Abstract
Rationale: Approximately 40% of people worldwide are exposed to household air pollution (HAP) from the burning of biomass fuels. Previous efforts to document health benefits of HAP mitigation have been stymied by an inability to lower emissions to target levels.
Objectives: We sought to determine if a household air pollution intervention with liquefied petroleum gas (LPG) improved cardiopulmonary health outcomes in adult women living in a resource-poor setting in Peru.
Methods: We conducted a randomized controlled field trial in 180 women aged 25–64 years living in rural Puno, Peru. Intervention women received an LPG stove, continuous fuel delivery for 1 year, education, and behavioral messaging, whereas control women were asked to continue their usual cooking practices. We assessed for stove use adherence using temperature loggers installed in both LPG and biomass stoves of intervention households.
Measurements and Main Results: We measured blood pressure, peak expiratory flow (PEF), and respiratory symptoms using the St. George’s Respiratory Questionnaire at baseline and at 3–4 visits after randomization. Intervention women used their LPG stove exclusively for 98% of days. We did not find differences in average postrandomization systolic blood pressure (intervention – control 0.7 mm Hg; 95% confidence interval, −2.1 to 3.4), diastolic blood pressure (0.3 mm Hg; −1.5 to 2.0), prebronchodilator peak expiratory flow/height2 (0.14 L/s/m2; −0.02 to 0.29), postbronchodilator peak expiratory flow/height2 (0.11 L/s/m2; −0.05 to 0.27), or St. George’s Respiratory Questionnaire total score (−1.4; −3.9 to 1.2) over 1 year in intention-to-treat analysis. There were no reported harms related to the intervention.
Conclusions: We did not find evidence of a difference in blood pressure, lung function, or respiratory symptoms during the year-long intervention with LPG.
Clinical trial registered with www.clinicaltrials.gov (NCT02994680).
Keywords: household air pollution, lung function, blood pressure, respiratory symptoms
At a Glance Commentary
Scientific Knowledge on the Subject
Household air pollution (HAP), resulting from the burning of biomass fuels for cooking, is a highly prevalent environmental exposure worldwide. HAP was estimated to be responsible for 1.6 million premature deaths and 77.2 million disability-adjusted life years lost in 2017. Previous intervention trials that have used cleaner-burning biomass stoves or clean fuels have failed to consistently demonstrate reductions in HAP below World Health Organization established target concentrations. Additionally, improvements in health outcomes are inconsistent or of unclear clinical significance. Potential reasons for the lack of associated improvements in health outcomes include insufficient reductions in personal exposures to HAP, insufficient abandonment of biomass-burning stoves, or high ambient air pollution.
What This Study Adds to the Field
This is the first randomized controlled trial to document exclusive liquefied petroleum gas stove use for 98% of follow-up days in intervention households resulting in average personal exposures to fine particulate matter below World Health Organization target concentrations when compared with controls. This trial was done in a resource-poor population of Peru where biomass burning is prevalent and ambient air pollution is low. Data from 180 adult women aged 25–64 years contributed 2,157 person-months to the intention-to-treat analysis. Despite high adherence with liquefied petroleum gas stove use and low fine particulate matter exposures during a 12-month period among intervention women, we did not find evidence of lower blood pressure, improved peak expiratory flow, or decreased respiratory symptoms when compared with controls.
Household air pollution (HAP) is a prevalent environmental exposure with an estimated 40% of the world population using biomass fuels for cooking (1). HAP is the result of incomplete combustion when a biomass fuel such as wood, charcoal, dung, or agricultural crop waste is burned inside or around the home for cooking. HAP exposes 3 billion people to air pollutants, including particulate matter, carbon monoxide, and nitrogen oxides (2). HAP was estimated to be responsible for 1.6 million premature deaths and 77.2 million disability-adjusted life years lost in 2017 (3).
Despite its ubiquity, HAP received relatively little attention from the scientific community until about three decades ago. Much of the evidence linking HAP exposure with multiple health outcomes stems from observational studies conducted in a variety of geographical settings (4–8). Previous intervention trials that have used either cleaner-burning biomass stoves or clean fuels have failed to consistently demonstrate reductions in HAP exposures below World Health Organization established target concentrations (9, 10).
We sought to evaluate the effect of a cleaner energy intervention on cardiovascular and pulmonary health in adult women living in a resource-limited setting of Peru. Our chosen intervention combined the use of liquefied petroleum gas (LPG) stoves, continuous fuel distribution, education, and behavioral messaging to maximize adherence with stove use and achieve the best possible reductions in HAP. We hypothesized that adult women who received the intervention would have a lower blood pressure, better lung function, and fewer respiratory symptoms throughout the year-long intervention.
Methods
Study Setting
The CHAP (Cardiopulmonary outcomes and Household Air Pollution) trial took place in eight rural communities in the Department of Puno, Peru, located near the shores of Lake Titicaca, at an elevation of 3,825 m above sea level (see Study setting in the Methods section of the online supplement).
Study Design
The trial was registered (ClinicalTrials.gov NCT02994680), and the protocol was published before the study began (11) (see Main Study Protocol in the online supplement). We followed CONSORT guidelines (Main Study Protocol: CONSORT checklist in the online supplement). Briefly, we conducted an individually randomized, open-label controlled trial (RCT) in 180 adult women. Here, we report on three of the eight primary outcomes. We drew a simple random sample of 569 households from a census of the study area to screen for eligible women (see Methods: Eligibility criteria in the online supplement). Enrollment was staggered in groups of 14–16 women each month between January 19, 2017, and January 20, 2018. Randomization followed baseline assessments between March 1, 2017, and February 15, 2018. Participants were assigned to intervention or control with a 1:1 ratio using randomly permuted block sizes of two, four, and six. A computerized randomization schedule was created by an investigator not involved in screening or enrollment. Assignment was concealed in sealed envelopes until baseline measurements were completed and the envelope was opened (see Methods: Randomization in the online supplement).
Intervention
The intervention was complex and multifaceted, consisting of an LPG stove, a waist-high table (see Figure E1A in the online supplement), continuous fuel delivery and installation by study staff, education, and behavioral messaging. Formative work conducted before the trial (12) established that most women preferred having a three-burner LPG stove. We contracted a local manufacturer (Industrias Surges) to build them because three-burner stoves were not commercially available at the time of our study. We invited intervention participants to join one cooking demonstration session with local ingredients and hands-on practice to teach participants how to safely use the LPG stove (see Cooking Demonstration and Education in the online supplement). Cooking demonstrations took place at randomization, which was staggered monthly over 1 year. During these demonstrations, we also reviewed the benefits of LPG stoves and disadvantages of biomass stoves (see Main Study Protocol: Guide to Education and Behavioral Messaging in the online supplement). Demonstrations followed a standard script and used the same educational banners. Following the demonstration, study staff delivered and installed the LPG stove, a table, a full LPG tank, and calendars that contained the messages from the demonstration (Figure E1B) to each participant’s home. Behavioral messages were developed as part of formative work (12).
Study staff delivered LPG fuel refills to intervention homes approximately every 2 weeks depending on rate of fuel consumption, checked for gas leaks or problems with the stoves, and reinforced behavioral messages from the calendars and demonstrations to encourage exclusive LPG use. We monitored for adverse events in intervention (death, burns from LPG stove, and tank explosions) and control participants (death and burns from a traditional biomass-burning cookstove).
Outcomes
This paper presents data for three of the eight primary outcomes in this trial (11): blood pressure, peak expiratory flow (PEF), and respiratory symptoms as measured by the St. George’s Respiratory Questionnaire (SGRQ). Systolic blood pressure (SBP) and diastolic blood pressure (DBP) were measured in triplicate at 5-minute intervals and in the sitting position using an automatic blood pressure monitor (HEM-7231T-Z Model BP786N; Omron). Participants were asked to rest for 5 minutes before the first measurement (see Manual of Procedures: Blood pressure and pulse oximetry). SBP and DBP (in mm Hg) were calculated as the mean of the second and third measurements. We measured PEF with a handheld spirometer (EasyOn PC; ndd), which has an ultrasonic flow reader that is not affected by air density and is suitable for use at high altitudes. Spirometry assessment followed standard guidelines (13) (see Methods: Spirometry in the online supplement). We used the maximum PEF (in L/s) and divided it by height2 (in meters) for analysis. We measured respiratory symptoms using a Spanish-validated version of the SGRQ (14) (see Methods: St. George’s Respiratory Questionnaire in the online supplement).
Blood pressure, spirometry, and SGRQ were measured at baseline and at 3, 6, 9 (blood pressure and SGRQ only), and 12 months after randomization. Assessments were done at a central location in each of the communities or outside of the participant’s home to keep field staff who performed clinical assessments and surveys blinded to assignment. Data were collected on tablets using the Research Electronic Data Capture software (REDCap; Vanderbilt University Medical Centre).
Assessment of Household Air Pollution and Stove Use Adherence
We measured 48-hour particulate matter that is ≤ 2.5 μm in aerodynamic diameter (PM2.5) with the Enhanced Child MicroPEM (RTI International, Research Triangle Park); cumulative black carbon as the optical attenuation of infrared light (OT21 Sootscan transmissometer; Magee Scientific) in postsampled PM2.5 filters; and carbon monoxide (CO) concentrations with the EasyLog Carbon Monoxide data logger (EL-USB-CO; Lascar Electronics) at baseline and at 1 (in a subset of 90 participants), 3, 6, and 12 months after randomization. We collected concurrent gravimetric (filter-based) and ambient temperature and relative humidity–corrected nephelometric (direct-reading) samples with the Enhanced Child MicroPEM.
We also measured 24-hour kitchen area concentrations of nitrogen dioxide (NO2) at baseline, and at 3, 6, and 12 months after randomization in a subset of 100 households using portable direct-reading monitors with NO2 sensor heads (Aeroqual Series 500; Aeroqual Limited). Kitchen area concentrations were measured by placing the monitors inside a bird cage hung 1 m from the stoves, 1.5 m above the floor and at least 1 m from doors and windows, when possible (Appendix, Supplemental Methods: Figure E1C). Personal exposures were measured concurrently. Participants wore monitors in a custom-made apron (Figure E1D) during awake hours and colocated them near their beds when sleeping or bathing. Details regarding exposure assessment are found elsewhere (see Methods: Assessment of household air pollution in the online supplement). To calculate 24-hour concentrations from the 48-hour measurements for PM2.5 and CO, we either averaged concentrations obtained over the first and second 24 hours if at least 20 hours of exposure assessment were collected on both days or used the concentrations obtained on the first 24 hours if at least 20 hours of exposure assessment were collected on Day 1 but not on Day 2. If fewer than 20 hours of exposure assessment were collected on both days, we did not calculate an average and the 24-hour concentration for that 48-hour measurement was considered missing.
We placed temperature loggers (Digit-TL; LabJack Corporation) near both LPG and biomass stoves of intervention homes to monitor adherence, and near biomass stoves in all control homes to monitor use. In a subset of 24 control homes, we also placed a Digit-TL on previously owned LPG stoves to monitor use (i.e., LPG stoves that were not provided by our trial). Digit-TLs were hung under the middle burner of LPG stoves and at 1 m or less above biomass stoves in the smoke stream (Figures E1E and E1F). Intervention adherence was calculated as the number of days with exclusive LPG use divided by the number of days when any cooking events were registered. Biomass stove use in control households was calculated as the number of days with exclusive biomass stove use divided by the number of days when any cooking events were registered.
Statistical Analysis
Sample size calculations and the statistical analysis plan were reported elsewhere (11) (see Main Study Protocol: Sample Size and Statistical Analysis Plan in the online supplement). To detect an effect size of one-half SD in the three primary outcomes listed above, 85 evaluable women were required in each arm to achieve 90% power with 5% type I error. Effect sizes were smaller or similar in magnitude to those in previous observational studies or RCTs (8, 15). For one of the eight primary outcomes to be considered statistically significant between intervention and control arms at a familywise error of 5%, the P value would have to be less than 0.00625.
The primary analysis was based on intention-to-treat. We used linear random effects models (16) to examine the effects of the intervention (i.e., intervention – control) on subject-specific levels of SBP, DBP, pre- and postbronchodilator PEF/height2 and SGRQ total, and symptoms scores collected at postrandomization visits (see Methods: Intention-to-treat analysis in the online supplement). The difference between the intervention and control arms was estimated as the coefficient corresponding to the parameter identifying the intervention group, which represents the between-arm differences (intervention – control) averaged over the 3-, 6-, 9-, and 12-month visits. As a sensitivity analysis, we added the baseline clinical outcomes as covariates (see Main Study Protocol: Statistical Analysis Plan in the online supplement). We conducted subgroup analysis by age (⩾50 yr or <50 yr), LPG ownership at baseline, previous or current Fondo de Inclusión Social Energético program participation (governmental LPG subsidization program), and ownership of pigs and dogs (given the common practice of cooking for these animals in our setting). We also conducted exposure–response analyses by ignoring allocation and visit (see Methods: Exposure– response analysis in the online supplement).
We used the Kolmogorov-Smirnov test to compare postrandomization average kitchen area concentrations and personal exposures to HAP by arm; Chi-square or Fisher exact tests to compare the proportions of HAP exposures below target concentrations by arm; Chi-square test to compare proportions between groups; and t tests to compare differences in continuous measurements between groups. We used R version 3.6.2 (Dark and Stormy Night) and the packages lme, mgcv, ggplot2,itsadug for statistical analyses and visualizations (www.r-project.org).
Ethics Review
The trial was approved by the Institutional Review Boards of A.B. PRISMA (CE2402.16) and Universidad Peruana Cayetano Heredia (66780) in Lima, Peru, and by the Johns Hopkins Bloomberg School of Public Health (7128) in Baltimore. All participants provided informed consent with a waiver of documentation.
Results
Participant Characteristics
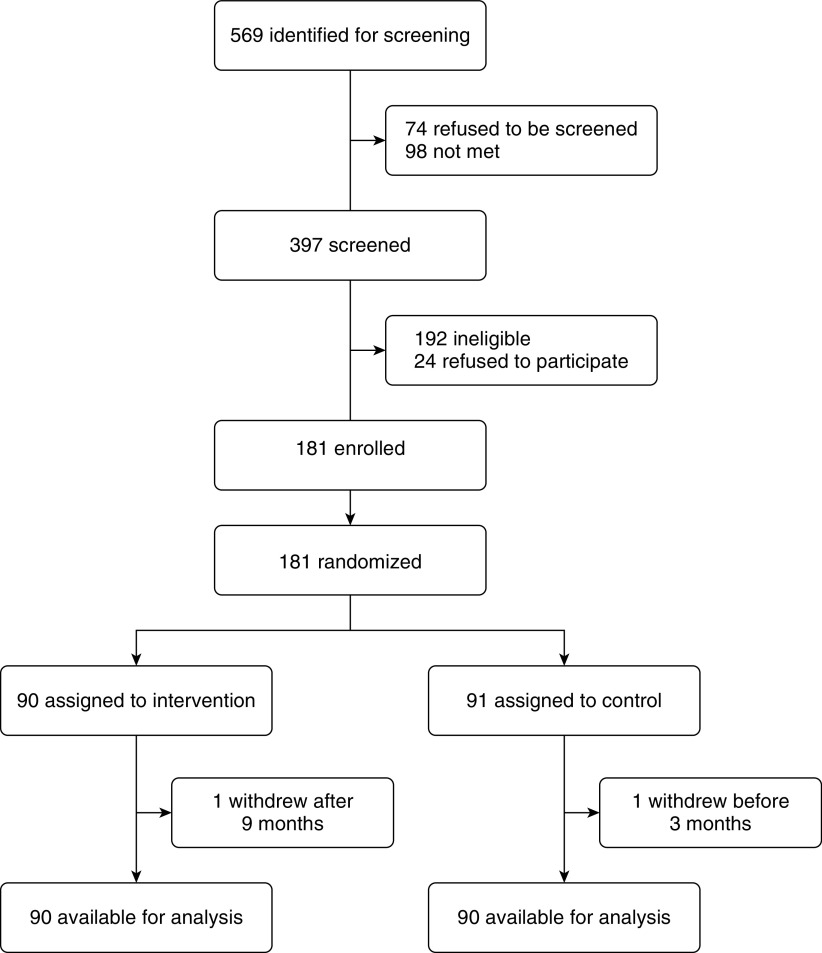
Between January 19, 2017, and January 20, 2018, we identified 569 households for screening and approached 397 participants (Figure 1). Of these, 192 did not meet eligibility criteria and 24 refused to participate. The main reasons for ineligibility (not mutually exclusive) were as follows: 131 did not use an indoor biomass stove daily and 46 did not have a kitchen separate from sleeping areas. We subsequently enrolled and randomized 181 participants through February 15, 2018. One control participant withdrew from the study before completing the 3-month evaluations, which left an intention-to-treat sample of 180 participants. These participants were followed longitudinally for 2,157 person-months (one intervention participant withdrew at 9 mo after randomization). The last participant's year-long follow-up was conducted on February 15, 2019. Overall, there were no or few missing data for primary outcomes (Figure E2), kitchen area concentrations and personal exposures to PM2.5 or black carbon (Figure E3), and potential confounders used in the exposure–response analysis (Figure E4). Trial arms had similar averages or proportions of demographic and socioeconomic characteristics, HAP exposures, and primary outcomes at baseline (Table 1). Although the intervention arm had a higher proportion of households with higher income (Chi-square P = 0.21), a lower average NO2 (t test of log-transformed values P = 0.11), a higher average SBP (t test P = 0.26), and a lower average SGRQ (t test P = 0.35) than the control arm at baseline, none of these differences were statistically significant.
Figure 1.
Screening, randomization, and follow-up (Consolidated Standards of Reporting Trials [CONSORT] flow diagram).
Table 1.
Sociodemographic Features, HAP Exposure, and Primary Outcomes of Participants at Baseline
| Characteristic | Control Arm (n = 90) | Intervention Arm (n = 90) |
|---|---|---|
| Demographics | ||
| Age, yr, mean (SD) | 47.9 (11.1) | 48.7 (9.1) |
| Married, n (%) | 63 (70) | 66 (73.3) |
| Literate, n (%) | 73 (81.1) | 75 (83.3) |
| Years of education, mean (SD) | 6.4 (3.3) | 6.1 (3.4) |
| Spouse’s years of education, mean (SD) | 9.1 (2.9) | 9.0 (3.4) |
| Socioeconomics | ||
| Income ⩾250 soles/mo (75 USD/mo), n (%) | 27 (30) | 36 (40) |
| Owns LPG stove, n (%) | 68 (75.6) | 64 (71.1) |
| Previous or current Fondo de Inclusión Social Energético program participation, n (%) | 46 (51.1) | 42 (46.7) |
| Wealth quintile, mean (SD) | 1.5 (0.6) | 1.5 (0.6) |
| Number of people who sleep in house, mean (SD) | 3.6 (1.5) | 3.8 (1.7) |
| Number of rooms, mean (SD) | 2.6 (0.9) | 2.6 (0.9) |
| Piped water inside home, in patio, or public standpipe, n (%) | 32 (35.6) | 32 (34.4) |
| Piped sewage or improved pit latrine, n (%) | 68 (75.6) | 64 (70) |
| Owns dogs, n (%) | 57 (63.3) | 66 (73.3) |
| Owns pigs, n (%) | 54 (60) | 52 (57.8) |
| Anthropometry | ||
| Height, cm, mean (SD) | 152.0 (4.7) | 150.8 (5.3) |
| BMI, kg/m2, mean (SD) | 26.9 (4.1) | 26.8 (4.2) |
| Kitchen area concentrations of HAP, mean (SD) | ||
| PM2.5, μg/m3 | 1,223 (1,007) | 1,187 (876) |
| BC, μg/m3 | 182 (120) | 206 (152) |
| CO, ppm | 53.4 (48.5) | 50.2 (40.8) |
| NO2, ppb | 150.7 (115.9) | 108.1 (73.9) |
| Personal exposures to HAP, mean (SD) | ||
| PM2.5, μg/m3 | 126 (214) | 104 (100) |
| BC, μg/m3 | 19 (17) | 21 (22) |
| CO, ppm | 6.6 (8.2) | 7.1 (8.4) |
| NO2, ppb | 20.4 (13.9) | 23 (18.9) |
| Primary outcomes, mean (SD) | ||
| Systolic blood pressure, mm Hg | 102.3 (11) | 100.4 (11.9) |
| Diastolic blood pressure, mm Hg | 68.1 (8.3) | 67.7 (8.7) |
| Prebronchodilator peak expiratory flow/height2, L/s/m2 | 2.91 (0.67) | 2.95 (0.64) |
| Postbronchodilator peak expiratory flow/height2, L/s/m2 | 3.09 (0.62) | 3.16 (0.61) |
| SGRQ total score | 10.3 (15.2) | 8.5 (10.2) |
| SGRQ clinical symptoms score | 14.7 (16.4) | 13.7 (13.7) |
| SGRQ activity score | 12.2 (20.7) | 9.3 (15.4) |
| SGRQ impact score | 7.9 (15.3) | 6.5 (9.4) |
Definition of abbreviations: BC = black carbon; BMI = body mass index; CO = carbon monoxide; HAP = household air pollution; LPG = liquefied petroleum gas; NO2 = nitrogen dioxide; PM2.5 = particulate matter that is ≤ 2.5 μm in aerodynamic diameter; SGRQ = St. George’s Respiratory Questionnaire.
Adherence with the Intervention
We obtained stove use surveillance on 97% of days in intervention households. We plotted stove use data for intervention households as a graphical matrix (Figure E5). Women in the intervention arm used their LPG stove exclusively for 98% of days when cooking events were registered and either used their biomass stove exclusively for 0.1% of days or stacked use with the LPG stove for 1.9% of days. They also used their biomass stoves or stacked use with the LPG stove for an average of 2.7% of days in the first 3 months compared with an average of 1.7% of days in the subsequent 9 months of the intervention (P = 0.05). We obtained biomass stove use surveillance in 95.4% of days in control households. Control women used their biomass stoves for 72.4% of monitored days. The subset of 24 control households with previously owned LPG stoves that we monitored used them at least once in 92.5% of days when they did not use their biomass stove.
Effects of the Intervention on Environmental Exposures
The intervention consistently reduced postrandomization 48-hour kitchen area concentrations (Figure 2) and personal exposures to HAP (Figure 3) in intervention participants compared with controls during the year-long intervention. When we averaged all postrandomization measurements of 48-hour time-weighted means by trial arm, we found lower kitchen area concentrations of PM2.5 (58 vs. 1,246 μg/m3; Kolmogorov-Smirnov P < 0.001) and lower personal exposures to PM2.5 (30 vs. 98 μg/m3; Kolmogorov-Smirnov P < 0.001) in intervention participants when compared with controls. Postrandomization kitchen area concentrations of PM2.5 were less than 35 μg/m3 in 23% of intervention households and 0% of control households (Fisher exact test P < 0.001), and postrandomization personal exposures to PM2.5 were less than 35 μg/m3 in 78% of intervention and 13% of control participants (Chi-square P < 0.001). Additional pollutant difference is presented elsewhere (see Results: Effects of the intervention on environmental exposures in the online supplement).
Figure 2.
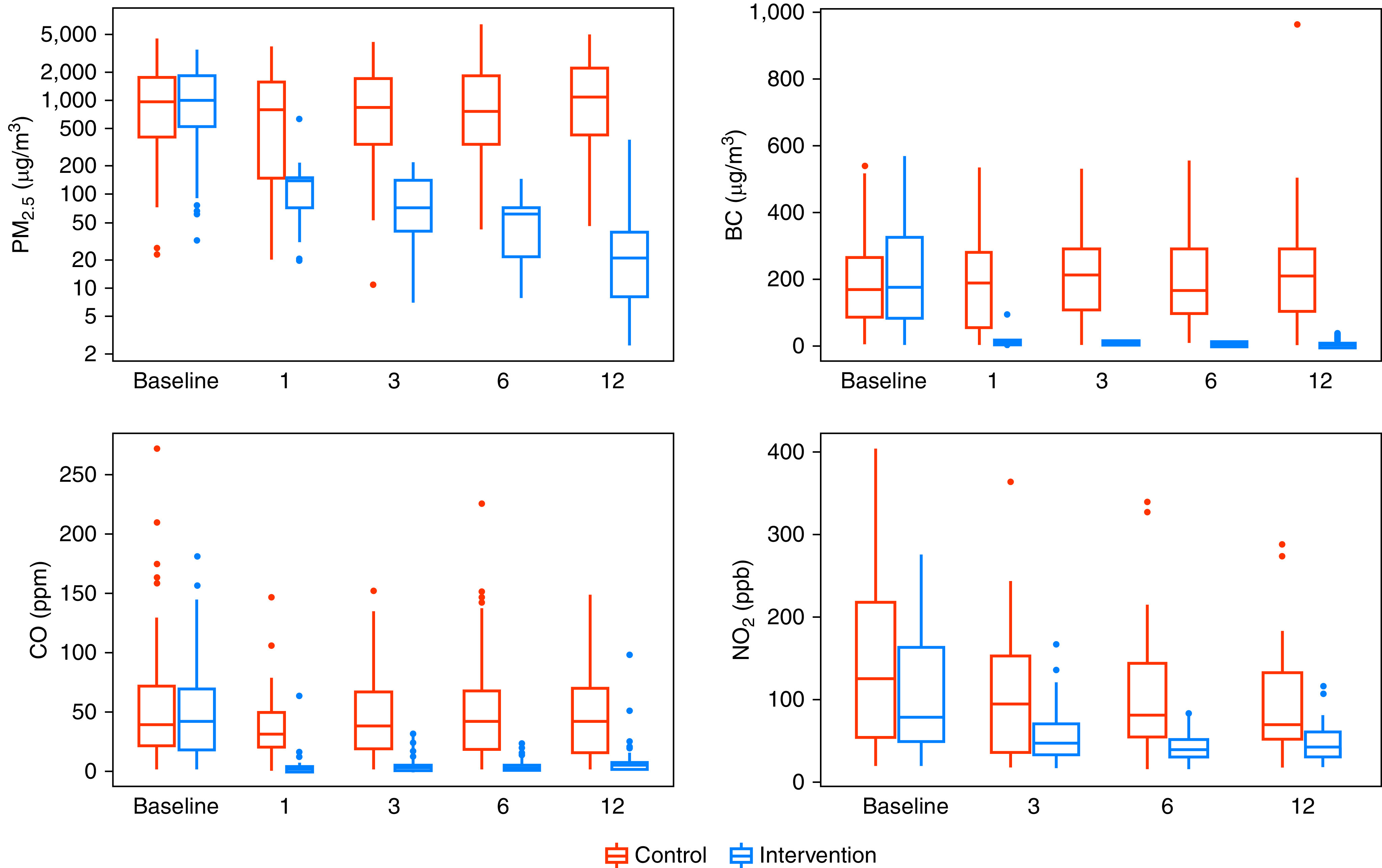
Boxplots of kitchen area concentrations of particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), black carbon (BC), carbon monoxide (CO), and nitrogen dioxide (NO2) by trial arm and visit. In each panel, control households are represented by red-colored boxplots and intervention households by blue-colored boxplots. The width of the barplots in each panel is proportional with sample size. The sample size for baseline and 1-, 3-, 6-, and 12-month visits for control households are as follows: PM2.5 and BC (n0 = 89, n1 = 42, n3 = 89, n6 = 88, n12 = 89), CO (n0 = 84, n1 = 42, n3 = 84, n6 = 86, n12 = 86), and NO2 (n0 = 34, n3 = 36, n6 = 33, n12 = 35). The sample size for baseline and 1-, 3-, 6-, and 12-month visits for intervention households are as follows: PM2.5 and BC (n0 = 89, n1 = 43, n3 = 90, n6 = 90, n12 = 87), CO (n0 = 85, n1 = 41, n3 = 80, n6 = 82, n12 = 77), and NO2 (n0 = 48, n3 = 41, n6 = 33, n12 = 29).
Figure 3.
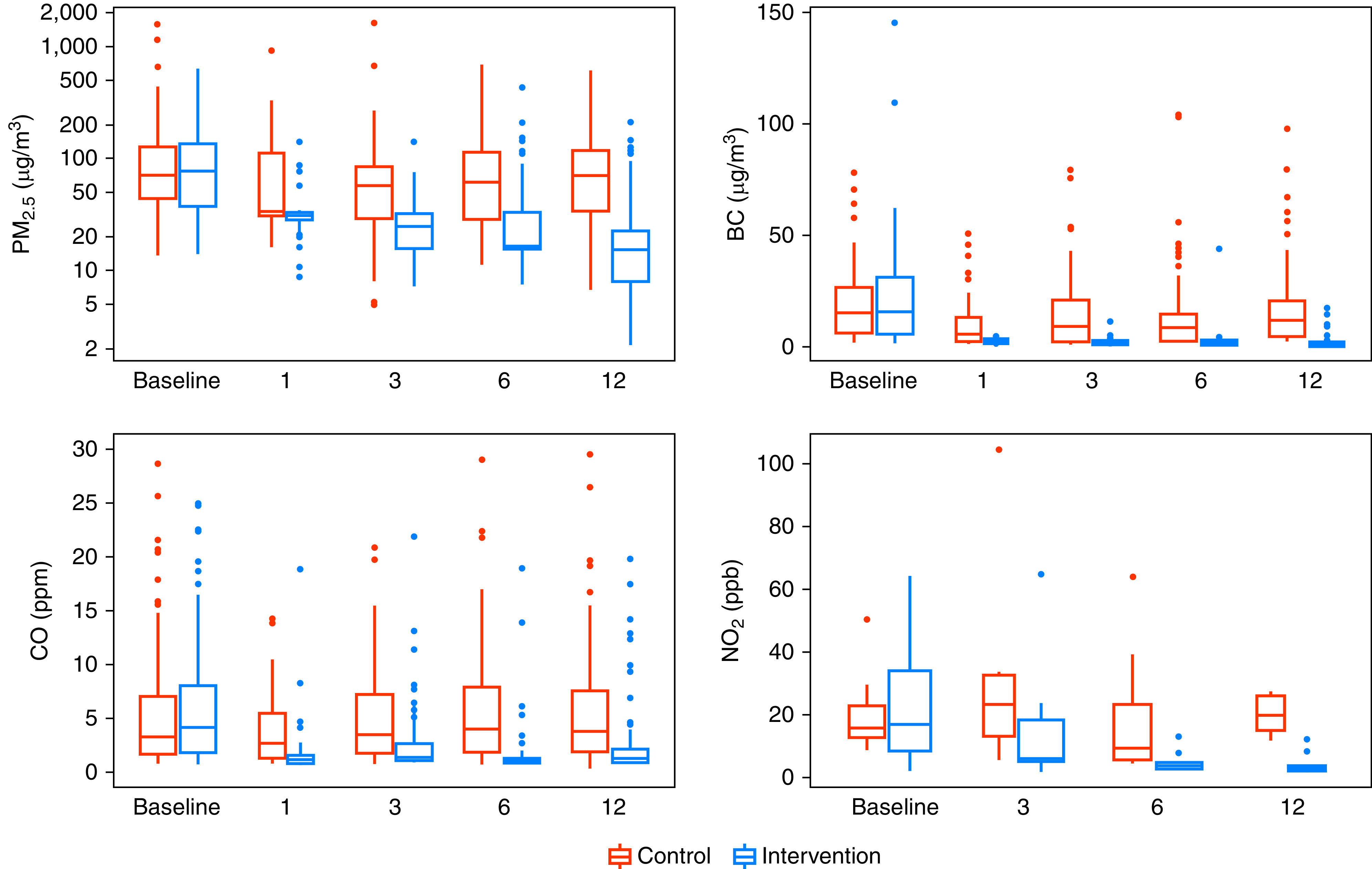
Boxplots of personal exposure to particulate matter ≤ 2.5 μm in aerodynamic diameter (PM2.5), black carbon (BC), carbon monoxide (CO), and nitrogen dioxide (NO2) by trial arm and visit. In each panel, control women are represented by red-colored boxplots and intervention women by blue-colored boxplots. The width of the barplots in each panel is proportional with sample size. The sample sizes for baseline and 1-, 3-, 6-, and 12-month visits for control households are as follows: PM2.5 and BC (n0 = 90, n1 = 40, n3 = 87, n6 = 90, n12 = 90), CO (n0 = 81, n1 = 41, n3 = 79, n6 = 81, n12 = 81), and NO2 (n0 = 8, n3 = 6, n6 = 9, n12 = 5). The sample sizes for baseline and 1-, 3-, 6-, and 12-month visits for intervention households are as follows: PM2.5 and BC (n0 = 90, n1 = 45, n3 = 90, n6 = 89, n12 = 87), CO (n0 = 79, n1 = 38, n3 = 76, n6 = 79, n12 = 79), and NO2 (n0 = 11, n3 = 13, n6 = 13, n12 = 9).
Intention-to-Treat Analyses for Primary Outcomes
We plotted mean values and corresponding 95% confidence intervals (95% CIs) for SBP, DBP, pre- and postbronchodilator PEF/height2, and SGRQ total and symptoms scores by arm and visit and summarized estimated postrandomization between-arm differences (intervention – control) for each primary outcome in Figure 4. PEF/height2 values were on average slightly higher in intervention participants when compared with control participants at any postrandomization visit; however, we did not find statistically significant between-arm differences in any of the primary outcomes. Our findings were unchanged when adjusted for baseline outcomes (see Results: Sensitivity analysis in the online supplement). We did not identify any subgroups with clinically significant differences favoring the intervention for any of the primary outcomes (Figures E6–E8).
Figure 4.
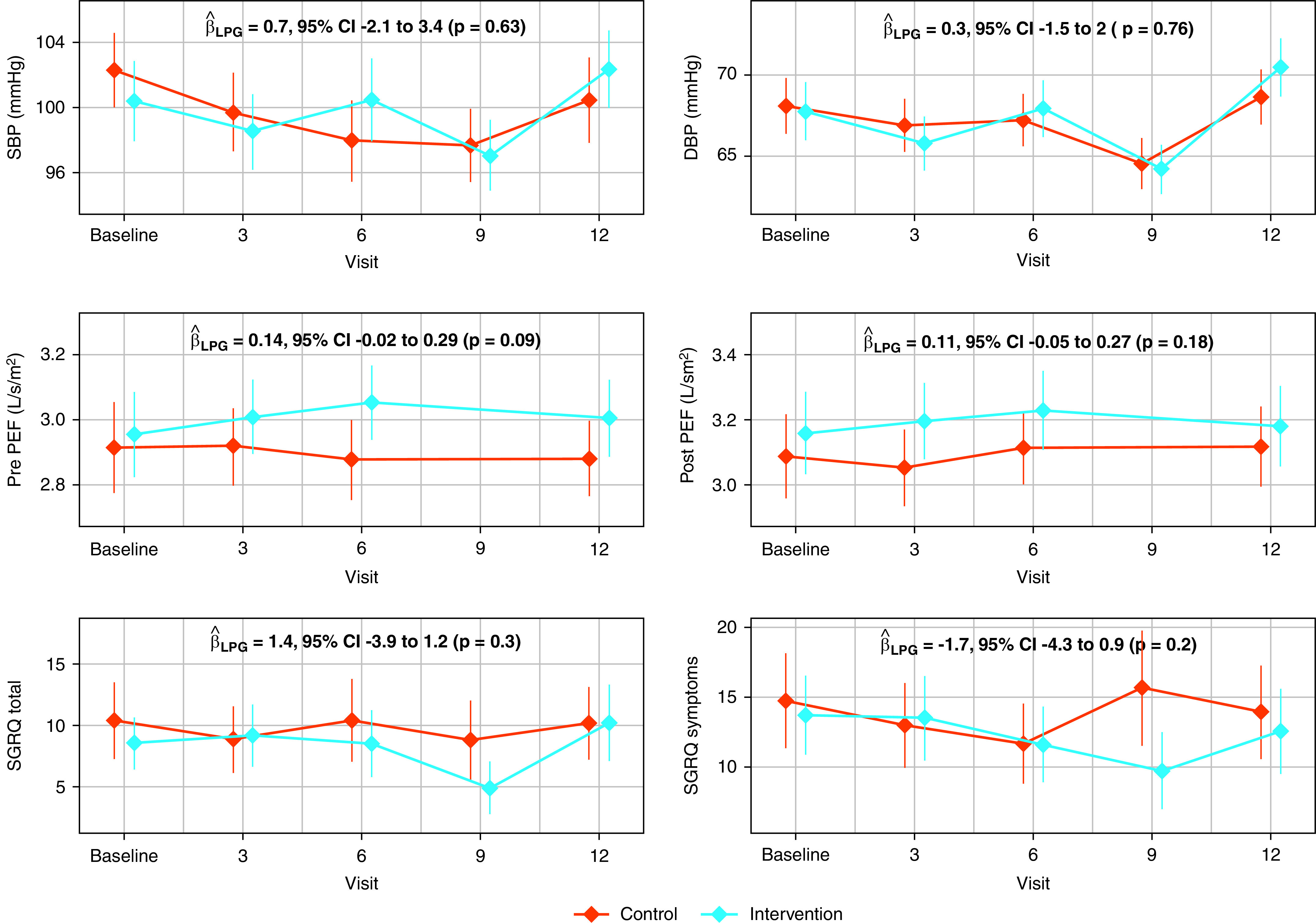
Average blood pressure, peak expiratory flow, and respiratory symptoms scores by trial arm and visit. We summarized mean systolic blood pressure (SBP), diastolic blood pressure (DBP), pre- and postbronchodilator height-adjusted peak expiratory flow (PEF/height2), and the St. George’s Respiratory Questionnaire (SGRQ) total and symptoms scores with a filled diamond. The vertical lines represent 95% confidence intervals (95% CIs). In each panel, outcomes for intervention women are represented by blue-colored diamonds and lines and control households by red-colored diamonds and lines. We also present the between-arms difference (intervention – control), the 95% CI, and corresponding P value estimated from a linear-mixed model for each of the primary outcomes within each panel. The sample sizes for postrandomization visits in the control women were as follows: SBP and DBP (n0 = 90, n3 = 90, n6 = 90, n9 = 90, n12 = 90), prebronchodilator PEF/height2 (n0 = 89, n3 = 90, n6 = 90, n12 = 89), postbronchodilator PEF/height2 (n0 = 89, n3 = 90, n6 = 90, n12 = 90), and SGRQ total and symptoms scores (n0 = 90, n3 = 90, n6 = 90, n9 = 90, n12 = 90). The sample sizes for postrandomization visits in the intervention women were as follows: SBP and DBP (n0 = 90, n3 = 90, n6 = 90, n9 = 90, n12 = 89), pre- and postbronchodilator PEF/height2 (n0 = 90, n3 = 90, n6 = 90, n12 = 88), and SGRQ total and symptoms scores (n0 = 90, n3 = 90, n6 = 90, n9 = 90, n12 = 89). LPG = liquefied petroleum gas.
Exposure–Response Analyses for Primary Outcomes
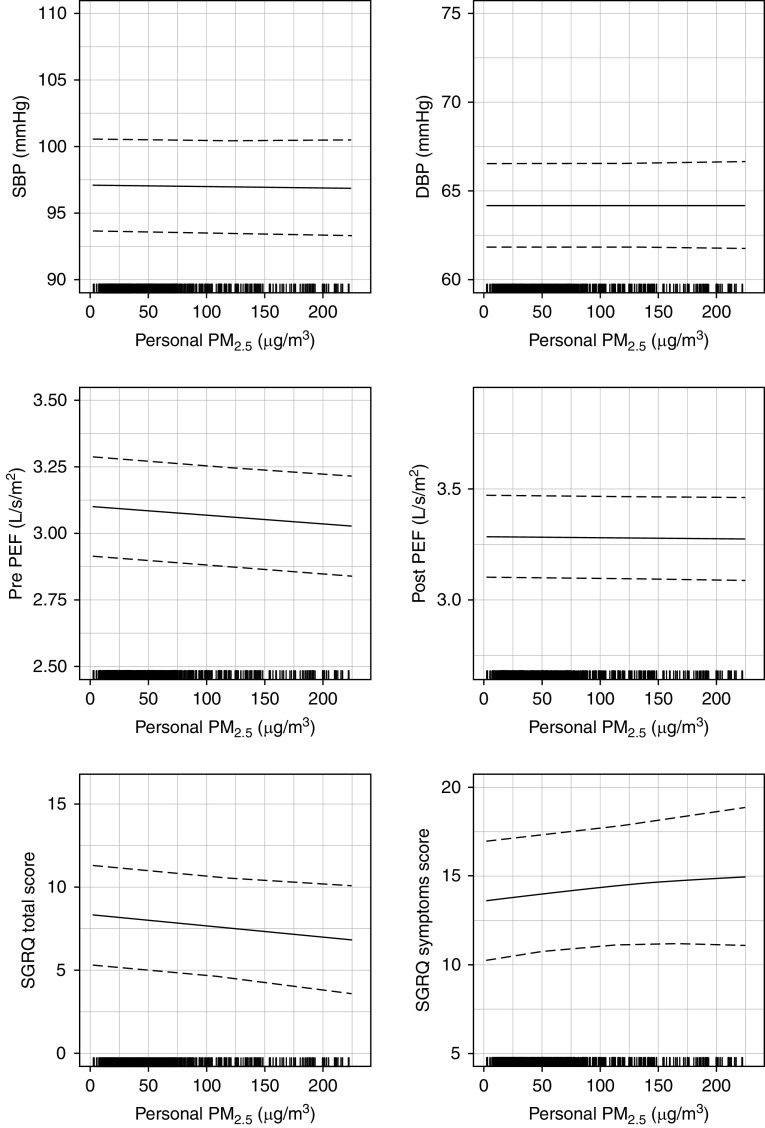
We did not observe any consistent relationships in most exposure–response analyses between primary outcomes and either personal exposures to HAP (Figure 5) or kitchen area concentrations (Figure E9). When we estimated the difference in primary outcomes between the 90th percentile (174 μg/m3) and the 10th percentile (13 μg/m3) of personal exposures to PM2.5, we found that SBP was 0.1 mm Hg lower (95% CI, −1.1 to 0.8) at higher exposures when compared with lower exposures, DBP was 0.0 mm Hg lower (95% CI, −0.7 to 0.8), prebronchodilator PEF/height2 was 0.05 L/s/m2 lower (95% CI, −0.09 to −0.02), postbronchodilator PEF/height2 was 0.01 L/s/m2 lower (95% CI, −0.04 to 0.02), SGRQ total score was 1.1 points lower (95% CI, −2.4 to 0.3), and SGRQ symptoms score was 1.0 points higher (95% CI, −1.2 to 3.2). The exposure–response curve for prebronchodilator PEF/height2 was significantly different between the 90th and 10th percentiles of personal exposures to PM2.5, which corresponds to a 6.8-L/min increase in prebronchodilator PEF in a lesser exposed woman who is 1.51 m tall compared with one of the same height who is more exposed.
Figure 5.
Exposure–response relationships between personal exposures to particulate matter that is ≤ 2.5 μm in aerodynamic diameter (PM2.5) and primary cardiopulmonary outcomes. The 180 participants contributed 713 pairs of blood pressure or St. George’s Respiratory Questionnaire (SGRQ) and personal exposure to PM2.5, and 711 pairs of peak expiratory flow (PEF) and personal exposure to PM2.5 data to these analyses. The six panels correspond to the association between personal exposures to PM2.5 and either blood pressure (SBP and DBP), PEF (pre- and postbronchodilator PEF/height2), and SGRQ (total and symptoms scores). In each of the six panels, we plot the estimated thin plate regression splines that describe the adjusted association between PM2.5 and each cardiopulmonary outcome (mean = solid line, 95% confidence interval = broken lines). We visually restricted the plots to show up to the 95th percentile of personal exposures to PM2.5 (230 μg/m3). We show a rug plot of the distribution of personal exposures to PM2.5. DBP = diastolic blood pressure; SBP = systolic blood pressure.
Adverse Events
There were no tank explosions during the year-long intervention. One intervention participant’s pressure cooker exploded while cooking with the LPG stove. No people were harmed but the LPG stove and table were destroyed and had to be replaced. Another intervention participant had a fire start in her kitchen after using her biomass stove. No people were harmed, but the LPG stove and table were burnt and had to be replaced. Both episodes were reported as adverse events.
Discussion
In this RCT involving adult women who reported using biomass fuels daily for cooking in Puno, Peru, we found that the intervention resulted in 98% adherence with exclusive LPG stove use and in consistently lower kitchen area concentrations and personal exposures to HAP compared with controls during the year-long intervention. However, despite important reductions in HAP, we did not find differences in the primary outcomes during the year-long intervention in either intention-to-treat or exposure–response analyses.
To our knowledge, this is the first RCT of an HAP intervention to document reductions in personal exposures to PM2.5 below World Health Organization recommended interim air quality guidelines in most participants. Two systematic reviews that evaluated previous cookstove interventions with either cleaner-burning biomass stoves or clean fuel stoves also found that these interventions were effective at reducing kitchen area concentrations of PM2.5 anywhere between 18% (9) and 40–85% (10), and at reducing personal exposures to PM2.5 by 24% (9). However, postintervention concentrations of PM2.5 remained well above recommended air quality guidelines in most of the reviewed studies (10). Interventions in recent RCTs in Malawi (17, 18), Nigeria (19), and Nepal (20) that were not included in these systematic reviews also failed to achieve a sufficiently large exposure contrast between intervention and control arms.
There are a couple of reasons why we believe our intervention was successful at reducing HAP exposures. First, we combined continuous delivery and installation of fuel tanks by our staff with behavioral messaging to ensure adherence with LPG stove use. When fuel was free and available, participants used the LPG stove with low to no biomass stove stacking, achieving >98% adherence with exclusive LPG stove use. Second, there are few other sources of air pollution in our study setting; ambient air pollution in Puno city is between 18 and 29 μg/m3 (21). Moreover, the distance between households in the rural area is approximately 100 m, so our study participants are less likely to be affected by HAP from biomass-burning neighbors than in settings with higher population density. Although many households owned LPG stoves at the start of the study, none used LPG exclusively. We also found that ownership of an LPG stove alone was not sufficient for sustained use in formative work (12). Our complex intervention established high adherence and thus efficacy of the intervention. Third, poverty is a major reason why people use biomass fuels. Indeed, adherence to the intervention was high because costly items such as the LPG stove and gas were provided for free.
Air pollution has been linked to worse cardiovascular and pulmonary health (22–24). Our achieved reductions in kitchen area concentrations and personal exposures to HAP should therefore have reduced local and systemic inflammation and resulted in less oxidative stress (24), as suggested by previous studies (25, 26). Given the degree in reduction of HAP exposure, we would have expected a measurable difference in blood pressure, PEF, or respiratory symptoms; however, instead we found no significant evidence of an effect. One potential concern may be that our sample size (90 intervention and 90 control participants) was too small. Although we were correctly powered to measure effects of one-half SD (11), it is possible that the effects of HAP exposure on blood pressure and PEF are smaller; however, the effect size is similar to or smaller than those reported in previous observational studies in Puno or RCTs in other locations (8, 15). The modest differences seen at the extreme ends of our exposure–response analysis may lend support to that hypothesis. Another potential concern is that a 1-year intervention may not provide sufficient time to reverse chronic inflammation from HAP exposure or measure meaningful changes in health outcomes. However, other HAP interventions targeting blood pressure or lung function have been able to effectively demonstrate reductions during similar short study periods (15, 27). It is also possible that our eligibility criteria selected too healthy a population. Average SBP and prebronchodilator PEF in our study sample were 101 mm Hg and 403 L/min, respectively. Moreover, we excluded women with hypertension and chronic obstructive pulmonary disease who may be most affected by HAP exposures and who could benefit the most from the intervention (28, 29).
Kitchen area concentrations and personal exposure to PM2.5 became progressively lower among intervention participants over time even when compared with concentrations among control participants. There are some possible explanations for this observation. Indeed, women in the intervention arm used their biomass stove or stove stacked more frequently earlier in the trial and less frequently the longer they had access to LPG. Second, there may have been less accumulation of soot in the ceiling and walls over time and consequently less resuspension of soot the longer intervention participants used their LPG stoves. Although there was a trend toward lower SBP between baseline and Month 9 measurements among control participants, these values were not significantly different from that among intervention participants at any point after randomization.
Our trial has several strengths. First, we successfully monitored both LPG and biomass stoves for 97% of days in intervention households. This completeness in stove monitoring increases confidence that our intervention was successful in achieving exclusive LPG stove use during the year-long intervention. Second, given this high level of adherence with exclusive LPG use, most intervention participants had personal exposures to HAP that were below recommended target levels. Indeed, there was a complete separation of distributions of kitchen area concentrations and personal exposures to HAP with lower concentrations in the intervention compared with the control arm. Third, the use of three-burner LPG stoves in our trial played a role in stimulating supply and demand for three-burner models, which are now available in our study setting. Our trial also has some limitations. First, women in the control arm did not cook daily with biomass as reported at baseline. Instead, control women used their biomass stove use for 72% of days during the year-long intervention. Days without biomass stove use could be because of use of an unmonitored stove, or failure of the temperature logger to capture the cooking event. Differences in cooking practices by season may explain unmonitored stove use in control households. Controls may have cooked daily with biomass at baseline but increased LPG use during the rainy season (30). Moreover, control households may have started using LPG more just by being in the study. Despite that, we still observed high kitchen area concentrations of HAP and personal exposures to HAP in repeated observations in control households. We cannot discount the possibility that control women may have changed their behavior to cook with the biomass stove and intervention women to cook with the LPG stove during the days of HAP exposure monitoring. Second, a large proportion of intervention and control women had LPG stoves at baseline. We were unable to monitor LPG stoves in all control households because many participants did not agree to have their own LPG stoves monitored. Third, although our intervention was successful at reducing PM2.5 and CO concentrations, kitchen area concentrations of NO2 remained above target levels for most households. Because exposure to NO2 is linked to lung inflammation, reduced lung function, and worsened cough and wheezing (31), it is possible that failing to reduce NO2 concentrations below target levels may have tempered the effect of the intervention on respiratory outcomes. Fourth, our population setting is unique in its sparse population density, high elevation, low ambient air pollution, and average distance between households of greater than 100 m. Therefore, our findings may not be generalizable to other higher-population-density, sea-level populations with higher levels of ambient air pollution. Fifth, with the relatively small size of this proof-of-concept study, for some outcomes we could only detect differences of half an SD, so some clinically important differences might have been missed. Finally, it is possible that the study endpoints chosen, although solid and clearly associated with morbidity and mortality, may not be sensitive enough to detect acute improvements in cardiopulmonary health. Other measures such as ambulatory blood pressure and lung function reversibility should also be considered.
In conclusion, our HAP intervention resulted in high adherence with exclusive LPG stove use, and subsequently reduced kitchen area concentrations and personal exposures to HAP among intervention participants. However, in this proof-of-concept RCT, we did not find evidence that these reduced HAP exposures lowered blood pressure, improved PEF, or decreased respiratory symptoms during a year-long intervention. A larger, multicountry RCT that is evaluating the effect of an LPG stove and fuel distribution intervention on blood pressure and respiratory symptoms with longer follow-up is currently underway and will either validate or disprove our study findings (32).
Footnotes
Joshua Rosenthal, Theresa Aguilar, Vanessa Burrowes, Elizabeth C. Fung, Phabiola Herrera, Alexander Lee, Kathryn A. Lee, Mitra Moazzami, Saachi Nangia, Laura Nicolaou, Carolyn O’Brien, Timothy Shade, Lena Stashko, Ariadne Villegas-Gomez, Abigail Winiker, Gary Malpartida, Lisa de las Fuentes, Dana Barr Boyd, Maria Jolly, and Angela Rozo
A complete list of CHAP trial Investigators may be found before the beginning of the References.
The research reported in this publication was supported by the NIH through the following institutes and centers: Fogarty International Center, National Institute of Environmental Health Sciences, National Cancer Institute, and Centers for Disease Control and Prevention under award numbers U01TW010107 and U2RTW010114 (Multiple Principal Investigators [MPIs]: W.C., G.F.G., L.P.N., N.K.S.). This trial was additionally supported in part by the Clean Cooking Alliance of the United Nations Foundation UNF-16-810 (Principal Investigator: W.C.). K.N.W., J.L.K., and C.H.M. were supported by NIH Research Training Grant D43TW009340 (MPIs: Pierre Buekens, W.C., Benjamin Chi, Khofi Kondwani) funded by the NIH through the following institutes and centers: Fogarty International Center, National Institute of Neurological Disorders and Stroke, National Institute of Mental Health, NHLBI, and the National Institute of Environmental Health Sciences. J.L.K., K.N.W., M.F.-D.-R., and D.G. were supported by a Global Established Multidisciplinary Sites award from the Center for Global Health at Johns Hopkins University (PI: W.C.). J.L.K. was further supported by the National Institute of Environmental Health Sciences of the NIH under Award Number T32ES007141 (PI: Marsha Wills-Karp). K.N.W. was supported by the NHLBI of the NIH under Award Number T32HL007534 (PI: Robert Wise). S.S. was supported by the NHLBI of the NIH under Award Numbers T32HL007534 (PI: Robert Wise) and F32HL143909 (PI: S.S.) and the Lietman Fellowship award of the Center for Global Health at Johns Hopkins University. M.F.-D.-R. was supported by the David Leslie Swift Fund of the Bloomberg School of Public Health, Johns Hopkins University. Our Global Non-Communicable Disease Research and Training field center in Puno, Peru, also received generous support from Mr. William and Bonnie Clarke III and the COPD Discovery Award from Johns Hopkins University.
Author Contributions: All authors participated in the trial design and conduct and participated in conference calls and in-person meetings to discuss trial findings. W.C. conducted statistical analyses with input from N.K.S. and L.H.M. and wrote the first draft with input from K.N.W., J.L.K., and M.F.-D.-R. G.F.G., L.P.N., S.A.H., V.G.D.-R., D.G., C.T.-M., C.H.M., S.S., M.C., R.T.C., and K.K. contributed to the interpretation of results and writing of the paper.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.202006-2319OC on December 11, 2020
Author disclosures are available with the text of this article at www.atsjournals.org.
Cardiopulmonary outcomes and Household Air Pollution (CHAP) trial Investigators
Steering Committee: William Checkley (Johns Hopkins University, Baltimore, MD), Gustavo F. Gonzales (Universidad Peruana Cayetano Heredia, Lima, Peru), Luke Naeher (University of Georgia, Athens, GA), Joshua Rosenthal (NIH, Bethesda, MD), N. Kyle Steenland (Emory University, Atlanta, Georgia).
Johns Hopkins University Investigators: Theresa Aguilar, Vanessa Burrowes, Magdalena Fandiño-Del-Rio, Elizabeth C. Fung, Dina Goodman, Steven A. Harvey, Phabiola Herrera, Josiah L. Kephart, Kirsten Koehler, Alexander Lee, Kathryn A. Lee, Catherine H. Miele, Mitra Moazzami, Lawrence Moulton, Saachi Nangia, Laura Nicolaou, Carolyn O’Brien, Suzanne Simkovich, Timothy Shade, Lena Stashko, Ariadne Villegas-Gomez, Kendra N. Williams, Abigail Winiker.
Asociación Benéfica PRISMA Investigators: Marilu Chiang, Gary Malpartida, Carla Tarazona-Meza.
Washington University Investigators: Victor Davila-Roman, Lisa de las Fuentes.
Emory University Investigators: Dana Barr Boyd, Maria Jolly, Angela Rozo.
RTI International: Ryan Chartier.
References
- 1. Bonjour S, Adair-Rohani H, Wolf J, Bruce NG, Mehta S, Prüss-Ustün A, et al. Solid fuel use for household cooking: country and regional estimates for 1980-2010. Environ Health Perspect . 2013;121:784–790. doi: 10.1289/ehp.1205987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhang J, Smith KR. Indoor air pollution: a global health concern. Br Med Bull . 2003;68:209–225. doi: 10.1093/bmb/ldg029. [DOI] [PubMed] [Google Scholar]
- 3. GBD 2017 Risk Factors Collaborators. Global, regional, and national comparative risk assessment of 84 behavioral, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet . 2018;392:1923–1994. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Thakur M, Nuyts PAW, Boudewijns EA, Flores Kim J, Faber T, Babu GR, et al. Impact of improved cookstoves on women’s and child health in low and middle income countries: a systematic review and meta-analysis. Thorax . 2018;73:1026–1040. doi: 10.1136/thoraxjnl-2017-210952. [DOI] [PubMed] [Google Scholar]
- 5. Po JY, FitzGerald JM, Carlsten C. Respiratory disease associated with solid biomass fuel exposure in rural women and children: systematic review and meta-analysis. Thorax . 2011;66:232–239. doi: 10.1136/thx.2010.147884. [DOI] [PubMed] [Google Scholar]
- 6. Kurmi OP, Semple S, Simkhada P, Smith WCS, Ayres JG. COPD and chronic bronchitis risk of indoor air pollution from solid fuel: a systematic review and meta-analysis. Thorax . 2010;65:221–228. doi: 10.1136/thx.2009.124644. [DOI] [PubMed] [Google Scholar]
- 7. Arku RE, Ezzati M, Baumgartner J, Fink G, Zhou B, Hystad P, et al. Elevated blood pressure and household solid fuel use in premenopausal women: analysis of 12 Demographic and Health Surveys (DHS) from 10 countries. Environ Res . 2018;160:499–505. doi: 10.1016/j.envres.2017.10.026. [DOI] [PubMed] [Google Scholar]
- 8. Burroughs Peña M, Romero KM, Velazquez EJ, Davila-Roman VG, Gilman RH, Wise RA, et al. Relationship between daily exposure to biomass fuel smoke and blood pressure in high-altitude Peru. Hypertension . 2015;65:1134–1140. doi: 10.1161/HYPERTENSIONAHA.114.04840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Quansah R, Semple S, Ochieng CA, Juvekar S, Armah FA, Luginaah I, et al. Effectiveness of interventions to reduce household air pollution and/or improve health in homes using solid fuel in low-and-middle income countries: a systematic review and meta-analysis. Environ Int . 2017;103:73–90. doi: 10.1016/j.envint.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 10. Pope D, Bruce N, Dherani M, Jagoe K, Rehfuess E. Real-life effectiveness of ‘improved’ stoves and clean fuels in reducing PM2.5 and CO: systematic review and meta-analysis. Environ Int . 2017;101:7–18. doi: 10.1016/j.envint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 11. Fandiño-Del-Rio M, Goodman D, Kephart JL, Miele CH, Williams KN, Moazzami M, et al. Cardiopulmonary outcomes and Household Air Pollution trial (CHAP) Trial Investigators. Effects of a liquefied petroleum gas stove intervention on pollutant exposure and adult cardiopulmonary outcomes (CHAP): study protocol for a randomized controlled trial. Trials . 2017;18:518. doi: 10.1186/s13063-017-2179-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hollada J, Williams KN, Miele CH, Danz D, Harvey SA, Checkley W. Perceptions of improved biomass and liquefied petroleum gas stoves in Puno, Peru: implications for promoting sustained and exclusive adoption of clean cooking technologies. Int J Environ Res Public Health . 2017;14:182. doi: 10.3390/ijerph14020182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J . 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 14. Jones PW, Quirk FH, Baveystock CM, Littlejohns P. A self-complete measure of health status for chronic airflow limitation: the St. George’s respiratory questionnaire. Am Rev Respir Dis . 1992;145: 1321–1327. doi: 10.1164/ajrccm/145.6.1321. [DOI] [PubMed] [Google Scholar]
- 15. Romieu I, Riojas-Rodríguez H, Marrón-Mares AT, Schilmann A, Perez-Padilla R, Masera O. Improved biomass stove intervention in rural Mexico: impact on the respiratory health of women. Am J Respir Crit Care Med . 2009;180:649–656. doi: 10.1164/rccm.200810-1556OC. [DOI] [PubMed] [Google Scholar]
- 16. Laird NM, Ware JH. Random-effects models for longitudinal data. Biometrics . 1982;38:963–974. [PubMed] [Google Scholar]
- 17. Mortimer K, Ndamala CB, Naunje AW, Malava J, Katundu C, Weston W, et al. A cleaner burning biomass-fuelled cookstove intervention to prevent pneumonia in children under 5 years old in rural Malawi (the Cooking and Pneumonia Study): a cluster randomised controlled trial. Lancet . 2017;389:167–175. doi: 10.1016/S0140-6736(16)32507-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Mortimer K, Lesosky M, Semple S, Malava J, Katundu C, Crampin A, et al. Pneumonia and exposure to household air pollution in children under the age of 5 years in rural Malawi: findings from the cooking and pneumonia study. Chest . 2020;158:501–511. doi: 10.1016/j.chest.2020.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Alexander DA, Northcross A, Karrison T, Morhasson-Bello O, Wilson N, Atalabi OM, et al. Pregnancy outcomes and ethanol cook stove intervention: a randomized-controlled trial in Ibadan, Nigeria. Environ Int . 2018;111:152–163. doi: 10.1016/j.envint.2017.11.021. [DOI] [PubMed] [Google Scholar]
- 20. Tielsch JM, Katz J, Khatry SK, Shrestha L, Breysee P, Zeger S, et al. Effect of an improved biomass stove on acute lower respiratory infections in young children in rural Nepal: a cluster-randomized, step-wedge trial. Lancet Glob Health . 2016;4(Suppl 1):S19. [Google Scholar]
- 21. Pollard SL, Williams DL, Breysse PN, Baron PA, Grajeda LM, Gilman RH, et al. CRONICAS Cohort Study Group. A cross-sectional study of determinants of indoor environmental exposures in households with and without chronic exposure to biomass fuel smoke. Environ Health . 2014;13:21. doi: 10.1186/1476-069X-13-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Brook RD, Franklin B, Cascio W, Hong Y, Howard G, Lipsett M, et al. Expert Panel on Population and Prevention Science of the American Heart Association. Air pollution and cardiovascular disease: a statement for healthcare professionals from the expert panel on population and prevention science of the American Heart Association. Circulation . 2004;109:2655–2671. doi: 10.1161/01.CIR.0000128587.30041.C8. [DOI] [PubMed] [Google Scholar]
- 23. Adam M, Schikowski T, Carsin AE, Cai Y, Jacquemin B, Sanchez M, et al. Adult lung function and long-term air pollution exposure: ESCAPE: a multicentre cohort study and meta-analysis. Eur Respir J . 2015;45:38–50. doi: 10.1183/09031936.00130014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Doiron D, de Hoogh K, Probst-Hensch N, Fortier I, Cai Y, De Matteis S, et al. Air pollution, lung function and COPD: results from the population-based UK Biobank study. Eur Respir J . 2019;54:1802140. doi: 10.1183/13993003.02140-2018. [DOI] [PubMed] [Google Scholar]
- 25. Quinn AK, Ae-Ngibise KA, Jack DW, Boamah EA, Enuameh Y, Mujtaba MN, et al. Association of carbon monoxide exposure with blood pressure among pregnant women in rural Ghana: evidence from GRAPHS. Int J Hyg Environ Health . 2016;219:176–183. doi: 10.1016/j.ijheh.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. McCracken JP, Smith KR, Díaz A, Mittleman MA, Schwartz J. Chimney stove intervention to reduce long-term wood smoke exposure lowers blood pressure among Guatemalan women. Environ Health Perspect . 2007;115:996–1001. doi: 10.1289/ehp.9888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Alexander D, Northcross A, Wilson N, Dutta A, Pandya R, Ibigbami T, et al. Randomized controlled ethanol cookstove intervention and blood pressure in pregnant Nigerian women. Am J Respir Crit Care Med . 2017;195:1629–1639. doi: 10.1164/rccm.201606-1177OC. [DOI] [PubMed] [Google Scholar]
- 28. Clark ML, Bachand AM, Heiderscheidt JM, Yoder SA, Luna B, Volckens J, et al. Impact of a cleaner-burning cookstove intervention on blood pressure in Nicaraguan women. Indoor Air . 2013;23:105–114. doi: 10.1111/ina.12003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Baumgartner J, Zhang Y, Schauer JJ, Huang W, Wang Y, Ezzati M. Highway proximity and black carbon from cookstoves as a risk factor for higher blood pressure in rural China. Proc Natl Acad Sci USA . 2014;111:13229–13234. doi: 10.1073/pnas.1317176111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Pollard SL, Williams KN, O’Brien CJ, Winiker A, Puzzolo E, Kephart JL, et al. An evaluation of the Fondo de Inclusión Social Energético program to promote access to liquefied petroleum gas in Peru. Energy Sustain Dev . 2018;46:82–93. doi: 10.1016/j.esd.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.US Environmental Protection Agency. Research Triangle Park, NC: U.S. Environmental Protection Agency; 2016. Integrated science assessment for oxides of nitrogen–health criteria. [Google Scholar]
- 32. Clasen T, Checkley W, Peel JL, Balakrishnan K, McCracken JP, Rosa G, et al. HAPIN Investigators. Design and rationale of the HAPIN study: a multicountry randomized controlled trial to assess the effect of liquefied petroleum gas stove and continuous fuel distribution. Environ Health Perspect . 2020;128:47008. doi: 10.1289/EHP6407. [DOI] [PMC free article] [PubMed] [Google Scholar]