrition.30
HIV History:
HIV-related history was obtained via participant and parent interview and through chart review. Information collected included age at ART initiation, current and past ART, current and lowest recorded CD4 count, current and worst recorded Word Health Organization (WHO) Clinical Stage, past hospitalizations, and history of opportunistic infections. Measures of current and historical HIV disease severity (current and lowest recorded CD4 counts; current and worst recorded WHO Stage; and number of previous hospitalizations) were combined into a disease severity index.
Negative Life Events:
A ten question survey developed in Zambia regarding negative life events over the prior 12 months was administered to each participant.26 The survey includes questions related to illness, hospitalization and death of family members, change in family structure, change of residence, violence and abuse, and other negative life events. The survey generates a negative life events score of 0-10, with higher scores indicating a greater number of negative life events.
Neuropsychological Assessment
Cognitive testing:
Details on cognitive testing have previously been described.26 Briefly, cognition across eight cognitive domains was tested, including attention, set shifting, inhibition, immediate recall, processing speed, motor speed, verbal fluency, and nonverbal reasoning. An age-and-sex-adjusted Z-score was calculated for each cognitive domain. Individual domain Z-scores were then added together and a Z-score taken of this total score, referred to as an NPZ8 score, was calculated as a summary measure of cognition. For our primary analysis of cognitive impairment, we defined cognitive impairment as an NPZ8 score more than 1 standard deviation below the mean for HEU controls. We also performed sensitivity analyses using alternate definitions of cognitive impairment including a global deficit score approach30-31 and using Frascati criteria32, to determine whether different conceptualizations of cognitive impairment would be more or less sensitive to the effects of depression. For our definition of cognitive impairment by global deficit score criteria we used the standard cutoff with a global deficit score of 0.5 or greater indicating impairment.30-31
Mood Assessment:
For our primary measurement of depressive symptoms we utilized the NIH Toolbox Sadness self-report module, as this was noted to be the instrument with the best performance characteristics in a simultaneously performed mixed-methods study used for adapting and validating the instruments (unpublished data). Depressed mood was also assessed using the NIH Toolbox Sadness parent-report modules33-36, and the parent-proxy and child self-report Patient Health Questionnaire, nine item (PHQ-9) Modified form for Adolescents.37 In a secondary analysis, we combined information from each of the assessment instruments for depressed mood into an index. Semi-structured qualitative interviews were conducted with each participant expressing depressive symptoms to assess whether symptoms were clinically significant and to assess participants’s interpretation of the cause of their depressive symptoms. We defined depressed mood as an NIH Toolbox Sadness score greater than the 90th percentile in the HEU control population (Sadness T-score>60), while “Clinically significant depressed mood” was defined as depressed mood resulting in self-reported functional impairment. Participants identified by any of the study assessments to be at-risk for harm due to suicidality, violence or abuse, or with clinically significant depressed mood were referred to a psychiatrist for evaluation and treatment.
Statistical Methods:
All statistical analyses were performed using Stata 16.1 (StataCorp 2020, College Station, Tx, USA). Dichotomous variables were compared between groups using a Chi-Squared test, while continuous variables were compared using the Kruskall-Wallis test for non-normally distributed variables and T-tests for normally distributed continuous variables. Risk factors for dichotomous outcomes were evaluated using multivariable logistic regression models. The linearity of the relationship between NPZ8 score and level of depressive symptoms was explored using bivariable and multivariable regression splines using the “mvrs” package in Stata.38 All initial regression models included the prespecified variables age, sex, socioeconomic status index, self-reported poor health, growth stunting, and negative life event index. Models for participants with HIV also included disease severity index as an exposure variable. Final models were generated using a manual stepwise backward selection procedure. Missing data were handled by pairwise deletion.
Ethics Statement:
This study was approved by the institutional review boards of the University of Rochester (protocol #00068985), the University of Zambia (reference #004-08-17), and the National Health Research Association of Zambia. Verbal and written informed consent was sought from the parents of all participants for participation in the study, and verbal and written assent was sought from all participants over the age of 12.
Results
Demographics:
A total of 416 participants were enrolled, including 208 with HIV and 208 HEU controls. 3 participants with HIV and 17 participants without HIV could not be assessed due to missing data. Due to stratified sampling, age, sex, and neighborhood of residence were similar between participants with HIV and HEU controls (see Table 1). HIV-infected participants had higher socioeconomic status than controls, were more likely to attend school, and were less likely to report difficulty accessing enough food. Participants with HIV were more likely to have a history of malnutrition, and more likely to have a deceased mother. All participants with HIV were treated with ART, with the most common regimen being tenofovir, lamivudine, and efavirenz, a common first line regimen in this age group in Zambia. The majority were adherent to ART by both self-report and provider report, and most had undetectable viral loads at the time of enrollment (see Table 2). The mean duration of time on ART was 7.5 years. Most participants with HIV had relatively high CD4 counts, and almost all participants were asymptomatic according to WHO Staging11.
Table 1:
Baseline Characteristics of Study Participants in the HANDZ Study, Stratified by HIV Status
| Variable | HIV-exposed uninfected |
HIV- infected |
P- value* |
|---|---|---|---|
| Mean age in years (SD) | 12 (2.8) | 11.6 (2.4) | 0.16 |
| Male Sex (%) | 48% | 55% | 0.13 |
| Mean Socioeconomic Status Index (SD) | 4.8 (2.0) | 6.0 (2.7) | <0.001 |
| Median Negative Life Event Index (IQR) | 1 (0-3) | 1 (0-3) | 0.45 |
| Poor health by self-report (%) | 6% | 10% | 0.13 |
| Deceased mother (%) | 0.0% | 9.5% | <0.001 |
| History of Malnutrition (%) | 4% | 31% | <0.001 |
| Difficulty accessing enough food (%) | 69% | 54% | 0.003 |
| Proportion currently attending school (%) | 86% | 92% | 0.04 |
| Mean NPZ8 Score (SD) | 0.2 (1.0) | −0.2 (1.0) | <0.001 |
| Median Global Deficit Score (IQR) | 0 (0-0.38) | 0.25 (0-.63) | <0.001 |
All p-values calculated using a chi-squared test for dichotomous variables, and Kruskal-Wallis rank test or t-test for continuous variables. P-values <=0.05 are in bold. SD=standard deviation; IQR=interquartile range; NPZ8=Neuropsychological test summary Z-Score
Table 2:
Baseline Characteristics of Participants with HIV in the HANDZ Study, Stratified by Presence of Depressed Mood
| Variable | No Depressed Mood (n=159) |
Depressed Mood (n=46) |
P-value* |
|---|---|---|---|
| Mean age (SD) | 11.6(2.2) | 12.0 (2.6) | 0.34 |
| Sex (% male ) | 55% | 55% | 0.92 |
| Poor health by self-report | 8% | 29% | 0.004 |
| Median Negative Life Event Index (IQR) | 1 (0-2) | 2 (1-3) | <0.001 |
| Socioeconomic Factors | |||
| Mean SESI (SD) | 5.9 (2.7) | 6.2(2.8) | 0.55 |
| Non-parental caretaker (%) | 20% | 15% | 0.53 |
| Living mother (%) | 90% | 96% | 0.23 |
| History of Malnutrition (%) | 30% | 33% | 0.74 |
| School Attendance (%) | 93% | 89% | 0.37 |
| Difficulty accessing enough food (%) | 44% | 57% | 0.14 |
| HIV-specific characteristics | |||
| Median disease severity index (IQR) | 3 (2-4) | 3 (2-4) | 0.95 |
| Viral Load Undetectable (%) | 88% | 80% | 0.14 |
| Adherence (%) | 94% | 91% | 0.43 |
| Mean age at starting ART, in years (SD) | 5.1(1.2) | 5.0(1.3) | 0.57 |
| Current CD4 (mean (SD)) | 774(340) | 769(386) | 0.93 |
| Nadir CD4 (mean (SD)) | 589(323) | 548(332) | 0.48 |
| Current WHO Stage, n in stage 1/2/3/4 | 147/2/5/0 | 46/0/0/0 | 0.16 |
| Worst WHO Stage, n in stage 1/2/3/4 | 15/23/41/72 | 10/5/11/18 | 0.36 |
All p-values calculated using a chi-squared test for dichotomous variables, and Kruskall-Wallis rank test or t-test for continuous variables. SESI, socioeconomic status index; ART, antiretroviral therapy; WHO, World Health Organization.
Risk factors for depression:
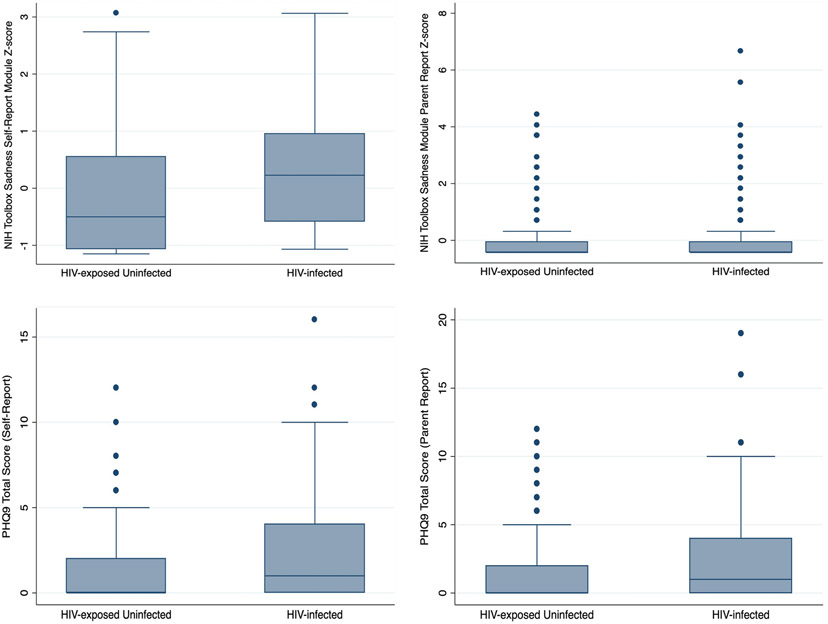
Participants with HIV had higher levels of depressive symptoms on all instruments utilized except for the parent-report module of the NIH Toolbox (see Figure 1; mean NIH Toolbox Sadness T-Score 50 vs. 44, p<0.01; mean PHQ-9 score 2.0 vs. 1.5, p=0.03). The association between HIV and depressive symptoms remained significant when all instruments were combined into a depression index (Depression index Z-score −0.23 vs. 0.21, p<0.001). A significantly greater proportion of participants with HIV demonstrated depressed mood (22% vs. 9%, p=0.03) and clinically significant depressed mood (10% vs. 2%, p=0.003.) HIV status remained significantly associated with NIH Toolbox Sadness T-Score in a multivariable linear regression model after adjusting for age, sex, socioeconomic status, negative life events, and self-reported poor health (ß=5.6; p<0.001). In the bivariable analysis evaluating risk factors for clinically significant depression including both HIV-Infected and HEU participants, risk factors included HIV status, self-reported poor health, and negative life event index (see Table 3). Neither age, sex, nor socioeconomic status was associated with depression. In a multivariable model including all prespecified variables, variables noted to be significant in the bivariable analysis (HIV status, poor health, and negative life events) remained significant in the multivariable model, with odds ratios similar to the univariate analysis. Removing age, sex, and socioeconomic status from the model in the backwards selection procedure did not significantly affect the odds ratios for the remaining variables, or the pseudo-R2 of the model (pseudo R2 for both full and reduced model=0.14). When separate models were fit for HIV-infected and HEU participants, odds ratios for each risk factor remained similar to those seen in the combined model with the exception of self-reported poor health, which was much more strongly associated with depression among HEU participants (OR 19.2; p<0.001) than among HIV-infected participants (OR 2.9; p=0.03). We did not identify any other significant differences between participants with and without depression (see Table 2). Participants with HIV most commonly attributed their depressive symptoms to fears of illness or death from HIV (in 27%), and to HIV-related stigma (in 25%). HEU participants most commonly attributed depressive symptoms to negative life events (in 35%) and to family stress related to poverty (in 35%).
Figure 1:
Depressive symptoms by HIV status as measured by the NIH Toolbox Sadness Module Self-report and Parent Report and the Patient Health Questionnaire 9 (PHQ-9). Depressive symptoms were significantly higher on all instruments utilized except for the Parent Report Version of the PHQ-9.
Table 3:
Bivariable Logistic Regression Analysis Identifying Risk Factors for Cognitive Impairment, Depressed Mood, and Both Cognitive Impairment and Depressed Mood.
| Outcome Variable | Exposure Variable | Odds Ratio (95% CI) | P-value |
|---|---|---|---|
| Depressed Mood | |||
| Age | 1.0 (0.8-1.1) | 0.69 | |
| Male Sex | 0.7 (0.3-1.4) | 0.33 | |
| HIV Status | 4.0 (1.5-10.9) | 0.006* | |
| Socioeconomic Status Index | 1.0 (0.8-1.2) | 0.97 | |
| Self-reported poor health | 7.8 (3.1-19.3) | <0.001* | |
| Negative Life Event Index | 1.3 (1.1-1.7) | 0.004* | |
| Growth Stunting | 1.3 (0.5-3.1) | 0.55 | |
| Cognitive Impairment | |||
| Age | 1.0 (1.0-1.1) | 0.43 | |
| Male Sex | 1.0 (0.6-1.7) | 0.89 | |
| HIV Status | 2.9 (1.7-4.8) | <0.001* | |
| Socioeconomic Status Index | 0.8 (0.7-0.9) | <0.001* | |
| Self-reported poor health | 3.6 (1.7-7.7) | 0.001* | |
| Negative Life Event Index | 1.0 (0.9-1.2) | 0.68 | |
| Growth Stunting | 5.9 (3.5-9.9) | <0.001* | |
| Cognitive Impairment + Depressed Mood | |||
| Age | 1.1 (0.9-1.3) | 0.27 | |
| Male Sex | 1.0 (0.4-2.6) | 0.93 | |
| HIV Status | 5.1 (1.4-17.6) | 0.011* | |
| Socioeconomic Status Index | 0.8 (0.6-1.0) | 0.046* | |
| Self-reported poor health | 10.4 (3.8-28.3) | <0.001* | |
| Negative Life Event Index | 1.4 (1.1-1.9) | 0.007* | |
| Growth Stunting | 2.8 (1.1-7.0) | 0.033* |
All odds ratios and p-values calculated using bivariable logistic regression.
Risk factors for cognitive impairment:
Participants with HIV had significantly lower mean NPZ8 scores (−0.2 in HIV+ vs. 0.2 in HEU, p<0.001) and significantly higher global deficit scores (median 0.25 in HIV+ vs 0.0 in HEU; p<0.001) than HEU controls, indicating poorer cognitive function. A significantly greater proportion of participants with HIV met criteria for cognitive impairment no matter which definition of cognitive impairment (NPZ8, Frascati Criteria, or GDS) was utilized. By NPZ8 criteria, 30% of participants with HIV vs. 13% of HEU controls had cognitive impairment (p<0.001). Risk factors for cognitive impairment included HIV status, self-reported poor health, socioeconomic status index, and history of growth stunting (see Table 3).
Association between depressed mood and cognitive function:
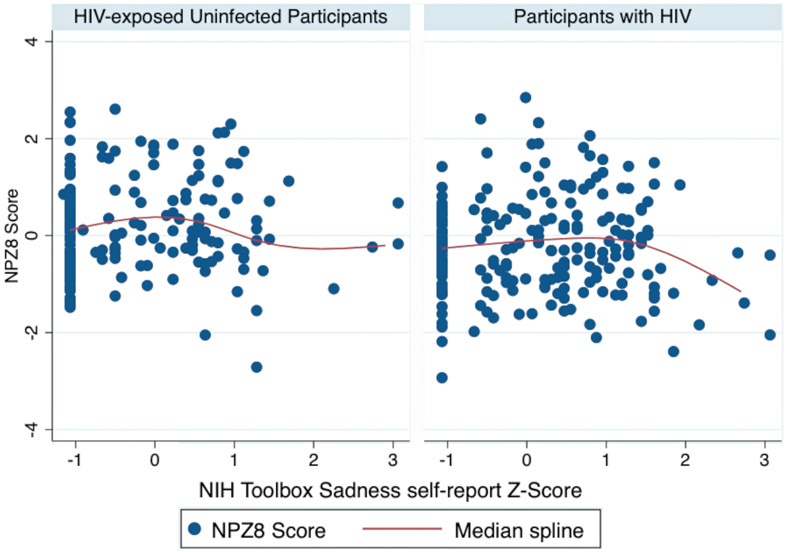
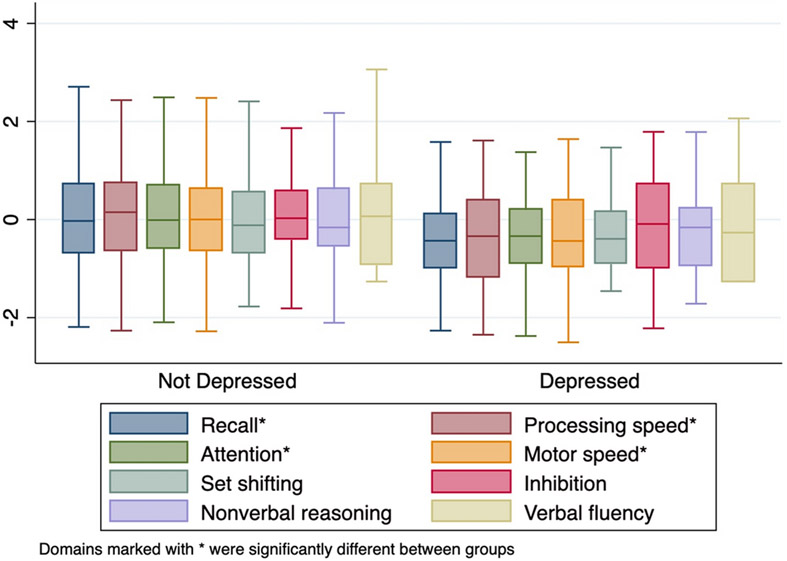
In the bivariable analysis, clinically significant depressed mood was strongly associated with cognitive impairment (Unadjusted OR=3.3, 95% CI 1.5-7.5, p=0.004) in the combined HIV-infected and HEU population. However, this was driven by a strong relationship in the participants with HIV (OR 2.9, 95% CI 1.2-7.2, p=0.02) with a nonsignificant relationship in the HEU group (OR 1.7, 95% CI 0.2-15.7, p=0.6). In a multivariable model controlling for age, sex, HIV status, poor health, and socioeconomic status, the relationship between depressed mood and cognitive impairment remained significant (Adjusted OR 2.8, 95% CI 1.04-7.3, p=0.04). Using multivariable adaptive regression splines we identified a nonlinear association between depression and cognition in participants with HIV but not HEU controls (see Figure 2). In participants with HIV, there was a threshold effect, or “knot,” in which participants with NIH Toolbox Sadness T-Scores ≥59 had a stronger relationship between sadness scores and lower NPZ8 scores (ß for values >=59=−0.21, p; p=0.002; ß for values <59 =−0.007, p=0.9). The cognitive domains with the strongest association with depression were immediate recall, attention, processing speed and motor speed, with nonsignificant effects on set shifting, inhibition, nonverbal reasoning and verbal fluency (see Figure 3). Sensitivity analysis showed that the relationship between depression and cognition was relatively consistent no matter which definition of cognitive impairment was utilized (Odds ratio for impairment by Frascati criteria 3.1, 95% CI 1.3-7.5, p=0.01; Odds ratio for impairment by global deficit score criteria 2.9, 95% CI 1.2-6.9, p=0.01).
Figure 2:
Scatter plot with superimposed median spline plot of depressive symptoms on the NIH Toolbox Sadness Self-report module vs. NPZ8 score, demonstrating stronger relationship between depressive symptoms and poor cognitive performance in participants with HIV vs. HEU controls.
Figure 3:
Cognitive domain scores by depression status, ordered by difference between depressed and non-depressed participants. Immediate recall, processing speed, attention, and motor speed were all significantly worse in participants with HIV compared to HEU controls.
Discussion
In this study we evaluated the relationship between depressed mood and cognitive function among children and adolescents with and without HIV in Lusaka, Zambia. Similar to prior studies in adults, we found higher rates of depressive symptoms among participants with HIV compared to a demographically similar HIV-exposed uninfected population. This difference in depressive symptoms was found on three of the four instruments used, with the notable exception of the parent report of the NIH Toolbox sadness module. Overall, parents scored their child lower on the sadness and/or depression scale than the children scored themselves. Our qualitative interviews suggested that parents may tend to downplay depressive symptoms in their children, and that this was more pronounced on the parent report version of the NIH Toolbox Sadness module compared to the PHQ-9 as the questions reference more subjective assessments of mood as compared to objective descriptors of behaviors. Differences between HIV-infected and HEU groups on depressive symptoms were not driven by any measured variables in the study such as socioeconomic status, and in fact, socioeconomic status was significantly higher in the HIV-infected group. This difference in socioeconomic status was likely due to two factors. First, wealthier and more educated people with HIV might choose to bring their children to PCOE due to its reputation for high-quality care, and might be more likely to participate in a study of cognition. Second, wealthier and more educated people in Zambia without HIV may have been less likely to be out in the communities from which participants were recruited, and may have been less likely to participate in a longitudinal study with a significant time commitment. Despite the fact that the HIV+ group was wealthier and had better food security, it was notable that malnutrition, stunting, and wasting, were all more common in the HIV+ population, with chart review and growth curves suggesting that this was likely due to cachexia and wasting that occurred prior to starting ART.
Risk factors for depressed mood were generally similar in the HIV-infected and HEU groups, and as we had hypothesized included both health-related factors and negative life events. Contrary to our hypothesis, measures of HIV-specific disease severity (e.g. CD4 count, viral load, WHO stage) had no correlation with depressed mood either alone or in combination. This may be because most participants with HIV were relatively healthy at the time of enrollment and thus mood was not strongly affected by their HIV history. However, qualitative interviews suggested that some of the increase in depressive symptoms in participants with HIV were likely due to fears of illness and death from HIV and to HIV-related stigma.
Strengths of the study include the relatively large sample size, well-defined population, and use of a multi-informant and multi-method assessment of depressive symptoms. This study complements prior work on depression and cognition in adults and extends these findings into adolescents and younger children. In a recent review by Rubin and colleagues7, 85% of cross-sectional studies also found a link between depression and cognitive impairment in participants with HIV. However, the majority of these investigations focused on adults, with only a single study of adolescents ages 15-18, and none of these studies included children younger than 15. Our data suggest that the relationship between depressive symptoms and cognition is non-linear in participants with HIV. That is, depressive symptoms above a certain threshold are associated with worse cognition, while below that threshold there is little to no relationship. This makes intuitive sense, in that there is little reason to believe that being relatively happier or sadder within the spectrum of normal mood would effect cognitive performance, while having a severely depressed mood might be expected to affect a range of cognitive processes such as memory, attention, and processing speed, and motor speed, which were, in fact, the cognitive processes most affected in this study. In addition, the fact that this nonlinear relationship was seen in participants with HIV but not in HEU controls is suggestive of the possibility that depression may be part of the spectrum of HIV-associated cognitive impairment.
In this cross-sectional study, we were not able to evaluate the causal relationship between risk factors, depression, and cognitive function. Depression may impair cognition, but it is possible that this relationship is reversed, and in fact poorer cognitive function causes depressive symptoms, or that both depression and poor cognition are explained by another factor. For example, both depression and cognitive impairment may be part of the spectrum of HIV-associated Neurocognitive Disorders (HAND). There is extensive published literature on the relationship between chronic inflammation and depression39-51, and chronic inflammation from HIV may contribute to both HAND and depression17-21. Further investigation of the causal relationship between variables will be evaluated in our longitudinal study in which methods better suited for assessment of causality such as structural equation modeling can be utilized.
Limitations, Bias, and Generalizability:
This study was conducted in a single center at a referral center in the capital city of Lusaka. Patients with HIV infection who are followed at referral centers tend to have greater medical needs than those who are randomly sampled from the community, and to the extent that poor health might contribute to both depression and poor cognitive function, sampling from a referral center might be more likely to identify a strong relationship between these factors. However, HIV-infected participants in our study were relatively healthy with high CD4 counts and few had a history of opportunistic infections, suggesting that referral bias is unlikely to be a major driver of our results. Our study excluded patients with no living biological parents, and it is possible that depression is more common and more severe in children with HIV without living parents, although the data on this are mixed5,52-54 With these caveats, we would anticipate that the study would generalize well to other urban centers in Sub-Saharan Africa.
All participants in this study spoke English. However, some questions and concepts utilized in the study were beyond the English language skills of some participants, and standard translations into local languages were utilized. However, there is no word in Nyanja or other commonly spoken local languages that is exactly equivalent to the English word for “depression,” and it is not clear that the cultural construct we are measuring is identical to the western construct of depression.55-58 There is also no validated definition of major depressive disorder in Zambia, and so instead we used the term “Clinically significant depressed mood,” to refer to the construct of high levels of depressive symptoms associated with depression-related impairment in everyday function. This may not map completely onto the construct of major depressive disorder.59-62 We have attempted to address this limitation through qualitative interviews to identify similarities and potential differences in these constructs. One additional limitation is that we did not utilize instruments to quantitate HIV-related stigma, although this will be measured and analyzed in our longitudinal analysis.
Conclusion
Our study establishes that clinically significant depressive symptoms are common among children and adolescents with HIV in Zambia, and that depressed mood is associated with cognitive dysfunction in this population. The relationship between depressed mood and cognitive impairment is stronger in children with HIV than in HEU controls. It remains unclear whether depressed mood causes cognitive impairment in children with HIV, or whether some other factor such as chronic immune activation leads to both depressed mood and cognitive impairment. Optimal treatment strategies for depression in children and adolescents with HIV remain to be determined, and the extent to which cognitive impairment is reversible with interventions to treat depression will need to be assessed in future studies.
Acknowledgments
Funding Source: This work was supported by the University of Rochester Center for AIDS Research (CFAR), an NIH funded program (P30 AI 045008); the McGowan Foundation; and the National Institute of Neurological Disorders and Stroke of the National Institutes of Health under Award Number R01NS094037 and K23NS117310. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest: All authors have no relevant conflicts of interest to disclose.
Data presented in this paper have previously been reported in abstract form at the American Academy of Neurology Annual Meeting, Philadelphia, PA, May 5, 2019.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
References
- 1.Buch S, Chivero ET, Hoare J et al. Proceedings from the NIMH symposium on “NeuroAIDS in Africa: neurological and neuropsychiatric complications of HIV”. J Neurovirol. 2016;22 (5):699–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen S, Ter Stege JA, Geurtsen GJ et al. Poorer cognitive performance in perinatally HIV-infected children versus healthy socioeconomically matched controls. Clin Infect Dis. 2015;60 (7): 1111–1119. [DOI] [PubMed] [Google Scholar]
- 3.Boivin MJ, Barlow-Mosha L, Chernoff MC et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS. 2018;32 (2):189–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilmshurst JM, Hammond CK, Donald K, Hoare J, Cohen K, Eley B. NeuroAIDS in children. Handb Clin Neurol. 2018;15299–116. [DOI] [PubMed] [Google Scholar]
- 5.Abubakar A Biomedical risk, psychosocial influences, and developmental outcomes: lessons from the pediatric HIV population in Africa. New Dir Child Adolesc Dev. 2014;2014 (146):23–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thakur KT, Boubour A, Saylor D, Das M, Bearden DR, Birbeck GL. Global HIV neurology: a comprehensive review. AIDS. 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin LH, Maki PM. HIV, Depression, and Cognitive Impairment in the Era of Effective Antiretroviral Therapy. Curr HIV AIDS Rep. 2019; 16: 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carvalhal A, Baril JG, Crouzat F et al. Recognizing cognitive and psychiatric changes in the post-highly active antiretroviral therapy era. Can J Infect Dis Med Microbiol. 2012; 23: 209–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah S, Sinharay S, Matsuda K et al. Potential Mechanism for HIV-Associated Depression: Upregulation of Serotonin Transporters in SlV-Infected Macaques Detected by 11C-DASB PET. Front Psychiatry. 2019; 10: 362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rivera-Rivera Y, Vázquez-Santiago FJ, Albino E, Sánchez MD, Rivera-Amill V. Impact of Depression and Inflammation on the Progression of HIV Disease. J Clin Cell Immunol. 2016; 7: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tymchuk S, Gomez D, Koenig N, Gill MJ, Fujiwara E, Power C. Associations between Depressive Symptomatology and Neurocognitive Impairment in HIV/AIDS. Can J Psychiatry. 2018; 63: 329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rabkin JG, Ferrando SJ, van Gorp W, Rieppi R, McElhiney M, Sewell M. Relationships among apathy, depression, and cognitive impairment in HIV/AIDS. J Neuropsychiatry Clin Neurosci. 2000; 12: 451–457. [DOI] [PubMed] [Google Scholar]
- 13.Saylor D, Kumar A, Nakigozi G et al. Interleukin-6 is associated with mortality and neuropsychiatric outcomes in antiretroviral-naïve adults in Rakai, Uganda. J Neurovirol. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kalungwana L, Elafros MA, Siddiqi OK et al. Cognitive impairment and psychiatric morbidity in HIV+ Zambians with new-onset seizure. Am J Trop Med Hyg. 2014;91(6):1254–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McGuire JL, Kempen JH, Localio R, Ellenberg JH, Douglas SD. Immune markers predictive of neuropsychiatric symptoms in HIV-infected youth. Clin Vaccine Immunol. 2015; 22: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Norcini Pala A, Steca P, Bagrodia R et al. Subtypes of depressive symptoms and inflammatory biomarkers: An exploratory study on a sample of HIV-positive patients. Brain Behav Immun. 2016; 56: 105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rivera-Rivera Y, García Y, Toro V et al. Depression Correlates with Increased Plasma Levels of Inflammatory Cytokines and a Dysregulated Oxidant/Antioxidant Balance in HIV-1-Infected Participants Undergoing Antiretroviral Therapy. J Clin Cell Immunol. 2014; 5: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McGuire JL, Kempen JH, Localio R, Ellenberg JH, Douglas SD. Immune markers predictive of neuropsychiatric symptoms in HIV-infected youth. Clin Vaccine Immunol. 2015; 22: 27–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Del Guerra FB, Fonseca JL, Figueiredo VM, Ziff EB, Konkiewitz EC. Human immunodeficiency virus-associated depression: contributions of immuno-inflammatory, monoaminergic, neurodegenerative, and neurotrophic pathways. J Neurovirol. 2013; 19: 314–327.20. [DOI] [PubMed] [Google Scholar]
- 20.Haines C, Loades ME, Coetzee BJ, Higson-Sweeney N. Which HIV-infected youth are at risk of developing depression and what treatments help? A systematic review focusing on Southern Africa. Int J Adolesc Med Health. 2019; [DOI] [PubMed] [Google Scholar]
- 21.Wilmshurst JM, Hammond CK, Donald K, Hoare J, Cohen K, Eley B. NeuroAIDS in children. Handb Clin Neurol. 2018; 152: 99–116. [DOI] [PubMed] [Google Scholar]
- 22.Benton TD, Kee Ng WY, Leung D, Canetti A, Karnik N. Depression among Youth Living with HIV/AIDS. Child Adolesc Psychiatr Clin N Am. 2019; 28: 447–459. [DOI] [PubMed] [Google Scholar]
- 23.Hoare J, Phillips N, Brittain K, Myer L, Zar HJ, Stein DJ. Mental Health and Functional Competence in the Cape Town Adolescent Antiretroviral Cohort. J Acquir Immune Defic Syndr. 2019; 81: e109–e116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Young-Wolff KC, Sarovar V, Sterling SA et al. Adverse childhood experiences, mental health, substance use, and HIV-related outcomes among persons with HIV. AIDS Care. 2019; 31: 1241–1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cuca YP, Shumway M, Machtinger EL et al. The Association of Trauma with the Physical, Behavioral, and Social Health of Women Living with HIV: Pathways to Guide Trauma-informed Health Care Interventions. Womens Health Issues. 2019; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Adams HR, Mwanza-Kabaghe S, Mbewe EG et al. The HIV-Associated Neurocognitive Disorders in Zambia (HANDZ) Study: Protocol of a research program in pediatric HIV in sub-Saharan Africa. J HIV AIDS Infect Dis 5: 1–18 [Google Scholar]
- 27.Nakazwe C, Michelo C, Sandøy IP, Pylkesnes K. Contrasting HIV prevalence trends among young women and men in Zambia in the past 12 years: data from demographic and health surveys 2002-2014. BMC Infect Dis. 2019; 19: 432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saito S, Chung H, Mahy M et al. Pediatric HIV Treatment Gaps in 7 East and Southern African Countries: Examination of Modeled, Survey, and Routine Program Data. J Acquir Immune Defic Syndr. 2018; 78Suppl 2: S134–S141. [DOI] [PubMed] [Google Scholar]
- 29.Khan S, Hancioglu A. Multiple Indicator Cluster Surveys: Delivering Robust Data on Children and Women across the Globe. Stud Fam Plann. 2019; 50: 279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Onis M, Garza C, Victora CG, Onyango AW, Frongillo EA, Martines J. The WHO Multicenter Growth Reference Study: Planning, study design and methodology. Acta Pediat Supp. 2006;447:12–24 [DOI] [PubMed] [Google Scholar]
- 31.Carey CL, Woods SP, Gonzalez R et al. Predictive validity of global deficit scores in detecting neuropsychological impairment in HIV infection. J Clin Exp Neuropsychol. 2004; 26: 307–319. [DOI] [PubMed] [Google Scholar]
- 32.Antinori A, Arendt G, Becker JT et al. Updated research nosology for HIV-associated neurocognitive disorders. Neurology. 2007; 69: 1789–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salsman JM, Butt Z, Pilkonis PA et al. Emotion assessment using the NIH Toolbox. Neurology. 2013; 80: S76–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paolillo EW, McKenna BS, Nowinski CJ, Thomas ML, Malcarne VL, Heaton RK. NIH Toolbox® Emotion Batteries for Children: Factor-Based Composites and Norms. Assessment. 2018; 1073191118766396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Babakhanyan I, McKenna BS, Casaletto KB, Nowinski CJ, Heaton RK. National Institutes of Health Toolbox Emotion Battery for English- and Spanish-speaking adults: normative data and factor-based summary scores. Patient Relat Outcome Meas. 2018; 9: 115–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kabundelo P, Mbewe M, Mwanza-Kabaghe S, Adams H, Bearden DR. Validation of the NIH Toolbox, an iPad-based Test of Cognition, in Children and Adolescents in Zambia. Abstract, Evidence for Impact Conference. Lusaka, Zambia: 2018. [Google Scholar]
- 37.Allgaier AK, Pietsch K, Frühe B, Sigl-Glöckner J, Schulte-Körne G. Screening for depression in adolescents: validity of the patient health questionnaire in pediatric care. Depress Anxiety. 2012; 29: 906–913. [DOI] [PubMed] [Google Scholar]
- 38.Royston P, Sauerbrei W. Multivariable model-building: a pragmatic approach to regression analysis based on fractional polynomials for continuous variables. Ch. 9.3, p. 203–205. Wiley, Chichester; 2008. [Google Scholar]
- 39.Musinguzi K, Obuku A, Nakasujja N et al. Association between major depressive disorder and pro-inflammatory cytokines and acute phase proteins among HIV-1 positive patients in Uganda. BMC Immunol. 2018; 19: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beumer W, Gibney SM, Drexhage RC et al. The immune theory of psychiatric diseases: a key role for activated microglia and circulating monocytes. J Leukoc Biol. 2012; 92: 959–975. [DOI] [PubMed] [Google Scholar]
- 41.Chiang JJ, Cole SW, Bower JE et al. Depressive symptoms and immune transcriptional profiles in late adolescents. Brain Behav Immun. 2019; 80: 163–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ferrari P, Parisi MM, Colombo R et al. Depression and Mania Induce Pro-inflammatory Activation of Macrophages Following Application of Serum from Individuals with Bipolar Disorder. Clin Psychopharmacol Neurosci. 2018; 16: 103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Grosse L, Carvalho LA, Wijkhuijs AJ et al. Clinical characteristics of inflammation-associated depression: Monocyte gene expression is age-related in major depressive disorder. Brain Behav Immun. 2015; 44: 48–56. [DOI] [PubMed] [Google Scholar]
- 44.Grosse L, Hoogenboezem T, Ambrée O et al. Deficiencies of the T and natural killer cell system in major depressive disorder: T regulatory cell defects are associated with inflammatory monocyte activation. Brain Behav Immun. 2016; 54: 38–44. [DOI] [PubMed] [Google Scholar]
- 45.Johnson JD, Barnard DF, Kulp AC, Mehta DM. Neuroendocrine Regulation of Brain Cytokines After Psychological Stress. J Endocr Soc. 2019; 3: 1302–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim YK, Na KS, Shin KH, Jung HY, Choi SH, Kim JB. Cytokine imbalance in the pathophysiology of major depressive disorder. Prog Neuropsychopharmacol Biol Psychiatry. 2007; 31: 1044–1053. [DOI] [PubMed] [Google Scholar]
- 47.Liberman AC, Trias E, da Silva Chagas L et al. Neuroimmune and Inflammatory Signals in Complex Disorders of the Central Nervous System. Neuroimmunomodulation. 2018; 25: 246–270. [DOI] [PubMed] [Google Scholar]
- 48.Lisi L, Camardese G, Treglia M et al. Monocytes from depressed patients display an altered pattern of response to endotoxin challenge. PLoS One. 2013; 8: e52585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazza MG, Lucchi S, Tringali AGM, Rossetti A, Botti ER, Clerici M. Neutrophil/lymphocyte ratio and platelet/lymphocyte ratio in mood disorders: A meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry. 2018; 84: 229–236. [DOI] [PubMed] [Google Scholar]
- 50.McKim DB, Weber MD, Niraula A et al. Microglial recruitment of IL-1β-producing monocytes to brain endothelium causes stress-induced anxiety. Mol Psychiatry. 2018; 23: 1421–1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miklowitz DJ, Portnoff LC, Armstrong CC et al. Inflammatory cytokines and nuclear factor-kappa B activation in adolescents with bipolar and major depressive disorders. Psychiatry Res. 2016; 241: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Demoze MB, Angaw DA, Mulat H. Prevalence and Associated Factors of Depression among Orphan Adolescents in Addis Ababa, Ethiopia. Psychiatry J. 2018; 2018: 5025143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar SP, Dandona R, Kumar GA, Ramgopal S, Dandona L. Depression among AIDS-orphaned children higher than among other orphaned children in southern India. Int J Ment Health Syst. 2014; 8: 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shiferaw G, Bacha L, Tsegaye D. Prevalence of Depression and Its Associated Factors among Orphan Children in Orphanages in Ilu Abba Bor Zone, South West Ethiopia. Psychiatry J. 2018; 2018: 6865085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lackey GF. “Feeling blue” in Spanish: a qualitative inquiry of depression among Mexican immigrants. Soc Sci Med. 2008; 67: 228–237. [DOI] [PubMed] [Google Scholar]
- 56.Brown AD, Mentha R, Rowley KG, Skinner T, Davy C, O’Dea K. Depression in Aboriginal men in central Australia: adaptation of the Patient Health Questionnaire 9. BMC Psychiatry. 2013; 13: 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bröer C, Besseling B. Sadness or depression: Making sense of low mood and the medicalization of everyday life. Soc Sci Med. 2017; 183: 28–36. [DOI] [PubMed] [Google Scholar]
- 58.Bolton P Cross-cultural validity and reliability testing of a standard psychiatric assessment instrument without a gold standard. J Nerv Ment Dis. 2001; 189: 238–242. [DOI] [PubMed] [Google Scholar]
- 59.Lee B, Kaaya SF, Mbwambo JK, Smith-Fawzi MC, Leshabari MT. Detecting depressive disorder with the Hopkins Symptom Checklist-25 in Tanzania. Int J Soc Psychiatry. 2008; 54: 7–20. [DOI] [PubMed] [Google Scholar]
- 60.Mendenhall E, Rinehart R, Musyimi C, Bosire E, Ndetei D, Mutiso V. An ethnopsychology of idioms of distress in urban Kenya. Transcult Psychiatry. 2019; 56: 620–642. [DOI] [PubMed] [Google Scholar]
- 61.Omoro SA, Fann JR, Weymuller EA, Macharia IM, Yueh B. Swahili translation and validation of the Patient Health Questionnaire-9 depression scale in the Kenyan head and neck cancer patient population. Int J Psychiatry Med. 2006; 36: 367–381. [DOI] [PubMed] [Google Scholar]
- 62.Tsai AC, Scott JA, Hung KJ et al. Reliability and validity of instruments for assessing perinatal depression in African settings: systematic review and meta-analysis. PLoS One. 2013; 8: e82521. [DOI] [PMC free article] [PubMed] [Google Scholar]