Abstract
Objective
India is experiencing an increasing prevalence of type 2 diabetes and cardiovascular diseases. Mobile health technology may be a strategy to reduce the risk of cardiometabolic disorders. This paper reports on the effect of a mobile health intervention on cardiometabolic risk factors.
Methods
The mobile health and diabetes intervention was a 12-week reality television-based mobile health program application delivered via videos, short message service and infographics through a smartphone application followed-up weekly by health coach calls. mobile health and diabetes was conducted in a randomized control trial mode randomized controlled trial methodology in three Indian cities (Chennai, Bengaluru and New Delhi) with participants recruited via community screening events. This paper looks at the pre–post changes in cardiometabolic risks among the participants and the place of demography in influencing these.
Results
The mobile health and diabetes intervention group experienced a small reduction in waist circumference (1.8 cm) compared to the control group (0.5 cm, p < 0.05) and a greater decrease in systolic blood pressure (2.7 mmHg) compared to the control group (p < 0.05). The improvements in cardiometabolic risk factors were more pronounced in individuals with obesity, although overall effects were very modest
Conclusions
Cardiometabolic risk factors can be reduced with a mobile health application using human coaching, especially in obese individuals, but the improvements are small. To be more effective and clinically meaningful, intensive engagement with the participants is probably required.
Keywords: mHealth, diabetes prevention, cardiometabolic risk factors, mHealth application, eHealth, obesity, lifestyle
Introduction
The prevalence of type 2 diabetes (T2DM) in India is rising rapidly and it is predicted that there would be 134.3 million people with diabetes by 2045.1 Cardiovascular disease (CVD) is also an important cause of morbidity and mortality in India and other developing countries. The increase in the prevalence of T2DM and CVD points to the urgent need for prevention and management strategies for cardiometabolic risk factors.2
Mobile phones provide a convenient method to take health messages to the community at large. Reviews on the use of mobile health (mHealth) technology for the prevention and management of T2DM suggests that this can be used quite effectively.3,4 In a short message service (SMS) study by Arora et al.,5 the authors reported that healthy behaviors that help to prevent diabetes prevention increased just by sending three text messages per day. Moreover, in an Indian SMS study, the authors reported a decrease in cumulative diabetes incidence in the SMS group compared to the control group.6
Mobile health and diabetes (mDiab) was a randomized controlled trial, using 12-weeks of reality television-based videos delivered via a mobile health application to individuals at a high risk of developing diabetes. The aim of this paper was to look at changes in cardiometabolic risk factors including, waist circumference, body fat percent, blood pressure (BP), plasma glucose and serum lipids after 12 weeks of intervention using mDiab. Additionally, we also report how the parameters have changed post-intervention in individuals at high risk, i.e. those with prediabetes, obesity or both.
Methods
mDiab, a mobile health application that was developed based on the learnings from a prior study, diabetes community lifestyle improvement program (D-CLIP), was used in this randomized control trial. The D-CLIP adapted the very successful US diabetes prevention program (DPP) and culturally modified it to suit the Indian population and delivered the 4 months (16 weeks) intensive intervention plus 8 months maintenance in a face-to-face group setting.7 mDiab, used the D-CLIP experiences, and translated the 16 weeks intensive lesson plans to 12 weeks (3 months) of video lessons, SMS, infographics and human coach calls. Two important and unique aspects of the mDiab program were – (1) The 12 weeks of video lessons were delivered in a reality television-based format. (2) Multiple modes/resources were available for the users to catch up on missed video lessons; (a) A summary of the weekly video content was delivered to the participant by a health coach via a phone call. (b) The participants could also interact through the application by messaging the health coaches and receive guidance on technical issues with regards to application navigation but also receive healthy lifestyle text messages and infographics. This trial has been registered with the Clinical Trials Registry of India (CTRI), prospectively, on 16 July 2015. (Identifier: CTRI/2015/07/006011).
Ethics approval
The study protocol8 was approved by the Institutional Ethics Committee at Madras Diabetes Research Foundation, All India Institute of Medical Sciences (Reference number: IEC/NP-232/05.02.2015) and the Human Research Ethics Committee at Deakin University, Australia (Reference number: DU-HREC 2015-167). Written consent was obtained from all participants. At baseline testing, all participants were also given a plain language statement (PLS) of the study.
Participants and study period
This study was a multicenter, randomized control trial, the methodology of which has been published previously8 but is briefly described here. The study participants were recruited from three cities in India, Chennai, Bangalore and New Delhi. Recruitment strategies included community screening events in parks, companies, residential colonies, religious sites and through direct clinic references. The inclusion criteria for the trial were participants aged 20–65 years, owning an Android smartphone and had prediabetes and/or obesity. Prediabetes was defined as fasting plasma glucose between 100 and 125 mg/dL (5.6–6.9 mmol/L) according to the American Diabetes Association (ADA) criteria9 and obesity if body mass index ≥25 kg/m2 according to the World Health Organization (WHO) body mass index cut-offs for Asians.10
During screening, demographic data (age, gender, smoking status, occupation, etc.) were collected while at baseline, anthropometry and clinical biomarkers including waist circumference, body fat, fasting plasma glucose (FPG), systolic blood pressure (SBP), diastolic blood pressure (DBP), total cholesterol, triglycerides (TGL), high-density lipoprotein (HDL) and low-density lipoprotein (LDL) were collected. The participants were either randomized into the intervention group, which received the mDiab application in their smartphones and weekly health coach calls or into the control group, which received usual care. Individuals randomized to the usual care arm received a consultation with a nutritionist and were provided handouts that reinforced the prevention of T2DM through increased physical activity and weight loss.
Randomization was carried out on a weekly basis. The study coordinators at each of the three study sites de-identified the participants using study IDs and sent a list of eligible participants’ study IDs to the study manager at the central randomization unit. The study manager randomized the participants using a random number generator computer program to allocate them into the intervention or control groups and then sent the weekly randomized lists back to the site coordinators. The study coordinators then informed the participants of their group. Due to the nature of the trial, it was not possible to blind the participants, investigators or staff to the randomization process.
All measurements were done at baseline and after 12 weeks of intervention. Body fat percent was measured using a body fat analyzer (Omron HBF-306 Body Fat Monitor). Waist circumference using a standard non-stretchable inch tape measure and was calibrated everyday. While calibrating, it was ensured that the difference between two readings was not greater than 0.2 cm. In case of the measuring tape being damaged, whether stretched, twisted or if graduations have been erased, the tape was replaced with a new one. The study participant was requested to stand with their feet together and arms by their side with the palms facing inward. The technician then located the inferior margin of the last rib and the crest of the ileum and identified the midpoint. After ensuring proper placement of the tape around the waist, measurement to the nearest 0.1 cm was recorded. Two measurements were recorded and the average of the two were taken. In any case, a difference of >0.2 cm between the two readings resulted in repeating the process. SBP and DBP were measured using an electronic BP apparatus, Omron machine (Omron Corporation, Tokyo, Japan). Two readings were taken 5 min apart and their mean was taken as the BP and the other clinical biomarkers were collected as blood samples post 10−12 h of overnight fasting. The following cut-off points were used to measure outcomes in this research: waist circumference – <90 cm (men) and <80 cm (women); percent body fat – <25% in men and <35% in women; BP, systolic: 120 mmHg and diastolic 80 mmHg; FPG <100 mg/dL; total cholesterol <200 mg/dL; serum triglycerides <150 mg/dL; LDL <100 mg/dL; HDL >40 mg/dL.
Statistical analysis
Analyses were conducted using Windows-based Stata Statistical Software: Release 14. College Station, TX: StataCorp LP. All outcome variables were tested for normality using histogram analysis. The clinical and anthropometric biomarkers were analyzed using within and between-group differences. Chi-square and independent-sample t-tests were carried out for descriptive analysis depending on whether the variables were categorical or continuous. Paired t-tests were used to establish differences between baseline and post-intervention time points. Analysis of variance (ANOVA) was used to establish between-group differences. All analyses were considered significant if the p-value was <.05.
Results
Baseline characteristics of participants
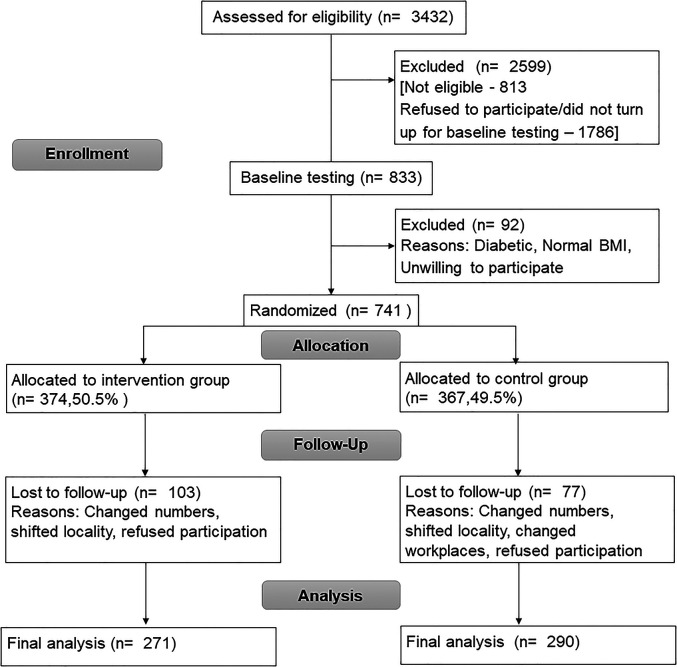
A total of 741 individuals were randomized into the study, of which 374 individuals were in the intervention group and 367 individuals in the control group. There was a 28% and 21% dropout in the intervention and control arms, respectively, leaving a total of 561 (271 – Intervention, 290 – control) individuals for the trial analysis. Table 1 presents the baseline demographic characteristics including age, gender, city of recruitment, smoking and alcohol habits, occupation, education levels and marital status. Table 1 also includes the baseline measures of the clinical and anthropometry variables. No statistically significant differences were found in terms of the variables between the intervention and control groups (Figure 1).
Table 1.
Baseline characteristics of the participants.
| Characteristic | Intervention (n = 271) | Control (n = 290) | p-value |
|---|---|---|---|
| Age in years, mean ± SD | 37.8 ± 9.2 | 37.8 ± 9.6 | .99a |
| Gender (n, %) | |||
| Female | 119(43.9) | 122(42.1) | .66b |
| Male | 152(56.1) | 168(57.9) | |
| Location (n, %) | |||
| Chennai | 89(32.8) | 117(40.3) | .18b |
| Bengaluru | 89(32.8) | 86(29.7) | |
| New Delhi | 93(34.3) | 87(30.0) | |
| Smoking status (n, %) | |||
| Smoker | 40(14.8) | 41(14.1) | .83b |
| Non-smoker | 231(85.2) | 249(85.8) | |
| Alcohol use (n, %) | |||
| Drinker | 94(34.7) | 82(28.3) | .10b |
| Non-drinker | 177(65.3) | 208(71.7) | |
| Education level (n, %) | |||
| No schooling | 3(1.1) | 3(1.0) | .66b |
| Primary school completed (5th grade) | 6(2.2) | 2(0.7) | |
| High School (10th grade) | 19(7.0) | 17(5.9) | |
| Higher School (12th grade) | 29(10.7) | 35(12.0) | |
| Technical education | 21(7.8) | 16(5.5) | |
| Undergraduate degree | 101(37.3) | 112(38.6) | |
| Postgraduate or above | 92(34.0) | 104(35.9) | |
| Preferred not to answer | 0(0.0) | 1(0.3) | |
| Occupation level (n, %) | |||
| Not working/unemployed/retired | 17(6.3) | 19(6.6) | .59b |
| Household and domestic work | 39(14.4) | 28(9.7) | |
| Agriculture/self employed | 10(3.7) | 16(5.5) | |
| Clerical | 8(3.0) | 12(4.1) | |
| Professional/executive/manager | 130(48.0) | 148(51.0) | |
| Sales/service | 16(5.9) | 15(5.2) | |
| Other | 51(18.8) | 52(17.9) | |
| Marital status (n, %) | |||
| Currently married | 191(70.5) | 213(73.5) | .33b |
| Divorced | 1(0.4) | 0(0.0) | |
| Living as married | 19(7.0) | 17(5.9) | |
| Separated | 0(0.0) | 4(1.4) | |
| Single | 58(21.4) | 54(18.6) | |
| Widowed | 2(0.7) | 2(0.7) | |
| Waist circumference (cm)c | 97.7 ± 10.0 | 97.3 ± 10.2 | .60a |
| Weight (kg)c | 78.2 ± 11.8 | 77.7 ± 11.8 | .63a |
| Body mass index (kg/m2)c | 29.4 ± 3.8 | 29.3 ± 4.2 | .74a |
| Percent body fat (%)c | 35.2 ± 8.8 | 35.9 ± 9.2 | .36a |
| Systolic blood pressure (mm/Hg)c | 120.9 ± 14.5 | 120.3 ± 14.4 | .62a |
| Diastolic blood pressure (mm/Hg)c | 81.1 ± 9.9 | 81.1 ± 10.2 | .93a |
| Fasting plasma glucose (mmol/L)c | 93.5 ± 10.6 | 93.5 ± 9.7 | .93a |
| Serum cholesterol (mg/dL)c | 180.8 ± 33.7 | 182.5 ± 36.3 | .56a |
| Serum triglycerides (mg/dL)c | 137.3 ± 59.4 | 132.8 ± 62.6 | .39a |
| High density lipoprotein (mg/dL)c | 40.5 ± 9.1 | 42.0 ± 10.3 | .07a |
| Low density lipoprotein (mg/dL)c | 112.7 ± 29.7 | 113.9 ± 31.9 | .65a |
Body mass index was calculated using the formula: weight in kg/height in m2.
Alcohol use– individuals were individuals who consumed >three alcoholic drinks for a minimum of once a week. Non-drinkers are individuals who had never had alcohol. Smoking – smokers included individuals who used tobacco products in any form and non-smokers included individuals exposed to tobacco smoke but were not active smokers.
Low-density lipoprotein was measured and not calculated.
Independent sample t-tests for continuous variables.
Chi-square test for categorical variable.
Indicates values are presented as mean ± SD.
Figure 1.
CONSORT flowchart of participant recruitment and follow-up.
Table 2 presents the change in the cardiometabolic risk factors within intervention and control groups over time and change between the groups. At the end of the study, the intervention group experienced a 1.8 cm mean reduction in waist circumference (p < 0.01) compared to a 0.5 cm mean reduction in the control group (p = 0.16). The overall difference between the groups was significant (p = 0.01). The difference in the body fat percentage was similar in the intervention group (0.7%) and in the control groups (1.0%) and, the differences between groups were not significant (p = 0.48). SBP significantly decreased in the intervention group (2.9 mmHg, p < 0.001), but not in the control group (0.8 mmHg, p = 0.26) resulting in a significant difference between the groups (p = 0.04). There was no significant decrease in DBP, fasting plasma glucose, serum cholesterol, serum triglycerides, HDL cholesterol and LDL cholesterol in either group.
Table 2.
Differences in cardiometabolic risk factors between intervention and control groups.
| Variable | Timeline | Intervention | Control | Difference between groupsb |
|---|---|---|---|---|
| Mean (SD) | Mean (SD) | p-value | ||
| Weight (kg) | Baseline | 78.2 ± 11.8 | 77.7 ± 11.8 | .01 |
| 12 weeks | 77.1 ± 11.4 | 77.3 ± 12.1 | ||
| Changea | −1.1 ± 3.0 | −0.3 ± 2.9 | ||
| p-valuec | <0.01 | 0.05 | ||
| Body mass index (kg/m2) | Baseline | 29.4 ± 3.9 | 29.3 ± 4.2 | .002 |
| 12 weeks | 29.0 ± 3.7 | 29.2 ± 4.4 | ||
| Changea | −0.4 ± 1.1 | −0.1 ± 1.1 | ||
| p-valuec | <0.01 | 0.17 | ||
| Waist circumference (cm) | Baseline | 97.8 ± 10.0 | 97.3 ± 10.2 | .01 |
| 12 weeks | 96.0 ± 8.8 | 96.8 ± 9.7 | ||
| Changea | −1.8 ± 5.6 | −0.5 ± 6.1 | ||
| p-valuec | <0.01 | 0.16 | ||
| Percent body fat (%) | Baseline | 35.2 ± 8.8 | 35.9 ± 9.2 | .48 |
| 12 weeks | 34.5 ± 8.5 | 35.0 ± 9.0 | ||
| Changea | −0.7 ± 5.5 | −1.0 ± 5.8 | ||
| p-valuec | 0.05 | <0.01 | ||
| SBP (mm/Hg) | Baseline | 120.9 ± 14.5 | 120.3 ± 14.4 | .04 |
| 12 weeks | 118.0 ± 13.6 | 119.5 ± 15.3 | ||
| Changea | −2.9 ± 11.1 | −0.8 ± 12.1 | ||
| p-valuec | <0.001 | 0.26 | ||
| DBP (mm/Hg) | Baseline | 81.1 ± 10.0 | 81.1 ± 10.2 | .17 |
| 12 weeks | 80.6 ± 10.0 | 81.6 ± 10.7 | ||
| Changea | −0.5 ± 8.1 | 0.5 ± 9.7 | ||
| p-valuec | 0.29 | 0.36 | ||
| FPG (mmol/L) | Baseline | 93.5 ± 10.6 | 93.4 ± 9.7 | .30 |
| 12 weeks | 93.2 ± 11.9 | 94.1 ± 13.5 | ||
| Changea | −0.4 ± 10.9 | 0.7 ± 12.7 | ||
| p-valuec | 0.60 | 0.36 | ||
| Serum cholesterol (mg/dL) | Baseline | 180.8 ± 33.7 | 182.5 ± 36.3 | .90 |
| 12 weeks | 180.3 ± 34.6 | 182.3 ± 35.4 | ||
| Changea | −0.5 ± 23.5 | −0.2 ± 25.3 | ||
| p-valuec | 0.74 | 0.88 | ||
| Serum TGL (mg/dL) | Baseline | 137.3 ± 59.4 | 132.8 ± 62.6 | .42 |
| 12 weeks | 135.2 ± 58.2 | 134.1 ± 62.3 | ||
| Changea | −2.2 ± 47.2 | 1.3 ± 50.7 | ||
| p-valuec | 0.46 | 0.68 | ||
| HDL (mg/dL) | Baseline | 40.5 ± 9.1 | 42.0 ± 10.3 | .44 |
| 12 weeks | 41.1 ± 9.0 | 42.1 ± 10.2 | ||
| Changea | 0.6 ± 6.9 | 0.1 ± 7.5 | ||
| p-valuec | 0.16 | 0.79 | ||
| LDL (mg/dL) | Baseline | 112.7 ± 29.7 | 114.0 ± 31.9 | .81 |
| 12 weeks | 111.7 ± 29.8 | 113.3 ± 30.4 | ||
| Changea | −1.1 ± 20.3 | −0.6 ± 22.4 | ||
| p-valuec | 0.40 | 0.64 |
SBP: systolic blood pressure; DBP: diastolic blood pressure; TGL: triglycerides; HDL: high density lipoprotein; LDL: low density lipoprotein.
A negative number is indicative or reduction.
Change = week 12 − baseline.
Analysis of variance (ANOVA).
Paired t-test.
Table 3 presents the changes in cardiometabolic risk factors (pre–post) between intervention and control groups in individuals at high risk for T2DM, classified as (a) individuals with both prediabetes and obesity and (b) individuals with obesity alone. The total number of individuals in these two cohorts equal to 533. We have not included individuals with prediabetes alone as the number of prediabetic individuals were too less to make a comparison.
Table 3.
Differences in cardiometabolic risk factors by high-risk groups.
| Parameters | Prediabetes and obese (n = 113) | Obese (n = 420) | ||||
|---|---|---|---|---|---|---|
| Interventiona (n = 51) |
Controla (n = 62) |
p-value | Interventiona (n = 212) |
Controla (n = 208) |
p-value | |
| Waist circumference (cm) | −1.5 ± 4.5 | 0.1 ± 6.3 | .14 | −1.8 ± 5.8 | −0.7 ± 5.9 | .05 |
| Weight (kg) | −0.6 ± 2.5 | 0.02 ± 2.0 | .12 | −1.1 ± 3.1 | −0.3 ± 3.0 | .004 |
| Body mass index (kg/m2) | −0.2 ± 1.0 | 0.02 ± 0.7 | .09 | −0.4 ± 1.1 | −0.1 ± 1.1 | .003 |
| Percent body fat (%) | −0.7 ± 5.8 | 0.5 ± 4.1 | .21 | −0.6 ± 5.4 | −1.2 ± 5.7 | .28 |
| Systolic blood pressure (mm/Hg) | −3.1 ± 9.8 | −0.6 ± 12.4 | .24 | −2.6 ± 11.3 | −0.8 ± 11.9 | .10 |
| Diastolic blood pressure (mm/Hg) | 0.4 ± 7.4 | 1.3 ± 8.5 | .56 | −0.6 ± 8.1 | 0.2 ± 9.8 | .37 |
| Fasting plasma glucose (mmol/L) | 4.2 ± 8.8 | 5.5 ± 10.7 | .49 | −2.2 ± 9.2 | −2.4 ± 7.8 | .80 |
| Serum cholesterol (mg/dL) | 2.3 ± 23.5 | 0.3 ± 22.9 | .63 | −1.4 ± 23.1 | −0.6 ± 25.6 | .75 |
| Serum triglycerides (mg/dL) | 10.9 ± 44.3 | −7.5 ± 56.5 | .06 | −6.0 ± 46.8 | 4.2 ± 46.6 | .03 |
| High density lipoprotein (mg/dL) | 0.9 ± 6.1 | 0.5 ± 5.0 | .70 | 0.3 ± 7.0 | −0.3 ± 8.2 | .43 |
| Low density lipoprotein (mg/dL) | −−1.1 ± 21.5 | 1.5 ± 18.0 | .47 | −0.9 ± 19.8 | −1.2 ± 23.2 | .88 |
Indicates that data were presented as mean ± SD.
In individuals who had both prediabetes and obesity, there was no statistically significant difference between the groups for any of the other variables. Among individuals with obesity, there was a statistically significant reduction in waist circumference (1.8 cm; p-value: 0.05) and serum triglycerides (6 mg/dL; p-value: 0.03) in the intervention group, compared to the control group (decrease in waist circumference: 0.7 cm; increase in serum triglycerides: 4.2 mg/dL).
Discussion
The aim of this paper was to look at changes in cardiometabolic risk factors after 12 weeks of intervention using mDiab, a mobile health application. Additionally, we also studied the changes in cardiometabolic risk factors within high-risk groups at 12 weeks of delivering the intervention.
The results of this study indicate that at 12 weeks of intervention, using a multi-modal mobile health application interlaced with human coach calls can lead to modest but significant reductions in waist circumference and SBP, but no changes in body fat, fasting plasma glucose, serum cholesterol, serum triglycerides, LDL and increase in HDL. However, owing to the small sample size more research is required with larger sample sizes in order to confirm these outcomes. In a similar study11 that used a multi-modal mHealth approach in individuals with prediabetes, the mean reduction in waist circumference in the intervention group was 4.56 cm (95% CI: –4.69 to–4.43) compared to 2.22 cm (95% CI: –2.36 to–2.09) in the control group (p < 0.001) supporting the waist circumference reduction in this intervention. However, these results were at 6 months of exposing the participants to the intervention. In another intervention12 that adapted the diabetes prevention program curriculum, the intervention group reported significantly better outcomes in BP and waist circumference compared to the control group. The population studied were all overweight individuals at risk for T2DM.
As the intervention period was short, these results suggest that changes may have been happening in the right direction and significant changes were only observed in markers most likely to change first The DESIR study13 that reported long-term consequences of change in waist circumference on cardiometabolic risk factors reported that a even a 3 cm decrease in waist circumference had a beneficial effect on cardiometabolic health and abdominally obese individuals were more receptive to interventions that targeted change in waist circumference. Longer-term follow-up studies are needed to see whether medium-term changes in cholesterol, triglycerides and lipoproteins were observed as a result of the mDiab intervention. Most of the improvements seemed to be greater in individuals with obesity. In other words, the intervention appears to be most effective among those classified as obese. This is also supported by the D-CLIP study results which reported that diabetes risk reduction was greater in individuals with obesity than in individuals with lower BMI.14 This is not surprising as those who have more weight to lose are the ones likely to do so. Within the control group, there was a statistically significant reduction of the body fat percentage but have not associated it with lesser reduction in waist circumference. However, if we had used other measurements of visceral adiposity such as calipers or a CT scan we could have made this association.
A study in Iran by Golshahi et al.15 that aimed at lowering cardiovascular risks, reported that providing the intervention face-to-face showed better improvements in BP than the use of a mHealth technology (SMS). In our study, the control arm participants who were provided usual care also had improvements in most of the biomarkers studied. Usual care in mDiab was one-time face-to-face counseling by a nutritionist on a healthy lifestyle including improving diet and physical activity. There is evidence to show that face-to-face diabetes prevention programs have better outcomes than mHealth interventions. The DMagic trial conducted in rural Bangladeshi population reported that face-to-face intervention worked better to prevent T2DM compared to the mHealth intervention.16
Some studies showed that just by using SMS, diabetes could be prevented in up to 30% of individuals with prediabetes.6 We are unable to reproduce those findings. The mHealth intervention that was delivered in this study had a multi-modal approach where the intervention was delivered by, video lessons, SMS, infographics and human health coach calls. Although it would be worth investigating the mode(s) of intervention that could have led to these improvements, it is safe to say that the use of a mHealth intervention that incorporates both a mHealth component and human interaction aided in alleviating cardiometabolic risks. This is supported by an intervention that tested the use of conventional (face-to-face) versus mobile versus blended for weight loss and reported that it is effective to use a combination of mHealth and face-to-face for efficacy.17
One of the strengths of this study was that the study used a randomized controlled trial design. Secondly, a wide panel of metabolic risk factors were measured. However, a short timeline of the study precludes assessment of the long-term sustainability of intervention effects and this was one of the limitations of the study. One of the study's main limitations was the high dropout rate (Intervention group – 28% and control group – 21%). Some of the reasons for this were changed phone numbers, workplaces and shifting to another locality. Although this study was unable to achieve the sample size reported in the methodology, a moderate effect was obtained with a power of 80%. The sample size was not directly powered to observe a difference in weight, cardiometabolic risk outcomes and improvements in physical activity. This was due to a lack of known population evidence from trials conducted in India at the time of sample size calculation. This limited the ability to perform more complex analysis, as extrapolating the data of an underpowered sample could result in exaggerated outcomes or false positives.
Another limitation is that the participants were primarily screened with a criterion of ‘owning an Android smartphone’ instead of looking into the electronic health (eHealth) literacy18 of participants, which might have enabled a better understanding of the population at hand. The dropout rate in the intervention group was greater than the control group. This may be a potential area for further research in order to understand plausible reasons for high dropout rates in mHealth studies. This may help strengthen the development of future mHealth intervention.
Mobile health interventions seem to be only modestly effective in reducing cardiometabolic risk. Individuals with obesity had a greater chance of showing improvements in a mHealth intervention. However, these results need to be interpreted with caution as it was observed in an urban population from three metropolis cities in India and hence cannot be generalized to the whole country as rural areas may respond differently.
While urbanization is related to the increasing prevalence of T2DM and obesity,19 future studies could explore the differential effects of a mHealth intervention between a rural and urban population. It is, however, important to remember that even the level of urbanicity and eHealth literacy can differ among cities in a massive country like India. Hence, some formative research to assess the eHealth literacy may help shape the intervention better. This will could potentially enhance participant engagement with the mHealth technology in use.
Conclusions
The global rising prevalence of obesity and T2DM needs to be curtailed using ways that are novel, adaptable, reproducible and sustainable. Our mobile health application (mDiab) shows that while it helped in reducing abdominal obesity (waist circumference) and in reducing SBP, these effects were modest and were not clinically significant. A long-term and more intensive mHealth intervention appears to be required to reduce cardiometabolic risk factors and for the prevention of diabetes.
Supplemental Material
Supplemental material, sj-docx-1-dhj-10.1177_20552076211039032 for Change in cardiometabolic risk factors among Asian Indian adults recruited in a mHealth-based diabetes prevention trial by Shruti Muralidharan, Harish Ranjani, Ranjit Mohan Anjana, Yashdeep Gupta, Samita Ambekar, Varsha Koppikar, N Jagannathan, Sidhant Jena, Nikhil Tandon, Steven Allender and Viswanathan Mohan in Digital Health
Footnotes
Contributorship: SJ, VM, HR, RMA and NT researched literature and conceived the study. HR, SA, SM, YG, SA, VK, JN was involved in protocol development, gaining ethical approval, patient recruitment and data analysis. SM wrote the first draft of the manuscript. All authors reviewed, edited the manuscript and approved the final version of the manuscript.
Declaration of conflicting of interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Ethical approval: The study was approved by the Institutional Ethics Committee at Madras Diabetes Research Foundation, All India Institute of Medical Sciences (Reference number: IEC/NP-232/05.02.2015) and the Human Research Ethics Committee at Deakin University, Australia (Reference number: DU-HREC 2015-167).
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship and/or publication of this article: This work was supported by the Government of India under the Biotechnology Industry Partnership Programme of the Department of Biotechnology.
Guarantor: VM.
ORCID iD: Shruti Muralidharan https://orcid.org/0000-0003-2254-0262
Supplemental material: Supplemental material for this article is available online.
References
- 1.International Diabetes Federation. IDF Diabetes atlas, 8th ed.Brussels, Belgium: International Diabetes Federation. 2017. [Google Scholar]
- 2.World Health Organization. Global status report on noncommunicable diseases 2014. Switzerland: World Health Organization. 2014. [DOI] [PubMed] [Google Scholar]
- 3.Muralidharan S, Ranjani H, Anjana R, et al. Mobile health technology in the prevention and management of type 2 diabetes. Indian J Endocrinol Metab 2017; 21: 334–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hartz J, Yingling L, Powell-Wiley TM. Use of mobile health technology in the prevention and management of diabetes mellitus. Curr Cardiol Rep 2016; 18: 130. [DOI] [PubMed] [Google Scholar]
- 5.Arora S, Peters AL, Agy C, et al. A mobile health intervention for inner city patients with poorly controlled diabetes: proof-of-concept of the TExT-MED program. Diabetes Technol Ther 2012; 14: 492–496. [DOI] [PubMed] [Google Scholar]
- 6.Ramachandran A, Snehalatha C, Ram J, et al. Effectiveness of mobile phone messaging in prevention of type 2 diabetes by lifestyle modification in men in India: a prospective, parallel-group, randomised controlled trial. Lancet Diabetes Endocrinol 2013; 1: 191–198. [DOI] [PubMed] [Google Scholar]
- 7.Weber MB, Ranjani H, Meyers GC, et al. A model of translational research for diabetes prevention in low and middle-income countries: the diabetes community lifestyle improvement program (D-CLIP) trial. Prim Care Diabetes 2012; 6: 3–9. [DOI] [PubMed] [Google Scholar]
- 8.Muralidharan S, Mohan V, Anjana RM, et al. Mobile health technology (mDiab) for the prevention of type 2 diabetes: protocol for a randomized controlled trial. JMIR Res Protoc 2017; 6: e242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet 2004; 363: 157–163. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. 2. Classification and diagnosis of diabetes: standards of medical care in diabetes—2019. Diabetes Care 2019; 42(Suppl 1): S13–S28. [DOI] [PubMed] [Google Scholar]
- 11.Block G, Azar KM, Romanelli RJ, et al. Diabetes prevention and weight loss with a fully automated behavioral intervention by email, web, and mobile phone: a randomized controlled trial among persons with prediabetes. J Med Internet Res 2015; 17: e240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fukuoka Y, Gay CL, Joiner KL, et al. A novel diabetes prevention intervention using a mobile app: a randomized controlled trial with overweight adults at risk. Am J Prev Med 2015; 49: 223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Balkau B., Picard P, Vol S, et al. Consequences of change in waist circumference on cardiometabolic risk factors over 9 years. Diabetes Care 2007; 30: 1901–1903. [DOI] [PubMed] [Google Scholar]
- 14.Weber MB, Ranjani H, Staimez LR, et al. The stepwise approach to diabetes prevention: results from the D-CLIP randomized controlled trial. Diabetes Care 2016; 39: 1760–1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golshahi J, Ahmadzadeh H, Sadeghi M, et al. Effect of self-care education on lifestyle modification, medication adherence and blood pressure in hypertensive adults: randomized controlled clinical trial. Adv Biomed Res 2015; 4: 204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fottrell E, Ahmed N, Morrison J, et al. Community groups or mobile phone messaging to prevent and control type 2 diabetes and intermediate hyperglycaemia in Bangladesh (DMagic): a cluster-randomised controlled trial. Lancet Diabetes Endocrinol 2019; 7: 200–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hurkmans E, Matthys C, Bogaerts A, et al. Face-to-face versus mobile versus blended weight loss program: randomized clinical trial. JMIR Mhealth Uhealth 2018; 6: e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Norman CD, Skinner HA. Ehealth literacy: essential skills for consumer health in a networked world. J Med Internet Res 2006; 8: e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Allender S, Lacey B, Webster P, et al. Level of urbanization and noncommunicable disease risk factors in Tamil Nadu, India. Bull World Health Organ 2010; 88: 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-dhj-10.1177_20552076211039032 for Change in cardiometabolic risk factors among Asian Indian adults recruited in a mHealth-based diabetes prevention trial by Shruti Muralidharan, Harish Ranjani, Ranjit Mohan Anjana, Yashdeep Gupta, Samita Ambekar, Varsha Koppikar, N Jagannathan, Sidhant Jena, Nikhil Tandon, Steven Allender and Viswanathan Mohan in Digital Health