Abstract
Seizures and interictal epileptiform discharges (IEDs) have been described during sevoflurane. We prospectively estimated their incidence in 54 otherwise neurologically healthy infants by obtaining the full-head video-electroencephalogram (EEG). No infants had clinical seizures but one had an electrographic seizure; three others had focal IEDs: 7.4% (95% CI 2.1%, 17.9%). We detected no differences in demographic or clinical characteristics between normal versus abnormal EEG groups. Diffuse slowing was the most common initial EEG change followed by fast (alpha, beta) activity in all head leads. Larger studies with more statistical power are needed to further investigate the hypotheses generated with this research.
INTRODUCTION
While their incidence in infants is unknown, seizures and interictal epileptiform discharges (IEDs) in the electroencephalogram (EEG) have been described in children and adults “without a history of epilepsy” during administration of sevoflurane for general anesthesia, with an incidence as high as 88% during induction1–8. Gamma-aminobutyric acid (GABA) receptor activation can be excitatory in immature brains due to changes in intracellular chloride ion concentrations during development9,10. Plausibly, sevoflurane’s action at GABA-ergic receptors could induce IEDs or seizures at very young ages11,12. Therefore, we hypothesized that during sevoflurane induction in very young infants, when there is routine, rapid up-titration to as high as 8% fraction inspired sevoflurane, excitatory brain activity and seizures may be observed. Accordingly, we prospectively recorded unprocessed, International 10–20 System full-head montage EEG with synchronized video during sevoflurane induction. Our goal was to provide an estimate of the incidence of abnormalities in the electroencephalogram and correlate this to any clinical behavior recorded in synchronized video. This approach differs from prior work in which: (1) subjects were older, (2) limited, processed electrode arrays were used, (3) clinical assessment was non-standardized, (4) subjects were premedicated with midazolam and (5) nitrous oxide was used.
METHODS
The study protocol was approved by the Institutional Review Board of the Albert Einstein College of Medicine and written informed consent was obtained from the parent or legal guardian for all subjects. We recruited patients less than 3 years of age and excluded those with prematurity, neurologic injury, epilepsy, or planned intracranial surgery. Sevoflurane was the sole anesthetic agent used for inhalational induction. No subjects were premedicated or received nitrous oxide. Anesthetic management was otherwise not restricted in order to be as clinically applicable as possible.
EEG was recorded using the microEEG® system, a Food and Drug Administration approved, 26-channel EEG collection and wireless data transfer device (Biosignal Group Corp., Acton, MA)13 (Supplemental Figure 1). We obtained baseline EEG and video-EEG of the sevoflurane induction. Two neurologists (AL, EY) rated the quality of data independently, described subject movements, and analyzed the EEG using Persyst software (Persyst Corp., Solana Beach, CA). A third neurologist (SS) reconciled any disagreements.
Statistical Analysis:
Baseline demographics were reported as medians and interquartile ranges (IQR) or counts and percentages. The proportion with abnormal EEG was estimated along with its 95% confidence interval by the Clopper-Pearson exact method. Univariate analysis of clinical characteristics by presence of EEG abnormalities was performed using Wilcoxon Rank-Sum test for continuous variables or Fishers Exact test for categorical variables. P values <0.05 was considered statistically significant. All tests were two tailed. In this pilot observational study, we set a goal of obtaining 50 interpretable recordings a priori without formal power analysis. All analyses were done using STATA 13.1 (STATA Corp., College Station, TX).
RESULTS
Between 9/21/16 and 6/8/18, 134 eligible subject families were identified and contacted (Supplemental Figure 2). In all, 54 subject recordings were eligible for analysis, with median age 7.6 months, IQR (4.9, 9.8), of whom 41 were male (76%) and 13 were female (24%), (Supplemental Table 1). The vast majority of clinicians initiated inhalational induction with 8% sevoflurane in high flow oxygen with none utilizing controlled ventilation or rapid over-pressure techniques. There was no difference in first recorded fraction inspired sevoflurane or induction time between subjects with normal versus abnormal EEG.
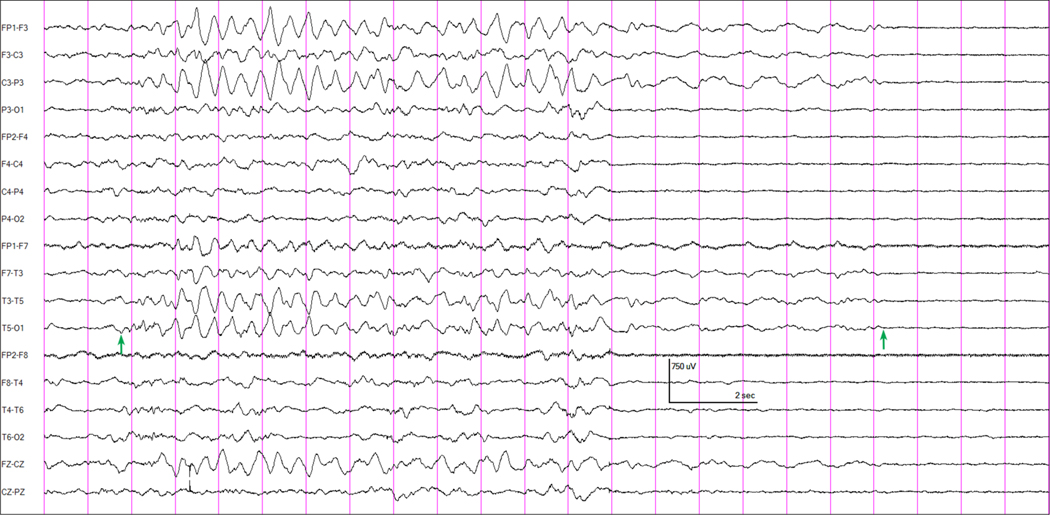
Most patients exhibited movements during sevoflurane induction characterized as “fighting,” “kicking,” “arm extension,” “arching,” or “back stiffening”. None were myoclonic jerks or seizure-like movements and none had an abnormal EEG correlate. Four subjects, 7.4% (95% CI 2.1%, 17.9%), demonstrated EEG abnormalities following sevoflurane induction. One subject, an otherwise healthy, former full-term, 7.9 month old boy undergoing chordee repair, had a focal left hemispheric electrographic seizure lasting approximately 17.5 seconds approximately 9 minutes into anesthesia induction with sevoflurane (Figure 1), when a bolus dose of propofol (3 mg/kg) was administered prior to laryngeal mask airway insertion. The patient had not received any other anesthetics and the etSEV at the time of propofol bolus was 3.65%. Prior to this, his EEG had not shown any abnormalities. No clinical seizure activity was noted in the video. Three others had focal IEDs – right frontal sharp waves, left frontocentral sharp waves, or multifocal spikes (Supplemental Figure 3).
Figure 1 –
EEG showing attenuation of all EEG activity associated with the administration of a bolus intravenous dose of propofol in a 7 month old boy having surgery for chordee repair. Anesthesia had been induced with sevoflurane approximately 9 minutes earlier. In this patient (and in no others), an evolving electrographic seizure pattern was recorded at the time of the onset of the propofol effect. The arrows indicate the onset and end of the left hemispheric seizure discharge. Longitudinal bipolar montage.
The initial EEG change during sevoflurane induction was most often diffuse increased background slowing (76%); sometimes this was diffuse high voltage delta slowing (Supplemental Figure 4B). This was most often (93%) followed by fast beta and alpha frequency activity over the entire brain, and slow wave activity decreased in amplitude (Supplemental Figure 4C). In some subjects, the slow wave activity almost disappeared, leaving an EEG consisting predominantly of fast (beta and alpha frequency) activity with only a small amount of slow wave activity (Supplemental Figure 4D). Some patients continued to move during the initial prominent delta slowing, but this ceased after the EEG reached the pattern of predominantly fast (beta and alpha frequency) activity. In 10 subjects (19%), the background slowing was preceded by transient beta activity, though beta did not follow the slowing in all of these. Overall, increased beta was present at some point in 51 of 54 subjects (94%). This occurred in 7 of 9 infants <4 months of age (78%) and in 44 of 45 (98%) of infants 4 months or older. If an intravenous bolus of propofol was given after the sevoflurane induction, a marked attenuation of all EEG activity was typically observed (Figure 1), followed by return of activity in a burst-suppression pattern in some subjects.
Interestingly, one subject, an otherwise healthy, former full-term, 13 month old female having surgery for removal of a superficial occipital skull dermoid cyst, had a focal right temporal electrographic seizure in the baseline EEG recording, before any anesthetic had been given (Supplemental Figure 5). On neuroimaging, the cyst did not extend into the intracranial space. No clinical seizure activity was noted during the baseline recording.
We compared clinical characteristics between subjects with normal EEG (n=50) to those with abnormal EEG during sevoflurane induction (n=4) (Table 1). On univariate testing, there were no differences between groups in terms of age, gender, maximum etSEV, etCO2, or the proportion in whom IV anesthetics (propofol and fentanyl) were administered. However, the study had low power to detect small but potentially clinically important differences. Among 20 subjects who received any propofol, 3 (15%) had EEG abnormalities compared to one of the 34 subjects (2.9%) who did not, p=0.14. However, 2 of the 3 had IEDs prior to propofol administration.
Table 1 –
Comparison of clinical factors between subjects with normal versus abnormal sevoflurane induction EEG
| Normal EEG | Abnormal EEG | p value | |
|---|---|---|---|
| (n = 50) | (n = 4) | ||
|
| |||
| Age (months) | 7.5 (4.9, 9.8) | 5.1 (2.0, 10.2) | 0.39 |
| Male gender (no.) | 38 (76%) | 3 (75%) | >0.99 |
| Initial inSEV (%) | 6.7 (6.0, 7.2) | 6.5 (6.2, 7.5) | 0.83 |
| Max etSEV (%) | 6.1 (5.4, 6.6) | 5.9 (5.6, 6.0) | 0.75 |
| EtCO2 min (mmHg) | 16 (11, 20) | 12.5 (9.5, 18) | 0.59 |
| EtCO2 max (mmHg) | 36 (31, 43) | 36.5 (30.5, 42.5) | 0.19 |
| Propofol given (no.) | 17 (34%) | 3 (75%)* | 0.14 |
| Fentanyl given (no.) | 27 (54%) | 1 (25%) | 0.34 |
| Induction time (minutes)♯ | 6 (3, 9) | 5 (4, 8) | 0.74 |
two of three subjects demonstrated IEDs prior to propofol administration
defined as the time beginning from first recorded fraction inspired sevoflurane concentration until any one of the following recorded times – (a) peripheral venous cannula insertion or first recorded intravenous medication administration, (b) anesthesia “ready” indicated by the provider, or (c) securing of the airway (e.g. LMA insertion or endotracheal intubation) – whichever came first.
Wilcoxon Mann-Whitney U test and Fishers Exact test used
DISCUSSION
In summary, the goal of our study was to prospectively estimate the incidence of abnormalities in the electroencephalogram in a cohort of infants presenting for routine surgery requiring sevoflurane induction. Commonly observed movements during induction suggestive of seizure activity were not associated with abnormalities on the EEG. However, we observed a 7.4% incidence (95% CI 2.1%, 17.9%) of epileptiform changes without clinical correlate in this otherwise neurologically healthy cohort of infants. Without electrophysiological monitoring of the brain, these abnormalities would not have otherwise been detected, but the etiology and clinical impact of these findings is unknown at this time. Although EEG abnormalities are present at a certain frequency in healthy children14, minor EEG abnormalities are very rare among infants, even those with epilepsy15. Recruiting a considerably larger sample size will be necessary to increase the precision of our incidence estimation.
Our findings were similar to previous reports in which the EEG was measured in infants during maintenance of surgical state of anesthesia (MOSSA)16 and emergence17, in which diffuse increased background slowing and diffuse increase in fast activity (beta and alpha frequency) were also observed. We observed fast activity in the vast majority of subjects including subjects less than 4 months of age. It was somewhat less prevalent in the younger age group but still occurred in 78% of those infants. In one study, fast activity was not observed in infants less than 4 months of age but patients received (1) nitrous oxide for induction (54.5% in those <4 months of age and 76.7% overall) and up to 15 mg/kg of propofol cumulatively overall and (2) substantially lower doses of sevoflurane during EEG recording (as this was outside the period of induction)17. Therefore, the anesthetic conditions and the phases of the anesthetic under examination differ from our study.
Anesthetic mediated EEG abnormalities might be associated with clinically meaningful outcomes. Fritz et al found an association between intraoperative EEG changes (burst suppression) and postoperative delirium in adult patients18. Plausibly, intraoperative epileptiform changes and burst suppression in pediatric patients might be associated with postoperative emergence delirium. In addition, while infant subjects in our cohort continued to move during the initial EEG slowing, spontaneous movements ceased after the EEG transitioned to a pattern of predominantly fast (beta and alpha frequency) activity. Whether this EEG transition or some other parameter might be used to influence anesthesia titration during induction to minimize the risk of unwanted airway reflexes (or guide timing of stimulating procedures such as peripheral venous cannulation or airway manipulation) awaits further study.
Supplementary Material
Supplemental Figure 1 – Infant wearing a MicroEEG® headpiece with biosensors in place – 44–48 cm head circumference model shown. This is a stock photo from the manufacturer and not an actual patient; image courtesy of Biosignal Group Corp., http://biosignalgroup.com/product-service/hydrodot-biosensors.
Supplemental Figure 2 – Participant flowchart.
Supplemental Figure 3 – Interictal epileptiform discharges recorded during sevoflurane induction. A: Right frontal sharp waves maximal at electrode F4 (green arrows) in a 10 week old boy having a liver biopsy due to cholestasis. The smaller green arrows demonstrate a field of the sharp waves to the Fz electrode B: Left frontocentral sharp waves (green arrows) in a 12 month old boy having a thoracoscopic resection of a mediastinal mass, a neuroblastoma. C: Multifocal spikes in a 13 month old girl having a thoracoscopic lower lobectomy for a congenital pulmonary airway malformation. Bifrontal spikes are indicated by green arrows, right central spikes by red arrows, and left occipitotemporal spikes by diagonal blue arrows. All EEG samples are displayed using a longitudinal bipolar montage; some noisy EEG channels are not shown in B and C. Vertical calibration bar = 300 μV in A and B, 500 μV in C.
Supplemental Figure 4 – EEG changes during induction of anesthesia with 8% sevoflurane in an 8 month old boy who had been born at full term. A: Prior to induction. B: At 2 min, 14 sec after mask on, the EEG shows high voltage delta slowing. C: At 4 min, 47 sec after mask on, increased fast activity appears as the slow wave activity decreases in voltage. D: At 9 min, 17 sec after mask on, the EEG is mostly fast (beta frequency) activity with some slower frequencies. All EEG segments are shown at the same voltage and time scales, calibration bars at lower left, filter settings 1 Hz to 70 Hz.
Supplemental Figure 5 – Baseline EEG recorded in a 13 month old girl who would be having surgery for excision of an occipital skull dermoid cyst, showing a focal seizure discharge over the right temporal region (green bracket) which evolves over time to a slower frequency (double-headed green arrow) but remains as a focal discharge over the same region. No medications had been given at the time of this baseline recording. Longitudinal bipolar montage.
Acknowledgments
Funding Disclosures: The research described was supported by institutional and departmental sources (the Department of Anesthesiology and The Saul R. Korey Department of Neurology, Montefiore Medical Center, Albert Einstein College of Medicine) as well as NIH/National Center for Advancing Translational Science (NCATS) Einstein-Montefiore CTSA Grant Number UL1TR001073.
GLOSSARY OF TERMS
- IED
Interictal epileptiform discharge
- EEG
Electroencephalogram
- CI
Confidence interval
- GABA
Gamma-aminobutyric acid
- IQR
Interquartile range
- MOSSA
Maintenance of surgical state of anesthesia
Footnotes
Conflicts of Interest: None.
Clinical trial number and registry URL: Not applicable.
Contributor Information
Jerry Y. Chao, Department of Anesthesiology, Montefiore Medical Center, Albert Einstein College of Medicine
Alan D. Legatt, The Saul R. Korey Department of Neurology, Dominick P. Purpura Department of Neuroscience, Montefiore Medical Center, Albert Einstein College of Medicine
Elissa G. Yozawitz, The Saul R. Korey Department of Neurology, Department of Pediatrics, Montefiore Medical Center, Albert Einstein College of Medicine
David C. Adams, Department of Anesthesiology, Montefiore Medical Center, Albert Einstein College of Medicine
Ellise S. Delphin, Department of Anesthesiology, Montefiore Medical Center, Albert Einstein College of Medicine
Shlomo Shinnar, The Saul R. Korey Department of Neurology, Department of Pediatrics, Department of Epidemiology & Population Health, Montefiore Medical Center, Albert Einstein College of Medicine
REFERENCES
- 1).Constant I, Seeman R, Murat I. Sevoflurane and epileptiform EEG changes. Pediatr Anaesth. 2005;15:266–274. [DOI] [PubMed] [Google Scholar]
- 2).Gibert S, Sabourdin N, Louvet N, et al. Epileptogenic effect of sevoflurane: determination of the minimal alveolar concentration of sevoflurane associated with major epileptoid signs in children. Anesthesiology. 2012;117(6):1253–6. [DOI] [PubMed] [Google Scholar]
- 3).Adachi M, Ikemoto Y, Kubo K, Takuma C. Seizure-like movements during induction of anaesthesia with sevoflurane. Br J Anaesth. 1992;68:214–215. [DOI] [PubMed] [Google Scholar]
- 4).Haga S, Shima T, Momose K, Andoh K, Hasimoto Y. Anesthetic induction of children with high concentration of sevoflurane. Masui. 1992;41:1951–1955. [PubMed] [Google Scholar]
- 5).Komatsu H, Taie S, Endo S, et al. Electrical seizures during sevoflurane anesthesia in two pediatric patients with epilepsy. Anesthesiology. 1994;81:1535–1537. [DOI] [PubMed] [Google Scholar]
- 6).Woodforth IJ, Hicks RG, Crawford MR, Stephen JP, Burke DJ. Electroencephalographic evidence of seizure activity under deep devoflurane anesthesia in a nonepileptic patient. Anesthesiology. 1997;87:1579–1589. [DOI] [PubMed] [Google Scholar]
- 7).Constant I, Dubois MC, Piat V, Moutard M, McCue M, Murat I. Changes in electroencephalogram and autonomic cardiovascular activity during induction of anesthesia with sevoflurane compared with halothane in children. Anesthesiology. 1999;91(6):1604–1615. [DOI] [PubMed] [Google Scholar]
- 8).Vakkuri A, Yli-Hankala A, Sarkela M, et al. Sevoflurane mask induction of anaesthesia is associated with epileptiform EEG in children: Acta Anaesthesiol Scand. 2001;45:805–811. [DOI] [PubMed] [Google Scholar]
- 9).Brohan J, Goudra BG. The role of GABA receptor agonists in anesthesia and sedation. CNS Drugs. 2017;(10):845–856. [DOI] [PubMed] [Google Scholar]
- 10).Ben-Ari Y, Khalilov I, Kahle KT, Cherubini E. The GABA excitatory/inhibitory shift in brain maturation and neurological disorders. Neuroscientist. 2012;18(5):467–486. [DOI] [PubMed] [Google Scholar]
- 11).Avoli M. GABA-mediated synchronous potentials and seizure generation. Epilepsia. 1996;37(11):1035–42. [DOI] [PubMed] [Google Scholar]
- 12).Voss LJ, Sleigh JW, Barnard JP, Kirsch HE. The howling cortex: seizures and general anesthetic drugs. Anesth Analg. 2008;107(5):1689–703. [DOI] [PubMed] [Google Scholar]
- 13).Grant AC, Abdel-Baki SG, Omurtag A, et al. Diagnostic accuracy of microEEG: a miniature, wireless EEG device. Epilepsy Behav. 2014;34:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14).Borusiak P, Zilbauer M, Jenke AC. Prevalence of epileptiform discharges in healthy children – new data from a prospective study using digital EEG. Epilepsia. 2010;51(7):1185–8. [DOI] [PubMed] [Google Scholar]
- 15).Niedermeyer R, Yarworth S. Scarcity of minor EEG abnormalities during the first two years of life. Clinical Electroencephalogr. 1978;(1):20–8. [DOI] [PubMed] [Google Scholar]
- 16).Akeju O, Pavone KJ, Thum JA, Firth PG, Westover MB, Puglia M, Shank ES, Brown EN, Purdon PL. Age-dependency of sevoflurane-induced electroencephalogram dynamics in children. British Journal of Anaesthesia 2015;115 Suppl 1:i66–i76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17).Cornelissen L, Kim SE, Purdon PL, Brown EN, Berde CB. Age-dependent electroencephalogram (EEG) patterns during sevoflurane general anesthesia in infants. Elife 2015;4:e06513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18).Fritz BA, Kalarickal PL, Maybrier HR, Muench MR, Dearth D, Chen Y, Escallier KE, Abdallah AB, Lin N, Avidan MS. Intraoperative electroencephalogram suppression predicts postoperative delirium. Anesthesia & Analgesia. 2016;122(1):234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1 – Infant wearing a MicroEEG® headpiece with biosensors in place – 44–48 cm head circumference model shown. This is a stock photo from the manufacturer and not an actual patient; image courtesy of Biosignal Group Corp., http://biosignalgroup.com/product-service/hydrodot-biosensors.
Supplemental Figure 2 – Participant flowchart.
Supplemental Figure 3 – Interictal epileptiform discharges recorded during sevoflurane induction. A: Right frontal sharp waves maximal at electrode F4 (green arrows) in a 10 week old boy having a liver biopsy due to cholestasis. The smaller green arrows demonstrate a field of the sharp waves to the Fz electrode B: Left frontocentral sharp waves (green arrows) in a 12 month old boy having a thoracoscopic resection of a mediastinal mass, a neuroblastoma. C: Multifocal spikes in a 13 month old girl having a thoracoscopic lower lobectomy for a congenital pulmonary airway malformation. Bifrontal spikes are indicated by green arrows, right central spikes by red arrows, and left occipitotemporal spikes by diagonal blue arrows. All EEG samples are displayed using a longitudinal bipolar montage; some noisy EEG channels are not shown in B and C. Vertical calibration bar = 300 μV in A and B, 500 μV in C.
Supplemental Figure 4 – EEG changes during induction of anesthesia with 8% sevoflurane in an 8 month old boy who had been born at full term. A: Prior to induction. B: At 2 min, 14 sec after mask on, the EEG shows high voltage delta slowing. C: At 4 min, 47 sec after mask on, increased fast activity appears as the slow wave activity decreases in voltage. D: At 9 min, 17 sec after mask on, the EEG is mostly fast (beta frequency) activity with some slower frequencies. All EEG segments are shown at the same voltage and time scales, calibration bars at lower left, filter settings 1 Hz to 70 Hz.
Supplemental Figure 5 – Baseline EEG recorded in a 13 month old girl who would be having surgery for excision of an occipital skull dermoid cyst, showing a focal seizure discharge over the right temporal region (green bracket) which evolves over time to a slower frequency (double-headed green arrow) but remains as a focal discharge over the same region. No medications had been given at the time of this baseline recording. Longitudinal bipolar montage.